Burden for 10 CFR Part 26 associated with Risk-Informed, Technology-Inclusive Regulatory Framework for Advanced Reactors Proposed Rule
Burden for 10 CFR Part 26 associated with Risk-Informed, Technology-Inclusive Regulatory Framework for Advanced Reactors Proposed Rule
DG-5073 - Fitness-For-Duty Programs For Commercial Nuclear Plants And Manufacturing Facilities Licensed Under 10 CFR Part 53
Burden for 10 CFR Part 26 associated with Risk-Informed, Technology-Inclusive Regulatory Framework for Advanced Reactors Proposed Rule
OMB:
U.S. NUCLEAR REGULATORY COMMISSION |
||
|
DRAFT REGULATORY GUIDE DG-5073 |
|
Proposed new Regulatory Guide 5.94, Revision 0 |
|
|
Issue Date: October 2024 Technical Lead: Brian Zaleski |
||
FITNESS-FOR-DUTY PROGRAMS FOR
COMMERCIAL
NUCLEAR PLANTS AND MANUFACTURING FACILITIES LICENSED UNDER 10 CFR
PART 53
Purpose
This regulatory guide (RG) describes an approach that is acceptable to the staff of the U.S. Nuclear Regulatory Commission (NRC) to meet regulatory requirements for fitness-for-duty (FFD) programs at commercial nuclear plants (CNPs) licensed under Title 10 of the Code of Federal Regulations (10 CFR) Part 53, “Risk-Informed, Technology-Inclusive Regulatory Framework for Commercial Nuclear Plants” (Ref. 1). Licensees, applicants, and other entities (as defined in 10 CFR 26.5) who implement FFD programs may consider this guidance when preparing an application for a 10 CFR Part 53 operating license, manufacturing license, combined license, limited work authorization, construction permit, or early site permit and when implementing the FFD program during construction, operation, and decommissioning.
Applicability
This RG applies to applicants and holders of a license under the provisions of 10 CFR Part 53 that implement an FFD program under 10 CFR Part 26, “Fitness for Duty Programs” (Ref. 2), Subpart M, “Fitness for Duty Programs for Facilities Licensed under 10 CFR Part 53.”
Applicable Regulations
10 CFR Part 26 describes the requirements and standards for the establishment, implementation, and maintenance of FFD programs.
For all FFD programs implemented under 10 CFR Part 26, Subpart M, the following requirements and subparts are applicable:
10 CFR 26.3(f) applies the 10 CFR Part 26 requirements to CNPs licensed under 10 CFR Part 53 and holders of a manufacturing license under 10 CFR Part 53.
10 CFR 26.4, “FFD program applicability to categories of individuals,” specifies the categories of individuals who are subject to 10 CFR Part 26 FFD programs.
10 CFR 26.5, “Definitions,” explains the relevant terminology.
10 CFR 26.23, “Performance objectives,” describes five performance objectives that every FFD program must meet.
10 CFR Part 26, Subpart A, “Administrative Provisions,” provides the requirements and standards for the establishment, implementation, and maintenance of FFD programs.
10 CFR Part 26, Subpart I, “Managing Fatigue,” contains the requirements for the management of fatigue for certain individuals who are subject to the FFD program.
10 CFR Part 26, Subpart M, “Fitness for Duty Programs for Facilities Licensed under 10 CFR Part 53,” provides the FFD requirements for 10 CFR Part 53 applicants, licensees, and other entities.
10 CFR Part 26, Subpart O, “Inspections, Violations, and Penalties,” provides the requirements that enable NRC inspection and enforcement of licensed activities to accomplish the purposes of 10 CFR Part 26.
In addition to the requirements and subparts that all licensees and other entities under 10 CFR Part 26, Subpart M, must implement, for FFD programs1 implemented under 10 CFR 26.605(a) and (b), a licensee or other entity must, and in some cases may, at their own discretion (as described in the Subpart M requirements), implement the following requirements and subparts:
10 CFR Part 26, Subpart C, “Granting and Maintaining Authorization,” establishes the requirements for a licensee or other entity to grant an individual or maintain an individual’s authorization for the types of access or perform the duties or responsibilities making them subject to 10 CFR Part 26. The requirements in this subpart apply to the FFD programs of licensees and other entities identified in 10 CFR 26.3(f) that elect not to implement the requirements in subpart M for the categories of individuals in 10 CFR 26.4 and those licensees and other entities that elect to implement the requirements in 10 CFR 26.605(b).
10 CFR Part 26, Subpart D, “Management Actions and Sanctions To Be Imposed,” establishes requirements and the minimum actions when an individual has violated the drug and alcohol provisions of an FFD policy or shows indications that he or she may not be fit to safely and competently perform his or her duties. The requirements in this subpart apply to the FFD programs of licensees and other entities identified in 10 CFR 26.3(f) that elect not to implement the requirements in subpart M for the categories of individuals in 10 CFR 26.4 and those licensees and other entities that elect to implement the requirements in 10 CFR 26.605(b).
10 CFR Part 26, Subpart E, “Collecting Specimens for Testing,” provides the requirements for the collection and testing for alcohol and collecting urine specimens for drug testing unless the licensee or other entity elects to use the U.S. Department of Health and Human Services’ “Mandatory Guidelines for Federal Workplace Drug Testing Programs” (HHS Guidelines) (Ref. 3) for urine testing. Licensees or other entities that collect urine specimens for drug testing and implement an FFD program in 10 CFR 26.605 must implement the following Subpart E requirements: 10 CFR 26.115, “Collecting a urine specimen under direct observation,” and 10 CFR 26.119, “Determining ‘shy’ bladder.” Licensees or other entities that collect urine specimens for conducting alcohol tests must implement the following Subpart E requirements: 10 CFR 26.91, “Acceptable devices for conducting initial and confirmatory tests for alcohol and methods of use,” 26.93, “Preparing for alcohol testing,” 26.95, “Conducting an initial test for alcohol using a breath specimen,” 26.97, “Collecting oral fluid specimens for alcohol and drug testing,” 26.99, “Determining the need for a confirmatory test for alcohol,” 26.101, “Conducting a confirmatory test for alcohol,” and 26.103, “Determining a confirmed positive test result for alcohol.”.
10 CFR Part 26, Subpart G, “Laboratories Certified by the Department of Health and Human Services,” contains a provision in 10 CFR 26.163(a)(2) that permits the conduct of special analyses of dilute specimens. Licensees and other entities who implement an FFD program under 10 CFR 26.605 and use a urine specimen for drug testing are required to implement special analysis testing under 10 CFR 26.163(a)(2).
10 CFR Part 26, Subpart H, “Determining Fitness-for-Duty Policy Violations and Determining Fitness,” contains requirements for determining whether a donor has violated the FFD policy and for making a determination of fitness. The requirements in this subpart apply to the FFD programs of licensees and other entities identified in 10 CFR 26.3(f) that elect not to implement the requirements in subpart M for the categories of individuals in 10 CFR 26.4 and those licensees and other entities that elect to implement the requirements in 10 CFR 26.605(b).
10 CFR Part 26, Subpart N, “Recordkeeping and Reporting Requirements,” provides recordkeeping and reporting requirements. The requirements in this subpart must be implemented by a licensee or entity that implements an FFD program in 10 CFR 26.605(b).
10 CFR Part 53 provides an alternative risk-informed and technology-inclusive regulatory framework for the licensing, construction, operation, and decommissioning of CNPs .
10 CFR 53.610, “Construction,” under 10 CFR Part 53 requires, in part, that licensees ensure the development and implementation of an FFD program under 10 CFR Part 26, to manage and control the construction activities.
10 CFR 53.620, “Manufacturing,” under 10 CFR Part 53 requires, in part, that holders of manufacturing licenses ensure the development and implementation of an FFD program, in accordance with 10 CFR Part 26, to manage and control the manufacturing activities within the scope of the manufacturing license.
10 CFR 53.860, “Security programs,” under 10 CFR Part 53 requires, in part, that each holder of an operating license or combined license develop, implement, and maintain an FFD program under 10 CFR Part 26.
10 CFR Part 73, “Physical Protection of Plants and Materials” (Ref. 4), prescribes requirements for the establishment and maintenance of a physical protection system that will be capable of protecting special nuclear material (SNM) at fixed sites and in transit and protecting plants in which SNM is used.
10 CFR 73.100, “Technology-inclusive requirements for physical protection of licensed activities at commercial nuclear plants against radiological sabotage,” provides requirements for the physical protection of CNPs licensed under 10 CFR Part 53.
10 CFR 73.120, “Access authorization program for commercial nuclear plants,” provides requirements for granting, maintaining, and denying authorization to individuals seeking unescorted access to a CNP licensed under 10 CFR Part 53.
49 CFR Part 40, “Procedures for Transportation Workplace Drug and Alcohol Testing Programs” (Ref. 5), tells all parties that conduct drug and alcohol tests required by U.S. Department of Transportation (DOT) regulations how to conduct these tests and which procedures to use. This part concerns the activities of transportation employers, safety-sensitive transportation employees (including self-employed individuals, contractors, and vendors as covered by DOT regulations), and service agents.
Related Guidance
RG 5.77, “Insider Mitigation Program” (Ref. 6), provides guidance for monitoring the initial and continuing trustworthiness and reliability of individuals granted or retaining unescorted access authorization to a protected or vital area, and implementation of defense-in-depth methodologies to minimize the potential for an insider to adversely affect, either directly or indirectly, the licensee’s capability to prevent significant core damage and spent fuel sabotage.
RG 5.84, “Fitness-for-Duty Programs at New Reactor Construction Sites” (Ref. 7), provides guidance for implementing 10 CFR Part 26, Subpart K, “FFD Program for Construction.”
RG 5.89, “Fitness for Duty Programs for Commercial Power Reactor and Category I Special Nuclear Material Licensees” (Ref. 8), provides guidance for the FFD programs implemented at commercial power reactors and Category I SNM licensees.
Draft Regulatory Guide (DG)-5078 (proposed new RG 5.99), “Fatigue Management for Nuclear Power Plant Personnel at Commercial Nuclear Plants Licensed under 10 CFR Part 53,” (Ref. 9) provides guidance for implementing 10 CFR Part 26, Subpart I, “Managing Fatigue.”
U.S. Department of Health and Human Services’ “Mandatory Guidelines for Federal Workplace Drug Testing Programs” (HHS Guidelines) provides proposed or final drug testing guidelines for the collection, shipment, storage, and testing of urine, oral fluid, and hair specimens and the medical review officer (MRO) evaluation of the laboratory test results.
RGs 5.77 and 5.84 were developed for CNP and Category I SNM licensees subject to the regulatory requirements in 10 CFR 26.3(a) through (d) and licensed under 10 CFR Parts 50, 52, or 70 and did not include (or consider) CNPs licensed under 10 CFR Part 53. However, these RGs provide information for sections in 10 CFR Part 26 that applicants, licensees, and other entities under 10 CFR Part 53 may want to review in developing and implementing their FFD program.
Purpose of Regulatory Guides
The NRC issues RGs to describe methods that are acceptable to the staff for implementing specific parts of the agency’s regulations, to explain techniques that the staff uses in evaluating specific issues or postulated events, and to describe information that the staff needs in its review of applications for permits and licenses. Regulatory guides are not NRC regulations and compliance with them is not required. Methods and solutions that differ from those set forth in RGs are acceptable if supported by a basis for the issuance or continuance of a permit or license by the Commission.
Paperwork Reduction Act
This RG provides voluntary guidance for implementing the mandatory information collections in 10 CFR Parts 26, 53, and 73 that are subject to the Paperwork Reduction Act of 1995 (44 U.S. Code (USC) 3501 et. seq.). These information collections were approved by the Office of Management and Budget (OMB), under control numbers 3150-0146, 3150-XXXX, and 3150-0002, respectively. Send comments regarding this information collection to the FOIA, Library, and Information Collections Branch (T6-A10M), U.S. Nuclear Regulatory Commission, Washington, DC 20555-0001, or by email to [email protected], and to the OMB Office of Information and Regulatory Affairs, Attn: Desk Officer for the Nuclear Regulatory Commission, 725 17th Street, NW Washington, DC 20503.
Public Protection Notification
The NRC may not conduct or sponsor, and a person is not required to respond to, a collection of information unless the document requesting or requiring the collection displays a currently valid OMB control number.
Purpose of Regulatory Guides 5
Public Protection Notification 5
Consideration of International Standards 8
C. STAFF REGULATORY GUIDANCE 10
1. 10 CFR 26.601, “Applicability.” 10
2. 10 CFR 26.4, “FFD program applicability to categories of individuals.” 10
a. Holders of a Manufacturing License 10
b. Transportation of a Manufactured Reactor 13
c. Licensees or Other Entities of a Commercial Nuclear Plant 13
d. Risk-informed Evaluation Process 14
3. 10 CFR 26.603(a), FFD Program Description 15
a. 10 CFR 26.603(a)(1), Description of Analysis Performed under 10 CFR 26.603(c) 15
b. 10 CFR 26.603(a)(2), Type of FFD Program to Be Implemented 16
c. 10 CFR 26.603(a)(3), Program Applicability to Individuals 16
d. 10 CFR 26.603(a)(4), Drug and Alcohol Testing and Fitness Determinations 17
e. 10 CFR 26.603(a)(5), Performance Monitoring and Review Program 17
4. 10 CFR 26.603(c), Criterion and Analysis for an FFD program 18
5. 10 CFR 26.603(d), FFD Performance Monitoring and Review 18
d. Defining Acceptable Performance (i.e., FFD program success) 23
f. 10 CFR 26.603(d)(1), Performance Measures and Thresholds 30
6. 10 CFR 26.603(e), FFD Program Change Control 57
7. 10 CFR 26.605, “FFD program requirements for facilities that do not implement § 26.604” 58
a. 10 CFR 26.605(b), FFD Program for Operation of a Commercial Nuclear Plant 58
8. 10 CFR 26.606, “Written policy and procedures.” 59
a. 10 CFR 26.606(a), Contents of Written Policies 59
b. 10 CFR 26.606(b), Contents of Written Procedures 63
9. 10 CFR 26.607, “Drug and alcohol testing.” 64
a. 10 CFR 26.607(b)(1), Pre-access Testing 66
b. 10 CFR 26.607(b)(2), Random Testing 67
c. 10 CFR 26.607(b)(3), For-cause Testing 73
d. 10 CFR 26.607(b)(4), Post-event Testing 75
e. 10 CFR 26.607(b)(5), Follow-up Testing 77
f. 10 CFR 26.607(c), Urine and Oral Fluid Specimens 77
g. 10 CFR 26.607(d), Privacy and Integrity 78
h. 10 CFR 26.607(e), Collection Facility 78
i. 10 CFR 26.607(f), Initial Testing 79
j. 10 CFR 26.607(g), Oral Fluid 79
k. 10 CFR 26.607(h), Point of Collection Testing and Assessment 82
l. 10 CFR 26.607(i), Hair Testing 82
m.10 CFR 26.607(j), Portal Area Monitor Screening 83
o. 10 CFR 26.607(k), Blood Specimens 84
p. 10 CFR 26.607(l), Custody-and-Control Form 85
q. 10 CFR 26.607(m), MRO Review and Training 85
10. 10 CFR 26.608, “FFD program training.” 86
11. 10 CFR 26.609, “Behavioral observation.” 89
12. 10 CFR 26.610, “Sanctions.” 92
a. Determining Sanction Groups by Roles and Responsibilities 92
b. Sanctions Based on Risk Significance 93
13. 10 CFR 26.611, “Protection of information.” 95
a. 10 CFR 26.611(a), Protecting Information 95
b. 10 CFR 26.611(b), Consent 97
14. 10 CFR 26.613, “Appeal process.” 98
15. 10 CFR 26.615, “Audits.” 99
a. 10 CFR 26.615(a), General 99
b. 10 CFR 26.615(b), Frequency 100
c. 10 CFR 26.615(c), Joint Audits or Accepting Party Audits 101
16. 10 CFR 26.617, “Recordkeeping and reporting.” 101
a. 10 CFR 26.617(a), Recordkeeping 101
b. 10 CFR 26.617(b)(1), Reporting to the NRC Operations Center 104
c. 10 CFR 26.617(b)(2), Annual Program Performance Reports 106
d. 10 CFR 26.617(c), Sharing of FFD-Related Information 106
17. 10 CFR 26.619, “Suitability and fitness determinations.” 106
Reason for Issuance
This document provides guidance for the FFD program requirements detailed in 10 CFR Part 26, Subpart M. These details involve, in part, policies, procedures, training, drug and alcohol testing, laboratory testing processes, behavioral observation, MRO responsibilities, fitness determinations, reporting, and recordkeeping. The FFD program for facilities licensed under 10 CFR Part 53 also includes requirements for a performance monitoring and review program (PMRP), and an FFD program change control process. Licensees and other entities who are not implementing an FFD program under 10 CFR Part 26, Subpart M, should use the guidance in the documents listed in section A, “Introduction,” sub-section “Related Guidance,” of this RG.
Background
The requirements in 10 CFR Part 26 establish a regulatory framework under which FFD programs that comply with these requirements meet the performance objectives in 10 CFR 26.23, “Performance objectives,” including providing reasonable assurance that individuals subject to these FFD programs are trustworthy and reliable, as demonstrated by the avoidance of substance abuse, and are not under the influence of any substance or mentally or physically impaired from any cause that would in any way adversely affect their ability to perform their duties safely and competently. The NRC amended 10 CFR Part 26 in the final rule that also created 10 CFR Part 53. Those amendments produced 10 CFR Part 26, Subpart M, for use by 10 CFR Part 53 licensees and other entities. Licensees and other entities that comply with the Subpart M framework meet the 10 CFR 26.23 performance objectives.
The requirements in 10 CFR Part 26, Subpart M, are performance-based and risk-informed consistent with the risk associated with the facility and human activities necessary to (1) effectively operate, maintain, surveil, decommission, and protect the facility, materials, and sensitive information (e.g., classified, safeguards, medical, and private information), (2) prevent or mitigate radiological consequences should the facility experience a structure, system, or component (SSC) failure, a reactor transient or accident, or other abnormal occurrence, and (3) detect, assess, and respond to an internal or external security incident or an adverse environmental condition (e.g., hazardous chemicals, earthquake, flooding).
The Subpart M framework supplements the access authorization program under 10 CFR 73.120 for CNPs licensed under 10 CFR Part 53. The regulations in 10 CFR 73.120 establish the general performance objective that individuals subject to the access authorization program are trustworthy and reliable, such that they do not constitute an unreasonable risk to public health and safety or the common defense and security, including the potential to commit radiological sabotage. The defense in depth afforded by the FFD and access authorization programs provide reasonable assurance that individuals who maintain unescorted access to the protected area,2 SNM, or sensitive information are trustworthy, reliable, and fit for duty.
Consideration of International Standards
The International Atomic Energy Agency (IAEA) works with member states and other partners to promote the safe, secure, and peaceful use of nuclear technologies. The IAEA develops Safety Requirements and Safety Guides for protecting people and the environment from harmful effects of ionizing radiation. This system of safety fundamentals, safety requirements, safety guides, and other relevant reports, reflects an international perspective on what constitutes a high level of safety. To inform its development of this RG, the NRC considered IAEA Safety Requirements and Safety Guides pursuant to the Commission’s International Policy Statement (Ref. 10) and Management Directive and Handbook 6.6, “Regulatory Guides” (Ref. 11).
The staff reviewed IAEA Safety Guide No. NS‑G‑2.8, “Recruitment, Qualification and Training of Personnel for Nuclear Power Plants,” issued 2004 (Ref. 12), as it is pertinent to this RG. The safety guide states, “A programme to identify personnel with a tendency towards drug or alcohol abuse should be established. Personnel prone to drug or alcohol abuse should not be employed for safety related tasks.” This RG describes a method for implementing the NRC’s requirements for specific elements of such a program.
The terms “screen” and “screening
test” for drugs or alcohol are used in this guide to describe
when a biological specimen is collected and assessed by FFD program
personnel using a point of collection testing (POCT) device or an
instrument that passively collects (but does not store) and analyzes
a biological specimen. The
word “test” for the drug and alcohol testing under 10
CFR 26.605 describes the process when a biological specimen is
collected by FFD program personnel and is (1) sent to an
HHS-certified laboratory for drug testing and analysis or (2)
analyzed for alcohol by an evidentiary breath testing device.
A licensee or other entity in 10 CFR 26.3(f) may choose to establish, implement, and maintain an FFD program that meets the requirements of 10 CFR Part 26, Subpart M, and apply this program to the individuals specified in 10 CFR 26.4. If the licensee or other entity does not implement an FFD program under 10 CFR Part 26, Subpart M, then the licensee or other entity is required to establish, implement, and maintain an FFD program that meets the requirements of Subparts A through I, N, and O of 10 CFR Part 26. A licensee or other entity covered by 10 CFR 26.3(f) may not implement 10 CFR Part 26, Subpart K, unless it requests an exemption from the requirements in 10 CFR Part 26, Subpart M, to implement Subpart K and the NRC grants the exemption request.
a. Holders of a Manufacturing License
The holder of a manufacturing license for the assembly or testing of a reactor under 10 CFR Part 53 should consider applying its FFD program to all individuals who—
implement the FFD program.
are granted unescorted access to the reactor during its assembly or testing;
operate or direct the operation of SSCs that are needed to assemble or test a reactor;
perform maintenance or surveillance or direct the maintenance or surveillance of SSCs of the reactor;
perform design changes on SSCs of the reactor;
perform quality assurance or quality verification activities; or
perform security duties as an armed security force officer, alarm station operator, response team leader, or watchman, hereafter referred to as “security personnel.”
Based on the duties and responsibilities described above, the holder of a manufacturing license should implement an FFD program that should apply to individuals who manage, direct, or perform functions that include, but are not limited to, the following:
activities during the assembly or testing the reactor necessary to meet NRC‑required codes and standards from the American Society of Mechanical Engineers or Institute of Electrical and Electronics Engineers;
assembling, installing, testing, or operating an SSC used for the following: control of reactivity, temperature, pressure, or coolant flow; reactor operation; accident or transient response or mitigation; heat transfer and management; or radiation and chemical detection and monitoring;
maintaining, testing, monitoring, and upgrading the cybersecurity and information technology services used for the assembly or testing of a reactor;
assembling, installing, testing, or operating the SSCs for the management of radioactive or hazardous materials for or during assembly or testing of the reactor;
controlling access to the protected area or foreign material exclusion areas during the assembly or testing of the reactor;
performing inspections, tests, analyses, and acceptance criteria or otherwise implementing the quality assurance program required by 10 CFR Part 53;
participating in the day-to-day operations of the FFD program, as defined by the licensee’s or other entity’s procedures.
The regulation in 10 CFR 26.4(g) refers to the following individuals as “FFD program personnel” if they are involved in the day-to-day operations of the program, as defined by the procedures of the licensees and other entities:
All persons who can link test results with the individual who was tested before an FFD policy violation determination is made, including, but not limited to the MRO.
These persons could include those who receive, read, manage (e.g., store, file), or communicate drug or alcohol test results (i.e., test results from an HHS-certified laboratory). The MRO should be considered as FFD program personnel if involved in the day-to-day operations of the program, regardless of full- or part‑time employment, or location of employment because the MRO must review positive test results obtained from an HHS-certified laboratory and should assist FFD program staff in the evaluation of subversion attempts. Also, and if applicable to the FFD program, 10 CFR 26.183(c) states that the MRO is responsible for identifying any issues associated with collecting and testing specimens, which may inform a licensee’s or other entity’s issuance of an FFD policy violation. FFD program personnel would not include individuals who are assigned to monitor portal area screening devices or conduct drug and alcohol screening tests or maintain FFD program information and computer systems.
Persons who make determinations of fitness.
These persons would not include medical or clinical professions who make determinations of fitness only occasionally and are not otherwise involved in the day-to-day operations of the FFD program.
Persons who make authorization decisions.
These persons should include individuals who have the title “reviewing official” as defined in 10 CFR 26.5 and would not include contractors/vendors (C/Vs) that perform reviews, activities, and investigations for the licensee or other entity to support a Reviewing Official’s determination whether to grant, maintain, or deny authorization for any individual.
Persons involved in selecting or notifying individuals for testing.
This would include the FFD program staff who select individuals subject to pre-access screening using hair or a point of collection testing and assessment (POCTA) device, random screening or testing, and follow-up testing. This would not include individuals who direct others to be subject to a for-cause or post-event test. For example, supervisors who direct staff members to report to the collection facility for a for-cause or post-event test would not be FFD program personnel because the initiating condition would be an observation or event that warrants the test. Also, administrative personnel, managers, or supervisors who are not part of the FFD program staff but who may receive direction from the FFD program staff to notify a particular person to report for a drug or alcohol screening or test, are not FFD program personnel.
FFD program personnel must be subject to random testing. To help ensure the integrity of the random testing process, licensees and other entities should place their FFD program personnel into a random testing pool managed by a different licensee or other entity. This would help ensure that FFD program personnel would be unable to predict when they would be subject to a random test.
All persons involved in the collection or onsite testing of specimens.
All persons involved in the collection of specimens should include individuals who perform multiple roles and responsibilities that include the collection of specimens, even if collections are not performed on a day-to-day basis.
Individuals who do not routinely collect specimens and do not perform FFD program activities on a day-to-day basis would not be considered FFD program personnel. These individuals could be managers, supervisors, or other licensee- or other entity-designated personnel who are trained under 10 CFR 26.608, “FFD program training,” to collect specimens and directed to collect one or more specimens during a particular time, shift, or day. These individuals would typically be called upon to conduct collections for the random testing program and may be used for all test conditions.
All persons involved with the collection of specimens would include those licensee- or other entity-designated individuals who are responsible for the packaging, temporary storage, and shipment of specimens to an HHS-certified laboratory or the storage of POCTA devices before and after their use (if used to inform a determination of fitness or suitability determination). These persons should include individuals responsible for maintaining specimen integrity, for example, individuals who ensure that specimens or POCTA and other screening devices or instrumentation are controlled to prevent tampering. Technicians who maintain or surveil the SSCs that store specimens and POCTA devices would typically not be considered FFD program personnel.
Individuals assigned to a license or other entity facility (like a loading dock) where packaged specimens are awaiting shipment (e.g., by U.S. postal service or private shipping companies) to an HHS-certified laboratory should be subject to the FFD program. Individuals who work at offsite collection facilities not owned or operated by the licensee or other entity would not be subject to the FFD program and would not be FFD program personnel.
The individuals involved in the onsite testing of specimens should include those licensee- or other entity-designated individuals who are directed to facilitate the use of a POCTA device (which would include an evidentiary breath testing device) to screen individuals for drugs, drug metabolites, and alcohol.
b. Transportation of a Manufactured Reactor
For the transportation of a manufactured reactor, the holder of the manufacturing license should ensure that the operators and standby operators that conduct the transport, as a conveyance,3 are subject to the DOT drug and alcohol testing program in 49 CFR Part 40. A licensee or other entity may assess whether the operators of the conveyance had any drug or alcohol violations while subject to the DOT’s Federally mandated drug and alcohol testing. This information may be obtained from the DOT’s website at https://clearinghouse.fmcsa.dot.gov.
Individuals subject to DOT drug and alcohol testing may be randomly tested at frequencies below that established in 10 CFR Part 26. The DOT random testing rates may change based on the mode of transportation or by calendar year.
If an individual conducting the conveyance appears impaired, either before or after entry in the NRC-licensed facility (e.g., protected area), the licensee and other entity should take timely action to coordinate with the conveyor the implementation of corrective actions before the potentially impaired individual attempts to perform the conveyance. Under its own discretion, a licensee or other entity could implement a contractual requirement with the conveyor to require drug and alcohol screening to help inform a licensee’s or other entity’s immediate evaluation of the individual’s ability to safely and competently perform the conveyance.
c. Licensees or Other Entities of a Commercial Nuclear Plant
A licensee or other entity under 10 CFR Part 53 must apply its FFD program to the categories of individuals described in 10 CFR 26.4. Operating experience from the large light-water reactor (LLWR) community should be used to assist in the application of the FFD program to individuals. For example, the NRC understands that it is common that licensees and other entities of LLWRs licensed under 10 CFR Part 50, “Domestic Licensing of Production and Utilization Facilities” (Ref. 13), apply their FFD program to all individuals who direct, perform, or could direct or perform those duties and responsibilities described in 10 CFR 26.4 or maintain unescorted access to the NRC-licensed facility (i.e., not just the protected area). In fact, the NRC has been informed by some licensees of LLWRs that every individual possessing a licensee- or other entity-issued badge to enter (i.e., gain access to) the site or an emergency response facility (whether onsite or offsite), or have access (whether the access is physical or electronic) to an SSC required for facility operations, would be subject to the FFD program or would be escorted.
d. Risk-informed Evaluation Process
The requirements in 10 CFR 26.4(a)(1) and (4) state that the FFD program must be applied to those individuals who operate and maintain or direct the operation and maintenance, respectively, of systems and components “that a risk-informed evaluation process or alternative method for evaluating safety significance has shown to be significant to public health and safety.” In lieu of applying the FFD program to all individuals, the following references may assist a licensee or other entity in its evaluation process or alternate method used to determine whether to apply the FFD program to certain individuals, but not others, based on the risk-significance of the component or structure.
10 CFR 50.69, “Risk-informed categorization and treatment of structures, systems and components for nuclear power reactors.”
Regulatory Guide 1.201, “Guidelines for Categorizing Structures, Systems, and Components in Nuclear Power Plants According to Safety Significance” (Ref. 14)
Nuclear Energy Institute (NEI) guidance document NEI 00-04, “10 CFR 50.69 SSC Categorization Guidelines” (Ref. 15)
“A new method for safety classification of structures, systems and components by reflecting nuclear reactor operating history into importance measures,” J. Cheng et al., Nuclear Engineering and Technology, Vol. 54, Issue 4, April 2022. Publicly available through www.ScienceDirect.com.
If the licensee or other entity elects to perform a risk-informed evaluation process or alternative method for evaluating safety significance, then the licensee or other entity should assess the below list of SSCs. The evaluation process should include an assessment of the safety‑significant function(s) of the SSC and the roles and responsibilities of any individual required to perform any action required for the SSC to fulfill its intended safety function. For this guide, a safety-significant function is one whose degradation or loss could result in a significant adverse effect on defense in depth, safety margin, or risk. This function would be accomplished by SSCs that are relied on to remain functional during and following design-basis and licensing-basis events other than design-basis accidents. These functions ensure the integrity of the reactor system (e.g., reactor containment vessel or shell and its thermodynamic heat cycle boundary); the capability to shut down the reactor and maintain it in a safe‑shutdown condition; and the capability to detect, prevent, or mitigate the consequences of accidents that could have onsite and offsite dose consequences. The SSCs should include those that are needed for the following:
containment;
nuclear fuel loading, recovery, or removal, or configuration control;
monitoring, maintaining, or controlling nuclear reactivity (e.g., fuel, poison, reflector, or moderator control), or coolant temperature, pressure, or flow;
system isolation and pressure, temperature, and flow management performed by SSCs not associated with controlling nuclear reactivity;
detecting, assessing, or responding to normal operations, transients, and abnormal conditions;
maintaining or restoring steady-state operation or the thermodynamic heat cycle, or causing and maintaining reactor shutdown;
detecting, assessing, or responding to radiation or contamination levels or hazardous chemical conditions; or
detecting, assessing, or responding to unauthorized access to the reactor, its SSCs, and licensee‑designated control areas.
10 CFR 26.603(a) requires certain 10 CFR Part 53 applicants to include a description of their FFD programs in their final safety analysis reports. This description informs the NRC inspection process and discussions with the applicant’s, licensee’s, or other entity’s FFD program staff. The entities that are required to submit these FFD program descriptions are those applicants that must comply with the application requirements in 10 CFR Part 53, Subpart H. In Subpart H, 10 CFR 53.1309(a)(6) requires an applicant for a construction permit to provide a description of its FFD program in its PSAR. Under 10 CFR 53.1279(b)(4), 53.1369(x), and 53.1416(a)(24), an applicant for a manufacturing license, operating license, and combined licensee, respectively, is required to provide a description of its FFD program in its final safety analysis report.
If the licensee or other entity performed the analysis under 10 CFR 26.603(c) and the analysis demonstrates that the facility and its operation4 satisfy the criterion in 10 CFR 53.860(a)(2), then the licensee or other entity must include in its FFD program a summary of the analysis, including the assumptions, methodology, conclusion, and references. If the analysis is equivalent to the analysis performed for the physical security, access authorization, other 10 CFR Part 53 program, then the licensee or other entity may reference the analysis performed for a different program; however, for the FFD program, the analysis must include a description of the facility and its operation. The licensee or other entity must maintain the analysis until permanently ceasing operations under 10 CFR Part 53.
The description must include the assumptions associated with the operation of the facility. This description will support an NRC review of the human performance elements necessary to safely construct, operate, maintain, decommission, and secure the facility. These assumptions should include those associated with (1) safety and security margins, (2) the principal individuals who must be on shift and the human actions required to operate and maintain (e.g., monitor, surveil, and repair) the facility in a safe operating or shutdown condition, (3) the principal individuals assigned on shift to perform or direct the performance of human actions to secure and protect the facility and control sensitive information (without providing safeguards information), (4) individuals assigned to offsite monitoring or control stations (including stations to implement physical protection) who are assigned to supervise, observe, or direct individuals on shift at the CNP site, and (5) individuals assigned to implement actions after a design basis event or accident occurs.
