Explanation for Program Changes or Adjustments Detailed
D2. Explanation for Program Changes or Adjustments 2024-0920-0666.docx
[NCEZID] The National Healthcare Safety Network (NHSN)
Explanation for Program Changes or Adjustments Detailed
OMB: 0920-0666
D2. Explanation for Program Changes or Adjustments 2024 National Healthcare Safety Network (NHSN)
OMB Control No. 0920-0666
Revision Request September 2024
57.103 Patient Safety Component--Annual Hospital Survey NHSN Patient Safety Component (PSC) Annual Survey collects facility-level data from the previous calendar year and is completed by all facilities enrolled in the NHSN Patient Safety Component. The Annual Survey data is used to calculate healthcare associated infection (HAI) Standardized Infection Ratio (SIR) risk adjustment models and track HAI incidence in facilities. The data is also used to support decision making, program planning, and research across CDC. The SIR is available for use for CMS Quality Reporting for select HAI and facility types, state health departments, other organizations, or groups (i.e., Leapfrog) and CDC in national surveillance reports. The survey is collected electronically on an annual basis via the NHSN application.
By updating the PSC Annual Survey, NHSN is ensuring improved relevance, enhanced data quality, alignment with industry standards and regulations, increased efficiency, and expanded analysis capabilities within CDC. |
||||||||||||||||
Type of Change |
Changed From |
Changed To |
Justification |
Impact to Burden |
||||||||||||
Revision |
*2. For the following organisms, indicate which methods are used for: (1) Primary susceptibility testing and (2) Secondary, supplemental, or confirmatory testing (if performed).
If your laboratory does not perform susceptibility testing, indicate the methods used at the outside laboratory.
|
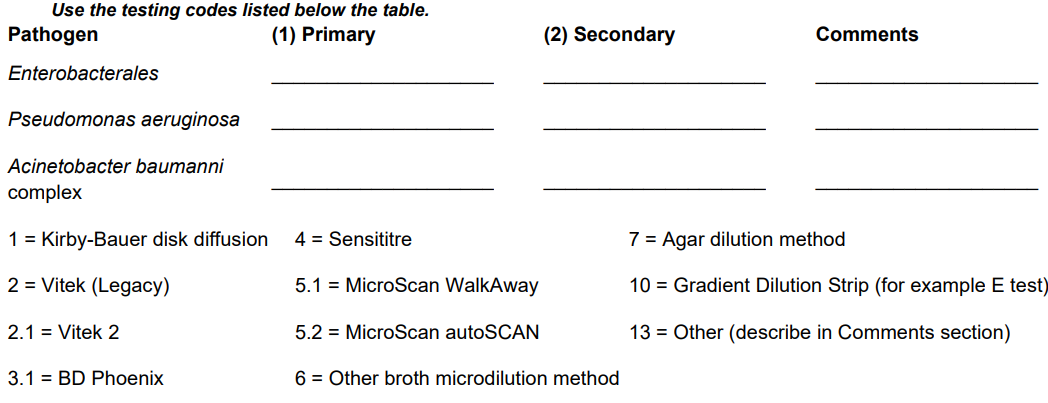
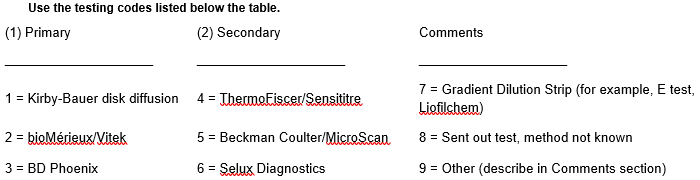
*2. For Enterobacterales, Pseudomonas aeruginosa and/or Acinetobacter baumannii complex, indicate which methods are used for: (1) Primary susceptibility testing and (2) Secondary, supplemental, or confirmatory testing (if performed).
If your laboratory does not perform susceptibility testing, indicate the methods used at the outside laboratory.
|
Simplified the question to have facilities respond only 1 time (not per organism). Updated the response options to reflect currently used lab tests |
0.5 minute decrease |
||||||||||||
revision |
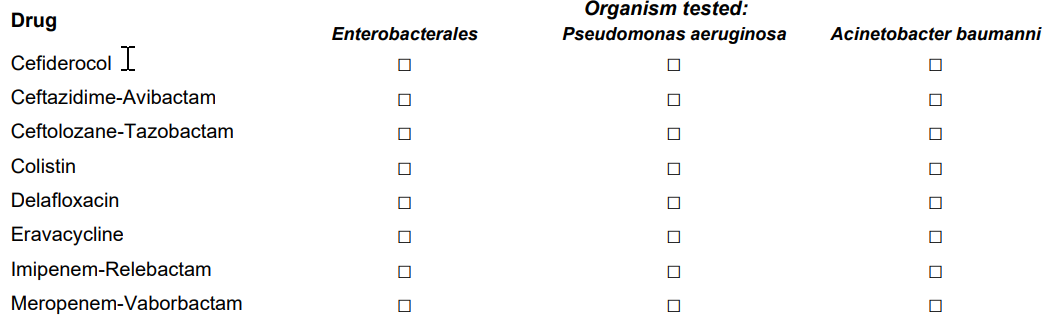
*3. Does either primary or secondary/supplemental antimicrobial susceptibility testing (AST) include the following (check all that apply):
|
*3. Does either primary or secondary/supplemental antimicrobial susceptibility testing (AST) include the following (check all that apply):
|
Simplified the question to have facilities respond only 1 time per drug (not per organism). Updated the response options to reflect drugs of interest. |
No change |
||||||||||||
revision |
|
*4. Has the laboratory implemented revised breakpoints recommended by CLSI for the following: a. Third Generation Cephalosporin and monobactam (i.e. aztreonam) breakpoints for Enterobacterales in 2010 □ Yes □ No b. Carbapenem breakpoints for Enterobacterales in 2010 □ Yes □ No c. Ertapenem breakpoints for Enterobacterales in 2012 □ Yes □ No d. Carbapenem breakpoints for Pseudomonas aeruginosa in 2012 □ Yes □ No e. Fluroquinolone breakpoints for Pseudomonas aeruginosa in 2019 □ Yes □ No f. Fluroquinolone breakpoints for Enterobacterales in 2019 □ Yes □ No g. Aminoglycoside breakpoints for Enterobacterales in 2023 □ Yes □ No h. Aminoglycoside breakpoints for Pseudomonas aeruginosa in 2023 □ Yes □ No i. Piperacillin-tazobactam breakpoints for Pseudomonas aeruginosa in 2023 □ Yes □ No j. Piperacillin-tazobactam breakpoints for Enterobacterales in 2022 □ Yes □ No |
to monitor the uptake of up-to-date CLSI breakpoints among clinical laboratories and interpret antimicrobial surveillance data which reuse hospital interpretations of antimicrobial susceptibility testing results. The additional organism-drug combos are the those that CLSI recently updated the breakpoints on. |
0.5 minute increase |
||||||||||||
Revision |
*5. Does the laboratory test bacterial isolates for presence of carbapenemase? (this does not include automated testing instrument expert rules) □ Yes □ No |
*5. Does the laboratory test bacterial isolates for presence of a carbapenemase? (this does not include automated testing instrument expert rules) □ Yes □ No |
Grammar update |
No change |
||||||||||||
Revision |
|
5b. If Yes, which test is routinely performed to detect carbapenemase: (check all that apply)
□ Nucleic Acid Amplification Test (for example, PCR, Cepheid) □ NG-Test Carba-5 (or other lateral flow assay) □ Modified Hodge Test □ Carba NP □ mCIM/CIM □ Other_________ |
Update of tests to more accurately reflect tests in use. |
No change |
||||||||||||
Deletion of question |
*9. Does your facility perform extended-spectrum beta-lactamase (ESBL) testing for E. coli Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis routinely or using a testing algorithm? □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
not needed anymore |
0.5 minute decrease |
||||||||||||
Revision |
*14. Does the laboratory employ any molecular tests to identify Candida from blood specimens? □ Yes □ No □ Unknown |
*13. Does the laboratory employ any PCR molecular tests to identify Candida from blood specimens? □ Yes □ No □ Unknown
|
Revised question wording to increase clarity. |
No change |
||||||||||||
Revision |
|
13a. If yes, which PCR molecular tests are used to identify Candida from blood specimens? (check all that apply) □ T2Candida Panel □ BioFire BCID □ GenMark ePlex BCID □ Other, specify: _________________ □ Unknown |
Revised question wording to increase clarity. |
No change |
||||||||||||
Revision |
*16. What method is used for antifungal susceptibility testing (AFST), excluding Amphotericin B? (check all that apply)
|
*15. What methods are used for antifungal susceptibility testing (AFST), excluding Amphotericin B? (check all that apply) |
Grammar update |
No change |
||||||||||||
Revision |
*17. What method is used for antifungal susceptibility testing (AFST) of Amphotericin B? (check all that apply)
|
*16. What methods are used for antifungal susceptibility testing (AFST) of Amphotericin B? (check all that apply) |
Grammar update |
No change |
||||||||||||
Revision |
*22. Indicate the primary and definitive method used to identify microbes from blood cultures collected in your facility. (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, OmniLog, Sherlock, etc.) □ Non-automated Manual Kit (for example, API, Crystal, RapID, etc.) □ Rapid Identification (for example, Verigene, BioFire FilmArray, PNA-FISH, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
*21. Which of the following methods serve as the primary method used for bacterial identification at your facility? (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, etc.) □ Non-automated Manual Kit (for example, API 20C, biochemicals) □ Rapid Identification (for example, NAAT/PCR, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
Updated question to more accurately reflect what we'd like facilities to answer. |
No change |
||||||||||||
revision |
*23. Indicate any additional secondary methods used for microbe identification from blood cultures collected in your facility (for example, a rapid method that is confirmed with the primary method, a secondary method if the primary method fails to give an identification, or a method that is used in conjunction with the primary method). (check all that apply) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, OmniLog, Sherlock, etc.) □ Non-automated Manual Kit (for example, API, Crystal, RapID, etc.) □ Rapid Identification (for example, Verigene, BioFire FilmArray, PNA-FISH, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
*22. Which of the following methods serve as the secondary or backup method used for bacterial identification at your facility? (for example, a secondary method if the primary method fails to give an identification, or if the primary method is unavailable). (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, etc.) □ Non-automated Manual Kit (for example, API 20C, biochemicals) □ Rapid Identification (for example, NAAT/PCR, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
Updated question to more accurately reflect what we'd like facilities to answer. |
No change |
||||||||||||
Revision |
*33. Does the facility routinely perform screening testing (culture or non-culture) for MRSA for any patients admitted to NICU settings? □ Yes □ No |
*32. Does the facility routinely perform screening testing (culture or non-culture) for MRSA for any patients admitted to NICU settings? □ Yes □ No □ N/A, facility does not have a NICU |
adding a N/A option as not all hospitals have a NICU |
No change |
||||||||||||
Deletion of question |
*36. Was this section completed in collaboration with your facility’s neonatal or newborn patient care team? For example, was input sought from a neonatal or newborn patient care team member, such as a NICU Medical Director, Lead Neonatal Physician, Neonatal Nurse Manager, Lead Neonatal Nurse Practitioner? □ Yes □ No □ N/A, my facility does not provide neonatal or newborn patient care services at any level (specifically, my facility does not provide delivery services, Level 1 well newborn care, Level II special care, or neonatal intensive care) |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Added new question |
N/A |
*35. Does your facility provide neonatal or newborn patient care services at any level (specifically, does your facility provide delivery services, Level 1 well newborn care, Level II special care, or neonatal intensive care)? □ Yes □ No |
We don't use the data on whether input was sought so felt that portion could go. We do, however, need to know whether neonatal care is provided for the skip pattern. |
0.5 minute increase |
||||||||||||
Deletion of question |
*42. Did the antibiotic stewardship leader(s) participate in responding to these questions? (Check one.) □ Yes, pharmacist lead □ Yes, physician lead □ Yes, both pharmacist and physician leads □ Yes, other lead □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
NA |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
□ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
55. Antibiotic stewardship activities are integrated into quality improvement and/or patient safety initiatives. □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
56. Our facility accesses targeted remote stewardship expertise (for example, tele-stewardship to obtain facility-specific support for our antibiotic stewardship efforts). □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
57. Our stewardship program works with the microbiology laboratory to implement the following interventions: (Check all that apply) □ Selective reporting of antimicrobial susceptibility testing results □ Placing comments in microbiology reports to improve prescribing □ None of the above |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
58. Which committees or leadership entities provide oversight of your facility’s antibiotic stewardship efforts? (Check all that apply)
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Revision |
|
53b. If Yes: This program or committee includes the following healthcare personnel: (Check all that apply; check at least one) □ Physician □ Quality improvement staff member □ Nurse □ Case manager □ Pharmacist □ Microbiology staff member or Laboratory staff member □ Advanced practice provider (for example, Physician Assistant, Nurse Practitioner □ Discharge planner □ Hospital Epidemiologist or Infection prevention professional □ Patients/families/caregivers □ Phlebotomist □ Outpatient clinicians □ Social worker □ None of the above |
Changes made reflect the final draft of the hospital sepsis core elements document. |
No change |
||||||||||||
Revision |
61. Facility leadership has demonstrated commitment to improving sepsis care by: (Check all that apply; check at least one.) □ Providing sepsis program leader(s) with sufficient specified time to manage the hospital sepsis program. □ Providing sufficient resources, including data analytics and information technology support, to operate the program effectively. □ Ensuring that relevant staff from key clinical groups and support departments have sufficient time to contribute to sepsis activities. □ Appointing a senior leader to serve as an executive sponsor for the sepsis program. □ Identifying sepsis as a facility priority and communicating this priority to hospital staff. □ None of the above.
