NSF-1700 Medical History
Medical Clearance Process for Deployment to the Polar Regions
NSF 1700 APR24
OMB: 3145-0177
 PIPELINE
#
PIPELINE
#
NOTE: Red boxes indicate required fields.
The forms will not be accepted if these fields are blank.
PARTICIPANT NAME:
MEDICAL HISTORY
The
PARTICIPANT
COMPLETES
this
Medical
History
form
prior
to
any
exam.
CONTACT INFORMATION (INCLUDE AREA CODES): |
||||||||||||
Name last, first, middle (must match official ID): |
Age: |
Birthdate: (MM/DD/YYYY) |
Sex Assigned at Birth, on original birth certificate: 



M F FFF FFF
|
|||||||||
Nickname: |
Maiden Name: |
Previous Name or Other Legal Name: |
||||||||||
Street Address: |
E-Mail: |
|||||||||||
City: |
State: |
Zip: |
Country: |
|||||||||
Day Telephone: |
Evening Telephone: |
Mobile: |
Fax: |
|||||||||
EMERGENCY POINT OF CONTACT: |
||||||||||||
Name: |
Address:
|
|||||||||||
Phone Number: |
||||||||||||
DEPLOYMENT INFORMATION |
||||||||||||
Job Title: |
Estimated Deployment Dates: (MM/YYYY) From: To: |
Prior Polar Deployment (Arctic or Antarctic)? (MM/YYYY) Location: From: To: |
||||||||||

Affiliation: 

NSF Science Event Company Name |
|

Technical Event 
Other |
|
|
||||||||
Proposed Antarctic Season 

Summer (Sep-Feb) Summer Extended (WINFLY and/or Mar, May) 

Winter (Mar-Oct) 
(dates) |
Worksite 


McMurdo Station South Pole Station Palmer Station Vessel 

Traverse |

Dates

|
Field Camp :
Other (specify):

Two Year PQ |
|
||||||||
Proposed Arctic Season 
Summer (Mar-Sep) 
Winter (Oct-Feb) |
Worksite 
Summit 
Raven |

Dates 
|
Field Camp: Two Year PQ |
|
||||||||
MEDICAL HISTORY |
|||||||||||||||||||

CURRENT MEDICATIONS - (Check box if None) |
|||||||||||||||||||
Name |
Dose |
Frequency |
Name |
Dose |
Frequency |
||||||||||||||
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
||||||||||||||

DRUG ALLERGIES - (Check box if None) |
|

FOOD ALLERGIES - (Check box if None) |
|
||||||||||||||||
Name |
Type of Reaction |
Name |
Type of Reaction |
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
PAST HOSPITALIZATIONS - (Check box if None) |
|
|
|||||||||||||||||
Condition |
Date |
(YYYY) |
|
Condition |
Date (YYYY) |
||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||

PAST SURGERIES - (Check box if None) |
|||||||||||||||||||
Condition |
Date |
(YYYY) |
|
Condition |
Date (YYYY) |
||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
||||||||||||||||

MEDICAL TESTING / PROCEDURES IN PREVIOUS 3 YEARS - (Check box if None) |
|||||||||||||||||||
Type (specify body location) |
Date |
(YYYY) |
Describe reason for test procedure and result: |
|
|||||||||||||||
MRI |
|
|
|||||||||||||||||
CT |
|
||||||||||||||||||
Ultrasound |
|
||||||||||||||||||
Angiogram |
|
||||||||||||||||||
Biopsy |
|
||||||||||||||||||
Catheterization (Cardiac, Renal, etc) |
|
||||||||||||||||||
Other: |
|
||||||||||||||||||
VACCINATION HISTORY |
|||||||||||||||||||
Most recent vaccination Date |
(YYYY) |
Most recent vaccination Date |
(YYYY) |
||||||||||||||||
COVID-19(Current Deployment Season) |
|
Other |
|
||||||||||||||||
Influenza (Current Deployment Season) |
|
Bacillus - Calmette (BCG) Vaccine (Given in childhood in countries with high rates of TB) |