The description must include the methodology used in the site‑specific analysis to demonstrate that the facility and its operation satisfy the criterion in 10 CFR 53.860(a)(2). This description should include any details of the analysis, analytical software (e.g., software name, version, and vendor), and calculational assumptions that the applicant, licensee, or other entity used that would assist the NRC in its evaluation of the analysis.
The description must describe any references used to support the analysis. This should include bibliographic information for technical studies, manuals, guidance, standards, and supporting documentation used in the analysis.
The regulation in 10 CFR Part 26, Subpart M, gives 10 CFR Part 53 licensees and other entities flexibility in selecting the set of FFD program requirements they will implement. In 10 CFR 26.603(a)(2), the NRC requires the licensee’s or other entity’s FFD program description to include a statement declaring which set of requirements the licensee or other entity will implement.
A licensee or other entity may implement the following sets of requirements:
all 10 CFR Part 26 requirements except those in Subparts K and M;
10 CFR 26.604, “FFD program requirements for facilities that satisfy the § 26.603(c) criterion,” if they are a licensee or other entity whose facility and its operation satisfy the criterion in 10 CFR 26.603(c); or
10 CFR 26.605, “FFD program requirements for facilities that do not implement § 26.604,” if they are a licensee or other entity whose facility and its operation satisfy the criterion in 10 CFR 26.603(c); a licensee or other entity who does not satisfy the criterion in 10 CFR 26.603(c); or a holder of a manufacturing license that allows the assembly or testing of a manufactured reactor.
Knowledge of which FFD program requirements the licensee or other entity intends to implement will inform the NRC’s inspection plan and review of human actions to construct, operate, maintain, decommission, and secure the facility.
The FFD program description must discuss which individuals described in 10 CFR 26.4 are subject to the licensee’s or other entity’s FFD program. This description should inform the NRC of any substantial differences from 10 CFR 26.4 the licensee or other entity expects in its categories of individuals who will be subject to the FFD program. Although the descriptions in 10 CFR 26.4 are for the LLWRs licensed under 10 CFR Part 50 or 10 CFR Part 52, “Licenses, Certifications, and Approvals for Nuclear Power Plants” (Ref. 16), they are applied to facilities licensed under 10 CFR Part 53 through 10 CFR 26.3(f) because the roles and responsibilities described in 10 CFR 26.4 apply to individuals at CNPs licensed under 10 CFR Part 53. Understanding the licensee’s or other entity’s FFD program applicability to individuals would enhance the NRC’s ability to assess the contribution of the FFD program to human performance in the conduct of duties and responsibilities necessary to operate, maintain, surveil, secure, and decommission the facility, if applicable. This information would also be used to inform NRC inspection of those categories of individuals who perform their principal roles and responsibilities onsite or offsite (i.e., at a remote operations or monitoring station), including individuals who may not be afforded physical unescorted access to the NRC-licensed facility, SNM, or sensitive information, but should be subject to the FFD program, such as individuals who operate or maintain cybersecurity and information systems.
The FFD program description must detail the licensee’s or other entity’s drug and alcohol testing and fitness determinations process. The description should discuss the following: whether the licensee or other entity plans to implement the drug testing requirements as provided in 10 CFR Part 26, such as Subparts E and H, or use provisions from the HHS Guidelines in its procedures; the collection and testing facilities to be used (including names and locations if not the licensed facility for which this description is being provided for); the biological specimens to be collected; planned use of a POCTA device for either oral fluid or urine and any instrumentation that may be used to passively detect drugs, alcohol or both; the suitability and determination of fitness process; and the sanctions to be imposed on one and two confirmed FFD policy violations. The description should include the manufacturer’s name and unique identification number of any POCTA device and passive screening instrumentation planned for use at the NRC‑licensed facility.
Regarding the description of the suitability and fitness determination process, these processes are similar, but they are not equivalent. Although they are both evaluations to determine whether to assign individuals to the duties specified in 10 CFR 26.4, a suitability determination is typically focused on whether the licensee or other entity should assign an individual a particular duty or responsibility or grant authorization. For example, the results of pre-access testing would inform the licensee’s or other entity’s decision whether to grant an individual authorization as defined in 10 CFR 26.5. A suitability evaluation would also include cases where an individual exhibits, for example, claustrophobia (such that the individual should not work in confined spaces); facial or respiratory performance considerations that may prevent proper donning or use of personnel protective equipment (e.g., an oxygen breathing apparatus); or acrophobia (such that the individual should not be assigned to work in elevated positions). This suitability determination would help provide assurance that such individuals, subject to the FFD program, are fit for duty to safely and competently perform their duties and responsibilities. Since this suitability determination could be site-, facility-, duty-, or responsibility-specific, it should be performed by an individual with detailed knowledge of the individual’s condition, the site and facility, and the duties and responsibilities to be performed by the individual. The applicant, licensee, or other entity should describe its planned suitability process.
A determination of fitness is typically the process entered when there are indications that an individual specified in 10 CFR 26.4 may be in violation of the licensee’s or other entity’s FFD policy or procedure; For example, the individual was identified as using an illegal substance or is a member of a group acting or advocating for an unlawful change to the U.S. government or violence to a particular ethnic, religious, or cultural group. A determination of fitness may require the use of a medical or clinical professional, called on by the licensee or other entity, to evaluate the individual, formulate a treatment plan, and recommend whether the individual’s authorization should be reinstated. The types of professionals called upon to make this determination should be educated, accredited, or trained in the specific area(s) of concern (e.g., drug or alcohol abuse, psychosis, etc.). The applicant, licensee, or other entity should describe its determination of fitness process including, if applicable, its planned use of the requirements in 10 CFR 26.187, “Substance abuse expert,” and 26.189, “Determination of fitness.”
In the summary of its PMRP, the licensee or other entity must inform the NRC of the initial set of performance measures and thresholds to be used in the PMRP. Summaries should state whether the measures and thresholds apply to the whole population subject to the FFD program or individuals in a particular employment or labor category. The description should also contain the information used to justify the measure or threshold; for example, how the measure or threshold was developed, which comparable facilities were part of the comparative assessment, and whether an FFD program or industry data were used to establish the threshold.
This section states that for a licensee or other entity to implement an FFD program under 10 CFR 26.604, the licensee or other entity must perform a site-specific analysis to demonstrate that the facility and its operation satisfy the criterion in 10 CFR 53.860(a)(2). In Section C.3.a in this RG, guidance is provided for the description of the human performance actions necessary to, in part, operate the facility and implement actions after a design basis event or accident occurs. 10 CFR 26.603(c) also requires that the licensee or other entity must maintain the analysis, including updates to reflect changes made to the staffing, FFD programs, or offsite support resources described in the analysis, to show that the facility and its operation continues to satisfy the criterion, until permanent cessation of operations under 10 CFR 53.1070.
The changes made to the licensee or other entity staff could include those individuals who perform those duties and responsibilities identified in Section C.3.a. Changes made to the FFD program could involve program changes in which the actions implemented to mitigate a potential reduction in program effectiveness were not effective and resulted or could have resulted in adverse human performance of the individuals who perform those duties and responsibilities identified in Section C.3.a. Changes in offsite support services could include changes to the methods, structures, systems, or equipment used by the individuals assigned to implement actions after a design basis event or accident occurs that adversely affect the human performance of those individuals.
The objective of the PMRP is to help ensure that the FFD program remains effective over time as program changes are implemented or substance abuse patterns change among the individuals subject to 10 CFR Part 26. An FFD program would remain effective if FFD performance data, reviews, and audits demonstrate that the licensee or other entity continues to meet the 10 CFR 26.23 performance objectives and adverse trends are not occurring that exceed established thresholds for identified performance measures. The PMRP should help maintain FFD performance at levels comparable to the historic FFD performance levels established by LLWR and Category I SNM facilities.
The PMRP framework is based, in part, on 10 CFR 26.41, “Audits and corrective action,” 10 CFR 26.415, “Audits,” 10 CFR 26.717, “Fitness-for-duty program performance data,” and 10 CFR 26.719, “Reporting requirements”; performance-based requirements in 10 CFR Part 50; and NRC guidance. These regulations and guidance are listed below to inform the licensee or other entity of regulatory requirements that are similar to elements within a PMRP. The licensees and other entities that must implement a PMRP could communicate with those that are now implementing these requirements to learn of operating experience to help in the development and implementation of the PMRP. The following performance-based requirements may have generated operating experience that could inform the development of the PMRP: 10 CFR 26.41(a); 10 CFR 26.41(b); 10 CFR 26.415(a) and (b); and 10 CFR 26.717(c) and (d).
The licensee or other entity can review the performance monitoring programs under 10 CFR Part 50 to inform its PMRP. Two such programs are 10 CFR 50.48(c)(2)(vii), which allows the use of performance-based methods to select fire protection program elements and establishes an NRC review and approval framework, and 10 CFR 50.65, which establishes the requirements for monitoring the effectiveness of maintenance at LLWRs.
The Reactor Oversight Process (ROP) is the NRC’s program to inspect, measure, and assess the safety and security performance of operating LLWRs and to respond to any decline in their performance. Licensees and other entities can find information on ROP program elements and performance monitoring considerations at https://www.nrc.gov/reactors/operating/oversight.html. For example, the information at this website discusses measuring nuclear power plant performance; the use of performance indicators and how the NRC assesses plant performance through inspection; enforcement of NRC requirements; and communications and making information available to the public. The inspection of FFD programs is in the Security cornerstone of the Safeguards strategic performance area and could involve the cross‑cutting areas of human performance, problem identification and resolution, and safety-conscious work environment.
NUREG/BR-0303, “Guidance for Performance-Based Regulation,” issued December 2002 (Ref. 17), provides information on the elements the NRC uses to establish a performance-based regulatory framework. It states, in part, “Performance-based approaches focus primarily on results. They can improve the objectivity and transparency of NRC decision-making, promote flexibility that can reduce licensee burden, and promote safety by focusing on safety-successful outcomes.”
The PMRP is designed, in part, to address the scenario in which a licensee or other entity meets all applicable 10 CFR Part 26 requirements, and its rate or number of FFD policy violations continues to increase. Although 10 CFR Part 26, Subparts B through K, have many audit-related requirements, no requirement compels a licensee or other entity to evaluate, for example, the question, “What random testing positivity rate is considered unacceptably high, such that the licensee or other entity no longer meets a 10 CFR 26.23 performance objective?” The PMRP requires the licensee or other entity to evaluate the random testing positive rate (and the other quantitative performance measures) annually, measure its performance data against its threshold (which was established based on site, fleet-level, and industry performance data), and assess whether corrective actions should be implemented. Licensees and other entities implementing Subpart M should consider the total risk associated with the population of individuals subject to the random testing program but who were not tested (see “Testing Rates and Deterrence under Subpart K FFD Program” (Ref. 18)).5
As a second example, a licensee or other entity identifies an increased number of individuals demonstrating signs of impairment while they are allowed unescorted access to the facility. A cause could be that a particular element of the pre-access screening process is not as effective as it once was. For example, the process is not identifying individuals who are subverting the testing process, or suitable inquiries are not identifying potentially disqualifying FFD information (PDI).6 This assessment of pre-access screening and an increased occurrence of FFD policy violations identified through random testing could provide information for the licensee or other entity to assess the safety culture of its staff (see the NRC’s Safety Culture Policy Statement (Ref. 19)).7 Figure 1 presents an example of declining FFD performance when pre-access or the protected area portal area performance does not correlate to FFD program performance inside the protected area.
FFD performance levels established by LLWR facilities and Category I SNM facilities provide a model for effective FFD program performance and performance measures. To maintain an effective FFD program, the PMRP regulations in 10 CFR 26.603(d) require that the licensee or other entity measure its performance and compare it to its past performance and its fleet-level and industry performance. If the data in a particular performance measure meets established by the licensee or other entity, corrective actions would be required to restore performance. Unlike audits that provide a discrete assessment at a particular place and time, the PMRP enables an organization to continuously assess its FFD program as it receives performance data.
The PMRP requirements do not compel a licensee or other entity to continuously improve performance. For example, a licensee or other entity is not required to continuously work to lower positivity rates to achieve zero positive test results or no subversion attempts. The PMRP requires corrective actions only when thresholds are met. Based on discussions within the NRC’s Safety Culture Policy Statement, the licensee’s or other entities’ instructions for PMRP implementation could:
enable continuous assessment;
ensure the program and corrective actions are timely implemented and verified as being effective; and
communicate performance and successes to the staff and other stakeholders and hold the staff accountable for performance.
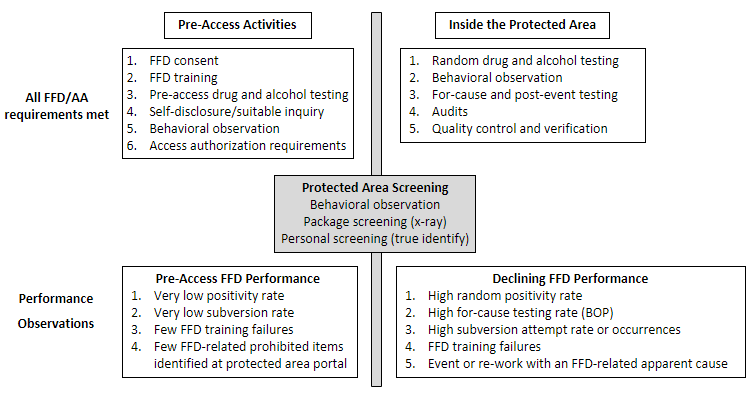
Figure 1. FFD Declining Performance: Pre-access Performance versus Protected Area Performance
However, nothing prevents a licensee or other entity from seeking to improve FFD performance. Such initiatives should be documented to inform any future program change, auditor, or NRC inspector, for example, as to the reason for the initiative and the baseline performance level at the time of the change. As an example, a licensee or other entity decides to implement drug screening using hair as the biological specimen for a specific group of C/Vs performing nonroutine but safety-sensitive maintenance or engineering design changes. The use of hair screening would provide additional assurance that the contracted workforce is trustworthy and reliable as demonstrated by their avoidance of Schedule I or II drugs as classified by the Drug Enforcement Administration.8 This would be considered as a licensee or other entity initiative to enhance the pre-access testing process for a specific group of individuals and the licensee or other entity could suspend this initiative without incurring a reduction in FFD program effectiveness.
As discussed in the Commission white paper “Risk-Informed and Performance-Based Regulation,” dated March 1, 1999 (See Ref. 20) one of the attributes of a risk-informed, performance-based regulation is the incorporation of safety margins. The Commission stated that, in a performance-based regulatory framework, the failure to meet a performance criterion, while undesirable, will not in and of itself constitute or result in an immediate safety concern. In this construct, margin can be the difference between a baseline level of performance (e.g., a required level of performance) and the actual level of performance above the baseline. Therefore, a licensee’s performance can decrease and the amount of margin would correspondingly decrease, yet the licensee’s performance could remain in excess of the required level of performance. Margin enables flexibility in program implementation because it allows variations in FFD performance above the baseline level of performance; this adds realism to the PMRP. Figure 2 illustrates FFD margin.
Margin within the FFD program is both quantitative and qualitative. Quantitative margin is the operating space under the licensee- or other entity-selected threshold for the particular performance measure where the threshold is quantitative, developed from FFD performance data, and indicates when margin is becoming too small. Qualitative margin within the FFD program is established from licensee and other entity implementation of the 10 CFR Part 26 requirements that establish defense in depth and by the licensee’s and other entity’s policies and procedures that go above the minimum regulatory requirements.
FFD qualitative margin is established from the FFD program elements that provide defense in depth above the assurances gained through performance monitoring of FFD policy violations and other quantitative indicators of FFD program performance (e.g., identification of PDI). These FFD program elements are PMRP qualitative reviews in 10 CFR 26.603(d)(1)(iv)(A) though (C); audits under 10 CFR 26.607(c)(4) and (e) and 10 CFR 26.615; self-disclosures under either 10 CFR 73.56 or 10 CFR 73.120, which can be used in the quantitative performance measure for PDI as listed in Tables 1 through 4; suitable inquiries under 10 CFR Part 26, Subpart C, if applicable, and either 10 CFR 73.56 or 10 CFR 73.120; protection of sensitive information under 10 CFR 26.611, “Protection of information;” fitness determinations under 10 CFR 26.619, “Suitability and fitness determinations,” or 10 CFR 26.189, as applicable; and the use of HHS-certified laboratories under 10 CFR 26.607 or 10 CFR 26.31, “Drug and alcohol testing,” as applicable.
Qualitative margin should not be used as an excuse to justify no action for exceeding a quantitative performance measure threshold. For example, if the random testing positivity rate is set at 1.0 percent for short-term C/Vs, and the CNP plans for a large influx of C/Vs to perform maintenance and expects the random testing positivity rate to go up because the C/Vs do not have a history of working in the nuclear industry, the random testing positivity rate threshold should not be increased. The threshold should remain as is, and the licensee or other entity should help ensure that the C/V workforce is fit for duty and trustworthy and reliable during their pre-access process and while subject to the random testing process This guidance is consistent with the ROP performance indicators.
The number and significance of occurrences in which an individual’s fitness, trustworthiness, or reliability was a root or contributing cause to an event or condition could inform a licensee’s or other entity’s assessment of FFD margin within the PMRP. For example, a licensee or other entity should assess quantitative margin if substantial rework or increased quality assurance and quality verification findings were attributed to a number of individuals who were subsequently identified as being in violation of the FFD policy, yet the threshold for the FFD policy violation performance measure was not exceeded. Additionally, qualitative margin should be assessed if these same individuals cause an SSC to be inoperable and the inoperable condition was not identified during post-maintenance testing; in this case, the defense in depth established to return an SSC to an operable condition was insufficient to identify the SSC performance deficiency. Other indicators of decreased margin could include the following: declines in FFD training scores, increased occurrences of PDI after an individual has been granted unescorted access to the protected area, and recurrent MRO or laboratory performance deficiencies. If the licensee or other entity determines that FFD margin has decreased based on the number and significance of events, then the performance measure should be evaluated even though a threshold may not have been exceeded. Since there is margin built into a licensee-established performance threshold, licensees should use the margin to strike a balance among the following: (1) managing an effective performance‑based FFD program, (2) involving management in the consideration of how qualitative factors (e.g., audit findings, staff size) may influence FFD performance assessments, and (3) issuing corrective actions when FFD data indicate a trend toward degraded performance.
Licensees and other entities should define acceptable performance in their PMRPs in terms of the performance standards in 10 CFR 26.23. For example, the following illustrates the relationship between the performance measures in Table 1 and the 10 CFR 26.23 performance objectives.
The behavioral observation performance measure relates to 10 CFR 26.23(a), (b), (c), (d) and (e).
The identification of disqualifying information performance measure relates to 10 CFR 26.23(a) and (c).
The identification of prohibited FFD items performance measure relates to 10 CFR 26.23(a), (b), (c), and (d).
The FFD policy violation performance measures for licensee employees, C/V, and labor category relate to 10 CFR 26.23(a), (b), (d) and (e).
Licensees and other entities are in an excellent position to define and measure success. The licensee or other entity could assess whether its facility is sited in a geographical location that may be more prone to substance abuse, will be operated using a large C/V workforce, or will be located in a community that does not have ready access to consistent mail services or medical or clinical professionals or clinics, which may challenge site specific implementation of FFD program requirements. Unless new to 10 CFR Part 26 implementation, the licensee or other entity knows its site‑specific and fleet-level performance, if applicable. Furthermore, the licensee or entity would be aware of its audit findings, changes to protected area portal area monitoring and screening, if applicable, the scope of its FFD training program, and other site‑specific FFD program elements.
The performance measures and thresholds in this guide are designed to help monitor important FFD program elements and are based on FFD performance information submitted by LLWRs and Category I SNM facilities to the NRC since approximately calendar year 2009 through the agency’s reporting requirements in 10 CFR 26.417(b)(2) and 10 CFR 26.717. The NRC maintains each FFD program performance report submitted to the agency in its Agencywide Documents Access and Management System (ADAMS). The NRC evaluates the information in these performance reports annually. Reports submitted under 10 CFR 26.417 and 26.719 inform the NRC of HHS-laboratory performance issues and specific FFD program weaknesses and FFD policy violations. NRC inspectors also receive these performance data. The public may search for this data in NRC ADAMS by searching for NRC Form 891, “Annual Reporting Form for Drug and Alcohol Tests.” This search process can be used to view FFD-related events reports under 10 CFR 26.719.
A review of this operating experience and the NRC-issued violations related to FFD shows that LLWRs and Category I SNM facilities have historically met the 10 CFR 26.23 performance objectives and that this FFD performance has contributed to public health and safety and the common defense and security. The data demonstrate low random, for-cause, and post-event positivity testing rates in safety-sensitive labor categories subject to 10 CFR Part 26 (and other safety-sensitive industries)9 and very few abnormal conditions (e.g., plant operational occurrences, human errors, human-related accidents, and rework) caused by human impairment (see Ref. 21, Ref. 22, and Ref. 23). Furthermore, since issuance of the 10 CFR Part 26 final rule in 2008 (Ref. 35) there has been only one violation of the 10 CFR Part 26 requirements that had a significance determination of greater than green.10 Also, very few occurrences have been brought to the attention of the NRC in which licensees or other entities acted to remove individuals from NRC-licensed sites because the individuals caused or could have caused a significant condition adverse to safety, security, or quality or were acting in manner that could harm themselves, others, or the facility. Therefore, the FFD regulatory framework, its implementation, and record of performance in the LLWR and Category I SNM facilities communities have contributed to the (1) deterrence of undesirable human actions that may erode the Commission’s defense-in-depth regulatory framework and (2) detection of individuals who may have been or were impaired or determined to be not trustworthy and reliable. Based on operating experience, past FFD program success within the LLWR fleet includes, at a minimum, the following FFD program characteristics:
Effective training on the FFD policy and procedures is demonstrated by test results from the administration of a comprehensive test.
A signed consent is completed and a pre-access drug and alcohol test with negative test results is verified before an individual is granted unescorted access to the protected area.
All individuals provide complete and accurate information in a self-disclosure, and an effective suitable inquiry is performed.
Random drug and alcohol testing is conducted at an annual random testing rate greater than or equal to 50 percent for the population of individuals subject to testing and there is no prior notice to report for random testing. Notification to test should be made only when an individual (including FFD program personnel) is in a work status and has the time to report to the collection site within the time-to-report metric established by the licensee or other entity. Random testing is conducted during all shifts and on all days as described in this guide. Following completion of the random screening with a negative indication or test (meaning that the test result was obtained from an HHS‑certified laboratory and was evaluated by the MRO), the individual is immediately placed back into the random testing pool.
All individuals participate in the behavioral observation program (BOP), are subject to behavioral observation, and report all FFD concerns to the representative designated by the licensee or other entity. The BOP includes all individuals subject to 10 CFR Part 26 whether working onsite or remotely.
All individuals are held responsible for being fit for duty, trustworthy, and reliable, and they are empowered to report FFD concerns about themselves (e.g., fatigue or adverse effect from the use of a prescription medication) or others without retaliation.
All individuals who indicate a presumptive positive test result on an initial drug or alcohol test, show signs of physiological or psychological impairment, demonstrate characteristics of being untrustworthy or unreliable, or act or threaten to act in a manner contrary to plant or human safety and security are immediately removed from all access to protected areas, SNM, and sensitive information, and the duties and responsibilities making them subject to the FFD program. If confirmed to have violated the FFD policy, these individuals must be issued an FFD‑required sanction under 10 CFR 26.610, “Sanctions,” or 10 CFR 26.75, “Sanctions”; should be evaluated medically, clinically, or both before returning to duty under 10 CFR 26.619 or 10 CFR Part 26, Subpart H; and should be subject to FFD retraining.
The FFD program personnel timely updates site-specific FFD performance data to enable continual assessment and prompt communication of results to management for consideration of corrective actions.
The FFD program personnel and MRO perform a timely review of drug and alcohol test results, promptly remove individuals from the workplaces if PDI about the individual has been identified or the individual is found with a prohibited FFD item (e.g., a substance that could be used to subvert a drug test, a beverage or consumable containing alcohol, or consumable containing marijuana (specifically delta-9 tetrahydrocannabinol (Δ-9 THC)11 or cannabidiol)), and communicate with HHS-certified laboratories and forensic toxicologist(s) on test result discrepancies, program weaknesses, and proposed program changes.
The workplaces and individuals subject to 10 CFR Part 26 are free from the presence and effects of alcohol and illegal drugs, and that training informs individuals subject to 10 CFR Part 26 of other potentially impairing substances like illegal substances, illegal drugs, and illicit substances.
All sensitive information is controlled to prevent unauthorized disclosure.
Privacy and due process are effectively afforded to all individuals.
Applicants, Newly Licensed Facilities, and Unique Facilities
During the licensing application phase of a 10 CFR Part 53 facility, the applicant is required to provide FFD program information to the NRC. This information includes, in part, the initial performance measures and thresholds the applicant plans to use to assess FFD program performance when the program is first implemented. Since this PMRP is new for the licensed facility and program implementation has not yet occurred at the site, there is no historical site-specific FFD performance data available for the licensee or other entity to use in its PMRP. In this case, the FFD performance data may be gathered from the fleet-level program, if available; LLWR facilities; and other 10 CFR Part 53 facilities.
Unique sites should obtain data from comparable sites. A comparable site is one that may be similar in design, operation, and the size of the staff that is subject to 10 CFR Part 26; may have a comparable ratio of licensee employees to C/Vs; and may be in a comparable location to that of the facility being licensed. Assessing the licensee employee to C/V ratio is important because operating experience from the LLWR fleet demonstrates that C/Vs result in about 4 times as many drug and alcohol positive test results as that of licensee employees and contribute to more than 97 percent of all subversion attempts.12 Understanding the location of the facility may be important because of differences in societal substance abuse profiles. For example, local geography, demographics, and other similar factors may contribute to the substance abuse profile for a facility. Licensees or other entities hiring C/Vs from communities surrounding the NRC-licensed facility may therefore experience differences in drug and alcohol positivity rates based on the location of the facility.
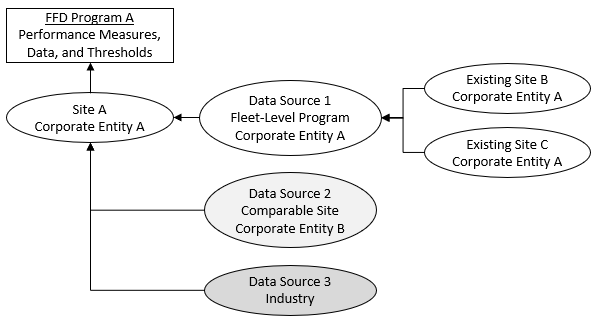
Figure 3. Data Sources for the PMRP
All Facilities Must Gather Data for the PMRP
Figure 3 shows several potential sources of data. As a first step, the licensee or other entity would use FFD performance data generated from the site and fleet-level program performance. Based on operating experience, a dataset from a fleetwide set of policies, procedures, training curriculum, and audit plans would enhance the effectiveness of performance comparisons. A fleet-level FFD program may also attempt to use the same C/Vs to accomplish work at all its sites, which could contribute to consistently effective FFD performance.
The second source of data the licensee or other entity should use is FFD performance data from comparable sites that are operating under a different FFD program implemented by a different licensee or corporate entity. This dataset could be relatively small compared to that of the LLWR community; however, it could provide a baseline of performance to inform the licensee’s or other entity’s measures and thresholds. Furthermore, if the 10 CFR Part 53 facility and the comparable sites have a very small population subject to the FFD program, they might enter into a drug testing consortium, which could be comparable to a fleet-level FFD program. The use of a drug and alcohol testing consortium could enhance FFD program effectiveness and enable implementation consistency across the sites within the consortium.
The third source of FFD data would be industry FFD performance data. As discussed in regulatory position C.5.d, operating experience shows generally low positive testing rates for drug and alcohol testing across the LLWR industry and the Category I SNM facilities when compared to other safety‑sensitive industries. This 10 CFR Part 26 industry performance level helps licensees and other entities meet the 10 CFR 26.23 performance objectives and helps justify using past performance to inform a licensee’s or other entity’s PMRP.
Figures 4 and 5 present historical data from the LLWR and Category I SNM facilities and illustrate that individuals should not immediately conclude that, because a site appears to be an FFD performance outlier, the performance at that site was unacceptable. Performance is a function of the activities occurring at a particular site, such as refueling or maintenance outages; engineering design changes; labor sources; total staff size; ratio of licensee employees to C/Vs; geographical location; and safety culture. For example, if the site has a large influx of C/Vs to perform a maintenance outage, then the pre-access, random, for-cause, post-event, and subversion testing rates may increase because the contracted workforce may not share the safety culture usually present at the site. In figures 4 and 5, the vertical bars represent the total number of licensee employees or C/Vs who received a random drug and alcohol test as measured from the left “y” axis; the black single line is the positivity rate at the NRC‑licensed facility as measured from the right “y” axis; and the “x” axis is the name of the facility. Calendar year 2011 performance data and site names are presented here for illustration to demonstrate relative FFD performance and should not be used in a PMRP.
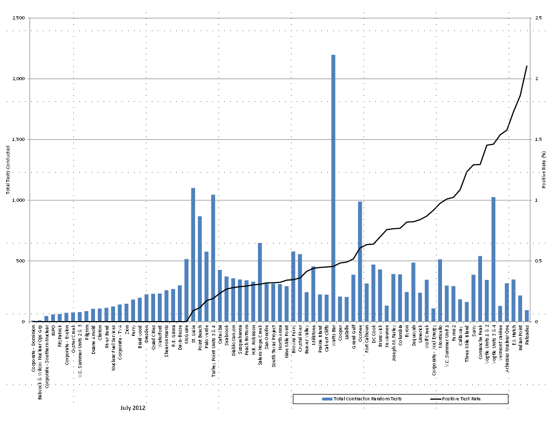
Figure 4. Random Testing by Site—C/Vs (Calendar Year 2011)
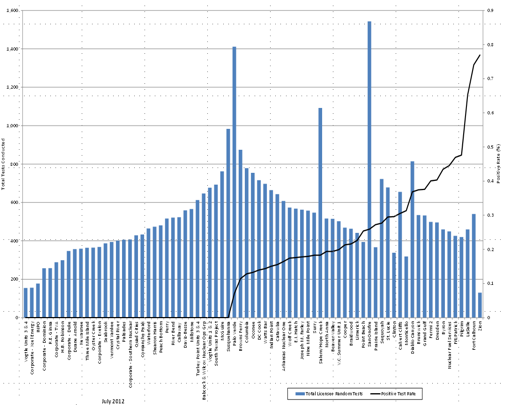
Figure 5. Random Testing by Site—Licensee Employees (Calendar Year 2011)
Data Reporting and Quality
In 10 CFR Part 53, the NRC requires licensees and other entities to submit FFD performance data, either under 10 CFR 26.617(b)(2) or 10 CFR 26.717(e), in accordance with the requirements in 10 CFR 26.11, “Communications.” Since calendar year 2014, the entire LLWR fleet has used NRC Form 890, “Single Positive Test Form” (Ref. 24), and Form 891, “Annual Reporting Form for Drug and Alcohol Tests” (Ref. 25), to report FFD performance information through the NRC’s Electronic Submissions System, General Submission Portal. This has resulted in a large FFD database of performance information that is publicly available in ADAMS and available for incorporation into a PMRP.
Licensees and other entities under 10 CFR Part 53 are required to use NRC-provided forms for the submission of performance data to the NRC. These are NRC Form 893, “10 CFR Part 26, Subpart M, Single Positive Test Form” (Ref. 26) and NRC Form 894, “10 CFR Part 26, Subpart M, Annual Reporting Form” (Ref. 27). Licensees and other entities may familiarize themselves with the instructions posted on the NRC website https://www.nrc.gov/ at “Electronic Submittals Application” located at the bottom of the home page. Licensees or other entities under 10 CFR Part 53 may also submit fatigue management information to the NRC using NRC Form 892. “Annual Fatigue Reporting Form.”
Licensees and other entities also maintain FFD data for their site or sites. These datasets are not publicly available. Licensees or other entities may control FFD performance information collected from 10 CFR Part 26 implementation as “business sensitive”; however, 10 CFR 26.617(c) requires them to share this information with 10 CFR Part 53 licensees and other entities. Information could be shared for the biennial PMRP, audits, and authorization determinations. When sharing occurs, it should be done in good faith and within a reasonable time to support PMRP implementation. Private or personally identifiable information (PII) should not be shared unless it is needed to meet a 10 CFR Part 26, 10 CFR 73.56, or 10 CFR 73.120 authorization requirement. Information sharing should include any site-specific activities or considerations within the data period that would inform the shared dataset, performance measures, or thresholds. The following activities or considerations should be assessed when reviewing FFD performance information from other sites:
Was a different drug testing process implemented or biological specimen used?
Was a different panel of drugs and drug metabolites or cutoffs used?
Was a new maintenance contract awarded that required authorization for individuals who have not worked in the commercial nuclear industry?
Did the facility undergo a refueling or maintenance outage?
Did the facility install or remove a manufactured reactor?
Was the facility staff adversely impacted by disease, or were there occurrences that may have affected the psychological or physiological well-being of the workforce?
Were the facility and its staff adversely affected by abnormal environmental conditions?