|
*55. Facility leadership has demonstrated commitment to improving sepsis care by: (Check all that apply; check at least one.) □ Providing sepsis program leader(s) with sufficient specified time to manage the hospital sepsis program. □ Providing sufficient resources, including data analytics and information technology support, to operate the program effectively. □ Ensuring that relevant staff from key clinical groups and support departments have sufficient time to contribute to sepsis activities. □ Appointing a senior leader to serve as an executive sponsor for the sepsis program. □ Identifying sepsis as a facility priority and communicating this priority to hospital staff. □ Having a sepsis coordinator who oversees day-to-day implementation of sepsis program activities □ None of the above. |
Changes made reflect the final draft of the hospital sepsis core elements document. |
No change |
||||||||||||
revision |
*64. Our facility uses the following approaches to promote evidence-based management of patients with sepsis: (Check all that apply; check at least one.) □ Hospital guideline or care pathway for management of sepsis □ Hospital order set for management of sepsis □ Structured template for documentation of sepsis treatment □ Standardized process for verbal hand-off of sepsis treatment □ Sepsis Response Team □ Rapid Response Team with training in sepsis management □ None of the above
|
*58. Our facility uses the following approaches to promote evidence-based management of patients with sepsis: (Check all that apply; check at least one.) □ Hospital guideline or care pathway for management of sepsis □ Hospital order set for management of sepsis □ Structured template for documentation of sepsis treatment □ Standardized process for verbal hand-off of sepsis treatment □ Sepsis Response Team □ Rapid Response Team with training in sepsis management □ Use of “Code Sepsis” protocol for facilitating prompt recognition and team-based care of sepsis □ None of the above |
Changes made reflect the final draft of the hospital sepsis core elements document. |
No change |
||||||||||||
Revision |
*69. Describe your facility’s use of manual chart review for sepsis performance evaluation and improvement: (Check one.) □ We review all sepsis hospitalizations □ We review all sepsis hospitalizations with adverse outcomes (e.g., all hospitalizations with in-hospital mortality) □ We review a sample of sepsis hospitalizations (e.g., a random sample) □ We do not complete routine chart reviews of sepsis hospitalizations
|
*63. Describe your facility’s use of chart review for sepsis performance evaluation and improvement: (Check all that apply.) □ We routinely review some or all sepsis hospitalizations to influence clinical care in real-time. □ We routinely review some or all sepsis hospitalization within 48 hours to provide positive feedback to individual clinicians on areas where care excelled. □ We routinely review some or all sepsis hospitalization within 48 hours to provide constructive feedback to individual clinicians on areas where care could be improved. □ We routinely review some or all sepsis hospitalizations to evaluate performance or to inform quality improvement work (e.g., root-cause analysis). □ We review charts for other purposes. □ We do not complete routine chart reviews of sepsis hospitalizations. |
Facilities have other methods besides manual. We wanted this question to be more inclusive of other electronic means.
|
0.5 minute increase |
||||||||||||
Deletion of a question |
*71. Clinicians receive feedback regarding their care of specific patients with sepsis: (Check all that apply; check at least one) □ Yes, positive feedback is provided for good sepsis care □ Yes, constructive feedback is provided for areas of improvement □ Neither of the above |
N/A |
Incorporated into another question |
0.5 minute decrease |
||||||||||||
revision |
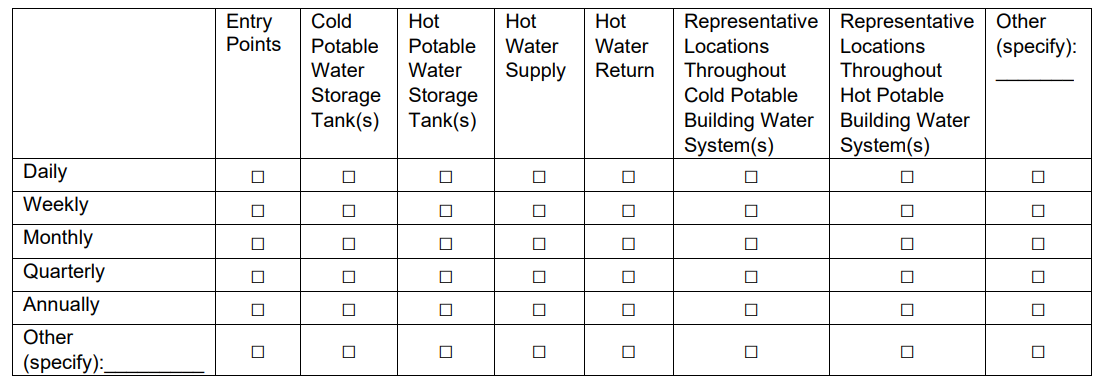
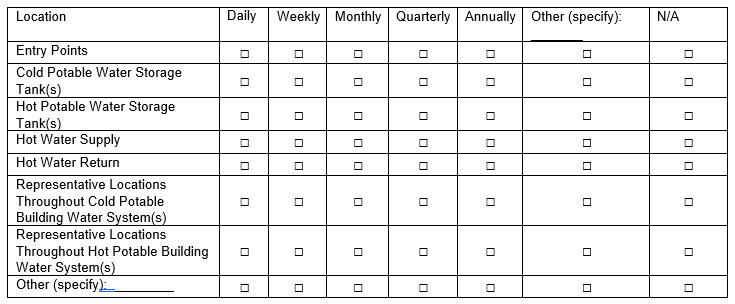
77b. If Yes, where and how frequently does your facility monitor disinfectant(s)? (Check all that apply)
|
70b. If Yes, where and how frequently does your facility monitor disinfectant(s)? (Check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
77d. If Yes, where and how frequently does your facility monitor water temperature? (check all that apply)
|
70d. If Yes, where and how frequently does your facility monitor water temperature? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
77f. If Yes, where and how frequently does your facility monitor water pH? (check all that apply)
|
70f. If Yes, where and how frequently does your facility monitor water pH? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
77h. If Yes, where and how frequently does your facility perform HPC testing? (check all that apply)
|
70h. If Yes, where and how frequently does your facility perform HPC testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
revision |
77j. If Yes, where an how frequently does your facility perform Legionella testing? (check all that apply)
|
70j. If Yes, where an how frequently does your facility perform Legionella testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
77l. If Yes, where an how frequently does your facility perform Pseudomonas testing? (check all that apply)
|
70l. If Yes, where an how frequently does your facility perform Pseudomonas testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Added new question |
N/A |
72. Our facility uses the following venous thromboembolism (VTE) prevention practices (select all that apply, and select at least one) □ Our facility has a VTE prevention policy. □ Our facility has a multidisciplinary team that addresses VTE prevention. □ Our facility has a facility-wide VTE prevention protocol that includes VTE and bleeding risk assessments linked to clinical decision support for appropriate VTE prophylaxis options. □ Our facility has embedded the VTE prevention protocol in admission order sets. □Yes □ No □ Our facility provides VTE prevention education for clinicians annually. □ Our facility provides VTE prevention education for patients during their stay at our facility. □ Our facility performs audits to determine whether patients are on risk-appropriate VTE prophylaxis and provides clinician feedback for quality improvement. □ Our facility tracks the incidence of VTE that develops during a patient’s stay at our facility (VTE not present on admission). □ Our facility does not use any of the above VTE prevention practices. |
provide data (baseline and annually) on VTE prevention practices in hospitals/facilities and help identify gaps between evidence-based guidelines for VTE prevention and implementation of those guidelines in practice. The baseline data would also be helpful in the evaluation of future VTE prevention initiatives.
|
1.0 minute increase |
||||||||||||
Added new question |
N/A |
*73. Our facility utilizes a checklist or bundle for prevention of the following HAIs. (Check all that apply) □ CLABSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□CAUTI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□CDI LabID Event At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□MRSA Bacteremia LabID Event At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□COLO SSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□HYST SSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown |
For the purposes of the Consensus Based Entity measure endorsement process, validity testing demonstrates the measure score (in our case, the SIR) correctly reflects the quality of care provided, adequately identifying differences in quality. The goal of these questions is to correlate process measures (for example, implementation of HAI prevention strategies) with the outcome measures of the NHSN SIRs. |
2.0 minute increase |
||||||||||||
Added new question |
N/A |
74. Did your facility (or any part of your facility) implement a new HAI prevention strategy within the last calendar year? *The following prevention strategies are examples from HAI prevention guidance documents (for example, 2022 SHEA/IDSA/APIC Practice Recommendations - Compendium of Strategies) and are supported by varying levels of evidence. □Yes □No □Unknown
If yes, check all HAIs that apply.
□CLABSI (check all that apply) □Documentation of daily assessment for central line necessity □Bundling of central line insertion supplies to ensure efficient access to supplies in convenient location for aseptic central line insertion □Use of chlorhexidine-containing dressings for central lines in patients >2 months of age □Use of antiseptic-containing caps/covers for central line ports □Use of antiseptic- or antimicrobial- impregnated central lines □Other (specify): _______
□CAUTI (check all that apply) □Documentation of daily assessment for indwelling urinary catheter necessity □Bundling of indwelling urinary catheter insertion supplies in convenient location to ensure efficient access to supplies for aseptic indwelling urinary catheter insertion □Implementation of a nurse-driven indwelling urinary catheter removal protocol or implementation of automatic stop orders requiring review of current indications and renewal of order for continuation of an indwelling urinary catheter □Process for consideration of bladder management alternatives to indwelling urethral catheterization in selected patients when appropriate □Incorporation of appropriate indications for urine culturing into electronic medical record system, as part of standardized institutional protocol for diagnostic stewardship □Other (specify): ________
□CDI LabID Event (check all that apply) □Use of an EPA-registered (EPA List K) sporicidal disinfectant for environmental cleaning/disinfection or use of additional disinfection of CDI patient rooms with no- touch technologies (for example, UV light disinfection) □Establish process in collaboration with environmental services to routinely assess adequacy of room cleaning □Restriction of antibiotics with the highest risk for CDI (for example, fluoroquinolones, carbapenems, 3rd and 4th generation cephalosporins) □Implementation of laboratory protocol to ensure testing of only appropriate specimens (for example, unformed stool) or a clinical decision support system to help reduce unnecessary Clostridioides difficile testing □Implementation of laboratory alert system to immediately report positive C. difficile results to clinical care providers and infection control personnel □Other (specify): ________
□MRSA Bacteremia LabID Event (check all that apply) □Process for monitoring and validation of compliance of daily CHG bathing in applicable patient populations (for example, adult ICU patients) □Process for multidisciplinary review of occurrences of hospital-onset MRSA bacteremia (for example, root cause analysis) to assess modifiable risk factors □Establish process in collaboration with environmental services to routinely assess adequacy of room cleaning □Implementation of a laboratory-based alert system that immediately notifies clinical care providers and infection control personnel of new MRSA-colonized and/or MRSA-infected patients □Implementation of universal gowns and gloves upon entry into adult ICU patient rooms, regardless of MRSA status □Other (specify): _______
□COLO SSI (check all that apply) □Use of combination of parenteral and oral antimicrobial prophylaxis with mechanical bowel prep, unless contraindicated, prior to elective colorectal surgery □Monitor compliance with antimicrobial prophylaxis guidelines being appropriately provided □Use of impervious plastic wound protectors for GI surgery □Implementation of preoperative warming for at least 30 minutes prior to surgery to prevent intraoperative hypothermia □Use of negative pressure dressings in patients who may benefit □Use of antiseptic-impregnated sutures □Other (specify): _______
□HYST SSI (check all that apply) □Use antiseptic-containing preoperative vaginal preparatory agents for patients undergoing elective hysterectomy □Monitor compliance with antimicrobial prophylaxis guidelines being appropriately provided □Implementation of preoperative warming for at least 30 minutes prior to surgery to prevent intraoperative hypothermia □Use of negative pressure dressings in patients who may benefit □Use of antiseptic-impregnated sutures □Other (specify): _______ |
For the purposes of the Consensus Based Entity measure endorsement process, validity testing demonstrates the measure score (in our case, the SIR) correctly reflects the quality of care provided, adequately identifying differences in quality. The goal of these questions is to correlate process measures (for example, implementation of HAI prevention strategies) with the outcome measures of the NHSN SIRs. |
3.0 minute increase |
||||||||||||
Added new question |
N/A |
*75. Does your facility provide training and/or education on HAI prevention to healthcare personnel as it relates to their role? □Yes □No □Unknown If yes, check all HAIs that apply.
□CLABSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□CAUTI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□CDI LabID Event At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□MRSA Bacteremia LabID Event At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□COLO SSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□HYST SSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
|
For the purposes of the Consensus Based Entity measure endorsement process, validity testing demonstrates the measure score (in our case, the SIR) correctly reflects the quality of care provided, adequately identifying differences in quality. The goal of these questions is to correlate process measures (for example, implementation of HAI prevention strategies) with the outcome measures of the NHSN SIRs. |
1.0 minute increase |
||||||||||||
57.122 HAI Progress Report State Health Department Survey To collect information from all states and territory health departments on healthcare associated infection (HAI) reporting requirements and data validation activities that were in place during the 2023 calendar year. Information collected from this survey is used to populate technical tables in the annual release of the National and State Healthcare Associated Infection Progress Report. The report helps identify the progress that is being made in the prevention of HAIs at the state and national level. Information from the survey is juxtaposed with state level data that monitors the number of facilities reporting and number of total HAI events. Understanding whether the state has validated their HAI data or has a state mandate to report such HAI data, is very helpful when interpreting the state-level HAI incidence data presented in CDC’s report. Data collection form will be electronic via REDCap. |
||||
Type of Change |
Changed From |
Changed To |
Justification |
Impact to Burden |
New |
|
Name State/Province Email address |
To ensure one form is completed per state. If there are multiple submissions per state, we may need to contact the completers to resolve. |
Increase |
Revision to questions 1-27, 30-35 |
2017 |
2023 |
Update to calendar year of data collection interest |
None |
Revision to questions 1-26 |
‘legislative’ |
‘legislation’ |
Updated for consistency across data collection |
None |
Revision to response options for questions 1-20, 23-26 |
‘No mandate (e.g., legislative or state-required mandate at any facility types)’ |
‘No reporting mandates (e.g., legislation or policy) for any facility types’ |
Updated for specificity/clarity and consistency across this response option |
None |
Revision to questions 2-26 |
‘mandate…’ |
‘reporting requirement…’ |
Updated for specificity/clarity |
None |
Revision to questions 5,6,13-16,19,20 |
Removed ‘Inpatient Rehabilitation Facility (IRF)’ as response option |
|
Response option is not applicable |
Decrease |
Revision to questions 7,8 |
Removed ‘Critical Access Hospital (CAH)’ as response option |
|
Response option is not applicable |
Decrease |
Revision to question 21 |
Did your state have a mandate (e.g., legislation or policy including reportable conditions) for acute care hospitals (ACH) to report SSI data to NHSN from any of the following procedure types at any time during 2017? (check all that apply)? |
Did your state have a reporting requirement (e.g., legislation or policy including reportable conditions) for acute care hospitals (ACH) to report SSI data to NHSN from any of the following procedure types at any time during 2023? If your state has no mandates, please only respond to the first option. |
Updated for specificity/clarity and consistency across this response option |
None |
Revision to question 21 |
Removed response options ‘APPY’, XLAP |
Added response options ‘CHOL’, ‘FX’ |
Procedure options updates given change in HAI reporting trends |
None |
Revision to question 21,22 |
Column header ‘Was this reporting mandate in effect on January 1, 2017?’ |
Column header ‘This mandate was in effect on January 1, 2023’ |
Changed question to statement to reduce confusion |
None |
Revision to question 22 |
Did your state have a mandate (e.g., legislation legislative or state-required mandate) for critical access hospitals (CAH) to report inpatient SSI data to NHSN from any of the following procedure types during 2017? (check all that apply)? |
Did your state have a reporting requirement (e.g., legislation or state-required mandate) for critical access hospitals (CAH) to report SSI data to NHSN from any of the following procedure types during 2023? If your state has no mandates, please only respond to the first option. |
Updated for specificity/clarity and consistency across this response option |
None |
Revision to question 22 |
Removed response options ‘AAA’, ‘APPY’, ‘CARD’, ‘CBGB/CBGC’, ‘CSEC’, ‘FUSN’, HPRO’ |
|
Procedure options not applicable for facility type |
Decrease |
Revision to question 23-26 |
Did your state have a mandate (e.g., legislative state-required mandate) for healthcare facilities to report…. |
Did your state have a reporting requirement (e.g., legislation or policy including reportable conditions) for healthcare facilities to report… |
Updated for consistency with similar questions |
None |
New |
|
(27) Did your state use the NHSN External Validation Toolkit to perform validation on 2023 NHSN data prior to June 1, 2024? Yes/No |
To evaluate use of CDC materials when conducting HAI data validation |
Increase |
New |
|
(28) Please select the HAI(s) that were validated using the NHSN External Validation Toolkit CLABSI, CAUTI, SSI-COLO, SSI-HYST, MRSA LabID Event, C. difficile LabID Event, or None |
To evaluate use of CDC materials when conducting HAI data validation |
Increase |
New |
|
(29) Please select the facility type(s) that were validated using the NHSN External Validation Toolkit Acute Care Hospital (ACH), Critical Access Hospital (CAH), Long Term Acute Care Facility (LTAC), Inpatient Rehabilitation Facility (IRF), or None |
To evaluate use of CDC materials when conducting HAI data validation |
Increase |
Revision to question 30,31 instructions |
(Please select a response for each HAI listed below) |
Check all that apply. If your state has [no access to any data listed] or [performs no data quality of any HAI’s listed], please only respond to the first option. |
Instructions updated to match format updates to response table |
None |
Revision to questions 30, 31,33,35 response table |
Each data cell listed by facility type and performance type |
Facility types moved as header and performance question moved to first row |
Increase readability of table |
None |
Revision to question 31 table row |
No data quality checks performed for any facility type for HAIs listed below |
No data quality checks performed for any facility type for HAIs listed below |
Updated for specificity/clarity |
None |
Revision to question 33 |
Has your state health department completed an external audit (medical record review of any HAI, or a review of laboratory records for MRSA or C. difficile LabID Events) of 2017 NHSN data from any of the following facility types prior to August 1, 2018? |
Has your state health department or partner organization completed an external audit (medical record review of any HAI, or a review of laboratory records for MRSA or C. difficile LabID Events) of 2023 NHSN data from any of the following facility types prior to June 1, 2024? |
External audits may be completed by parties outside of the state health department. |
None |
Deletion |
(34) Which HAIs and facility types were validated during the external audit? (Please answer all fields) |
|
Question not needed given data table already allowed reporting by the facility types. This question was redundant. |
None |
Revision to question 35 |
Please select the HAIs for each facility that had a state mandate (e.g., legislation or policy) to conduct an external audit of NHSN data during 2017. (Please answer all fields) |
Please select the required HAIs for each facility type that had a state mandate (e.g., legislation or policy including reportable conditions) to conduct an external audit of NHSN data during 2023. If your state does not have a mandate to conduct an annual external audit, please skip this question. |
Updated for consistency with similar questions |
None |
Revision to question 35 |
|
If you need space to clarify or comment on any of your survey responses, please do so here |
Not required. Respondent can provide any additional details relevant if desired. These data will be reviewed and appropriate follow-up as needed |
None |
57.137 Long-Term Care Facility Component – Annual Facility Survey The NHSN Annual Facility survey for long-term care facilities (LTCFs) is required for facilities that currently, or plan to, report healthcare associated infections (urinary tract infections), laboratory-identified events for C. difficile and/or multidrug resistant organisms, and/or prevention process measures. There are four new questions that will be added to the Annual Facility Survey effective January 2025. The new questions will provide additional information about the facility Infection Preventionist (IP) role.
|
||||
Type of Change |
Changed From |
Changed To |
Justification |
Impact to Burden |
Addition of a new question: Question #5 |
Variable/question not currently on form |
*5. In addition to the Infection Preventionist (IP) role, how many other roles is the IP responsible for? Select all that apply:
Other __________ |
To obtain additional information about the Infection Preventionist role at the facility. |
Increase to burden because it is an additional question. Estimated average 2 minutes to complete question. |
Addition of a new question: Question #6 |
Variable/question not currently on form |
*6. If your Infection Preventionist (IP) has more than 1 role (as reported above), what percentage of their time is dedicated to the IP role? (Check one)
We have a full-time position for an IP |
To obtain additional information about the Infection Preventionist role at the facility.
|
Increase to burden because it is an additional question. Estimated average 2 minutes to complete question.
|
Addition of a new question: Question #7 |
Variable/question not currently on form |
*7. What formal training has your Infection Preventionist received? Select all that apply
Other |
To obtain additional information about the Infection Preventionist role at the facility.
|
Increase to burden because it is an additional question. Estimated average 2 minutes to complete question.
|
Addition of a new question: Question #8 |
Variable/question not currently on form |
*8. How many times in the past year have you had to find a new employee to take over the Infection Preventionist (IP) role? In other words, how many times has this position “turned over”? (Check one)
Four or more |
To obtain additional information about the Infection Preventionist role at the facility.
|
Increase to burden because it is an additional question. Estimated average 2 minutes to complete question.
|
Revision: Shift existing questions down. |
#5 - #25 |
Change numbering to #9- #29 |
Question numbers are shifted down to accommodate the four new questions.
|
No burden change |
57.150 LTAC Annual Survey NHSN PSC Annual Survey collects facility-level data from the previous calendar year and is completed by all facilities enrolled in the NHSN Patient Safety Component. The Annual Survey data is used to calculate HAI Standardized Infection Ratio (SIR) risk adjustment models and track HAI incidence in facilities. The data is also used to support decision making, program planning, and research across CDC. The SIR is available for use for CMS Quality Reporting for select HAI and facility types, state health departments, other organizations, or groups (i.e., Leapfrog) and CDC in national surveillance reports. It will be collected electronically once annually via the NHSN application.
By updating the PSC Annual Survey, we ensure improved relevance, enhanced data quality, alignment with industry standards and regulations, increased efficiency, and expanded analysis capabilities within the CDC.
|
||||||||||||||||
Type of Change |
Changed From |
Changed To |
Justification |
Impact to Burden |
||||||||||||
Revision |
*2. For the following organisms, indicate which methods are used for: (1) Primary susceptibility testing and (2) Secondary, supplemental, or confirmatory testing (if performed).
If your laboratory does not perform susceptibility testing, indicate the methods used at the outside laboratory.
|
*2. For Enterobacterales, Pseudomonas aeruginosa and/or Acinetobacter baumannii complex, indicate which methods are used for: (1) Primary susceptibility testing and (2) Secondary, supplemental, or confirmatory testing (if performed).
