CMS-10639 Supporting Statement A NHSN CY 2025
CMS-10639 Supporting Statement A NHSN CY 2025.docx
National Healthcare Safety Network (NHSN) Data Validation Study for the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) (CMS-10639)
OMB: 0938-1340
Supporting Statement – Part A
National Healthcare Safety Network (NHSN) Data Validation for the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) (CMS-10639)
Background
Pursuant to section 1881(h) of the Social Security Act (the Act) as amended by section 153(h) of the Medicare Improvements for Patients and Providers Act (MIPPA), the Centers for Medicare & Medicaid Services (CMS) established the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) starting in 2011. The ESRD QIP is the first nationally implemented value-based purchasing program established by CMS, and it is aimed at promoting patient health by providing a financial incentive for renal dialysis facilities to deliver high-quality care.
In implementing the ESRD Quality Incentive Program, CMS believes that a successful quality incentive program will promote the delivery of high-quality health care services in the renal dialysis facility setting. Under section 1881(h)(2) of the Act, the Secretary is required to specify quality measures for evaluating the quality of care ESRD patients receive at renal dialysis facilities. While the Act outlines a few mandatory measure topics, the Secretary is authorized to adopt measures on specified areas of medical topics determined appropriate by the Secretary (§ 1881(h)(2) of the Act). The ESRD QIP began in calendar year (CY) 2011 with an initial set of three quality measures and has increased and refined the measure set over the intervening years through notice and comment rulemaking.
To score facility performance on quality measures, CMS must be able to collect data on these measures. CMS collects these data from multiple sources, including Medicare claims (OMB Control Number 0938-1197) and other tools such as the Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) Dialysis Event Protocol. To further expand the measures used to evaluate the quality of care provided to ESRD patients in renal dialysis facilities, CMS also collects data using the ESRD Quality Reporting System (EQRS), formerly known as CROWNWeb. The information collection requirements associated with the ESRD QIP measures that use data collected in EQRS are covered under OMB Control Number 0938-1289. The information collection requirements associated with the NHSN Bloodstream Infection Modules are captured under OMB Control Number 0920-0666 (the National Healthcare Safety Network).
One of the critical elements of the ESRD QIP’s success is ensuring that the data submitted to calculate measure scores and Total Performance Scores (TPS) are accurate. In the CY 2015 ESRD PPS final rule, CMS finalized a feasibility study for Payment Year (PY) 2017 and PY 2018 to validate data reported to the CDC’s NHSN Dialysis Event Module for the NHSN Bloodstream Infection clinical measure. The purpose of this study was to compare the accuracy and frequency of dialysis event data reported to the NHSN system, to what was recorded in patient medical records for those years. Healthcare-Acquired Infections (HAI) are relatively rare, and CMS finalized that the feasibility study would target records with a higher probability of including a dialysis event, because this would enrich the validation sample while reducing the burden on facilities. For CY 2015, the feasibility study examined records from only 9 ESRD facilities.
The Feasibility Study in PY 2017 was conducted as follows: the validation contractor mailed CMS-approved formal request letters and packets to the nine selected facilities and asked for lists of all CY 2015 positive blood cultures, associated medical records, completed surveys, and patient cover sheets. The medical records were considered the “gold standard” for comparison purposes. Trained medical reviewers abstracted the data from the patient medical records. All nine selected facilities participated in the survey and submitted their positive blood culture templates. A total of 16 positive blood cultures were identified from those facilities. After abstracting the 16 patient charts, reviewers identified 21 reportable events. Due to the relatively small sample size, there were no instances where the chart reviewed was without an event and there were no events reported in NHSN for that patient during the time period. That would constitute a true negative. The facilities selected were also asked to complete a survey to provide information related to their participation with the feasibility study. They provided information regarding the processes used to track Dialysis Events as well as how their denominators were calculated. This information helped generalize findings and identify potential deficiencies and improvement opportunities. Based on the results of the feasibility study and the survey responses, the validation contractor made several recommendations for improvement opportunities. Specifically, they recommended:
Additional training for Dialysis Event abstraction covering complex multiple dialysis events reporting as well as commonly found reporting errors.
A revision of the definition of a “match,” considering a match of the data if the report is within +/- 3 days of the event.
Using a more robust study methodology, possibly expanding analysis from only one Dialysis Event to include the other two types of events and increasing the sample size to identify areas of required adjustment.
CMS took these recommendations and developed a more robust data validation to be implemented in the PY 2019 ESRD QIP. Due to the changes in the sample size and the methodology, described below, CMS developed a PRA package specific to the NHSN Data Validation work being done for the ESRD QIP beginning with the PY 2019 Program. CMS updated this PRA Package for the PY 2020 ESRD QIP, the PY 2021 ESRD QIP, the PY 2022 ESRD QIP, the PY 2023 ESRD QIP, the PY 2024 ESRD QIP, the PY 2025 ESRD QIP, the PY 2026 ESRD QIP, and now CMS is updating the PRA Package for the PY 2027 ESRD QIP, as discussed more fully below.
