1273 PRAMS SSA 11_13_24_clean
1273 PRAMS SSA 11_13_24_clean.docx
[NCCDPHP] Pregnancy Risk Assessment Monitoring System (PRAMS)
OMB: 0920-1273
Pregnancy Risk Assessment Monitoring System (PRAMS)
Revision [OMB Control No. 0920-1273, Exp. 11/30/2022]
Supporting Statement
Part A
Contact Person:
Brenda L. Bauman, MSPH
Telephone: 770-488-3756
E-mail: [email protected]
Division of Reproductive Health
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Atlanta, Georgia
October 26, 2022
Updated March 21, 2023
Updated November 13, 2024
Contents
1. Circumstances Making the Collection of Information Necessary 5
2. Purpose and Use of Information Collection 7
3. Use of Improved Information Technology and Burden Reduction 10
4. Efforts to Identify Duplication and Use of Similar Information 12
5. Impact on Small Businesses or Other Small Entities 15
6. Consequences of Collecting the Information Less Frequently 15
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 15
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 15
9. Explanation of any Payment/Gift to Respondents 17
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents 19
11. Institutional Review Board (IRB) and Justification for Sensitive Questions 28
12. Estimates of Annualized Burden Hours and Costs 29
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 35
14. Annualized Cost to Federal Government 35
15. Explanation for Program Changes or Adjustments 35
16. Plans for Tabulation and Publication and Project Time Schedule 36
17. Reason(s) Display of OMB Expiration Date is Inappropriate 38
18. Exceptions to Certification for Paperwork Reduction Act Submissions 38
Attachments
Attachment 1a. Authorizing Legislation-Public Health Service Act
Attachment 1b. Authorizing Legislation-Safe Motherhood and Infant Health Authorization
Attachment 1c. Authorizing Legislation-The Prematurity Research Expansion and Education for
Mothers who deliver Infants Early (PREEMIE) Reauthorization Act of 2018
Attachment 2a. PRAMS Phase 8 Web Survey - MD
Attachment 2b. PRAMS Phase 8 Web Survey – PR
Attachment 2ca. PRAMS Phase 8 Web Survey – SC
Attachment 2d. PRAMS Phase 8 Web Survey – VA
Attachment 2e. PRAMS Phase 8 Web Survey - WY
Attachment 2f. PRAMS Phase 8 Web Mode Data Collection Metrics
Attachment 3a. 60-Day Federal Register Notice
Attachment 3b. Program Response to Comments 60day Federal Register Notice
Attachment 4. PRAMS Questionnaire Development Process
Attachment 5. PRAMS Response Gifts and Incentives by Jurisdiction
Attachment 6. Privacy Impact Assessment
Attachment 7. PRAMS Phase 8 and Phase 9 Questionnaire Topic Reference
Attachment 8a. PRAMS Livebirth Phase 8 Core Mail Questionnaire (English)
Attachment 8b. PRAMS Livebirth Phase 8 Core Mail Questionnaire (Spanish)
Attachment 8c. PRAMS Livebirth Phase 8 Core Phone Questionnaire (English)
Attachment 8d. PRAMS Livebirth Phase 8 Core Phone Questionnaire (Spanish)
Attachment 8e. PRAMS Livebirth Phase 9 Core Mail Questionnaire (English)
Attachment 8f. PRAMS Livebirth Phase 9 Core Mail Questionnaire (Spanish)
Attachment 8g. PRAMS Livebirth Phase 9 Core Phone Questionnaire (English)
Attachment 8h. PRAMS Livebirth Phase 9 Core Phone Questionnaire (Spanish)
Attachment 8i. PRAMS Livebirth Phase 9 Core Web Questionnaire (English)
Attachment 8j. PRAMS Livebirth Phase 9 Core Web Questionnaire (Spanish)
Attachment 9a. PRAMS Stillbirth Mail Questionnaire (English)
Attachment 9b. PRAMS Stillbirth Mail Questionnaire (Spanish)
Attachment 9c. PRAMS Stillbirth Phone Questionnaire (English)
Attachment 9d. PRAMS Stillbirth Phone Questionnaire (Spanish)
Attachment 10a. PRAMS Livebirth Phase 8 Standard Mail Modules (English)
Attachment 10b. PRAMS Livebirth Phase 8 Standard Mail Modules (Spanish)
Attachment 10c. PRAMS Livebirth Phase 8 Standard Phone Modules (English)
Attachment 10d. PRAMS Livebirth Phase 8 Standard Phone Modules (Spanish)
Attachment 10e. PRAMS Livebirth Phase 9 Standard Mail Modules (English)
Attachment 10f. PRAMS Livebirth Phase 9 Standard Mail Modules (Spanish)
Attachment 10g. PRAMS Livebirth Phase 9 Standard Phone Modules (English)
Attachment 10h. PRAMS Livebirth Phase 9 Standard Phone Modules (Spanish)
Attachment 10i. PRAMS Livebirth Phase 9 Standard Web Module (English)
Attachment 10j. PRAMS Livebirth Phase 9 Standard Web Module (Spanish)
Attachment 11a. PRAMS Social Determinants of Health Supplement Mail and Phone
Attachment 11b. PRAMS Social Determinants of Health Supplement Web
Attachment 11c. PRAMS COVID-19 Vaccine Supplement Mail and Phone
Attachment 11d. PRAMS COVID-19 Vaccine Supplement Web
Attachment 11e. PRAMS COVID-19 Experiences Supplement Mail and Phone
Attachment 11f. PRAMS COVID-19 Experiences Supplement Web
Attachment 11g. PRAMS Opioid Use Supplement Mail and Phone
Attachment 11h. PRAMS Opioid Use Supplement Web
Attachment 11i. PRAMS Marijuana Use Supplement Mail and Phone
Attachment 11j. PRAMS Marijuana Use Supplement Web
Attachment 11k. PRAMS Disabilities Supplement Mail and Phone
Attachment 11l. PRAMS Disabilities Supplement Web
Attachment 12a. PRAMS Livebirth Mail Introduction and Informed Consent (English)
Attachment 12b. PRAMS Livebirth Mail Introduction and Informed Consent (Spanish)
Attachment 12c. PRAMS Stillbirth Mail Introduction and Informed Consent (English)
Attachment 12d. PRAMS Stillbirth Mail Introduction and Informed Consent (Spanish)
Attachment 12e. PRAMS Livebirth Phone Introduction and Informed Consent (English)
Attachment 12f. PRAMS Livebirth Phone Introduction and Informed Consent (Spanish)
Attachment 12g. PRAMS Stillbirth Phone Introduction and Informed Consent (English)
Attachment 12h. PRAMS Stillbirth Phone Introduction and Informed Consent (Spanish)
Attachment 12i. PRAMS Livebirth Web Introduction and Informed Consent (English)
Attachment 12j. PRAMS Livebirth Web Introduction and Informed Consent (Spanish)
Attachment 13. IRB Approval
Attachment 14. PRAMS Livebirth Respondent Counts by Jurisdiction
Attachment 15a. PRAMS Field Testing Methodology
Attachment 15b. PRAMS Field Testing Questionnaire English and Spanish
Attachment 15c. PRAMS Field Testing Questionnaire Informed Consent (English)
Attachment 15d. PRAMS Field Testing Questionnaire Informed Consent (Spanish)
Attachment 16. Summary of PRAMS Core Question Changes from Phase 8 to Phase 9
Attachment 17. PRAMS Phase 9 Cognitive Testing Report
Attachment 18. PRAMS Phase 9 Field Testing Report
Attachment 19. PRAMS Nonresponse Bias Assessment of 2019 Data
Attachment 19a. PRAMS Nonresponse Bias Assessment of 2020 and 2021 Data
Summary
Goal of the project: The goal of the Pregnancy Risk Assessment Monitoring System (PRAMS) is to collect jurisdiction-specific (states, cities, and U.S territories), population-based data on maternal behaviors and experiences before, during, and shortly after pregnancy.
Intended use of the resulting data: PRAMS is a jurisdiction customized survey conducted in 50 jurisdictions in the United States (U.S.). The data are used to identify groups of women and infants at high risk for health problems, monitor changes in health status, and measure progress towards state and national objectives in improving the health of mothers and infants. PRAMS data are also used by researchers to investigate emerging issues in the field of reproductive health and by federal, state, and local governments to plan and review programs and policies aimed at reducing health problems among mothers and babies.
Methods to be used to collect the data: Information is collected 2-6 months after live birth or stillbirth by mail and web survey, with telephone follow-up for non-responders. Call back surveys may be implemented as a follow up to the initial survey to gather additional information on post-pregnancy experiences and infant and toddler health. Because PRAMS uses standardized data collection procedures and instruments, it allows data to be compared among jurisdictions. Respondents are selected through a stratified systematic sample pulled monthly from the vital records birth certificate or fetal death files in each participating jurisdiction. Ad-hoc sampling strategies such as hospital-based may be used when needed, such as during an emergency response.
Subpopulation: The subpopulation to be studied for PRAMS is women who recently delivered a live born or stillborn infant.
How data will be analyzed: CDC analyzes aggregated or multi-site data to produce estimates on selected maternal and infant health indicators. Descriptive analysis methods are used to estimate prevalence and 95% confidence intervals while bivariate and multivariable regression methods are used to identify relationships between health behaviors and experiences and health outcomes via odds ratios or prevalence risk ratios. Grantees also employ similar analytic methods to analyze jurisdiction specific data.