If your laboratory does not perform susceptibility testing, indicate the methods used at the outside laboratory.
|
Simplified the question to have facilities respond only 1 time (not per organism). Updated the response options to reflect currently used lab tests |
0.5 minute decrease |
||||||||||||
revision |
*3. Does either primary or secondary/supplemental antimicrobial susceptibility testing (AST) include the following (check all that apply):
|
*3. Does either primary or secondary/supplemental antimicrobial susceptibility testing (AST) include the following (check all that apply):
|
Simplified the question to have facilities respond only 1 time per drug (not per organism). Updated the response options to reflect drugs of interest. |
No change |
||||||||||||
revision |
f. Fluroquinolone breakpoints for Enterobacterales in 2019 □ Yes □ No |
*4. Has the laboratory implemented revised breakpoints recommended by CLSI for the following: a. Third Generation Cephalosporin and monobactam (i.e. aztreonam) breakpoints for Enterobacterales in 2010 □ Yes □ No b. Carbapenem breakpoints for Enterobacterales in 2010 □ Yes □ No c. Ertapenem breakpoints for Enterobacterales in 2012 □ Yes □ No d. Carbapenem breakpoints for Pseudomonas aeruginosa in 2012 □ Yes □ No e. Fluroquinolone breakpoints for Pseudomonas aeruginosa in 2019 □ Yes □ No f. Fluroquinolone breakpoints for Enterobacterales in 2019 □ Yes □ No g. Aminoglycoside breakpoints for Enterobacterales in 2023 □ Yes □ No h. Aminoglycoside breakpoints for Pseudomonas aeruginosa in 2023 □ Yes □ No i. Piperacillin-tazobactam breakpoints for Pseudomonas aeruginosa in 2023 □ Yes □ No j. Piperacillin-tazobactam breakpoints for Enterobacterales in 2022 □ Yes □ No |
to monitor the uptake of up-to-date CLSI breakpoints among clinical laboratories and interpret antimicrobial surveillance data which reuse hospital interpretations of antimicrobial susceptibility testing results. The additional organism-drug combos are the those that CLSI recently updated the breakpoints on. |
0.5 minute increase |
||||||||||||
revision |
|
*5. Does the laboratory test bacterial isolates for presence of a carbapenemase? (this does not include automated testing instrument expert rules) □ Yes □ No |
Grammar update |
No change |
||||||||||||
|
|
5b. If Yes, which test is routinely performed to detect carbapenemase: (check all that apply) □ Nucleic Acid Amplification Test (for example, PCR, Cepheid) □ NG-Test Carba-5 (or other lateral flow assay) □ Modified Hodge Test □ Carba NP □ mCIM/CIM |
Update of tests to more accurately reflect tests in use. |
No change |
||||||||||||
Deletion of question |
*9. Does your facility perform extended-spectrum beta-lactamase (ESBL) testing for E. coli Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis routinely or using a testing algorithm? □ Yes □ No
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
not needed anymore |
0.5 minute decrease |
||||||||||||
Revision |
*14. Does the laboratory employ any molecular tests to identify Candida from blood specimens? □ Yes □ No □ Unknown |
*13. Does the laboratory employ any PCR molecular tests to identify Candida from blood specimens? □ Yes □ No □ Unknown
|
Revised question wording to increase clarity. |
No change |
||||||||||||
Revision |
|
13a. If yes, which PCR molecular tests are used to identify Candida from blood specimens? (check all that apply) □ T2Candida Panel □ BioFire BCID □ GenMark ePlex BCID □ Other, specify: _________________ □ Unknown |
Revised question wording to increase clarity. |
No change |
||||||||||||
Revision |
*16. What method is used for antifungal susceptibility testing (AFST), excluding Amphotericin B? (check all that apply)
|
*15. What methods are used for antifungal susceptibility testing (AFST), excluding Amphotericin B? (check all that apply) |
Grammar update |
No change |
||||||||||||
Revision |
*17. What method is used for antifungal susceptibility testing (AFST) of Amphotericin B? (check all that apply)
|
*16. What methods are used for antifungal susceptibility testing (AFST) of Amphotericin B? (check all that apply) |
Grammar update |
No change |
||||||||||||
revision |
*22. Indicate the primary and definitive method used to identify microbes from blood cultures collected in your facility. (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, OmniLog, Sherlock, etc.) □ Non-automated Manual Kit (for example, API, Crystal, RapID, etc.) □ Rapid Identification (for example, Verigene, BioFire FilmArray, PNA-FISH, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None
|
*21. Which of the following methods serve as the primary method used for bacterial identification at your facility? (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, etc.) □ Non-automated Manual Kit (for example, API 20C, biochemicals) □ Rapid Identification (for example, NAAT/PCR, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
Updated question to more accurately reflect what we'd like facilities to answer. |
No change |
||||||||||||
revision |
*23. Indicate any additional secondary methods used for microbe identification from blood cultures collected in your facility (for example, a rapid method that is confirmed with the primary method, a secondary method if the primary method fails to give an identification, or a method that is used in conjunction with the primary method). (check all that apply) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, OmniLog, Sherlock, etc.) □ Non-automated Manual Kit (for example, API, Crystal, RapID, etc.) □ Rapid Identification (for example, Verigene, BioFire FilmArray, PNA-FISH, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None
|
*22. Which of the following methods serve as the secondary or backup method used for bacterial identification at your facility? (for example, a secondary method if the primary method fails to give an identification, or if the primary method is unavailable). (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, etc.) □ Non-automated Manual Kit (for example, API 20C, biochemicals) □ Rapid Identification (for example, NAAT/PCR, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
Updated question to more accurately reflect what we'd like facilities to answer. |
No change |
||||||||||||
Deletion of question |
*35. Did the antibiotic stewardship leader(s) participate in responding to these questions? (Check one.) □ Yes, pharmacist lead □ Yes, physician lead □ Yes, both pharmacist and physician leads □ Yes, other lead □ No
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
□ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
48. Antibiotic stewardship activities are integrated into quality improvement and/or patient safety initiatives. □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
49. Our facility accesses targeted remote stewardship expertise (for example, tele-stewardship to obtain facility-specific support for our antibiotic stewardship efforts). □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
50. Our stewardship program works with the microbiology laboratory to implement the following interventions: (Check all that apply) □ Selective reporting of antimicrobial susceptibility testing results □ Placing comments in microbiology reports to improve prescribing □ None of the above
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
51. Which committees or leadership entities provide oversight of your facility’s antibiotic stewardship efforts? (Check all that apply)
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Revision |
55b. If Yes, where and how frequently does your facility monitor disinfectant(s)? (Check all that apply)
|
49b. If Yes, where and how frequently does your facility monitor disinfectant(s)? (Check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55d. If Yes, where and how frequently does your facility monitor water temperature? (check all that apply)
|
49d. If Yes, where and how frequently does your facility monitor water temperature? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55f. If Yes, where and how frequently does your facility monitor water pH? (check all that apply)
|
49f. If Yes, where and how frequently does your facility monitor water pH? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55h. If Yes, where and how frequently does your facility perform HPC testing? (check all that apply)
|
49h. If Yes, where and how frequently does your facility perform HPC testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55j. If Yes, where an how frequently does your facility perform Legionella testing? (check all that apply)
|
49j. If Yes, where an how frequently does your facility perform Legionella testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55l. If Yes, where an how frequently does your facility perform Pseudomonas testing? (check all that apply)
|
49l. If Yes, where an how frequently does your facility perform Pseudomonas testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Added new question |
N/A |
51. Our facility uses the following venous thromboembolism (VTE) prevention practices (select all that apply, and select at least one) □ Our facility has a VTE prevention policy. □ Our facility has a multidisciplinary team that addresses VTE prevention. □ Our facility has a facility-wide VTE prevention protocol that includes VTE and bleeding risk assessments linked to clinical decision support for appropriate VTE prophylaxis options. □ Our facility has embedded the VTE prevention protocol in admission order sets. □Yes □ No □ Our facility provides VTE prevention education for clinicians annually. □ Our facility provides VTE prevention education for patients during their stay at our facility. □ Our facility performs audits to determine whether patients are on risk-appropriate VTE prophylaxis and provides clinician feedback for quality improvement. □ Our facility tracks the incidence of VTE that develops during a patient’s stay at our facility (VTE not present on admission). □ Our facility does not use any of the above VTE prevention practices. |
provide data (baseline and annually) on VTE prevention practices in hospitals/facilities and help identify gaps between evidence-based guidelines for VTE prevention and implementation of those guidelines in practice. The baseline data would also be helpful in the evaluation of future VTE prevention initiatives.
|
1.0 minute increase |
||||||||||||
Added new question |
N/A |
*52. Our facility utilizes a checklist or bundle for prevention of the following HAIs. (Check all that apply) □ CLABSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□CAUTI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□CDI LabID Event At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□MRSA Bacteremia LabID Event At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□COLO SSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□HYST SSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown |
|
2.0 minute increase |
||||||||||||
Added new question |
N/A |
53. Did your facility (or any part of your facility) implement a new HAI prevention strategy within the last calendar year? *The following prevention strategies are examples from HAI prevention guidance documents (for example, 2022 SHEA/IDSA/APIC Practice Recommendations - Compendium of Strategies) and are supported by varying levels of evidence. □Yes □No □Unknown
If yes, check all HAIs that apply.
□CLABSI (check all that apply) □Documentation of daily assessment for central line necessity □Bundling of central line insertion supplies to ensure efficient access to supplies in convenient location for aseptic central line insertion □Use of chlorhexidine-containing dressings for central lines in patients >2 months of age □Use of antiseptic-containing caps/covers for central line ports □Use of antiseptic- or antimicrobial- impregnated central lines □Other (specify): _______
□CAUTI (check all that apply) □Documentation of daily assessment for indwelling urinary catheter necessity □Bundling of indwelling urinary catheter insertion supplies in convenient location to ensure efficient access to supplies for aseptic indwelling urinary catheter insertion □Implementation of a nurse-driven indwelling urinary catheter removal protocol or implementation of automatic stop orders requiring review of current indications and renewal of order for continuation of an indwelling urinary catheter □Process for consideration of bladder management alternatives to indwelling urethral catheterization in selected patients when appropriate □Incorporation of appropriate indications for urine culturing into electronic medical record system, as part of standardized institutional protocol for diagnostic stewardship □Other (specify): ________
□CDI LabID Event (check all that apply) □Use of an EPA-registered (EPA List K) sporicidal disinfectant for environmental cleaning/disinfection or use of additional disinfection of CDI patient rooms with no- touch technologies (for example, UV light disinfection) □Establish process in collaboration with environmental services to routinely assess adequacy of room cleaning □Restriction of antibiotics with the highest risk for CDI (for example, fluoroquinolones, carbapenems, 3rd and 4th generation cephalosporins) □Implementation of laboratory protocol to ensure testing of only appropriate specimens (for example, unformed stool) or a clinical decision support system to help reduce unnecessary Clostridioides difficile testing □Implementation of laboratory alert system to immediately report positive C. difficile results to clinical care providers and infection control personnel □Other (specify): ________
□MRSA Bacteremia LabID Event (check all that apply) □Process for monitoring and validation of compliance of daily CHG bathing in applicable patient populations (for example, adult ICU patients) □Process for multidisciplinary review of occurrences of hospital-onset MRSA bacteremia (for example, root cause analysis) to assess modifiable risk factors □Establish process in collaboration with environmental services to routinely assess adequacy of room cleaning □Implementation of a laboratory-based alert system that immediately notifies clinical care providers and infection control personnel of new MRSA-colonized and/or MRSA-infected patients □Implementation of universal gowns and gloves upon entry into adult ICU patient rooms, regardless of MRSA status □Other (specify): _______
□COLO SSI (check all that apply) □Use of combination of parenteral and oral antimicrobial prophylaxis with mechanical bowel prep, unless contraindicated, prior to elective colorectal surgery □Monitor compliance with antimicrobial prophylaxis guidelines being appropriately provided □Use of impervious plastic wound protectors for GI surgery □Implementation of preoperative warming for at least 30 minutes prior to surgery to prevent intraoperative hypothermia □Use of negative pressure dressings in patients who may benefit □Use of antiseptic-impregnated sutures □Other (specify): _______
□HYST SSI (check all that apply) □Use antiseptic-containing preoperative vaginal preparatory agents for patients undergoing elective hysterectomy □Monitor compliance with antimicrobial prophylaxis guidelines being appropriately provided □Implementation of preoperative warming for at least 30 minutes prior to surgery to prevent intraoperative hypothermia □Use of negative pressure dressings in patients who may benefit □Use of antiseptic-impregnated sutures □Other (specify): _______ |
|
3.0 minute increase |
||||||||||||
Added new question |
N/A |
*54. Does your facility provide training and/or education on HAI prevention to healthcare personnel as it relates to their role? □Yes □No □Unknown If yes, check all HAIs that apply.
□CLABSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□CAUTI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□CDI LabID Event At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□MRSA Bacteremia LabID Event At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□COLO SSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□HYST SSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other |
|
1.0 minute increase |
||||||||||||
57.151 Rehab Annual Survey NHSN PSC Annual Survey collects facility-level data from the previous calendar year and is completed by all facilities enrolled in the NHSN Patient Safety Component. The Annual Survey data is used to calculate HAI Standardized Infection Ratio (SIR) risk adjustment models and track HAI incidence in facilities. The data is also used to support decision making, program planning, and research across CDC. The SIR is available for use for CMS Quality Reporting for select HAI and facility types, state health departments, other organizations, or groups (i.e., Leapfrog) and CDC in national surveillance reports. It will be collected electronically once annually via the NHSN application.
By updating the PSC Annual Survey, we ensure improved relevance, enhanced data quality, alignment with industry standards and regulations, increased efficiency, and expanded analysis capabilities within the CDC.
|
||||||||||||||||
Type of Change |
Changed From |
Changed To |
Justification |
Impact to Burden |
||||||||||||
Revision |
*2. For the following organisms, indicate which methods are used for: (1) Primary susceptibility testing and (2) Secondary, supplemental, or confirmatory testing (if performed).
If your laboratory does not perform susceptibility testing, indicate the methods used at the outside laboratory.
|
*2. For Enterobacterales, Pseudomonas aeruginosa and/or Acinetobacter baumannii complex, indicate which methods are used for: (1) Primary susceptibility testing and (2) Secondary, supplemental, or confirmatory testing (if performed).