NHSN Data Validation for the ESRD QIP
CMS finalized a new methodology for the NHSN Data Validation work conducted in PY 2019 and later finalized a policy continuing use of that methodology with slight modifications to the sampling methodology. Additionally, for PY 2019, CMS finalized that the validation would consist of 35 facilities—a policy that was continued for PY 2020.
For PY 2021, CMS continued use of the PY 2020 methodology, with some modifications. CMS increased the number of participating facilities from 35 to 150 facilities for the validation conducted in CY 2019 for the PY 2021 validation. Beginning with the PY 2021 validation, CMS also increased the number of collected records from 10 to 20 records per quarter over the first two quarters. For PY 2022, CMS finalized a policy to continue use of the PY 2021 validation methodology with one modification; CMS increased the number of participating facilities from 150 to 300 facilities for the validation conducted in CY 2020 for the PY 2022 ESRD QIP. Further details on the methodology finalized for use in the PY 2022 ESRD QIP are provided below. The purpose of this validation is to compare the data entered by facilities into the CDC’s NHSN system against what is reported in medical records.
For the PY 2022 validation, CMS finalized a policy to select 300 facilities to participate in NHSN Dialysis Event validation by submitting 20 patient records per quarter covering two quarters of data reported in CY 2020, for a total of 40 patient records per year. For the PY 2023 validation and continuing thereafter, CMS finalized an update to this policy to reflect that facilities selected to participate in the NHSN validation can submit a total of 20 records across any 2 quarters, and are not restricted to the first 2 quarters of the calendar year. A CMS contractor will send the selected facilities requests for medical records for all patients with “candidate events” during the evaluation period (i.e. patients who had any positive blood cultures; received any intravenous antimicrobials; had any pus, redness, or increased swelling at a vascular access site; and/or were admitted to a hospital during the evaluation period). Facilities will have 60 calendar days to respond to the request for medical records based on candidate events either electronically or on paper. If the contractor determines that additional medical records are needed to reach the 20-record threshold from a facility to validate whether the facility accurately reported the dialysis events, then the contactor will send a request for additional, randomly selected patient records from the facility. The facility will have 60 calendar days from the date of the letter to respond to the request. Through collaboration with the CDC for system and data access, the CMS contractor will utilize the methodology described above for reviewing and validating records from candidate events and randomly selected patients, to determine whether the facility reported dialysis events for those patients in accordance with the NHSN Dialysis Event Protocol. If a facility is selected to participate in the validation but does not provide CMS with the requisite lists of positive blood cultures within 60 calendar days of receiving a request, then CMS will deduct 10 points from the facility’s TPS. Information from the validation may also be used to inform our consideration of future program policies that would incorporate NHSN data accuracy into the scoring process.
Justification
Need and Legal Basis
Dialysis facilities, researchers, and patient advocacy groups, as well as other stakeholders who have previously submitted public comments on the ESRD PPS Proposed Rule, have expressed significant concerns about facilities not reporting dialysis events when they should be reported. These public comments, as well as a thorough review of data reported for the PY 2016 NHSN BSI clinical measure, and results from the NHSN data validation feasibility study, suggest that 23 percent of dialysis events are under-reported, and have clarified the delicate tradeoffs associated with incentivizing facilities to report and prevent dialysis events. Due to the small sample size of facilities that are validated through the ESRD QIP data validation, it is difficult to pinpoint exactly why the underreporting rate is high. CMS believes that the leading cause for underreporting is due to a lack of clear and consistent communication between hospitals and dialysis facilities. Complete and accurate reporting is critical to maintaining the integrity of the NHSN surveillance system, enables facilities to implement their own quality improvement initiatives, and enables the CDC to design and disseminate prevention strategies. To gain a more accurate understanding of the patient population and the data being submitted to NHSN, it is imperative that the data validation be expanded to include a greater number of facilities. As noted above, we have finalized incremental expansion of the sample for NHSN validation over two years (PY 2021 and PY 2022), and CMS believes that this expansion is necessary to ensure that NHSN data are accurate and complete. Based on statistical analyses conducted by CDC, with an expected accuracy of 80% of dialysis events from facilities and setting the precision of the NHSN validation to 95% confidence and 1% margin of error, we estimate that a total of 303 facilities and 6,072 chart reviews would be necessary to achieve the appropriate statistical power for the data validation. However, because that increase in sample size would represent a nearly tenfold increase in sampled facilities compared to the PY 2020 ESRD QIP, CMS finalized an incremental expansion of the sample size incrementally over two years (PY 2021 and PY 2022). This PRA package focuses on the PY 2027 validation.
