0920-1238 EDN Tuberculosis Follow-Up Worksheet for Newly-Arrived P
[NCEZID] US Tuberculosis Follow-up Worksheet for Newly Admitted Persons with Overseas Tuberculosis Classifications
2023 EDN TB Follow-Up Worksheet_Revised_Clean
EDN TB Follow-up Worksheet
OMB: 0920-1238
Form approved
OMB Control
No. 0920-1238
Expiration Date 03/31/2026 The
EDN
Tuberculosis
Follow-Up
Worksheet
for
Newly-Arrived
Persons
with
Overseas
Tuberculosis
Classifications
A.
Demographic
A1.
Name
(Last,
First,
Middle):
A2.
Alien #:
A3.
Visa
type:
A4.
Initial
U.S.
entry
date:
A5. Age:
A6. Sex:
A7. DOB: / /
A8.
TB
Class
Based
on
Technical
Instructions
for
Panel
Physicians:
A9. Country of examination:
A10. Country of birth:
A11a. Name
in care
of:
A11b. Phone
number: A11c. Address:
A12a. Sponsor
agency name:
A12b. Phone
number: A12c. Address:
B.
Jurisdictional Information
B1. Arrival
jurisdiction: B2.
Current jurisdiction:
C.
U.S.
Evaluation
C1. Date of first U.S. test or provider/clinic visit:
/ /
Mantoux
Tuberculin Skin Test
(TST) in
U.S.
Interferon-Gamma
Release Assay (IGRA)
in U.S.
C2a. Was a
TST administered in
the U.S.? Yes No Unknown If
YES,
C2b.
TST placement date:
/ / Placement
date unknown
C2c. TST mm: Unknown
C2d. TST interpretation:
Positive Negative
Unknown C2e.
History of Previous Positive TST: Yes No Unknown
C3a. Was
IGRA performed
in the
U.S.? Yes No Unknown If
YES,
C3b. Date collected:
/ / Date
unknown IUs/Spots C3c.
IGRA brand:
QuantiFERON® T-SPOT
Other, specify:
C3d.
Result: Positive Negative Indeterminate,
Borderline, or Invalid Unknown Equivocal C3e.
History of previous positive IGRA: Yes No Unknown
U.S
Review
of Pre-Immigration/I-693 CXR
U.S.
Domestic
CXR
Comparison
C4. Pre-immigration
CXR/I-693 available?
Yes No Unknown
C6a. U.S.
domestic CXR
done? Yes No Unknown If
YES,
C6b.
Date of U.S. CXR:
/ /
C8. U.S. domestic CXR comparison to pre-immigration/I-693
CXR:
Stable Worsening Improving
Unknown
C5.
U.S. interpretation of
pre-immigration/I-693 CXR:
Normal (Negative
for TB) Abnormal
Suggestive of TB Non-TB
Condition Poor
Quality/Not Interpretable Unknown
C7.
Interpretation of U.S.
CXR:
Normal (Negative
for TB) Abnormal
Suggestive of TB Non-TB
Condition Poor
Quality/Not Interpretable Unknown
Public
reporting
burden
of
this
collection
of
information
is
estimated
to
average 30
minutes
per
individual,
including
the
time for
reviewing
instructions,
searching
existing
data
sources,
gathering
and
maintaining
the
data
needed,
and
completing
and reviewing
the
collection
of
information.
An
agency
may
not
conduct
or
sponsor,
and
a
person
is
not
required
to
respond
to
a collection of
information unless it displays a currently valid OMB control
number. Send comments regarding this burden estimate
or
any
other
aspect
of
this
collection
of
information,
including
suggestions
for
reducing
this
burden
to
CDC/ATSDR
Information Collection Review Office, 1600 Clifton Road NE, MS
D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-1238).



















