If your laboratory does not perform susceptibility testing, indicate the methods used at the outside laboratory.
|
Simplified the question to have facilities respond only 1 time (not per organism). Updated the response options to reflect currently used lab tests |
0.5 minute decrease |
||||||||||||
revision |
*3. Does either primary or secondary/supplemental antimicrobial susceptibility testing (AST) include the following (check all that apply):
|
*3. Does either primary or secondary/supplemental antimicrobial susceptibility testing (AST) include the following (check all that apply):
|
Simplified the question to have facilities respond only 1 time per drug (not per organism). Updated the response options to reflect drugs of interest. |
No change |
||||||||||||
revision |
|
*4. Has the laboratory implemented revised breakpoints recommended by CLSI for the following: a. Third Generation Cephalosporin and monobactam (i.e. aztreonam) breakpoints for Enterobacterales in 2010 □ Yes □ No b. Carbapenem breakpoints for Enterobacterales in 2010 □ Yes □ No c. Ertapenem breakpoints for Enterobacterales in 2012 □ Yes □ No d. Carbapenem breakpoints for Pseudomonas aeruginosa in 2012 □ Yes □ No e. Fluroquinolone breakpoints for Pseudomonas aeruginosa in 2019 □ Yes □ No f. Fluroquinolone breakpoints for Enterobacterales in 2019 □ Yes □ No g. Aminoglycoside breakpoints for Enterobacterales in 2023 □ Yes □ No h. Aminoglycoside breakpoints for Pseudomonas aeruginosa in 2023 □ Yes □ No i. Piperacillin-tazobactam breakpoints for Pseudomonas aeruginosa in 2023 □ Yes □ No j. Piperacillin-tazobactam breakpoints for Enterobacterales in 2022 □ Yes □ No |
to monitor the uptake of up-to-date CLSI breakpoints among clinical laboratories and interpret antimicrobial surveillance data which reuse hospital interpretations of antimicrobial susceptibility testing results. The additional organism-drug combos are the those that CLSI recently updated the breakpoints on. |
0.5 minute increase |
||||||||||||
revision |
*5. Does the laboratory test bacterial isolates for presence of carbapenemase? (this does not include automated testing instrument expert rules) □ Yes □ No |
*5. Does the laboratory test bacterial isolates for presence of a carbapenemase? (this does not include automated testing instrument expert rules) □ Yes □ No |
Grammar update |
No change |
||||||||||||
revision |
|
5b. If Yes, which test is routinely performed to detect carbapenemase: (check all that apply) □ Nucleic Acid Amplification Test (for example, PCR, Cepheid) □ NG-Test Carba-5 (or other lateral flow assay) □ Modified Hodge Test □ Carba NP □ mCIM/CIM □ Other __________ |
Update of tests to more accurately reflect tests in use. |
No change |
||||||||||||
Deletion of question |
*9. Does your facility perform extended-spectrum beta-lactamase (ESBL) testing for E. coli Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis routinely or using a testing algorithm? □ Yes □ No
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
not needed anymore |
0.5 minute decrease |
||||||||||||
Revision |
*14. Does the laboratory employ any molecular tests to identify Candida from blood specimens? □ Yes □ No □ Unknown |
*13. Does the laboratory employ any PCR molecular tests to identify Candida from blood specimens? □ Yes □ No □ Unknown
|
Revised question wording to increase clarity. |
No change |
||||||||||||
Revision |
|
13a. If yes, which PCR molecular tests are used to identify Candida from blood specimens? (check all that apply) □ T2Candida Panel □ BioFire BCID □ GenMark ePlex BCID □ Other, specify: _________________ □ Unknown |
Revised question wording to increase clarity. |
No change |
||||||||||||
Revision |
*16. What method is used for antifungal susceptibility testing (AFST), excluding Amphotericin B? (check all that apply)
|
*15. What methods are used for antifungal susceptibility testing (AFST), excluding Amphotericin B? (check all that apply) |
Grammar update |
No change |
||||||||||||
Revision |
*17. What method is used for antifungal susceptibility testing (AFST) of Amphotericin B? (check all that apply)
|
*16. What methods are used for antifungal susceptibility testing (AFST) of Amphotericin B? (check all that apply) |
Grammar update |
No change |
||||||||||||
Revision |
*22. Indicate the primary and definitive method used to identify microbes from blood cultures collected in your facility. (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, OmniLog, Sherlock, etc.) □ Non-automated Manual Kit (for example, API, Crystal, RapID, etc.) □ Rapid Identification (for example, Verigene, BioFire FilmArray, PNA-FISH, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None
|
*21. Which of the following methods serve as the primary method used for bacterial identification at your facility? (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, etc.) □ Non-automated Manual Kit (for example, API 20C, biochemicals) □ Rapid Identification (for example, NAAT/PCR, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
Updated question to more accurately reflect what we'd like facilities to answer. |
No change |
||||||||||||
revision |
*23. Indicate any additional secondary methods used for microbe identification from blood cultures collected in your facility (for example, a rapid method that is confirmed with the primary method, a secondary method if the primary method fails to give an identification, or a method that is used in conjunction with the primary method). (check all that apply) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, OmniLog, Sherlock, etc.) □ Non-automated Manual Kit (for example, API, Crystal, RapID, etc.) □ Rapid Identification (for example, Verigene, BioFire FilmArray, PNA-FISH, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None
|
*22. Which of the following methods serve as the secondary or backup method used for bacterial identification at your facility? (for example, a secondary method if the primary method fails to give an identification, or if the primary method is unavailable). (check one) □ MALDI-TOF MS System (Vitek MS) □ MALDI-TOF MS System (Bruker Biotyper) □ Automated Instrument (for example, Vitek, MicroScan, Phoenix, etc.) □ Non-automated Manual Kit (for example, API 20C, biochemicals) □ Rapid Identification (for example, NAAT/PCR, Gene Xpert, etc.) □ 16S rRNA Sequencing □ Other (specify): _________________ □ None |
Updated question to more accurately reflect what we'd like facilities to answer. |
No change |
||||||||||||
Deletion of question |
*25. Number of fraction of full-time employees (FTEs) for a designated hospital epidemiologist (or equivalent role) affiliated with your facility: _____ |
N/A |
Not needed anymore |
0.5 minutes decrease |
||||||||||||
Deletion of question |
*35. Did the antibiotic stewardship leader(s) participate in responding to these questions? (Check one.) □ Yes, pharmacist lead □ Yes, physician lead □ Yes, both pharmacist and physician leads □ Yes, other lead □ No
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
□ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
48. Antibiotic stewardship activities are integrated into quality improvement and/or patient safety initiatives. □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
49. Our facility accesses targeted remote stewardship expertise (for example, tele-stewardship to obtain facility-specific support for our antibiotic stewardship efforts). □ Yes □ No |
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
50. Our stewardship program works with the microbiology laboratory to implement the following interventions: (Check all that apply) □ Selective reporting of antimicrobial susceptibility testing results □ Placing comments in microbiology reports to improve prescribing □ None of the above
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Deletion of question |
51. Which committees or leadership entities provide oversight of your facility’s antibiotic stewardship efforts? (Check all that apply)
|
N/A |
Not needed anymore |
0.5 minute decrease |
||||||||||||
Revision |
55b. If Yes, where and how frequently does your facility monitor disinfectant(s)? (Check all that apply)
|
48b. If Yes, where and how frequently does your facility monitor disinfectant(s)? (Check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55d. If Yes, where and how frequently does your facility monitor water temperature? (check all that apply)
|
48d. If Yes, where and how frequently does your facility monitor water temperature? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55f. If Yes, where and how frequently does your facility monitor water pH? (check all that apply)
|
48f. If Yes, where and how frequently does your facility monitor water pH? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55h. If Yes, where and how frequently does your facility perform HPC testing? (check all that apply)
|
48h. If Yes, where and how frequently does your facility perform HPC testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55j. If Yes, where an how frequently does your facility perform Legionella testing? (check all that apply)
|
48j. If Yes, where an how frequently does your facility perform Legionella testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Revision |
55l. If Yes, where an how frequently does your facility perform Pseudomonas testing? (check all that apply)
|
48l. If Yes, where an how frequently does your facility perform Pseudomonas testing? (check all that apply)
|
Added “N/A” column for those who do not test certain locations |
No change |
||||||||||||
Added new question |
N/A |
50. Our facility uses the following venous thromboembolism (VTE) prevention practices (select all that apply, and select at least one) □ Our facility has a VTE prevention policy. □ Our facility has a multidisciplinary team that addresses VTE prevention. □ Our facility has a facility-wide VTE prevention protocol that includes VTE and bleeding risk assessments linked to clinical decision support for appropriate VTE prophylaxis options. □ Our facility has embedded the VTE prevention protocol in admission order sets. □Yes □ No □ Our facility provides VTE prevention education for clinicians annually. □ Our facility provides VTE prevention education for patients during their stay at our facility. □ Our facility performs audits to determine whether patients are on risk-appropriate VTE prophylaxis and provides clinician feedback for quality improvement. □ Our facility tracks the incidence of VTE that develops during a patient’s stay at our facility (VTE not present on admission). □ Our facility does not use any of the above VTE prevention practices. |
provide data (baseline and annually) on VTE prevention practices in hospitals/facilities and help identify gaps between evidence-based guidelines for VTE prevention and implementation of those guidelines in practice. The baseline data would also be helpful in the evaluation of future VTE prevention initiatives.
|
1.0 minute increase |
||||||||||||
Added new question |
N/A |
*51. Our facility utilizes a checklist or bundle for prevention of the following HAIs. (Check all that apply) □ CLABSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□CAUTI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□CDI LabID Event At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□MRSA Bacteremia LabID Event At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□COLO SSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown
□HYST SSI At what minimum, regular frequency is adherence to the checklist/bundle monitored/measured? Check one. □Weekly □Monthly □Quarterly □Yearly □PRN □Other □Not regularly monitored/measured
Is checklist/bundle adherence shared routinely with the clinical team? □Yes □No □Unknown |
|
2.0 minute increase |
||||||||||||
Added new question |
N/A |
52. Did your facility (or any part of your facility) implement a new HAI prevention strategy within the last calendar year? *The following prevention strategies are examples from HAI prevention guidance documents (for example, 2022 SHEA/IDSA/APIC Practice Recommendations - Compendium of Strategies) and are supported by varying levels of evidence. □Yes □No □Unknown
If yes, check all HAIs that apply.
□CLABSI (check all that apply) □Documentation of daily assessment for central line necessity □Bundling of central line insertion supplies to ensure efficient access to supplies in convenient location for aseptic central line insertion □Use of chlorhexidine-containing dressings for central lines in patients >2 months of age □Use of antiseptic-containing caps/covers for central line ports □Use of antiseptic- or antimicrobial- impregnated central lines □Other (specify): _______
□CAUTI (check all that apply) □Documentation of daily assessment for indwelling urinary catheter necessity □Bundling of indwelling urinary catheter insertion supplies in convenient location to ensure efficient access to supplies for aseptic indwelling urinary catheter insertion □Implementation of a nurse-driven indwelling urinary catheter removal protocol or implementation of automatic stop orders requiring review of current indications and renewal of order for continuation of an indwelling urinary catheter □Process for consideration of bladder management alternatives to indwelling urethral catheterization in selected patients when appropriate □Incorporation of appropriate indications for urine culturing into electronic medical record system, as part of standardized institutional protocol for diagnostic stewardship □Other (specify): ________
□CDI LabID Event (check all that apply) □Use of an EPA-registered (EPA List K) sporicidal disinfectant for environmental cleaning/disinfection or use of additional disinfection of CDI patient rooms with no- touch technologies (for example, UV light disinfection) □Establish process in collaboration with environmental services to routinely assess adequacy of room cleaning □Restriction of antibiotics with the highest risk for CDI (for example, fluoroquinolones, carbapenems, 3rd and 4th generation cephalosporins) □Implementation of laboratory protocol to ensure testing of only appropriate specimens (for example, unformed stool) or a clinical decision support system to help reduce unnecessary Clostridioides difficile testing □Implementation of laboratory alert system to immediately report positive C. difficile results to clinical care providers and infection control personnel □Other (specify): ________
□MRSA Bacteremia LabID Event (check all that apply) □Process for monitoring and validation of compliance of daily CHG bathing in applicable patient populations (for example, adult ICU patients) □Process for multidisciplinary review of occurrences of hospital-onset MRSA bacteremia (for example, root cause analysis) to assess modifiable risk factors □Establish process in collaboration with environmental services to routinely assess adequacy of room cleaning □Implementation of a laboratory-based alert system that immediately notifies clinical care providers and infection control personnel of new MRSA-colonized and/or MRSA-infected patients □Implementation of universal gowns and gloves upon entry into adult ICU patient rooms, regardless of MRSA status □Other (specify): _______
□COLO SSI (check all that apply) □Use of combination of parenteral and oral antimicrobial prophylaxis with mechanical bowel prep, unless contraindicated, prior to elective colorectal surgery □Monitor compliance with antimicrobial prophylaxis guidelines being appropriately provided □Use of impervious plastic wound protectors for GI surgery □Implementation of preoperative warming for at least 30 minutes prior to surgery to prevent intraoperative hypothermia □Use of negative pressure dressings in patients who may benefit □Use of antiseptic-impregnated sutures □Other (specify): _______
□HYST SSI (check all that apply) □Use antiseptic-containing preoperative vaginal preparatory agents for patients undergoing elective hysterectomy □Monitor compliance with antimicrobial prophylaxis guidelines being appropriately provided □Implementation of preoperative warming for at least 30 minutes prior to surgery to prevent intraoperative hypothermia □Use of negative pressure dressings in patients who may benefit □Use of antiseptic-impregnated sutures □Other (specify): _______ |
|
3.0 minute increase |
||||||||||||
Added new question |
N/A |
*53. Does your facility provide training and/or education on HAI prevention to healthcare personnel as it relates to their role? □Yes □No □Unknown If yes, check all HAIs that apply.
□CLABSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□CAUTI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□CDI LabID Event At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□MRSA Bacteremia LabID Event At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□COLO SSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
□HYST SSI At what frequency is training or education is provided? Check all that apply. □Upon hire □When new product or processes are implemented □Quarterly □Yearly □PRN □Other
|
|
1.0 minute increase |
||||||||||||
57.500 Outpatient Dialysis Center Practices Survey Dialysis center survey questions help in understanding practices followed in the dialysis facilities as well as provide data for future analysis of infection control initiatives.
|
||||
Q3 - Removal of Joint Commission option |
Is your facility accredited by an organization other than CMS? Yes No
Joint Commission National Dialysis Accreditation Commission (NDAC) Accreditation Commission for Health Care (ACHC) Other (specify) _______________
|
Is your facility accredited by an organization other than CMS? Yes No
National Dialysis Accreditation Commission (NDAC) Accreditation Commission for Health Care (ACHC) Other (specify) _______________
|
Joint Commission no longer used |
Zero impact |
Q4 - Deletion of verbiage |
What types of dialysis services does your center offer (certified and non-certified)? (select all that apply): In-center daytime hemodialysis Home Peritoneal Dialysis Home Hemodialysis In-center nocturnal hemodialysis In Center Peritoneal Dialysis
|
a. What types of dialysis services does your center offer? (select all that apply): In-center daytime hemodialysis Home Peritoneal Dialysis Home Hemodialysis In-center nocturnal hemodialysis In Center Peritoneal Dialysis
|
The certified and non-certified verbiage was removed from the question to provide clarification.
|
Zero impact |
Q8 - language updated |
Is there someone at your dialysis center in charge of infection control? Yes No
|
Is there someone at your dialysis center in charge of infection control training or oversight? Yes No
|
Language updated for clarity. |
Zero impact |
Q9 - NEW Question added |
In the past year, has your clinic been cited for infection control breaches in a state/certification/recertification survey? Yes No |
|
To ascertain any infection control breaches citations. |
3 additional minutes |
Q10 – Acronyms spelled out |
Does your center provide dialysis services within long-term care facilities (e.g., staff-assisted dialysis in nursing homes or skilled nursing facilities; not long-term acute care hospitals)? Yes No
HD in LTC PD in LTC
|
Does your center provide dialysis services within long-term care facilities (e.g., staff-assisted dialysis in nursing homes or skilled nursing facilities; not long-term acute care hospitals)? Yes No
Hemodialysis in LTC Peritoneal Dialysis in LTC
|
Acronyms spelled out for clarity. |
Zero impact |
Q11 – verbiage updated |
Is there a dedicated vascular access nurse/coordinator (either full or part-time) at your center?
Yes No |
Which staff are responsible for ensuring permanent vascular access placement and maintenance? (to decrease CVC use in hemodialysis patients)? Dedicated vascular access coordinator Nephrologist who oversees patient education and coordinates patient care related to vascular access Relationship with or access to a surgeon skilled in access placement (or a process to refer patients to a surgeon that is skilled in access placement) Cannulation expert Relationship with or access to interventional nephrologists or interventional radiologist Other, specify: ________________
|
Question was updated to capture additional data on staff whom ensure vascular access placement and maintenance. |
Zero impact |
Q14 reworded and additional options |
Are patients routinely isolated or cohorted for treatment within your center for any of the following conditions? (if yes, select all that apply) No, none Hepatitis C Active tuberculosis (TB disease) Vancomycin-resistant Enterococcus (VRE) Methicillin-resistant Staphylococcus aureus (MRSA) Clostridioides difficile (C. diff.) Other, specify: ________________
|
Are patients routinely isolated or cohorted for treatment within your center for any of the following pathogens? (if yes, select all that apply) No, none Hepatitis C Vancomycin-resistant Enterococcus (VRE) Methicillin-resistant Staphylococcus aureus Clostridioides difficile (C. diff.) Any carbapenem- resistant organism [(i.e., carbapenem-resistant Enterobacterales (CRE), carbapenem-resistant Acinetobacter (CRAB), carbapenem-resistant Pseudomonas aeruginosa (CRPA)] Candida auris Other, specify: ________________
|
Question reworded and options added for clarity and better surveillance of isolated or cohorted patients. |
Zero impact |
Q15 – Question being removed |
In the past year, where have you dialyzed patients with SARS-COV-2 infections? (Select all that apply) Isolation room Covid shift Covid Unit Separate area on treatment floor while other non-COVID patients are dialyzed Not Applicable
|
|
Question removed upon expiration of public health emergency |
2 minute savings |
Q17 added |
|
Does your facility have an airborne infection isolation room (AIIR) to isolate patients infected with pathogens that are transmitted through the airborne route (for example, active tuberculosis)? Yes No
|
Question added to obtain additional data on isolated patients. |
1 minute additional |
Q23 – Additional subcategories added |
How many MAINTENANCE, NON-TRANSIENT ESRD and AKI PATIENTS were assigned to your center during the first week of February (2/1 through 2/7)? ________
Of these, indicate the number who received:
|
How many MAINTENANCE, NON-TRANSIENT ESRD and AKI PATIENTS were assigned to your center during the first week of February (2/1 through 2/7)? ________
Of these, indicate the number who received:
a1. No. of pediatric patients: ______
b1. No. of pediatric patients: _________
c1. No. of pediatric patients: __________
|
The addition to this question allows us to capture data on the pediatric patients served in the dialysis facilities. |
2 additional minutes |
Q24 - updated from optional to required |
Optional: Based on the number of patients that treated in the first week of February (2/1 through 2/7), please indicate the number of patients per Race:
|
Required: Based on the number of patients that treated in the first week of February (2/1 through 2/7), please indicate the number of patients per Race:
Declined to response: ___________ |
Optional question added to obtain data on patient race |
5 additional minutes |
Q25 - updated from optional to required |
Optional: Based on the number of patients that treated in the first week of February (2/1 through 2/7), please indicate the number of patients per Ethnicity
Declined to respond: _______ |
Required: Based on the number of patients that treated in the first week of February (2/1 through 2/7), please indicate the number of patients per Ethnicity
Declined to respond: _______ |
Optional question added to obtain data on patient ethnicity |
5 additional minutes |
Q27 - options updated |
Of the patient care staff members counted in question 26, how many received: a. A completed series of hepatitis B vaccine (ever)? ________ b. The influenza (flu) vaccine for the current/most recent flu season? ________
|
Of the patient care staff members counted in question 26, how many received: a. A completed series of hepatitis B vaccine (ever)? ________ b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. Annual COVID-19 vaccine?
|
Addition of common vaccines will be beneficial for infection surveillance. |
5 minutes additional |
Q29 – question reworded |
Does your center have a respiratory program for annual fit testing on your healthcare personnel? Yes No
If yes: a. Which staff do you fit test?? (select all that apply) Nurse/Nurse Assistant Dietitian Dialysis Patient-Care Technician Physicians/Physician Assistant Dialysis Biomedical Technician Nurse Practitioner Social Worker Other: ___________________
|
Does your center have a respiratory protection program for annual respirator use/training of your healthcare personnel? Yes No
If yes: a. Which staff are trained to use respirators? (select all that apply) Nurse/Nurse Assistant Dietitian Dialysis Patient-Care Technician Physicians/Physician Assistant Dialysis Biomedical Technician Nurse Practitioner Social Worker Other: ___________________
|
The questions were reworded because not all respirators require annual fit testing. |
Zero impact |
Q34 - options updated |
Of the In-Center Hemodialysis patients in question #31, how many received: a. A completed series of hepatitis B vaccine (ever)? ________ b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. At least one dose of pneumococcal vaccine (ever)? ________
|
Of the In-Center Hemodialysis patients in question #31, how many received: a. A completed series of hepatitis B vaccine (ever)? ________ b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. At least one dose of pneumococcal vaccine (ever)? ________ d. Annual COVID-19 vaccine? ______ _______
|
Question options updated to include annual COVID-19 vaccine. |
5 additional minutes |
Q42 – options updated |
Of the Peritoneal Dialysis patients in question #41, how many received: a. A completed series of hepatitis B vaccine (ever)? ________ b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. At least one dose of pneumococcal vaccine (ever)? _______
|
Of the Peritoneal Dialysis patients in question #41, how many received: a. A completed series of hepatitis B vaccine (ever)? ________ b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. At least one dose of pneumococcal vaccine (ever)? _______ d. Annual COVID-19 vaccine?
|
Question updated to include the Annual COVID-19 vaccine. |
5 additional minutes. |
Q49 - options updated |
Of the Home Hemodialysis patients from question #46, how many received:
a. A b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. At least one dose of pneumococcal vaccine (ever)? _______
|
Of the Home Hemodialysis patients from question #46, how many received:
a. A b. The influenza (flu) vaccine for the current/most recent flu season? ________ c. At least one dose of pneumococcal vaccine (ever)? _______ d. Annual COVID-19 vaccine? |
Question updated to include the Annual COVID-19 vaccine. |
5 additional minutes |
Q60b – options updated |
CDC Making Dialysis Safer for Patients Coalition – facility-level participation CDC Making Dialysis Safer for Patients Coalition – corporate or other organization-level participation The Standardizing Care to improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative Peritoneal Dialysis Catheter-related Infection Project SCOPE Collaborative Hemodialysis Access-related Infection Project None of the above ___
|
CDC Making Dialysis Safer for Patients Coalition – facility-level participation CDC Making Dialysis Safer for Patients Coalition – corporate or other organization-level participation The Standardizing Care to improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative Peritoneal Dialysis Catheter-related Infection Project SCOPE Collaborative Hemodialysis Access-related Infection Project None of the above Other (please specify) ________________
|
‘Other’ added to the options to allow for learning if there are other initiatives we may not be aware of. |
Zero impact |
Q62 - options updated |
Which of the following CDC Core Interventions does your center apply for prevention of blood stream infections? (Check all that apply)
Surveillance and feedback using NHSN Hand hygiene observations Catheter/vascular access care observations Staff education and competency Patient education/engagement Catheter reduction Chlorhexidine for skin antisepsis Catheter hub disinfection Antimicrobial ointment or chlorhexidine-impregnated dressing None
|
Which of the following CDC Core Interventions does your center apply for prevention of blood stream infections? (Check all that apply)
Surveillance and feedback using NHSN Hand hygiene observations Catheter/vascular access care observations Staff education and competency Patient education/engagement Catheter reduction Chlorhexidine with alcohol Catheter hub disinfection Antimicrobial ointment Chlorhexidine-impregnated dressing None
|
Options revised as chlorhexidine w alcohol is recommended. |
Zero impact |
Q76 - options updated |
Are antimicrobial lock solutions used to prevent hemodialysis catheter infections in your center? Yes, for all catheter patients Yes, for some catheter patients No
a. If yes, which lock solution is most commonly used? (select one) Sodium citrate Gentamycin Vancomycin Taurolidine Ethanol Multi-component lock solution or other, specify: ___________
|
Are antimicrobial lock solutions used to prevent hemodialysis catheter infections in your center? Yes, for all catheter patients Yes, for some catheter patients No
a. If yes, which lock solution is most commonly used? (select one) Sodium citrate Gentamycin Vancomycin Taurolidine Ethanol Taurolidine and heparin (DefencathTM) Multi-component lock solution or other, specify: ___________
|
Defencath was recently approved by the FDA. |
Zero impact. |
Q79 - verbiage updated |
Does your center provide hemodialysis catheter patients with supplies to allow for changing catheter dressings outside the dialysis center? Yes, routinely for all or most patients with a catheter Yes, only for select patients with a catheter No
|
Does your center provide in-center hemodialysis catheter patients with supplies to allow for changing catheter dressings outside the dialysis center? Yes, routinely for all or most patients with a catheter Yes, only for select patients with a catheter No
|
Updated question to ensure clarity that question is asking about in-center only patients |
Zero impact |
57.507 Home Dialysis Center Practices Survey In the effort to review all data collection forms in the NHSN OMB package to ensure compliance, we are submitting updates to form 57.507 Home Dialysis Center Practices Survey, as the data collection form on the OMB webpage does not match what is currently collected by NHSN.