Yes |

No |
|||||||||||||||
DT/Tdap |
|
||||||||||||||||||
MMR |
|
||||||||||||||||||
Hepatitis A |
|
||||||||||||||||||
Hepatitis B |
|
||||||||||||||||||
Additional Space (Please attach proof of vaccination for required vaccinations as noted on “Dear Doctor” section):
LIFESTYLE |
|||||||||||||||||||
Tobacco |
Yes |
No |
Describe: Packs/Day Total yrs. |
Year last |
|||||||||||||||
Do you currently use tobacco products? |
|
|
|
|
|
|
|||||||||||||
Have you used tobacco products in the past? |
|
|
|||||||||||||||||
MEDICAL LIMITATIONS- SAFETY AND HEALTH
|
YES |
NO |
Do you need assistance to climb a ladder? |
|
|
Do you have difficulty climbing 2 flights of stairs? |
|
|
Do you have difficulty walking for 30 minutes without resting? |
|
|
Would you have difficulty jumping safely from a 2-foot height? |
|
|
Do you have problems at high altitude? |
|
|
Do you have difficulty wearing a tightly fitting respirator/mask? |
|
|
Explanations: Explain any “YES” answer from above. Please describe the condition and/or the approximate date of occurrence.
|
||
Alcohol |
Yes |
No |
If abstinent, please enter date of your last alcoholic beverage (or NONE): (YYYY or NONE) |
||||||||||
Do you drink alcohol? |
|
|
|
|
|
|
|||||||
Have you ever felt you should decrease your alcohol consumption? |
|
|
Describe frequency and type of alcohol:
Describe “yes” answers to alcohol questions: |
||||||||||
Have you ever received a DUI, DWAI or court ordered treatment for alcohol? |
|
|
|||||||||||
Have you ever been diagnosed and/or treated with an addiction or alcohol related disorder? |
|
|
|
|
|
|
|||||||
|
|
||||||||||||
Exercise and conditioning |
Yes |
No |
Describe frequency and type of exercise: |
||||||||||
Do you have a regular exercise program? |
|
|
|||||||||||
Have you had a cardiovascular stress test? |
|
|
Date of last Exercise Stress Test (CST): (MM/YYYY) |
||||||||||
Reason for CST:
|
|||||||||||||
GENERAL MEDICAL HISTORY |
|||||||||||||
New Government regulations require that you be informed of the following: “The Genetic Information Nondiscrimination Act of 2008 (GINA) prohibits employers and other entities covered by GINA Title II from requesting or requiring genetic information of an individual or family member of the individual, except as specifically allowed by this law. To comply with this law, we are asking that you not provide any genetic information when responding to this request for medical information. ‘Genetic information’ as defined by GINA, includes an individual's family medical history, the results of an individual's or family member's genetic tests, the fact that an individual or an individual's family member sought or received genetic services, and genetic information of a fetus carried by an individual or an individual's family member or an embryo lawfully held by an individual or family member receiving assistive reproductive services.” Therefore, you should not forward any information related to your family’s medical history and only submit answers to these questions regarding your own personal/individual history. |
|||||||||||||
ANSWER THE FOLLOWING QUESTIONS REGARDING YOUR PRESENT OR PAST MEDICAL HISTORY |
|||||||||||||
Condition |
Yes |
No |
Condition |
Yes |
No |
||||||||
1 |
Neurology |
|
|
2D |
Congestive heart failure |
|
|
|
|||||
1A |
Cerebrovascular accident (CVA) |
|
|
2E |
Coronary angioplasty/stent/bypass |
|
|
|
|||||
1B |
Concussion |
|
|
2F |
Coronary artery disease |
|
|
|
|||||
1C |
Dizziness/Loss of Consciousness |
|
|
2G |
Heart murmur/valvular heart disease |
|
|
|
|||||
1D |
Headaches (Migraine) |
|
|
2H |
Hypertension (high blood pressure) |
|
|
|
|||||
1E |
Headaches (Other) |
|
|
2I |
Myocardial Infarction (MI) |
|
|
|
|||||
1F |
Multiple sclerosis |
|
|
2J |
Supraventricular tachycardia (SVT) |
|
|
|
|||||
1G |
Peripheral neuropathy |
|
|
2K |
Other cardiac condition |
|
|
|
|||||
1H |
Seizures |
|
|
3 |
Vascular disease |
|
|
|
|||||
1I |
Transientischemic attack (TIA) |
|
|
3A |
Abdominal aneurysm |
|
|
|
|||||
1J |
Traumatic brain injury (TBI) |
|
|
3B |
Arterial emboli |
|
|
|
|||||
1K |
Other neurological disorder |
|
|
3C |
Cerebral aneurysm |
|
|
|
|||||
2 |
Cardiology |
|
|
3D |
Deep venous thrombosis (DVT) |
|
|
|
|||||
2A |
Angina/chest pain |
|
|
3E |
Venous stasis ulcers |
|
|
|
|||||
2B |
Atrial fibrillation |
|
|
3F |
Other vascular condition |
|
|
|
|||||
2C |
Cardiac pacemaker/defibrillator |
|
|
|
|
|
|
|
|||||
For all “yes” answers provide details to include line number, age of onset, frequency of event, date of last episode, current medications, other therapies and current status of the condition. |
|||||||||||||
GENERAL MEDICAL HISTORY |
||||||||||||
ANSWER THE FOLLOWING QUESTIONS REGARDING YOUR PRESENT OR PAST MEDICAL HISTORY |
||||||||||||
|
Condition |
Yes |
No |
|
Condition |
Yes |
No |
|||||
4 |
Rheumatologic & Autoimmune disease |
|
|
8I |
Irritable bowel syndrome (IBS) |
|
|
|
||||
4A |
Fibromyalgia |
|
|
8J |
Pancreatitis |
|
|
|
||||
4B |
Osteoarthritis |
|
|
8K |
Peptic ulcer disease |
|
|
|
||||
4C |
Rheumatoid arthritis |
|
|
8L |
Ulcerative colitis |
|
|
|
||||
4D |
Systemic Lupus erythematosis |
|
|
8M |
Other gastrointestinal disease |
|
|
|
||||
4E |
Other Rheumatologic/Autoimmune condition |
|
|
9 |
Dermatology |
|
|
|||||
9A |
Dermatitis |
|
|
|
||||||||
5 |
Ears, Nose and Throat |
|
|
9B |
