0920-1357 GAIN Study Visit Survey - Revised 09SEP2022
[NCHHSTP] The GAIN (Greater Access and Impact with NAT) Study: Improving HIV Diagnosis, Linkage to Care, and Prevention Services with HIV Point-of-Care Nucleic Acid Tests (NATs)
Att 11_GAIN study visit survey_screenshots_changes accepted_082922
Participants in Cross-sectional Comparison of Several POC NATs - Study Visit Survey
OMB: 0920-1357
Form
Approved
OMB
No. 0920-1357
Expiration
Date: 12/31/2024
Public
reporting burden of this collection of information is estimated to
average 15 minutes per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information. An agency may not conduct or sponsor,
and a person is not required to respond to a collection of
information unless it displays a currently valid OMB control number.
Send comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton
Road NE, MS D-74, Atlanta, Georgia 30333; Attn: OMB-PRA (0920-1357)

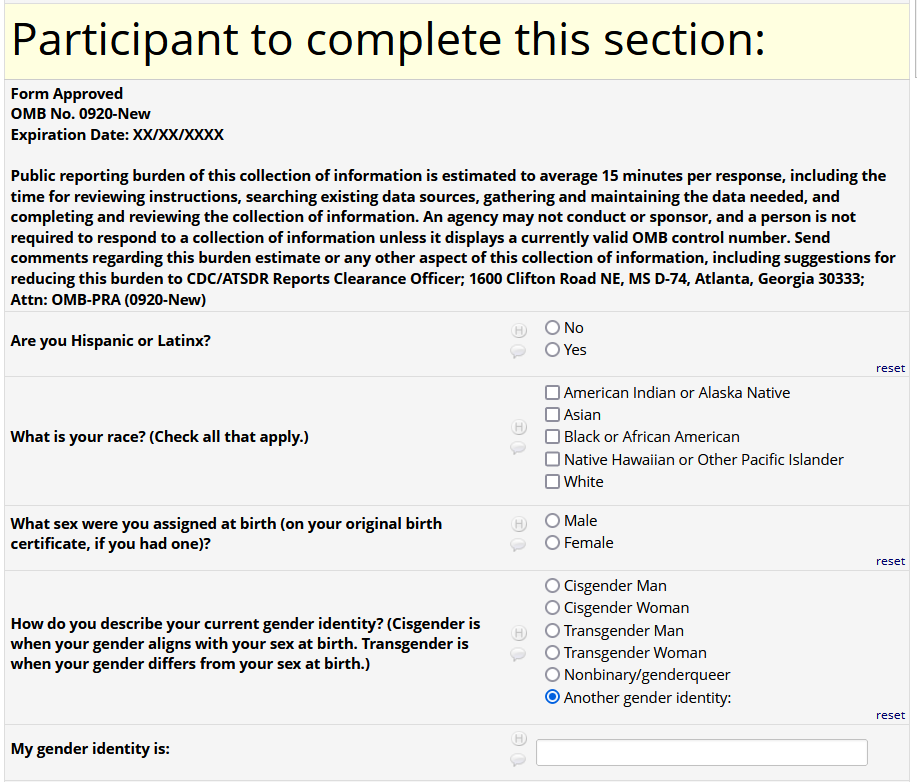

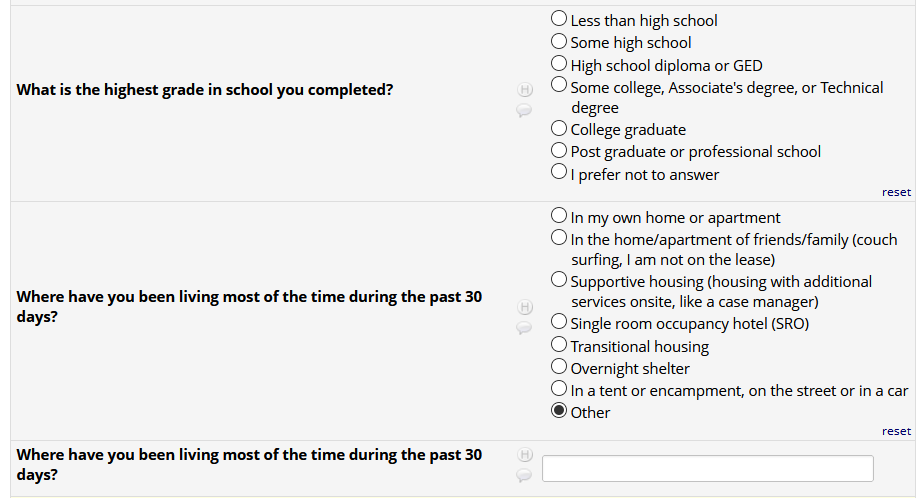

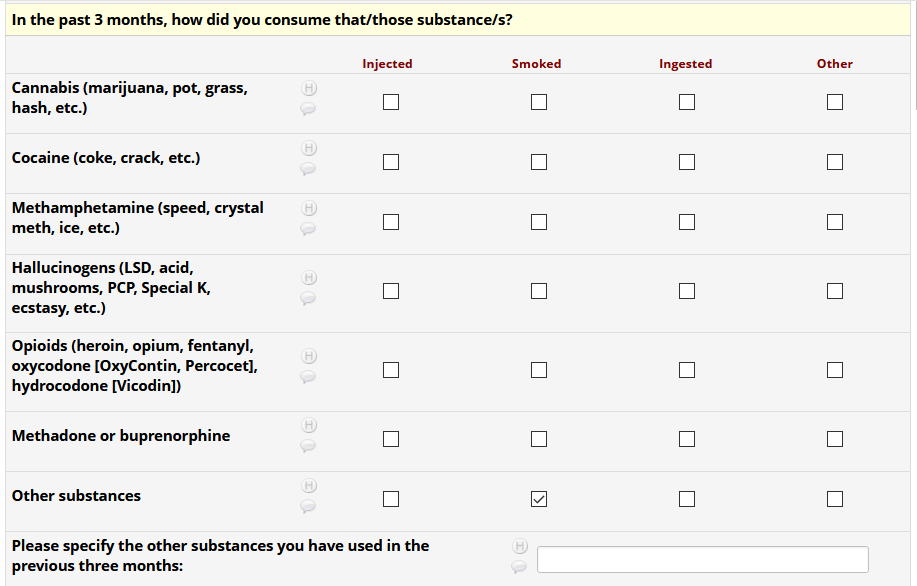
If HIV status negative or unknown:
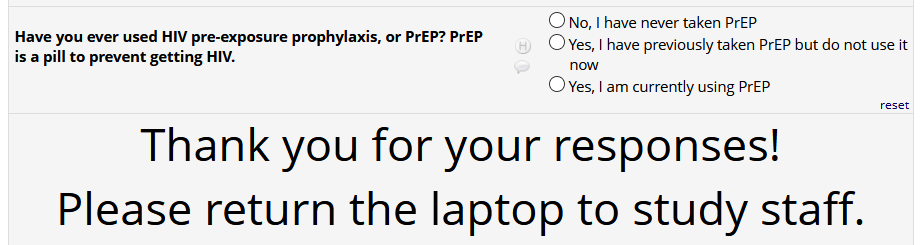
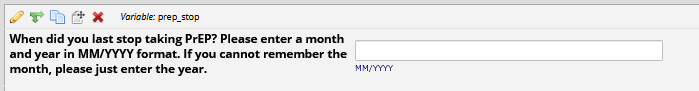
 If
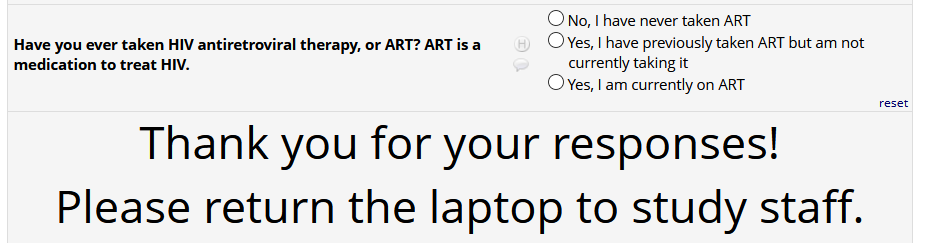
HIV status positive:
If
HIV status positive:
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lisa A Niemann |
| File Modified | 0000-00-00 |
| File Created | 2023-11-03 |
© 2026 OMB.report | Privacy Policy