Justification
This Information Collection Request (ICR) is for a revision of a previous ICR (OMB #0920-1273, Expiration 11/30/2022) to continue the implementation of the Pregnancy Risk Assessment Monitoring System (PRAMS) for three more years. PRAMS is joint surveillance effort between CDC and participating jurisdictions, funded by cooperative agreement DP21-001. CDC is collecting data for Phase 8 of the PRAMS survey until March 2023. CDC is seeking a revision due to inclusion of a new version of the PRAMS survey (Phase 9) to be used beginning with April 2023. In addition, a revision is requested to implement a web mode of data collection for all participating jurisdictions for Phase 9 of PRAMS.
PRAMS data supports CDC’s Safe Motherhood and Infant Health activities to enhance the understanding of maternal behaviors and experiences and their relationship with adverse pregnancy and infant outcomes. PRAMS is a population-based surveillance system that provides data not available from other sources and complements information provided by birth certificates with more details on behaviors and experiences from the pre-pregnancy, prenatal and postpartum period. PRAMS uses a stratified systematic sample of women who recently delivered a live birth or stillbirth; with statistical weighting, PRAMS becomes representative of the jurisdictions’ live birth or fetal death population. PRAMS data is used by participating states and jurisdictions for needs assessment and to inform program development and evaluation, health care services and policy.
PRAMS has a core set of questions common across all state and other jurisdictions (i.e., large cities and U.S. territories) grantees (henceforth referred to as “jurisdictions”). There are optional standard modules that jurisdictions can choose to include from a CDC developed bank of questions to address state-specific priorities or special topics (e.g., adverse childhood experiences, emerging tobacco products). In addition, jurisdictions can choose to field state-added questions that are independently jurisdiction-designed. In most jurisdictions, PRAMS is an ongoing survey that produces annual, jurisdiction-level estimates. Jurisdictions not intending to implement the survey on an ongoing basis can alternatively employ a point-in-time survey for one year of data collection.
Increasingly, PRAMS infrastructure is used to support special-purpose information collection (supplemental modules). PRAMS has the capability of rapidly adding supplemental modules to address emerging issues (e.g., COVID-19, social determinants of health, prescription opioid use). PRAMS has also been used to implement call back surveys to gather additional information on post-pregnancy experiences and infant health and toddler health (e.g., opioid call back survey). Consistent with terms of clearance for PRA approval for PRAMS and the Behavioral Risk Factor Surveillance System (BRFSS), CDC will use the change request mechanism for approval of supplemental modules or call back surveys that collect additional data on topics covered by the Phase 9 PRAMS survey. If the content is not consistent with what is included in the Phase 9 survey and is deemed an emergency, OMB will consider an emergency clearance. Per the discussion in section 12, anticipated burden hours are included in the burden table. Cognitive and field testing will be done before the implementation of new or revised supplemental modules or call back surveys.
Ongoing surveillance efforts that quantify disease and risk factors and identify opportunities for prevention are central to CDC’s planning and evaluation efforts. CDC’s authority to collect information for this purpose is provided by the Public Health Service Act (PHSA; Attachment 1a), and PRAMS is authorized under section 317(k) (Attachment 1b). Furthermore, PRAMS data is essential to informing CDC’s Safe Motherhood and Infant Health activities. An additional example of legislative support for PRAMS can be seen in the Prematurity Research Expansion and Education for Mothers who deliver Infants Early (PREEMIE) Act, which supports the use of PRAMS to track pregnancy outcomes and help prevent preterm birth (Attachment 1c).
PRAMS is an ongoing, population-based surveillance system based on a stratified systematic sample designed to produce annual estimates of selected maternal experiences and behaviors that occur during the months prior to and during pregnancy, as well as the first months after pregnancy for each site. Data for PRAMS are collected by funded jurisdictions from women who recently gave birth to a live born or stillborn infant. As of May 2021, PRAMS is funded in 50 jurisdictions. The births in the 50 jurisdictions that participate in PRAMS surveillance are 81% of all live births in the United States. Jurisdictions that collect data on women with recent livebirths include 46 states, New York City, Washington, DC, Puerto Rico, and the Commonwealth of the Northern Mariana Islands. Utah is the only site also funded to collect data on women with a recent stillbirth.
Findings from PRAMS are used to enhance the understanding of maternal behaviors and their relationship with adverse pregnancy outcomes. PRAMS data can also be used by participating jurisdictions for needs assessment and to inform program development and evaluation, as well as health care services and policy. PRAMS data are used to monitor various targets in Healthy People 2030, preconception health and health care indicators, and selected performance measures for various programs and initiatives (e.g., Title V Maternal and Child Health Program and The Collaborative Improvement and Innovation Network to Reduce Infant Mortality).
PRAMS data from 2017-2018 was used as an evaluation component by the Maternal and Child Health Bureau (MCHB) in the Health Resources and Services Administration (HRSA) for the Healthy Start program evaluation. At the time of the evaluation, 63 of the 75 continuing Healthy Start grantees eligible for selection were located in jurisdictions that conduct the PRAMS survey. From among these grantees, MCHB/HRSA randomly selected 15 jurisdictions with Healthy Start grantee to participate for the one-time oversampling (oversampling of Healthy Start participants for 2017- 2018 only). The goal of the evaluation was to determine the effect of the Healthy Start program on changes in participant-level characteristics (e.g., health services utilization, preventive health behaviors, health outcomes). For one component of the overall program evaluation, HRSA compared Healthy Start participants and non-participants using PRAMS and linked vital records for key benchmarks and outcomes such as low birth weight; preterm birth; perinatal depression screening; breastfeeding initiation; safe sleep practices; and health care access into the postpartum period. Future collaborations with federal partners to augment PRAMS to oversample special populations will be submitted as a change request for approval.
PRAMS data is used to inform maternal and infant health programs and health policy to reduce maternal and infant morbidity and mortality related to several purposes by a diverse set of users. The primary uses of the data are listed below:
PRAMS data, combined with California’s Maternal and Infant Health Assessment (MIHA) survey, will be used to track several Healthy People 2030 objectives related to Maternal, Infant, and Child Health. The live births in the 50 jurisdictions that participate in PRAMS and California MIHA are 96% of all U.S. live births. Current Healthy People 2030 objectives using PRAMS data include:
MICH-14 – Increase the proportion of infants who are put to sleep on their backs
Three developmental objectives
MICH-D01 - Increase the proportion of women who are screened for postpartum depression at their postpartum checkup postpartum depression screening
MICH-D02 – Reduce the proportion of pregnant women who use illicit opioid pain relievers during pregnancy
MICH-D03 – Increase the proportion of infants who are put to sleep in a safe sleep environment
Jurisdiction health departments will use PRAMS data for needs assessment (e.g., HRSA Title V needs assessments), planning and reviewing programs, and policies aimed at reducing health problems among mothers and babies.
PRAMS data will be used by jurisdiction maternal and child health program staff, policy makers, and health providers to identify gaps in health care provision or utilization.
PRAMS data will be used by researchers to investigate emerging issues in the field of reproductive health.
PRAMS is a key data source for preconception indicators and will be used by a variety of agencies in planning maternal and infant health programs.
Researchers can request a multi-site dataset with de-identified individual-level data according to the guidelines on the PRAMS website at PRAMS Data | PRAMS | CDC. This multi-site PRAMS dataset is updated annually and consists of weighted data from jurisdictions that participate in the PRAMS Automated Research File for the surveillance year. The multi-site dataset is used to provide jurisdiction-level estimates for maternal and child health indicators, health risk, and protective behaviors. Data will be appropriate for trend analyses; tests of differences among (demographic) subpopulations; multivariate analyses of health outcomes; and other statistical analyses. The dataset can be used to calculate aggregate estimates for the included jurisdictions, but is not weighted to provide national estimates of health indicators. The aggregate estimates are not nationally representative, but instead reflect the combined estimates from jurisdictions included in the dataset and the estimates are proportional to the number of live births in each jurisdiction.
An annual summary of maternal and child health indicators derived from PRAMS data are available in aggregate and by jurisdiction for public health reporting at https://www.cdc.gov/prams/php/data-research/mch-indicators-by-site.htm. Findings from PRAMS are also used by jurisdictions to inform program and policy that improve maternal and child behaviors and outcomes as described PRAMS Data to Action Success Stories | PRAMS | CDC .
Field testing data under this approval will be used to identify issues that may affect implementation of the questionnaire.
The PRAMS data are collected using the PRAMS Integrated Data Collection System (PIDS). PIDS is used to schedule and track data collection activities; record data on mail, web, and telephone operations (e.g., when survey was completed by mail or phone) manage call attempts for telephone interviews; and record survey responses and capture additional comments provided by mothers. The PIDS system consolidated several standalone data collections systems for mail data entry, telephone interviewing, and tracking activities into a secure web-based single point-of-entry centralized database. PIDS allows centralized data storage and real-time updates on participants’ survey completion status. PIDS is implemented using Commercial off-the-shelf [COTS] software tools that encompasses the latest technology.
PRAMS is currently a mail survey with telephone follow–up. Due to the flexibility of the PIDS data collection system, women have the option of completing the survey by mail or telephone interview. The combination of multiple contacts and mixed data collection modes has proven effective in increasing response rates in many populations. The specific modes selected for PRAMS complement one another to maximize response rates while minimizing cost.
Telephone surveys offer a cost-effective method of data collection to engage individuals who do not have a permanent address or who prefer telephone interviews to self-administered paper surveys. Telephone data collection can accommodate the respondent’s schedule by arranging to conduct interviews at a convenient time. Interviewers use Computer Assisted Telephone Interview (CATI) software to enter data directly into the centralized database. Use of CATI software promotes efficiency in two ways: skip patterns are programmed in the survey instrument to route interviewers to ask respondents only questions that they are eligible to answer, and real-time quality control checks can be used to eliminate errors which may have been caused by manual data entry procedures.