Melanoma |
|
|
|
||||
5A |
Hearing impairment |
|
|
9C |
Psoriasis/Eczema |
|
|
|
||||
5B |
Nosebleeds |
|
|
9D |
Skin cancer |
|
|
|
||||
5C |
Seasonal Allergies |
|
|
9E |
Other skin condition |
|
|
|
||||
6 |
Ophthamology |
|
|
10 |
Orthopedic |
|
|
|||||
6A |
Glaucoma |
|
|
10A |
Cervical spine injury |
|
|
|
||||
6B |
Visual impairment |
|
|
10B |
Chronic pain |
|
|
|
||||
6C |
Other eye condition |
|
|
10C |
Dislocation |
|
|
|
||||
6D |
Lasik/restorative surgery |
|
|
10D |
Fractures |
|
|
|
||||
7 |
Pulmonary |
|
|
10E |
Low back injury |
|
|
|
||||
7A |
Altitude sickness |
|
|
10F |
Joint replacement/pins/plates |
|
|
|
||||
7B |
Asthma after 10 years of age |
|
|
10G |
Other orthopedic condition |
|
|
|
||||
7C |
Chronic bronchitis/bronchiectasis |
|
|
11 |
Metabolic |
|
|
|
||||
7D |
Chronic obstructive pulmonary disease |
|
|
11A |
Adrenal insufficiency |
|
|
|
||||
7E |
Dyspnea (shortness of breath) |
|
|
11B |
Diabetes Type I |
|
|
|
||||
7F |
Obstructive sleep apnea |
|
|
11C |
Diabetes Type II |
|
|
|
||||
7G |
Pulmonary embolism |
|
|
11D |
Gout |
|
|
|
||||
7H |
Positive TB Test/Treatment |
|
|
11E |
Hypercholesterolemia |
|
|
|
||||
7I |
Chronic cough (greater than 3 weeks) |
|
|
11F |
Hyperthyroidism |
|
|
|
||||
7J |
Night sweats |
|
|
11G |
Hypothyroidism |
|
|
|
||||
7K |
Unexplained weight loss |
|
|
11H |
Pituitary insufficiency |
|
|
|
||||
7L |
Exposed to anyone with known TB |
|
|
11I |
Other hormonal disorder |
|
|
|
||||
7M |
Other pulmonary condition |
|
|
12 |
Gynecology-female |
|
|
|
||||
8 |
Gastro intestinal disease |
|
|
12A |
Menstrual period over 30 days ago? |
|
|
|||||
8A |
Black tarry stools/Blood in stool |
|
|
12B |
Date of last PAP smear |
|
|
|||||
8B |
Cholelithiasis (gall stones) |
|
|
12C |
Premenstrual syndrome (PMS) |
|
|
|
||||
8C |
Crohn’s disease |
|
|
12D |
Endometriosis |
|
|
|
||||
8D |
Frequent or persistent diarrhea |
|
|
12E |
Severe menstrual cramps |
|
|
|
||||
8E |
Gastroesophageal reflux (GERD) |
|
|
12F |
Ovarian cysts |
|
|
|
||||
8F |
Hemorrhoids |
|
|
12G |
Sexually transmitted disease |
|
|
|
||||
8G |
Hepatitis (describe below) |
|
|
12H |
Other gynecological conditions |
|
|
|
||||
8H |
Hernia |
|
|
12I |
HIV |
|
|
|
||||
|
|
|
|
|
|
|
|
12J |
Use of hormonal medication |
|
|
|
For all “yes” answers provide details to include line number, age of onset, frequency of event, date of last episode, current medications, other therapies and current status of the condition. Please indicate reason for hormonal medication if used for other than contraception. |
||||||||||||
GENERAL MEDICAL HISTORY |
|||||||
ANSWER THE FOLLOWING QUESTIONS REGARDING YOUR PRESENT AND PAST MEDICAL HISTORY |
|||||||
Condition |
Yes |
No |
|
Condition |
Yes |
No |
|
13 |
Psychiatric |
|
|
15 |
Hematology/Oncology |
|
|
13A |
Addiction |
|
|
15A |
Anemia |
|
|
13B |
Anxiety/panic attacks |
|
|
15B |
Cancer (describe type below) |
|
|
13C |
Attention deficit disorder/ ADHD |
|
|
15C |
Leukemia |
|
|
13D |
Bipolar |
|
|
15D |
Lymphoma – Hodgkins |
|
|
13E |
Depression |
|
|
15E |
Lymphoma – non Hodgkins |
|
|
13F |
Eating disorder (bulimia/anorexia) |
|
|
15F |
Platelet disorder |
|
|
13G |
ER/Hospitalization visit (psych condition) |
|
|
15G |
Hemochromatosis |
|
|
13H |
Post-traumatic stress disorder |
|
|
15I |
Other Hematologic/Oncologic |
|
|
13I |
Schizophrenia |
|
|
16 |
Genitourinary - male |
|
|
13J |
Suicidal thoughts or attempts |
|
|
16A |
Prostate disease or prostate cancer |
|
|
13K |
Other psychiatric condition |
|
|
16B |
Sexually transmitted disease |
|
|
14 |
Renal disease |
|
|
16C |
Testicular abnormality |
|
|
14A |
Chronic Renal Disease |
|
|
16D |
Other genitourinary condition |
|
|
14B |
Frequent urinary tract infections |
|
|
16E |
HIV |
|
|
14C |
Hematuria (blood in urine) |
|
|
17 |
Diving |
|
|
14D |
Kidney stones |
|
|
17A |
Are you a diver for the USAP? |
|
|
14E |
Other kidney condition |
|
|
17B |
Have you had the bends? (describe) |
|
|
|
18 |
Any other medical condition NOT listed above |
|
|
|||
For all “yes” answers provide details to include line number, age of onset, frequency of event, date of last episode, current medications, other therapies and current status of the condition. |
|||||||
I certify that the information contained herein is complete and accurate to the best of my knowledge. I will inform the contractor’s medical staff of ALL medical/health changes, including medications that occur after submitting this form. I understand that failure to provide any or all of the requested information may result in a denial of my application for assignment to the Polar Regions. I also understand that willfully providing false statements to a Federal agency or its representatives is a criminal offense.
Print Name Signature Date |
|||||||
NPI
#
Client
Bill #
Age:
Sex Assigned at Birth, on original birth certificate:
Dear Lab Collection (LabCorp or Physician),
This person is being considered for participation in the National Science Foundation’s Arctic or Antarctic program. Collect specimens for the indicated laboratory analyses: All results need to be translated to English.
Participant: Do not eat or drink for 8 hours before labs.
Required Polar Panel
(LabCorp Summer Panel #)
Additional Labs (Labcorps Winter Panel #)
 Complete
Blood Count with Differential
Complete
Blood Count with Differential

