CMS-10668 CDI Validation Template
Quality Measures and Administrative Procedures for the Hospital-Acquired Condition Reduction Program (CMS-10668)
FY27_CDI_ValTemp.xlsx
OMB: 0938-1352
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0938-1352 can be found here:
Document [xlsx]
Download: xlsx | pdf
Template
NHSN Location
FY2027 Submission Instructions
Overview
DefinitionsTemplate
NHSN Location
FY2027 Submission Instructions
Sheet 1: Definitions
| Clostridioides Difficile Infection (CDI) Validation Template |
||
| In support of the Centers for Medicare & Medicaid Services (CMS) Hospital-Acquired Condition (HAC) Reduction Program inpatient data validation efforts | ||
| for the Fiscal Year (FY) 2027 program year: | ||
| • Each hospital selected for CDI validation is to produce a list of all final results for stool specimens that are toxin positive for CDI during an inpatient episode of care. | ||
| • The list may include specimens collected in the ED and/or 24-hour observation locations collected prior to an inpatient admission; however, if the patient | ||
| was only seen in the ED and/or 24-hour observation and never admitted as an inpatient status, do not include these on the Validation Template. These are | ||
| scenarios where CMS and the National Healthcare Safety Network (NHSN) reporting differ. | ||
| • The line list should include stool specimens that are toxin positive for CDI from unformed stools only. | ||
| Exclusions include: C-diff antigen positive, antigen only detected, no toxin detected | ||
| FY 2027 - CDI Validation Template | ||
| (Use this template for 1Q 2024 through 4Q 2024 stool specimens toxin positive for CDI - all quarters must be submitted on separate templates) | ||
| FIELD (* indicates required field) | DESCRIPTION | SECTION |
| NHSN Facility ID* | The National Healthcare Safety Network (NHSN)-assigned facility ID under which your hospital submits NHSN data. | |
| Provider ID/CCN* | Hospital's 6-digit CMS Certification Number (CCN). Do not include any hyphens. | Hospital Information Section |
| Hospital Name* | Hospital Name associated with CCN. | Complete the first row in the |
| State* | Enter the 2 character abbreviation for the state in which the hospital is located. | spreadsheet. The information |
| Calendar Quarter* | Select from the drop-down list the calendar quarter to which the CDI Validation Template pertains. | provided in the first row will be applied to all toxin positive stool |
| Hospital Contact Name* | Hospital contact name for CMS to contact with questions. | specimens listed on the |
| Contact Phone* | Phone number for hospital contact listed. | template. |
| Contact Email* | Email address for hospital contact listed. | |
| Assay Type* | The type of test used to detect CDI. | |
| Stool Specimens Toxin Positive for C. difficile (Y/N)* | Select Yes or No from the drop-down list. Does the hospital have any final stool cultures toxin positive for CDI for patients in the calendar quarter referenced? | |
| Patient Identifier* | The patient identifier assigned by the hospital. Use the same patient identifier that would be submitted to NHSN if the episode of care (EOC) would be reported as a laboratory-identified CDI event. | |
| Birthdate* | The patient date of birth using MM/DD/YYYY format. | |
| Sex* | Select Female, Male or unknown from the drop-down list to indicate the sex of patient. | |
| Admit Date* | Enter date of patient’s inpatient admission to hospital in MM/DD/YYYY format. Date of admission = date that the patient is physically admitted to an inpatient location. Time spent in the Emergency Department or other outpatient locations before admission are NOT used to set the Date of Admission. See table below for additional information. | Patient & Stool Specimen Section |
| Discharge Date* | Enter date patient was discharged from the hospital in MM/DD/YYYY format. If a patient has not been discharged from the hospital enter "Not Discharged" for the Discharge Date field. Discharge dates that fall within the reporting quarter will be eligible for validation. |
Complete for every final specimen toxin positive for CDI. |
| First Name | First name of patient. | |
| Last Name | Last name of patient. | |
