Change Request September 2023
Change Request OMB Memo September 2023.docx
Premarket Tobacco Product Applications and Recordkeeping Requirements
Change Request September 2023
OMB: 0910-0879
US Food and Drug administration
Center for Tobacco Products
“Premarket Tobacco Product Applications and Recordkeeping Requirements”
(OMB Control Number 0910-0879)
Change Request Collection Overview and Justification
Date: September 2023
The Food and Drug Administration (FDA)/Center for Tobacco Products (CTP) is submitting this non substantive change request for updates to the Premarket Tobacco Product Application (PMTA) forms to assist industry applicants to complete their submissions more efficiently and accurately. There are two updated forms included in this change request, Form FDA 4057, Form FDA 4057b, and one new voluntary Tobacco Product Grouping Spreadsheet Validator tool. The results of these changes are an overall net decrease in the burden to this collection.
This change request is organized into one section for each specific item in the collection. As a collective group, the updates made to the two forms and the new validator tool are an effort by FDA to reduce the administrative burden on both the industry applicants and FDA. These new and updated resources for PMTA submissions will:
Increase FDA compliance with the Plain Writing Act of 2010
Improve the data quality for PMTA submissions leading to more accurate reporting, analysis, and data management
Minimize the chances of PMTA submission being rejected due to incomplete or incorrect data
Reduce the amount of time that FDA and industry applicants spend reviewing and correcting submission information and product data
Supporting Statement edits

Justification and Change Log for Form FDA 4057
Justification:
The changes to Form FDA 4057 will allow industry users to complete this form more efficiently and accurately. With a more accurately completed Form FDA 4057, it will reduce the time and effort for CTP to process data from the form into our product data and review systems. CTP seeks to be good stewards of the public’s time and mitigate confusion with duplicative requests for information. Our reorganization of the form was done in the spirit of the Paperwork Reduction Act’s goal to reduce the amount of time it will take industry applicants to complete this form.
The content in Form FDA 4057 has not significantly changed. We have removed fields that led to duplicate data collection and reorganized the form to follow a more logical and sequential flow for the applicant. For example, we have consolidated all applicant, representative, and point of contact data collection into one section. Previously, these data fields were spread throughout the form in a way that was not logical to the industry applicant.
The structural and data field changes listed below reduced the length and complexity of the form and moved most of the detailed instructions and completion tips to the appendices to be used a resource without confusing or burdening the industry applicant.
Additionally, some of the language used on the existing form and in the instructions were unclear. To adhere to the requirements of the Plain Writing Act of 2010, the form has been updated to be clearer and more concise for the industry applicant to understand and complete all required fields correctly.
We anticipate a reduction of ten minutes per response in burden hours to complete Form FDA 4057 as a result of these form updates.
Form FDA 4057 Change Log:
Structural Changes:
Part Headers
Added “Part [X]:” to part headers
E.g. “Applicant Information” -> “Part A: Applicant Information”
Changed background color to blue to distinguish levels (e.g., Section, Part, etc.)
Added relevant instructions in italics
![]()
![]()
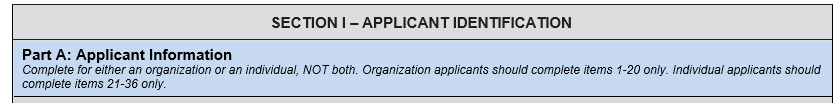
Separated “Name of Applicant” section’s fields into separate sections: 1) “If applicant is an organization…” and 2) “If applicant is an individual…”
Created a numbering system
Numbered every data field; numbering restarts with each new Part (Part A: 1-36, Part B: 1-18, etc.)
Updated PRA related information:
Average burden time was updated from 45 minutes to 35 minutes
Page number of PRA Statement is located within the form was updated from 22 to 29
![]()
-->
![]()
Added warning and instructions to beginning of form
“Marketing without a Marketing Granted Order (MGO) is illegal and may be subject to enforcement.”
“Please carefully read the instructions located in the Appendix before completing this form.”
Updated language of second footnote and cross-referenced fields for both first and second footnotes
Previous second footnote language:
“FDA generally expects that product samples will be a required part of a PMTA and that an applicant should be prepared to submit them in accordance with FDA instructions within 30 days after submitting a PMTA; however there may be situations in which sample submission may not be necessary (see Appendix C for additional information)”
Updated second footnote language:
“FDA generally expects that product samples will be a required part of a PMTA and that an applicant should be prepared to submit them in accordance with FDA instructions.”