The crosswalk below lists all the updates that are being made to the form.
|
||||
Type of Change |
Changed From |
Changed To |
Justification |
Impact to Burden |
Q3 – Revision of options Joint commission removed |
Is your facility accredited by an organization other than CMS? Yes No
Joint Commission National Dialysis Accreditation Commission (NDAC) Accreditation Commission for Health Care (ACHC) Other (specify) _______________
|
Is your facility accredited by an organization other than CMS? Yes No
National Dialysis Accreditation Commission (NDAC) Accreditation Commission for Health Care (ACHC) Other (specify) _______________
|
Joint Commission no longer used |
Zero impact |
Q4 – deletion of verbiage |
a. What types of dialysis services does your center offer (certified and non-certified)? (select all that apply):
|
What types of dialysis services does your center offer? (select all that apply): Home Peritoneal Dialysis Home Hemodialysis
|
the certified and non-certified verbiage was removed from the question to provide clarification".
|
Zero impact |
Q4 – additional verbiage for clarity |
Peritoneal Dialysis Home Hemodialysis |
Home Peritoneal Dialysis Home Hemodialysis |
Added “home” before peritoneal dialysis for clarity |
Zero impact |
Q7 - NEW Question added |
|
Within the last 3 years, has your facility/organization been surveyed by CMS or a CMS approved accrediting organization (i.e., state survey agency, Accreditation Commission for Health Care [ACHC], National Dialysis Accreditation Commission [NDAC])? Yes No |
To ascertain any infection control breaches citations. |
3 additional minutes |
Q8 - Sub- question 8a added |
Does your center provide dialysis services within long-term care facilities (e.g., staff-assisted dialysis in nursing homes or skilled nursing facilities; not long-term acute care hospitals)? Yes No
|
8a. Does your center provide dialysis services within long-term care facilities (e.g., staff-assisted dialysis in nursing homes or skilled nursing facilities; not long-term acute care hospitals)? Yes No 8b. If yes, what types of dialysis services are provided within long-term care facilities? (check all that apply): HD in LTC PD in LTC
|
Sub-Question was added for clarity |
1 additional minute |
Revised Patient Section |
Combined Patient/Staff Census |
Patient Census |
To keep all patient questions combined and separate from staff questions |
Zero impact |
Q12 - Language modified |
How many MAINTENANCE, NON-TRANSIENT PATIENTS were assigned to your center during the first week of February (2/1 through 2/7)? |
How many ADULT MAINTENANCE, NON-TRANSIENT ESRD and AKI PATIENTS were assigned to your center during the first week of February (2/1 through 2/7)? |
Provides clarity to the question |
Zero impact |
Q13 – New question |
|
If MIXED Population or PEDIATRIC Population was selected in question 4, how many Maintenance, Non-Transient ESRD and AKI PEDIATRIC PATIENTS were assigned to your center the first week of February (2/1 through 2/7) _________
Peritoneal Dialysis: ___________ |
To allow for capture of pediatric patient data |
5 minutes additional |
Q14 added NEW |
|
Based on the number of patients that treated in the first week of February (2/1 through 2/7), please indicate the number of patients per Race:
Declined to response: ___________ |
Optional question added to obtain data on patient race |
5 additional minutes |
Q15 added NEW |
|
Based on the number of patients that treated in the first week of February (2/1 through 2/7), please indicate the number of patients per Ethnicity
Declined to respond: _______ |
Optional question added to obtain data on patient ethnicity |
5 additional minutes |
NEW Staff Section |
Combined Patient/Staff Census |
Staff Census |
To keep all staff questions combined |
Zero impact |
Q16 new to staff section |
Added to new Staff Section from Patient/Staff Census section |
How many patient care STAFF (full time, part time, or affiliated with) worked in your center during the first week of February (2/1 through 2/7)? Include only staff who had direct contact with dialysis patients or equipment: _________
Of these, how many were in each of the following categories? a. Nurse/nurse assistant: __________ b. Dialysis patient-care technician: __________ c. Dialysis biomedical technician: __________ d. Social worker: __________ e. Dietitian: _________ f. Physicians/physician assistant: _________ g. Nurse practitioner: _________ h. Other: _________
|
Moved from Patient/Staff Census section to keep all staff questions in one section |
Zero impact |
Directions clarified |
Please respond to the following questions based on information from your center in the first week of February (2/1 through 2/7). This applies to current or most recent February relative to current date. |
Please respond to the following questions based on your peritoneal dialysis patients in the first week of February (2/1 through 2/7). This applies to current or most recent February relative to current date. |
Provides clarity in the directions for the section |
Zero impact |
New Peritoneal Dialysis Patient Section Added |
New Peritoneal Dialysis Patient Section added |
|
Created new section to keep all Peritoneal Dialysis Patient-related questions together |
Zero impact |
Q18 - New question under Peritoneal Dialysis Patient Section |
|
Number of maintenance, non-transient ESRD and AKI Peritoneal Dialysis patients that were assigned to your center during the first week of February (2/1 through 2/7 |
To obtain additional information on peritoneal dialysis patients |
Zero impact – question auto-populates from a prior question in the survey |
Directions clarified |
Please respond to the following questions based on information from your center in the first week of February (2/1 through 2/7). This applies to current or most recent February relative to current date. |
Please respond to the following questions based on your home dialysis patients in the first week of February (2/1 through 2/7). This applies to current or most recent February relative to current date. |
Provides clarity in the directions for the section |
Zero impact |
New Home Hemodialysis Patients Section Added |
|
|
To keep all home hemodialysis patient-related questions together |
Zero impact |
Q22 - New Question added to Home Hemodialysis Patient section |
|
Number of maintenance, non-transient ESRD and AKI Home Hemodialysis patients that were assigned to your center during the first week of February (2/1 through 2/7): |
To obtain additional data on home hemodialysis patients. |
Zero impact – question auto-populates from a prior question in the survey |
Q24 - Modified language |
Does your home hemodialysis facility perform buttonhole cannulation |
Does your dialysis facility utilize buttonhole cannulation techniques for Home Hemodialysis patients? Yes No
a. Of the AV fistula patients from question #22a, how many had buttonhole cannulation? ________
b. When buttonhole cannulation is performed for home hemodialysis patients: i. Who most often performs it?
|
To clarify question |
Zero impact |
Q25 – Moved from Vaccine Section to Home Hemodialysis Patients section |
Of the Home Hemodialysis patients counted in question #21, how many received: a. A complete series of hepatitis B vaccine (ever)? __________ b. The influenza (flu) vaccine for the current/most recent flu season? ______________ c. At least one dose of pneumococcal vaccine (ever)? ______________
|
Of the Home Hemodialysis patients counted in question #21, how many received: a. A complete series of hepatitis B vaccine (ever)? __________ b. The influenza (flu) vaccine for the current/most recent flu season? ______________ c. At least one dose of pneumococcal vaccine (ever)? _________ d. The annual COVID-19 vaccine |
Placement of question within the survey moved as well as the addition of the Annual COVID-19 vaccine as a new option. |
Zero impact |
Q26 Moved from Surveillance section to Home Hemodialysis Patients section |
Which of the following events in your Home Hemodialysis patients does your center routinely track? |
Which of the following events in your Home Hemodialysis patients does your center routinely track? Bloodstream infection Needle/access dislodgement Vascular access site Air embolism infection Catheter breakage or bloodline separation Other (specify): ____________ |
Wording did not change, just placement within the survey. |
Zero impact |
Q27 – Options Updated |
Which type of pneumococcal vaccine does your center offer to patients? (choose one)
Polysaccharide (i.e., PPSV23) only Conjugate (e.g., PCV13) only Both polysaccharide & conjugate Neither offered
|
Which type of pneumococcal vaccine does your center offer to patients? (choose one) New Conjugate (PCV20) only New Conjugate (PCV15) and Polysaccharide (PPSV23) Both New Conjugate (Either PCV20 or PCV15) and Polysaccharide (PPSV23) Other (please specify) Neither offered
|
Updated pneumococcal vaccine options added. |
3 additional minutes |
Q32 – verbiage modified |
Is your center actively participating in any of the following prevention initiatives (select all that apply):
None of the above |
Has your center participated in any national or regional infection prevention-related initiatives in the past year? Yes No
a. If yes, what is the primary focus of the initiative(s)? (if >1 initiative, select all that apply) Catheter reduction Hand hygiene Bloodstream infection prevention Patient education/engagement for infection prevention Increase vaccination rates Decrease/improve use of antibiotics Improve general infection control practices Improve culture of safety Other, specify: _________________________________________________
b. If yes, is your center actively participating in any of the following prevention initiatives (select all that apply): CDC Making Dialysis Safer for Patients Coalition – facility-level participation CDC Making Dialysis Safer for Patients Coalition – corporate or other organization-level participation The Standardizing Care to improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative Peritoneal Dialysis Catheter-related Infection Project SCOPE Collaborative Hemodialysis Access-related Infection Project None of the above Other, specify
|
Revised question to obtain better, more complete data. |
5 additional minutes |
Q33 – Added as new |
|
a. What education do you provide to patients in your center when they start dialysis? (check all that apply): Vascular access care Hand hygiene Risks related to catheter use Recognizing signs of infection Instructions for access management when away from the dialysis unit Different dialysis modalities (i.e., home dialysis or peritoneal dialysis) Other, specify: ______________________________ None
b. What education do you provide to your patients regularly (at least annually) (check all that apply): Vascular access care Hand hygiene Risks related to catheter use Recognizing signs of infection Instructions for access management when away from the dialysis unit Different dialysis modalities (i.e., home dialysis or peritoneal dialysis) Other, specify: __________________ None
|
To obtain data on the types of education provided to patients |
10 additional minutes |
Q34 - Added as New |
|
Does your center provide training for staff on infection prevention and control at least once annually? Yes No |
To obtain data on staff training on infection control measures |
5 additional minutes |
Q35 - Added as New |
|
Does your center perform staff knowledge assessments for infection prevention and control (select all that apply) At least annually One or more times each year At least once a year When new equipment or procedures are introduced |
To obtain data on staff training on infection control measures |
5 additional minutes
|
Section Title Updated |
Vascular Access |
Arteriovenous (AV) Fistulas or Grafts |
For clarity of the questions under the section |
Zero impact |
Q36 - verbiage updated |
Before prepping the fistula or graft site for rope-ladder cannulation, what is the site most often cleansed |
Before prepping the fistula or graft site for cannulation, what is the access site most often cleansed with (either by patients or staff upon entry to the clinic)? Soap and water Alcohol-based hand rub Antiseptic wipes Other, specify: ____________ Nothing
|
For clarity of the data requested under the question |
Zero impact |
Q37 - verbiage updated |
Before rope-ladder cannulation of a fistula or graft, what is the site most often prepped with? (select the one most commonly used) |
Before cannulation of a fistula or graft, what is the skin most often prepped with? (select one) Alcohol Chlorhexidine without alcohol Chlorhexidine with alcohol (e.g., Chloraprep™, PDI Prevantics®) Povidone-iodine (or tincture of iodine) Sodium hypochlorite solution (e.g., ExSept®, Alcavis) without alcohol Sodium hypochlorite solution (e.g., ExSept®, Alcavis) followed by alcohol Other, specify: _________________ Nothing |
For clarity of the data requested under the question |
Zero impact |
Q43 – Added new option |
Are antimicrobial lock solutions used to prevent hemodialysis catheter infections? Yes, for all catheter patients Yes, for some catheter patients No a. If yes, which lock solution is most commonly used? (select one) Sodium citrate Taurolidine Gentamicin Ethanol Vancomycin Multi-component lock solution or other, specify: __________
|
Are antimicrobial lock solutions used to prevent hemodialysis catheter infections? Yes, for all catheter patients Yes, for some catheter patients No a. If yes, which lock solution is most commonly used? (select one) Sodium citrate Taurolidine Gentamicin Ethanol Vancomycin Multi-component lock solution or other, specify: __________ Taurolidine and heparin (DefencathTM) |
Added new antimicrobial lock approved by FDA |
Zero impact |
Q45 – Old Q45 Removed |
Does your center provide hemodialysis catheter patients with supplies to allow for changing catheter dressings outside the dialysis center? Yes, routinely for all or most patients with a catheter Yes, only for select patients with a catheter No |
|
Question removed to avoid confusion as to where home patients would obtain their supplies (would be sent directly to patient home, not provided by center) |
Zero impact |
57.701 Glycemic Control Module-HYPO Annual Survey The Medication Safety Annual Hospital Survey collects facility-level data from the previous calendar year and is completed by all facilities enrolled in the Medication Safety Component. The data will be used in analysis of data collected within the modules included in the Medication Safety Component, as well as used to support decision making, program planning, and research across CDC. Annual survey data will be collected electronically once annually via the NHSN application. The crosswalk below lists all the updates that are being made to the form.
|
|||||
Changed From |
Changed To |
Justification |
Impact to Burden |
||
Glycemic Control Module Annual Hospital Survey |
Medication Safety Component – Annual Hospital Survey |
A revision to the title reflects additional opioid-related topics added to the facility survey such that the survey encompasses topics related to all NHSN Medication Safety Component modules.
|
None |
||
|
6. *Select the module(s) for which your facility currently reports or intends to report data: □ Glycemic Control Module □ Opioid-Related Adverse Events (ORAE) Module |
Addition of question to allow facility to indicate which NHSN Medication Safety Component modules they will participate in. This will allow facilities to be prompted only to complete survey questions that correspond to the modules they will participate in. |
None |
||
3. *Does your facility have an inpatient glycemic control quality improvement or safety program in place as demonstrated by: (Check all that apply.)