| NHSN Location* | Select from the drop-down list, the NHSN location to which the patient was assigned when the stool specimen was collected. Only locations from the drop-down will be accepted; do not use a hospital-assigned location. |
|
| Lab ID* | Lab ID, accession number or specimen number corresponding to toxin positive for CDI stool specimen. | |
| Stool Specimen Collection Date* | Provide the date the stool specimen was collected in MM/DD/YYYY format. | |
| Stool Specimen Collection Time | Provide the time the stool specimen was collected if easily available. | |
| For additional information, view the appropriate CDI Abstraction Manual posted on the Inpatient Data Validation Resources | ||
| page of QualityNet (direct link): | https://qualitynet.cms.gov/inpatient/data-management/data-validation/resources | |
| For the purposes of CMS inpatient data validation, please note the differences between NHSN data submission | ||
| and validation template/medical record submission, as described below: | ||
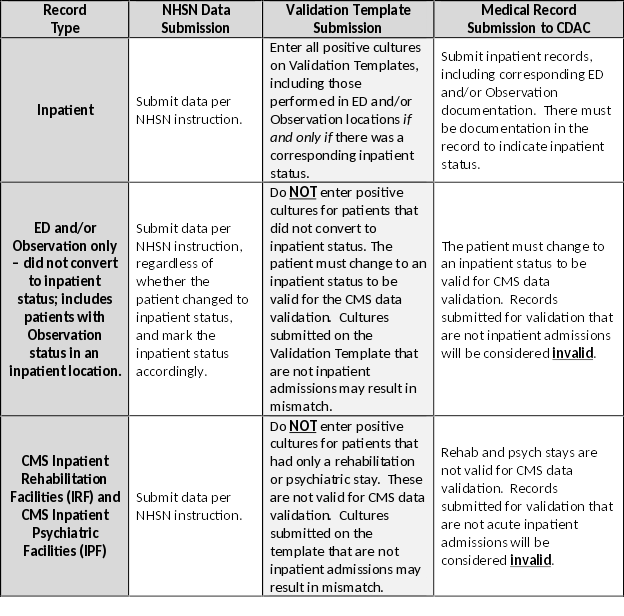
|
||
| According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this | ||
| information collection is 0938-1352 (Expires 01/31/2026). The time required to complete this information collection is estimated to average 10 hours per response, including the time to review instructions, | ||
| search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for | ||
| improving this form, please write to CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850. ****CMS Disclosure**** Please do not send | ||
| applications, claims, payments, medical records or any documents containing sensitive information to the PRA Reports Clearance Office. Please note that any correspondence not pertaining to the | ||
| information collection burden approved under the associated OMB control number listed on this form will not be reviewed, forwarded, or retained. | ||
| If you have questions or concerns regarding where to submit your documents, please contact the Validation Support Contractor at [email protected]. | ||
Sheet 2: Template
| NHSN Facility ID* | Provider ID/CCN* | Hospital Name* | State* | Calendar Quarter* | Hospital Contact Name* | Contact Phone* | Contact Email* | Assay Type* | Stool Specimens Toxin Positive for C. difficile (Y/N)* | Patient Identifier* | Birthdate* | Sex* | Admit Date* | Discharge Date* | First Name | Last Name | NHSN Location* | Lab ID* | Stool Specimen Collection Date* | Stool Specimen Collection Time |
Sheet 3: NHSN Location
| Below is a list of NHSN Locations applicable for CMS inpatient data validation CDI reporting. | ||
| CDC LOCATION LABEL | CDC LOCATION CODE | LOCATION DESCRIPTION |
| Inpatient Adult Critical Care Units | ||
| Burn Critical Care | IN:ACUTE:CC:B | Critical care area for the care of patients with significant/major burns. |
| Medical Cardiac Critical Care | IN:ACUTE:CC:C | Critical care area for the care of patients with serious heart problems that DO NOT require heart surgery. |
| Medical Critical Care | IN:ACUTE:CC:M | Critical care area for the care of patients who are being treated for nonsurgical conditions. |
| Medical-Surgical Critical Care | IN:ACUTE:CC:MS | Critical care area for the care of patients with medical and/or surgical conditions. |
| Neurologic Critical Care | IN:ACUTE:CC:N | Critical care area for the care of patients with life- threatening neurologic diseases. |
| Neurosurgical Critical Care | IN:ACUTE:CC:NS | Critical care area for the surgical management of patients with severe neurologic diseases or those at risk for neurologic injury as a result of surgery. |
| Oncology Medical Critical Care | IN:ACUTE:CC:ONC_M | Critical care area for the care of oncology patients who are being treated for nonsurgical conditions related to their malignancy. |
| Oncology Surgical Critical Care | IN:ACUTE:CC:ONC_S | Critical care area for the evaluation and management of oncology patients with serious illness before and/or after cancer-related surgery. |
| Oncology Medical-Surgical Critical Care | IN:ACUTE:CC:ONC_MS | Critical care area for the care of oncology patients with medical and/or surgical conditions related to their malignancy. |
| Onsite Overflow Critical Care | IN:ACUTE:CC:OF_ONSITE | Area previously used for non-patient care which has been repurposed to care for critically ill or injured patients. |
