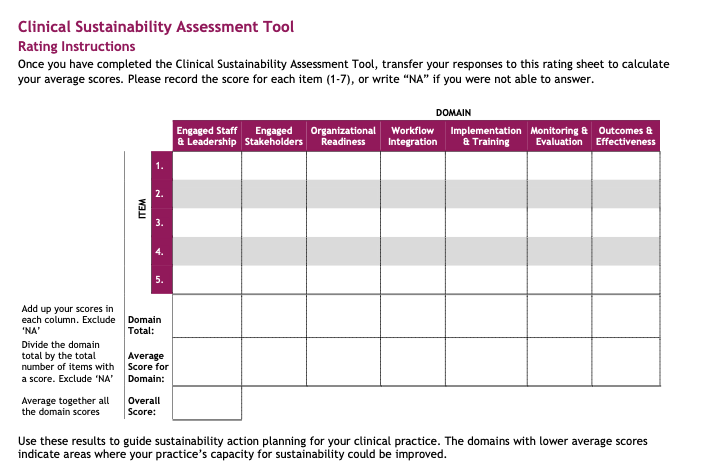
Form 8 Clinical Sustainability Assessment Tool
Implementation and Testing of Diagnostic Safety Resources
Attachment I - Clinical Sustainability Assessment Tool (CSAT).2024_5_24
8. Attachment I - Clinical Sustainability Assessment Tool (CSAT)
OMB:
Attachment I: Clinical Sustainability Assessment Tool (CSAT)
Form
Approved
OMB No. 0935-XXXX
Exp. Date
XX/XX/20XX
This
survey is authorized under 42 U.S.C. 299a. This
information collection is voluntary and the
confidentiality of your responses to this survey is protected by
Sections 944(c) and 308(d) of the Public Health Service Act [42
U.S.C. 299c-3(c) and 42 U.S.C. 242m(d)]. Information that could
identify you will not be disclosed unless you have consented to that
disclosure. Public reporting burden for this collection of
information is estimated to average
15 minutes
per response, the estimated time required to complete the survey. An
agency may not conduct or sponsor, and a person is not required to
respond to, a collection of information unless it displays a
currently valid OMB control number. The data you provide will help
AHRQ’s mission to produce evidence to make health care safer,
higher quality, more accessible, equitable, and affordable. Send
comments regarding this burden estimate or any other aspect of this
collection of information, including suggestions for reducing this
burden, to: AHRQ Reports Clearance Officer Attention: PRA, Paperwork
Reduction Project (0935-xxxx) AHRQ, 5600 Fishers Lane, Room #07W42,
Rockville, MD 20857, or by email to the AHRQ MEPS Project Director
at [email protected].
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Danielle Schlang |
| File Modified | 0000-00-00 |
| File Created | 2024-07-22 |
© 2026 OMB.report | Privacy Policy