To further the use of improved information technology, CDC, through a contractor, has developed a web mode in PIDS that is integrated alongside the mail and telephone features for livebirths data collection. The intent is for participants to have the option of three modes by which to complete the PRAMS survey; respondents can complete the survey by mail, phone or web depending on their own needs and preferences. The web mode may better reach some populations that have lower response rates in PRAMS data and result in an increase in the response rate overall and improved representativeness of survey respondents. Web mode will not be offered in stillbirth data collection.
In the spring of 2022, OMB approved implementation of the web survey mode in five sites (Maryland, Puerto Rico, South Carolina, Virginia, and Wyoming). Web versions of the Phase 8 survey were programmed in PIDS for these five early adopter sites (Attachment 2a-2e) and deployed in May 2022. The May 2022 batch completed the 90-day data collection cycle at the end of July 2022. Analyses comparing metrics for the May 2022 web batch with the April 2022 batch (for which participants did not have the web mode option) examined changes in response rates, response rates by subpopulation, data quality (measured by item non- response), and grantee resources and costs. Results are summarized below with additional details provided in Attachment 2f.
Across each metric examined, the post-web metrics indicated modest overall improvement relative to their pre-web counterparts. After the web mode was implemented, there was, on average, a 2 percentage point increase in response rates, improved response rates for all categories of race, ethnicity, and education level examined, reduced item nonresponse as compared to mail and telephone modes, reduced postage costs by 4% to 13%, reduced staff hours expended by 6% to 41% in all but one site, and no change in the number of technical support queries from sampled mothers. CDC continues to monitor response rates and other performance measures by mode of data collection for the five early adopter sites to identify strategies and technical assistance needs for jurisdictions to address response rates when implementing the web mode. Based on these findings from Phase 8 web mode of data collection, PRAMS will add the web mode to Phase 9 data collection in April 2023. Jurisdictions will be onboarded for web mode in phases so each jurisdiction will receive adequate technical assistance should they need it at the start of implementation. By September 2023, all 50 jurisdictions will be offering web mode, in addition to mail and telephone data collection.
PRAMS regularly collaborates with other federal agencies to identify other sources of maternal and infant data that may be duplicative and identifies opportunities to leverage and learn from other data collection systems. Because only 1-5% of the general population is pregnant or postpartum at any time, there is a need for data that purposively samples from this population to provide stable estimates that can be stratified by population subgroup as well as to provide jurisdictional-specific estimates of maternal experiences and behaviors that occur before, during, and shortly after pregnancy.
Data on maternal health may be available at the national level but are not designed specifically to create jurisdiction-based estimates. National-level surveys such as the National Survey of Children’s Health, the National Survey of Family Growth (NSFG; OMB No. 0920-0314, exp. 12/31/2024), the National Health Interview Survey (NHIS, OMB No. 0920-0214, exp. 12/31/2023), the National Health and Nutrition Examination Survey (NHANES; OMB No. 0920-0950, exp. 4/30/2023), among others, collect data on maternal health (e.g., pregnancy history) to produce prevalence estimates at the national level. PRAMS differs in that its sample is at the jurisdiction level and produces direct (i.e., not modeled) estimates for jurisdictions.
Other jurisdiction-based surveillance systems such as the Behavioral Risk Factor Surveillance System (BRFSS; OMB No. 0920-1061, exp. 12/31/2024) and the Youth Risk Factor Surveillance System (YRBS; OMB No. 0920-0493, exp. 11/30/2023) collect jurisdiction-level population-based estimates that may be relevant to maternal health such as use of family planning methods. While BRFSS is a sample of the adult general population in each jurisdiction and YRBS is a sample of middle and high school students in each jurisdiction, PRAMS is the only data system specific to women with a recent live birth or stillbirth. New mothers and women currently pregnant may be found among BRFSS- or YRBS-sampled population, but they constitute a population subgroup that is usually too small for detailed analysis. This limitation expresses itself in two ways: small numbers generally preclude making statistically reliable estimates for various topics on the general survey that are specific to this subgroup; and their rarity in the sample makes it inefficient to ask questions specific to pregnancy and childbirth, as the questions would rarely be applicable to respondents.
The National Institutes of Health (NIH) hosts a passive data collection system called PregSource. PregSource uses a crowd-sourcing approach, asking pregnant women to enter information regularly and directly about their pregnancies throughout gestation and the early infancy of their babies into online surveys and trackers via a website and/or mobile application. PregSource does not collect state-level population-representative data nor collect the breathe of information to inform indicators of national or state interest.
CDC actively engages with partners for feedback on survey priority topics and new questions. During 2021 and 2022, CDC engaged in a highly collaborative process to develop Phase 9 of the PRAMS survey. Over 300 federal, state, local, academic, and professional organizations, were invited to submit proposals to add new PRAMS questions or modify the existing questions. In response, PRAMS received 155 unique requests to add new questions and 146 requests to modify existing PRAMS questions for Phase 9. These requests, along with questions from the current phase (Phase 8) questions, were evaluated by subject matter experts and selected through multiple rounds of review for inclusion in the Phase 9 questionnaire. All PRAMS grantees and the Division of Reproductive Health questionnaire revision workgroup were invited to participate in the review process. Furthermore, PRAMS regularly consulted with other federal agencies and a range of centers and divisions within CDC to ensure new and existing PRAMS survey questions align with current standards and practices for specific topics. Examples of collaborations include the Health Resources and Services Administration for infant sleep environment and food insecurity questions; Food and Drug Administration for cigarette and e-cigarette use during pregnancy; National Institute of Child Health and Human Development (National Institute of Health) for disability; CDC’s Office of Minority Health for experiences of racism and discrimination; CDC’s National Center for Birth Defects and Developmental Disabilities for alcohol use during pregnancy; CDC’s National Center for Immunization and Respiratory Diseases for maternal vaccination; CDC’s Division of Heart Disease and Stroke Prevention for blood pressure monitoring; CDC’s Division of Violence Prevention for intimate partner violence and adverse childhood experiences; and CDC’s Division of Oral Health for dental care utilization during pregnancy.
Additionally, to ensure that duplicative information is not collected, data elements (e.g., demographic information) that are already available from the existing source datasets (e.g., birth certificates or fetal death records) for the sample are not collected by PRAMS. To determine the accuracy of select PRAMS self-reported survey data and birth certificate data, CDC compared these data to hospital delivery and prenatal care records in 2009. Based on the findings of this internal validity study, modifications were made to the core PRAMS survey to improve efficiency. The PRAMS team used the results to improve and/or remove select items from the current PRAMS survey. For example, past PRAMS surveys asked women about the number of prior live births and whether their last birth resulted in a low birthweight and/or preterm infant--information also captured on the birth certificate. For the current, Phase 8 PRAMS survey, these items were removed from the core survey due to the high validity on the birth certificate; therefore, reducing the length of the PRAMS survey, limiting participant burden, and saving the government time and money. Other items, such as length of hospital stay, were kept on the survey when it was found that PRAMS data provided accurate supplemental information not collected on the birth certificate.
This approach continued in the development of the Phase 9 PRAMS survey. To further reduce participants’ burden, we looked to eliminate questionnaire redundancy with birth certificate data by examining additional indicators that are common to both the PRAMS survey and the birth certificate. PRAMS reviewed nine published validations studies of PRAMS data against the birth certificate data, compared select indicators on the PRAMS survey against those obtained from the birth certificate, and consulted with colleagues from the National Center for Health Statistics to discuss the viability of removing certain PRAMS survey questions and using birth certificate data instead. Based on these findings, questions on height, weight, vitamin use, preconception care, infant date of birth, and timing of first prenatal care visit were dropped from the Phase 9 survey as this information can be accurately captured from birth certificate data. Certain questions were also made shorter by collapsing the responses options for questions on e-cigarette use, smoking, alcohol use, health insurance coverage (preconception, prenatal, and postpartum), life stressors, and income into fewer response categories.
There will be no impact on small business.
PRAMS is an ongoing surveillance system providing annual estimates of maternal behaviors and experiences before, during, and shortly after pregnancy. These data are used for the purposes of monitoring trends; allowing state-by-state comparisons; and used by states and federal agencies for reporting of performance measures and other benchmarks. Collection of data less frequently would prohibit calculation of these annual estimates and inhibit the utility of the data. Trend analyses created from annual estimates and the ability to monitor health impact changes would be interrupted by less frequent data collection. Furthermore, ongoing data collection maintains the infrastructure to monitor the health impacts of any state policy changes and to collect data on emerging issues.
There are no special circumstances. This request complies with the regulation of 5 CFR 1320.5.
A 60-day notice was published in the Federal Register on July 5, 2022 (Vol. 87, No. 127, 39837-39839) to make the public aware of this information collection (Attachment 3a). Two comments were received: one non-substantive and one in support of the data collection; a response was developed (Attachment 3b); and no modifications were needed to the information collection plan in response to these comments. A 30-day notice will be published in the Federal Register to allow for additional public and affected agency comments.
The PRAMS questionnaire development process is described in Attachment 4. Efforts to consult within and outside of the agency are meant to ensure relevant core and emerging topics are captured; obtain external partner feedback on the survey questions; and ensure continued relevance and utility. Consulted organizations are listed in Table A.8-1.