| Prenatal Critical Care | IN:ACUTE:CC:PNATL | Critical care area for the care of pregnant patients with complex medical or obstetric problems requiring a high level of care to prevent the loss of the fetus and to protect the life of the mother. |
| Respiratory Critical Care | IN:ACUTE:CC:R | Critical care area for the evaluation and treatment of patients with severe respiratory conditions. |
| Surgical Cardiothoracic Critical Care | IN:ACUTE:CC:CT | Critical care area for the care of patients following cardiac and/or thoracic surgery. |
| Surgical Critical Care | IN:ACUTE:CC:S | Critical care area for the evaluation and management of patients with serious illness before and/or after surgery. |
| Trauma Critical Care | IN:ACUTE:CC:T | Critical care area for the care of patients who require a high level of monitoring and/or intervention following trauma or during critical illness related to trauma. |
| Inpatient Pediatric Critical Care Units | ||
| ONC Pediatric Critical Care | IN:ACUTE:CC:ONC_PED | Critical care area for the care of oncology patients ≤18 years old who are being treated for surgical or nonsurgical conditions related to their malignancy. |
| Pediatric Burn Critical Care | IN:ACUTE:CC:B_PED | Critical care area for the care of patients ≤18 years old with significant/major burns. |
| Pediatric Surgical Cardiothoracic Critical Care | IN:ACUTE:CC:CT_PED | Critical care area for the care of patients ≤18 years old following cardiac and thoracic surgery. |
| Pediatric Medical Critical Care | IN:ACUTE:CC:M_PED | Critical care area for the care of patients ≤18 years old who are being treated for nonsurgical conditions. |
| Pediatric Medical-Surgical Critical Care | IN:ACUTE:CC:MS_PED | Critical care area for the care of patients ≤18 years old with medical and/or surgical conditions. |
| Pediatric Neurosurgical Critical Care | IN:ACUTE:CC:NS_PED | Critical care area for the surgical management of patients ≤18 years old with severe neurologic diseases or those at risk for neurologic injury as a result of surgery. |
| Pediatric Respiratory Critical Care | IN:ACUTE:CC:R_PED | Critical care area for the evaluation and treatment of patients ≤18 years old with severe respiratory conditions. |
| Pediatric Surgical Critical Care | IN:ACUTE:CC:S_PED | Critical care area for the evaluation and management of patients ≤18 years old with serious illness before and/or after surgery. |
| Pediatric Trauma Critical Care | IN:ACUTE:CC:T_PED | Critical care area for the care of patients ≤18 years old who require a high level of monitoring and/or intervention following trauma or during critical illness related to trauma. |
| Inpatient Neonatal Units | ||
| Well Newborn-- Nursery (Level I) | IN:ACUTE:WARD:NURS | Hospital area for evaluation and postnatal care of healthy newborns. May include neonatal resuscitation and stabilization of ill newborns until transfer to a facility at which specialty neonatal care is provided. |
| Special Care Nursery (Level II) | IN:ACUTE:STEP:NURS | The capabilities of Level II, listed below, are from the American Academy of Pediatrics definitions of levels of neonatal care.1 Level II special care nursery Level I capabilities plus: -Provide care for infants born ≥32 wks. gestation and weighing ≥1500 g who have physiologic immaturity or who are moderately ill with problems that are expected to resolve rapidly and are not anticipated to need subspecialty services on an urgent basis -Provide care for infants convalescing after intensive care -Provide mechanical ventilation for brief duration (<24 h) or continuous positive airway pressure or both -Stabilize infants born before 32 wks. gestation and weighing less than 1500 g until transfer to a neonatal intensive care facility |
| Neonatal Critical Care (Level II/III) | IN:ACUTE:CC_STEP:NURS | Combined nursery housing both Level II and III newborns and infants, as per the NHSN level definitions above and below. This is analogous to a mixed acuity unit specifically for Neonatal Critical Care patients. |