Information Users
Section 1881(h) of the Act requires the Secretary, generally, to adopt a set of quality measures and assess the quality of care provided by renal dialysis facilities using those measures. The information that comes out of the validation will be used by CMS and others to monitor and assess the accuracy of data reported to NSHN as part of the ESRD QIP. The information will also be used by CMS to identify areas where data accuracy could be improved and to direct its contractors to engage in activities designed to improve data accuracy in those areas (e.g., the development of targeted trainings to help facilities improve the accuracy of data reporting). While facilities selected for participation in a given year will receive confidential feedback, non-selected facilities, beneficiaries, and the public do not have access to validation results.
Use of Information Technology
The NHSN data validation allows facilities to submit their quarterly data either electronically or on paper. These expenses would not exceed what is normally expended for a typical healthcare facility infection surveillance program. Paper forms are provided to facilities for data collection, but they are not required to use them for entry of data into NHSN or for submission to CMS for the data validation.
Duplication of Efforts/Similar Information
The purpose of the validation is to review the medical records of the selected patients to ensure that the facility accurately reported any dialysis event data to the NHSN system. The patient medical record is the most accurate source of information regarding each patient’s condition and symptoms. The validation does not require facilities to duplicate their effort and report identical data to CMS, rather it requires facilities to submit the complete patient record so that CMS can independently review and identify whether the facility properly reported dialysis events into NHSN according to the CDC’s dialysis event protocol.
Small Businesses
Facilities treating 10 or fewer patients are excluded from NSHN Dialysis Event reporting. Individuals on peritoneal dialysis are also not included. Information collection requirements were designed to impose minimal burdens on small renal dialysis facilities subject to the ESRD QIP. Specifically, the NHSN system was created to allow small renal dialysis facilities to enter data via their web-based application rather than using paper-based data submission or employing a full electronic health record, which can be prohibitively expensive for these facilities. As a result, this effort facilitates small renal dialysis facilities’ collection and reporting of required data.
Less Frequent Collection
Each month, facilities report the number of maintenance hemodialysis outpatients who were dialyzed in the facility on the first two working days of the month, using the Denominators for Outpatient Dialysis form. This count is used to estimate the number of patients at the facility who are at risk of HAIs. Each month, facilities use a Dialysis Event form to report the details of each of three infection related dialysis events (IV antimicrobial starts, positive blood cultures, and evidence of local access site infection) that occurred among their patients. Due to the seasonal variability of bloodstream infections, it is absolutely essential for facilities to report the full 12 months of data to reflect performance over the course of the entire performance period.
While the CDC requires that facilities report these data monthly under a previously approved PRA package (OMB Control Number 0920-0666), the data validation policy requires selected facilities to submit lists of dialysis events across any two quarters of a given year. At this time, CMS is only able to validate two quarters of data due to operational constraints regarding when the data are available and when the scores and possible TPS reductions are calculated. Per the ESRD QIP guidelines, facilities are required to complete reporting for each quarter before the end of the subsequent quarter. This requirement hinders CMS’s ability to collect additional data and compute final scores prior to the start of the preview period.
Special Circumstances
The data validation methodology is designed to collect quarterly data from dialysis facilities. Without this quarterly data, the CMS contractor will not be able to complete the data validation in accordance with CMS’s data validation policy. CMS therefore believes that quarterly collection is most appropriate in order to appropriately complete the NHSN data validation for the ESRD QIP.
Federal Register Notice/Outside Consultation
The CY 2025 ESRD PPS proposed rule served as the 60-day Federal Register notice and published on July 5, 2024 (89 FR 55760).
Payment or Gift to Respondent
Dialysis facilities are required to submit measure data to CMS as part of the Conditions for Coverage of End-Stage Renal Disease Facilities (see 42 CFR 494.180(h)). No additional payments or gifts will be given to respondents for compliance with the requirements of the NHSN Data Validation for the ESRD QIP. As noted above, if a facility is selected to participate in the validation but does not provide CMS with the requisite medical records within 60 calendar days of receiving a request, then we would deduct 10 points from the facility’s TPS. This ten-point reduction may subject the facility to a payment penalty under the Program.
Confidentiality
CMS adheres to all confidentiality-related statutes, regulations, and agency policies. All information collected under ESRD QIP will conform to all applicable Federal laws and regulations and Federal, HHS, and CMS policies and standards as they relate to information security and data privacy. The laws and regulations that may apply include, but are not limited to: The Privacy Act of 1974; the Federal Information Security Management Act of 2002; the Computer Fraud and Abuse Act of 1986; the Health Insurance Portability and Accountability Act of 1996; the EGovernment Act of 2002, the Clinger Cohen Act of 1996; the Medicare Modernization Act of 2003, and the corresponding implementing regulations. OMB Circular A–130, Management of Federal Resources, Appendix III, Security of Federal Automated Information Resources also applies. Federal, HHS, and CMS policies and standards include but are not limited to: All pertinent National Institute of Standards and Technology publications; the HHS Information Systems Program Handbook and the CMS Information Security Handbook.