Table A.8-1. Consulted organizations for questionnaire development process
Organizations Consulted (2014-present) |
|
Name/Organization |
Subject Matter Expertise Provided |
CDC, Division of Reproductive Health |
Contraception, safe infant sleep, weight gain during pregnancy, chronic conditions, disaster preparedness, sexually transmitted infections |
CDC, Division of Nutrition, Physical Activity, and Obesity |
Breastfeeding, food insecurity |
CDC, Division of Oral Health |
Dental care utilization |
CDC, Emergency Operations Center |
Zika |
CDC, Immunization Services Division |
Influenza vaccine, Tdap, COVID-19 |
CDC, National Institute for Occupational Safety and Health |
Occupation |
CDC, National Center for Injury Prevention and Control |
Opioid use/misuse; intimate partner violence, adverse and positive childhood experiences |
CDC, National Center for Birth Defects and Developmental Disabilities |
Infant development, folic acid, alcohol use |
CDC, Office on Smoking and Health |
E-cigarette and hookah use |
CDC, National Center for Heart Disease and Stroke Prevention |
Blood pressure monitoring |
FDA |
E-cigarette and hookah use |
HRSA |
Infant health care visits, safe infant sleep, food insecurity |
NIH |
Disability |
CMS, SAMSHA |
Opioid use/misuse |
Jurisdiction Health Department PRAMS/MCH Programs Consulted for Phase 9 Questionnaire Development (2022) |
|
Alabama |
Nebraska |
Alaska |
Nevada |
Arizona |
New Hampshire |
Arkansas |
New Jersey |
California |
New Mexico |
Colorado |
New York City |
Connecticut |
New York State |
Delaware |
North Dakota |
District of Columbia |
Northern Mariana Islands |
Florida |
Oklahoma |
Georgia |
Oregon |
Hawaii |
Pennsylvania |
Illinois |
Puerto Rico |
Indiana |
Rhode Island |
Iowa |
South Carolina |
Kansas |
South Dakota |
Kentucky |
Tennessee |
Louisiana |
Texas |
Maine |
Utah |
Maryland |
Vermont |
Massachusetts |
Virginia |
Michigan |
Washington |
Minnesota |
West Virginia |
Mississippi |
Wisconsin |
Missouri |
Wyoming |
Montana |
|
PRAMS has historically worked with grantees to identify best strategies to improve response rates and has offered grantees the option of providing gifts and response incentives since 1989. Jurisdictions--not CDC--individually decide whether to provide a gift, which is offered to all sampled mothers, and/or response incentives, which is offered to mothers who respond to the survey. Due to the geographic, cultural, demographic, and economic diversity of the United States, jurisdictions also choose the type of gift and/or incentive that they provide. Each jurisdiction documents their choice of gift and/or incentive in their PRAMS Protocol that is submitted for IRB review at the jurisdiction level. Most PRAMS jurisdictions offer gifts to all sampled mothers and incentive to sampled mothers who responded to the survey. A few jurisdictions offer either a gift or an incentive, but not both. In general, gifts and incentives have been found to be important for encouraging participation in federal surveys, especially for more reluctant responders.1,2,3 For PRAMS, increasing motivation to respond readily is particularly salient: this survey focuses on a special population during a limited time period following the birth of an infant when women indicate that participating in even simple activities is constrained by lifestyle changes, financial constraints, childcare duties, and fatigue.4,5 Given these constraints, jurisdiction -level gifts and incentives have been found to be important and useful for PRAMS, so that they can be tailored to those less likely to respond in a given jurisdiction (e.g., from racial and ethnic minority groups, and women with lower education or literacy levels, lower income, .6) as well as respondents that require additional follow up (phone responders).
PRAMS jurisdictions have historically conducted experiments to determine what kind of gift or incentive is most effective in motivating survey response as part of the PRAMS protocol.7,8 Gifts are often of nominal value and are sent to all sampled mothers, usually in the first survey mailing. A gift is provided prior to filling out the survey and is independent of participation. Examples of these gifts include pens, refrigerator magnets, thermometers, pocket calendars, and baby bibs. Response incentives are provided to individuals who complete the survey. Jurisdictions may employ different strategies in offering an incentive to maximize participation. Some jurisdictions offer the same incentives to all individuals who complete the survey, and some offer a choice of incentives. Those individuals who do not respond to the three mailings may be more difficult to recruit for participation and, as such, a few jurisdictions offer slightly larger incentives to those who complete a phone interview (i.e., phone contacts and interviews only begin after a mother has been sent three mailings and has not responded). Some jurisdictions only provide incentives to those that complete the survey through a phone interview. Incentives tend to be higher in value than gifts. Examples of incentives include $10-$30 gift cards to grocery stores or other local retail stores, birth certificates, diaper packs, music CDs, and tote bags. At the time of the initial Paperwork Reduction Act approval for PRAMS, jurisdictions were providing a range of incentives which, in some cases, are valued over $25. Changing incentives already in place in jurisdictions could detrimentally effect response rates. In the event a jurisdiction would like to offer a new response incentive greater than r $25, CDC will consult with OMB. CDC will track the relationship between incentive size and response rates, particularly for populations that are less likely to respond. CDC will also work with jurisdictions to conduct experiments to assess the impact of changes to incentive size.
Jurisdictions have conducted experiments to establish appropriate incentive levels. In 2009, in order to increase telephone response rates, Missouri PRAMS experimented with adding a $20 gift card for women who responded to the telephone survey. They compared six months of data collection without this incentive and six months of data collection with the incentive. The telephone response rate increased by 6.5 after the implementation of the incentive. During this same time period, mail responders were continued to be offered a $10 gift card with no change. There was no observed increase in the mail response. Louisiana conducted an experiment in 2014 to compare the effect of raising the value of their gift card incentive from $10 to $20 on response rates. They observed an increase of 5 percentage points in overall response and a significant increase of 12 percentage points in mail response using the higher value incentive. Among subgroups, response rates increased for: 1) Black mothers living in Orleans parish (14 percentage point increase); 2) Black mothers living outside of Orleans parish with low birth weight infants (13 percentage point increase); and 3) Black mothers living outside of Orleans parish with normal birth weight infants (11 percentage point increase). The results also indicated the higher value incentive was more cost effective than devoting resources to increase follow-up efforts.
Women with a recent stillbirth experienced a recent pregnancy loss and they will still be grieving the loss of their stillborn child; thus, gifts and/or incentives will be differentiated from mothers with a recent livebirth for whom their infant has died. Sensitivity considerations are built into all components of the data collection methodology.
A full list of gifts and incentives by jurisdiction is included in Attachment 5. An incentive, valued at $25, will be offered to individuals participating in field testing (Attachments 15a).
Privacy Act Determination
PRAMS data collection activities rely on the PRAMS Integrated Data Collection System, also known as PIDS. All PRAMS grantees are required to use PIDS. PIDS contains information from jurisdiction birth/fetal death certificates which is used by the jurisdictions to contact sampled mothers for data collection and to link to the birth certificate file for data weighting. The National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) Information Systems Security Officer determined that the Privacy Act does apply to PRAMS because of the use of personally identifiable information (PII) from the birth certificate that is collected in PIDS (Attachment 6). As a result, the PRAMS questionnaires will include a Privacy Act notice.
A text file is created at the jurisdiction level that contains personally identifiable information (PII) from the birth/fetal death certificate; the text file is imported into PIDS data system. Monthly batch files are imported by PRAMS jurisdictions into PIDS. Batch files contain PII such as mother’s name, date of birth, address, telephone number, and the infant’s name and date of birth. To identify the sample for PRAMS, CDC receives from the jurisdictions through PIDS the birth certificate number and dates of birth. Once the sample is selected, a PRAMS ID is assigned to individual records and the web UserID and passcode are also assigned for each sampled mother for the web data collection mode. The jurisdictions, but not CDC, have name and addresses for moms selected for the sample. PII maintained in the system is used by jurisdictions to generate letters and labels to be mailed to respondents for PRAMS surveillance. Similarly, PII is used to verify a respondent’s identity when a phone interview is conducted. If a respondent elects to complete the survey on the web, mother’s year of birth is used to verify a respondent’s identity once logged into the web mode of data collection. Because of PII uploaded and retrieved by PIDS, the system is categorized as a moderate level system for security purposes. Mothers are assigned a unique ID in PIDS for all data collection functionality including linkages for follow-up surveys. The unique ID is randomly generated and cannot be used to identify respondents. PII maintained at the jurisdiction level is destroyed when the annual weighted data set is received.
Personally identifiable information (PII) data are transmitted and loaded into PIDS from jurisdictions to CDC through a secure, encrypted https protocol. All PIDS data are stored and accessed in accordance with industry standard procedures. No information in identifiable form (IIF) will be filed or retrieved by the name of the individual or other unique respondent identifier such as social security number. PII Data stored in PIDS are only accessible to select jurisdiction users and contract PIDS developers. CDC staff members, outside of those who conduct statistical weighting and PIDS system maintenance, do not have access to PII data. The System of Records Notice (SORN) being used for PIDS is 09-20-0160: http://www.cdc.gov/SORNnotice/09-20-0160.htm.
Overview of the Data Collection System
The PRAMS sample is drawn from the birth certificate or fetal death record file monthly by collaborators in the jurisdiction’s Vital Records Department. It is delivered to the jurisdiction PRAMS staff usually within the first week of each month. Jurisdiction PRAMS staff clean up the file, making sure addresses, phone numbers, or any other missing information are completed, before uploading the sample file into PIDS. Jurisdiction PRAMS staff initiate the data collection process, starting with contacting sampled mothers by mail. In the pre-survey letter and the three mailings that include paper questionnaires, sampled mothers can complete the survey by paper (mail) or web. Information on how to complete the survey by web is included in the pre-survey letter and in the three mailings that include the questionnaire. After the three mailings, sampled mothers who did not respond by mail or web are contacted by telephone.