| Neonatal Critical Care (Level III) | IN:ACUTE:CC:NURS | A hospital neonatal intensive care unit (NICU) organized with personnel and equipment to provide continuous life support and comprehensive care for extremely high- risk newborn infants and those with complex and critical illness. The capabilities of Level III, listed below, are from the American Academy of Pediatrics definitions of levels of neonatal care.1 Level III NICU Level II capabilities plus: -Provide sustained life support -Provide comprehensive care for infants born < 32 wks. gestation and weighing <1500 g and infants born at all gestational ages and birth weights with critical illness -Provide prompt and readily available access to a full range of pediatric medical subspecialists, pediatric surgical specialists, pediatric anesthesiologists, and pediatric ophthalmologists -Provide a full range of respiratory support that may include conventional and/or high-frequency ventilation and inhaled nitric oxide -Perform advanced imaging, with interpretation on an urgent basis, including computed tomography, MRI, and echocardiography |
| Neonatal Critical Care (Level IV) | IN:ACUTE:CC:NURS_IV | Critical care area for the care of newborns and infants with serious illness requiring Level IV care; area is supervised by a neonatologist Level IV Level III capabilities plus: -Located within an institution with the capability to provide surgical repair of complex congenital or acquired conditions -Maintain a full range of pediatric medical subspecialists, pediatric surgical subspecialists, and pediatric subspecialists at the site -Facilitate transport and provide outreach education |
| Inpatient Specialty Care Areas (SCA) | ||
| Dialysis Specialty Care Area | IN:ACUTE:SCA:DIAL | Specialty care area for the care of patients who require acute dialysis as a temporary measure. |
| Pediatric Dialysis Specialty Care Area | IN:ACUTE:SCA:DIAL_PED | Specialty care area for the care of patients ≤18 years old who require acute dialysis as a temporary measure. |
| Pediatric Solid Organ Transplant Specialty Care Area | IN:ACUTE:SCA:SOTP_PED | Specialty care area for the postoperative care of patients ≤18 years old who have had a solid organ transplant (for example, heart/lung, kidney, liver, pancreas). |
| Solid Organ Transplant Specialty Care Area | IN:ACUTE:SCA:SOTP | Specialty care area for the postoperative care of patients >18 years old who have had a solid organ transplant (for example, heart/lung, kidney, liver, pancreas). |
| Inpatient Adult Wards | ||
| Antenatal Care Ward | IN:ACUTE:WARD:ANTENAT | Hospital area for observation, evaluation, treatment or surgery of high-risk pregnancy patients. |
| Behavioral Health/Psych Ward | IN:ACUTE:WARD:BHV | Area for the evaluation and treatment of patients with acute psychiatric or behavioral disorders. |
| Burn Ward | IN:ACUTE:WARD:B | Area for the evaluation and treatment of patients who have burns. |
| Ear, Nose, Throat Ward | IN:ACUTE:WARD:ENT | Area for the evaluation, treatment, or surgery of patients with ear, nose, or throat disorders. |
| Gastrointestinal Ward | IN:ACUTE:WARD:GI | Area for the evaluation, treatment, or surgery of patients with disorders of the gastrointestinal tract. |
| Genitourinary Ward | IN:ACUTE:WARD:GU | Area for the evaluation, treatment, or surgery of patients with disorders of the genitourinary system. |
| Gerontology Ward | IN:ACUTE:WARD:GNT | Area for the evaluation, treatment, or surgery of patients with age-related diseases. |
| Gynecology Ward | IN:ACUTE:WARD:GYN | Area for the evaluation, treatment, or surgery of female patients with reproductive tract disorders. |
| Jail Unit | IN:ACUTE:WARD:JAL | Overnight stay patient care area of a hospital or correctional facility used only for those who are in custody of law enforcement during their treatment. |
| Labor and Delivery Ward | IN:ACUTE:WARD:LD | Area where women labor and give birth. |
| Labor, Delivery, Recovery, Postpartum Suite | IN:ACUTE:WARD:LD_PP | Suite used for labor, delivery, recovery and postpartum care -- all within the same suite. |
| Medical Ward | IN:ACUTE:WARD:M | Area for the evaluation and treatment of patients with medical conditions or disorders. |
| Medical-Surgical Ward | IN:ACUTE:WARD:MS | Area for the evaluation of patients with medical and/or surgical conditions. |
| Neurology Ward | IN:ACUTE:WARD:N | Area for the evaluation and treatment of patients with neurologic disorders. |
| Neurosurgical Ward | IN:ACUTE:WARD:NS | Area for the care of patients whose primary reason for admission is to have neurosurgery or to be cared for by a neurosurgeon after head or spinal trauma. |