Data Field Changes:
SECTION I: APPLICATION IDENTIFICATION |
||||||
Original Field |
New Field and Location |
Description of Change |
||||
N/A |
Other Organization Names (if applicable) Part A.2 |
Added a new field to list out other names the organization may use or be known by |
||||
Date of Submission |
Submit Date Part. A.5,24 |
Changed to “Submit Date” for language clarity |
||||
Company Headquarters’ FDA-assigned Facility Establishment Identifier (FEI) Number |
Organization Headquarters’ FDA-assigned Facility Establishment Identifier (FEI) Number Part A.3 Part D.3 Part E.3 |
Changed “company” to “organization” for consistent use of “organization” throughout the form. |
|
|||
Company Headquarters’ D&B DUNS® Number |
Organization Headquarters’ D&B DUNS® Number Part A.4. Part D.4 Part E.4. |
Changed “company” to “organization” for consistent use of “organization” throughout the form. |
|
|||
Primary Address (Street Address, P.O. Box) |
Street Address Line 1 Part A.6,28 Part B.10 Part C.10 Part D.6 Part E.7 |
Deleted details that are specified in instructions. Changed name of field to reduce confusion about “primary”. |
|
|||
Telephone (Include Country Code if applicable) |
Phone Number Part A.18, 34 Part B.16 Part C.15 Part D.18 Part E.19
|
Deleted details that are specified in the instructions. Updated language for clarity. |
|
|||
FAX |
Fax Number Part A.19,35 Part B.16 Part C.16 Part D.19 Part E.20 |
Updated language for clarity. |
|
|||
M.I. |
Middle Initial Part A.13,22 Part B.4 Part C.3 Part D.13 Part E.14 |
Wrote out the abbreviation “M.I.” for clarity thereby reducing the need for additional content in the instructions. |
|
|||
Prefix (e.g., Mr., Ms., Dr.) |
N/A |
Deleted field |
||||
Generational Suffix (e.g., Jr., III) |
Generational Suffix Part A.15,25 Part B.6 Part C.5 Part D.15 Part E.16 |
Deleted details that are specified in instructions |
||||
Professional Suffix (e.g., MD, Ph.D.) |
Professional Suffix Part A.16,26 Part B.7 Part C.6 Part D.16 Part E.17 |
Deleted details that are specified in instructions |
||||
Authorized Representative Information (Page 2) + U.S. Agent Information (Page 3) |
“Select if authorized representative or U.S. agent is same…” Part B.1 |
Added a checkbox for applicant to indicate if the authorized representative or U.S. agent is the same as applicant. If the same, this will eliminate data collection duplication. |
|
|||
Authorized Representative Information (Page 2) + U.S. Agent Information (Page 3) |
Authorized Representative or U.S. Agent Information Part B.2 |
Combined Authorized Representative Information and U.S. Agent Information into one section to reduce duplicative data gathering. An applicant will have either an authorized representative or U.S. agent so there is no need to have two sections for data collection. |
|
|||
Alternate Point of Contact |
Part C. Alternate Point of Contact Information (Optional) |
Updated section title to indicate that this section is optional. |
|
|||
Alternate Point of Contact checkboxes |
Select alternate: Part C.1 |
Revised checkboxes to put “Manufacturer (Other than Applicant), “Other, Regulatory”, “Other, Technical” into one “Other” checkbox category. “Manufacturer (Other than the Applicant)” point of contact option was moved under Part D.12-20. |
|
|||
Manufacturer Information |
Part D: Manufacturer Information Part D.1 |
Added a checkbox for applicant to indicate if manufacturer is the same as applicant. If the same, this will eliminate data collection duplication. |
|
|||
Manufacturer Information |
Part D: Manufacturer Information |
Manufacturer information was moved after Parts A-C to create a logical hierarchy for contact information for the applicant. Manufacturer information was also moved to group it sequentially with Part E: Manufacturer/Packaging/Sterilization Sites Information |
|
|||
Section V – Manufacturing/Packaging/Sterilization Sites Relating to a Submission |
Part E: Manufacturer/Packaging/Sterilization Sites Information |
Manufacturer/Packaging/Sterilization Sites Information was moved to Section I – Applicant Identification because the data collected is contact information and should be grouped with other sections collecting similar information. |
|
|||
Section V – Manufacturing/Packaging/Sterilization Sites Relating to a Submission – Company/Institution Name |
Organization Name Part E.2
|
Changed “company” to “organization” for consistent use of “organization” throughout the form. |
|
|||
Section V – Manufacturing/Packaging/Sterilization Sites Relating to a Submission – Checkbox question “The Manufacturing/Packaging/Sterilization Site is ready for inspection” |
Is the manufacturing/packaging/sterilization site ready for inspection? Part E.6 |
Reformatted to a question to match the yes/no checkboxes. Moved the question to middle of section to reduce likelihood it is overlooked. |
|
|||