To help ensure the quality of FFD performance data used in the PMRP, the FFD program personnel should periodically verify (by conducting and documenting a review or audit under 10 CFR 26.202(f), 26.603(d)(2), or 26.615, as applicable) the origination and processing of FFD performance data. This can be done by reviewing custody and control forms (CCFs) and correlating CCF information with the types of drug and alcohol testing and screening processes implemented by the licensee or other entity. Data quality would also be ensured by using properly trained collectors, informed donors, nonexpired specimen test or screening kits, forensic toxicologist reviews, and verification that MRO recommendations and findings are consistent with laboratory test results. The qualitative review in 10 CFR 26.603(d)(1)(iv)(B), which is discussed in section C.5.f(4)(b) of this RG, further describes this topic related to HHS laboratories and the MRO.
Performance Data and Program Considerations
The following should be considered in the development and implementation of the PMRP:
Data Characteristics. Site- and fleet-specific performance data should be simple to obtain, objective, and measurable.
Timely data and assessment. The PMRP procedure should require timely data and assessment because this would inform authorization determinations to enable individuals to return to duty or commence work faster and investigations of events and accidents and issuance of corrective actions to correct causes.
Data accuracy. The PMRP procedure should ensure that the data used in the PMRP is accurate. For an FFD program that implements drug testing, data should be accurate because most data used in this element of the PMRP will be from laboratory test results that have been reviewed by a Certifying Scientist and the MRO for the FFD program. Similarly, qualitative data obtained through audits and self-assessments should be accurate because the audit should be reviewed by licensee or other entity management before issuance. For audits, qualitative performance assessments and performance measures for behavioral observation, assessment of PDI, and identification of prohibited items, the assurance of data accuracy for these sources should be assured by documented descriptions of the particular occurrence.
Ownership. Performance measures and the associated data should have an owner who is held accountable for performance measure effectiveness. The owner should be empowered by licensee or other entity management to take actions (i.e., corrective actions) to improve the effectiveness of performance measure effectiveness and data quality. The owner should be empowered to propose more complex solutions to licensee or other entity management.
Gaming the PMRP. Individuals should not be able to manipulate the PMRP to prevent approaching or exceeding a threshold or allow qualitative considerations to adversely skew data evaluations. For example, if an individual is discovered with a prohibited FFD item (such as synthetic urine), then this event should count towards the behavioral observation and prohibited item performance measures, and not just one or the other should there be more room before the threshold is exceeded. Licensees or other entities may want to assign two or more owners to monitor the PMRP. An individual who games the system is not acting in a trustworthy and reliable manner.
General Considerations – 10 CFR 26.603(d)
A performance measure is a description of a particular FFD program element that is being monitored because FFD performance data or review findings exist to evaluate the program element. A threshold is a quantitative value of the FFD performance data where corrective actions must be taken because of an unacceptable decrease in margin for a performance measure. FFD performance must be monitored and reviewed. Performance measures and thresholds are not established for the reviews under 10 CFR 26.603(d)(1)(iv)(A) through (C).
10 CFR 26.603(d)(1) requires the PMRP to be documented and maintained. 10 CFR 26.603(d)(1)(i) prescribes the performance measures that must be implemented; however, licensees and other entities are encouraged to establish their own additional site-specific or fleet‑level performance measures.
The PMRP bounds the number of quantitative FFD-related performance deficiencies to thresholds that are developed from actual performance data generated by FFD programs. Many of the thresholds listed in Tables 3 and 4 are at zero and one occurrence. Historically, actual FFD-related occurrences have not resulted in conditions inimical to public health and safety or the common defense and security; however, the question remains as to how many occurrences contrary to the FFD policy (and at what level of significance) are needed to demonstrate that an FFD program would no longer meet the FFD performance objectives. As an example, effective FFD performance would not be demonstrated if 13 percent (2 of 15) NRC-licensed operators were in violation of the FFD policy for substance abuse over the last 12 months and this rate of FFD policy violations for this labor category meets its performance measure threshold. Additionally, if a facility has only a few individuals per shift to operate, maintain, and secure the facility and an individual were impaired or not trustworthy and reliable, a mitigating strategy should be described in a procedure if the ability to maintain a minimum staff number in a key functional area (e.g., operation, maintenance, or security) is necessary for safety and security. This becomes more important if a licensee or other entity relies on a single individual to maintain, surveil, or secure an SSC or provide supervisory oversight of another individual or shift of individuals. The thresholds described in Tables 3 and 4 reflect this guidance. These actions may include operating, monitoring, surveilling, maintaining, protecting, securing, or responding to transients, accidents, or events or directing any of these actions. Therefore, with smaller staff sizes, even with automated SSCs, it may become increasingly important that each individual is not impaired or appears untrustworthy and unreliable.
Even if there are relatively long timelines to accomplish actions, the risk of an impaired individual does not always decrease with time. For example, fatigue increases with time and may be considered cumulative as time passes (meaning fatigue gets worse with time). The potential for substance-induced impairment may also increase with time as illustrated in figures 7 and 8.
As illustrated in figure 7, the potential for alcohol impairment increases for the first hour following ingestion as the alcohol disperses throughout the body and undergoes metabolism by the liver. Studies have demonstrated a strong link between blood alcohol concentration, its potential to cause impairment, and its removal from the blood stream. This is best illustrated by the time-dependent alcohol limits in 10 CFR 26.103, “Determining a confirmed positive test result for alcohol.”
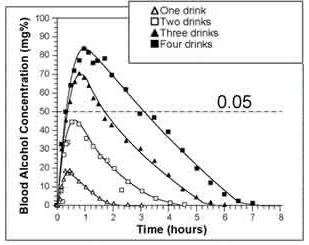
Figure 7. Alcohol Metabolism (Ref. 28)
The potential impairment caused by inhalation or ingestion of marijuana (Δ-9 THC) is more complex than that of alcohol. As shown in figure 8 with the graph line using triangles, human performance as described in the report Marijuana-impaired Driving - A Report to Congress (Ref. 29) did not correlate to THC concentration in the blood. The relatively long slow decline in performance was also identified by others such as in the report Cannabis and Driving (Ref. 30).13 In this report, the researchers cited research in which “plasma THC concentrations increase rapidly, peaking at ~3-10 minutes after the first inhalation . . .” and “then fall rapidly as the drug is absorbed and within about 20–30 min[utes] reach a low, relatively stable plateau that persists over several hours. THC-induced impairment on many measures declines slowly for ~5–6 h[ours] following acute dose in a manner that is generally unrelated to this post-peak THC blood level.” This report also stated that “[o]ral absorption is slower and less efficient than with smoking, with a significantly more delayed onset of drug effect, and with intoxication that is then more sustained, with lower peak THC concentrations than those that follow smoking.”
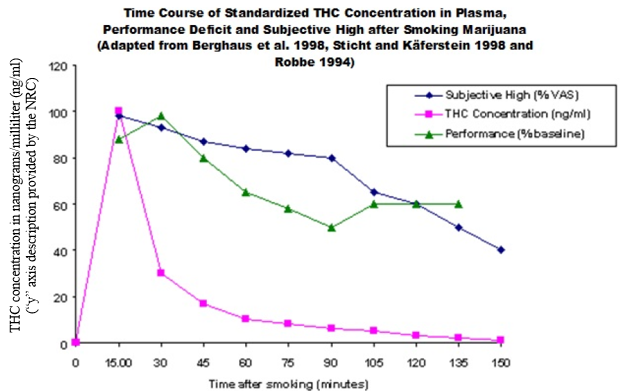
Figure 8. Time Course of Standardized THC in Plasma
The performance measures and thresholds in this guide include facilities subject to 10 CFR Part 26, Subpart M, and the thresholds are not a simple arithmetic function of LLWR FFD performance. Expert panel review and statistical analyses were performed to account for holders of a manufacturing license and 10 CFR Part 53 CNPs that may present and implement staffing plans (e.g., staff size and labor categories), markedly different than those of the current LLWR community licensed under 10 CFR Part 50 or 10 CFR Part 52. The thresholds in this guide were scaled down to be more representative of the staff size at a 10 CFR Part 53 facility (using a 3 to 1 staff size ratio from that of an LLWR facility).
Figure 6 provides an overview of the three types of FFD programs and their corresponding performance measures under a 10 CFR Part 26, Subpart M, FFD program. Tables 1 and 2 summarize the quantitative performance measures. These performance measures are risk-informed in that they focus on human performance outcomes that could result in potential adverse consequences if they occurred while the individual is performing or directing those duties and responsibilities or having those types of access that make the individual subject to the FFD program.
Performance Measure Overview
Figure 6 illustrates the framework for the quantitative performance measures.
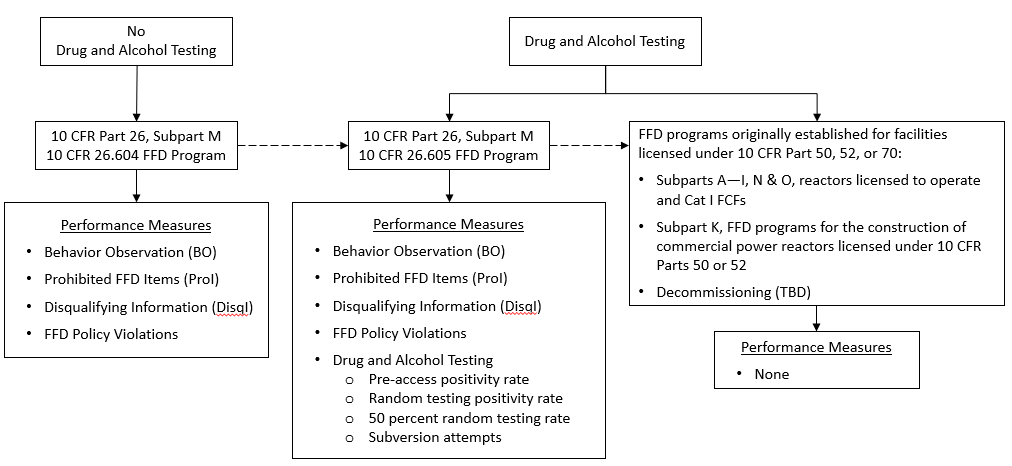
Figure 6. Overview of FFD Programs and Performance Measures
Table 1 lists the performance measures for an FFD program under 10 CFR 26.604, which does not require drug and alcohol testing. These measures are focused on measuring the number of aberrant performance issues (behavioral observation (BO), identification of a prohibited FFD item (ProI), identification of disqualifying information (DisqI), and other FFD policy violations categorized by employment and labor category). Table 2 is for an FFD program implemented under 10 CFR 26.605 and includes the performance measures in Table 1. If a licensee or other entity implements an FFD program under 10 CFR 26.604 and performs drug and alcohol testing, then the licensee or other entity should consider using the Table 2 performance measures to inform their PMRP as to whether the drug and alcohol testing program is meeting the intended outcome of detection and deterrence.
For Tables 1 and 2, the performance measures separate the licensee employee , C/Vs, and labor categories to granulate a licensee’s or other entity’s risk-informed review of the FFD-related occurrence. This granularity should help focus corrective actions to maintain or improve FFD program effectiveness. Tables 1 and 2 also present the data sources that should be used to inform the establishment and revision of performance measures and thresholds.
Table 1. Quantitative Performance Measure Summary—No Drug and Alcohol Testing (10 CFR 26.604)
Table 2. Quantitative Performance Measure Summary—Drug and
Alcohol Testing
(10 CFR 26.605)
|
|
Reviews Performed |
||
Identifier |
Performance Measure |
Site Specific |
FFD Program |
Industry |
BO DisqI ProI |
Behavioral observation Disqualifying information Prohibited FFD item |
Yes |
Yes |
See Table 1 |
PVLE PVCV PVLC |
FFD policy violations by licensee employee FFD policy violations by C/V FFD policy violations by labor category |
Yes |
Yes |
Yes |
PAPR PASA |
Pre-access positivity rate for C/Vs Pre-access subversion attempts by C/Vs |
Yes |
Yes |
No |
RTRLE RTRCV |
Random testing rate for licensee employees Random testing rate for C/V |
Yes |
No |
No |
RTPRLE RTPRCV |
Random testing positivity rate for licensee employees Random testing positivity rate for C/V |
Yes |
Yes |
Yes |
SALE SACV SALC |
Subversion attempts by licensee employees Subversion attempts by C/Vs Subversion attempts by labor category |
Yes |
Yes |
Yes Yes No |
Performance Measure Thresholds
The PMRP enables a licensee or other entity to identify the significant FFD program elements, measure the effectiveness of these elements, and implement corrective actions when the number of FFD deficiencies within a performance measure meets its established threshold or when assessment or audits identify performance deficiencies. This framework is like the actions a licensee or other entity would take for performance deficiencies in other program areas such as safety, fire protection, radiation protection, security, or quality assurance.
Table 3. Quantitative Performance Measure and Threshold
Summary—
No Drug and Alcohol Testing (10 CFR 26.604)
Identifier |
Performance Measure |
Thresholds |
BO DisqI ProI |
Behavioral observation Disqualifying information Prohibited FFD item |
One occurrence per year. One occurrence per year. One occurrence per year. |
PVLE
PVCV
PVLC |
FFD policy violation by licensee employee
FFD policy violations by C/V
FFD policy violations by labor category |
PVLE and PVCV—Determined by the applicant, licensee, or other entity.
PVLC—One occurrence each for NRC‑licensed operators, NRC‑required security officers, quality assurance/quality verification personnel, chemistry technician, cybersecurity and information technology services personnel, and individuals who perform safety- or security‑significant activities and the supervisors of these individuals. Zero occurrences if there is only one individual on shift (solo operations) within a particular labor category required for operation or security of the facility.
PVLC—Zero occurrences for FFD program personnel, SSNM transporters, and radiation protection technicians. |
Table 4. Quantitative Performance Measure and Threshold
Summary—
Drug and Alcohol Testing (10 CFR 26.605)
Identifier |
Performance Measure |
Thresholds |
BO DisqI ProI |
Behavioral observation Disqualifying information Prohibited FFD item |
One occurrence per year One occurrence per year One occurrence per year |
PAPRLE
PASACV |
Pre-access positivity rate for licensee employees
Pre-access subversion attempts by C/V |
Determined by the applicant, licensee, or other entity |
RTRLE
RTRCV
RTPRLE
RTPRCV |
Random testing rate for licensee employees
Random testing rate for contractor vendors
Random testing positivity rate for licensee employees
Random testing positivity rate for C/V |
50 percent
50 percent
0.5 percent
1.0 percent |
PVLE
PVCV
PVLC |
FFD policy violations by licensee employee
FFD policy violations by C/V
FFD policy violations by labor category |
PVLE and PVCV—Determined by the applicant, licensee, or other entity.
PVLC—One occurrence each for NRC‑licensed operators, NRC-required security officers, quality assurance/quality verification personnel, chemistry technician, cybersecurity and information technology services personnel, and individuals who perform safety- or security-significant activities and the supervisors of these individuals. Zero occurrences if there is only one individual on shift (solo operations) within a particular labor category required for operation or security of the facility.
PVLC—Zero occurrences for FFD program personnel, SSNM transporters, and radiation protection technicians. |
SALE
SACV
SALC |
Subversion attempts by licensee employee
Subversion attempts by C/V
Subversion attempts by labor category |
SALE—Zero occurrences.
SACV—Two occurrences.
SALC—Zero occurrences for licensee employees and two for C/Vs by company affiliation. |
Performance Measures for Behavioral Observation, Prohibited FFD Items, and Disqualifying Information
The performance measures for behavioral observation, prohibited FFD items, and disqualifying information are risk-informed because they are a direct assessment of an individual’s fitness, trustworthiness, and reliability while they are performing or directing those duties and responsibilities or maintaining the types of access that make them subject to the FFD program.
Like all performance measures, the behavioral observation measure is not meant to be punitive. The identification of individuals who may be impaired or not trustworthy and reliable, whether onsite or off site, promotes safety and security by protecting the individual if the impairment requires medical intervention and protects others, and the facility if the individual is identified with aggressive or harmful intent. Protecting the individual, others, and the facility is further served by the individual being subject to a sanction for the FFD policy violation, which would enable a determination of fitness and longer-term actions such as counseling, treatment, or training. Therefore, FFD program effectiveness is maintained as individuals are identified, and corrective action can be taken to address root and apparent causes for any performance deficiency.
A comparative measure for behavioral observation could be for-cause testing. Under 10 CFR 26.31(c)(2), for‑cause testing is conducted in response to an individual’s observed behavior or physical condition indicating possible substance abuse or after receiving credible information that an individual is engaging in substance abuse. However, the behavioral observation measure is not dependent on the conduct of a for-cause test. The behavioral observation measure should include any observation of performance that indicates the individual may not be fit for duty and trustworthy and reliable and should be counted when the individual is removed, either temporarily or permanently, from assigned duties and responsibilities and access to the facility, SNM, or sensitive information. So, although a behavioral observation can be compared to for-cause testing, the comparison would not be conservative. Behavioral observation would not identify as many individuals as the for-cause testing condition because the for-cause testing condition includes an individual's observed behavior or physical condition indicating possible substance abuse or after receiving credible information that an individual is engaging in substance abuse.
For an FFD program under either 10 CFR 26.604 or 10 CFR 26.605, an occurrence for the behavioral observation performance measure should be counted if the individual is on duty and is inattentive, slurring words, unable to read, stumbling, not following instructions or procedures, or acting in an unnatural emotional or physiological state compared to the individual’s baseline mannerisms and performance. If the FFD program implements drug and alcohol testing, behavioral observation is still a principal method to ascertain impairment. However, a confirmed positive drug test conducted for cause would assist in the determination of impairment and a confirmed positive alcohol test coupled with the behavioral observation should result in a determination of impairment. For an individual off site, an individual’s actions contrary to fitness (such as sale, use or possession of illegal substances or impairment by alcohol) would be counted within the behavioral observation performance measure.
For the behavioral observation performance measure, the following occurrence would not count: an individual subject to the FFD program takes a drug or alcohol screening test, and the results indicate a presumptive positive (for drugs, drug metabolites, alcohol, adulterants, or biological indicators), but the individual does not show any signs of impairment from any cause. However, this occurrence would be counted within the FFD policy violation performance measure after the receipt of a confirmed positive test from the laboratory and after the MRO review or if the individual shows signs of impairment even with a negative screen (as discussed in the previous paragraph).
The observation of impairment need not be “confirmed” by an observation conducted by a second person, because identifying and preventing an individual from causing a condition adverse to safety and security is the goal of a BOP. Seeking a second opinion on an observation of impairment or actions or communications indicating that an individual may be impaired or not trustworthy and reliable is contrary to safety and security, because it would delay the removal of the individual from duties, responsibilities, or access and may actually harm the individual because medical attention could be delayed.
As part of the PMRP, licensees or other entities must review, document, and evaluate the effectiveness of their behavioral observation performance measure. This review would help licensees or other entities implementing 10 CFR 26.604 ascertain whether to supplement their FFD program with a drug and alcohol testing program or for all licensees and other entities whether to implement other corrective actions. The following factors should be considered when reviewing and evaluating the behavioral observation measure and its performance data.
Evaluate the BOP training to ensure that it is effective, understood, and implemented by all individuals subject to 10 CFR Part 26. This can be accomplished through independent assessment of the training and monitoring of training test results.
Evaluate whether a planned behavioral observation of an individual should be performed. A planned behavioral observation is one that is performed by shift, oversight or management personnel who are independent of the human performance activity being performed. Planned behavioral observations could include planned observations of plant tours, shift changes, facility operations, surveillance, maintenance, and security activities.
Evaluate whether a planned behavioral observation of facility operations should be conducted during swing shifts and midshifts, holidays, and weekends. These observations help ensure that all individuals are subject to the BOP, thereby helping deter conduct detrimental to the effectiveness of an FFD program.
Evaluate whether behavioral observation through activities such as teamwork, peer checks, or management oversight had a reasonable opportunity to prevent plant transients, operational occurrences, human errors, or occurrences related to a work-related injury or illness. A work-related injury or illness was described in an Occupational Safety and Health Administration interpretation (https://www.osha.gov/laws-regs/standardinterpretations/2014-02-28):
Section 1904.5(a) [of 29 CFR] provides that injuries and illnesses must be considered work-related if an event or exposure in the work environment either caused or contributed to the resulting condition or significantly aggravated a pre-existing condition. Work-relatedness is presumed for injuries or illnesses resulting from events or exposures in the work environment unless an exception in section 1904.5(b)(2) specifically applies. Accordingly, for a case to be work-related there must be a causal connection between the injury or illness and an event or exposure at work. For OSHA recordkeeping purposes, causality is established if work is a cause. The work event or exposure need only be a cause of the injury or illness; it need not be the sole or predominant cause. See, the preamble to the final rule revising OSHA's recordkeeping regulation 66 Federal Register 5929-32, 5946 and 5948. Also, "it is not necessary that the injury or illness result from conditions, activities, or hazards that are uniquely occupational in nature." 66 Federal Register 5929.
Identify whether security features or processes could be enhanced to protect FFD-related information (e.g., suitable inquiries, CCFs, MRO notes, drug and alcohol testing results) and better identify potentially impaired individuals before their entry into the protected area or before they perform or direct those activities that make them subject to the FFD program.
Review how behavioral observation data (e.g., indications of impairment, physical or emotional characteristics, and untrustworthy and unreliable behavior) are documented for review by FFD program personnel or a reviewing official designated by the licensee or other entity and effectively protected from unauthorized disclosure.
For the prohibited FFD item performance measure, a prohibited FFD item is anything that if used or possessed could result in the individual causing or contributing to a situation that is contrary to the 10 CFR Part 26 performance objectives or being in violation of the FFD policy established by the licensee or other entity. Prohibited FFD items that could cause impairment include, but are not limited to, alcohol‑based consumables (i.e., food, drink, confectionaries, sauces, etc.); nonmedical inhalants; and substances containing other illegal substances, illegal drugs, and illicit substances such as lotions, ointments, tinctures, oils, vapors, or other products containing either Δ8-, Δ9-, or Δ10-THC, whether synthetic or natural. The licensee or other entity should provide guidance on the use and possession of these and other illegal substances, illegal drugs, and illicit substances and whether possession or use of the product within the protected area is a prohibited FFD item performance deficiency. The use of any impairing substance before or during the conduct of roles and responsibilities making the individual subject to the FFD program is a performance deficiency.
Licensees and other entities should establish guidance on the products containing cannabidiol, a psychologically inactive chemical from the cannabis plant, and whether possession or use of this product within the protected area is a prohibited FFD item performance deficiency. A study of commercially available products containing cannabidiol determined that many products do not accurately list key ingredients or their concentration (Ref. 31). So, even if the product label states that the cannabidiol was derived from the hemp plant and does not exceed the Δ9-THC concentration of the Farm Bill (Ref. 32), the product may contain a quantity of Δ9-THC (or other cannabinoid) that may cause impairment.
Prohibited FFD items also include paraphernalia that could subvert a drug or alcohol test. These include, but are not limited to, synthetic or naturally generated biological specimens not produced by the donor; any substance that could be added to urine or oral fluid or applied to hair follicles before or during the urine, oral fluid, or hair collection process14 such as, but not limited to, nitrites, pyridinium chlorochromate, chromium (VI), bleach, iodine/iodide, halogens, peroxidase, and peroxide; and any device that stores or delivers urine, oral fluid, or hair not directly produced and delivered by the donor or a chemical or other substance not required for an MRO‑reviewed physiological condition.
For facilities using or planning to use C/Vs, the behavioral observation and prohibited FFD item thresholds for the C/Vs should be kept at one occurrence for each performance measure. This threshold aligns with the performance standard that all individuals subject to 10 CFR Part 26 are fit for duty and trustworthy and reliable while they are on the job and the scaling of LLWR FFD performance data to the estimated population size of a facility licensed under 10 CFR Part 53. However, LLWR FFD performance data do show that C/Vs account for the majority of all subversion attempts and positive drug and alcohol testing results. Therefore, as discussed earlier, if the use of C/Vs is planned and the licensee or other entity effectively implements the access authorization and FFD programs and the number of C/V FFD-related performance deficiencies increases, this may have been expected and planned, but the PMRP would still require this situation to be reviewed and corrective actions taken when the threshold is met.
The behavioral observation and disqualifying information measures do not apply to pre-access testing or reviews of individuals who self‑identified or announced their possible impairment, FFD policy violation, or condition that may have caused them to be untrustworthy and unreliable, even if they were in the protected area, unless the self-identification occurred after an event, human error, drug or alcohol screening or test, a behavioral observation conducted by another person, or other identification of an FFD policy violation. Individuals, licensees, and other entities should not be penalized if an individual takes the initiative to self-announce a potentially adverse condition and possibly seek medical, clinical, or other help as this demonstrates a healthy safety culture before an adverse event occurs.
The threshold for the disqualifying information measure is one occurrence per year as indicated in tables 3 and 4. The disqualifying information measure includes those occurrences when the disqualifying information was verified or validated and the individual was maintaining unescorted access. Individuals with disqualifying information should either be temporarily or permanently removed from maintaining unescorted access and those duties and responsibilities making them subject to the FFD program. Restoration of unescorted access or any role or responsibility described in 10 CFR 26.4 should be permitted only after the licensee or other entity evaluates the information for significance and the individual successfully completes any assigned corrective actions. Unlike the behavioral observation measure, which is impairment on the job and has a direct nexus to safety and security, a disqualifying information occurrence may not likely present itself as an immediate condition adverse to safety, security, or quality, and the 10 CFR Part 53 framework does not penalize a licensee or other entity that identifies an individual through behavioral observation, disqualifying information, or prohibited FFD item.
The disqualifying information performance measure includes two types of information: PDI in 10 CFR 26.5 and information that could be used to help the licensee or other entity satisfy the 10 CFR 26.23 performance objectives, specifically paragraphs (b) and (c). Paragraph (b) states that the FFD program must—
Provide reasonable assurance that individuals are not under the influence of any substance, legal or illegal, or mentally or physically impaired from any cause, which in any way adversely affects their ability to safely and competently perform their duties.
Paragraph (c) states that the FFD program must “Provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program.”
The guidance illustrates the physiological and psychological indicators that an individual may be “mentally or physically impaired from any cause” or may not be “fit to perform the duties that require them to be subject to the FFD program.” The examples also illustrate when an individual may not be trustworthy and reliable to maintain access or perform those duties as assigned or when directed by others, procedures, training, or facility conditions.
The individual—
caused or threatened to cause harm to the NRC-licensed facility, to themselves, or to others (e.g., workplace violence or hostilities);
was argumentative or not following instructions for a specimen collection or assigned duty or responsibility, yet the occurrence was not egregious enough to warrant a permanent denial of authorization;
exhibited emotional outbursts that caused concern among coworkers, supervisors, or management;
deceitfully provided incorrect information in response to a suitable inquiry;
falsified records in the conduct of construction, operation, maintenance, surveillance, security, or other facility activity or was deliberately negligent in the performance of duties and responsibilities (e.g., tampering with equipment, disregard for instructions or records); or
operated a vehicle under the influence of an illegal substances, illegal drugs, and illicit substances, whether convicted or not, as determined by State or local law.
Performance Measures for Policy Violations (PVLE, PVCV, and PVLC)
Tables 3 and 4 list three performance measures for policy violations. Since many situations could result in an FFD policy violation, the PVLE and PVCV measures aggregate all the different types of occurrences by employment category, and the PVLC takes measurement one step further by measuring policy violations within certain labor categories. This framework should enable a dataset that is easily compared to that from other sites to inform a PMRP. In the policy violation category, a licensee or other entity will have to double count some performance data because occurrences that count under the behavioral observation, prohibited FFD item, disqualifying information, random testing positivity rate for licensee employees and C/Vs (RTPRLE and RTPRCV), and subversion attempts by licensee employees and C/Vs are all FFD policy violations. Furthermore, if drug testing is performed, positive test results would be included in the dataset for the policy violation performance measure.
The policy violation by labor category performance measure is focused on risk. The measure counts only the FFD policy violation in certain labor categories where the duties and responsibilities of the individuals represent a higher risk to safety and security if the individual were impaired or determined not to be trustworthy and reliable. There are two categories of individuals within the PVLC measure: those that typically work in a team environment and those that typically implement solo operations (FFD program personnel, strategic special nuclear material (SSNM) transporters, and radiation protection technicians). Individuals who typically work in a team environment (NRC-licensed operators, NRC‑required security officers, cybersecurity and information technology services personnel, chemistry technicians, quality assurance/quality verification personnel, and individuals who perform safety- or security-significant activities and the supervisors of these individuals) generally have a second individual providing some oversight or second checking. For example, security officers are monitored by central alarm station oversight, periodic radio checks, post rotations, and supervisory tours, and individuals performing solo operations and cybersecurity and information technology services personnel are also monitored (either remotely or locally) as the activities are accomplished (e.g., instrument or system functional changes, computer or instrumentation indications). For individuals who perform solo operations and whose performance could result in conditions adverse to safety and security (whether immediate or latent and undetected), a zero threshold should be established as shown in Tables 3 and 4. Furthermore, if the licensee identifies other labor categories that perform safety- or security-significant activities in a solo environment on shift and a replacement individual is not available to immediately perform the roles and responsibilities of the individual who has been determined to be impaired or not trustworthy and reliable, then the threshold should also be established at zero occurrences.
The policy violation performance measure should include the occurrences or situations in which an individual—
used, sold, or possessed illegal drugs on or off site;
abused legal drugs, alcohol, or both on or off site;
misused a prescription or over-the-counter drug, resulting in impairment on site or could not report to work due to impairment;
failed to report an FFD concern (i.e., failed to conduct behavioral observation) about another individual to the licensee- or other entity-designated representative;
failed to report legal actions, as defined in 10 CFR 26.5;
retaliated against an individual who reported an FFD concern;
failed to notify the individual designated by the licensee or other entity that he or she is or may become impaired due to fatigue, prescription medication use, or other physiological or psychological condition;
cheated on an FFD or other NRC-required test conducted for training;
consumed an alcoholic beverage at the NRC-licensed facility or was determined to be impaired by the consumption of any alcohol while at the NRC-licensed facility as determined either by the results of an evidentiary breath testing device or a laboratory test result with MRO review;
failed one or more elements of a licensee- or other entity-directed return-to-duty program implemented to prevent recurrence of an FFD policy violation or other condition warranting medical or clinical assistance; or
failed to effectively communicate (orally or in writing) to the MRO or FFD program personnel any issue that could potentially be regarded as or was an FFD policy violation.
If drug and alcohol testing is conducted, the FFD policy violation performance measure should include occurrences in which the individual—
was notified that they have been selected for random screening or testing and does not report to the collection site within the time period specified;
refused to provide a specimen for screening or testing;
inhaled or orally consumed or used a substance forbidden by the licensee or other entity’s procedures (i.e., a prohibited FFD item) before or during the conduct of the drug or alcohol screen or test (such as hand sanitizer before a protected area screening test); or
subverted or attempted to subvert the screening or testing process, including the circumvention of a passive drug or alcohol screening device.
Performance Measures for the Pre-access Process (PAPRCV and PASACV)
The purpose in monitoring the C/V pre-access positivity rate and C/V pre-access subversion attempt rate is to enable a comparative analysis with the FFD performance observed in the protected area (i.e., the FFD performance of individuals who maintain unescorted access), as illustrated in Figure 1. A secondary purpose is to enable the qualitative assessment of the individuals to whom the licensee or other entity intends to grant unescorted access to the NRC-licensed facility, SNM, or sensitive information.
The NRC is not providing a threshold for the C/V pre-access positivity rates for policy violations or subversion attempts. Licensees and other entities should monitor their rates or occurrences (per year or per facility event or activity) and compare them against other FFD performance measures (quantitative and qualitative) at their site and other sites. This comparison could provide insights into the effectiveness of a number of FFD program areas. For example, the licensee or other entity could identify potential weaknesses in the following:
the process used to obtain accurate information on suitable inquiries and evaluate this information;
the contracting process that screens the contracted workforce to prevent unnecessary challenges to the NRC’s FFD program requirements;
FFD training and the pre-access drug and alcohol testing process if implemented; and
the diligence of specimen collectors in identifying subversion attempts.
This analysis could provide insight into whether the 10 CFR Part 26, 10 CFR 73.56, or 10 CFR 73.120 authorization requirements are effectively implemented to prevent individuals from being granted and maintaining unescorted access to the facility, SNM, and sensitive information. A low pre-access positivity rate and a high random testing positivity rate (leading to a high ratio between the two) may indicate that individuals are abstaining from drug and alcohol use before their pre-access drug or alcohol test and resuming the drug or alcohol use after being granted authorization. A hair screening test could be an effective corrective action to address this apparent performance deficiency or a corporate initiative to more successfully screen individuals. A low ratio could also indicate a change from historical performance. For example, if the pre-access positivity rate is constant yet individuals working in the facility and subject to 10 CFR Part 26 are identified as self-medicating in a manner contrary to the FFD program policy, then a potential performance deficiency may be in the pre-access testing process because these individuals would be identified after they have been granted authorization. This comparison should be performed when there is a large and temporary C/V work force on site.
The secondary purpose of this performance measure is to enable the licensee or other entity to identify and address any potential differences between pre-access positivity rates among groups of C/Vs and licensee employees. The NRC staff expects that C/Vs may be contracted to perform work at multiple sites within the same fleet‑level FFD program, or perhaps different C/V companies may be contracted by different licensees and other entities but shared to reduce costs or improve C/V effectiveness. Comparing the pre-access positivity rates will provide insight into the safety culture of the contracted workforces, which may lead to mitigative strategies (i.e., corrective actions) if a particular workforce is violating the FFD policy at a higher rate than peer work groups.
Performance Measures for the Random Testing Rate (RTRLE and RTRCV)
The threshold for the two random testing rate performance measures for licensee employees and C/Vs is the number of random tests performed annually equal to at least 50 percent of the population subject to testing. The licensee or other entity should establish a random testing pool for each employment category.