| Oncology Leukemia Ward | IN:ACUTE:WARD:ONC_LEUK | Area for the evaluation and treatment of patients with leukemia. |
| Oncology Lymphoma Ward | IN:ACUTE:WARD:ONC_LYMPH | Area for the evaluation and treatment of patients with lymphoma. |
| Oncology Leukemia/Lymphoma Ward | IN:ACUTE:WARD:ONC_LL | Area for the evaluation and treatment of patients with leukemia and/or lymphoma. |
| Oncology Solid Tumor Ward | IN:ACUTE:WARD:ONC_ST | Area for the evaluation and treatment of oncology patients with solid tumors. |
| Oncology Hematopoietic Stem Cell Transplant Ward | IN:ACUTE:WARD:ONC_HSCT | Area for the care of patients who undergo stem cell transplant for the treatment of cancers, immune effector cell therapy, and/or blood or immune system disorders. |
| Oncology General Hematology-Oncology Ward | IN:ACUTE:WARD:ONC_HONC | Area for the evaluation and treatment of patients with cancer and/or blood disorders. |
| Ophthalmology Ward | IN:ACUTE:WARD:OPH | Area for the care of patients whose primary reason for admission is to have eye surgery or to be cared for by an ophthalmologist after eye trauma. |
| Orthopedic Ward | IN:ACUTE:WARD:ORT | Area for the evaluation, treatment, or surgery on bones, joints, and associated structures by an orthopedist. |
| Orthopedic Trauma Ward | IN:ACUTE:WARD:T_ORT | Area for the evaluation and treatment of patients with orthopedic injuries or disorders. |
| Onsite Overflow Ward | IN:ACUTE:WARD:OF_ONSITE | Area previously used for non-patient care which has been repurposed to care for non-critically ill or injured patients |
| Plastic Surgery Ward | IN:ACUTE:WARD:PLS | Area for the care of patients who have reconstructive surgery performed by a plastic surgeon. |
| Postpartum Ward | IN:ACUTE:WARD:PP | Area for the care of patients recovering from childbirth. |
| Pulmonary Ward | IN:ACUTE:WARD:PULM | Area for the evaluation and treatment of patients with respiratory system conditions or disorders. |
| Rehabilitation Ward (within Hospital) | IN:ACUTE:WARD:REHAB | Area for the evaluation and restoration of function to patients who have lost function due to acute or chronic pain, musculoskeletal problems, stroke, or catastrophic events resulting in complete or partial paralysis. |
| School Infirmary | IN:ACUTE:WARD:IFM | Overnight stay patient care area of a school infirmary or health center (for example, private residential school or college campus). |
| Stroke (Acute) Ward | IN:ACUTE:WARD:STRK | Area for the evaluation, stabilization, and treatment of patients who have experienced an acute stroke. |
| Surgical Ward | IN:ACUTE:WARD:S | Area for the evaluation and treatment of patients who have undergone a surgical procedure. |
| Telemetry Ward | IN:ACUTE:WARD:TEL | Hospital area dedicated to providing evaluation and treatment of patients requiring continuous cardiac monitoring |
| Vascular Surgery Ward | IN:ACUTE:WARD:VS | Area for the evaluation and treatment of patients who have undergone vascular surgery. |
| Chemical Dependency Ward | IN:ACUTE:WARD:CD | Area for the evaluation and treatment of patients with chemical dependency. |
| Inpatient Pediatric Wards | ||
| Adolescent Behavioral Health Ward | IN:ACUTE:WARD:BHV_ADOL | Area for the evaluation and treatment of patients 13-18 years old with acute psychiatric or behavioral disorders. |
| Oncology Pediatric Hematopoietic Stem Cell Transplant Ward | IN:ACUTE:WARD:ONC_HSCT_PED | Area for the care of patients ≤18 years old who undergo stem cell transplant for the treatment of cancers and/or blood or immune system disorders. |
| Oncology Pediatric General Hematology/Oncology Ward | IN:ACUTE:WARD:ONC_HONC_PED | Area for the evaluation and treatment of patients ≤18 years old with cancer and/or blood disorders. |
| Pediatric Behavioral Health Ward | IN:ACUTE:WARD:BHV_PED | Area for the evaluation and treatment of patients ≤18 years old with acute psychiatric or behavioral disorders. |
| Pediatric Burn Ward | IN:ACUTE:WARD:B_PED | Area for the evaluation and treatment of patients ≤18 years old who have tissue injury caused by burns. |
| Pediatric Ear, Nose, Throat Ward | IN:ACUTE:WARD:ENT_PED | Area for the evaluation and treatment of patients ≤18 years old with disorders of the ear, nose, and/or throat. |