 Blood
Chemistries (Na;
K; Cl;
Ca; Glucose,
Serum; Creatinine, Serum; GFR/BUN)
Blood
Chemistries (Na;
K; Cl;
Ca; Glucose,
Serum; Creatinine, Serum; GFR/BUN)
Hepatic Panel (Alkaline Phosphatase; Total Bilirubin;
AST (SGOT); ALT (SGPT))

 Lipid
Panel (Cholesterol;
HDL; LDL;
Triglycerides) Hepatitis B Total Core Antibody
Lipid
Panel (Cholesterol;
HDL; LDL;
Triglycerides) Hepatitis B Total Core Antibody
Hepatitis C Antibody RPR (Syphilis)
Blood Type (ABO and Rh)
PSA (LabCorp Test #)

 Males
50
and
older
and
wintering
at
South
Pole HgA1c
(LabCorp Test #)
Males
50
and
older
and
wintering
at
South
Pole HgA1c
(LabCorp Test #)
Diabetes I or II or borderline glucose level
 Quantiferon
TB
(LabCorp
Test
#)
Quantiferon
TB
(LabCorp
Test
#)
 Positive
TB
skin
test
or
BCG
vaccine MMR
Titer (LabCorp Test
#)
Positive
TB
skin
test
or
BCG
vaccine MMR
Titer (LabCorp Test
#)
Required for all first time participants
 HIV
(LabCorp Pane
#)
HIV
(LabCorp Pane
#)
 Winter
at South
Pole or
volunteering for
the walking blood bank
Winter
at South
Pole or
volunteering for
the walking blood bank
TSH (LabCorp Test #)
 Winter
at
South
Pole
or
hyper/hypothyroidism
Ferritin(LabCorp
Test #)
Winter
at
South
Pole
or
hyper/hypothyroidism
Ferritin(LabCorp
Test #)
Winter at South Pole
 Uric
Acid
(LabCorp
Test
#)
Uric
Acid
(LabCorp
Test
#)
 Winter
at South Pole or diagnosed with Gout Other:
Quantiferon
TB
(LabCorp
Test
#)
Winter
at South Pole or diagnosed with Gout Other:
Quantiferon
TB
(LabCorp
Test
#)