SORN #: 09-70-0520 – ESRD Program Management and Medical Information System (PMMIS) published 6/17/2002 (67 FR 41244), updated 5/8/2007 (72 FR 26126), and revised 6/26/2009 (74 FR 30606).
Sensitive Questions
There are no questions of a sensitive nature being collected as part of this data validation.
Burden Estimates
Consistent with the currently approved PRA, CMS continues to use the following equation to estimate burden associated with the NHSN Data Validation for PY 2027:
Burden =
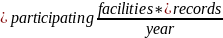 *
*
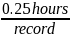 *
*
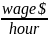
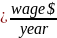

Table A. NHSN Data Validation Burden Estimate Elements
Burden Estimate Element |
PY 2027/CY 2025 |
Number of facilities participating in the NHSN Data Validation, annually |
300 |
Estimated number of medical records per facility per year |
20 |
Time spent for record collection and submission per facility |
5 hours (approx. 0.25 hours per record) |
Hourly wage per hour engaged in data collection and submission plus overhead and benefits |
$45.38 |
Under the NHSN data validation process previously finalized for PY 2027, CMS will sample records from 300 facilities. A CMS contractor will send these facilities quarterly requests.
To derive wage estimates, CMS used data from the U.S. Bureau of Labor Statistics’ (BLS) May 2022 National Occupational Employment and Wage Estimates.1 CMS anticipates that the labor required to collect and submit these data will be completed by either Medical Records Specialists or similar administrative staff. The median hourly wage of a Medical Records Specialist is $22.69. Fringe benefits and overhead are calculated at 100% using current HHS department-wide guidance on estimating the cost of fringe benefits and overhead. These are necessarily rough adjustments both because fringe benefits and overhead costs vary significantly from employer to employer and because methods of estimating these costs vary widely from validation to validation. Nonetheless, there is no practical alternative and CMS believes that these are reasonable estimation methods.
Using the assumptions described above, CMS estimates that an hourly labor cost of $45.38 as the basis of the wage estimates for all collection of information calculations in the ESRD QIP. CMS also estimates the total annual burden for the CY 2025/PY 2027 validation is $68,070.
Table B. NHSN Data Validation Burden Per Facility, PY 2027/CY 2025
NHSN Data Validation Facilities CY 2024 |
Number of Facilities |
Number of Records Per Year |
Estimated Time Per Record |
Estimated Wage Plus Benefits Per Hour for Record Collection |
Annual Hour Burden Per Facility |
Annual Burden Per Facility |
NHSN Data Validation |
300 |
20 |
0.25 |
$45.38 |
5 |
$226.90 |
Table C. NHSN Total Data Validation Burden, PY 2027/CY 2025
-
Basis
Annual Hour Burden
Annual Burden
Each Facility
5
$226.90
National
1,500
$68,070.00
Capital Cost
There are no capital costs.
Cost to Federal Government
The cost to the Federal Government includes costs associated with the collection and validation of the data. The validation costs are an estimated $165,000 (FY) annually for the validation contract. The CDC maintains the NHSN system. The estimated cost to operate the validation contract includes 20 percent of one CMS staff at the GS-13 Step 5 Level (approximate salary is $117,516). This results in a total estimated cost of $188,503 ($165,000 + 0.20 x $117,516) annually.
Changes to Burden
This is a revision to an information collection request (ICR). The currently approved PRA associated with the PY 2026 validation estimates a total burden of 1,500 hours at a cost of $68,070 for 300 facilities to submit a total of 20 records per year (using updated wage rates). There is no change in burden in this PRA for PY 2027. The annual burden associated with the PY 2027 validation is 1,500 hours at a cost of $68,070.
Publication/Tabulation Date
NHSN is an ongoing data collection system, and as such, does not have an annual timeline for the collection of medical record information. The data are reported on a continuous basis by participating institutions and aggregated by CDC into a national database that is analyzed for two main purposes: to describe the epidemiology of healthcare-associated adverse events, and to provide comparative data for populations with similar risks. Comparative data can be used by participating and non-participating healthcare institutions that collect their data using NHSN methodology.
Expiration Date
CMS will display the expiration date and the new OMB control number on the collection instruments.
Explain any exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
There are no exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
1 https://www.bls.gov/oes/2022/may/oes292072.htm.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lallemand, Nicole |
| File Modified | 0000-00-00 |
| File Created | 2024-09-06 |
© 2026 OMB.report | Privacy Policy