| Pediatric Genitourinary Ward | IN:ACUTE:WARD:GU_PED | Area for the evaluation and treatment of patients ≤18 years old with disorders of the genitourinary system. |
| Pediatric Medical Ward | IN:ACUTE:WARD:M_PED | Area for the evaluation and treatment of patients ≤18 years old with medical conditions or disorders. |
| Pediatric Medical-Surgical Ward | IN:ACUTE:WARD:MS_PED | Area for the evaluation and treatment of patients ≤18 years old with medical and/or surgical conditions. |
| Pediatric Neurology Ward | IN:ACUTE:WARD:N_PED | Area for the evaluation and treatment of patients ≤18 years old with neurologic disorders. |
| Pediatric Neurosurgical Ward | IN:ACUTE:WARD:NS_PED | Area for care of patients ≤18 years old whose primary reason for admission is to have neurosurgery or to be cared for by a neurosurgeon after head or spinal trauma. |
| Pediatric Orthopedic Ward | IN:ACUTE:WARD:ORT_PED | Area for the evaluation and treatment of patients ≤18 years old with orthopedic injuries or disorders. |
| Pediatric Rehabilitation Ward (within Hospital) | IN:ACUTE:WARD:REHAB_PED | Area for the evaluation and restoration of function to patients ≤18 years old who have lost function due to acute or chronic pain, musculoskeletal problems, stroke, or catastrophic events resulting in complete or partial paralysis. |
| Pediatric Surgical Ward | IN:ACUTE:WARD:S_PED | Area for the evaluation and treatment of patients ≤18 years old who have undergone a surgical procedure. |
| Inpatient Step Down Units | ||
| Adult Step Down Unit | IN:ACUTE:STEP | Area for adult patients who are hemodynamically stable and can benefit from close supervision and monitoring, such as frequent pulmonary toilet, vital signs, and/or neurologic and neurovascular checks. |
| Oncology Step Down Unit | IN:ACUTE:STEP:ONC | Area for oncology patients who are hemodynamically stable and can benefit from close supervision and monitoring, such as frequent pulmonary toilet, vital signs, and/or neurologic and neurovascular checks. |
| Pediatric Step-Down Unit | IN:ACUTE:STEP:PED | Area for patients ≤18 years old who are hemodynamically stable and can benefit from close supervision and monitoring, such as frequent pulmonary toilet, vital signs, and/or neurologic and neurovascular checks. |
| Inpatient Mixed Acuity Units | ||
| Adult Mixed Acuity Unit | IN:ACUTE:MIXED:ALL_ADULT | Hospital area for the evaluation and treatment of adult patients whose conditions are of varying levels of acuity (for example, critical care, ward-level care, step-down type care, etc.). Such a care area may be comprised of patients followed by different hospital services (for example, coronary, medical, surgical, etc.). This care area may or may not include ''acuity adaptable'' or ''universal'' beds (specifically, this model of patient care allows a patient to stay in same bed during all phases of his care, from critical care through lower levels of care). |
| Pediatric Mixed Acuity Unit | IN:ACUTE:MIXED:ALL_PEDS | Hospital area for the evaluation and treatment of pediatric patients whose conditions are varying levels of acuity (for example, critical care, ward-level care, step down type care, etc.). Such a care area may be comprised of patients followed by different hospital services (for example, coronary, medical, surgical, etc.). This care area may or may not include “acuity adaptable” or “universal” beds (specifically, this model of patient care allows a patient to stay in the same bed during all phases of his care, from critical care through lower levels of care). |
| Mixed Age Mixed Acuity Unit | IN:ACUTE:MIXED:ALL | Hospital area for the evaluation and treatment of a mixture of adult and pediatric patients whose conditions are of varying levels of acuity (for example, critical care, ward-level care, step-down type care, etc.). Such a care area may be comprised of patients followed by different hospital services (for example, coronary, medical, surgical, etc.). This care area may or may not include ''acuity adaptable'' or ''universal'' beds (specifically, this model of patient care allows a patient to stay in same bed during all phases of his care, from critical care through lower levels of care). |