Additional Information:
If our Medical Director’s NPI # or account # was used to collect the lab work (UTMB) will be able to access these results directly from LabCorp. You can get a copy of your labs at http://patient.labcorp.com Please allow a few days to get your lab into their system. Once you have signed up it may take a few hours before you see your results.
Dear Medical Provider:
DOB:
Age:
Sex Assigned at Birth, on original birth certificate:
This person is being considered for participation in the National Science Foundation’s Arctic or Antarctic program. Polar medical facilities have limited diagnostic and therapeutic capabilities. In the event of a severe injury or medical emergency, transportation to a modern hospital or clinic may take several days or longer.
Environmental conditions in the Polar Regions may be harsh. Temperatures range from 30 degrees above to 100 degrees below zero Fahrenheit. Physiologic altitude varies from 0 to over 10,000 feet above mean sea level.
Participants may live in close quarters for extended periods of time in constant daylight or darkness. Your clinical assessment will be used to determine the person's physical qualification for deployment to the Polar Regions.
Conduct the indicated tests and provide the results to the Participant in English.
Vaccinations
![]() TDap (Pertussis)
TDap (Pertussis)
Influenza vaccine: Yearly (The United States Antarctic Program will require injections (shots) as the only method of compliance for the flu vaccine requirement.)
![]() COVID-19 (Must meet up to date status with the current CDC
recommendations)
COVID-19 (Must meet up to date status with the current CDC
recommendations)
Testing (Summer, Baseline)
Medical Self History (sign pages 5 and 13, initial pages 12 and 13 after printing)
![]() Polar Physical Examination (pages 8-9 of this form)
Polar Physical Examination (pages 8-9 of this form)
![]() Quantiferon. Chest
x-ray does not take place
Quantiferon. Chest
x-ray does not take place
EKG - 12 lead with tracing (new participants; every five years if aged 40-49, and yearly if 50+)
![]() Exercise Stress
Test with
MD Interpretation
(if wintering
at South
Pole: every two
years if
50-59, yearly
if 60+) (Bruce
Protocol - must complete 9 minutes, stage 3, 85% max heart rate)
Exercise Stress
Test with
MD Interpretation
(if wintering
at South
Pole: every two
years if
50-59, yearly
if 60+) (Bruce
Protocol - must complete 9 minutes, stage 3, 85% max heart rate)
![]() Pulmonary Function
Test, Pre/Post
Bronchodilator (Asthma
after 10
years of
age)
Pulmonary Function
Test, Pre/Post
Bronchodilator (Asthma
after 10
years of
age)
![]() Occupational Pulmonary Function Test (Spirometry)
Occupational Pulmonary Function Test (Spirometry)
Guaiac Stool Test (yearly 50+ yrs or older)
![]() Lab Collection
Lab Collection
Testing (Winter)
![]() Pap Smear Cytology Report with Endocervical Cell Report
(South Pole wintering over required
every 3
years for
women ages
21-65)
Pap Smear Cytology Report with Endocervical Cell Report
(South Pole wintering over required
every 3
years for
women ages
21-65)
![]() Mammogram Radiology Report (South Pole wintering Over
required every 2 years for women ages 40 and over)
Mammogram Radiology Report (South Pole wintering Over
required every 2 years for women ages 40 and over)
![]() Chest X-ray:
2
View
AP/LAT
(Every 5
years if
there is
a history
of smoking
>15 years;
or if
wintering over
at South
Pole) (Final Radiology Report Only: do
not submit x-ray films)
Chest X-ray:
2
View
AP/LAT
(Every 5
years if
there is
a history
of smoking
>15 years;
or if
wintering over
at South
Pole) (Final Radiology Report Only: do
not submit x-ray films)
Gallbladder Ultrasound (Required for wintering at South Pole, McMurdo, and Summit Station) (fast for a minimum of 6 hours before test; report only)
![]() Lab Collection
Lab Collection
Prescription medications (type and quantity) are limited at all Polar medical facilities. Participants are required to bring a sufficient supply of medications for the duration of their deployment or make the necessary arrangements for shipment of medication in accordance with provided guidelines found within the Polar Physical Qualification Important Information attachment.
After the examination, return the Medical History, Polar Physical Examination Form and ALL results to the Participant so they can include it with this packet. It’s the responsibility of the Participant to return all results to Center for Polar Medical Operations, Antarctic Support Contract, University of Texas Medical Branch (UTMB) at Galveston, in English. FAX: 409-772-3600. For questions, please contact UTMB at [email protected] or 1-855-300-9704 (toll free). Thank you, University of Texas Medical Branch –Center for Polar Medical Operations
POLAR PHYSICAL EXAMINATION
MUST BE COMPLETED BY M.D., D.O., P.A., OR N.P.
Name: |
Date of Birth: |
Blood Type: |
||||||
New Government regulations require that you be informed of the following: “The Genetic Information Nondiscrimination Act of 2008 (GINA) prohibits employers and other entities covered by GINA Title II from requesting or requiring genetic information of an individual or family member of the individual, except as specifically allowed by this law. To comply with this law, we are asking that you not provide any genetic information when responding to this request for medical information. ‘Genetic information’ as defined by GINA, includes an individual's family medical history, the results of an individual's or family member's genetic tests, the fact that an individual or an individual's family member sought or received genetic services, and genetic information of a fetus carried by an individual or an individual's family member or an embryo lawfully held by an individual or family member receiving assistive reproductive services.” Therefore, you should not forward any information related to the patient’s family’s medical history and only submit answers to those questions regarding this patient’s personal/individual history. |
||||||||
VITAL SIGNS |
VISION |
|||||||
Height: Weight: |
Without Correction |
With Correction
DIST R
L |
NEAR
|
|||||
|
DIST NEAR |
|||||||
BP: / Pulse: |
R |
|||||||
Framingham |
|
|||||||
BMI: Risk Score/ASCVD: |
L |
|||||||
Finding |
Normal |
Abnormal |
Finding |
Normal |
Abnormal |
|||
General appearance |
|
|
Abdomen |
|
|
|||
Head and neck |
|
|
Inguinal, include hernia (Male Only) |
|
|
|||
Eyes |
|
|
Genitalia male (Not Deferrable) |
|
|
|||
Ears |
|
|
Spine |
|
|
|||
Nose |
|
|
Upper extremities |
|
|
|||
Mouth |
|
|
Lower extremities |
|
|
|||
Thyroid |
|
|
Skin (include body) |
|
|
|||
Lymph nodes |
|
|
Vascular |
|
|
|||
Chest and lungs |
|
|
Neurologic |
|
|
|||
Breasts male/female (Not Deferrable) |
|
|
Emotional Status |
|
|
|||
Heart |
|
|
Pelvic exam (South Pole wintering, female) |
|
|
|||
Guaiac Test (annually, age 50 and over): |
Influenza Vaccination is Mandatory for Deployment (annually; must be for the flu season that corresponds to deployment):
Date |
|||||||
Result Date |
||||||||
COVID-19 Vaccination is Mandatory for Deployment (meets CDC’s recommendations for up-to-date status):
Dates |
||||||||
TDap Vaccination (every 10 years): |
|
|||||||
Must include lot number, expiration date, manufacturer, |
|
|||||||
date of injection. Please attach CDC compliant proof of |
Date |
|||||||
vaccination. |
|
|||||||
 POLAR
PHYSICAL
EXAMINATION
FORM
POLAR
PHYSICAL
EXAMINATION
FORM
PIPELINE #
PARTICIPANT NAME:
Examiner – Comment on all abnormal findings |
Examiner – Comment on overall fitness and health conditions that might interfere with the Participant’s ability to participate in a remote polar deployment. 
Overall fitness of the participant is good. Participant is able to participate and complete duties in a remote polar environment. Participant will require further evaluation prior to clearance. (Comment or Recommendation) |
Examiner’s Name (printed with credentials) Signature Date This exam is void without credentials.
Examiner Street Address:
City: State: Zip Code:
Office Phone: Office Fax: Return the completed examination form and results of the requested tests to the Participant. |
 PIPELINE
#
PIPELINE
#
Dear Dentist:
PARTICIPANT NAME:
This person is being considered for participation in the National Science Foundation’s Arctic or Antarctic program. The Polar Regions are isolated and lack dental facilities. Participants must be free of dental disease. There must be no caries, active periodontal disease, potential endodontic disease, prosthetic deficiencies, potentially symptomatic wisdom teeth, or any uncompleted treatment. All dental work must be completed and documented. All results are to be given to the Participant so he or she can return the results to the Center for Polar Medical Operations, Antarctic Support Contract, University of Texas Medical Branch (UTMB) at Galveston and/or University of Colorado Polar Medicine.
Following the dental exam, the candidate should provide documentation of:
I. DENTAL EXAM |
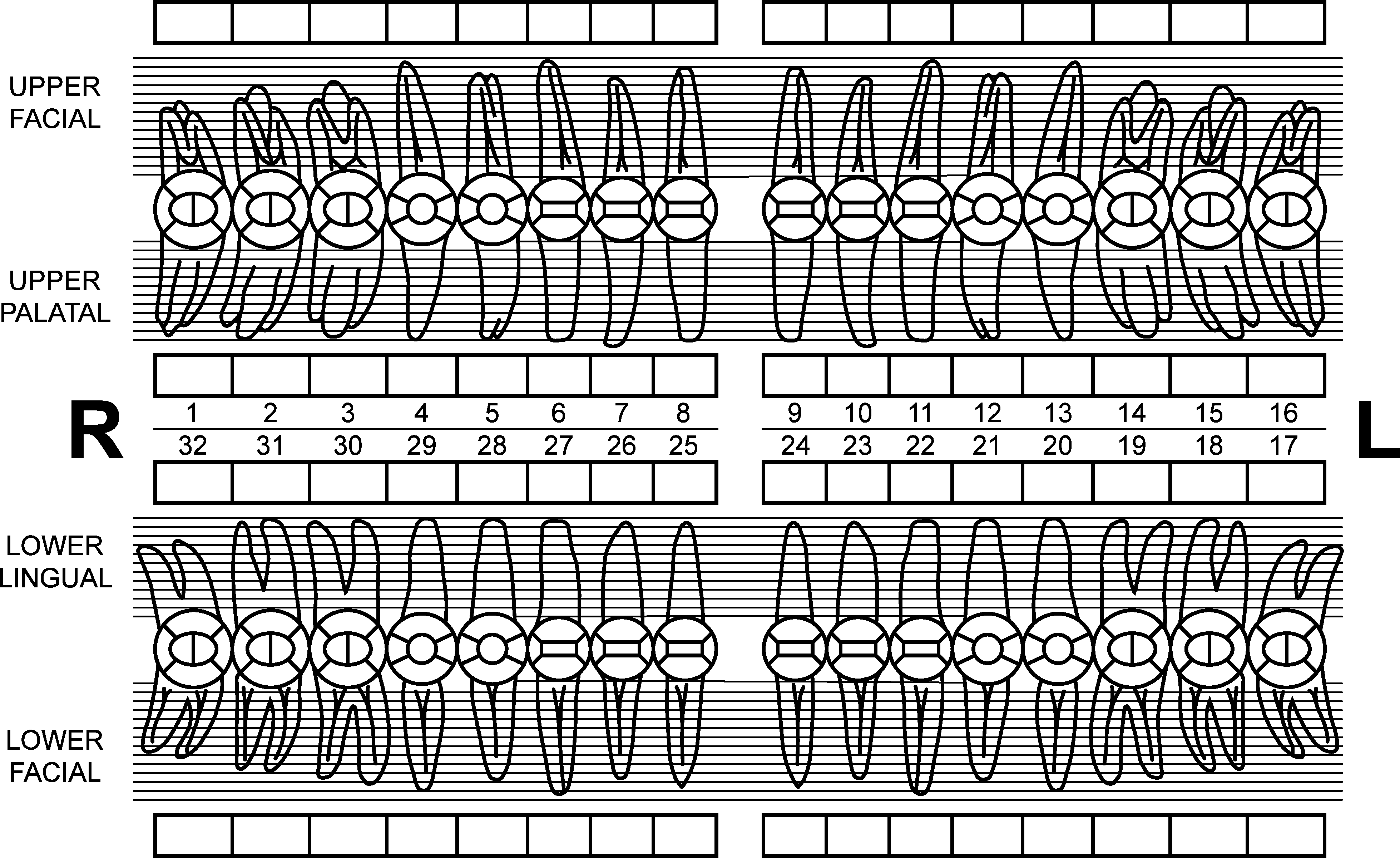
Chart all existing restorations, missing teeth, and endodontically treated teeth only on the Dental Examination Form. The treating dentist must sign the Dental Examination Form and document all completed work. Please include the completed periodontal evaluation chart to accompany dental exam form and digital x-rays. |
II. THIRD MOLARS |
Treatment must be completed three weeks prior to deployment in order for the dental condition to stabilize before deployment. Third molars must be extracted only if they are symptomatic or any of the following are present:
|
III. RADIOGRAPHS |
Digital radiographs can be e-mailed in high-resolution JPEG format (preferred) to [email protected]. Original mounted radiographs can be included with the Dental Exam Form. Copies or poor quality radiographs will not be accepted. Radiographs become a part of the participant’s USAP record and WILL NOT BE RETURNED. You may wish to use a double film pack to retain original radiographs for yourself. Necessary radiographs include:
|
IV. ORTHODONTICS |
Orthodontic care is not available in the Polar Regions; so, Participants with fixed orthodontic appliances or undergoing any active treatment may be considered for short deployments, but only with written approval from the attending provider and approval from the ASC Dental Reviewer.
|
V. PERIODONTAL EVALUATION |
Must be completed and periodontal disease must be treated if or any of the following are present: 1. Advanced periodontal disease. 2. Bleeding pockets 5mm or more mm depth. 3. Mild bone loss. |
After the examination, return the Polar Dental Examination Form, Periodontal chart, X-rays and ALL results to the Participant. It is the responsibility of the Participant to return all results to Center for Polar Medical Operations, Antarctic Support Contract, University of Texas Medical Branch (UTMB) at Galveston. For additional questions, contact UTMB at [email protected] or 1-855-300-9704 (toll free).
Thank you,
University of Texas Medical Branch – Center for Polar Medical Operations
 POLAR
DENTAL
EXAM
FORM
POLAR
DENTAL
EXAM
FORM
PIPELINE #
PARTICIPANT NAME:
POLAR DENTAL EXAMINATION
Name: |
Date of Birth: |
Age: |
|||||
Day Telephone #: |
Email Address: |
||||||
Last Deployment Dates: From: |
To: |
Estimated Deployment Dates: From: To: |
|||||
Chart existing restorations, missing, and endodontically treated teeth only:
|
PERIODONTAL EVALUATION 

