0920-1423 Aim 2a - Cohort Screeeer_English
[NCHHSTP] Expanding PrEP in Communities of Color (EPICC)
Att 4f_Aim2aCohortScreenerEnglish_clean
Aim 2a Cohort Screener (English/Spanish)
OMB: 0920-1423
Form Approved
OMB No. 0920-1423
Expiration Date: 12/31/2026
Expanding PrEP in Communities of Color (EPICC+)
Attachment 4f
Aim 2a Cohort Screener English
Public reporting burden of this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; Attn: OMB-PRA (0920-New)
Cohort Screener

Thank you for your interest in our research study – Expanding PrEP In Communities of Color (EPICC+)! The goal of EPICC+ is to improve PrEP adherence and PrEP use over time among young cisgender (cis) men and people who identify as non-binary including gender nonconforming, and genderqueer who take PrEP. We are currently looking for young cis men and non-binary people on PrEP who can take part in a study lasting at least 12 months and up to 18 months. Participation will include an enrollment session and follow-up activities at 3, 6, 9, and 12 months. Some participants may also complete follow-up activities at 15 and 18 months. Study activities include: surveys at the enrollment and follow-ups and collection of blood samples. Some participants will also be asked to complete an in-depth interview at the end of their participation. Participants enrolled for 12 months may earn up to $500 for completing app download, all 5 surveys, 3 blood samples, and an exit interview. Participants enrolled for 18 months may earn up to $650 for completing app download, all 7 surveys, 4 blood samples, and an exit interview.
Interested in joining the study? Please answer the following questions as part of the study screening process to see whether you are eligible to participate. It takes about 5 minutes to complete, and you are welcome to message our study team with any questions you may have. If you consent to be screened and screen eligible, clinic staff from the clinic where you report getting care will verify your eligibility by confirming you receive care at that clinic and reviewing your medical record to confirm you have an active PrEP prescription.
The screener asks questions about topics like age, gender identity, sexual orientation, and HIV status. We understand that these are personal questions and can be uncomfortable to answer in a survey. Please note that the process is voluntary, and you can decide to quit at any time. You may also choose "Decline to answer" on any questions that make you feel uncomfortable; however, choosing that option may affect our ability to determine if you are eligible for the study. If you do complete the survey and want to learn more about this study or future research, please enter your contact information so that the study team can call you regarding next steps. Any information shared in this survey is confidential and will only be used for research purposes. All information will be kept on a secure server. If you choose not to be contacted for future studies, your information will be deleted.
The possible risks of screening for the study include risk of breach of confidentiality. Data is encrypted on this survey website to minimize this risk. When study staff receive your information, it will be kept on a secure server. You may not benefit personally from being in this research study. All participants will receive financial incentives to support study participation.
We want to acknowledge that some of the language used in our study questions may include some outdated language or lack the diversity of experiences that we now understand exist. Although we do our best to use measures that reflect emerging language, at times the items available in research are not where they need to be and are drawn from items developed ten (or
more) years ago. Wherever possible, we have updated the language or are working with developers to get new versions.
If you have questions, or concerns, you may contact the study coordinator at (448) 488-9069 or you may email the study team at [email protected]. If you have questions or concerns about your rights as a research subject, you may contact FSU Institutional Review Board (IRB) 850-644-7900 or by email to [email protected]. Please provide the FSU IRB# 00003652 for this study.

Do you consent to be screened and have your eligibility confirmed by clinic staff if you screen eligible?
Yes (1)
No (2)

 Page
Break
Page
Break
Sorry, if you do not consent to be screened, you cannot continue the screener. Thank you for your time.
What is your date of birth? (dd/mm/yyyy)

Decline to answer

4) What is your race and/or ethnicity? Select all that apply and enter additional details in the spaces below.
American Indian or Alaska Native –
Enter, for example, Navajo Nation, Blackfeet Tribe of the Blackfeet Indian Reservation of Montana, Native Village of Barrow Inupiat Traditional Government, Nome Eskimo Community, Aztec, Maya, etc._________________________
Asian – Provide details below.
Chinese
Asian Indian
Filipino
Vietnamese
Korean
Japanese
Enter, for example, Pakistani, Hmong, Afghan, etc. ____________________
Black or African American - Provide details below.
African American
Jamaican
Haitian
Nigerian
Ethiopian
Somali
Enter, for example, Trinidadian and Tobagonian, Ghanaian, Congolese, etc._____________
Hispanic or Latino – Provide details below.
Mexican
Puerto Rican
Salvadoran
Cuban
Dominican
Guatemalan
Enter, for example, Colombian, Honduran, Spaniard, etc. ___________________
Middle Eastern or North African – Provide details below.
Lebanese
Iranian
Egyptian
Syrian
Iraqi
Israeli
Enter, for example, Moroccan, Yemeni, Kurdish, etc._______________
Native Hawaiian or Pacific Islander – Provide details below.
Enter, for example, Chuukese, Palauan, Tahitian, etc.______________________
White – Provide details below.
English
German
Irish
Italian
Polish
Scottish
Enter, for example, French, Swedish, Norwegian, etc. _________________