SECTION II: NEW TOBACCO PRODUCT INFORMATION |
|
|
||||
All fields within Section II (e.g., “Check here if you are submitting a co-packaged product.”, “New Tobacco Product Name”, “Product Category/Sub-Category:” |
N/A |
All fields were deleted in Section II and were replaced with instructions to complete required Form FDA 4057b – Premarket Tobacco Product Application Grouping Product Submission Spreadsheet. Form FDA 4057b is now where applicants will provide new product information. |
|
|
||
SECTION III: SUBMISSION INFORMATION |
|
|
||||
Specify submission type – Select one submission type |
Part A: General Submission Information |
Changed “Specify submission type” and checkboxes for “This PMTA is for an individual new tobacco product” and “This is a group of PMTAs covering multiple new tobacco products” to “Part A: General Submission Information.” |
|
|
||
(check only one)
|
N/A |
Deleted checkboxes. |
|
|
||
For products that were previously commercially marketed in the U.S., provide the product names and corresponding marketing date(s):
|
For products that were previously commercially marketed in the U.S., provide the product names and corresponding marketing date(s): Part A.2
|
Moved question from end of Section III to second question in Part A (Part A.2) to keep like questions together. Updated question text for clarity. Old: For products that have been previously commercially marketed in the U.S., please list the date(s) during which the tobacco product was marketed. New: For products that were previously commercially marketed in the U.S., provide the product names and corresponding marketing date(s):
|
|
|
||
Cross-referenced Content |
Cross-reference information (Optional) Part B |
Table contents and layout changed. Language and layout updated for clarity. Contents/questions updated to capture relevant information in a cleaner and clearer format. |
|
|
||
Related Submissions |
N/A |
Deleted field – Related Submission question block |
|
|
||
N/A |
Referenced Tobacco Product Master File(s) (TPMF) (Optional) Part C |
Added “Referenced Tobacco Product Master File(s) (TPMF) (Optional)” question block with the following new fields to capture relevant information in a table format. New table and layout created for ease of understanding and clarity of language consisting of five questions and a combination of open text fields and yes/no checkboxes as response options. Three tables with identical questions provided for applicant entry. |
|
|
||
N/A |
Referenced Tobacco Product Master File(s) (TPMF) (Optional) Part C |
New questions/items being collected in Part C: C.1. TPMF Owner (open text response) C.2. TPMF STN (assigned by FDA): (open text response) C.3. Is the content applicable to all products within this submission? (Yes/No (list all applicable product name(s)): checkbox and open text response) C.4. Information and sections to be referenced (e.g., all sections, section I-III) (open text response) C.5. Right of reference included (Yes/No checkbox response) |
|
|
||
Formal Meetings Held with FDA pertaining to this tobacco product (For each meeting, as needed, enter the STN number and meeting held date.) |
Part D. Formal Meetings Held with FDA Pertaining to the New Product(s) (Optional) |
Reformatted table layout and removed fields not necessary as it relates the Formal Meeting Held information. Updated “Select if this Meeting is relevant to all bundled products” with checkbox response to |
|
|
||
New Tobacco Product Name (Provide product name if meeting is relevant to a specific product) |
N/A |
Deleted Field |
|
|
||
Select if this Meeting is relevant to all bundled products |
3. Is the meeting relevant to all products within this submission? Part D.3 |
Updated “Select if this Meeting is relevant to all bundled products” with checkbox response to “Is the meeting relevant to all products within this submission?” Response type changed from single checkbox to Yes/No (list all applicable product name(s)): checkbox with open text field entry. |
|
|
||
For products that have been previously commercially marketed in the U.S., please list the date(s) during which the tobacco product was marketed. |
Part A.2 |
Moved this field to Part A, keeping with similar question. Minimal Update to question text for clarity. |
|
|
||
SECTION IV: APPLICATION CONTENTS |
|
|
||||
Section IV – Application Contents
|
Section IV – Application Contents
|
Reformatted table: Added headers and highlighting to provided distinction between parts. Added “Location” field to indicate file name and location of application contents under each application content item Combined “Comprehensive Index” and “Table of Contents” for consistency with other FDA CTP forms. (Part A.2.) Removed New parts are as follows: Administrative --> Part A: Administrative Content Labeling and Marketing Plans --> Part B: Labeling and Marketing Plans Inspections --> Part C: Inspections Scientific Content --> Part D: Scientific Content
|
|
|
||
SECTION V: STATEMENT OF COMPLIANCE WITH THE FEDERAL FOOD, DRUGS AND COSMETIC (FD&C) ACT |
|
|
||||
Section VI – Statement of Compliance with the Federal Food, Drug and Cosmetic (FD&C) Act |
Section V – Statement of Compliance with the Federal Food, Drug and Cosmetic (FD&C) Act |
Renamed to Section V from Section VI. Section V from original form (Manufacturer/Packaging/Sterilization Sites Information) now Part E in Section I. |
|
|
||
Includes a brief description of how the PMTA satisfies content requirements of section 910(b)(1) of the FD&C Act (specify in the table of contents where the brief description is located) |
|
Updated language for clarity and conciseness. Field number changed to V1. Open text entry field added. |
|
|
||
Includes a brief description of how marketing the new tobacco product would be appropriate for the protection of public health as determined with respect to the population as a whole, including users and non-users of the tobacco product, and taking into account the following (specify in the table of contents where the brief description is located)
a. The increased or decreased likelihood that existing users of tobacco products will stop using such products and; b. The increased or decreased likelihood that those who do not use tobacco products will start using such products
|
|
Updated language for clarity and conciseness. Field number changed to V1. Open text entry field added. |
|
|
||
SECTION VI: CERTIFICATION STATEMENTS |
|
|
||||
Section VII - Certification Statements |
Section VI – Certification Statements |
Updated section number from VII to VI |
|
|
||
“The application must contain...” |
“Applications must contain...” |
Verbiage in first sentence of instructions has been slightly adjusted here |
|
|
||
N/A |
“For the following section, insert...”
|
An additional paragraph of instructions was added for clarity on how to complete the certification statements. Clarifies that the name of “the responsible official” they should insert should be the authorized representative. Provides details on printing and saving file appropriately to ensure all available content is available to FDA to process. |
|
|
||
1. General Application Certification Statement for all applications.* |
i. Certification Statement for Standard PMTAs: |
Updated numbering (1. -> i.) and title for first Certification Statement |
|
|
||
“name of responsible official” |
“insert name of responsible official” VI.i VI.iii VI.iv |
Updated from “name of responsible official” to “insert name of responsible party” for clarity. |
|
|
||
“name of applicant” |
“applicant name” VI.i VI.ii |
Moved “(applicant name)” to follow response entry field. “_____________(applicant name)” to maintain consistency withing form. |
|
|
||
“Signature” and “Date” |
1. Signature and date (mm/dd/yyyy) VI.i.1. VI.ii.1. VI.iii.1. VI.iv.1. VI.v.1. |
Combined signature and date field to also include expected date format for clarity. |
|
|
||
2.Modified tobacco product certification for supplemental PMTAs.* |
ii. Modified Tobacco Product Certification for Supplemental PMTAs. |
Updated numbering (2. -> ii.) and title for second Certification Statement |
|
|
||
“name of original tobacco product” |
“insert name of previously submitted tobacco product(s) |
Updated “name of original tobacco product” to “insert name of previously submitted tobacco product(s)” for clarity. |
|
|
||
STN of PMTA for the original product |
STN of previously submitted PMTA |
Updated language for clarity. |
|
|
||
name of original tobacco product |
name of previously submitted PMTA(s) |
Updated language for clarity. |
|
|
||
21 CFR 1114.45 |
21 CFR 1114.47 VI.ii VI.iii VI.iv |
Changed to updated CFR |
|
|
||
3.Same tobacco product certification for resubmission* |
iii. Same Tobacco Product Certification for Resubmission |
Updated numbering (3. -> iii.) and title for third Certification Statement |
|
|
||
4.Different tobacco product certification for resubmission |
iv. Different Tobacco Product Certification for Resubmission |
Updated numbering (4. -> iv.) and title for fourth Certification Statement |
|
|
||
5.Financial Interest and Arrangements of Clinical Investigators Certification Statement |
v. Financial Interest and Arrangements of Clinical Investigators Certification Statement: |
Updated numbering (5. -> v.) and title for fifth Certification Statement |
|
|
||
“name of company” |
“name of Company” |
Updated “name of company” to “name of Company” to maintain consistency throughout form. |
|
|
||
SECTION VII - APPENDICES |
|
|
||||
SECTION VIII - APPENDICES |
SECTION VII - APPENDICES |
Updated number section number (VIII -> VII) |
|
|
||
N/A |
CONTINUATION PAGES |
Added header to beginning of Appendix to identify purpose |
|
|
||
Appendix A: New Tobacco Product Details |
N/A |
Deleted section |
|
|
||
N/A |