Performance Measures for the Random Testing Positivity Rates (RTPRLE and RTPRCV)
Based on analysis across the LLWR industry over multiple years, while crediting margin and defense in depth, the recommended RTPRLE and RTPRCV thresholds are established at 0.5 and 1.0 percent, respectively. However, licensees and other entities should still evaluate these thresholds against historical performance for the site, fleet-level program, and industry.
Like other performance measures, RTPRLE and RTPRCV could be influenced by the activities being performed at the site. However, an increasing random testing positivity rate could indicate a declining safety culture and reduced effectiveness of the FFD training program, and it may also suggest poor behavioral observation and an ineffective pre-access screening program.
This RG does not provide a performance measure for the random testing positivity rate by labor category. However, such a review by a licensee or other entity could identify FFD program challenges. The granularity of this performance measure would focus on those labor categories that direct or perform duties and responsibilities important to safety and security. Like the PVLC and SALC performance measures, increased occurrences or rates could indicate diminishing safety culture within the labor category. If needed, corrective actions could be focused on specific work groups.
Performance Measures for Subversion Attempts (SALE, SACV, and SALC)
As shown in Table 4, the threshold for the subversion attempt performance measures is either one or zero occurrences based on the employment and labor category of the individual. There is zero tolerance for any individual who attempts to subvert the testing process, as demonstrated by the required permanent denial of authorization for a subversion attempt. However, this measure is also risk-informed in that the licensee or other entity must monitor and establish a threshold for subversion attempts as required by 10 CFR 26.603(d)(1)(i)(B). This includes attempts by individuals who do not have unescorted access to the facility because the rule is not prescriptive in where the screening or testing is conducted. For example, subversion attempts may occur during pre-access screening tests using hair or pre-access testing using urine or oral fluid. Test results from the pre-access process provide information to the licensee or other entity on whether the individuals seeking access to the facility are trustworthy and reliable.
There is no recommended threshold for the subversion attempts occurring during the pre-access process. However, the number and rate of subversion attempts must still be monitored and assessed, and corrective actions implemented as identified by the licensee or other entity under 10 CFR 26.603(d). The identification of individuals subverting a test during the pre-access process demonstrates vigilance by the collector and MRO and the effectiveness of the FFD program. It may also provide insight into the licensee’s or other entity’s contracting and hiring practices, suitable inquiries, and FFD training.
Occurrences that are counted with the subversion attempt measure could warrant more comprehensive corrective actions than corrective actions that would be implemented for measures like behavioral observation and disqualifying information. This is because subversion, as defined in 10 CFR 26.5, is, in part, a willful act to avoid being tested or to bring about an inaccurate drug or alcohol test result, a very clear indication that the individual may not be trusted and relied upon. Instead, occurrences for other measures may not be willful and not indicate a lack of trustworthiness and reliability. More comprehensive corrective actions should also be considered if two or more subversion attempts occur by licensee employees or within a particular labor category because individuals could be acting to helps other to subvert the testing process. This situation could indicate a negative safety culture and a potentially more widespread failure of others to follow the FFD policy and procedure. In this case, monitoring of the subversion attempt performance measures by the licensee or other entity will help track whether the subversion attempts share commonalities in (1) the use of similar urine adulteration kits, (2) failure to report FFD concerns about others, and (3) the possession, use, or sale of illegal substances, illegal drugs, and illicit substances.
In this category of performance measures, licensees and other entities may need to double count some performance data. For SALE and SACV, the measure counts every occurrence within the performance measure category based on employment category. However, if the FFD policy violation involves an individual in one of the labor categories (provided in Table 5), then that individual is also counted in the SALC performance measure.
Monitoring Program—10 CFR 26.603(d)(1)(iii)
As FFD performance data are received, licensees and other entities must monitor the performance of their FFD programs against their established performance measures and thresholds to determine whether a threshold has been met. This typically means conducting reviews at least weekly when random testing is conducted, as soon as reasonably practical if a for-cause or post-event test is conducted, and during the period of in-processing of licensee employees and C/Vs who are expecting to be granted authorization. The data to be monitored and reviewed include not only the results of drug and alcohol testing, if conducted, but also times when individuals are observed to be not fit for duty, trustworthy, or reliable. Such cases include situations in which an individual is physiologically or psychologically impaired from any cause, cannot be trusted or relied on to safely and competently perform assigned duties and responsibilities, or presents an FFD concern or has disqualifying information that has not been evaluated by the licensee or other entity.
FFD program personnel should maintain awareness of FFD policy violations as they occur because of their potential contribution to performance data approaching a threshold and as a potential precursor to support reporting information to the NRC. For example, reports to the NRC (see 10 CFR 26.617, “Recordkeeping and reporting,” and 10 CFR 26.719, “Reporting requirements”) are required for program weaknesses and FFD policy violations caused by individuals in certain labor categories. These events are of potential safety or security consequence requiring notification to the NRC and assessment by the licensee because the aberrant performance of the individual’s duties and responsibilities could be adverse to safety or security.
In preparation for and during refueling, significant maintenance, manufactured reactor installation or replacement, and other events, the population of individuals subject to the FFD program may change. This may cause an expected or unexpected change in FFD program performance. PMRP monitoring may enable timely corrective actions if a condition (like the use of a large number of C/Vs) causes an increase in FFD policy violations. Corrective actions for this occurrence could consist of FFD-related poster boards, internal website notifications and reminders, planned behavioral observation of certain labor categories or work activities based on risk significance, FFD training, or conduct of audits. Meeting a threshold because of a change to staffing (e.g., the number of licensee employees and/or C/Vs) in and of itself is not an adverse condition if the licensee or other entity planned this occurrence (i.e., there was effective work force planning and management). However, an increase in the number of FFD policy violations because C/Vs were brought on site does not justify increasing a threshold or accepting a temporary reduction in program effectiveness. This guidance is no different than that provided for the ROP performance indicators, which do not change based on staffing, training, qualification, or other internal or external event or condition.
Monitoring also includes the situation in which FFD program performance appears to be improving without licensee or other entity action, as first presented in Figure 1. FFD program personnel should assess the cause of the program improvement because there may be two disparate reasons, as illustrated below. Reviewing such a situation demonstrates an effective safety culture.
Examples of Program Improvements that May Have Caused an Apparent Improvement in FFD Performance
Improved specimen collections and collector attentiveness—Improvements have enhanced deterrence or detection of subversion attempts.
Improved training and communications—Improvements have enhanced safety culture and knowledge of the FFD program.
Enhanced availability of a licensee’s or other entity’s employee assistance programs—More individuals are being helped.
Improved background checks, suitable inquiries, and pre-access screening—Individuals with a more robust safety culture are being afforded access to the facility, SNM, or sensitive information.
Enhanced contracting practices—Improved screening of contracted companies and their employees possibly reduces use of illegal substances, illegal drugs, and illicit substances in the contracted workforce.
Implementation of corrective actions from audits, self-assessments, or other initiatives.
Examples of Performance Deficiencies that May Have Caused an Apparent Improvement in FFD Performance.
Degradation in behavioral observation—Fewer for-cause tests are conducted for individuals who may present a behavior or physical condition indicating possible substance abuse or after receiving credible information that an individual is engaging in substance abuse, as defined in 10 CFR 26.5; poor management oversight; and fewer FFD concerns identified. Any of the three could indicate a degraded safety culture.
Inattentive specimen collectors—Fewer subversions are identified.
Random testing biased to test licensee employees because they are on site full time, with fewer C/Vs being tested because they are on site for only short periods—The FFD program may not be meeting the annualized 50 percent random testing rate for both the licensee employee and C/V populations.
MRO performance issues—Positive drug tests are not being confirmed as positive when justified under HHS Guidelines or the requirements in 10 CFR Part 26, Subpart H, or use of prescription medications is not being critically assessed.
Alcohol testing issues—Evidential breath testing device is misoperated or miscalibrated.
Laboratory performance issues—Drug panels or cutoffs are being inaccurately applied to test instruments or there are performance issues involving certifying scientist or laboratory equipment.
Inaccurate point of collection testing and assessment devices—The tested drugs, drug metabolites, adulterants, and biomarkers; cutoffs; precision; and accuracy are not maintaining program effectiveness.
Quantitative and Qualitative Reviews—10 CFR 26.603(d)(1)(iv)
The PMRP requires documented quantitative and qualitative reviews of certain FFD program elements. In 10 CFR 26.603(d)(1)(iv), the NRC outlines the three elements that must be assessed: (1) worker protections, (2) laboratory test results and MRO performance, and (3) the change control process. The principal focus of the qualitative review is to assess whether the licensee or other entity effectively implemented the FFD program element to maintain FFD program performance. If the licensee of other entity elects to review the effectiveness of their corrective actions under the PMRP, this review should include an assessment of whether the corrective actions were commensurate with the severity of the performance deficiency and would help preclude recurrence. This review could also be used to verify that the performance deficiencies were not programmatic weaknesses requiring a report to the NRC under 10 CFR 26.617 or 10 CFR 26.719.
Reviewing Worker Protections—10 CFR 26.603(d)(1)(iv)(A)
The PMRP requires a documented assessment of worker protections. The importance of this review is to ensure that individuals subject to the FFD program are afforded privacy and due process consistent with the intent of the rule as described in Section IV, “Discussion of Final Action,” of the Federal Register notice for the 2008 Part 26 Final Rule (Ref. 33) Goal 7 of that rulemaking was to “[p]rotect the privacy rights and other rights (including due process) of individuals who are subject to 10 CFR Part 26.” Furthermore, the NRC stated that protecting individual rights under 10 CFR Part 26 is of the “highest importance.”
10 CFR 26.603(d)(1)(iv)(A) requires the review of worker protections to include a performance assessment of the following three elements:
Protections Afforded by 10 CFR 26.606(b)(1)(iii)
Worker protections afforded by 10 CFR 26.606(b)(1)(iii) include protecting the privacy of an individual who provides a specimen, protecting the integrity of the specimen, and ensuring that the test results are valid and attributable to the correct individual.
Privacy
This review should examine whether all individuals are afforded a reasonably equivalent level of privacy during the collection of a biological specimen. This level of privacy changes with the type of biological specimen collected and where the specimen is collected. The qualitative performance outcome of this review should be that all donors and affected individuals in the immediate vicinity of the specimen collection are provided an appropriate and reasonably equivalent level of privacy. Procedures should state the levels of privacy that will be afforded given the type and location of collections being performed. In establishing the levels of privacy, the licensee or other entity should consider the information provided in paragraphs B through D below, the HHS Guidelines, and 10 CFR 26.87(b) and (d), 26.107(a), 26.115, 26.153(f)(3), 26.403(b)(1), 26.405(e), and 26.411(a).
Privacy for Oral Fluid Specimens
The overall goal of allowing POCTA using oral fluid or urine during a random test in the workplace15 is to obtain a relatively immediate indication of an individual’s fitness through the detection of drugs, drug metabolites, alcohol, adulterants, and other biological indicators and to allow the individual to immediately return to work with a negative immunoassay screening result if there are no signs of physiological or psychological impairment. Therefore, if a licensee or other entity uses a POCTA oral fluid device in the workplace, meaning that the individual does not need to leave an assigned duty location (i.e., office, station, post, desk, work location) to provide the specimen at a collection facility, privacy still should be afforded. For the donor, privacy could be as simple as providing an oral fluid specimen standing away from other workers or behind a curtain or partition while being observed by a collector, which would provide visual and some audible privacy. For individuals in the immediate vicinity who are not subject to a test, privacy extends to them as well, in that they may not desire to hear (donor expectorating) or watch (expectorating or placing an absorbent oral fluid swab in the donor’s mouth) an individual submit to an oral fluid collection. The visual indication, and any discussion of a POCTA positive, adulterated, substituted, or dilute screening result, should be treated as private medical information and not disclosed to others who do not have a need to know. This type of privacy consideration should also be applied to the collection of exhaled breath for alcohol testing and the reading of its test results as described in 10 CFR 26.87(b).
Privacy for Hair Specimens
Similar to privacy during a collection of oral fluid using a POCTA device, donor privacy during the collection of hair specimens could be as simple as ensuring visual privacy (e.g., using a curtain or partition) while cutting the hair. This facilitates collection of head hair from individuals who may desire privacy for various reason (e.g., alopecia, baldness, skin conditions, hair weaves, religious practice) and extends privacy to others in the immediate vicinity since cutting hair in an NRC-regulated workplace environment is not a social norm. Furthermore, unlike the use of a POCTA oral fluid device in the workplace, the goal of a hair specimen is to facilitate a retrospective analysis of previous drug use, not an immediate indication of whether the individual may be fit to perform assigned duties and responsibilities safely and competently. Additionally, hair testing is limited to pre-access screening only, which would probably not be conducted in a workplace.
Privacy for Urine Specimens
During the collection of urine for drug screening or testing, the donor should have visual privacy. This privacy should prevent any individual who is outside the urine collection cube, stall, or toilet area from observing the area between the donor’s knees and shoulders, unless a directly observed collection is being performed. An acceptable practice is for the collector to observe the floor of the collection cube, stall, or toilet area for indications that the donor may be attempting to subvert the drug screening or testing process. Audible privacy is not required as it is not a social norm, and certain noises may indicate that the donor may be attempting to subvert the drug testing process.
Specimen Collection Facilities
The review performed under 10 CFR 26.603(d)(1)(iv)(A) is separate from that performed under 10 CFR 26.607(e), which requires the licensee or other entity to audit its use of a collection facility that is not part of the NRC‑licensed facility (e.g., a local hospital or other facility licensed and audited by the State (or State‑designated entity)). The PMRP review is an assessment of whether appropriate protections are being afforded to all individuals subject to screening or testing at an offsite facility. Indicators of deficient performance may include complaints of inadequate facilities, instructions, and protections afforded to the individuals subject to testing; questionable custody and control of biological specimens, paperwork, packaging, or quality control; and cleanliness. The length of time to conduct a test could also be a performance indicator since abnormally long collection times could indicate a lack of collector understanding of the collection procedure, inadequate facilities, a donor subversion attempt, or that the collection facility is too far away from the NRC-licensed facility. The licensee or other entity should evaluate any adverse impact of long collection times or long travel times on blood alcohol concentration levels at the time of initial and confirmatory testing.
Specimen Integrity and Valid Test Results
Three phases are associated with specimen integrity and valid test results. In Phase 1, the collector directly and continuously controls and monitors the donor throughout the collection process in accordance with a procedure; the donor follows the collector’s instructions; the CCF is properly filled out and legible; and the specimen collection containers are, if necessary, properly sealed, labeled, packaged, stored, and shipped to the HHS-certified laboratory. In Phase 2, the laboratory accurately accessions, tests, and evaluates test results and sends the results to the MRO staff or the collector accurately reads and documents the POCTA screening indication on a licensee- or other entity-generated CCF. In Phase 3, the MRO staff timely receives and reviews the laboratory test results; the MRO timely reviews positive, adulterated, substituted, invalid, or dilute test results and finds no documented laboratory discrepancies or discrepant biological markers; and the MRO has a timely discussion with the correct donor for the specimen being reviewed regarding the test result(s), if necessary. The qualitative performance outcome for this review should be that specimen integrity is maintained, and valid test results are obtained for all specimens.
Information Protection
The worker protection afforded by 10 CFR 26.611 is the effective control of information collected to comply with 10 CFR Part 26. The qualitative performance outcome of this review should be that all private and medical information is effectively controlled to prevent unauthorized disclosure. Should an information protection breach be identified, the documented elements of this performance review should include the following:
how the disclosure or breach occurred, for example, was it an electronic or hard-copy loss of information, a cyberattack, or a willful act by a licensee employee or C/V,
type and quantity of information involved, for example did it involve privacy, medical, or testing information,
number of individuals affected by the breach,
likelihood that individuals other than the licensee employees and C/Vs responsible for controlling the information had access or a probability of access to manipulate databases,
likelihood that the disclosure or breach harmed or could harm affected individual(s), for example, did it include information from which an individual’s identity could be stolen, which could cause financial harm, or medical information (such as drug and alcohol test results), which could cause emotional harm, and
corrective actions implemented to minimize harm and mitigate the impact of the disclosure or breach.
If the information breach involves employees or contractors of the NRC or other Federal agency, the licensee or other entity should consider promptly reporting the occurrence to the NRC Operations Center or resident inspector if assigned.
Appeals Process
The PMRP requires a biennial review of the licensee’s or other entity’s implementation of 10 CFR 26.613, “Appeals process.” This review must include an assessment of whether the licensee’s or other entity’s process is impartial and objective. An impartial review should not be biased by race, color, national origin, sex, gender, religion, age, employment, labor category, or length of service. An impartial review is established and maintained by the individual or individuals designated by the licensee or other entity to be the reviewing authority. An objective review should be based on verifiable and documented facts and not influenced by personal feelings or opinions.
A reviewing authority is the individual who hears the appeal and makes the final determination on whether the individual violated the FFD policy based on the validity of the information presented. To be impartial, the reviewing authority should not be assigned to the FFD program personnel group (see 10 CFR 26.31(b)), work in the same line of the organization as the individual who requested the review or be a close friend of the individual requesting the review. To ensure that the review is objective, the reviewing authority should understand the relevant requirements in 10 CFR Part 26 (some of which are listed after this paragraph below), the specimen collection technique and manufacturer’s device information, and the policy and procedures for the drug or alcohol test conducted. Additionally, the reviewing authority should use the requirements and guidance provided by the NRC or HHS, depending on whether 10 CFR Part 26 or the HHS Guidelines are being implemented through the licensee’s or other entity’s procedures. The reviewing authority may review guidance provided by the Drug Enforcement Administration, Food and Drug Administration, all institutes, centers, and offices of the National Institutes of Health, and organizations such as the American Association of Medical Review Officers, Medical Review Officer Certification Counsel, Drug and Alcohol Testing Industry Association, Society of Forensic Toxicologists, Society of Hair Testing, and the National Laboratory Certification Program. Because 10 CFR Part 26 is a Federal regulation, the reviewing authority’s conclusion and guidance used must not conflict with the requirements in 10 CFR Part 26, unless the guidance is verbatim from the HHS and pursuant to its guidelines as implemented by the licensee’s or other entity’s procedures. Below are some topics the reviewing authority should understand:
the licensee’s or other entity’s FFD policy and procedures, including those for custody and control of specimens, behavioral observation, disqualifying information, prohibited FFD item, drug and alcohol screening and testing, FFD policy violations and sanctions, and determinations of fitness;
acceptable conduct during drug and alcohol screening and testing;
characteristics of substance and fatigue impairment;
characteristics of not being trustworthy and reliable—the insider threat;
MRO evaluation of laboratory test results (e.g., 10 CFR 26.185, “Determining a fitness-for-duty policy violation,” or the equivalent section in the HHS Guidelines);
sanctions—licensee administered and NRC required;
granting and maintaining authorization or unescorted access; and
protecting privacy and unauthorized disclosure of sensitive information.
The PMRP review should examine whether the appeals process is initiated and completed in a timely manner and whether it allows sufficient time for the individual to respond to questions or present or submit relevant information within timeliness goals set by the licensee or other entity. Due process may not be served if the appeals process does not begin and end in a reasonable timeframe. The qualitative performance outcome for this review should be that each review conducted is impartial, objective, and completed on a timely basis by a knowledgeable reviewing authority.
Reviewing Laboratory Test Results and Medical Review Officer Performance—10 CFR 26.603(d)(1)(iv)(B))
For licensees and other entities that implement the FFD program described in 10 CFR 26.605, or if substance testing was part of an FFD program under 10 CFR 26.604, this review must include a documented assessment of laboratory test results reported under 10 CFR 26.169, “Reporting Results,” and the actions taken by the MRO under 10 CFR 26.185 or 10 CFR 26.619. The review is important for three reasons: (1) it protects the worker because FFD policy violations are based on MRO review of laboratory test results and a discussion with the donor, (2) it provides a performance-based assessment of both the laboratory and the MRO, and (3) it facilitates actions to improve laboratory performance through auditing or contracting, MRO training, or both, under 10 CFR 26.607(m). The qualitative performance outcome for this review should be that the MRO accurately reviews laboratory test results and determines confirmed positive test results, discrepant biological markers, or subversion attempts.
Based on operating experience, MRO errors are very infrequent. They typically occur during the review of a presumptive positive test result in which the MRO must evaluate the individual’s medications or indications of a possible subversion attempt. Consequently, this review should focus on a sampling of laboratory test results and MRO reviews that may involve the following:
Prescription medication. A prescription medication must be issued for a legitimate medical purpose by a practitioner acting in the usual course of their professional practice that was purported to cause the laboratory-determined positive drug test (see Ref. 34 and Ref. 35). A valid prescription for a Schedule II controlled substance should have been filled within 6 months or less, depending on State law. Prescriptions for other medications should typically be filled within a year. The Food and Drug Administration warns against using a prescription past its expiration date and provides information regarding off-label use of drugs.16
Over-the-counter medication. Over-the-counter medication, such as cough and cold medication containing codeine, has resulted in laboratory-determined positive drug testing results. In some states, medication containing codeine—a drug listed in the NRC’s panel of drugs to be tested—may be sold over the counter.
Amphetamine-based drugs. An MRO could request laboratory analysis of the d- and l- isomers of amphetamine to better inform the MRO decision as to illegal drug use.
Natural or synthetic cannabinoids. This review should include cannabidiol and any labeling or information purported to cause or contribute to a laboratory-determined positive drug test.
Operating experience has identified occurrences of MROs failing to fully evaluate subversion attempts. The NRC staff has posted guidance on the agency’s website, and the HHS has also posted MRO guidance that covers, in part, adulteration and substitution of biological specimens. Additionally, the American Association of Medical Review Officers and Medical Review Officer Certification Council have published guidance that can be used to inform MRO evaluation of subversion attempts. Although the NRC does not endorse these external sources of MRO guidance, use of this information can inform an MRO’s decisions as long as that decision does not conflict with the applicable requirements in 10 CFR 26.185 or 10 CFR 26.619, unless the guidance is verbatim from the HHS and pursuant to its guidelines as implemented by the licensee’s or other entity’s procedures. Below are nine examples that should be evaluated as a subversion attempt:
The donor reported to the drug or alcohol collection site later than the time period established by the licensee or other entity in its FFD procedure or did not report as soon as reasonably practicable.
The donor was argumentative, aggressive, or excessively nervous, resulting in a discrepant specimen collection.
The donor failed to follow FFD program personnel instructions or procedure requirements.
The donor feigned inability to produce a biological specimen for screening or testing.
The donor introduced material (liquid, solid, aerosol, etc.) into the mouth, into the collected oral fluid or urine specimen, or on the hair specimen before or during a collection, unless explicitly authorized by the collector, observer, or FFD procedure.
The donor refused to perform an additional collection (using either a screening device or collection container for a test) following a presumptive positive from a POCTA device; the donor was unable to produce an adequate volume of biological specimen for screening or testing; or there was a protected area portal screening instrument alarm or indication of a drug, drug metabolite, or alcohol detection above the instrument setpoint.
The donor’s urine temperature was outside the 90 to 100 degrees Fahrenheit temperature range established in the HHS Guidelines for urine and by the NRC in 10 CFR 26.111(a). Some licensees and other entities have used infrared technology to quantify urine temperature. The use of such instrumentation is considered a best practice if the instrument is properly operated and maintained (e.g., periodically calibrated and properly stored).
The collector or any other FFD program personnel identified subversion paraphernalia (i.e., a prohibited FFD item) whether used or not.
The MRO or collector identified mismatches or discrepancies in drug or drug metabolite concentrations, color, effervescence (i.e., bubbles), creatinine, pH, particulate, or clarity with (1) oral fluid oral fluid or urine specimens, including split specimens, (2) a collected specimen and a specimen collected under direct observation, or (3) a significant difference between a POCTA screening result and the HHS laboratory test result. A subversion attempt could also be determined by a single specimen collection if the specimen has invalid physical characteristics. Table 7 gives examples of invalid physical characteristics; this list is not inclusive, and the MRO should always evaluate questionable specimen characteristics.
Table 7. Examples of Specimen Physical Characteristics that Could Be Mismatched
Urine or Oral Fluid |
Hair |
Color |
Texture |
Effervescence |
Brittleness |
Precipitate |
Variable density |
Smell |
Smell |
Cloudiness |
— |
Specimen characteristics may change because of physiology, environment, or the donor’s action (e.g., excessive hydration or introduction of a chemical to subvert the drug test) or inaction (e.g., failure to wash hands or remove nonendogenous material from the mouth). To help prevent subversion attempts, the licensee or other entity should include guidance in its procedures on how a collector and MRO should evaluate specimen characteristics. This guidance may include a set of questions asking the donors if they take prescription or over-the-counter medications, went to their locker or car before the collection, had recently been exposed to chemicals that could adulterate a specimen (e.g., bleach), use cannabidiol or another analog of marijuana, etc. Answers to these questions may lead an MRO to determine that a subversion attempt occurred. For example, there are medications and liver and kidney conditions that can color a urine or oral fluid specimen; food products can add smell and color to urine or oral fluid specimens; and chemical straighteners, relaxers, texturizers, and coloring may affect the texture and brittleness of hair.
A subversion attempt review should compare the laboratory data reported to the data provided to the NRC using NRC-provided electronic reporting. The review should verify that the datasets correlate and that corrective actions are implemented to remedy the cause of any discrepant data from the laboratory, licensee, or other entity or the data reported to the NRC and, if applicable, shared with other licensees or entities.
This review must include a documented assessment of the changes the licensee or other entity made to its FFD policy, procedures, or processes. This review should help ensure that the aggregation of FFD program changes implemented over the life of the facility does not result in an undocumented decrease in FFD program effectiveness. Therefore, this review should include an evaluation of all changes to the FFD program since the biennial review was last performed and the aggregated impact of these changes on the following elements:
Maintaining an effective FFD program. This review should focus on the cumulative effect of changes made to behavioral observation, self-disclosure and suitable inquiry procedures, training, pre-access and random testing (if applicable), fitness determinations, fatigue management, and the FFD policy, sanctions, and appeals process.
Insider mitigation and access authorization programs (if applicable). This review should focus on whether the aggregation of FFD program changes adversely impacted a licensee’s or other entity’s ability to identify individuals who are not acting in a trustworthy and reliable manner. For example, the review should assess whether changes were made to behavioral observation training or procedures; the number of individuals on site and able to perform behavioral observation independently and objectively; the conduct of background investigations (criminal, employment, education, character, reputation, financial, etc.); instructions on consent and self-disclosure; conduct of a suitable inquiry; and determination and review of PDI. Such changes could adversely impact the effectiveness of the FFD, insider mitigation, and access authorization programs.
Quantitative performance measures or audit findings. This review should assess whether an FFD program change had the desired effect on a performance measure, FFD data, or FFD program element (e.g., collection, processing, evaluation).
NRC-licensed operators. The review should address whether FFD program changes or the aggregation of FFD program changes and licensed operator program changes reduced or has the potential to reduce assurance that NRC-licensed operators can safely and competently perform assigned duties and responsibilities and are trustworthy and reliable. This review should include assessing the licensed operator medical determinations made under 10 CFR 53.765, “Medical requirements,” against any 10 CFR Part 26 fitness determinations performed on the individual to determine whether all NRC requirements were implemented and whether the individual is in fact fit for duty.
NRC-required security officers. Similar to the review of NRC-licensed operators, this review should address whether FFD program changes or the aggregation of FFD program changes and changes to security officer medical and physical qualifications reduced or had the potential to reduce assurance that these individuals can safely and competently perform assigned duties and responsibilities and are trustworthy and reliable. This review should include correlating the security officer medical and physical determinations made in 10 CFR Part 73, Appendix B, “General Criteria for Security Personnel” (or other licensee training and qualification criteria) with any 10 CFR Part 26 fitness determination made for the security officer to determine whether all NRC requirements were implemented and whether the individual is in fact fit for duty.
This requirement states that “corrective actions shall be implemented to address when FFD performance meets a licensee-established performance threshold or to resolve a finding resulting from a qualitative review or audit in a manner that restores performance and corrects root and/or contributing causes.” Based on the concept of FFD margin as discussed in this guide, licensees or other entities may choose from a spectrum of actions to address a possible or actual FFD performance deficiency, from continual monitoring to obtaining additional FFD performance data to further the assessment of the potentially discrepant condition, including an evaluation to assess root and contributing causes.
The corrective action requirement states that the corrective actions should “[correct] root, contributing causes, or both.” The NRC staff acknowledges that a formal determination of root and contributing causes17 may be difficult and costly, and therefore may not result in an equivalent benefit to public health and safety. Consequently, these determinations could be generalized and qualitative, but it should contain enough detail to describe the apparent root or contributing cause, inform reviews of future occurrences, and avoid recurrences. The issuance of an NRC-required sanction is not a corrective action.
When FFD performance does not meet established thresholds or goals, timely corrective action should be implemented to correct the deficiency and preclude recurrence. The licensee or other entity would be required to document its corrective actions and should evaluate the effectiveness of its corrective actions under its PMRP. These actions could include, but are not limited to, the following:
changes to its FFD policy, procedures, or training;
changes to drug and alcohol testing;
posters or notifications describing the FFD policy or program or recent events;
additional qualitative reviews or audits;
employee assistance program and support groups;
communications with the staff regarding FFD program elements;
planned behavioral observation (e.g., peer review, supervisory oversight) of individuals performing or directing activities from a risk-informed determination that such activities are important to safety or security; and
outreach to other facilities or licensees to discuss FFD performance and operating experience.
PMRP Review Periodicity—10 CFR 26.603(d)(3)
Licensees and other entities are required to evaluate their performance measures and thresholds every 2 years and adjust them to maintain FFD program effectiveness based on site-specific performance, licensees’ or other entities’ fleet-level program performance, if applicable, and industry performance to identify areas for improvement. This review should be informed by results obtained from PMRP qualitative reviews and audits. The 2-year review frequency should enable 2 complete years of FFD performance data to be used in a biennial PMRP performance review. The 10 CFR 26.5 definition of “nominal” would not apply to this biennial periodicity because, under 10 CFR 26.603(d)(3)(ii), the PMRP review process must be completed by May 15 of every odd year.
The biennial review should consider the following questions and whether changes from the last review occurred or are expected in the next biennial review period:
Were there changes in FFD program personnel during the review period?
What were the names of the CNPs that were used for comparison analysis of site-specific FFD performance to inform the PMRP?
Has the licensee or other entity selected a different set of comparable sites than that used in the previous biennial review cycle and why?
What were the FFD program changes made this cycle and why?
What were the FFD program changes (i.e., corrective actions) from the last biennial period, and were they effective?
Is the documentary evidence sufficient to explain or justify a change to a performance measure, threshold, or program element?
What sample sets of FFD performance data were used to verify accurate accounting of FFD performance data within the performance measures?
What qualitative considerations may have affected the previous year and possibly the next PMRP cycle?
Were the corrective actions identified during PMRP qualitative reviews or audits effective?
Documentation of the biennial review should be sufficient for an independent reviewer to assess whether the PMRP was comprehensive enough to ensure that the FFD program remains effective. The licensee or other entity would need to complete and approve the program review, with PMRP program revisions (i.e., corrective actions) implemented before May 15 of the odd year in which the program review was conducted. This would help align the program review with the NRC data collection requirement in 10 CFR 26.617(b)(2) or 10 CFR 26.717, as applicable. Figure 9 illustrates this scheduling.
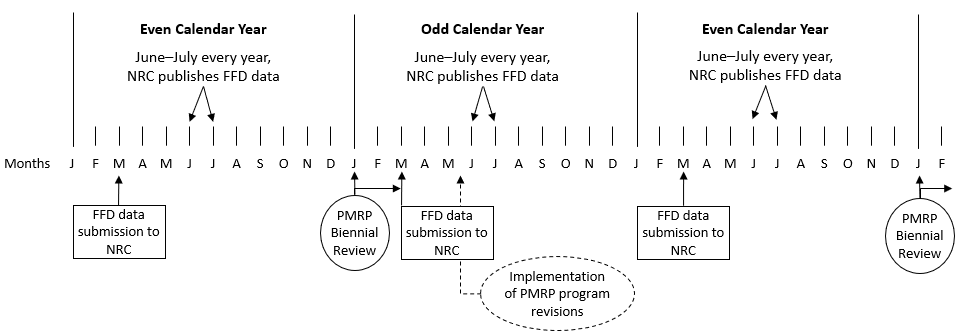
Figure 9. PMRP Biennial Review Schedule—10 CFR 26.603(d)(3)
FFD Reporting Schedule
Every year, licensees and other entities collect FFD program performance data for January 1 through December 31.
Before March 1 of every year, licensees and other entities submit FFD data to the NRC for the previous calendar year (10 CFR 26.617(b)(2) and 10 CFR 26.717(e)).
Before May 15 of every odd year, the licensee or other entity must implement approved changes to its PMRP (10 CFR 26.603(d)(3)(ii)).
The NRC works to publish the previous year’s FFD performance information in the June–July timeframe every year. In some cases, the NRC must call the data “draft” or “preliminary” because the NRC data quality checks may have identified discrepancies requiring licensee or other entity resubmission of data or further NRC assessment. However, based on operating experience, NRC‑maintained FFD datasets with a “draft” designation have differed only slightly from complete and accurate datasets, thereby enabling their use by a licensee or other entity in their PMRP.