| Oncology Mixed Acuity Unit (all ages) | IN:ACUTE:MIXED:ONC | Area for the evaluation and treatment of a mixture of adult and pediatric oncology patients whose conditions are of varying levels of acuity (for example, critical care, ward-level care, step-down type care, etc.). This care area may or may not include "acuity adaptable" or “universal” beds (specifically, this model of patient care allows a patient to stay in the same bed during all phases of care, from critical care through lower levels of care). |
| Inpatient Operating Rooms | ||
| Cardiac Catheterization Room/Suite | IN:ACUTE:OR:CATH | A room or rooms in a hospital equipped for the performance of heart catheterizations for diagnostic or therapeutic purposes. Operating Room requirements for air changes, temperature, humidity and surfaces must be met. |
| Cesarean Section Room/Suite | IN:ACUTE:OR:LD | A room or suite in a hospital equipped for the performance of obstetric and gynecologic surgeries and for the care of the neonate immediately after birth. Operating Room requirements for air changes, temperature, humidity and surfaces must be met. |
| Interventional Radiology | IN:ACUTE:OR:RAD | A room where diagnostic or therapeutic radiology procedures are done on outpatients or inpatients. Operating room requirements for air changes, temperature, humidity, and surfaces must be met. |
| Operating Room/Suite | IN:ACUTE:OR | A room or suite in a hospital equipped for the performance of surgical operations. Requirements for air changes, temperature, humidity, and surfaces must be met. |
| Post Anesthesia Care Unit/Recovery Room | IN:ACUTE:OR_STEP | Area designated for monitoring patients for immediate effects of anesthesia before either going home or on to an in-patient care area. |
| Acute Care Facilities General | ||
| 24-Hour Observation Area | OUT:ACUTE:WARD | Area where patients are monitored for suspected or non-life-threatening conditions for 24 hours or less. More than 50% of patients in this location must be outpatients who are not expected to be admitted to an inpatient unit. |
| Emergency Department | OUT:ACUTE:ED | Area that provides emergency medical services; top priority is given to those with life-threatening illness or injury. |
| Facility-Wide Locations | ||
| Facility-wide Inpatient (FacWideIN) | FACWIDEIN | Facility-wide Inpatient (FacWIDEIn) |
| Facility-wide Outpatient (FacWideOUT) | FACWIDEOUT | Facility-wide Outpatient (FacWIDEOut) |
Sheet 4: FY2027 Submission Instructions
| USER GUIDE AND SUBMISSION INSTRUCTIONS | ||||||||||||||||
| ---> | The FY 2027 Validation Template User Guide and Submission Instructions, along with supporting documentation, can be found on the CMS QualityNet website. | |||||||||||||||
| To access, select [Hospitals–Inpatient], and then [Data Management], followed by [Data Validation], and lastly [Resources]: | ||||||||||||||||
| https://qualitynet.cms.gov/inpatient/data-management/data-validation/resources | ||||||||||||||||
| The only acceptable method of sending HAI Validation Templates is through the CMS Managed File Transfer (MFT) application: | ||||||||||||||||
| https://qnetmft.cms.gov | ||||||||||||||||
| HAI Validation Templates contain Protected Health Information (PHI) and cannot be sent via email -- even if a template were sent encrypted from a secure | ||||||||||||||||
| workplace email, it would still be considered a security violation. | ||||||||||||||||
| It is recommended to submit HAI Validation Templates at least a week prior to the submission deadline in case there are difficulties with | ||||||||||||||||
| transmitting files, and to allow time for revisions/corrections when necessary. | ||||||||||||||||
| If you are unable to log in to the CMS MFT application, the first person to contact is your hospital's Security Official (SO). | ||||||||||||||||
| If your SO is unable to establish your access, you will need to contact the Center for Clinical Standards & Quality (CCSQ) Service Center by phone at 866-288-8912. | ||||||||||||||||
| It is recommended hospitals have two SOs at all times to ensure the ability to upload Validation Templates by the established submission deadlines. | ||||||||||||||||
| We suggest hospitals ask their IT department to add [email protected] to their ‘Safe Senders List’ to ensure validation-related email notifications are received. | ||||||||||||||||