The PIDS web-based data collection software system is provided by the CDC to all funded grantees (PRAMS jurisdictions) for collection of livebirths data. Data collection on stillbirths are completed in REDCap and not in PIDS. CDC, through the support of a contractor, programs each jurisdiction’s mail survey into PIDS for data entry, creates web survey screens for PIDS web data collection, and programs the telephone survey into the CATI interface within the PIDS system. Responses from the returned paper surveys (mail) are entered into PIDS’ mail data entry portal and telephone interview responses are entered into CATI by interviewers as they are conducting the interviews. For the web mode, respondents access the survey through a secure CDC website which interfaces with the PIDS system to record web responses. Data entry verification for information entered from the mail surveys is performed by the jurisdiction, where at least 10% of returned surveys are entered twice, during verification process, into PIDS to ensure that the initial mail data entry is correct. Data entry during the verification process are not saved in PIDS. Jurisdictions and CDC can view operational summary reports at any time in the PIDS system. Jurisdiction signal the end of data collection for a monthly batch by releasing the data to CDC using a batch expiration feature in the PIDS system, after the 90-day data collection period for each batch. At the end of data collection for a calendar year, all jurisdiction data are cumulated, and final cleaning and quality control checks are done at CDC. Once the data are cleaned and checked, data weighting is done. PRAMS does not report discordant self-reported data back to jurisdiction vital records for corrections to the birth certificate. As part of the final cleaning and quality control checks, for each sampled respondent, CDC verifies the variables captured on the finalized jurisdiction birth certificate file for the calendar year to ensure alignment with variables captured in the PRAMS data set.
Items of Information to be Collected
A complete PRAMS questionnaire topic reference is provided for Phase 8 and Phase 9 in Attachment 7. The questionnaires are laid out by CDC programmers in PIDS for each jurisdiction at the beginning of each data collection cycle (phase). PRAMS core questionnaire for Phase 8 livebirths (Attachments 8a-8d), Phase 9 livebirths (Attachments 8e-8j), and stillbirths (Attachments 9a-9d) includes questions on maternal behaviors and experiences before, during, and shortly after pregnancy and includes information on health conditions, utilization and content of health services, and risk behaviors. Demographic information such as race/ethnicity and age is not collected on the PRAMS questionnaire because that information is already available in the birth certificate or fetal death file from which the sample is drawn.
Standard module questions (for livebirths only) are selected by individual jurisdictions based on their information needs and are generally implemented as written. However, jurisdictions may elect to drop options from the list of responses for some questions if jurisdictions feel those responses are not applicable in their context. Phase 8 Standard modules (Attachment 10a-10d) and Phase 9 Standard modules (Attachment 10e-10j) cover a range of health topics. CDC programs each jurisdiction-specific survey into PIDS and jurisdictions administer their tailored version of the survey (core plus standard modules) without change throughout the questionnaire phase, which typically lasts between 3 and 5 years.
CDC periodically funds PRAMS jurisdictions to rapidly implement supplemental modules developed mid-phase by CDC to address emerging issues. Jurisdictions have the option to continue to collect CDC developed supplemental modules in the absence of funding. Supplemental modules that are being collected by jurisdictions for the remainder of Phase 8 include social determinants of health, COVID-19 vaccine, COVID-19 experiences, prescription and illicit opioid use, marijuana, and disability (Attachments 11a-11l). Jurisdictions may elect to include these supplements during Phase 9, except for the disability supplement which is now integrated into the core. These supplements can be added for one or more birth years but can be discontinued at the end of a year of data collection. If jurisdictions elect to add additional supplemental modules to the survey mid-phase to address emerging needs and priorities, questions are added as attached leaflets to the mail survey and added to the end of questionnaire in PIDS CATI for the phone data collection mode.
For jurisdictions implementing the web mode of data collection, web versions of the questionnaire for each jurisdiction collecting livebirths data are also programed in PIDS. The web mode of data collection is not available for stillbirth data collection. The full Phase 8 surveys for each of the five early adopter sites can be found in Attachment 2a-2e. For Phase 9, the programmed web survey includes the core PRAMS questions (Attachments8i-8j) in addition to the standard questions (Attachments 10i-10j) and supplemental modules (Attachments11b,11d,11f,11h, and 11j) selected by the individual jurisdictions. The version of each jurisdiction questionnaire is the same for all data collection modes (mail, phone, web). Core and standard questions remain the same for the questionnaire phase (PRAMS Phase 8 will remain in place for 2022 calendar year births (data collection ends June 2023) and Phase 9 will begin with 2023 calendar year births (data collection begins April 2023).
In addition, call back surveys may be implemented to gather additional information on post-pregnancy experiences and infant and toddler health. Women who respond to the PRAMS survey may be re-contacted (opt-out consent process used) at a later time period) to collect additional information about post-pregnancy experiences and infant and toddler health. In October 2019, an opioid call back survey was conducted by CDC at 9 months post birth, among jurisdictions with a high burden of opioid overdose deaths. Call back surveys may be developed to address other emergent issues as they arise and will be submitted as a change request for those topics consistent with what is currently included in Phase 9.
Field testing which may be conducted by CDC staff or through a contracted vendor will be used to refine questions and improve the quality and validity of the data collected. Field testing data will be used to identify issues that may affect implementation of the questionnaire. Women with young infants who are one year of age or less will be recruited in clinics or doctor’s offices per described field testing methodology (Attachment 15a) and screened to establish eligibility (Attachment 15b). Field testing respondents after completing questions being tested, will respond to a short survey and provide feedback on the quality of questions (Attachment 15b). Prior to field testing, new and revised survey questions may require cognitive testing to assess reading comprehension and detect response error. CDC will submit cognitive testing requests, as needed, under an appropriate generic clearances (e.g., CDC/NCCDPHP 0920-1291: Cognitive Testing and Pilot Testing for the National Center for Chronic Disease Prevention and Health Promotion, and CDC/NCHS 0920-0222: Collaborating Center for Questionnaire Design and Evaluation Research.
Planned Controls
The PIDS system including recent adaptions for web mode data collection was designed with the highest level of security to ensure data encryption and protection of information. The PRAMS model protocol specifies jurisdiction-level physical and IT-related security measures that must be implemented at each site. Jurisdictions may customize or enhance these recommended procedures, and they must be documented in the jurisdiction PRAMS protocol that is submitted to the local IRB for review. Compliance with these measures is evaluated during site visits that occur annually or every other year. All jurisdiction PRAMS staff are required to complete CDC PRAMS-developed Human Subjects Training prior to accessing the PIDS system or any participant information upon hiring, and annually thereafter. A central part of this training is related to ensuring respondents’ privacy. Jurisdictions must document attendance at the Human Subjects Training, and quarterly monitoring of trained telephone interviewers. This documentation must be submitted to CDC.
PRAMS staff at the jurisdiction level have access to personal identifying information (PII) from the birth certificate or fetal death file, such as birth certificate numbers, names, addresses and phone numbers. The birth certificate or fetal death file number is only made available to the CDC statistician who conducts data weighting for the purpose of conducting the weighting procedures. PII maintained at the jurisdiction level is destroyed when the annual weighted data set is received. PII data is encrypted and stored on the CDC network, and complies with all agency physical (e.g., gated campus and building access) and administrative (e.g., password-protected network) security measures. Access is managed through CDC's Secure Access Management Services [SAMS] portal and employs common and consistent enterprise controls for user identity management, identity proofing, authentication, and authorization. Access to the data center is limited only to select authorized personnel. Records at CDC are retained and disposed in accordance with the Scientific and Research Project Records Control Schedule.
How Information Will Be Shared and For What Purpose
Jurisdiction health departments and/or their designees are the data collectors for PRAMS (i.e., information will originate with the jurisdiction), and then data are released by the jurisdiction to CDC on a monthly basis. CDC does not transmit data from one jurisdiction to any other, and only provides annual weighted data sets to each jurisdiction through a secure file transfer system accessible through PIDS. CDC receives only de-identified records. Jurisdictions maintain responses to the PRAMS questionnaire separately from sample files. After data collection and receipt of the final weighted dataset, sample files are destroyed. Jurisdiction-level datasets are owned by individual jurisdictions, include all variables, and are used for jurisdiction purposes at the discretion of the jurisdiction. Historically (data through 2011), a subset of jurisdiction data was uploaded into PRAMStat, a public access data platform available to the public that displays only pre-determined tables of a subset of indicators from the full dataset. More recent years of data (2016-2022) for maternal and child health indicators are available in aggregate and by jurisdiction are available at Selected 2016-2022 Maternal and Child Health (MCH) Indicators | PRAMS | CDC .
On January 22, 2024, CDC PRAMS transitioned to an automated process for requesting access for a pre-approved list of variables (or data set), in order to disseminate data in a more timely and efficient manner. This pre-approved data set, called “PRAMS Automated Research File,” is a restricted use data set and has been stripped of several variables that could potentially identify a respondent (e.g., full date of birth, hospital of birth, county of residence), while other demographic variables also are only provided in grouped categories (e.g., age group). The data is available for download via a secure portal, CDC’s Secure Access Management System (SAMS). To access and download the data set, researchers must register with SAMS for identification verification, provide their contact information (name and email address), and acknowledge the PRAMS data sharing agreement on the terms of use of the data. This automated process for releasing data makes PRAMS data more readily available to researchers and public health practitioners while also helping reduce burden created by individual requests for data on jurisdictions and CDC PRAMS. A data use agreement (DUA) outlining the new process has been executed with jurisdictions that elect to participate in the Automated Research File. The DUA signed between CDC and each PRAMS grantee that participates in the Automated Research File specify how variables will be categorized for release and which variables are not to be released. For jurisdictions that could not sign this DUA, researchers can contact those jurisdictions for approval for access to their data.
Currently, jurisdiction data that meet the response threshold (currently 50%) may be released to individuals who access the PRAMS Automated Research File. Beginning with release of the first year of Phase 9 data (2023 birth cohort data, planned for February 2025), the response rate threshold requirement will be removed, and jurisdiction data will be released through the PRAMS Automated Research File portal regardless of the response rate. For previous calendar years of births (Phase 8 years 2016 to 2022), data for jurisdictions that had not been previously released based on a response rate threshold will be uploaded to the Automated Research File. Nonresponse bias analyses (Attachments 19 and 19a) provide justification for the removal of the response rate threshold requirement.
Results from field testing will be compiled in reports that will summarize findings on the performance of the questions as part of the questionnaire development process. The information in the reports will not reflect a summation of responses to the survey questions, but rather women’s impressions or difficulties with the questions. Demographic information on maternal age, education level, and race/ethnicity will be used to summarize testing results, but interview responses will not be attributed to specific participants.
Impact of the Proposed Collection on Respondents’ Privacy
PRAMS sample files include names, addresses, and some phone numbers. Email addresses may be provided by sampled women in the web mode. The names, addresses, email addresses, and phone numbers are maintained at the jurisdiction level and used solely for the purpose of contacting respondents. They are not provided to CDC and never included in any datasets released for analysis. Other information, including maternal age, race/ethnicity, and infant characteristics available from the birth certificate file, are released in grouped categories in the datasets that are released to requestors to protect the confidentiality of respondents. Other potentially identifying information such as birth certificate number or birth dates are not released to researchers. A Certificate of Confidentiality provides additional protections to respondents for sensitive questions such as substance use. Section 301(d) of the Public Health Service Act (PHS) Act, which authorizes the use of Certificates, was amended by the 21st Century Cures Act amends Section 301(d) of the Public Health Service Act (PHS) and automatically issues Certificates to biomedical, behavioral, clinical, or other research activities in which identifiable, sensitive information is collected.
How Individuals Are Informed That Providing Information Is Voluntary Or Mandatory
Individuals participating in PRAMS are informed that they do not have to participate and that they may refuse to answer any question. A description of the protections and limitations of the Certificate of Confidentiality is provided.
Opportunities to Consent
For the mailed survey, informed consent information is provided with each survey (Attachments 12a-12b [livebirth]; 12c-12d [stillbirth]). Completing and returning the self-administered booklet by mail is understood as consent to participate. Verbal consent is obtained during the introduction for telephone interviews (Attachments12e-12f [livebirth]; 12g-12h [stillbirth]). The introductory script, including the voluntary nature of the survey and ability to refuse to answer sensitive questions, precedes the phone survey questions with a prompt asking if it is okay to proceed with survey administration. For the livebirth web mode of data collection, a web screen containing informed consent information (Attachments 12i-12j) is displayed prior to accessing the survey. Women must actively consent before proceeding with the survey. Jurisdictions may modify the informed consent to reflect jurisdiction and local context (e.g., abuse reporting laws). These modifications are approved by the CDC (Attachment 13) and local IRBs.
If jurisdictions are participating in a call back survey, women who consent and respond to the PRAMS survey may be re-contacted at a later time to collect additional information about post-pregnancy experiences and infant and toddler health. The previously implemented opioid call back survey used an opt-out consent process. Any modifications to the consent process requires approval by CDC and local IRBs.
Field testing of questions will occur in relevant health department clinics and private pediatrician’s offices where women will be recruited in office waiting rooms to complete the survey. Each woman will be asked to read the informed consent form and provide verbal consent prior to participation. (Attachments 15c-15d).
How Information Will Be Secured
Access to jurisdiction datasets will be limited to the jurisdictions themselves and CDC contractors and staff who conduct weighting and data cleaning procedures. Security measures include: 1) Physical controls: CDC facilities are secure, ID-accessed buildings. Data will not be stored in hard copy formats; and 2) Technical controls: all electronic data are stored on secured servers protected with firewalls and passwords. All employees are trained on data security measures by taking appropriate HHS courses online. All data collection and records management practices and systems adhere to HHS and CDC IT policies and procedures.
Field testing may be conducted by CDC or a contracted vendor. Access to field test data sets will be limited to the entity (CDC or a contracted vendor) conducting the test and CDC staff who assist in the test. At CDC, security measures would include the same physical and technical controls described above. Contractors would be required to implement the same types of security measures. Results from field testing may be retained during the questionnaire development process but are destroyed within two years.
The PRAMS Protocol is submitted annually for continuing review to the CDC Institutional Review Board (Attachment 13) as well as local IRB in implementing jurisdictions (some jurisdictions have opted to implement provisions of the Revised Common Rule and not seek annual continuing review). All modifications to the data collection protocol, as well as changes to core and standard modules, and new supplemental modules and call-back surveys are submitted as amendments for review by the CDC IRB, as well as the jurisdiction IRBs. Additional jurisdiction specific modifications also receive local and CDC IRB review. The PRAMS core questionnaire has included some questions that may be considered sensitive such as those regarding substance use during pregnancy; experience of intimate partner violence, experience of stressful life events; experiences with depression or anxiety; and HIV testing. Standard questions are available for additional sensitive topics such as maternal substance use. Jurisdictions may be funded to collect data as supplemental modules and/or a call back survey that also contain sensitive data. This sensitive information reveals jurisdiction needs for health programs and services for emerging health issues. The informed consent preceding data collection expresses the voluntary nature of the survey and ability to refuse to answer sensitive questions. Per the 21st Centuries Cure Act, all federally funded research that collects sensitive data are automatically granted a Certificate of Confidentiality to provide additional protections to the privacy of research participant responding to sensitive questions. Sensitivity considerations are built into all components of the data collection methodology, especially as some respondents are grieving a stillbirth or recent loss of a live born child. Any questions of a sensitive nature are not asked during field testing when administering the verbal interview format to protect the confidentiality of respondents.
Burden estimates are based on data collection for Phase 8 which will conclude June 2023. Phase 9 will begin in April 2023 and has been designed to be approximately the same length as Phase 8. The core survey does not change during a given phase of implementation. Optional standard questions are selected at the beginning of each Phase by jurisdictions and do not change during a given phase of implementation. However, new supplemental modules may be introduced during the survey phase. No new supplemental modules or call back surveys will be implemented for the remainder of Phase 8.
For this estimation, resident birth totals for each participating jurisdiction are based on 2019 data, instead of the most recent year (2020) of data due to disruptions in surveillance operations caused by the COVID-19 pandemic in 2020. The base total number of interviews is similar across years and is assumed to be the same in subsequent years. Although the sample size varies from jurisdiction to jurisdiction—based on the stratification scheme and the number of births—annual sample sizes with anticipated completed number of surveys per jurisdiction range from approximately 500 to 1600 for continuous livebirths surveillance, for an estimated for a total of 51,556 survey respondents annually.
Each jurisdiction participating in PRAMS Phase 8 currently administers a mail survey and a telephone survey. Five early adopter jurisdictions also are administering the PRAMS survey through a web data collection mode for the last year of Phase 8. Based on the findings from these early adopter sites indicating the benefits of adding a web mode of data collection, for Phase 9, we plan to conduct data collection by mail and web mode with telephone follow-up for non-responders. All modes of data collection contain the same set of questions. Each jurisdiction has its own version of the survey that consists of a combination of core questions (used by all participating states) and jurisdiction-selected standard modules. Both English and Spanish language versions are available for each survey mode. Sites decide if they are going to use a Spanish version at the initiation of each Questionnaire Phase based on their jurisdiction’s Hispanic population. The total number of questions on the survey will vary by site, but all sites must adhere to a 14-page space limit on the English mail survey and use only corresponding questions on the Spanish mail survey. All sampled women are eligible to participate, so there are no screening procedures administered.
The PRAMS survey experiences a very low drop-off rate for phone respondents and very few partially completed mail surveys, and even fewer drop off with web mode among the five early adopter jurisdictions. Based on interview duration data from previous years of PRAMS administration, we estimate the average burden for the introduction and consent process for the livebirth mail (Attachments 12a-12b), telephone (Attachment12e-12f) and web modes (Attachments 12i-12j) of data collection is 1 minute. The burden for the core livebirth questions (Attachments 8a-8b [Phase 8]; 8e-8f [Phase 9]) is estimated to be 15 minutes. Questions from standard modules (Attachments10a-10b [Phase 8]; 10e-10f [Phase 9]) chosen by the jurisdiction is estimated to be 10 minutes. Total time estimated for women with a recent live birth completing the survey, inclusive of informed consent, is 26 minutes.
Periodically, the PRAMS surveillance infrastructure is used for fielding emerging public health priorities of interest or to address emergency response activities. Emerging priorities or emergency response activities may happen under rapidly evolving circumstances and cannot always be planned for. In the last year of Phase 8, 22 jurisdictions (19 funded and 3 unfunded) are collecting supplemental data on social determinants of health (AZ, CO, GA, KS, LA, ME, MA, MN, MS, MO, NH, NYS, OK, PA, RI, SC, SD, TN, UT, VT, WV, and WI); 11 jurisdictions are collecting supplemental data on COVID-19 Vaccine (AZ, DC, IL, NE, NJ, NYS, CNMI, OR, PR, SC, and WY); 10 jurisdictions are collecting supplemental data on COVID-19 experiences (AZ, DC, MD, NE, NJ, CNMI, PR, SC, VA, and WY); 6 states are collecting supplemental data on prescription pain reliever (opioid) use (AL, KS, MA, NV, SD, and VT); 2 states are collecting supplemental data on marijuana use (RI and VA); and one state is collecting supplemental data on disability (MA). Burden hours for supplemental modules are based on the estimated number of times a PRAMS survey respondent would complete an additional supplemental module (i.e., responses). Using the number of completed PRAMS interviews in 2019 and number of supplemental modules implemented in the last year of Phase 8, there will be an estimated 52,984 responses completed annually for supplemental modules (Attachment 14); it is anticipated that similar supplemental collections with a similar number of responses and average burden per response will be fielded during subsequent years of approval. In order to calculate burden hours, the number of estimated responses is used in place of the number of respondents because respondents in one jurisdiction may complete multiple supplemental modules whereas in another jurisdiction respondent may not complete any supplemental modules. The estimated average time for completion of each supplemental module is 8 minutes based on previous experience with supplements.
In addition, call back surveys may be implemented to gather additional information on post-pregnancy experiences and infant and toddler health. Women who respond to the PRAMS survey may be re-contacted (opt-out consent process used) at a later time period) to collect additional information about post-pregnancy experiences and infant and toddler health. In October 2019, a call back survey about opioid use was conducted at 9 months post birth, among jurisdictions with a high burden of opioid overdose.
Consistent with terms of clearance for PRA approval for PRAMS and the Behavioral Risk Factor Surveillance System (BRFSS), CDC will use the change request mechanism for approval of supplemental modules or call back surveys that collect additional data on topics covered by the Phase 9 PRAMS survey. If the content is not consistent with what is included in the Phase 9 survey and is deemed and emergency, OMB will consider an emergency clearance.
Though a call back survey is not currently being implemented or planned, this method may be used in the future to collect additional information about post-pregnancy experiences and infant and toddler health. To allow for the potential for call back survey collections, we include estimated burden hours annually for the period of approval. Given an average of 279 responses per jurisdiction from the opioid call back survey and participation in future call back surveys by approximately 10 jurisdictions, we estimate 2,790 responses and approximately 30 minutes to complete a call back survey (based on time to complete the opioid call-back survey). If a call back survey is developed, it will be submitted as a change request.
The stillbirth survey, administered in the state of Utah at the time of request for approval, only includes a core survey instrument. It is estimated that there will be approximately 160 responses for the stillbirth surveillance in Utah. The total time estimated for completing the introduction and consent is 1 minute (Attachments 12c-12d). The burden for the stillbirth survey (Attachments 9a-9d) is estimated to be 24 minutes. Total time estimated for women with a recent stillbirth completing the survey, inclusive of informed consent, is 25 minutes.
PRAMS conducts cognitive testing of new and modified questions from core survey, standard modules, supplemental modules, and call back surveys to ensure that respondents understand the questions correctly and can provide accurate responses. Cognitive testing for the Phase 9 core and optional standard questions were completed by the National Center for Health Statistics, Collaborating Center for Questionnaire Design Evaluation Research (OMB No. 0920-0222, expiration 9/30/24). The report on Phase 9 cognitive testing is described in Attachment 17. During the next three years, should PRAMS need to conduct cognitive testing for new or revised questions, CDC will submit testing requests, as needed, under a generic clearance.
PRAMS also conducts field testing of new and modified questions from core survey, standard modules, supplemental modules, and call back surveys for feedback on reading comprehension, clarity of instructions, appropriateness of responses options, and overall quality of the PRAMS questions. While field testing for the Phase 9 core and optional standard questions have been completed (Attachment 18) under the current PRAMS OMB approval (OMB No. 0920-1273, expiration 11/30/2022), the need may arise for field testing of new supplemental modules and call back surveys during Phase 9. Consistent with terms of clearance where CDC will use the change request mechanism for approval of supplemental modules or call back surveys on topics consistent with those covered by the Phase 9 survey, cognitive testing and field testing will be performed.
Procedures for field testing include a brief screening questionnaire followed by a request for verbal consent. Women are then offered a self-administered version of the field-testing survey or the survey will be offered in an interview format. Following the survey, women will be asked to provide feedback on the quality of the questions (Attachment 15a-15d). We recruit a total sample of no more than 50 women for field testing, allowing for approximately 3 field tests administered each year. The field-testing process inclusive of screening, verbal consent, survey administration and debriefing questions takes approximately 40 minutes to complete.
Each month, jurisdictions sample mothers from the monthly birth certificate file using a pre-programmed SAS file to create the data file and import it into PIDS to begin data collection. This process takes, on average, 30 minutes for each jurisdiction to complete each month. The annual estimate for 50 jurisdictions is 300 hours.
The total number of respondents are estimated at 52,016 (total number of women anticipated to participate in the PRAMS survey, field testing, or the PRAMS Stillbirth Questionnaire).
The burden estimate for PRAMS includes six types of information collection: (1) information collection associated with the PRAMS data collection for women with recent live births (PRAMS Phase8/Phase9 core questions and jurisdiction-selected standard modules) (Attachment 8a-8j); (2) supplemental modules (Attachment 11a-11l ); (3) call back survey; (4) PRAMS data collection for women with recent stillbirths (Attachment 9a-9d); (5) field testing data collection as part of finalizing question development/modification (Attachments 16a-16d), and (6) jurisdictions submission of data file to CDC. Participation is voluntary and there are no costs to respondents other than their time. In addition, there is a burden estimate for the amount of time for each of the 50 jurisdictions to randomly sample mothers, pull their data, and upload the pulled data into PIDS on a monthly basis. The total estimated annualized burden hours are 31,268.
Table A.12-1. Estimated Annualized Burden to Respondents
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (in hours) |
Total Burden Hours |
Women who recently delivered a live birth |
PRAMS Phase 8/Phase 9 (Core Questions plus jurisdiction selected standard modules) |
51,556 |
1 |
26/60 |
22,341 |
Supplemental Modules |
52,984* |
1 |
8/60 |
7,065 |
|
Call Back Surveys |
2,790** |
1 |
30/60 |
1,395 |
|
Field Testing |
150**** |
1 |
40/60 |
100 |
|
Women who recently delivered a stillbirth |
PRAMS Stillbirth Questionnaire |
160 |
1 |
25/60 |
67 |
Jurisdictions |
Submission of data file to CDC |
50 |
12 |
30/60 |
|
Total hours |
31,268 hours |
||||
*This value does not represent additional respondents, but the estimated number of responses based on supplemental modules implemented in jurisdictions for calendar year 2022 births. Estimated number of responses are based on the sum of the number of respondents from 2019 weighted data among jurisdictions that are participating in the following supplemental modules: The social determinants of health supplement (PRAMS respondents to complete = 22,573), the COVID-19 vaccine supplement (PRAMS respondents to complete = 12,938), the COVID-19 Experience supplement (PRAMS respondents to complete = 9,343), the prescription pain reliever (opioid) use supplement (PRAMS respondents to complete = 5,854), and the marijuana use supplement (PRAMS respondents to complete = 2,276).
**This value does not represent additional respondents, but the estimated number of responses to the planned call back surveys.
****Field testing will be conducted approximately three times per year, with no more than 50 respondents each time.
Annualized burden costs are summarized in the table below. These calculations assume the average hourly wage of $29.81 for all jurisdictions included in the PRAMS. Hourly rates were taken from the most recent publicly available Current Employment Statistics of the Bureau of Labor Statistics and are based upon the average hourly earnings for December 2020 from the Current Employment Statistics survey conducted by the Bureau of labor Statistics (available at http://data.bls.gov/cgi-bin/surveymost). We estimate the total annual burden cost to be $932,099.08.
Table A.12-2. Estimated Annualized Cost to Respondents
Type of Respondents |
Form Name |
No. of Respondents |
Total Burden (in hours) |
Average Hourly Wage Rate |
Total Cost Burden |
Women who recently delivered a live born infant |
PRAMS Phase 8/Phase 9 Questionnaire (Core Questions plus jurisdiction selected standard modules) |
51,556 |
22,341 |
$29.81 |
$665,985.21 |
Supplemental Modules |
52,984 |
7,065 |
$29.81 |
$210,607.65 |
|
Call Back Survey |
2,790 |
1,395 |
$29.81 |
$41,584.95 |
|
Field Testing |
150 |
100 |
$29.81 |
$2,981.00 |
|
Women who recently delivered a stillbirth |
PRAMS Stillbirth Questionnaire |
160 |
67 |
$29.81 |
$1,997.27
|
Jurisdictions |
Submission of data file to CDC |
50 |
300 |
$29.81 |
$8,943.00 |
Total |
$932,099.08 |
||||
There are no maintenance or capital costs to respondents.
Costs that are presented below include data collection, weighting and sampling, as well as data distribution (i.e., websites and production of data sets). These are based on the funds provided to jurisdictions for data collection as well as internal PRAMS costs.
Table A.14-1. Annualized Estimated Cost to the Federal Government
Estimated funds provided to jurisdictions |
$8,005,416 |
Estimated CDC PRAMS budget (costs for operations and contracts including PIDS) |
$4,839,369 |
Total |
$12,844,785 |
This request is for a revision. CDC is seeking a revision due to inclusion of a new version of the PRAMS survey (Phase 9) to be used beginning with April 2023. A summary of changes to PRAMS core survey questions from Phase 8 to Phase 9 is included in Attachment 16. In addition, a revision is requested to implement a web mode of data collection for all participating jurisdictions for Phase 9 of PRAMS. The revision also includes additional hours for jurisdiction to submit the data files of sampled mothers to CDC.