Appendix A: Alternate Point of Contact Information
|
Added Appendix A: Alternate Point of Contact Information to provide applicant additional entry fields for Section I Part C. |
|
|
||
Appendix B: Properties Needed to Uniquely Identify the Tobacco Product, by Category and Subcategory |
N/A |
Deleted section |
|
|
||
N/A |
Appendix B: Manufacturer Information |
Added Appendix B: Manufacturer Information to provide applicant additional entry fields for Section I Part E. |
|
|
||
Appendix C instructions for Completion of PMTA Form |
Appendix F: Instructions for Completion of PMTA Form |
Moved instructions on how to complete form from Appendix C to end of form. Appendix F contains detailed updated instructions for completing every field in the form as well as instructions related to form submission. |
|
|
||
N/A |
Appendix C: Cross-Reference Information |
Added Appendix C: Cross-Reference Information to provide applicant additional entry fields for Section III Part B. |
|
|
||
N/A |
Appendix D: Referenced Tobacco Product Master File(s) (TPMF) |
Added Appendix D: Referenced Tobacco Product Master File(s) (TPMF)to provide applicant additional entry fields for Section III Part C. |
|
|
||
N/A |
Appendix E: Formal Meetings Held with FDA Pertaining to the New Product(s) |
Added Appendix E: Formal Meetings Held with FDA Pertaining to the New Product(s) to provide applicant additional entry fields for Section III Part D. |
|
|
||
Justification for Security Updates to Form FDA 4057b
Justification:
FDA discovered vulnerabilities in the previous version of Form FDA 4057b. These updates to Form FDA 4057b add security features to the form. These minimal features include:
Preventing easy access to macros and other functionalities by external users by creating a stronger password protect for the spreadsheet. Previously, external users could break the macros or other functionalities in the spreadsheet and therefore compromise the functionality and integrity of the form.
Adding an instruction to explicitly say the form is only to be used for PMTA submissions
We anticipate a reduction of ten minutes per response in burden hours to complete Form FDA 4057b because of the use of the new validator tool. The validator tool will provide a list of all issues that must be corrected in a few minutes rather than applicants spending the time to review each field.
Justification for FDA Tobacco Product Grouping Spreadsheet Validator
Justification:
The FDA Tobacco Product Grouping Spreadsheet Validator is a free software that validates the content of FDA product grouping spreadsheets such as “Form FDA 4057b – Premarket Tobacco Product Application Product Grouping Spreadsheet”. The validator is available for voluntary use by the tobacco industry (sponsors, manufacturers, and importers) prior to submitting a product grouping spreadsheet to FDA.
The Tobacco Product Grouping Spreadsheet Validator will allow industry users to validate product attributes in their product grouping spreadsheet with the defined and accepted product data standards, and make corrections as needed. If there are no errors found in a spreadsheet, the Validator will produce a certificate of completion that can be saved locally and included with the applicants FDA submission voluntarily. If errors are found during validation, the Validator will provide the applicants with the error to the end of each impacted row of the spreadsheet, allowing applicants to make necessary changes.
The software and any output files reside locally on an applicant’s computer, allowing them to work on the product grouping spreadsheet offline. The Validator does not transmit any data across the web to FDA. FDA does not have the ability to access, review, or supplement the information on local computers through this application. We anticipate the use the validator tool will take an average of five minutes per response.
The use of the product validation tool will benefit the tobacco industry in the following ways:
Allowing industry applicants to submit their applications with confidence, knowing that product attributes have been validated.
Minimizing the chances of applications being rejected due to incomplete or incorrect data.
Minimizing delays in processing applications due to incorrect Form FDA 4057b.
The use of the product validation tool will benefit FDA in the following ways:
Reducing the time spent manually reviewing each submitted spreadsheet and working with industry users to make corrections.
Improving data quality will allow for more accurate reporting, analysis, and data management.
Improving data quality to allow FDA to uniquely identify tobacco products and reduce duplicate entries internally.
Spreadsheet Validator Screenshots

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | dgittleson |
| File Modified | 0000-00-00 |
| File Created | 2024-11-28 |
© 2026 OMB.report | Privacy Policy