| HAI VALIDATION TEMPLATE COMPLETION & SUBMISSION TIPS | ||||||||||||||||
| Prior to submitting HAI Validation Templates to CMS, it is recommended that quality assurance is performed on the data within the template. | ||||||||||||||||
| Review the [Definitions] tab to ensure correct information is entered in each field. | ||||||||||||||||
| ü | Do not add, delete, rename, or change the order of the tabs. | |||||||||||||||
| ü | Do not add, delete, or rename column headings. | |||||||||||||||
| ü | Do not leave the first row blank or skip rows between patient data. | |||||||||||||||
| ü | Make sure the Provider ID/CCN field is exactly 6 numeric characters (do not add a hyphen). | |||||||||||||||
| ü | Make sure the State field contains the 2 character abbreviation for your state, not the full state name. | |||||||||||||||
| ü | Verify the Calendar Quarter listed on each Validation Template is correct. | |||||||||||||||
| ü | Review all dates for accuracy and correct format as specified on the [Definitions] tab. | |||||||||||||||
| ü | If a patient has not been discharged from the hospital, enter ‘Not Discharged’ for the Discharge Date field. | |||||||||||||||
| ü | The 'Specimens Toxin Positive for C. diff' column cannot include rows listing both "Yes" and "No"; entering "No" indicates no positive cultures for the quarter. | |||||||||||||||
| ü | Ensure all NHSN locations are within the approved NHSN drop down on the template. Hospital-assigned locations will not be accepted. | |||||||||||||||
| ü | Be sure to populate all required fields on each consecutive row if there were multiple final positive cultures collected for the same patient. | |||||||||||||||
| ü | Perform quality check of data entered into this template against what was entered into NHSN; stay mindful of differing CMS and NHSN deadlines. | |||||||||||||||
| ü | Check to ensure any cases with a separate Inpatient Rehabilitation Facility (IRF) or Inpatient Psychiatric Facility (IPF) CCN are not included on the template. | |||||||||||||||
| ü | Append the file name with the 6-digit CMS Certification Number (CCN)/Provider ID, followed by an underscore and the quarter. | |||||||||||||||
| For example: 012345_1QYY_FYXX_CDI_ValTemp.xlsx | ||||||||||||||||
| • When submitting templates via the [Compose] button within the Mail area of the CMS MFT dashboard, input the subject of the message | ||||||||||||||||
| with the 6-digit CCN/Provider ID, Submission Quarter, and Template type(s) attached. | ||||||||||||||||
| For example: 012345 1QYY FYXX MRSA & CDI Validation Templates | ||||||||||||||||
| • When choosing a recipient, select the ellipsis button to the right of the To field and then select the [Groups] tab to locate the "Validation Support Contractor" group. | ||||||||||||||||
| Do NOT select any individual person(s) from the recipient list; only select the "Validation Support Contract" Group. | ||||||||||||||||
| Some individual accounts are not regularly monitored—sending to any one individual risks delay in processing. | ||||||||||||||||
| • Leave the 'Require Registered Users' box checked under Options. Un-checking this box puts the message at risk of not being processed. | ||||||||||||||||
| • We strongly encourage hospitals to add a check on the 'Read Receipt' box under Options. | ||||||||||||||||
| After a file has been downloaded by someone on the Validation Support Contractor team, it will be in the queue for processing. | ||||||||||||||||
| • It is suggested that users verify a message has been sent by clicking on the [Sent Items] button from the left-side navigation panel of the MFT dashboard. | ||||||||||||||||
| NOTE: It can take a couple minutes for messages to appear in the Sent Items folder. Please, do NOT re-send messages multiple times, | ||||||||||||||||
| as this significantly delays processing and requires version confirmation. | ||||||||||||||||
| • You will receive email confirmation (usually within 2 business days of being downloaded) from the Validation Support Contractor letting you know the Validation | ||||||||||||||||
| Templates were processed. If you do not receive a processing confirmation, please include your hospital's 6-digit CCN/Provider ID in an | ||||||||||||||||
| email to | [email protected] | |||||||||||||||
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy