Fast Track Supporting Statement
Fast Track Submission_System Usability Survey_101818.doc
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery
Fast Track Supporting Statement
OMB: 0935-0179
Request for Approval under the “Generic Clearance for the Collection of Routine Customer Feedback” (OMB Control Number: 0935-0179)
T ITLE
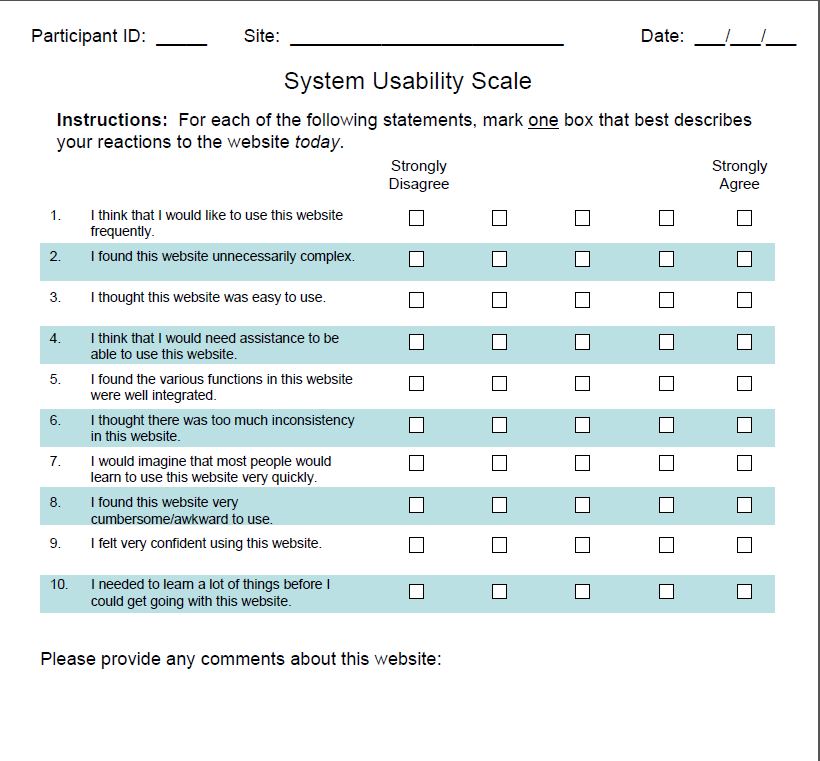
OF INFORMATION COLLECTION: System Usability Scale (SUS)
ITLE
OF INFORMATION COLLECTION: System Usability Scale (SUS)
PURPOSE The SUS is a brief questionnaire which is considered as the industry standard for evaluating usability of hardware, software, mobile devices, websites, and applications. The PRO app will be programmed to include the prompt requiring the 180 patients to complete the SUS right after they submit their PROMIS data.
DESCRIPTION OF RESPONDENTS:
Providers noted that data from a PRO app would be most useful when the PRO data are likely to have clinical significance and can guide treatment of the patient. As the SUS will be conducted following app usage, only patients who have completed the app will be eligible to complete the SUS.
TYPE OF COLLECTION: (Check one)
[ ] Customer Comment Card/Complaint Form [ ] Customer Satisfaction Survey
[ X] Usability Testing (e.g., Website or Software) [ ] Small Discussion Group
[ ] Focus Group [ ] Other: ______________________
CERTIFICATION:
I certify the following to be true:
The collection is voluntary.
The collection is low-burden for respondents and low-cost for the Federal Government.
The collection is non-controversial and does not raise issues of concern to other federal agencies.
The results are not intended to be disseminated to the public.
Information gathered will not be used for the purpose of substantially informing influential policy decisions.
The collection is targeted to the solicitation of opinions from respondents who have experience with the program or may have experience with the program in the future.
Name:_______Alexandra Burn___________
To assist review, please provide answers to the following question:
Personally Identifiable Information:
Is personally identifiable information (PII) collected? [ ] Yes [X ] No
If Yes, will any information that is collected be included in records that are subject to the Privacy Act of 1974? [ ] Yes [ ] No
If Yes, has an up-to-date System of Records Notice (SORN) been published? [ ] Yes [ ] No
Gifts or Payments:
Is an incentive (e.g., money or reimbursement of expenses, token of appreciation) provided to participants? [ ] Yes [ X ] No
BURDEN HOURS
Category of Respondent |
No. of Respondents |
Participation Time |
Burden |
Individuals |
180 |
5/60 |
14.4 |
Totals |
|
|
14.4 |
FEDERAL COST: The estimated annual cost to the Federal government is $350.50__
If you are conducting a focus group, survey, or plan to employ statistical methods, please provide answers to the following questions:
The selection of your targeted respondents
Do you have a customer list or something similar that defines the universe of potential respondents and do you have a sampling plan for selecting from this universe? [X ] Yes [ ] No
If the answer is yes, please provide a description of both below (or attach the sampling plan)? If the answer is no, please provide a description of how you plan to identify your potential group of respondents and how you will select them?
The universe of targeted respondents for the SUS is encompassed by those patients who have completed the OBERD PRO app. Given the focus on the physical function PROMIS measure for this pilot test the target population that may complete the app and thereby be eligible to participate in the SUS will include:
65+ (practices can target patients based on identifying Medicare enrollment)
Post-procedure, rehabilitating patients of any age
English proficient (the pilot app and interview will only be available in English
Administration of the Instrument
How will you collect the information? (Check all that apply)
[ ] Web-based or other forms of Social Media
[ ] Telephone
[ ] In-person
[ X ] Other, Explain: The SUS will be programmed on the app for patients to complete right after they answer the PROMIS questions.
Will interviewers or facilitators be used? [X ] Yes [ ] No
Please make sure that all instruments, instructions, and scripts are submitted with the request.
A
Form
Approved
OMB No. 0935-0179
Exp. Date 11/30/2020
Public
reporting burden for this collection of information is estimated to
average 5
minutes per response, the estimated time required to complete
the survey. An agency may not conduct or sponsor, and a person
is not required to respond to, a collection of information unless it
displays a currently valid OMB control number. Send
comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden, to: AHRQ Reports Clearance Officer Attention: PRA,
Paperwork Reduction Project (0935-0179) AHRQ, 5600 Fishers Lane, #
07W41A, Rockville, MD 20857.
| File Type | application/msword |
| File Title | Fast Track PRA Submission Short Form |
| Author | OMB |
| Last Modified By | SYSTEM |
| File Modified | 2018-10-29 |
| File Created | 2018-10-29 |
© 2026 OMB.report | Privacy Policy