The objective of the FFD change control requirement is to give licensees and other entities the ability to timely revise their FFD program and address program weaknesses. Changes to the program may become necessary as alterations occur in societal or workforce drug abuse, laboratory testing, collection technologies, the panel of drugs and drug metabolites to be tested and their cutoffs, and biological markers.
The need to establish change control is based on the 10 CFR Part 26, Subpart M, framework that implements objective- and performance-based requirements that enable certain flexibilities in program implementation. For example, a licensee or other entity may decide to use oral fluid, urine, or both in its drug screening and testing program but may decide to change the type of specimens it uses. A licensee or other entity may establish its own biological collection facility instead of using the collection facility it had been using. The licensee or other entity may elect to switch to one or more HHS Guidelines from the requirements in 10 CFR Part 26. Maintaining an accurate record of changes to its program and the justifications for these changes will help ensure that the FFD program remains effective and inform the PMRP. The detail and scope of a documented justification should be consistent with the significance of the change being made.
The following are examples of changes that would probably not result in a reduction in FFD program effectiveness. Although the licensee and other entity would still need to perform and retain an analysis demonstrating that its change did not reduce FFD program effectiveness, these examples are provided as a guide to assist a licensee or other entity in determining whether a change would require a mitigating strategy.
changes to the HHS Guidelines or HHS-certified laboratory testing processes or procedures (excluding a reduction in the panel of drugs and drug metabolites to be tested, besides phencyclidine or targeted drug metabolite for a particular drug) if a licensee or other entity commits to the use of a guideline or guidelines as described in Subpart M;
changes to a manufacture’s POCTA device that was reviewed by the licensee’s or other entity’s forensic toxicologist who found that the change(s) did not reduce device effectiveness;
alternating between drug testing using oral fluid and urine for the biological test specimen. The licensee or other entity should apply the same drug testing protocol to all individuals subject to the FFD program and not subject one group of individuals to urine testing and another to oral fluid testing.
the use of a different collection facility, HHS-certified laboratory, or MRO;
a change in the frequency of audits or training if the change was based on performance;
a change to the licensee’s or other entity’s credited technical analysis used to justify meeting the criterion in 10 CFR 26.603(c), as long as the facility and its operation continue to meet the criterion;
use of a hair specimen for pre-access screening before the granting of FFD authorization (i.e., unescorted access to the NRC-licensed facility, SNM, or sensitive information);
a manufacturer or National Highway Traffic Safety Administration change in the use, calibration, or maintenance of evidentiary breath testing devices or the manufacturer’s written procedure;
changes to protected area portal monitor screening instrumentation, including passive detection or POCTA devices, that detect drugs or alcohol or help prevent the introduction of prohibited FFD items into the protected area as long as the required forensic toxicologist review was conducted; and
establishment of procedure instructions for the collection of alternative biological specimens as ordered by the MRO for case-specific situations.
The following are examples of changes that may reduce FFD program effectiveness and must include a mitigating strategy to maintain FFD program effectiveness pursuant to 10 CFR 26.603(e)(2):
use of a POCTA for urine or oral fluid when the licensee’s or other entity’s forensic toxicologist cannot make a finding that the POCTA device panel of drugs, drug metabolites, cutoffs, adulterants, biological markers, and accuracy or precision, if applicable, are comparable to those established by the HHS or the NRC for drug or alcohol screening or testing;
collecting a urine specimen for testing at an HHS-certified laboratory after the individual screened positive on a POCTA using oral fluid or a portal area screening instrument that samples and evaluates sweat, exhaled breath, or iris physiology;
changes in the worker protections (e.g., changes to appeals or due process, MRO reviews and discussions with the donor, or the collection process that reduce privacy);
a statistically significant change to onsite staffing that could adversely impact behavioral observation or supervisory oversight; and
a change in an established threshold that would prevent FFD performance from meeting the threshold without an adequately justified technical basis based on FFD performance data.
The licensee or other entity should describe the following milestones in its procedures: (1) “before the loading of fuel onsite into a reactor vessel,” (2) “before receiving a manufactured reactor,” and (3) “before individuals subject to part 26 operate, test, perform maintenance of, or direct the maintenance or surveillance of safety- or security‑related SSCs or SSCs that a risk-informed evaluation process or alternative method for evaluating safety significance has shown to be significant to public health and safety.” Because of the variety of reactor and system designs, these phrases could have a different meaning at different sites. The following discussions show how the milestones could be based on the potential for radiological consequences.
(1) “before the loading of fuel onsite into a reactor vessel”—This phrase establishes a point in time during the transition from construction to operation, beyond which CNP activities could potentially result in adverse radiological consequences to people or the environment. For example, the phrase could mean (a) before placing the unirradiated fuel (e.g., a metallic fuel assembly, liquid fuel, or pelletized fuel) into an SSC, such as a storage vault, tank, rack, or transfer system that is used to load fuel into the reactor vessel or (b) before initiating a mechanical process that inserts fuel into a reactor vessel or core basket at either the 10 CFR Part 53 manufacturing licensee facility or CNP.
(2) “before receiving a manufactured reactor”—This milestone is intended to correspond to the point in time when the manufactured reactor enters the protected area and is placed in a position or location that enables its integration into the rest of the facility or the SSCs that contribute to nuclear criticality. This milestone is not intended to correspond to receipt of a manufactured reactor into a facility such as a warehouse for temporary storage of the unit.
(3) “before individuals subject to part 26 operate, test, perform maintenance of, or direct the maintenance or surveillance of safety- or security-related SSCs or SSCs that a risk-informed evaluation process or other alternative method for evaluating safety significance has shown to be significant to public health and safety”—The section of this RG addressing guidance for 10 CFR 26.4 provided guidance for this statement.
The FFD policy statement must be written in sufficient detail to provide affected individuals with information on what is expected of them and what consequences may result from a lack of adherence to the policy. Each licensee or other entity should clearly and concisely write its FFD policy to facilitate understanding by all individuals subject to the FFD program. Licensees and other entities are encouraged to communicate with one another and the licensees in the LLWR fleet to develop an FFD policy.
The FFD policy statement must be provided to all individuals who are subject to the program before they are subject to behavioral observation, drug and alcohol testing, or both. Licensees and other entities should establish a process and record to ensure that all individuals are informed of the policy, either through training, read-and-sign, or other means, and may consider obtaining the individual’s consent as well (see 10 CFR 26.611, “Protection of information”).
The written policy must address the FFD performance objectives of 10 CFR 26.23. The licensee or other entity should stress that the policy applies not only when an individual is on site and performing or directing those activities making them subject to 10 CFR Part 26, but also off site. For example, individuals should be aware that their actions will be evaluated as an FFD concern if identified by an individual subject to the FFD program and communicated to representatives designated by a licensee or other entity. FFD concerns would involve engaging in prohibited offsite activities, such as the sale, possession, or use of illegal drugs or acting in a manner that shows that the individual may not be trusted or relied on to be given unescorted access to the NRC-licensed facility, SNM, or sensitive information. Operation of a vehicle while under the influence of an illegal substance, illegal drug or illicit substance, whether convicted or not, or failure to follow laws established by local, State, and Federal governments also indicates FFD concerns and should be used as examples of potential FFD policy violations within the written FFD policy.
The FFD policy should describe how an individual’s effectiveness in implementing the FFD policy and procedures will be measured in the PMRP through various performance measures like those associated with behavioral observation, possession of prohibited FFD items or PDI, and unauthorized access to protected information. Individuals should be held accountable for their performance, and sanctions must be issued in the case of an FFD policy violation. Although a sanction is not meant to be punitive (except in the case of a subversion attempt), the occurrence of an FFD policy violation could indicate a potential weakness in FFD program implementation, communication, or training.
The policy should be written in sufficient detail to address the following topics:
The use, sale, or possession of illegal drugs on or off site, regardless of whether such conduct resulted in a conviction (see the definition of legal action in 10 CFR 26.5). The policy should be clear as to what it means to be “on site.” For example, “on site” could mean the area owned by the licensee or other entity upon which the NRC-licensed facility is sited, as well as any licensee- or other entity facility located away from the site, from which an individual may operate or direct the operation of SSCs required for safety or security.
The use, sale, or possession of alcohol while on site or in a duty status. The licensee or other entity should define when an individual is in a “duty status.” This definition should include any period of time in which the individual is on shift working for the licensee or other entity and maintaining unescorted access to the facility or performing a duty or responsibility making them subject to the FFD program, whether on site or off site. This should include remote facilities where safety-significant systems or components may be operated within the design basis of a licensed CNP or monitored by the licensees and other entities in 10 CFR 26.3(f) or emergency operations may be directed.
The consumption of alcohol within 5 hours of performing or directing the performance of work making the individual subject to the FFD program. Individuals should be informed that alcohol metabolism is a function of many variables and that 5 hours may not be sufficient to reduce in situ alcohol concentrations below the cutoffs in 10 CFR 26.101, “Conducting a confirmatory test for alcohol,” and 10 CFR 26.103, “Determining a confirmed positive test result for alcohol.” Regardless of when alcohol or any other substance was consumed, inhaled, or injected, a confirmed positive test result will result in an FFD policy violation and sanction in accordance with the FFD policy and procedures.
An individual’s failure to notify the licensee or other entity of any legal action on or off site involving drugs or alcohol. The failure to notify the licensee or other entity is an indication that the individual might not be trustworthy and reliable. Also, the licensee or other entity should establish in its procedures how quickly an individual should make such a notification (e.g., within 2 working days, next shift, as soon as reasonably practicable) and how the information should be communicated to a designated individual (email, written letter, phone call, or verbally in person).
An individual’s use of another individual’s prescription medication, whether on or off site, whether or not the individual was determined to be impaired by observation by a trained individual under 10 CFR Part 26 or a presumptive positive drug or alcohol screen or test.
The consumption, inhalation, injection, or application of any product that may cause impairment (e.g., the product contains a specific warning not to operate machinery or drive vehicles, or the product is not approved for human consumption, inhalation, injection, or application).
The failure to report to the licensee or other entity’s designated representative an FFD concern of another individual, whether on or off site, or retaliation against another individual who reported an FFD concern.
The possession or use of a material, chemical, or paraphernalia to subvert or attempt to subvert a drug or alcohol screen or test, or acting in a manner that prevented (e.g., failure to follow instructions) or significantly obstructing the collection or processing of a biological specimen for a drug or alcohol screen or test.
An individual who acted (verbally or physically) in a manner that threatened, harmed, or could harm individuals or SSCs within the NRC-licensed facility or offsite infrastructure supporting or required for the safe and secure operation of the facility.
An individual who acted (verbally or physically) in a manner that threatened or resulted in the theft of NRC-licensed material or unauthorized disclosure of sensitive information.
The policy should state that an individual may be subject to an FFD policy violation and a review of their FFD or access authorization if the individual—
committed a criminal activity, regardless of whether it resulted in a conviction, that would support a conclusion that the individual is not trustworthy and reliable;
cheated on a licensee-administered training exam test, falsified records, or purposely gained unauthorized access to a protected area, SNM, or sensitive information;
purposely failed to properly perform a licensee- or other entity-assigned duty, responsibility, or procedural requirement, including not informing the licensee or other entity of FFD concerns or that their failure to report to work was due to impairment caused by substance abuse;
advocated or engaged in any acts of terrorism or activities designed to overthrow the U.S. Government;
is a member of an organization dedicated to terrorism, either with an awareness of the organization’s dedication to that end, or with the specific intent to further such activities;
is a member of an organization dedicated to the use of violence or force to overthrow the U.S. Government; or
is a member of an organization that advocates or practices acts of force or violence to discourage others from exercising their rights under the U.S. Constitution or any State of the United States with the specific intent to further such action.
The policy should state that the NRC-required sanctions are the minimum sanctions to be applied and that the licensee or other entity may increase the severity of the sanction, up to and including permanent denial of authorization, based on the severity, circumstances, and number of FFD policy violations.
The policy should describe the requirement that individuals, who are notified that they have been selected for random, for-cause, or post-event testing, must report to the collection site within the time period specified by the licensee or other entity, including the regulatory requirement to report as soon as reasonably practicable which could occur before the licensee- or other entity-established time period to report.
The policy should describe the actions that constitute a refusal to provide a specimen for screening or testing, and state that the consequence of refusal is permanent denial of authorization. This explanation should include information that the FFD policy violation will be communicated to and used by other NRC licensees and other entities subject to 10 CFR Part 26 and 10 CFR Part 73.
The policy should describe the individual’s responsibility to report legal actions as defined in 10 CFR 26.5.
The policy should describe the responsibilities of all individuals to report FFD concerns and arrive at work unimpaired from any substance, fatigue, or physiological or psychological condition. This should include a statement that personal issues (financial, marital, family medical, etc.) may adversely impact an individual’s fitness.
The policy should describe the circumstances that constitute a human error and an event (both under 10 CFR 26.607(b)(4)) and the examples of observed behavior, physical condition, or credible information (see 10 CFR 26.607(b)(3)) that will result in a post-event or for‑cause drug or alcohol test, or both, or removal from the performance or directing of those duties and responsibilities or access making him or her subject to the rule if drug test, alcohol testing, or both is not performed.
The policy should describe the required medical or clinical treatment and follow-up testing for FFD policy violations and state that the individual will be held accountable for the successful completion of the treatment and testing.
The policy should describe the FFD violation appeals process and state that an individual’s FFD policy violation (omitting private information) will be shared with other licensees and other entities to be used in their FFD authorization (10 CFR Part 26, Subpart C) and access authorization (10 CFR 73.56 and 10 CFR 73.120) programs.
Licensees and other entities are required to develop, implement, and maintain written procedures that address the requirements in 10 CFR 26.606(b). The procedures should also detail the following, if applicable:
The development, approval, implementation, and revision of the FFD policy and procedures. This would support FFD change control.
The applicable 10 CFR Part 26 requirements that will be implemented by the licensee or other entity that are not described in Subpart M.
The applicable sections of the HHS Guidelines that will be implemented by the licensee or other entity, which cannot contradict the requirements in Subpart M.
The process that maintains or updates the analysis under 10 CFR 26.603(c).
The process for identifying those individuals who must be subject to the FFD program. This process should include the risk-informed determination evaluation process used to assess whether the roles and responsibilities of individuals make them subject to the FFD program.
The process of transitioning an FFD program for construction under 10 CFR 26.605(a) to an FFD program for CNP operation under 10 CFR 26.605(b).
The physiological and psychological (i.e., emotional) indicators that individuals should understand to be able to adequately ascertain whether someone is fit for duty, trustworthy, and reliable.
The potential adverse consequences that could result from small group dynamics or the siting of a 10 CFR Part 26 facility in a geographically remote location.
The process and elements of the BOP, including the individual’s responsibilities, and the purpose and implementation of behavioral observation conducted by electronic audible and visual surveillance by security personnel and any remote monitoring station.
The FFD program organization including the roles and responsibilities of FFD program personnel, including the FFD manager or supervisor, FFD program staff, MRO, and MRO staff.
The identification, communication, and management of individuals who are subject to drug and alcohol testing, whether on or off site, to ensure that they are timely tested under any test condition.
The establishment of measures to help prevent subversion of the drug and alcohol testing at all collection facilities.
The techniques to be used in collecting, testing, shipping, and temporary storage of biological specimens for drug and alcohol testing, including procedures for protecting the privacy of an individual who provides a specimen, for protecting the integrity of the specimen, and for ensuring that the test results are valid and attributed to the correct individual.
Operation and oversight of an onsite or offsite specimen collection facility.
How FFD procedures are made available for inspection pursuant to 10 CFR Part 26, Subpart O.
The process to be followed when individuals make a self-declaration that they are not fit to safely and competently perform their duties for any part of a working tour as a result of fatigue or substance use. The procedure should—
Describe the process to be followed when an individual makes a self- declaration.
Describe requirements for establishing controls and conditions under which individuals may be permitted or required to perform work after they declare that they are not fit because of fatigue.
Describe the process to be followed if the individual disagrees with the results of a fatigue assessment required under 10 CFR 26.211(a)(2).
The process for implementing the controls required under 10 CFR 26.205 for the individuals who are performing the duties listed in 10 CFR 26.4(a).
The process to be followed in conducting the supervisory reviews and fatigue assessments under 10 CFR Part 26, Subpart I.
The disciplinary actions that the licensee may impose on an individual following a fatigue assessment and the conditions and considerations for taking those disciplinary actions.
Subpart M of 10 CFR Part 26 gives licensees and other entities several flexibilities in implementing the drug and alcohol screening and testing requirements. These flexibilities are to help maintain FFD program effectiveness through the use of innovative technologies.
Option—Hair Screening Test. The properties of hair specimen testing make it possible to determine an individual’s illegal or illicit drug use weeks or months in the past. With such increased visibility into a licensee employee’s or C/V’s past behavior, the licensee or other entity can make a more informed decision as to the individual’s trustworthiness and reliability. Further, if a licensee employee or C/V knows their past drug use can be detected and evaluated with a hair test, the screening test would contribute to deterrence, and possibly the individual would elect not to request authorization to the NRC-licensed facility.
Periodic screening of hair for controlled substances could significantly enhance the effectiveness of an FFD program by complementing the authorization process. Under the licensee’s or other entity’s own authority for pre-access screening or if an exemption is submitted and approved by the NRC for random or follow-up testing, hair screening or testing could potentially enhance the effectiveness of the FFD program.
· Option—Innovative Technologies. A licensee or other entity may want to implement innovative drug and alcohol screening technologies at its protected area (such as passive drug and alcohol detection and analysis instrumentation) and in the random testing program using a POCTA screening device. Such devices may be able to determine the true identity of the individual, maintain custody and control of the screening results, and electronically notify management of a positive screening result. These systems could be used to screen 100 percent of all individuals entering and leaving the NRC‑licensed facility, thereby substantially improving FFD program effectiveness.
· Option—Drug and Alcohol Testing Consortium. The 10 CFR Part 26 framework does not prevent licensees and other entities from establishing a consortium to implement a single FFD program at multiple NRC-licensed facilities operated by different licensees or other entities. A consortium approach could allow licensees and other entities to aggregate enough individuals subject to testing to enable the implementation of a random testing program that is not challenged by a small staff size and possibly result in other program efficiencies, such as the random testing of FFD program personnel.
As discussed below for 10 CFR 26.607(b)(2), if the number of individuals subject to the FFD program becomes much smaller than about 100 individuals, licensees and other entities may begin experiencing challenges in implementing an effective random testing program. For example, the actual testing rate may be fewer than once per week, and it may be easy to count how many individuals were subject to a random test and therefore how many tests might be left to conduct
Consortium testing programs are established in the commercial transportation and maritime industries subject to DOT drug and alcohol testing. This could represent a programmatic opportunity to leverage other federally mandated drug and alcohol testing programs if a 10 CFR Part 53 CNP is sited in a geographically remote location. For example, collection site resources, MROs, and medical and clinical professionals and facilities, including employee assistance programs, could be shared among multiple licensees and other entities.
· Option—Increased Emphasis on Behavioral Observation. Such a program could be modeled from 10 CFR 26.406, “Fitness monitoring.” As of the publication of this guide, there has been no operating experience with the development and implementation of this type of program; however, with the emphasis of 10 CFR Part 26, Subpart M, on behavioral observation, implementation of fitness monitoring could be an option if the licensee or other entity receives NRC approval for an exemption to enable the use of 10 CFR 26.406.
· Option—Increasing Sanctions for an FFD Policy Violation. The sanctions listed in 10 CFR Part 26 are the minimum sanctions required. Licensees and other entities may increase the severity of a sanction above that of the NRC sanction. As described in the guidance for 10 CFR 26.610, sanctions should be risk informed. This means that if an individual is found to be in violation of the FFD policy and was performing or directing work that a risk-informed evaluation process has shown to be significant to public health and safety or the common defense and security, the consequences of this individual being impaired would justify a more severe sanction. Such a sanction would facilitate a more aggressive or comprehensive treatment plan (i.e., return‑to‑duty plan) should the licensee or other entity choose to retain the individual as an employee or C/V.
Individuals who seek unescorted access to the protected area of an NRC-licensed facility or will perform or direct individuals who perform the kinds of activities making them subject to the FFD program, must be subject to a pre-access drug and alcohol test no more than 14 days before being granted unescorted access. If the licensee or other entity is implementing an FFD program under 10 CFR 26.604, drug and alcohol testing is not required. The purpose of this requirement is to ensure that the individual is fit for duty, trustworthy, and reliable as confirmed by a negative drug and alcohol test result and completion of other authorization requirements before being granted authorization. The specimen used for a pre-access drug test must be oral fluid or urine under 10 CFR 26.607(b)(1) and be analyzed at an HHS-certified laboratory under 10 CFR 26.607(c)(4).
Based on FFD operating experience, most individuals who attempt to subvert the testing process are identified during a pre-access test, likely because this testing event is known, and individuals have time to prepare to subvert the test. Individuals subject to random, for-cause, post-event, and follow-up testing should have less of an opportunity to obtain subversion paraphernalia before reporting for testing; however, again, FFD performance data demonstrate that, even during these test conditions, individuals have attempted to subvert the testing process. The diligence of the FFD program personnel, especially the collector, is most important during any test condition. To help prevent a subversion attempt, licensees and other entities may take the following actions:
Conduct the pre-access test using an oral fluid or urine specimen. Based on operating experience, the use of oral fluid may be preferred because it is more difficult to subvert the test since the collector directly observes and collects an oral fluid specimen. A POCTA device may not be used for a pre-access drug or alcohol test but may be utilized to periodically screen individuals as they wait to be granted authorization for unescorted access to the facility.
Do not inform the individual when the pre-access test will be conducted, or which biological specimen will be collected. This will make it more difficult for the individual to plan a subversion attempt.
Conduct more than one pre-access drug and alcohol screen or test under the licensee’s or other entity’s sole discretion. This provides additional information on an individual’s possible use of illegal substances, illegal drugs, and illicit substances and trustworthiness and reliability.
Schedule the pre-access test to ensure that the HHS laboratory test results are received and reviewed by the MRO before the individual’s scheduled work but not so early that the test will not provide a good indication of whether the individual is unimpaired to commence work. Typically, licensees and other entities receive a negative test result from an HHS‑certified laboratory within 2 to 3 days and a positive test result within 6 days after laboratory receipt of a urine or oral fluid collection kit.
Use a hair specimen for pre-access screening. The use of hair as the biological matrix for testing for Scheduled I and II drugs and drug metabolites could be a significant deterrent and may identify PDI for the individual seeking authorization (see guidance for 10 CFR 26.607(i)).
Use innovative technologies to conduct pre-access screening at the protected area entry point. Literature indicates that technologies that use passive detection techniques can detect or measure drugs and alcohol from sweat (palm readers), breath exhalation, and iris scans for the assessment of certain drugs in vivo that may cause impairment. For example, upon alarm of a protected area screening instrument (see 10 CFR 26.607(j)), the licensee could administer a POCTA collection using oral fluid to further investigate the cause of the portal area alarm. An individual who screens positive on the POCTA device must be subject to a urine or oral fluid collection that is sent to an HHS-certified laboratory (a for-cause test). This second specimen collection should be an oral fluid specimen.
All individuals must report to the collection facility as soon as reasonably practicable after notification within the time period specified in the FFD program procedure. This means that an individual should not have an opportunity to delay their arrival for the random test; such overt actions should be considered a subversion attempt. All individuals should be informed that the necessity to report for testing as soon as reasonably practicable takes precedence over the need to report within the time period established by procedure.
Random screening and testing must be administered in a manner that provides reasonable assurance that individuals are unable to predict the time periods in which specimens will be collected under 10 CFR 26.607(b)(2)(i). To help meet this requirement, licensees and other entities should include the following guidance in their procedures:
An onsite collection facility should give the appearance that it is open for business, for example by keeping the lights on.
The licensee or other entity should take reasonable steps to conceal from the workforce when collections will be performed. For example, if individuals who collect specimens only arrive onsite to collect specimens (i.e., the NRC-licensed facility is not their normal work location), then the collection process should attempt to prevent consistency in collector arrival times so that individuals are unable to predict the time periods when random testing will be conducted.
Random testing should be randomly conducted on all shifts and days, including holidays and weekends. There should be no safe time or day.
The testing process should ensure that individuals cannot predict when they will be tested next based on tests conducted previously. The periodicity of random testing should be variable. For example, testing should be conducted three time per week with the testing days changing every week, instead of testing every Monday, Wednesday, and Saturday.
Licensees and other entities are required to establish a time period within which an individual must report to the collection facility. For ease of program implementation and understanding by individuals subject to the FFD program, a single sitewide time period should be established. However, this time period should be based on the time and distance an individual could travel to get to the collection facility; the time it takes an individual to be replaced by another individual, if necessary (e.g., a security officer or a control room operator); and the time to secure an individual who is involved with an in-progress work activity, if necessary. The time period should not be long enough to give an individual time to obtain materials designed to subvert a drug or alcohol test. For example, the individual should not have time to go to their personal locker or vehicle before the test.
Reasonable accommodations may be afforded if the individual has a known mobility disability or if the individual’s supervisor determines that the selected individual is performing safety- or security‑related work or other duties in which relief is not reasonably practicable or safe (i.e., the immediate disruption of such work would have a high likelihood of causing an unsafe or unsecure work environment). In such a situation, the supervisor should notify the FFD program personnel of this determination, including the time and reason for this decision, and inform the individual of their selection for a random test only after the individual can leave the worksite (e.g., during a break, shift change, stop in work, or completion of the work activity). Then the individual must submit to the random test as soon as reasonably practical. To reduce the opportunity for a subversion attempt, the selected individual should not be notified of a random test until able to report to testing.
The procedure should describe how the random screening or testing notification should be made. Based on operating experience, the NRC staff has observed that FFD program personnel will inform the individual’s supervisor, and the supervisor will assess the availability of the individual for testing and report back to the FFD program personnel. The FFD program personnel and the supervisor will then agree on the notification time, and the supervisor will notify the individual of their selection for a random test when the notification time occurs. Experience has demonstrated that supervisors should be informed to make the reasonable accommodation determination and should be held accountable for ensuring that the individuals in their work group are available for screening or testing.
The procedure should state that the individual should not excessively hydrate or eat immediately before an oral fluid or urine collection. This restriction should be implemented because excessive hydration could dilute the urine specimen and eating or placing an endogenous substance in one’s mouth immediately before an oral fluid collection could be an attempt to subvert the testing process. Similar to the pre-access process, FFD program personnel should not announce whether an oral fluid or urine screening or test will occur during the random testing process.
The population of individuals subject to random testing includes all persons subject to 10 CFR Part 26 as described in Subpart M. If an individual is not subject to random testing, the individual will not be permitted to perform or direct the performance of any activity (as described in 10 CFR 26.4,) or have the types of access that make that individual subject to Subpart M.
If an individual is selected for random testing and is not available to be tested (e.g., the individual is on vacation), the individual should be tested as soon as reasonably practicable upon returning to work. If the individual is off site and was selected for two or more random tests during the same unavailability period, the individual need only be subject to one unannounced random test as soon as reasonably practicable upon returning to work. The metric for “as soon as reasonably practicable upon returning to work” should be the first shift in which the individual returns to work.
If the individual is assigned to perform or direct activities making them subject to the FFD program at a site located significantly far from a collection facility or assigned specimen collectors, the licensee or other entity is still required to test this individual as soon as reasonably practicable. For this situation, the licensee or other entity should collect a urine or oral fluid specimen and test the specimen at an HHS‑certified laboratory. This process is recommended because the use of a POCTA device at a remote clinic or hospital would not provide assurance of testing validity and accuracy.
The licensee or other entity should ensure that the random testing pool is updated to account for fluctuations in site workforce population, such as C/Vs on site for a short duration. Therefore, the licensee or other entity should track all individuals through use of access controls, work schedules, or other means to ensure that individuals subject to the random testing process are notified to report for screening or testing and that the individual’s true identity is verified immediately before the specimen collection. The determination of true identity for the purposes of 10 CFR Part 26 should be as defined by the site security plan or as required by the access authorization requirements in the applicable portion of 10 CFR 73.56 or 10 CFR 73.120. For example, true identity may be determined by a nonexpired, unique identification badge issued by either a Federal or State authority or the licensee or other entity that has the individual’s photo and name or a fingerprint or iris scan meeting quality assurance requirements established by the licensee or other entity.
Licensees and other entities are encouraged to use commercially available random testing software to develop the list of individuals subject to random screening or testing. Operating experience with the use of this software has identified problems with some software packages, so licensees and other entities should discuss this with other NRC licensees and entities subject to 10 CFR Part 26.
The random testing requirement in 10 CFR 26.607(b)(2)(iv) states that “an individual completing a test is immediately eligible for another random test.” The phrase “completing a test” is not defined or described in 10 CFR Part 26. For an FFD program implemented under 10 CFR 26.605, defining this phrase in the licensee’s or other entity’s procedures would be important because of the variety of testing methods a licensee or other entity may use.
If a POCTA device is used for random screening, the individual must be immediately available for another random selection if the immunoassay indication is negative for all drugs, drug metabolites, and adulterants, and positive for biomarkers if applicable. If the POCTA device shows a positive indication for any drugs, drug metabolites, and adulterants or a negative indication for a biomarker if applicable, or both, then the individual should be removed from all duties, responsibilities, and access that make him or her subject to the FFD program and immediately retested using an oral fluid or urine collection device that can be analyzed at an HHS-certified laboratory.
If an individual was subject to an oral fluid or urine collection using a device to be sent to an HHS‑certified laboratory for random testing (meaning that a screening test was not conducted), this individual must be placed back in the random testing pool immediately after the collection only if the individual shows no signs of impairment. If the individual shows signs of impairment, then the individual must be removed from all duties, responsibilities, and access that make them subject to the FFD program until the individual is evaluated by the MRO.
The NRC created two different calculational methods (Figures 12 and 13) in Microsoft® Excel® for illustrative purposes to help inform licensee- or other entity-developed procedures that determine how many individuals must be selected per week to achieve an annualized random testing rate greater than or equal to 50 percent for all populations of individuals to be tested. The methods were designed so that licensees or other entities can enter their own custom values for the workforce size and the duration that the licensee employees and C/Vs will be on site. The methods also find the probability that an individual worker will be tested, which is an informational metric (not a regulatory requirement) that can be used to deter substance abuse by demonstrating there is a high chance each worker will be tested. Figures 10 and 11 below provide an example showing how the math works in the methods illustrated in Figures 12 and 13.

Figure 10. Tests to Achieve the 50 Percent Annual Random Testing Rate

Figure 11. Probability that an Individual will be Selected for Testing
Using the first random testing method, the licensee or other entity would use the green table in Figure 12 to look up its workforce statistics (i.e., number of workers, duration on site, and if a licensee employee or C/V) and then use the tan table to find the recommended number of weekly random tests it should give to reach the 50 percent annual random testing goal. It could also review in the blue table the associated probability that an individual worker will be sampled, which is again an informational metric to help deter substance abuse.
For example, a licensee with a C/V population of 50 to 150 workers who will be on site for more than 60 days would use cell D9 (column D, row 9), as shown in the box outlined in red within the green shaded area. In the box outlined in red in the tan table, the licensee would then look up the number of weekly tests that it should conduct to reach the 50 percent annual testing requirement. The blue table shows the probability that an individual worker will be tested, which is for informational purposes and does not have regulatory limits.
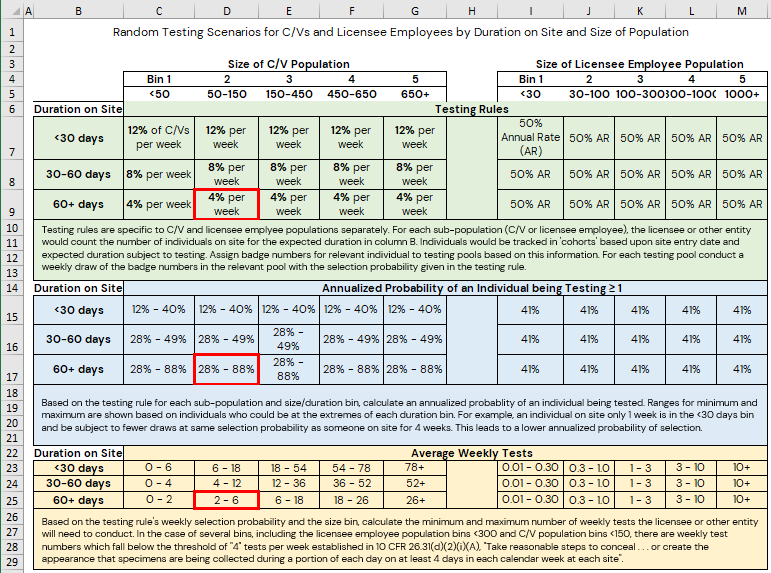
Figure 12. The First Calculational Method for Random Sampling
The second random test method uses formulas for custom inputs (Figure 13). The licensee or other entity would enter their workforce statistics in the green table and then review the testing results, which will automatically update in the pink table. This second method may be downloaded from NRC ADAMS located at https://adams.nrc.gov/wba/ by performing a “Document Title” search for “Random Testing calculational Methods for Reg Guide 5.94.”
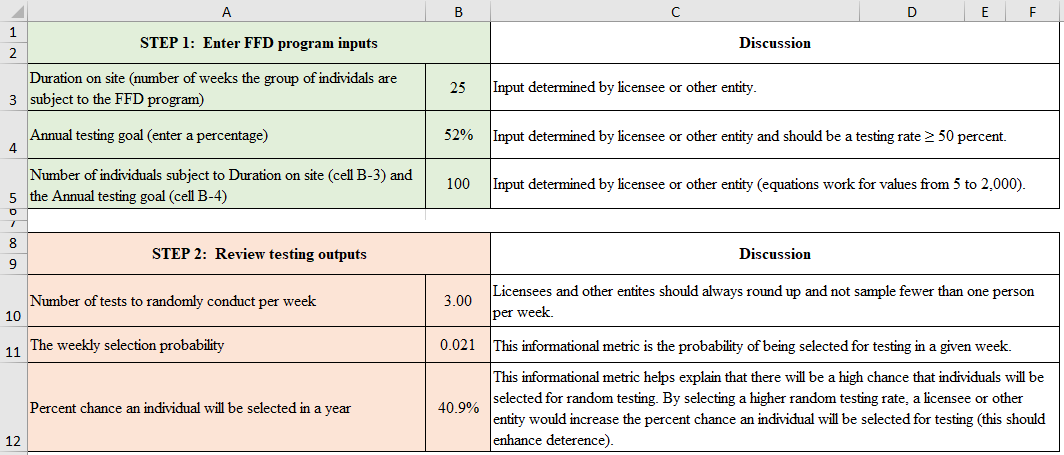
Figure 13. The Second Calculational Method for Random Sampling
Both methods make it easier for licensees and other entities to determine the number of random weekly screenings or tests they should conduct to hit the 50 percent annual random testing requirement. The tables also provide information on the probability that individuals at work will be tested.
One advantage of the first method is that it contains a printed copy for implementation, whereas the second method requires the use of an active Excel file. An advantage of the second method is that it precisely estimates the exact workforce numbers, whereas the first method uses a binning methodology. This gives licensees and other entities flexibility in program implementation.
When the number of weekly tests is not a whole number, licensees and other entities should round up. For example, if the number of weekly tests is 1.4 or 1.8 per week, then the value would be two tests per week. If the recommended number of weekly tests is less than one (e.g., 0.4 or 0.7), which will occur among small workforces, then one person should be selected per week. This guidance helps ensure that the 50 percent annual testing goal will be achieved.
For-cause testing for drugs and alcohol is required to be conducted when an individual’s behaviors or physical condition suggests possible substance abuse, or upon receipt of credible information (as described below) that an individual is engaging in substance abuse, as defined in 10 CFR 26.5. An individual subject to a for-cause test should not be permitted to return to those duties, responsibilities, or access that make them subject to the FFD program, until the individual’s observed behavioral or physical condition has been evaluated by a medical professional (see 10 CFR 26.619; 10 CFR 26.77, “Management actions regarding possible impairment”; and 10 CFR 26.189) and the drug and alcohol test results have been reviewed by the MRO. The discussions in this guide for 10 CFR 26.608, “FFD program training,” and 10 CFR 26.609 include examples of observed behaviors or physical conditions that create a reasonable suspicion of possible substance abuse.
Notwithstanding the above, if there is a smell of alcohol with no other behavioral or physical indications of impairment, then only an alcohol test should be necessary. However, a licensee or other entity may establish in its procedures that even if the smell of alcohol is apparent, a drug test could also be administered. This testing protocol could benefit the licensee or other entity because additional information could be obtained about the individual’s compliance with the FFD policy, and it could benefit the individual because the results may identify underlying conditions that may require medical or clinical attention or attendance within an employee assistance program.
For indications of possible impairment that do not create a reasonable suspicion of alcohol or substance abuse, the licensee or other entity should permit the individual to return to work only after the physiological or psychological condition is evaluated by the MRO and the MRO has determined that the individual is fit to perform his or her duties safely and competently (see 10 CFR 26.619, 10 CFR 26.77, and 10 CFR 26.189).
For indications of impairment due to fatigue, the requirements in 10 CFR Part 26, Subpart I, must be followed. However, since many drugs cause fatigue (e.g., benzodiazepines) and are not part of the 10 CFR Part 26 or HHS Guidelines drug testing panel, licensees and other entities should have procedures to evaluate such an individual for both sleep deprivation and fatigue caused by substance use. The NRC has issued DG-5078, “Fatigue Management for Nuclear Power Plant Personnel at Commercial Nuclear Plants Licensed Under 10 CFR Part 53,” for 10 CFR Part 26, Subpart I, implementation by a licensee or other entity licensed under 10 CFR Part 53.
As described in the guidance for 10 CFR 26.607(h), (i), and (j), a licensee or other entity would be permitted to use innovative technologies to screen individuals before their entry into the protected area. This drug and alcohol screening process may not be used for for-cause or post-event testing. However, if the innovative technology indicates a positive screening result for a drug, drug metabolite, or alcohol, the individual would be subject to either a POCTA or a specimen collection that must be analyzed by an HHS‑certified laboratory. If the POCTA indicates a positive result for a drug, drug metabolite, adulterant, or negative for a biomarker if applicable, then a for-cause test would need to be conducted using an oral fluid or urine collection that is sent to the HHS-certified laboratory for analysis. If a POCTA screen is not performed, then upon a positive indication by the passive screening instrument, the licensee or other entity would need to collect an oral fluid or urine for cause, and the specimen must be analyzed by an HHS‑certified laboratory.
The phrase “credible information” is used in the description of a for-cause test, but it is not defined or described in 10 CFR Part 26. Some examples of information that should be considered credible for purposes of Subpart M implementation are if the information was received from the following:
an individual subject to the FFD program;
law enforcement, court records, or a medical or clinical professional;
an arbiter assigned to a 10 CFR Part 26 appeal;
C/Vs performing authorization requirements (e.g., background investigations or suitable inquiries);
the licensee’s or other entity’s human resources representative;
FFD or access authorization program personnel from a different NRC licensee or other entity; or
an NRC or State representative or Federal agent.
Individuals subject to the FFD program because they maintain unescorted access to the facility under 10 CFR Part 26, and possibly 10 CFR 73.120, should be afforded the benefit of the doubt that the information they communicate is credible. However, operating experience has demonstrated that individuals in the workplace are capable of falsely accusing others of FFD concerns. Therefore, access authorization and FFD program personnel should receive, assess all information, and use their experience and judgment to determine whether they should independently verify the information as being true and accurate before taking any action (e.g., conduct a for-cause test or temporarily remove authorization) against the individual with the alleged FFD concern.
If information is learned about an individual from an open source (such as the internet, news media, social media sites) and the information concerns a possible FFD policy violation involving substance abuse, then personnel designated by the licensee or other entity should verify the truth and accuracy of the information based on other sources before initiating a for-cause test and a determination of fitness under 10 CFR Part 26.
If information learned about an individual from an open source is associated with a possible FFD policy violation related to trustworthiness or reliability but not substance abuse, then personnel designated by the licensee or other entity should verify the truth and accuracy of the information based on other sources before initiating actions that may remove the individual from performing or directing any activities or maintaining any access that makes them subject to the FFD program. Should the information represent a safety or security concern to the individual, other people, or any facility, the licensee or other entity should immediately remove the individual’s authorization, escort the individual to a safe location, and subject the individual to a determination of fitness.
Post-event testing must be conducted as soon as practical under the following two conditions:
Condition 1: As soon as practicable after an event that human error may have contributed to or caused.
Condition 2: Within 4 hours of events that result in adverse health consequences or damage to safety- or security-related SSCs, as defined in 10 CFR 26.5. Performing a test within 4 hours after the event will help ensure that the individual’s biological metabolism has not reduced the drug, drug metabolite, or alcohol concentrations below initial cutoff levels.
The licensee or other entity must define in its procedures what types of human errors will trigger a post-event test, since not all human errors should require testing. For example, a post-event test related to human error should be conducted when an individual or individuals improperly operate a component or system or fail to perform a duty or responsibility considered to be within the skill of the trade, craft, or profession that caused or could have caused harm to humans, SSCs, or safety or security. For example, the individual(s) caused the following:
an unplanned discharge of a firearm or protective spray;
improper or inaccurate computer entry for an SSC required for safety or security that went unmitigated for a period of time creating a cybersecurity vulnerability or reactor safety concern;
a vehicular accident with damage to the vehicle or an SSC of more than minor consequence;
an unplanned radiation exposure to an individual(s) or an unplanned radiological release;
a dropped heavy load, scaffolding failure, or crane overload; or
a lifted-load impact to another SSC.
The licensee or other entity should consider writing in its procedures examples of when a post-event test need not be conducted for a human error. In these cases, the licensee or other entity should assess whether the error could have reasonably caused harm to humans or SSCs, which should result in a post-event test related to human error. Additionally, defense in depth should be used to inform the decision-making process. For example, an improper weld could potentially result in a system breach, but the defective weld was identified and corrected; in this case, the welder would not be tested. Other examples include the following:
procedural errors (process, administrative, and insignificant errors of commission or omission);
incorrect operation of a valve, pump, dashpot, instrument, or other such item;
inadequate performance of a quality assurance or quality verification check;
improper welding, fabrication, assembly, installation, maintenance, or surveillance during the conduct of activities deemed to be within the skill of the trade, craft, or profession;
hurting one’s body, head, or appendage even if the injury (e.g., minor cuts, minor scraps, or minor blunt force trauma) did require some immediate onsite medical attention (e.g., band aides, cold packs, over-the-counter pain relievers); and
improper mathematical calculations, technical evaluations, or computer entries.
Post-event testing may be delayed only if it is necessary for the individual to obtain immediate medical intervention. The licensee or other entity should define or describe “immediate medical intervention” in its procedures. Interventions could include medical treatment that requires a medical doctor, paramedic, or nursing care for the following:
head trauma, including trauma to eyes, ears, nose, or mouth;
broken bones or blunt force trauma;
exposure, ingestion, or inhalation of a hazardous chemicals; and
burns and other significant wounds.
The conduct of a post-event test for human error under Condition 1 above, if conducted within 4 hours of the event, satisfies the post-event test requirement for a Condition 2 consequence-based post-event test. Two tests should not be conducted on a particular individual for a single event, and a for-cause or post-event test should not be used as a reason to delay medical treatment.
Based on operating experience, the licensee or other entity should not let complex or burdensome determinations of significance or monetary value be a determining factor in deciding whether to conduct a post-event test. Such determinations may delay the conduct of the drug and alcohol testing and result in a missed opportunity to identify an illegal substances, illegal drugs, and illicit substances because of metabolism of that substance.
Licensees or other entities must test the individual(s) who caused or contributed to the human error, event, or both and need not test individuals who were affected by the event and whose actions likely did not cause or contribute to the event.
The licensee or other entity is required to conduct follow-up testing to verify an individual’s continued abstinence from substance abuse as determined by the substance abuse expert (SAE) designated by the licensee or other entity. Site procedures should include instructions for the process to be used in the development of a testing plan for drugs, alcohol, or both, or a clinical treatment plan that, if completed successfully, would provide the licensee or other entity with additional information to inform its decision on whether to restore or maintain the individual’s authorization.
Licensees and other entities that are not required to implement 10 CFR Part 26, Subpart H, may use information from Subpart H, such as the conduct of follow-up testing for authorization in 10 CFR 26.69, “Authorization with potentially disqualifying fitness-for-duty information,” in its procedures. The following is guidance for the follow-up testing process:
If an individual is not terminated for the FFD policy violation issued for condition or occurrence that demonstrated the individual may not be trustworthy and reliable, the individual should be subject to a determination of fitness and a clinical plan that monitors, treats, and assesses the individual’s trustworthiness and reliability. This clinical plan could include drug and alcohol testing.
If the individual is not terminated for an FFD policy violation for substance abuse, then the individual should be subject to and satisfactorily complete a follow-up testing plan for drugs, alcohol, or both, in order to maintain or be granted authorization.
The follow-up testing process and any clinic or treatment plan should describe the medical or clinical professional(s) who is designated by the licensee or other entity to assess the individual, develop the plan, monitor the individual, and verify whether the individual satisfactorily completed the plan. The medical or clinical professionals should have the appropriate qualifications, training, or academics to assess the individual’s condition.
Licensees or other entities may implement a drug testing program using urine as the biological specimen for testing drugs and drug metabolites for all test conditions. This testing program must be detailed in procedures and be based on the requirements in 10 CFR Part 26, Subpart M. Licensees or other entities must require a urine specimen to be tested for validity (including biological markers) and whether the specimen was adulterated, substituted, or diluted. If the licensee or other entity discovers the specimen has been adulterated, substituted, diluted, or invalid, or a biological marker was not identified, then the situation warrants additional action by the laboratory and licensee to determine whether an FFD policy violation has occurred. If the specimen were adulterated or substituted, or a biological marker was not identified, then this occurrence is grounds for the licensee or other entity to determine that the test was subverted and issue the individual a permanent denial of authorization. If the specimen is determined to be dilute, then the licensee or other entity must require the laboratory to conduct the special analysis testing required by 10 CFR 26.163(a)(2). Licensees or other entities are enabled to test the urine specimen for biological markers other than those markers (e.g., pH, creatinine, specific gravity) used to typically test for validity, but this should be established in a procedure and consistently applied to all individuals.
To help ensure specimen integrity, the collector should verify the true identity of the donor, present the donor with a new collection container, take steps to ensure the integrity of the specimen, and properly fill out the CCF. The collector should explain the collection process to the donor, answer any questions the donor may have about the collection process, and inform the donor that the failure to follow collector instructions, act in a confrontational manner, or act to subvert the drug or alcohol test is grounds for a permanent denial of authorization. Collector responsibilities also include actions to ensure that the bathroom stall is ready for the collection (e.g., water sources are secured; a water bluing agent is added to the toilet water; water piping, ventilation, and ceiling areas are secured from unauthorized donor entry). A discussion of collector training appears in 10 CFR 26.85, “Collector qualifications and responsibilities,” the guidance for 10 CFR 26.608 in this RG and in the HHS Guidelines.
If FFD program personnel call for a direct observation collection, then the collector should be able to effectively observe all sides of the individual’s midsection to verify that the urine specimen is not subverted or adulterated and the urine exit the donor’s body or urostomy bag. To prevent a subversion attempt, before the collection of urine from a medical device, the donor should have informed the licensee or other entity, and the licensee or other entity should have verified the medical necessity of the device. Although an observed collection is performed, other privacy considerations should be afforded as discussed in this guide, such as all nonessential individuals should be prevented from being in the visual vicinity of the observed collection and the donor should be afforded visual privacy while re-dressing. The donor’s failure to provide a specimen or subvert the testing process must be immediately reported to FFD program personnel.
For confirmatory, split specimen, invalid, MRO-directed, or adulterated/subverted testing, licensees or other entities must use laboratories certified by HHS for the specific biological specimen to be tested in order to issue an NRC-required sanction. Licensees or other entities should refer to the laboratory certification procedures in HHS Guidelines for urine (or oral fluid) specimen laboratory testing certification, as applicable. The licensee or other entity must establish and maintain a contract with a primary and backup HHS‑certified laboratory (at a different location with a different certifying scientist) for the specimen(s) to be tested.
Licensees or other entities are required to develop, implement, and maintain written procedures that address the methods and techniques used to protect the privacy of an individual who provides a specimen, procedures for protecting the integrity of the specimen, and procedures to ensure that the test results are valid and attributable to the correct individual. The section of this guide addressing 10 CFR 26.603(d) discusses privacy and specimen integrity. Guidance on maintaining specimen integrity could also be developed from 10 CFR Part 26, Subpart E, and the HHS Guidelines.
Licensees or other entities may rely on a local hospital or facility licensed and audited by the State (or State-designated entity) to collect specimens for drug testing and perform alcohol testing. Licensees or other entities must audit these facilities on a biennial basis to confirm that the facility procedures are comparable to those described in 10 CFR Part 26, Subpart E, for the collection of urine or oral fluid specimens or the HHS Guidelines, as applicable. The contract between the licensee or other entity and the collection facility should identify these measures. The contract should enable NRC inspection of the collection facility. The offsite collection facility should be located near the facility to ensure that metabolism of a parent drug or alcohol does not result in a false negative test result. A documented review by a forensic toxicologist should inform this determination.
A licensee or other entity may use a POCTA to conduct initial testing during the random testing process for both drugs and alcohol or use a device to collect a urine or oral fluid specimen that is sent to an HHS-certified laboratory for initial and confirmatory drug testing. An evidentiary breath testing device may be used for initial and confirmatory alcohol testing.
A POCTA is medical device. The U.S. Food and Drug Administration states that “[m]edical devices range from simple tongue depressors and bedpans to complex programmable pacemakers, and closed loop artificial pancreas systems.” For example, a POCTA for drug screening should have the product classification of “Kit, Test, Multiple, Drugs of Abuse, Over the Counter.”18
Specimens that yield positive, adulterated, substituted, or invalid initial validity or drug test results or discrepant biological markers must be subject to confirmatory testing by an HHS-certified laboratory, certified for that biological specimen, except for invalid specimens that cannot be tested.
Licensees or other entities that collect oral fluid specimens for drug testing should follow the procedures outlined in the HHS Guidelines for oral fluid testing. These licensees or other entities must include in their procedures the name and revision of the specific HHS Guideline and a description of the specific sections in the guideline being implemented.
Cutoffs
The drugs, drug metabolites, and the initial and confirmatory testing cutoffs would be those established in the HHS Guidelines for oral fluid testing, except as required by 10 CFR Part 26, Subpart M, regarding the panel of drugs and drug metabolites that must be tested. If the licensee or other entity elects to change a cutoff or drug metabolite, a forensic toxicologist review conducted pursuant to 10 CFR 26.31(d)(1)(i)(D) would need to certify this change. Section 3.4 of Subpart C of the HHS Guidelines for using oral fluid specimens provides drug test cutoff concentrations for undiluted oral fluid. These oral fluid cutoffs result in a comparable program outcome as a drug testing program that uses urine and the cutoffs in the HHS Guidelines for urine specimens.
Comparisons of Oral Fluid and Urine Specimen Testing
Licensees or other entities should be aware of the relevant differences between urine or oral fluid specimens for drug testing. For example, each specimen presents a different window of detection, detection rate, subversion, validity testing, and collection methods. Licensees or other entities should consider how these differences contribute to or compromise the effectiveness of their FFD program. For example, for pre-access testing, using urine for drug testing provides a longer window of detection and therefore may be better to detect drug use, whereas the collection of an oral fluid specimen may help prevent a subversion attempt and better indicate the individual’s current condition of fitness. As discussed in this guide, if a screening test using oral fluid results in a presumptive positive test result, then a drug test using oral fluid must be conducted for HHS‑certified laboratory testing. Similarly, if a POCTA uses urine and indicates a presumptive positive screening result, then a urine specimen must be collected and sent to the HHS-certified laboratory for testing.
The route of administration of a potentially illegal substances, illegal drugs, and illicit substances influences the time that drug and metabolites appear in oral fluid. The oral procedure described in the HHS Guidelines, which requires an individual to abstain from oral consumption for 10 minutes before giving an oral fluid specimen, helps rid the mouth of environmentally obtained parent drugs. A licensee or other entity should consider in its procedures that as soon as an individual is notified to test, the individual should abstain from excessive hydration and the placement of any product in the mouth.
Once drugs (and metabolites) enter the bloodstream, they rapidly diffuse into oral fluid by excretion from highly blood-perfused salivary glands. Consequently, oral fluid tests generally are positive for the parent drug as soon as the drug is absorbed into the body. Therefore, oral fluid testing may be more reflective of real‑time concentrations of substances; however, drug and metabolite concentrations should not be used to determine impairment, and the increased window of detection associated with urine testing provides a benefit as well. Note that oral fluid testing presents a direct blood-oral fluid transport and measures in situ concentrations of the parent drug and drug metabolites, whereas urine involves only metabolites or products that could not be metabolized.
Sequential oral fluid testing (e.g., two tests over a period of time such as 30 to 60 minutes) and obtaining the quantitative test results from the HHS-certified laboratory could determine whether the drug or drug metabolite concentration in the individual is increasing or decreasing. This information would enable the MRO to make a more informed decision on the need to remove these individuals from safety- and security‑sensitive duties and to determine whether the individual used the drug while in a work status. Sequential testing of urine specimens could also be performed; however, obtaining a second or third urine specimen of adequate volume may be a challenge. Note that sequential testing for alcohol would also provide important information for the determination of fitness or issuance of an FFD policy violation.
Considerations for Oral Fluid Testing by Test Type
Oral fluid testing could more accurately quantify parent drug concentrations than urine testing and makes it harder for individuals to subvert the testing process. Additionally, oral fluid testing is less invasive than urine testing and is generally considered a faster method of collection. While licensees or other entities may conduct oral fluid testing for any testing type, the following are some testing and screening considerations:
Pre-access Testing. Oral fluid testing is generally preferred for pre-access testing. Although the detection windows for oral fluid are usually shorter than those for urine, oral fluid testing would help reduce subversion attempts, because oral fluid collections are directly observed by a collector.
For-cause Testing. To better inform an assessment of an individual’s potential impairment or trustworthiness and reliability in the workplace, oral fluid testing is generally preferred. For the very infrequent occasions in which a for-cause test cannot be administered within 24 hours of the observation of impairment or receipt of credible information, urine specimen testing is recommended because of its larger window of detection. This occasion would involve a situation in which medical treatment precludes the timely administration of a for-cause test or a significant failure of the FFD program prevents the timely administration of a for-cause test. The FFD program supervisor, MRO, and the licensee’s or other entity’s management should coordinate the decision as to the type of specimen to be collected and when the test should be administered.
Post-event Testing. To determine whether recent substance abuse was a potential cause of the event, oral fluid testing is recommended. Oral fluid gives a direct indication of the parent drugs present in the individual’s blood stream, which better shows recent use and potential impairment. Additionally, the predetection windows are shorter for oral fluid, making it more likely an oral fluid test would detect recent drug use. If a post-event test cannot be done promptly (for example, within 4 hours of the event requiring the test), then a urine specimen should be collected. Similar to the case of a for‑cause test, the FFD program supervisor, MRO, and the licensee’s or other entity’s management should coordinate decisions in this delayed testing situation.
Follow-up Testing. Licensees and other entities should use a urine specimen for follow‑up testing, because the use of urine testing generally enables a longer window of detection of substance abuse than oral fluid testing. Licensees and other entities could consider hair testing within a follow-up testing program, but there are two potential challenges to using hair. First, the use of hair for follow-up testing requires an approved exemption from the NRC. Second, because the large window of detection for hair testing enables the testing to occur less frequently, an individual could continue to have unescorted access to the facility within the periodicity of the hair testing plan. This scenario would present an opportunity for the individual to use drugs and potentially be impaired within the periodicity of the hair testing plan while being subject to the FFD program.
Random Testing. Licensees or other entities should consider randomly selecting between oral fluid and urine screening to provide benefits similar to those described under pre-access testing. urine testing will help to detect the use of drugs because it has a larger window of detection than oral fluid testing, while oral fluid will alert licensees to recent drug use and better help prevent subversions. The results of random screening that uses both biological specimens would provide the most comprehensive understanding of the testing pool’s drug use, help prevent subversion attempts, and provide information on the positivity and detection rates of both testing types. Information on the positivity and detection rates could significantly enhance a PMRP or inform a planned change to the FFD program through the change control process.
Limitations of Oral Fluid Testing
Oral fluid testing has several limitations, including decreased ability to reliably detect certain stimulants and cannabinoids. The use of these substances and others can cause reduced salivation and make it more difficult to collect an oral fluid specimen of adequate volume. Additionally, the relatively lower volume of specimen collected compared to urine can make it difficult to confirm and quantify multiple analytes in a single assay and, in some cases, to achieve a split specimen. Further, the pH in oral fluid can be relatively high, which may lead to lower drug concentrations for testing.
If a licensees or other entity uses oral fluid for drug or alcohol testing, then the collection, packaging, and temporary storage of the drug or alcohol test device, and shipment of an oral fluid specimen to an HHS-certified laboratory or the collection of an oral fluid specimen for alcohol testing must be performed in accordance with licensee- or other entity-established procedures based either on the requirements in 10 CFR part 26 or the procedures in HHS Guidelines identified by the licensee or other entity in 10 CFR 26.606(b)(1)(iv).Should there be a conflict between the HHS Guidelines and the manufacturer’s instructions for the oral fluid collection kit, the licensee or other entity should use its change control process and a forensic toxicologist review to determine whether the difference is substantial enough to warrant a mitigative strategy or the use of one instruction over the other.
A point of collection testing (POCT) device collects a biological specimen in a location that need not be a collection facility, hospital, or clinic. These types of devices are also called point of care testing devices and require the specimen to be analyzed in a laboratory to obtain drug or alcohol test results. A point of collection testing and assessment (POCTA) device provides for an immediate onsite (i.e., in the workplace) assessment of drug and alcohol screening results without the need for a laboratory analysis of the specimen. POCTA devices collect either urine or oral fluid specimens and typically use an immunoassay procedure to detect and measure substances through the assessment of antigens. The device will typically provide a visual indication to reveal whether a drug, drug metabolite, or alcohol exceeded its initial screening cutoff. Many devices also test for adulterants and biomarkers, thereby supplementing information for an MRO review. A POCTA for urine will need to test for adulterants and validity; devices for oral fluid and urine should test for biological markers as described in 10 CFR Part 26 or the HHS Guidelines. A forensic toxicologist review of the POCTA is required before its use to ensure that its use will not decrease FFD program effectiveness. If it does, then a mitigating strategy is needed to maintain FFD program effectiveness. POCTA is used only in the pre-access and random screening processes because FFD program requirements would ensure that the individuals are fit for duty, trustworthy, and reliable even if the POCTA device was later determined to be less effective than initially determined. For the use of a POCTA device during the pre-access process, this assurance is obtained because the individuals would be subject to a drug and alcohol test using a specimen that is sent to an HHS-certified laboratory for analysis before the individuals are afforded unescorted access to the protected area. For the random testing of individuals within the protected area using a POCTA device, individuals subject to this test have already met NRC requirements to be granted FFD authorization and been granted unescorted access and are subject to behavioral observation. If a POCTA device indicates that an initial screening cutoff is exceeded, that the specimen is substituted or adulterated, or that biomarkers are not present, then a second collection is required using a method that enables testing at an HHS-certified laboratory. As required by 10 CFR 26.607(h)(4)(i), the biological specimen to be collected and sent to the HHS-certified laboratory must be the same as that used for the screening.
A POCTA device for drugs and drug metabolites often indicates results within 10 minutes of collection, while alcohol POCTA devices may provide results in less than 1 minute. Rapid screening results provided by POCTA would allow for more expedient assessment of the individual and removal of the individual from assigned duties, responsibilities, and access if the initial results exceed the immunoassay initial screening cutoffs. It would also enable the individual to return to work more quickly if the screening was negative and there were no observable signs of impairment. POCTA devices that use sweat, breath, or other biological scanning and detection techniques would not be precluded by 10 CFR Part 26, Subpart M, and may present substantial flexibility for a licensee or other entity to use innovative technologies to ensure that its workplace is free from the presence and effects of drugs and alcohol. The guidance in this RG for 10 CFR 26.607(j) discusses this in more detail.
Licensees and other entities may collect hair specimens for drug or drug metabolite testing only to inform an FFD or access authorization determination of whether an individual is trustworthy and reliable, because a hair test presents a historical record of potential illegal substance, illegal drug, and illicit substance use. A confirmed positive test result for a hair specimen must be considered PDI, as defined in 10 CFR 26.5, until determined otherwise by a review under 10 CFR 26.613 or 10 CFR 26.185.
Under the 10 CFR Part 26, Subpart M, framework, a hair specimen may be collected only during the pre-access screening process. The results of a hair test would supplement the results of urine or oral fluid testing at an HHS-certified laboratory to satisfy the pre-access testing requirement and any POCTA screening of individuals as they wait before being granted unescorted access to the facility. The hair testing process should be described in the licensee or other entity’s FFD policy and procedure to ensure program effectiveness and consistency, which is a worker protection consideration.
Licensees and other entities may test hair specimens only for Schedule I and II drugs and drug metabolites listed in section 202 of the Controlled Substances Act. The panel of drugs is restricted to these two schedules because the 10 CFR Part 26 panel of drugs and drug metabolites are principally Schedule I and II controlled substances. Schedule I controlled substances have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. Schedule II controlled substances have a high potential for abuse that may lead to severe psychological or physical dependence.
Licensees and other entities that collect hair specimens for drug screening use the procedures outlined in the HHS Guidelines issued for hair specimens. Licensees and other entities must also test these specimens at HHS-certified laboratories. Therefore, the initial and confirmatory testing cutoffs are required to be equivalent to those in the HHS Guidelines. Should differences be identified between the HHS Guidelines and the panel of drugs and drug metabolites to be tested in an FFD program, a forensic toxicologist must evaluate the differences pursuant to 10 CFR 26.31(d)(1)(i)(D) before testing.
Like oral fluid and urine testing, guidance to inform an MRO’s assessment of an HHS-certified laboratory test result from a hair specimen should principally be that issued by the HHS or the NRC, but other sources could be used. These include guidance from the Federal Bureau of Investigation, the U.S. Department of Defense, and private laboratories.
A 10 CFR Part 26 sanction may not be issued for any test result from a hair specimen test unless the licensee or other entity determined that the individual subverted, or attempted to subvert (as defined in 10 CFR 26.5) the hair testing process, or the licensee or other entity was implementing an NRC‑approved exemption to use hair specimens for other test conditions.
A noninvasive POCTA device may be used to screen individuals before they enter or exit the protected area. The device or instrument should be noninvasive in that it may only collect and detect, and then analyze, for example, exhaled breath, sweat, or pupil characteristics (e.g., movement, blood flow). This would include passive monitoring and assessment of blood through the use of transdermal or iris detection technologies. This technology could include fluorescence measurement, electrospray ionization/mass spectrometry, liquid chromatography-mass spectrometry, or pupillometer technology.
Portal area screening would enable a licensee or other entity to proactively screen all individuals before they enter the NRC-licensed facility and upon their exit. This screening strategy would have a significant detection and deterrence value. Additionally, this would help provide substantial assurance that individuals did not subvert the passive screening when they entered the facility or consumed, inhaled, or used an illegal substances, illegal drugs, and illicit substances while on site. This screening strategy would directly support the performance objectives of 10 CFR 26.23(a), (b), and (c).
As described in this guide, some portal area monitors detect the true identity of the individual by scanning a fingerprint or conducting an iris scan. These instruments may also determine true identity through facial recognition and provide an electronic communication to a designated central monitoring, control station, or individual to inform specific individuals of the person who just screened positive on an instrument detecting a drug or alcohol. This could be most beneficial for facilities in geographically remote locations or facilities that have minimum staffing because a portal area instrument alarm could lock protected area turnstiles and prevent an individual’s access to the protected area. The refusal to perform a drug or alcohol screening or test is a refusal to test—a subversion attempt—and the individual must be issued a permanent denial of authorization in 10 CFR 26.610. Passive screening instruments that lock an entrance point should have a readily available manual override to avoid impacting the overall safety or security of the facility. Similarly, the exit should not be locked by a portal area screening instrument. If the portal area instrument detects a substance during exit screening, and the individual does not submit to the FFD program requirements upon an alarm, then the individual has violated the FFD policy or procedure and perhaps attempted to subvert the screening process.
A forensic toxicologist is required to review and document their evaluation that the instrument and setpoints used and maintained in the instrument are acceptable for use in the detection and screening of the drugs, drug metabolites, and alcohol selected for screening. Furthermore, the toxicologist must verify that the instrument will be operated in accordance with manufacturer’s specifications. If screening detects the presence of drugs, drug metabolites, or alcohol at or above the instrument setpoint(s), the individual is subject to a verification test using an oral fluid or urine specimen The test can be done using a POCTA device. The individual would not be allowed to re-screen through the portal area instrument in an attempt to clear the alarm because of subversion considerations.
The portal monitor instrument could also be used to detect the presence of other prohibited FFD items, such as explosives, incendiaries, and firearms. Operating experience has shown the diverse sensing capabilities of these innovative technologies.
For laboratory personnel, section 11.3 of the HHS Guidelines defines a forensic toxicologist, in part, as a person with documented scientific qualifications in analytical toxicology. In 10 CFR 26.31(d)(1)(i)(D), the NRC also provides information on a forensic toxicologist qualifications. For 10 CFR Part 26 implementation and as determined by the licensee or other entity in its procedures, this individual should meet the following qualifications:
certification or licensure in forensic or clinical toxicology by the State where the facility is located, a Ph.D. in one of the natural sciences, or training and experience comparable to a Ph.D. in one of the natural sciences with training and laboratory/research experience in biology, chemistry, and either pharmacology or toxicology;
experience in forensic toxicology with emphasis on the collection and analysis of biological specimens for drugs of abuse; and
experience in forensic applications of analytical toxicology (e.g., publications, court testimony, conducting research on the pharmacology and toxicology of drugs of abuse) or qualifications as an expert witness in forensic toxicology.
Blood specimens may be tested only under the order of the licensee- or other entity-designated MRO for a valid medical reason. MROs designated by a third party (e.g., hospitals) are prohibited from ordering or implementing blood specimen testing. An HHS-certified laboratory need not be used to analyze a blood specimen; however, the specimen should be tested at a facility licensed or certified by the State or other government entity for the analysis of blood specimens.
For the collection of urine, oral fluid, and hair specimens, the licensee or other entity is required to use a CCF that has been approved by the OMB. For the use of a POCTA device, the licensee or other entity would have to implement an approved and maintained (by the licensee or other entity) procedure that ensures the reliability of the tracking, handling, and storage of a specimen from the point of specimen collection to final disposition of the specimen. This requirement may also apply to aliquots sent to the HHS‑certified laboratory and the reliability of an identification system to uniquely assign the specimen to the donor. A CCF should not be used for any specimen collected and analyzed by a portal area monitor in 10 CFR 26.607(h) because these is no collector.
Some portal area monitors detect the true identity of the individual and may provide an electronic communication to a designated central monitoring, control station, or individual to inform specific individuals of the person who just screened positive on an instrument detecting a drug or alcohol. The licensee or other entity could determine whether the instrument communication could be used as a licensee-developed CCF if collection integrity and privacy is ensured. This could be most beneficial to link the screening result with the subsequent drug or alcohol test that would be required under 10 CFR 26.607(h)(4)(i) and for facilities sited in geographically remote locations or minimally staffed, or when an individual alarms the instrument and then immediately exits the facility. This electronic communication should be in accordance with the licensee’s or other entity’s procedures for cybersecurity and information technology services and should not provide quantitative values for the screening results.
When determining who may serve as an MRO, licensees or other entities should observe the procedures outlined in the HHS Guidelines and requirements in 10 CFR 26.183, “Medical review officer.” In general, an MRO must be a currently licensed physician who has knowledge of pharmacology and toxicology, has completed the required training, passed an initial examination, and completed a requalification course at certain intervals after initial certification.
The MRO must evaluate HHS-certified laboratory test results in accordance with licensee procedures. These procedures should reference or incorporate the requirements in 10 CFR 26.185, the HHS Guidelines, or both to ensure that the specimen is properly and accurately evaluated by the MRO.
Because the requirements in 10 CFR Part 26, Subpart M, for the collection, testing, and assessment of alternative biological specimens for drug testing are objective based and nonprescriptive, MROs should remain knowledgeable about drug testing and evaluation of all biological specimens in the licensee’s or other entity’s FFD program. MROs must attend and pass an initial medical- or clinical‑based training session to improve their knowledge of MRO duties and responsibilities, drug and alcohol testing processes and procedures, and evaluation of drug testing results. This course must be conducted by a nationally recognized MRO training and certification organization that has been assessed by the licensee or other entity to cover the major requirements in 10 CFR 26.183 and 10 CFR 26.185 or the equivalent requirements in the HHS Guidelines as implemented through licensee or other entity procedures. MROs must also attend a medical- or clinical-based training session on a triennial basis to improve their knowledge of changes in drug and alcohol testing processes and procedures and evaluation of drug testing results.
Licensees and other entities may consider adding FFD training to their systems approach to training program.
Licensees or other entities should require their biological specimen collectors to be trained in the collection and processing of urine, oral fluid, and hair, if part of the FFD program. This training would include both the use of POCTA devices and biological specimen collection kits designed to be sent to HHS‑certified laboratories for testing. Individuals with specific training, experience, credentials, or academic education in conducting biological specimen collections could lead this training. Initial training should include a written exam plus an instructor‑observed and graded simulated collection for all test types used in the FFD program. Following initial training, collector training should be periodic and could be in the form of online training or a read and sign. Based on operating experience outside of the 10 CFR Part 26 framework, the NRC recognizes the significant benefit of periodic, instructor‑observed simulated collections to assist collectors in maintaining skill, knowledge, and proficiency. The licensee or other entity procedures should incorporate information from the HHS Guidelines and Subpart E of 10 CFR Part 26.
Collector training, credentialing, and education could be obtained from nationally recognized organizations. Furthermore, the LLWR industry may have resources available to 10 CFR Part 53 licensees and other entities that implement drug and alcohol testing programs. Training and credentialing of a collector would help prevent disagreements between the collector and donor, contribute to the effectiveness of the FFD program, and protect the donor, and may help prevent the need for appeals.
The licensee or other entity must train all employees in the elements of its BOP. These elements include requiring that all individuals must conduct and be subject to behavioral observation and must report FFD concerns to the licensee or other entity. Behavioral observation training should be sufficient to inform all individuals subject to the FFD program of physiological and psychological indications that an individual may not be fit for duty or may not be trustworthy and reliable.
NUREG/CR-7183, “Best Practices for Behavioral Observation Programs at Operating Power Reactors and Power Reactor Construction Sites” (Ref. 36), is a research and comparative study conducted by Oak Ridge National Laboratory for the NRC. It documents best practices associated with BOPs used by Federal agencies and private entities. This report also discusses the need for effective BOPs at operating power reactors and those under construction and presents insights and recommendations to improve BOP performance.
A BOP best practice includes understanding the physiological and psychological indications of possible drug‑induced impairment; table 8 below shows these indications. As described in NUREG/CR‑7183, these indicators or combinations of them represent a symptomology matrix of the possible drug class affecting the observed individual. These drug classes include central nervous system depressants and stimulants, hallucinogens, dissociative anesthetics, narcotic analgesics, inhalants, and cannabis. Individuals without medical or clinical training can observe many of these indicators; however, to determine which chemical may be causing the physiological or psychological indication of a class of drugs, additional training should be conducted. It is not uncommon for individuals to try to mask indicators of potential impairment or substance abuse through unusual dress involving sunglasses; face or head coverings; coats or long sleeves in warmer seasons; abnormal use of cologne, perfume, or aftershave; or other means.
Table 8. Physiological and Psychological Indicators of Possible Impairment
Restlessness |
Uncoordinated movement |
Dazed appearance |
Horizontal gaze nystagmus |
Body tremors |
Grinding teeth |
Exaggerated reflexes |
Vertical gaze nystagmus |
Excited |
Euphoric |
Memory loss |
Lack of pupil convergence |
Talkative |
Slurred speech |
Difficulty answering questions |
Pupil size |
Difficulty walking |
Poor balance |
Disoriented |
Reaction to light |
Droopy eyes |
Nausea |
Dizziness |
Pulse rate |
Drowsiness |
Sluggishness |
Fatigued |
Blood pressure |
Poor perception of time or distance |
Flashbacks |
Body temperature |
Perspiring |
Runny nose |
Red nasal area |
Muscle tone |
Anxiety |
Hallucinations |
Paranoia |
Synesthesia |
- |
NUREG-2155, Rev. 2, “Implementation Guidance for 10 CFR Part 37, ‘Physical Protection of Category 1 and Category 2 Quantities of Radioactive Material’” (Ref. 37), is a technical report that provides guidance on the implementation of physical protection requirements. Although not written for facilities licensed under 10 CFR Part 53, the document provides guidance for evaluating an individual’s trustworthiness and reliability when deciding to allow unescorted access to certain radioactive materials. This document states that “[w]hen a person’s life history shows evidence of unreliability or untrustworthiness, a licensee may question if that person can be relied on and trusted to exercise the responsibility necessary for working with risk‑significant radioactive materials.” Individuals with unescorted access to facilities licensed under 10 CFR Part 53, like individuals who may be granted unescorted access to Category 2 types of materials, should not pose an unreasonable risk to the public health and safety, including the potential to commit or aid in the theft of SNM, or commit radiological sabotage. The licensee or other entity should consider training or informing their staff of the following indicators associated with a lack of trustworthiness and reliability:
willful or intentional acts of omission;
untruthfulness or falsification of records or communications;
sale, use, or possession of illegal substances;
behavioral changes, moodiness, or depression not previously observed;
abuse of legal substances (e.g., drugs, over-the-counter medications and consumer products, and alcohol);
impaired performance attributable to psychological or other disorders identified through medical or clinical evaluations (e.g., NRC-licensed operators and NRC-required security officers);
conduct that warrants referral for criminal investigation or results in an arrest or conviction;
attempted or threatened destruction of property or life, including hostility or aggression toward fellow workers, management, social or cultural groups, or ethnicity or race;
repeated absenteeism;
irresponsibility in the performance of assigned duties;
failure to comply with licensee or other entity policy, procedures, or work directives, including violation of safety or security procedures;
inability to deal with stress or the appearance of being under unusual stress;
suicidal tendencies or an attempt at suicide; and
recurring financial irresponsibility.
The following list presents examples of acts, qualities, or characteristics that would indicate that the individual should not be granted FFD authorization under 10 CFR Part 26.
Committed, attempted to commit, aided, or abetted another individual who committed or attempted to commit any act of sabotage, espionage, treason, sedition, or terrorism against the United States.
Publicly or privately advocated actions that may be inimical to the interest of the United States, or publicly or privately advocated the use of force or violence to overthrow the Government of the United States or the alteration of the form of government of the United States by unconstitutional means.
Knowingly established or continued a sympathetic association with a saboteur, spy, traitor, seditionist, anarchist, terrorist, or revolutionary; with an espionage agent or other secret agent or representative of a foreign nation whose interests may be inimical to the interests of the United States; or with any person who advocates the use of force or violence to overthrow the Government of the United States or the alteration of the form of government of the United States by unconstitutional means. Ordinarily, the licensee should not consider chance or casual meetings or contacts limited to normal business or official relations.
Knowingly joined or engaged in any activity in sympathy with, or in support of, any foreign or domestic organization, association, movement, group, or combination of persons who advocate or practice the commission of acts of force or violence to prevent others from exercising their rights under the Constitution or laws of the United States or any State or any subdivisions thereof by unlawful means or who advocate the use of force and violence to overthrow the Government of the United States or the alteration of the form of government of the United States by unconstitutional means. Ordinarily, the licensee should not consider chance or casual meetings or contacts limited to normal business or official relations.
Deliberately misrepresented, falsified, or omitted relevant and material facts from documentation provided to the licensee.
Had been convicted of a crime(s) that indicated poor judgment, unreliability, or untrustworthiness.
a. The licensee’s or other entity’s BOP is a principle method to detect behavior that may indicate possible use, sale, or possession of illegal drugs; use or possession of alcohol on site or while on duty; any physical impairment; or any condition that, if left unattended, may constitute an unreasonable risk to public health and safety or the common defense and security. All personnel are responsible for observing the behavior and reporting FFD concerns about individuals subject to the FFD program.
b. All personnel responsible for performing BOP functions should be trained to have sufficient awareness to detect degradation in performance that may be the result of being under the influence of any substance, legal or illegal, or a physical or mental impairment that in any way may adversely affect their ability to perform their duties safely and competently. Training must communicate the requirement to promptly report any onsite or offsite behaviors or activities by individual subject to the FFD program that may constitute an unreasonable risk to the safety or security of the NRC-licensed facility or SNM, or may cause harm to others, to the licensee‑designated personnel for appropriate evaluation and action in accordance with the FFD policy. This reporting must include any information on character or reputation indicating that the observed individual cannot be trusted or relied on to perform those duties and responsibilities or maintain FFD authorization (i.e., have access to NRC-licensed facilities or sensitive information).
c. Licensees or other entities should consider the following factors, among others, when designing an effective BOP. NUREG/CR-7183 describes many of these elements in more detail.
The BOP should have a clearly written purpose explaining why the program is necessary and should include the scope, performance objectives, and procedures of the program.
Planned observations of human performance should be tailored to the facility’s population size, level of direct (in‑person) management oversight, geographic location of the facility, and identification and assessment of SSCs that might require enhanced security. This means that proactive observations of human activities should be scaled to the physical and human conditions at the facility. For example, if C/Vs are brought on site for refueling or maintenance activities, licensee employees should be more vigilant in observing behavior and plan to observe individuals (perhaps through peer checking and oversight) because the baseline human performance characteristics of short-term C/Vs may not be known.
The BOP should list those behaviors related to threats, including physiological and psychological indicators, and train all individuals in the identification of characteristics or observable traits related to human performance that may represent a risk to human or facility safety or security.
The BOP should stress that all individuals must perform observations and that this contributes to human and facility safety and security, because actions could be taken to mitigate conditions adverse to plant safety and security and assist an impaired individual or prevent workplace violence.
The BOP should require that individuals making a behavioral observation participate in the documentation of the observed behavior, such as describing any patterns of concern.
The BOP should list the steps of how to report an observation, constructively help an impaired individual, and remove oneself from harm if the observed individual intends to cause harm.
The BOP should explain that this program is one layer of a comprehensive regulatory framework that includes access authorization, physical security, and cybersecurity to help thwart an insider threat. An insider threat includes not only individuals who may be psychologically impaired, but also individuals who may be knowingly trying to obtain sensitive information or gain access to NRC-licensed material without appropriate authorization or need to know, or who may want to harm the facility or its workers.
The BOP should integrate with the process for returning an observed individual to duty if the initial BOP assessment (e.g., conducted by a supervisor or FFD program personnel) determines that the FFD concern does not exist. This process should consider whether such an occurrence should be documented. This return‑to‑duty process could be similar to that implemented for fatigue assessments.
The BOP should have a process to escalate the assessment of an individual to a clinical, medical, or trained professional for a more comprehensive assessment of the individual using the determination of fitness process.
Supplementing the guidance regarding 10 CFR 26.608, all personnel are responsible for observing behavior and should receive training in the following topics:
Understanding the significant elements of the BOP described above.
Understanding that the BOP applies both on and off site, whether or not an individual is in a work status.
Understanding that there is no lower threshold associated with a potential FFD concern. Common sense is an element of a successful BOP. Individuals should understand that they do not need corroborating evidence or multiple examples of an FFD concern before reporting—only one indication is needed to conclude that a potential FFD concern exists. All individuals should be empowered to make that observation and determination because they have received BOP training. Waiting for corroborating evidence or another individual to assess the potential FFD concern could be contrary to safety and security.
Knowing examples of substances that are prohibited for use, sale, or possession while on or off site, including the possible recognition (e.g., odor, color, bottling, labeling, appearance) of potentially illegal drugs, other substances that could potentially cause impairment, and alcohol.
Recognizing the common containers or paraphernalia used to take illegal drugs or alcohol (e.g., bottles, flasks, small bags, pipes, needles) and other indicators.
Identifying behavior that may indicate the possession, use, or recent consumption of illegal drugs or alcohol. Table 8 lists physiological and psychological indicators of substance abuse.
Understanding that an individual’s baseline human performance characteristics may change if the individual is being adversely affected by a physiological or psychological ailment or is trying to hide something or do something that is not authorized.
Reporting and documenting a behavior observation for management review.
Understanding examples of when an observer should consider intervening (and how to intervene) if an individual represents an FFD concern.
Understanding how and when to seek emergency medical treatment or security support for an observed individual.
Understanding that making false (i.e., unjustified) claims of FFD concerns is a violation of the FFD policy and could be considered retaliation or workplace harassment.
BOP Effectiveness
To establish baseline human performance to aid in determining whether an FFD concern exists, licensees and other entities may implement proactive program elements such as the following:
Video recordings, voice monitoring, biometrics, and photographs could be used from security systems for comparative analysis of human performance. Movement analysis technique was employed more than a decade ago at the Mall of America in Bloomington, Minnesota.19
If a facility has a small staff, then periodically shifting individuals from one shift or team to another may help mitigate any negative group dynamics that could result from small groups and quell or identify aberrant behavior.
Periodic audio and visual contact with staff working in a geographically remote location using video conferencing may help detect changes in human attitudes (i.e., safety culture) or performance.
Periodic staff and management rotations to the site, corporate office, or central monitoring station could help build companywide teamwork, as well as better informed and more fungible employees.
Third parties could assess human performance, small group dynamics, and any negative performance issues that may be developing at a facility.
Licensees or other entities, while encouraged to use external site monitoring by video or teleconferencing, should be aware of its limitations, especially when an individual’s baseline has not been established. The value of video and teleconferencing technology is limited when evaluation through physical proximity may be required, an individual is already physically impaired or intoxicated, there is a language barrier, the call occurs during an emergency, or the technology fails because of connectivity or other access issues. Additionally, video or teleconferencing may limit an observer’s ability to identify important nonverbal communication cues (e.g., leg shaking, toe tapping, sweaty palms, dilated pupils).
Licensees or other entities should not exclusively rely on video or teleconferencing for behavioral observation. Licensees or other entities using video or teleconferencing to fulfill parts of their BOP objectives should include an in-person (face-to-face) baseline evaluation before an individual’s assignment to a plant site. This in-person evaluation could leverage the access authorization-required annual supervisory reviews, NRC-required psychological examinations, and the medical reviews conducted for NRC‑licensed operators and NRC-required security officers. These reviews and observations may be used to inform determinations of fitness required by 10 CFR Part 26.
The licensee or other entity is required to establish sanctions for FFD policy violations that, at a minimum, prohibit the individuals specified in 10 CFR 26.4 from being assigned to perform or direct those duties and responsibilities that make them subject to 10 CFR Part 26, Subpart M, unless or until the licensee determines that the individual’s condition or behavior does not pose a potential risk to public health and safety or the common defense and security. The sanction also needs to escalate with the number of occurrences and severity of the FFD policy violation.
The severity of the sanction should be based on three factors: the length of the issued sanction; the scope and detail of any treatment or clinical plan to help remedy the root or apparent cause(s) of the FFD policy violation; and the scope and length of a follow-up testing program for drugs, alcohol, or both.
The sanction must be long enough to act as a deterrent and, if the individual is retained as a licensee employee or C/V, enable the individual to complete counseling or treatment. Because of the wide variety of possible FFD policy violations and FFD programs, the licensee’s or other entity’s procedures should include instructions for meeting this requirement. For example, an individual tests positive for alcohol and is issued a 30-day denial of authorization as presented in table 9. Yet, the licensee- or other entity-assigned SAE knows that different people need different treatment plans for alcohol use disorders. The clinical assessment determines that the subject individual presents a situational drinking pattern that does not present any other adverse compounding factors (such as depression, abuse of other substances, family issues) and prescribes that the individual undergo six sessions of counseling over 3 weeks, plus a follow-up testing program. In this case, the licensee or other entity may restore the individual’s authorization upon completion of the 30-day sanction. If the assessment ordered 12 sessions over 6 weeks, the individual may still be sanctioned for 30 days, but the SAE may reassess the individual’s fitness, may find with reasonable assurance that the individual will remain fit for duty, and may recommend to the licensee or other entity restoration of authorization based on the reassessment, yet the individual still must complete the required counseling and follow‑up testing sessions, if assigned.
The guidance below is based on lessons learned from the LLWR community and informed by the expectation that facilities licensed under 10 CFR Part 53 may be licensed and operated with staff sizes markedly smaller than those of current LLWR facilities, and these facilities may be sited in geographically remote locations. Facilities with small staff sizes set up the paradigm that the relative contribution of an individual to safety and security may be greater than at facilities with larger staffs, despite advances in passive and automated technologies. For example, if a 10 CFR Part 53 CNP has one individual performing radiation protection, chemistry, or health physics activities, two NRC‑licensed individuals per shift, and a few onsite NRC-required security officers, it is possible that if one person in a particular labor category is impaired, the effectiveness of the team may be diminished. For this reason, the regulations and guidance escalate the severity of sanctions based on risk.
In determining the schedule of sanctions for violations of the FFD policy, licensees or other entities could assign individuals to one of three sanction groups (Group 1, 2, or 3), based on the risk significance level of their assigned duties and responsibilities. Group 1 could be the highest risk significance level and Group 3 the lowest. Licensees or other entities should perform a risk‑significance assessment and assign individuals to groups based on the results of that assessment. For guidance, individuals who perform the following duties and responsibilities, regardless of their other job duties, should be assigned to Group 1. These are the individuals who are empowered, entrusted, assigned, and possibly licensed by the NRC to perform or direct those duties and responsibilities that make them subject to the FFD program.
Operate or direct the operation of safety- or security-related equipment that a risk-informed evaluation process or alternative method for establishing safety significance has shown to be significant to public health and safety.
Maintain an NRC operator’s license.
Perform as an NRC-required security officer.
Perform quality control and quality verification activities.
Perform radiation protection, radiological survey, or reactor plant chemistry activities.
Perform cybersecurity or information technology services for the NRC-licensed facility.
Be designated as FFD program personnel.
Group 2 should include individuals who:
perform duties and responsibilities of a non-licensed operator (e.g., an Auxiliary Operator);
perform or direct the performance of maintenance or surveillance of equipment that a risk-informed evaluation process or alternative method for establishing safety significance has shown to be significant to public health and safety;
transport SSNM; or
perform or direct the performance of engineering evaluations or calculations or cybersecurity or information technology services for the NRC-licensed facility.
Group 3 should include individuals who are not listed in Groups 1 and 2, like individuals who direct or provide facility support services (e.g., janitorial, painting, landscaping, warehousing, food services, etc.).
As shown in Table 9, the first violation of the FFD policy should result in the immediate denial of authorization for at least 30 days from the date of the unfavorable determination if the individual is in Group 1 or 2. For individuals in Group 3, the first FFD policy violation should result in the immediate denial of authorization for at least 14 days. Any subsequent FFD policy violation should result in a denial of authorization for a minimum of 5 years if the individual is in Group 1, 3 years in Group 2, and 1 year for those in Group 3.
If it is determined that the individual violated an FFD policy a third time or subverted the collection or testing process, the person’s authorization (for both escorted and unescorted access) to the site should be permanently denied. A permanent denial of authorization should also be considered if the FFD policy violation involving an individual’s trustworthiness and reliability approaches the severity of the examples associated with criminal history record checks described in the guidance for 10 CFR 26.608.
Table 9. Risk-Informed Sanctions
Violation |
Group 1 |
Group 2 |
Group 3 |
First |
30 days |
30 days |
14 days |
Second |
5 years |
3 years |
1 year |
Third |
Permanent denial |
Permanent denial |
Permanent denial |
Subversion attempt |
Permanent denial |
Permanent denial |
Permanent denial |
The 30-day sanction for Groups 1 and 2 is based on affording time for the individual to enter and possibly complete clinical treatment to prevent recurrence before having authorization restored. The 30-day sanction is also based on the safety- or security-significance of the roles and responsibilities performed by the individuals in Groups 1 and 2 since there may be fewer individuals at a facility licensed under 10 CFR Part 53 to immediately replace the roles and responsibilities lost by the individual who violated the FFD policy.
The 14-day sanction for Group 3 is based on 10 CFR 26.75(e)(1) and operating experience in the LLWR community. This operating experience demonstrates that about 80 percent of all positive drug and alcohol testing results are from C/Vs every year, which represents about 4 times as many drug and alcohol positive test results as that of licensee employees.
The 5-, 3-, and 1-year sanctions for Groups 1, 2, and 3, respectively, for a second violation of the FFD policy are principally based on the safety- or security-significance of the duties and responsibilities performed by the individual in violation of the FFD policy. The 5-year denial for Group 1 is also based on 10 CFR 26.75(e)(2), which establishes a 5-year denial of authorization for a second FFD policy violation. The 3-year denial is risk informed because if an individual in Group 2 makes an error, there would be a reasonable probability that the error will be detected by individuals in Group 1. Accordingly, the error should not result in an immediate condition adverse to safety or security. The 1-year denial for an individual in Group 3 is based on operating experience that these individuals (even if impaired) would not have access to SSCs to cause an unidentified or latent condition adverse to safety or security.
The permanent denial of authorization for a subversion attempt or third violation of the FFD policy is based on 10 CFR 26.75(b) and (g), respectively.
The following examples should escalate the severity of the sanction issued for an FFD policy violation:
the individual was involved in the sale, use, or possession of illegal drugs onsite but outside the protected area or offsite;
the individual was involved in the distribution of illegal drugs on or off site;
the FFD program personnel, MRO, or other licensee- or other-entity designation individual makes the determination that the individual demonstrates characteristics warranting a more severe sanction, such as a lack of acknowledgement and remorse that the individual’s actions were in violation of the FFD policy or statements or actions that the individual cannot be trusted or relied upon.
If an individual’s condition or behavior caused an FFD policy violation or FFD concern, the individual’s authorization to the site should be denied until a suitability or fitness evaluation determines otherwise. The sanction should be administered from the date that the FFD policy violation was issued to the individual.
Licensees or other entities must establish and maintain a system of files and procedures to prevent unauthorized disclosure of personal information, whether electronic or hardcopy. Individuals may consent to allow the disclosure of personal information to any individual.
PII can be used to distinguish or trace an individual’s identity, either alone or when combined with other information that is linked or linkable to a specific individual. Because many different types of information can be used to distinguish or trace an individual’s identity, the term PII is necessarily broad. The definition of PII is not anchored to any single category of information or technology. Rather, it requires a case-by-case assessment of the specific risk that an individual can be identified using information that is linked or linkable to the individual. In performing this assessment, licensees and other entities should recognize that information that is not PII can become PII whenever additional information is made publicly available, in any medium and from any source.
For example, information can become PII when an individual’s name is used in combination with any of the following:
mother’s maiden name,
driver’s license number,
bank account information,
credit card information,
relatives’ names,
postal address,
email address,
home or cellular phone number,
personal characteristics,
Social Security number,
date or place of birth, or
other information that would make an individual’s personal identity easily traceable.
The following types of information can also be considered to be personal information, although not necessarily PII. This information could be acquired from FFD program implementation and could be provided to other licensees and entities under 10 CFR 26.617(c) to support authorization determinations.
frequency, date, and time of selection for testing;
reason for selection for testing;
type, quantity, and quality of the biological specimen collected;
test results (laboratory, POCTA, initial screening, and MRO reviewed);
other specific chemistry, biological factors, or chemical or biological indicators of the specimen collected, not specifically related to the panel of substances tested;
the discovery or indication of any underlying medical conditions;
any BOP reports and indicators;
the action taken in response to behavior observation indicators;
any observations or determinations made during the FFD or access authorization processes, such as self‑disclosures, reporting of legal actions, and results from background investigations, character and reputation reviews, and psychological assessments;
an individual’s medication, physiological or psychological ailments, diagnostic reports, or clinical reports or notes;
findings or results from an MRO or SAE evaluation and the contents of a clinical treatment plan; and
the information on a CCF (whether paper or electronic).
Information that is not personal includes the fact that an individual subverted or attempted to subvert the drug or alcohol test; was found to be in violation of the FFD policy; had one, two, or three FFD policy violations; had FFD authorization removed as a result of an FFD concern; or was issued a particular FFD‑required sanction. These types of information do not require protection because they are expected outcomes of FFD program implementation, are used to inform the access authorization program, are shared with other licensees and other entities subject to 10 CFR Part 26, and the individual consented to being subject to the FFD program. These elements of information do not contain privacy, medical, or PII; however, the details associated with these elements of information may need to be protected.
Licensee and other entity computer systems used for administration, human resources, access authorization, and FFD program purposes should be programmed to prevent unauthorized disclosure or viewing of sensitive (i.e., privacy, medical) information. The NRC has taken enforcement action against a licensee that operated an information system in which individuals making access authorization determinations could (but were not authorized to) view quantified drug testing results and the MRO clinical assessment. These individuals had no need to know, for example, quantitative test results.
Licensees and other entities must obtain a signed consent that documents the individual’s acceptance of being subject to the FFD program and authorizes the disclosure of the personal information collected and maintained under this subpart, except for disclosures to the individuals and entities specified in 10 CFR 26.37(b)(1) through (b)(6), (b)(8), and persons deciding matters under review in 10 CFR 26.613. The licensee or other entity may include other provisions in the consent form; however, the consent form should clearly state which provisions are required by the NRC and which are advanced by the licensee or other entity.
The consent form must be signed and dated before making the individual subject to the FFD program. This means that the consent should be obtained prior to informing the individual to report for a pre-access drug and alcohol test. If an individual refuses to consent to the NRC requirements, then the licensee or other entity would be precluded from granting or maintaining an individual’s authorization.
Licensees and other entities implementing 10 CFR Part 26, Subpart M, could communicate with the LLWR fleet to ascertain the contents of the consent form used at facilities licensed under 10 CFR Part 50 or 10 CFR Part 52; however, differences in requirements should be assessed. For example, 10 CFR Part 26, Subpart M, requires 10 CFR Part 53 licensees and other entities to share information on FFD program implementation and requires individuals to be informed of this information‑sharing requirement. The Subpart M requirements also enable the individual to be subject to a consortium random testing program instead of a site or corporate FFD program, which could involve a C/V that is not regulated by the NRC. Also, the consent form should note that 10 CFR 26.825, “Criminal penalties,” states that under section 223 of the Atomic Energy Act of 1954, as amended, the NRC may issue criminal sanctions for willful violation of, attempted violation of, or conspiracy to violate, any regulation issued under sections 161b, 161i, or 161o of the Act. For the purposes of section 223, all of the regulations in 10 CFR Part 26 are issued under one or more of sections 161b, 161i, or 161o, except for the sections listed in 10 CFR 26.825(b).
By definition, willful violations are of particular concern to the Commission because its regulatory program is based on licensees and their contractors, employees, and agents acting with integrity and communicating with candor. Willful violations cannot be tolerated by either the Commission or a licensee. Therefore, a violation may be considered more significant than the underlying noncompliance if it includes indications of willfulness. The term “willfulness” includes a spectrum of violations ranging from deliberate intent to violate or falsify to and including careless disregard for requirements. Willfulness does not include acts that do not rise to the level of careless disregard (e.g., negligence or inadvertent clerical errors in a document submitted to the NRC).
10 CFR Part 53 licensees and other entities may desire to implement a common consent form that could align with that used by licensees and other entities under 10 CFR Part 50 or 10 CFR Part 52. This could enhance worker protections by ensuring consistency as an individual may work at various NRC-licensed facilities subject to access authorization and FFD programs.
The individual’s signature on the consent form should be used as confirmation that the individual has read and understands the contents of the consent form and has voluntarily agreed to authorize the licensee or other entity to obtain FFD-related information and take action to comply with the provisions of 10 CFR Part 26.
Transferring Personal Information
The signed consent must authorize the disclosure of the personal information collected and maintained under the licensee or other entity’s FFD program. Signed consent must be obtained before disclosing the personal information, except for disclosures to the following persons:
a licensee or other entity’s representatives who have a need to access the information to perform their assigned duties under the FFD program, including determinations of fitness, FFD program audits, or some human resources functions;
NRC representatives;
assigned MROs and MRO staff;
appropriate law enforcement officials under court order;
the subject individual or their representative who has been designated in writing;
persons deciding matters on review or appeal;
the presiding officer in a judicial or administrative proceeding initiated by the subject individual; or
other persons pursuant to court order.
The licensee or other entity (transferor) should have the authority to transfer personal information to other NRC licensees or other entities (transferee) if the transferee demonstrates a specific need for the personal information consistent with the requirements in 10 CFR Parts 26, 53, or 73 and identifies the piece(s) of personal information required to meet that need. For example, such a specific need could include the transferee’s ability to verify an individual’s past employment history, testing status, completion of testing, presence or absence of sanctions required by the NRC or administered by the licensee or other entity, determination of past authorization status, or any other relevant criminal or substance abuse history, which the transferee represents is necessary to determine the granting or maintaining of authorization.
Once personal information is transferred, the transferor would remain responsible only for the protection of the copies, files, or version of records still under its physical or digital control. The transferee, upon receiving the transferred information, then assumes responsibility for the physical and digital custody of such information and assumes the obligations for its care under 10 CFR 26.611.
The licensee or other entity is required to have an appeal process that is objective and impartial. The licensee’s or other entity’s appeals process should not modify, subjugate, or abrogate any review rights that currently exist for individuals with their respective employers. The guidance for the PMRP auditing section under 10 CFR 26.603(d) in this document also addresses the appeals process.
An individual who has been denied authorization at the site or whose authorization has been terminated because of a violation of the FFD program should be provided with the following:
the basis for the denial of authorization within 90 days of denial;
the opportunity to provide additional information and correct inaccurate information contained in the record;
the opportunity to have a collective bargaining representative present during the presentation or review of findings and to be advised of their rights under any collective bargaining agreement. However, no collective bargaining representative may unduly influence or interfere with the review proceedings or the individual’s exercise of any particular right; and
the opportunity to have the decision, together with any additional information, reviewed in accordance with the appeal process detailed in the licensee’s or other entity’s procedures. For example, the appeal process may entail a separate review by another licensee or other entity manager who is equivalent or senior to and independent of the individual who made the decision to deny or terminate access to the site because of the program violation. The determination from this independent review is final.
On August 6, 2019, in SRM-SECY-19-0033, “Staff Requirements—SECY-19-0033—Discontinuation of Rulemaking—Access Authorization and Fitness-for-Duty Determinations” (Ref. 38), the Commission approved the staff’s proposal to discontinue rulemaking on third‑party arbitration of access authorization and FFD decisions. The Commission directed the staff to—
continue their persistent monitoring of trends and data related to human behavioral monitoring and fitness-for-duty programs at licensee facilities and promptly notify the Commission when circumstances arise that cause the staff to change its conclusions or further action is needed by the Commission to address this issue.
The Commission also directed the staff to inform the Commission of “any instance where a third‑party arbitrator overturned a licensee’s access authorization determination . . . .” An access authorization determination includes the determination that the individual is trustworthy and reliable, which is also a key element in an FFD program.
Licensees and other entities are encouraged to inform the NRC when an appeal under 10 CFR 26.613 results in a condition in which the subject individual was reinstated with unescorted access to the NRC-licensed facility, NRC-licensed material, or sensitive information and could represent a condition adverse to safety, security, or quality. This occurrence could also involve arbitrated changes to a determination of fitness and NRC-required minimum sanctions.
Licensees or other entities who implement an FFD program are required to perform audits to ensure the continuing effectiveness of the FFD program, including audits of FFD program elements that are provided by C/Vs, and the FFD programs of C/Vs that are accepted by the licensee or other entity. Audits can be combined, and audit dates managed in accordance with licensee procedures. Persons performing FFD audits should be independent of the FFD functional area being audited, except that FFD program personnel may audit an HHS-certified laboratory, offsite collection facility, or other FFD-related C/V. The following are examples of the potential subject of an audit:
the culture of safety and security demonstrated by the site workforce;
the sufficiency of procedure details for the conduct of drug and alcohol screening and testing through the incorporation of provisions from 10 CFR Part 26 or HHS Guidelines;
the methods and processes used in the BOP and its effectiveness in identifying disqualifying information and prohibited FFD items;
review of workplace events involving retaliation, intimidation, or harassment;
the effectiveness of the sanctions in deterring individuals from substance abuse and in facilitating treatment before the individual is returned to duty; and
the implementation of the random testing and POCTA screening programs.
Licensees or other entities are required to audit their programs at a frequency that ensures their continuing effectiveness and that corrective actions are taken to resolve any problems identified. The following supplements the guidance provided for the PMRP:
An audit of significant FFD program elements should be conducted before—
the start of any activity making the 10 CFR Part 53 CNP or manufacturing facility subject to 10 CFR Part 26; or
the initial loading of nuclear fuel into a reactor at a 10 CFR Part 53 CNP that completed construction (i.e., an audit conducted before the licensee or other entity transitions from an FFD program under 10 CFR 26.605(a) to an FFD program under 10 CFR 26.605(b).
FFD program elements should be audited:
before the start of activities that involve a large influx of licensee employees or C/Vs to accomplish a particular site activity, such as maintenance, engineering design change, refueling, or manufactured reactor refueling or replacement;
if FFD performance data indicate an adverse trend in multiple FFD program areas;
periodically and planned not to exceed a nominal 36-month period, if significant changes were made to an FFD program element that has not been assessed by the PMRP;
if a new C/V is hired to perform one or more FFD program elements;
when the primary and backup HHS-certified laboratories performing contracted activities required by the licensee’s or other entity’s FFD program are not inspected by the HHS’s National Laboratory Certification Program:
This audit should be conducted following an implementation period after contract issuance and periodically thereafter based on significant changes to the laboratory contract; changes in 10 CFR Part 26 laboratory requirements in Subpart G, if implemented by the licensee or other entity in lieu of those in the HHS Guidelines; and any MRO-laboratory performance deficiencies identified through the PMRP.
A licensee or other entity need not audit elements implemented by an HHS‑certified laboratory if the element is comparable to that in 10 CFR Part 26. For example, if the panel of drugs and drug metabolites to be tested in the FFD program is comparable to that for which the laboratory is certified by the National Laboratory Certification Program, the drug and drug metabolite testing process and its methodologies need not be audited. A forensic toxicologist must make the determination of “comparable.” An example of an element that is not comparable is the special analysis testing required by 10 CFR Part 26.
Note: Should a 10 CFR Part 53 licensee or other entity have unresolved performance issues with an HHS-certified laboratory that appear to be generic or are associated with implementation of the HHS Guidelines, the licensee or other entity should contact the NRC to facilitate discussions with the HHS to improve program effectiveness.
An audit of any hospital or other facility licensed by the State (or State-designated entity) to conduct specimen collections and perform alcohol testing must be conducted under the PMRP. Similar to that discussed for the auditing of an HHS-certified laboratory, the licensee or other entity should document its justification for using a hospital or other facility licensed by the State (or State-designated entity).
The audit report, findings, observations, and recommendations should be reported to management at a level above that of the FFD program manager or supervisor.
Licensees or other entities are permitted to conduct joint audits or accept audits conducted by others, so long as the audit addresses the relevant FFD program elements (e.g., C/V services). Before conducting a joint audit or accepting an audit conducted by others, the licensee or other entity should review the joint or other party audit procedure to determine whether the audit process could reveal deficiencies in programs, procedures, or reporting, and then assess whether the auditors had or would have qualifications to audit the particular FFD program area. For example, a licensee or other entity accepting an audit conducted by a third party should be wary of the effectiveness of the audit if an audit plan was not documented and followed, objectives and sample sizes were not described, interviews with relevant control staff were not included, no quantitative or qualitative data were assessed, or auditor qualifications were not defined.
Licensees or other entities sharing or conducting joint audits must protect personal, medical, and other private information in its possession in accordance with 10 CFR 26.611.
The licensee or other entity is required to ensure that records pertaining to the administration of the FFD program, which may be stored and archived electronically, are maintained so that they are available for NRC inspection and any review or legal proceedings resulting from the administration of the program. These records should also be available for PMRP implementation. The system of files or programs containing FFD program records should be identified to support FFD program implementation and protected against unauthorized access as described in guidance on this RG for 10 CFR 26.611. Records should be updated to reflect accurate information.
Retention until License Termination
Licensees and other entities that implement 10 CFR 26.617(a) must maintain records pertaining to the administration of the FFD program and FFD performance data until license termination. These records include the following:
the record of and amendments to the analysis in 10 CFR 26.603(c), if performed and used to justify implementation of an FFD program under 10 CFR 26.604;
records associated with the 10 CFR 26.603(d) PMRP;
records associated with the 10 CFR 26.603(e) change control process;
FFD performance data required by 10 CFR 26.717; and
reports required by 10 CFR 26.617(b).
5-Year Records Retention
The licensee or other entity that implements an FFD program under 10 CFR Part 26, Subpart M, should maintain the following records for a least 5 years after the licensee or other entity terminates or denies an individual’s authorization or until the completion of all related legal proceedings, whichever is later, or until license termination. This retention period supports the 10 CFR 26.603 change control process and the PMRP. These records do not pertain to the administration of the FFD program or FFD performance data.
records pertaining to individuals:
information used to make pre-access (i.e., authorization) decisions;
the determination of a violation of FFD policy and related management actions;
any MRO evaluations or suitability and fitness evaluations;
an individual’s FFD-required return-to-duty treatment plan, including the sanction, required training, and clinical or medical evaluations; and
appeals of FFD policy violations or other FFD program actions issued to an individual.
records pertaining to FFD program implementation:
the current and superseded versions of the written FFD policy and procedures, including the implementation of all FFD program elements;
a list of the random testing pool, a list of individuals selected for random testing for each day and shift of testing, including those individuals selected but not tested, and the reasons individuals were not tested. These records must be kept for all populations of individuals assigned to random testing pools (e.g., the random testing pools for licensee employees and C/Vs, and other pools that may be established for other groups of individuals like C/Vs in a particular labor category);
a record of whether the individual selected for random testing received a POCTA screening, a test that was sent to an HHS-certified laboratory for analysis, or both, and the type of biological specimen that was collected and analyzed;
a record of those individuals who were subject to a pre-access screening test using hair as the biological specimen and the test results obtained from the HHS‑certified laboratory;
a record of those individuals who screened positive on a portal area passive detection instrument and a record of the results obtained from a POCTA or a specimen collected and sent to an HHS‑certified laboratory;
audits performed under 10 CFR 26.615;
the MRO’s notes, communications with the donor, justifications for FFD determinations, and assessment of an individual’s use of prescription medication that resulted in a laboratory confirmed positive drug test, and whether the positive test result was confirmed or the presumptive positive was overturned by the MRO;
copies of the CCF and consent forms;
events reportable to the NRC Operations Center; and
the electronic forms or documents used by the licensee or other entity to record drug and alcohol test results and issuance of waivers under its fatigue management program.
3-Year Retention
The licensee or other entity that implements an FFD program under 10 CFR Part 26, Subpart M, should maintain records of the following for a minimum of 3 years or longer to support NRC inspection, unless the license is terminated. These records do not pertain to the administration of the FFD program or FFD performance data.
records of FFD training and examinations;
contracts with entities providing FFD support services (e.g., screening and testing kits and instrumentation, laboratories, collection facilities, collectors, MROs, and SAEs); and
other FFD program related records not mentioned in this section of the RG.
Retention of Shared Records
If the licensee or other entity implements both FFD and access authorization requirements, then the retention period for any record that is used in both the FFD and access authorization programs should be the longer of the periods required by the FFD and access authorization requirements. Examples of these records may include the consent, self-disclosure, results of an appeal, PDI, information learned from a pre-access hair screen, reportable occurrences, and determinations of fitness (e.g., a psychological assessment). Similarly, this retention guidance applies to information obtained from the medical reviews of NRC-licensed operators or NRC-required security officers and used in the FFD program.
Licensees and other entities under 10 CFR Part 53 and subject to 10 CFR Part 26 must report to the NRC Operations Center by telephone within 24 hours the discovery of any intentional act that casts doubt on the integrity of the FFD program and any programmatic failure, degradation, or discovered vulnerability of the FFD program that may permit undetected drug or alcohol use or abuse by individuals who are subject to the FFD program. These events are reported under 10 CFR Part 26, rather than under the provisions of 10 CFR 73.71, “Reporting of safeguards events.”
Licensees or other entities should exercise judgment in determining whether to report a particular FFD issue to the NRC Operations Center. Single occurrences or nonrelated multiple occurrences not representing a programmatic failure should not be reported. Also, the electronic reporting of a single FFD policy violation to the NRC using the NRC’s electronic submissions system will not satisfy the 24-hour telephone report to the NRC Operations Center. Similarly, a 24-hour telephone report to the NRC Operations Center will not satisfy making a report through the NRC’s electronic submissions system.
The following examples offer guidance to assist in determining reportability:
An “intentional act that casts doubt on the integrity of the FFD program” could involve, but is not limited to, the following examples:
the unauthorized notification of a person that he or she will be selected for drug or alcohol testing on a certain day or at a certain time, which would subvert the FFC program’s normal notification procedure;
any unauthorized diversion or subversion of a drug or alcohol specimen or its documentation while in licensee or other entity custody;
any actual tampering or unauthorized alteration of onsite instrumentation or equipment used to screen, analyze, or store a drug or alcohol specimen; and
any collusion between any person with FFD-related program management access or authorization (e.g., an instrument calibration technician, computer software/hardware technician, FFD program personnel, supervisors) and any person subject to drug or alcohol testing, which would subvert the testing process.
A “programmatic failure, degradation, or discovered vulnerability of the FFD program that may permit undetected drug or alcohol abuse by individuals who are subject to the FFD program” could involve, but is not limited to, the following examples of the failure to—
conduct behavioral observation or, if required, perform pre-access or random testing of a significant number of persons who constructed, operated, or directed the operation of safety- or security-related SSCs or perform other duties described in 10 CFR 26.4;
implement an effective suitability and fitness evaluation process under 10 CFR Part 26 that enabled individuals who were not determined to be fit for duty and trustworthy and reliable to operate or direct the operation of safety- or security‑related SSCs or perform other duties, or maintain those types of access making them subject to the FFD program;
provide reasonable assurance that one or more of the 10 CFR 26.23 performance objectives are met;
effectively monitor a performance measure with FFD performance data and conditions adverse to safety, security, or quality occurred; or
perform an analysis of changes to site conditions that demonstrates the licensee or other entity no longer meets the criterion in 10 CFR 26.603(c) and was implementing the FFD program described in 10 CFR 26.604.
Licensees or other entities should also consider making a report for the following persons, if found and confirmed not to be fit for duty, or not trustworthy and reliable during the conduct of the described duty(ies) or maintaining the types of access that make them subject to the FFD program:
a person who maintains or has applied to the NRC for an operator’s license who directed or performed a safety-significant activity (e.g., on shift);
an NRC-licensed operator or certified fuel handler who directed or manipulated nuclear fuel;
a security officer performing NRC-required duties;
a person who performed quality assurance or quality verification activities on SSCs designed for inspection, test, analysis, and acceptance criteria;
a radiation protection technician or health physicist who inaccurately or incompletely surveyed radiation areas or effluent release pathways, or who ineffectively controlled access to radiological control areas, causing conditions adverse to safety; or
FFD program personnel.
If a licensee or other entity identifies that an HHS-certified laboratory has a significant quality issue or a collector or laboratory personnel were determined not to be trustworthy and reliable, then the issue should be reported.
If a person entered the protected area in possession of an illegal substance of more than a personal‑use quantity (e.g., a quantity established by State law) of a State legalized but Federally controlled drug, then the issue should be reported.
FFD-reportable occurrences should be evaluated and documented (with appropriate protection of information) by the licensee or other entity for the implementation of corrective actions to ensure the continuing effectiveness of the FFD program and to enable NRC inspection.
Annual program performance reporting is required for the information specified in 10 CFR 26.717(b), as applicable. For the annual program performance report—
The licensee or other entity should submit its data for all individuals subject to the FFD program whether assigned to the NRC-licensed facility or its remote monitoring facility (e.g., a central monitoring station).
The licensee or other entity implementing 10 CFR Part 26, Subpart M, must use the NRC‑provided electronic reporting forms for each FFD policy violation occurrence and the annual and biennial reporting requirement in Subpart M.
PII, privacy information, and medical information should never be reported to the NRC on NRC‑provided forms, because the forms are made available to the public; this includes any PII within the licensee- or other entity-determined file name or description. Licensees and other entities should use a unique identifier, developed and maintained by the licensee or other entity, for the subject individuals. Licensees and other entities shall ensure that the information protection requirements of 10 CFR Part 26 are applied to all FFD-related communications and correspondence.
All licensees and other entities subject to 10 CFR Part 26 are required to submit annual performance data for January through December before March 1 of the following year.
Licensees and other entities are required to describe in sufficient detail an individual’s FFD policy violation or programmatic weakness to NRC licensees and other entities subject to 10 CFR Part 26 when requested by these licensees and other entities to support authorization determinations under 10 CFR Part 26, Subpart C, 10 CFR 73.120, or 10 CFR 73.56, or to support a licensee or other entity’s PMRP.
Under certain circumstances, a licensee or other entity licensed under 10 CFR Part 53 may use electronic communications to perform face-to-face determinations and assessments conducted in accordance with 10 CFR 26.619; 10 CFR 26.207, “Waivers and exceptions”; or 10 CFR 26.211, “Fatigue assessments.” To support the use of electronic communications, the NRC contracted the Pacific Northwest National Laboratory (PNNL), U.S. Department of Energy, to study whether a medical and mental health assessment via electronic communication could be an acceptable alternative to an in‑person, face-to-face assessment.
The outcome of the PNNL study was a report, “The Use of Electronic Communications to Perform Determinations of Fitness” (Ref. 39), that focused on whether the technology supports allowing for face-to-face fitness determinations to be conducted using electronic means. The literature discussed in the PNNL report indicated, in part, “many medical and mental health professions have accepted that electronic communication is effective and ethical for certain patients and circumstances.” The report also concluded that “the growing acceptance of teleconferencing in medicine and mental health indicates that enabling the use of teleconferencing within an appropriate regulatory framework and associated guidance may be beneficial for at least some for-cause determinations of fitness.” However, the report also found that “practitioners using electronic communications for assessment and diagnosis may often need the assistance of personnel who are physically present with the individual being assessed.” The report further concluded the following:
There are no established guidelines for determining whether an assessment conducted through electronic communications would be appropriate for a particular individual. In circumstances where the assessment might need to draw upon physical information (i.e., odor, pupil size, or overall appearance) electronic communications alone would not be appropriate.
The PNNL report also acknowledged that, in some telemedicine instances, “a qualified expert will not be available within a reasonable commuting distance or in time to observe a behavior or physical condition as it may be transitory.” The report continued that “[i]n such circumstances, it would be beneficial to allow an appropriate expert practitioner to conduct the for-cause determination of fitness using electronic communications as a consultant to a local ‘host’ practitioner.” Additionally, in November 2012, the National Laboratory of Medicine, National Institutes of Health, published a workshop summary entitled “The Evolution of Telehealth: Where Have We Been and Where Are We Going?” (Ref. 40). This summary found, in part, that “studies show good agreement between diagnosis and treatment plans with in-person mental health care and those developed using telehealth technologies.” Based on these studies, the NRC finds that, in such cases, an in-person element would be integral to the assessment process.
These studies also show that such remotely conducted determinations or assessments should be augmented by an individual who is present in person with the individual being assessed and who is trained in accordance with the requirements of either 10 CFR 26.29, “Training,” and 10 CFR 26.203(c) or 10 CFR 26.608 and 10 CFR 26.202(c), as applicable. This training should include detailed instruction on the behavioral observation elements described in Section C.10 of the guide which provides guidance for the implementation of a training program under 10 CFR 26.608. The individual assigned to be the observer should be very familiar with the physiological and psychological indicators of possible impairment in table 8 and should have the character and integrity to be trusted and relied upon to provide an honest, independent, and accurate observational assessment of the individual as directed by the MRO or other medical professional. Should the observer be determined to have, for example, purposely communicated inaccurate information, feigned a particular observation, or ignored a physical or psychological indication, etc. about the individual, the observer should be determined to have subverted the Federally mandated FFD program and permanently denied FFD authorization to the NRC-licensed facility.
Permitting the use of electronic communications is expected to help ensure FFD program effectiveness, especially when the 10 CFR Part 53 CNP is in a geographically remote location or when the facility has a small staff.
The NRC staff may use this regulatory guide as a reference in its regulatory processes, such as licensing, inspection, or enforcement. However, the NRC staff does not intend to use the guidance in this regulatory guide to support NRC staff actions in a manner that would constitute backfitting as that term is defined in 10 CFR 53.1590, “Backfitting,” and as described in NRC Management Directive 8.4, “Management of Backfitting, Forward Fitting, Issue Finality, and Information Requests” (Ref. 41), nor does the NRC staff intend to use the guidance to affect the issue finality of an approval under 10 CFR Part 53, Subpart H, “Licenses, Certifications, and Approvals.” The staff also does not intend to use the guidance to support NRC staff actions in a manner that constitutes forward fitting as that term is defined and described in Management Directive 8.4. If a licensee believes that the NRC is using this regulatory guide in a manner inconsistent with the discussion in this Implementation section, then the licensee may file a backfitting or forward fitting appeal with the NRC in accordance with the process in Management Directive 8.4.
ACRONYMS AND ABBREVIATIONS
ADAMS Agencywide Documents Access and Management System
BO behavioral observation
BOP behavioral observation program
CBD cannabidiol
CCF custody and control form
CFR Code of Federal Regulations
CNP commercial nuclear plant
C/V contractor/vendor
DG draft regulatory guide
DisqI disqualifying information
DOT U.S. Department of Transportation
FFD fitness for duty
FOIA Freedom of Information Act
FR Federal Register
GSA General Services Administration
HHS U.S. Department of Health and Human Services
IAEA International Atomic Energy Agency
LC labor category
LE licensee employee
LLWR large light-water reactor
MRO medical review officer
NRC U.S. Nuclear Regulatory Commission
OMB Office of Management and Budget
OF Oral fluid
PAPR pre-access positivity rate
PAPRCV pre-access positivity rate for contractor/vendor
PASA pre-access subversion attempt
PASACV pre-access subversion attempts by contractor/vendor
PDI potentially disqualifying information
PII personally identifiable information
PMRP Performance Monitoring and Review Program
PNNL Pacific Northwest National Laboratory
POCT point of collection testing
POCTA point of collection testing and assessment
ProI prohibited FFD item
PVCV policy violation by contractor/vendor
PVLC policy violation by labor category
PVLE policy violation by licensee employee
RG regulatory guide
ROP Reactor Oversight Process
RTPRCV random testing positivity rate for contractor/vendor
RTPRLE random testing positivity rate for licensee employee
RTRCV random testing rate for contractor/vendor
RTRLE random testing rate for licensee employee
SACV subversion attempt by contractor/vendor
SAE substance abuse expert
SALC subversion attempt by labor category
SALE subversion attempt by licensee employee
SAWC subversion attempt by work category
SNM special nuclear material
SRM staff requirements memorandum
SSC structures, systems, and components
SSNM strategic special nuclear material
THC tetrahydrocannabinol
U.S.C. United States Code
REFERENCES20
1 The term “FFD program” has two meanings in this guide. First, “FFD program” refers to a single program (i.e., policies, procedures, etc.) implemented by a licensee or other entity at a particular 10 CFR Part 53-licensed facility. An FFD program can also be one that is implemented by a fleet of facilities subject to 10 CFR Part 26 that are being constructed or operated by one licensee or corporate entity or many entities. Figure 3 of this guide illustrates this.
2 As used in this guide, protected area means both (1) an area encompassed by physical barriers and to which access is controlled (10 CFR 26.5) and (2) any vital area, material access area, or controlled access area where licensed material is used or stored (10 CFR 73.120(b)(1)(I)).
3 As defined in 10 CFR 71.4, “conveyance” means (1) for transport by public highway or rail, any transport vehicle or large freight container, (2) for transport by water, any vessel, or any hold, compartment, or defined deck area of a vessel including any transport vehicle on board the vessel, and (3) for transport by any aircraft.
4This RG and 10 CFR Part 26, Subpart M, use the phrase “facility and its operation” to communicate that the FFD program focuses on the human performance of individuals to construct, operate, maintain, decommission, and secure a facility, not just the fact that the radiological dose consequences are not exceeded, with or without human action.
5 The number of individuals who were not subject to random testing, but who may in fact be acting in a manner contrary to the FFD policy or are not trustworthy and reliable, is not simply the random testing positivity rate multiplied by 50 percent of the population. Based on a statistical analysis, individuals who work full time and are subject to an annualized 50 percent random testing rate have a 39.99 percent chance of being tested once per year. Therefore, the probability of actually testing an individual is about 10 percent lower than one would expect from a 50 percent random testing rate program. This 10 percent value represents the approximate percentage of the additional staff members who are unidentified (Ref. 15).
6 As defined in 10 CFR 26.5, PDI means information demonstrating that an individual violated a licensee's or other entity's FFD policy; had authorization denied or terminated unfavorably under 10 CFR 26.35(c)(2), 26.53(a), 26.63(d), 26.65(g), 26.67(c), 26.69(f), or 26.75(b) through (e); used, sold, or possessed illegal drugs; abused legal drugs or alcohol; subverted or attempted to subvert a drug or alcohol testing program; refused to take a drug or alcohol test; has been subjected to a plan for substance abuse treatment (except for self-referral); or had legal action or employment action, as defined in 10 CFR 26.5, taken for alcohol or drug use.
7 The Safety Culture Policy Statement (available at https://www.nrc.gov/about-nrc/safety-culture/sc-policy-statement.html) sets forth the Commission’s expectation that individuals and organizations establish and maintain a positive safety culture commensurate with the safety and security significance of their activities and the nature and complexity of their organizations and functions.
8 The Drug Enforcement Administration’s discussion of these substances can be viewed at https://www.dea.gov/drug-information/drug-scheduling.
9 See the Quest Diagnostics® Drug Testing Index® at https://www.questdiagnostics.com/business-solutions/employers/drug-screening/knowledge-center/drug-testing-index. See also DOT reported test results at https://www.transportation.gov/odapc/DOT_Agency_MIS_Data. DOT’s random testing rate requirements are found in 49 CFR 382.305, “Random testing,” with reported test results at https://www.transportation.gov/odapc/DOT_Agency_MIS_Data.
10 A “Green” inspection finding is a violation of an NRC requirement and indicates performance within an expected performance level where the associated cornerstone objectives are met. An NRC finding of “greater than green” is a violation of higher safety or security significance using a significance determination process that compares performance against risk-informed thresholds. The NRC then assesses the resulting information and determines an appropriate response using the guidelines in an action matrix. See the NRC’s website at https://www.nrc.gov/reactors/operating/oversight/actionmatrix-summary.html for more information.
11 The cannabis plant (i.e., marijuana) contains more than 100 cannabinoids (i.e., compounds), one of which is THC. THC can be impairing based on the location, in part, of its carbon-carbon double bonds.
12 NRC‑published FFD performance reports may be viewed at https://www.nrc.gov/reactors/operating/ops-experience/fitness-for-duty-programs/performance-reports.html.
13 The Cannabis and Driving report includes discussions about Δ-8 THC and Δ-10 THC, which also cause impairment.
14 When drug screening using a hair specimen, an example of a subversion attempt would be an individual who washes their hair at the NRC-licensed facility after the individual was informed of the need to collect a hair specimen. This subversion attempt determination is necessary because many products on the market can purposely (or unknowingly) damage, condition, or coat the hair making the specimen invalid for testing. Additionally, substances are specifically marketed to “purify” or “cleanse,” or “remove” medications, chemicals, and other impurities from the surface or within the hair shaft.
15 The licensee and other entity should assess whether an oral fluid collection should be performed in any contamination or radiological control area.
16 For information on using a drug past its expiration date see https://www.fda.gov/drugs/pharmaceutical-quality-resources/expiration-dates-questions-and-answers and for information regarding off-label use see https://www.fda.gov/regulatory-information/search-fda-guidance-documents/label-and-investigational-use-marketed-drugs-biologics-and-medical-devices.
17One type of formal causal analysis is called MORT, Management Oversight and Risk Tree, as briefly described NUREG‑1513, Integrated Safety Analysis Guidance Document (ADAMS ML011440260).
19The Mall of America implemented a risk assessment and mitigation program that trains security officers to look for behaviors in a mall setting that are not considered normal to help identify security risks.
20 Publicly available NRC documents are available electronically through the NRC Library on the NRC’s public website at https://www.nrc.gov/reading-rm/doc-collections/ and through the NRC’s Agencywide Documents Access and Management System (ADAMS) at https://www.nrc.gov/reading-rm/adams.html. The documents can also be viewed online or printed for a fee in the NRC’s Public Document Room (PDR) at 11555 Rockville Pike, Rockville, MD. For problems with ADAMS, contact the PDR staff at (301) 415‑4737 or (800) 397‑4209; fax (301) 415-3548; or e-mail [email protected]. Copies of
the non-NRC documents included in these references may be obtained from the publishing organization.
22 Copies of International Atomic Energy Agency (IAEA) documents may be obtained through its website at
https://www.iaea.org/ or by writing the International Atomic Energy Agency, P.O. Box 100, Wargamers Strasse 5, A-1400 Vienna, Austria; telephone (+431) 2600-0; fax (+431) 2600-7; or e-mail at [email protected].
1.U.S. Code of Federal Regulations (CFR), “Licensing and Regulation of Commercial Nuclear Plants,” Part 53, Chapter I, Title 10, “Energy.”
2. CFR, “Fitness for Duty Programs,” Part 26, Chapter I, Title 10, “Energy.”
3. U.S. Department of Health and Human Services, “Mandatory Guidelines for Federal Workplace Drug Testing Programs,” 82 FR 7920 (January 23, 2017) (for urine testing); “Mandatory Guidelines for Federal Workplace Drug Testing Programs—Oral/Fluid,”84 FR 57554 (October 25, 2019); “Mandatory Guidelines for Federal Workplace Drug Testing Programs,” 85 FR 56108 (September 10, 2020) (proposed guidelines for hair testing).
4. CFR, “Physical Protection of Plants and Materials,” Part 73, Chapter I, Title 10, “Energy.”
5. CFR, “Procedures for Transportation Workplace Drug and Alcohol Testing Programs,” Subtitle A, “Office of the Secretary of Transportation,” Part 40, Title 49, “Transportation.”
6. U.S. Nuclear Regulatory Commission (NRC), Regulatory Guide (RG) 5.77, “Insider Mitigation Program,” Washington, DC.
7. NRC, RG 5.84, “Fitness-for-Duty Programs at New Reactor Construction Sites,” Washington, DC.
8.NRC, RG 5.89, “Fitness-for-Duty Programs for Commercial Power Reactor and Category I Special Nuclear Material Licensees,” Washington, DC.
9. NRC, Draft Regulatory Guide DG-5078 (Proposed New RG 5.99), “Fatigue Management for Nuclear Power Plant Personnel at Commercial Nuclear Plants Licensed under 10 CFR Part 53,” Washington, DC.
10.NRC, “Nuclear Regulatory Commission International Policy Statement,” Federal Register, Vol. 79, No. 132, July 10, 2014, pp. 39415–39418.
11. NRC, Management Directive (MD) 6.6, “Regulatory Guides,” Washington, DC.
12. International Atomic Energy Agency, “Recruitment, Qualification and Training of Personnel for Nuclear Power Plants,” IAEA Safety Standards Series No. NS-G-2.8, IAEA, Vienna, 2004. 22
13.CFR, “Domestic Licensing of Production and Utilization Facilities,” Part 50, Chapter I, Title 10, “Energy.”
14 RG 1.201, “Guidelines for Categorizing Structures, Systems, and Components in Nuclear Power Plants According to Safety Significance,” Washington, DC.
15 NEI 00-04, “10 CFR 50.69 SSC Categorization Guidelines,” Rev. 0, dated July 2005 (ML053910035).
16.CFR, “Licenses, Certifications, and Approvals for Nuclear Power Plants,” Part 52, Chapter I, Title 10, “Energy.”
17.NRC, NUREG/BR-0303, “Guidance for Performance-Based Regulation,” Washington, DC, December 2002.
18.ICF International, “Testing Rates and Deterrence under Subpart K FFD Program,” November 1, 2007. (ML17192A061).
19.NRC, “Safety Culture Policy Statement,” Federal Register, Vol. 76, No. 114, June 14, 2011, pp. 34773–34778.
20. NRC, SRM-SECY-98-144, “Staff Requirements—SECY-98-144—White Paper on Risk Informed and Performance-Based Regulation,” Washington, DC, March 1, 1999.
21.Chang, J., U.S. NRC, “Risk Analysis of Fitness-For-Duty Programs and Spent Nuclear Fuel Transportation Security-redacted version” (ML22081A148).
22.Chang, J., U.S. NRC, “Risk Analysis of FFD Programs Data Sheet-redacted version” (ML22081A153).
23. Condon, C.A., P. Mirick, J.A. Baweja, and A.L. Bunn, “Performance Monitoring Program: Developing Comparative Metrics for Fitness-for-Duty Programs,” PNNL-32470, Rev. 1, Pacific Northwest National Laboratory, Richland, Washington, 2022.
24. NRC Form 890, “Single Positive Test Form,” Washington, DC. (ML22321A221).
25.NRC Form 891, “Annual Reporting Form for Drug and Alcohol Tests,” Washington, DC. (ML22321A193).
26. NRC Form 893, “10 CFR Part 26, Subpart M, Single FFD Policy Violation Form,” Washington, DC.
27. NRC Form 894, “10 CFR Part 26, Subpart M, Annual Reporting Form for FFD Performance Information,” Washington, DC.
28. Wilkinson et al., Journal of Pharmacokinetics and Biopharmaceutics 5(3):207-224, 1977.
29. Compton, R. (2017, July). Marijuana-Impaired Driving - A Report to Congress. (DOT HS 812 440). Washington, DC: National Highway Traffic Safety Administration.
30. Pearlson, G., et al., (2021, September). Cannabis and Driving. Front Psychiatry, doi: 10.3389/fpsyt.2021.689444.
31.Spindle, T., et al., “Study Shows Widespread Mislabeling of CBD Content Occurs for Over-the-Counter Products,” John Hopkins Medicine, https://www.hopkinsmedicine.org/news/newsroom/news-releases/study-shows-widespread-mislabeling-of-cbd-content-occurs-for-over-the-counter-products, July 20, 2022.
32.The Agriculture Improvement Act of 2018, Public Law No. 150-334, December 20, 2018, see section 10113.
33.NRC, “Fitness for Duty Programs,” Federal Register, Vol. 73, No. 62, March 31, 2008, pp. 16966–17235.
34. CFR, “Prescriptions,” Part 1306, Chapter II, Title 21, “Food and Drugs.”
35. Controlled Substances Act, 21 United States Code (U.S.C.) §§ 829, 844(a).
36.NRC, NUREG/CR-7183, “Best Practices for Behavioral Observation Programs at Operating Power Reactors and Power Reactor Construction Sites,” Washington, DC.
37.NRC, NUREG-2155, Rev. 2, “Implementation Guidance for 10 CFR Part 37, ‘Physical Protection of Category 1 and Category 2 Quantities of Radioactive Material,’” Washington, DC. (ML14189A355).
38.NRC, SRM-SECY-19-0033, “Staff Requirements—SECY-19-0033—Discontinuation of Rulemaking—Access Authorization and Fitness-for-Duty Determinations,” Washington, DC, August 6, 2019. (ML18360A185).
39.“The Use of Electronic Communications to Perform Determinations of Fitness,” Pacific Northwest National Laboratory, Richland, Washington, August 2017.
40.Nesbitt, T.S., “The Evolution of Telehealth: Where Have We Been and Where Are We Going?” National Laboratory of Medicine, November 3, 2012, https://www.ncbi.nlm.nih.gov/books/NBK207141/.
41.NRC, MD 8.4, “Management of Backfitting, Forward Fitting, Issue Finality, and Information Requests,” Washington, DC.
This RG is being issued in draft form to involve the public in the development of regulatory guidance in this area. It has not received final staff review or approval and does not represent an NRC final staff position. Public comments are being solicited on this RG and its associated regulatory analysis. Comments should be accompanied by appropriate supporting data. Comments may be submitted through the Federal rulemaking website, https://www.regulations.gov, by searching for draft regulatory guide DG-5073. Alternatively, comments may be submitted to the Office of the Secretary, U.S. Nuclear Regulatory Commission, Washington, DC 20555-0001, ATTN: Rulemakings and Adjudications Staff. Comments must be submitted by the date indicated in the Federal Register notice.
Electronic copies of this RG, previous versions of RGs, and other recently issued guides are available through the NRC’s public website under the Regulatory Guides document collection of the NRC Library at https://www.nrc.gov/reading-rm/doc-collections/reg-guides/index.html. The RG is also available through the NRC’s Agencywide Documents Access and Management System (ADAMS) at https://www.nrc.gov/reading-rm/adams.html, under Accession No. ML22200A037. The regulatory analysis is associated with a rulemaking and may be found in ADAMS under Accession No. ML24095A166.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | 5.84 |
| Author | Harris, Paul |
| File Modified | 0000-00-00 |
| File Created | 2024-11-01 |
© 2026 OMB.report | Privacy Policy