Probings 5 mm or greater YES NO Active Disease Noted YES NO 

Adv. Periodontal Disease YES NO Bone Loss YES NO |
||||||
THIRD MOLAR EVALUATION 

3rd Molars Present YES NO List partially erupted List impacted that can be probed
List fully impacted List potentially symptomatic
List implants List retained 1° teeth |
|||||||
Itemized Documentation of all treatment identified and rendered and original digital radiographs must accompany this form |
|||||||
DATES |
Diagnosis and treatment needed |
DATES |
List all treatment completed |
||||
|
|
|
|
||||

Attach the following ORIGINALS to this exam: PANO OR FULL MOUTH SERIES Required first deployment only. Date of last Pano or Full Mouth Series: |
BITEWING X-RAYS, SET OF FOUR MOUNTED 
✔ SHOWING ALL POSTERIOR TEETH Date taken: X-Ray cannot be older than 12 months at the time of dental review by UTMB |
||||||
I have thoroughly examined this candidate for travel to the Polar Regions. All necessary treatment has been performed; all evaluations completed; and the appropriate diagnostic radiographs will accompany this completed form as requested by the “Dear Dentist” letter. |
|||||||
DENTIST’S NAME (PLEASE PRINT) |
TELEPHONE NUMBER (include area code): |
||||||
|
STREET ADDRESS |
||||||
DENTIST’S SIGNATURE DATE |
|
||||||
|
CITY STATE ZIP |
||||||
ATTENTION EXAMINING DENTIST: |
POLAR MEDICAL STAFF USE ONLY |
||||||
Return this completed form, all documentation of treatment and all ORIGINAL X-rays (digital preferred) to the Participant. |