One revision from the last approval which will not result in a change of burden is an updated version of the PRAMS survey (core questions plus jurisdiction selected standard module) from Phase 8 to Phase 9. The Phase 9 survey will begin in April 2023 and has been designed to be the same length as Phase 8. The other revision that will not impact burden hours is the implementation of a web mode of data collection as an option in addition to existing mail mode when participants are contacted. Phone follow up will occur for mail and web non-respondents. The questions are the same for all modes of data collection and not expected to impact burden hours.
The remaining changes, which do impact the overall burden include 1) a slightly reduced estimate of the number of responses to the PRAMS survey (core questions plus jurisdiction selected standard module) based on responses received in 2019 (decrease of 223 hours), 2) a decrease in the anticipated number of supplemental module responses with an increase in the time to complete each module from 5 to 8 min based on current supplemental modules being implemented by jurisdictions (resulting in an overall decrease of 1,916 hours), 3) a decrease in the estimated annual burden for potential call back surveys (decrease of 586 hours) with current estimates based on responses to the most recent call back survey, and 4) an increase in the amount of time allotted for each field testing interview resulting in an overall increase for field testing from 20 to 40 minutes (increase of 50 hours). Additionally, we added a burden estimate of 300 hours annually for jurisdictions to randomly sample mothers each month, pull their data, upload the data to PIDS, and submit the data file to CDC. As a result of these five changes, total response burden changed from 33,641 hours (last approval) to 31,268 hours for a total decrease of 2,373 hours.
PRAMS data collection is ongoing. Data collection for each year of PRAMS begins in April of that year and continues to the end of June of the subsequent year. Births occurring in January are sampled in April to ensure all infants are at least two months old at the time the survey is completed. Data collection for December births starts in March of the subsequent year and continue through the end of June. Data is submitted monthly to CDC for editing and cleaning. Real-time data quality reports can be generated by jurisdictions using the PRAMS Integrated Data Collection System (PIDS). Editing, cleaning, and weighting of the data will be conducted by CDC, is ongoing, and usually begins in July of the subsequent year. In April 2023, PRAMS jurisdictions will begin data collection for the 2023 birth cohort, with 23 jurisdictions implementing web mode, along with mail and phone modes of data collection. By September 2023, all 50 jurisdictions will be implementing web mode data collection. A phased approach to implementation of the web mode is intended to ensure that each jurisdiction will receive adequate technical assistance.
Jurisdictions must ensure submission of birth files to CDC for use in the weighting process. Currently, 24 jurisdictions have enrolled in the State and Territorial Exchange of Vital Events (STEVE) to transmit their birth files in real-time on a weekly basis to CDC and the remaining 26 jurisdictions submit their final birth file via CDC’s SAMS portal. Participation in STEVE is voluntary. CDC will continue to enroll jurisdictions who wish to participate in STEVE in the next two years. Final weighted data sets will be returned to the jurisdictions as they are weighted. Jurisdiction data are released for use by internal and external researchers. Data availability by jurisdictions can be found on the PRAMS website PRAMS Data | PRAMS | CDC. Previously, researchers were required to submit a proposal to access the PRAMS analytic research file. In January 2024, PRAMS began releasing multi-jurisdiction data using the PRAMS Automated Research File, a pre-approved list of variables (or data set) in order to disseminate data in a more timely and efficient manner. Supporting technical documentation including a detailed description of PRAMS standardized data collection methodology, questionnaire and weighting process is available on the PRAMS website at Data Methodology | PRAMS | CDC . To access and download the pre-approved data set, researchers register with SAMS for identification verification, provide their contact information, and acknowledge the PRAMS data sharing agreement on the terms of use of the data. Additional documentation including formats, codebooks, and technical notes are available on the Automated Research File web portal. Instructions for accessing analytic files are found on the PRAMS website at https://www.cdc.gov/prams/php/data-research/index.html. The new automated process reduces the data access wait time from 2-3 months to 2-3 days. reduces the data access wait time from 2-3 months to 2-3 days.
For Healthy People 2030 measures, all PRAMS jurisdiction data are used without a response rate threshold.
Figure 1. PRAMS Data Collection Timeline for the Last Year of Phase 8 Data Collection
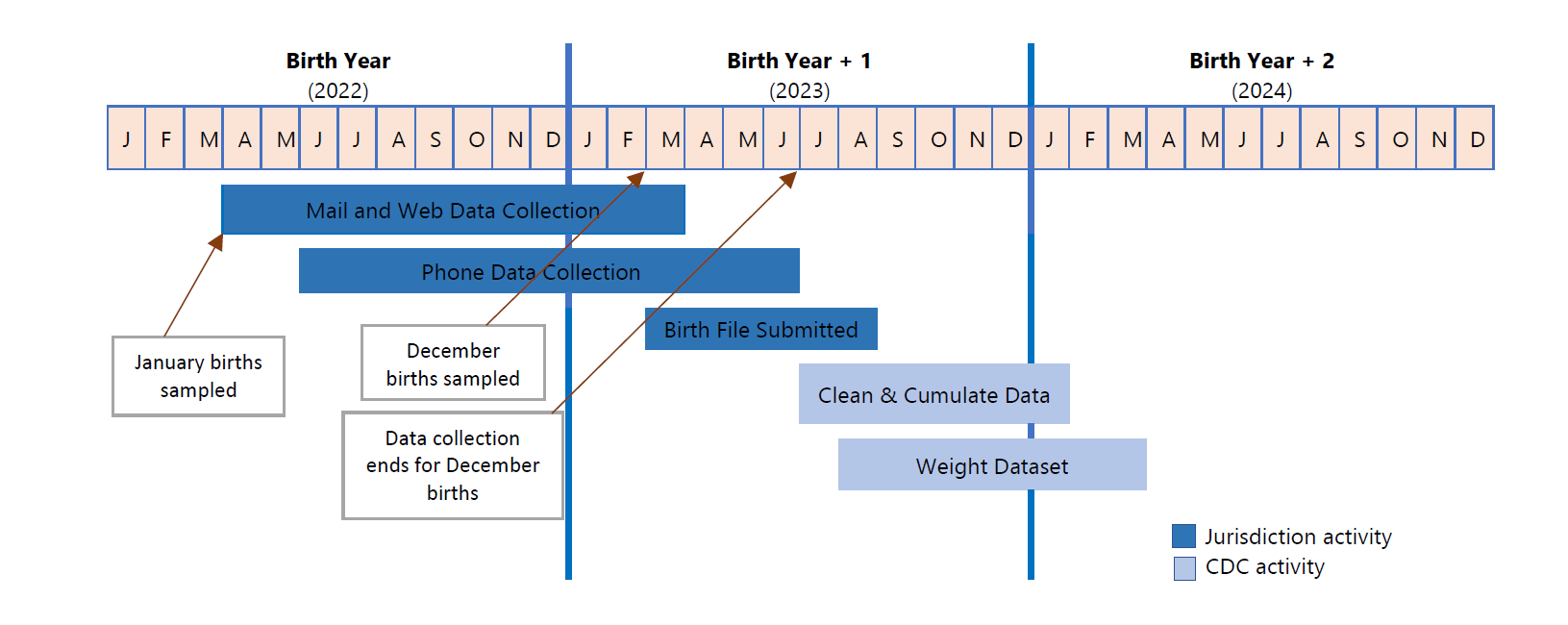
Not applicable. The expiration date of OMB approval is displayed on all PRAMS surveys.
There are no exceptions to the certification.
1 Berry SH, Pevar J, Zander-Cotugno M (2008). Use of Incentives in Surveys Supported by Federal Grants. Rand Corporation, March 2008:
http://www.copafs.org/seminars/use_of_incentives_in_surveys.aspx.
2 Singer E, Ye C. (2013) The Use and Effects of Incentives in Surveys. Annals of the American Association of Political and Social
Science, 645:112-141: http://journals.sagepub.com/doi/pdf/10.1177/0002716212458082.
3 Singer E, Kulka RA. (2002). Paying Respondents for Survey Participation. In Studies of Welfare Populations: Data collection and research issues.105-28. Washington DC: National Academy Press. https://aspe.hhs.gov/system/files/pdf/174381/04.pdf.
4 Van Ryswyk EM, Middleton PF, Hague WM, Crowther CA (2015). Women's views on postpartum testing for type 2 diabetes after gestational diabetes: Six month follow-up to the DIAMIND randomized controlled trial. Prim Care Diabetes. 2015 Aug 27. pii: S1751-9918(15)00100-X. doi: 10.1016/j.pcd.2015.07.003: https://www.ncbi.nlm.nih.gov/pubmed/26320407.
5 Nicklas JM, Zera CA, Seely EB, et al. (2011). Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a
history of gestational diabetes. BMC Pregnancy and Childbirth 11:23: https://www.ncbi.nlm.nih.gov/pubmed/25837258.
6 Kim SY, Tucker M, Danielson M, Johnson CH, Snesrud P, Shulman H. (2008). How can PRAMS survey response rates be improved among American Indian mothers? Data from 10 States. Matern Child Health J, 12(Supp 1):119-125: https://www.ncbi.nlm.nih.gov/pubmed/18350261.
7 Liu ST, Geidenberger X. (2011). Comparing incentives to increase response rates among African Americans in the Ohio pregnancy risk assessment monitoring system. Matern Child Health J, 15(4): 527-33: https://www.ncbi.nlm.nih.gov/pubmed/20428935.
8 CDC, Pregnancy Risk Assessment Monitoring System. PRAMS National Meeting Program Booklet, Abstract excerpt. Atlanta, GA, 2015.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | How to Write and Submit |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2024-11-24 |
© 2026 OMB.report | Privacy Policy