C9a. Completed
treatment pre-immigration/I-693?
Yes
No
Unknown
If YES,
C9b. Treated for TB disease Treated
for LTBI Treated,
but unknown if TB disease or LTBI If
Treated
for TB disease,
Treatment completed prior
to panel physician
or civil surgeon examination Treatment
completed
after
panel
physician or
civil surgeon
diagnosis
(DS
3030)
At DGMQ-designated
DOT site At
non-DGMQ-designated DOT
site Other,
specify:
C9c. Treatment start date:
/ /
Start
date
unknown C9d. Treatment end date:
/ / End
date unknown
C9e. Report
of treatment
administered prior
to panel
physician or civil surgeon examination: Treatment
documented on
overseas medical history
form (DS 3026)
Documented on
DS forms
& patient
reported at
panel physician
or civil surgeon examination
After U.S.
arrival only,
patient verbally
reported treatment completion Unknown
The
EDN
Tuberculosis
Follow-Up
Worksheet
for
Newly-Arrived
Persons
with
Overseas
Tuberculosis
Classifications
Alien
#
U.S.
Review of
Pre-Immigration/I-963 Treatment
C9f. Standard TB treatment regimen was administered? Standard
TB
treatment Non-standard
TB treatment Unable
to verify C10a.
Arrived to
the U.S.
on treatment?
Yes No
Unknown If
YES,
C10b. Treated
for TB
disease Treated
for
LTBI
C10c. Start date: / / Start
date
unknown C11a: Pre-Immigration/I-693
treatment concerns?
Yes No
If
YES,
C11b.
Select
all
that
apply:
Treatment duration
too short Incorrect treatment regimen
Inadequate information
provided
Lack of adequate diagnostics Unknown DOT/adherence status
Undocumented/unverified
treatment
Other, specify:
C12.
U.S.
Microscopy/Bacteriology* Sputa
collected in U.S.? Yes No
*Covers
all
results
regardless
of
sputa
collection
method.
#
Date Collected
AFB Smear
Sputum Culture
Drug Susceptibility Testing
1
/ /
Positive Negative Not
Done Unknown
NTM
Contaminated Not Done
MTB Complex Negative
Unknown
MDR-TB
Mono-INH No DR
Mono-RIF Other
DR Not Done
2
/ /
Positive Negative Not
Done Unknown
NTM
Contaminated Not Done
MTB Complex Negative
Unknown
MDR-TB
Mono-INH No DR
Mono-RIF Other
DR Not Done
3
/ /
Positive Negative Not
Done Unknown
NTM MTB Complex Contaminated Negative Not
Done Unknown
MDR-TB Mono-RIF Mono-INH Other
DR No
DR Not
Done
D.
Evaluation Disposition
in U.S.
D1a. Evaluation disposition date in U.S.:
/ / D1b.
State/jurisdiction of
evaluation disposition
in U.S.:
D2a.
Evaluation disposition
in U.S.:
Completed evaluation
D2b.
If
evaluation
was
completed,
was treatment recommended? Yes No LTBI Active
TB
Initiated Evaluation / Not completed Did
not initiate evaluation D2c.
If
evaluation
was
NOT
completed,
why
not?
Select
all
that
apply. Not
Located Moved
within
U.S.,
transferred
to:
State/jurisdiction
Lost to
Follow-Up Moved
outside U.S. Refused
Evaluation Died Unknown Other,
specify:
D3. Diagnosis Class
0 - No TB
exposure, not infected
or Class 1
- TB exposure, no
evidence of infection Class
2 - TB infection, no disease Class
3 - TB, TB disease Class
4 - TB, inactive disease Pulmonary Extra-pulmonary Both
sites Culture-confirmed Yes No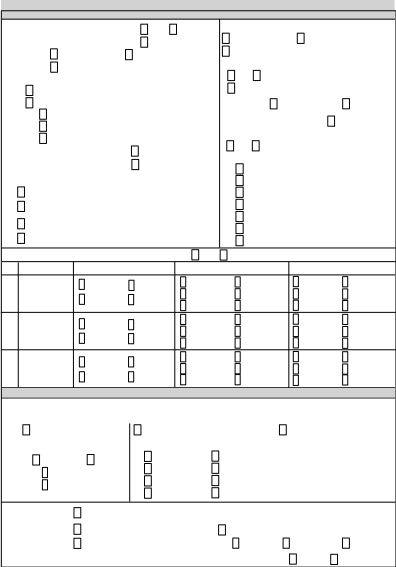















































































































The
EDN
Tuberculosis
Follow-Up
Worksheet
for
Newly-Arrived
Persons
with
Overseas
Tuberculosis
Classifications
Alien
#
D4.
If
diagnosed
with
TB
disease:
State Case
Number: RVCT
# unknown* RVCT
Reported* Year State RVCT
# /
TBLISS
# TBLISS
# unknown* TBLISS
Reported*
City/County Case
Number: Year State RVCT
# /
TBLISS # *Note:
Either the RVCT
or TBLISS number
may be reported.
E.
U.S.
Treatment
for
TB
Disease
or
TB
Infection
E1a. U.S.
treatment
initiated: Yes No Unknown E1b.
If
NO,
specify
the
reason.
Select
all
that
apply:
Patient declined
against medical
advice Lost
to follow-up Moved
within
U.S.,
transferred
to:
State/jurisdiction
Died Moved
outside
the
U.S. Prior
treatment completed
(year: ) Currently
on treatment Treatment
not offered
based on Unknown Contraindication
for treatment local
clinic
guidelines Other,
specify: E1c.
If
YES: Treated
for
TB
disease Treated
for LTBI E2.
Treatment start date: / / E3.
State/jurisdiction
of
treatment
in
U.S.:
E4. Specify
initial LTBI
regimen: Isoniazid (9
months; 9H)
Isoniazid (6
months; 6H)
Isoniazid/Rifapentine (3 months; 3HP) Isoniazid/Rifampin
(INH+RIF; 4
months) Rifampin (4 months; 4R)
Isoniazid/Rifampin/Ethambutol/Pyrazinamide
(RIPE; 2
months; suspected
TB disease)
Unknown
Other, specify: E5a.
U.S. treatment
completion status*
and dates: Completed
/ / Treatment
ongoing Treatment
discontinued/stopped
/ / Unknown
*Completed
refers
to
finished
treatment,
Treatment
ongoing
refers
to
treatment
that
is
initiated
but
not
yet
completed.
Treatment
discontinued/stopped
refers
to
initiated
treatment that is not completed. If
treatment
discontinued/stopped,
E5b.
Specify
the
reason.
Select
all
that
apply:
Patient declined
against medical
advice Lost
to
follow-up Moved
within
U.S.,
transferred
to:
Died Unknown State/
jurisdiction
Moved outside
the U.S.
Dying (treatment
stopped because Adverse
effect Other,
specify:
of imminent death,
regardless of
cause
of death) Not
TB disease Developed
TB [For
Provider decision Pregnancy
[For patient patient
diagnosed with
diagnosed with
LTBI] LTBI]
F.
Evaluation
Site
Information
G.
Treatment
Site
Information
Provider’s Name: Clinic Name: Telephone
Number:
Provider’s Name: Clinic
Name: Telephone
Number: Same
as evaluation
site information
H.
Comments




















































| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | WorksheetGen |
| Author | Dam, Amanda (CDC/NCEZID/DGMH) (CTR) |
| File Modified | 0000-00-00 |
| File Created | 2023-10-13 |
© 2026 OMB.report | Privacy Policy