PQ WINTER REVIEW NPQ |
||||||

PIPELINE #
PARTICIPANT NAME:
UNITED STATES ANTARCTIC PROGRAM/ARCTIC PROGRAM DEPLOYMENT CONSENT/AUTHORIZATION DOCUMENTS
IMPORTANT NOTICE FOR PARTICIPANTS IN THE UNITED STATES ANTARCTIC PROGRAM
Participants in the United States Antarctic Program (USAP) are expected to comport themselves in such a manner that their activities and demeanor reflect credit on themselves and their sponsoring organizations. The special circumstances and conditions prevailing in Antarctica and the Arctic require high standards of conduct.
The potential for mishap in the Polar Regions is a constant threat. Your ability to deal effectively with a mishap is reduced if you are under the influence of alcohol or other drugs. The National Science Foundation (NSF) will not condone abuse of alcohol or controlled substances at its facilities. Unauthorized or excessive use of such substances will not be tolerated.
The laws of the United States prohibit the possession, shipping, or mailing of illegal drugs. In addition, governments in New Zealand, South American countries, and Arctic countries have strict laws forbidding the possession or transportation through their countries of firearms, knives, pornographic materials, marijuana or non-prescription drugs. These laws are strictly enforced and penalties for violation are severe. Like any traveler, you must abide by applicable foreign law. If you are found in violation thereof, you are subject to prosecution in the courts of that country. Association with the Antarctic and Arctic programs affords neither preferential treatment nor immunity from prosecution.
Conviction for any criminal action under the laws of the United States or foreign countries may result in your removal from participation in the Antarctic and Arctic programs.
Initials
I have read and understand this Important Notice for Participants in the United States Antarctic Program and the Arctic Program.
MEDICAL RISKS FOR NSF-SPONSORED PERSONNEL TRAVELING TO ANTARCTICA AND THE ARCTIC
Travel to Antarctica and the Arctic imparts certain risks to the traveler. You may experience extremely cold (subzero) temperatures, high altitude and other environmental conditions that put you at risk for cold-related and/or other injuries. The limitations in the medical care available and difficulties, in emergencies, of providing timely evacuation to tertiary medical care facilities in the U.S. or other countries increase your risk of serious complications from exposure or lack of immediate medical care.
Extremes of daylight and darkness can impact sleep or other behaviors. Living in close quarters increases the likelihood of exposure to communicable diseases. Participants should consider these risks before deciding to deploy.
United States Antarctic Program. Virtually all medical care to USAP participants is provided through the USAP medical care system. Medical clinics operate at all three year-round stations (McMurdo, South Pole, and Palmer Stations). Emergency medical technicians and dispensary operations are available on the two oceanographic research vessels. First-aid/first responders support larger seasonal remote field camps. The three clinics are comparable to a small community hospital emergency room and ambulatory care facility, but without secondary or tertiary care facilities nearby for patient referral or specialist support. Radiography (X-rays) and selected laboratory tests are available in the clinics, but more sophisticated imaging procedures and diagnostic tests are not. Operating room surgical suites are not available at the stations, although each clinic has a triage/trauma room. The USAP does not maintain a frozen blood supply at each station, relying instead on a “walking blood bank” (where individual donors would provide fresh blood if transfusions were needed and blood types matched). The evacuation of critically ill or injured patients from Antarctic sites to sophisticated medical care off the continent (to New Zealand, South America, or the United States) is difficult during the austral summer and may be impossible during the austral winter (February through August).
Arctic. A contracted paramedic is on staff at Summit Station on the Greenland Ice Cap. Facilities for emergency care are available (although rarely used) at Kangerlussuaq (western) and Thule Air Base (northern) in Greenland. Virtual or other emergency health care support may be made available for certain remote Arctic locations; e.g., medical kits and access to medical advice via satellite telephone. Researchers and support personnel at other Arctic locations, such as Alaska, Canada, Russia, etc., are typically able to avail themselves of locally available commercial care. Partly because of these limitations, NSF requires medical and dental screening of personnel prior to deployment. These medical screening examinations are necessary to determine the presence of medical conditions that could threaten the health or safety of the individual while deployed. They are also necessary to determine whether medical conditions exist that cannot be effectively managed while the individual is deployed. Persons who fail to meet these medical/ dental screening criteria will be notified of the specific reason(s) for their disqualification. Disqualified individuals may request reconsideration by completing a waiver application (obtained from the University of Texas Medical Branch or University of Colorado Polar Medicine).
Participants should realize that serious accidents or injuries might challenge the medical care system. Therefore, individuals should recognize the limitations in the medical care system before they engage in any risk-taking behaviors (whether on-the- job or during recreational pursuits) that may result in accidents or injuries.
Data collected as a result of this medical screening requirement are maintained in accordance with the Privacy Act (5
U.S. Code 552a) and protected against unauthorized release, as described in the appended Privacy Notice found in the Polar Physical Qualification Important Information Packet. The collection of this information must display a currently valid OMB control number. You are not required to respond to the collection of this information unless it displays a currently valid OMB control number.
Initials
I have read and understand the Medical Risks for NSF-Sponsored Personnel Traveling to Antarctica and the Arctic.
MEDICAL SCREENING FOR BLOOD-BORNE PATHOGENS
As described above, medical clinics at the three U.S. Antarctic research stations and the NSF research stations in the Arctic do not have or maintain readily available supplies of frozen blood. In the event of the need for a transfusion, other individuals at the research station with matching blood types would be asked to donate fresh whole blood for the patient. In order to maintain a viable donor pool, NSF requests that U.S. Antarctic and Arctic program participants during the respective austral summer seasons voluntarily submit to testing for Human Immunodeficiency Virus (HIV) along with the required Hepatitis virus B and C as part of their medical screening process. Please note that HIV testing is required for candidates intending to spend the winter in Antarctica or in the Arctic. [Whether you are voluntarily consenting to this testing (summer only) or required to do so (winter deployment), you should take this form with you to your laboratory appointment to ensure that the tests are performed.]
CONSENT FOR HIV ANTIBODY BLOOD TEST
I have been informed that my blood will be tested for Human Immunodeficiency Virus (HIV) antibodies, the causative agent of Acquired Immune Deficiency Syndrome (AIDS). I understand that the testing involves the withdrawal of a small amount of my blood by venipuncture and subsequent testing of that blood sample via ELISA (Enzyme-Linked Immuno-Sorbent Assay) and Western Blot methods.
I understand that if I have any questions regarding the testing procedure or interpretation of results, I should discuss them with my health care provider. I understand that my examining physician will receive a copy of these test results and may be required, under State law, to report positive test results to state health department authorities, and I consent to these disclosures.
I understand that the results of this blood test will be incorporated into my USAP medical file. All information in that file is maintained in accordance with the Privacy Act (5 USC 552a) and protected against unauthorized release, as described in the appended Privacy Notice found in the Polar Physical Qualification Important Information Packet.

 I
volunteer for the
Walking Blood Bank,
should a medical
emergency develop while
I am on station that
requires a blood
I
volunteer for the
Walking Blood Bank,
should a medical
emergency develop while
I am on station that
requires a blood
donation to help save a human life.
Yes No
I have read and understand the above Medical Screening for Blood-Borne Pathogens information.
Initials

 Having
read and understood the above statements, I hereby GIVE DO
NOT GIVE
Having
read and understood the above statements, I hereby GIVE DO
NOT GIVE
Initials
Signature
my consent to the collection and testing of my blood to determine the presence of HIV antibodies if required.
I have read and understand the United States Antarctic Program/Arctic Program Deployment Consent/Authorization Documents.
AUTHORIZATION FOR TREATMENT OF FIELD-TEAM MEMBER/PARTICIPANT UNDER 18 YEARS OF AGE
I am the parent or legal guardian of , who is an underage participant in the National Science Foundation/Geosciences/Division of Polar Programs. Should any medical/dental care be required during his or her deployment to Antarctica or to the Arctic, I hereby give my authorization and consent to the National Science Foundation’s Division of Polar Programs’ medical care provider(s) for any medical care, treatment or procedures that are deemed medically necessary while my son or daughter is deployed to either the Arctic or the Antarctic.
Name of Parent or Legal Guardian
Street Address
City State Zip Code
Telephone Numbers
Daytime: Evening:
Print Name Signature Date
NSF
Form 1700 (rev April 2024)
OMB
CONTROL
NUMBER:
3145-0177 Expires:
(Previous
versions
are
not
authorized.)
Polar
Physical
Qualification
(PQ)
Packet
Page
Applicants:
Please
retain
one
copy
for
your
records




| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | ASC Tech Comm Group, Corey Anthony - form automation |
| File Modified | 0000-00-00 |
| File Created | 2024-07-20 |
© 2026 OMB.report | Privacy Policy