Special team(s) dedicated to consulting on patients with diabetes that actively assist in the management of inpatients with diabetes Senior executive who serves as a point of contact or “champion” to help ensure the glycemic control program has resources and support to accomplish its mission Clinician (physician, nurse, or pharmacist) leader with dedicated time to manage the program and conduct daily interventions Allocation of dedicated resources to support glycemic control activities Staff from key support departments and groups who contribute to glycemic control activities At least annual presentation of information on glycemic control activities and outcomes to facility leadership and/or board At least annual opportunity to address glycemic control resource needs with facility leadership and/or board Facility communication mechanisms about glycemic control activities, via email, newsletters, events, or other avenues Provision of facility staff training and development on glycemic control activities Documented statement of facility support for glycemic control activities (e.g., a written policy or statement approved by the board) Our facility does not have a glycemic control quality improvement or safety program in place Our facility has other glycemic control programmatic components, please describe briefly: __________________
|
7. *Does your facility provide leadership support and clinical resources specifically for inpatient glycemic control quality improvement or safety program activities as demonstrated by: (Check all that apply.)
Currently, our facility does not have leadership support or clinical resources specifically to address inpatient glycemic control as part of our patient safety and quality improvement activities |
Provides clarity to the data collection and streamlines facility response options |
None |
||
4.Does your facility have inpatient glycemic control quality improvement or safety practices as demonstrated by: (Check all that apply.)
Provider education Patient education Provider reminder systems Active surveillance for glucose control metrics, such as hypoglycemia/hyperglycemia events or other facilitated relay of clinical data to providers Audit and feedback on performance to providers Incentives, regulation, or policy that are provider- or health system-directed Insulin orders/protocols that are standardized across units or the facility Our facility does not have practices specific to glycemic control quality improvement or patient safety Our facility has other glycemic control practices, please describe briefly: ______________________________ |
8. *Does your facility promote inpatient glycemic control practices as part of your patient safety and quality improvement activities as demonstrated by: (Check all that apply.)
Offering provider education on glycemic control and best-practices for managing diabetic patients at least annually Offering prescriber (e.g., physician, nurse practitioner) education and/or training on glycemic control and best-practices for managing patients with diabetes at least annually Offering nurse education and/or training on glycemic control and best-practices for managing patients with diabetes at least annually Offering pharmacy education and/or training on glycemic control and best-practices for managing patients with diabetes at least annually Using facility communication to raise awareness about inpatient glycemic control activities via email, newsletters, events, or other avenues (e.g., grand rounds) Offering patient education Active surveillance for glucose control metrics, such as hypoglycemia/hyperglycemia events or other facilitated relay of clinical data to providers Insulin orders/protocols that are standardized across units or the facility Our facility uses other approaches to promote inpatient glycemic control practices, please describe : ______________________________ Currently, our facility does not have specific activities to promote inpatient glycemic control practices |
|
|
||
5. Describe the current state of hypoglycemia management / prevention protocols at your facility: (Check one.)
Nurse driven protocols for hypoglycemia management / prevention are not available at our facility Standardized nurse driven protocols for hypoglycemia management / prevention are available, but use of the protocols are not monitored Standardized nurse driven protocols for hypoglycemia management / prevention are available and use of the protocols are monitored
6. Describe the level of coordination between point of care glucose testing, insulin delivery, and nutrition delivery on the non-critical care wards at your facility. (Check one.)
• There is not a systematic mechanism or protocol to coordinate glucose testing, insulin administration, and meal/nutrition scheduling • There is a systematic mechanism or protocol to coordinate glucose testing, insulin administration, and meal/nutrition scheduling in some units but not all units • There is a systematic mechanism or protocol to coordinate glucose testing, insulin administration, and meal/nutrition scheduling in all units of the facility
7. Select the description that most accurately reflects the approach to glycemic control and insulin management in the non-critical care units at your facility: (Check one.)
No protocol is available in the non-critical care units at our facility Our facility has a protocol for insulin and hyperglycemia management (including subcutaneous insulin orders) that outlines preferred insulin choices for different situations; however, the protocol guidance is not embedded in order sets |
N/A (DELETION) |
These questions are no longer required; data collection is consolidated and streamlined. |
None |
||
|
9. *Does your facility use the following strategies to implement inpatient glycemic control and insulin management practices? (Check all that apply.) Our facility has a standardized protocol for insulin use and hyperglycemia management (including subcutaneous insulin orders) that outlines preferred insulin choices for different situations 9a. If this response is selected, please indicate how this protocol is implemented. (Check one.) • The insulin use protocol is available for use, but not embedded into any standardized (e.g., admission) order sets • The insulin use protocol is integrated into standardized (e.g., admission) order sets; however, providers must “opt in” • The insulin use protocol is integrated into standardized (e.g., admission) order sets that requires providers to “opt out”
Our facility has standardized nurse-driven protocols for monitoring for and responding to hypoglycemia events 9b. If this response is selected, please indicate where these protocols are used. (Check one.) • Nurse-driven glycemic control monitoring protocols are used only in critical care units • Nurse-driven glycemic control monitoring protocols are used in select medical or surgical units • Nurse-driven glycemic control monitoring protocols are used in all inpatient units • Nurse-driven glycemic control monitoring protocols are used elsewhere; please indicate:___________
Our facility has standardized nurse-driven protocols for monitoring for and responding to hyperglycemia events 9c. If this response is selected, please indicate where these protocols are used. (Check one.) • Nurse-driven glycemic control monitoring protocols are used only in critical care units • Nurse-driven glycemic control monitoring protocols are used in select medical or surgical units • Nurse-driven glycemic control monitoring protocols are used in all inpatient units • Nurse-driven glycemic control monitoring protocols are used elsewhere; please indicate:___________
Our facility has a standardized process/protocol to coordinate glycemic control monitoring (i.e. glucose testing, insulin administration) with meal/nutrition scheduling 9d. If this response is selected. Please indicate where these protocols are used. (Check one.) • Coordinating glycemic control with nutrition is done only in critical care units • Coordinating glycemic control with nutrition is done in select medical or surgical units • Coordinating glycemic control with nutrition is done in all inpatient units • Coordinating glycemic control with nutrition is done elsewhere; please indicate:___________________
Our facility uses a different strategy to implement inpatient glycemic control practices, please describe: ________________ Currently, our facility does not have any standardized protocols to support implementation of inpatient glycemic control practices
10. *Does your facility use the following approaches to monitor and report inpatient glycemic control and insulin management practices? (Check all that apply.)
Our facility monitors the use of standardized protocols for insulin use and hyperglycemia management for inpatients with diabetes Our facility performs active surveillance for hypoglycemia events on a daily basis to allow real-time correction of insulin use / diabetes management Our facility performs active surveillance for hyperglycemia events on a daily basis to allow real-time correction of insulin use / diabetes management Our facility performs retrospective review of hypoglycemia / hyperglycemia events on a regular (monthly or quarterly) basis to identify opportunities to improve insulin use / diabetes management Our facility reports unit-level results of glycemic control event monitoring Our facility shares feedback to providers on the glycemic control of their inpatients with diabetes Our facility uses a different approach to monitor inpatient glycemic control and insulin management practices, please describe: ________________ Currently, our facility does not monitor inpatient glycemic control and insulin management practices |
These questions consolidate previous data collection questions and more accurately collect data of interest. |
None |
||
9. Approximately what percentage of your inpatient population with diabetes is utilizing continuous glucose monitoring (CGM): (Check one.) ______ % Unsure |
12.*Approximately what percentage of your inpatient population with diabetes have a continuous glucose monitoring (CGM) device that is being used in the course of inpatient care: (Check one.) ______ % Unsure |
Revision Clarifies Question |
None |
||
|
Section 3a. Opioid Prescribing Safety Practices
13. *Does your facility have an inpatient opioid stewardship quality improvement program? (Check one.)
Yes No Other; please describe:
14. *Does your facility have any of the following practices in place within or outside of an opioid stewardship program: (Check all that apply.)
Leadership Commitment such as a senior executive who serves as a point of contact or “champion” to help ensure the opioid stewardship practices has resources and support to accomplish its mission. Maintain written policies and procedure that support opioid stewardship activities. Support clinical knowledge, expertise, and practice such as require ongoing clinician training, education, and engagement to support effective pain management and opioid stewardship for prescribers and care teams. Patient and Family Caregiver Education and Engagement, such as patient/family education related to pain management goals and modalities. Tracking, Monitoring, and Reporting of key quality metrics are used to identify opportunities for improvement and to assess the impact of opioid stewardship efforts. Accountability, such as set measurable goals for promoting, establishing, and maintaining a culture of opioid stewardship. Community Collaboration and coordination with community leaders and stakeholders Our facility does not have an opioid stewardship quality improvement or safety program in place. Our facility has other opioid safety practices, please describe briefly: __________________
Section 4b. Education
15. *Does your facility have opioid prescribing education programs or practices in place? (Check one.)
Yes No [If checked, skip questions 15a and 15b] Other; please describe: _______________ [If checked, skip questions 15a and 15b]
15a. If your facility has opioid prescribing education programs or practices in place, how frequently is education provided? (Check all that apply.)
At time of hire/orientation At least annually At least quarterly Other; please describe: __________________
15b. If your facility has opioid prescribing education programs or practices in place, what groups of healthcare workers are included in your opioid education programs or practices? (Check all that apply.)
Physicians and licensed independent practitioners authorized to prescribe in your state (e.g., physician assistants, nurse practitioners) Nursing staff Pharmacy staff Other staff; please describe: __________________ Section 4c. Quality Measurement 16. *What quality metrics are tracked, monitored and/or reported related to opioid safety or quality improvement? (Check all that apply.)
Opioid prescribing trends(e.g., provider, unit, patient-level Use of multi-modal pain management tools Opioid-related adverse events Our facility does not track, monitor, or report opioid quality metrics. [If checked, skip 16a – 16c] Our facility monitors other opioid quality/safety metrics, please describe briefly: ____________
16a. If opioid quality/safety metrics are tracked, monitored, and/or reported, at what level is data trended and/or reported? (Check one.) Physician-level Specialty-level Unit-level Facility-Level Other level; please describe: ____________
16b. What type of opioid-related adverse events are tracked in your facility? (Check all that apply.) Allergic adverse events (e.g., anaphylaxis) Other adverse drug events (e.g., constipation) confusion, delirium, respiratory depression) Events requiring administration of an opioid antagonist Events that result in a transfer to a higher level of care Events that result in patient death Our facility does not track, monitor, or report opioid-related adverse events Our facility monitors other opioid-related adverse events, please describe briefly: ____________
16c. If opioid-related events are tracked, what methods are used to identify potential opioid-related adverse events? (Check all that apply.) Voluntary reporting system Alerts for antagonist medication administration (e.g., naloxone administration) Code Blue/Medical Emergency Team activations Reports to quality/safety leadership Other methods, please describe briefly: _______________ |
Addition of questions to collect information about facility’s opioid prescribing safety practices, education, and quality measurement corresponding to facility data collected with the NHSN Opioid-Related Adverse Events (ORAE) Module. |
Increase in burden due to addition of new questions.
|
||
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Farrell, Paula (CDC/NCEZID/DHQP/SB) (CTR) |
| File Modified | 0000-00-00 |
| File Created | 2024-11-16 |
© 2026 OMB.report | Privacy Policy