Do you understand and are you comfortable speaking and reading English or Spanish?
Yes (1)
No (2)
Decline to answer

[If yes to previous question: Do you understand and are you comfortable speaking and reading English and/or Spanish]
What is your preferred language?
English (1)
Spanish (2)
Decline to answer
Do you have a personal cell phone with texting and internet?
Yes (1)
No (2)
Decline to answer

Are you willing to provide a mailing address within the 50 states or Puerto Rico where you can receive packages?
Yes (1)
No (2)
Decline to answer

Which of the following BEST represents how you think about yourself?
Lesbian or gay
Straight, that is not lesbian or gay
Bisexual
Something else:_____________
Decline to answer
[If American Indian or Alaskan Native is not checked]
How do you currently describe yourself? (Check all that apply)
Woman, including transgender woman
Man, including transgender man
Nonbinary, including gender nonconforming, and genderqueer
A different gender identity: ____________
Don’t know
Decline to answer
[If American Indian or Alaskan Native is checked]
How do you currently describe yourself? (Check all that apply)
Woman, including transgender woman
Man, including transgender man
Nonbinary, including gender nonconforming, and genderqueer
Two-Spirit
A different gender identity: ____________
Don’t know
What sex were you assigned at birth, on your original birth certificate?
Male
Female
Intersex
Decline to answer

 Page
Break
Page
Break
Have you ever had sex (as a top or bottom, insertive or receptive) with a person who has a penis?
Yes (1)
No (2)
Decline to answer

Are you living with HIV?
Yes (1)
No (2)
Decline to answer (4)
Are you currently taking PrEP?
Yes, I am currently taking PrEP (1)
No, I am not currently taking PrEP, but I have a PrEP prescription.
No, I am not currently taking PrEP and do not have a PrEP prescription.
Decline to answer
Do you get PrEP from one of the clinics listed below or a clinic affiliated with one of the clinics below?
Montefiore MAYS Clinic - Bronx, N
Walk-In Sexual Health (W.I.S.H.) Clinic – Bronx, NY
Adolescent Initiative, Children’s Hospital of Philadelphia - Philadelphia, PA Amity Medical Group - Charlottte, NC
Amity Medical Group Park Road Location
Amity Medical Group Harris Boulevard Location
Amity Medical Group Monroe Road Location
Amity Medical Group Dallas Hwy Location
Wake County Health Department (Sunnybrook Road) – Raleigh, NC Five Horizons Health Services
Tuscaloosa, AL
Montgomery, AL
Dothan, AL
Ybor Youth Clinic 1315 E 7th Ave STE 104 - Tampa, FL
USF Student Health and Wellness Center 12530 USF Bull Run Drive – Tampa, FL
USF Curran Children’s Medical Services 13101 Bruce B Downs Blvd – Tampa, FL
Texas Children’s Hospital or Harris Health PrEP Clinic at Thomas Street - Houston, TX
Yes (1)
No (2)
Decline to answer
Display This Question:
If previous question = 1
Which clinic or affiliated clinic do you get your PrEP from?
Montefiore MAYS Clinic - Bronx, NY
Walk-In Sexual Health (W.I.S.H.) Clinic – Bronx, NY
Adolescent Initiative, Children’s Hospital of Philadelphia - Philadelphia, PA Amity Medical Group - Charlotte, NC
Amity Medical Group Park Road Location
Amity Medical Group Harris Boulevard Location
Amity Medical Group Monroe Road Location
Amity Medical Group Dallas Hwy Location
Wake County Health Department (Sunnybrook Road) – Raleigh, NC Five Horizons Health Services
Tuscaloosa, AL
Montgomery, AL
Dothan, AL
Ybor Youth Clinic 1315 E 7th Ave STE 104 - Tampa, FL
USF Student Health and Wellness Center 12530 USF Bull Run Drive – Tampa, FL
USF Curran Children’s Medical Services 13101 Bruce B Downs Blvd – Tampa, FL
Texas Children’s Hospital or Harris Health PrEP Clinic at Thomas Street - Houston, TX
A valid email address is needed for us to communicate with you about enrolling in this study. Your contact information will be kept secure and will not be shared with anyone
outside of the designated study staff. If you choose not to participate in the study, your email and all contact information will not be stored without your permission.
After you enter your contact information and select your pronouns, proceed to the next screen for information about your eligibility.
Enter your contact information below:
Phone Number (1)
Email (2)
Name (5)
What are your pronouns (select all that apply)?
He/Him/His
She/Her/Hers
They/Them/Theirs
Other ________________________________________________
Decline to answer
Thank you for your response - it looks like you may be eligible to participate in the study, and our team will reach out to you about the next steps.
FOR THOSE ELIGIBLE
[Required question] Would you like to receive email updates in the future about other research study opportunities and/or research findings?
Yes, I want to receive email updates about opportunities to enroll in other research studies
Yes, I want to receive email updates about research findings
No, I do not want to sign up for this
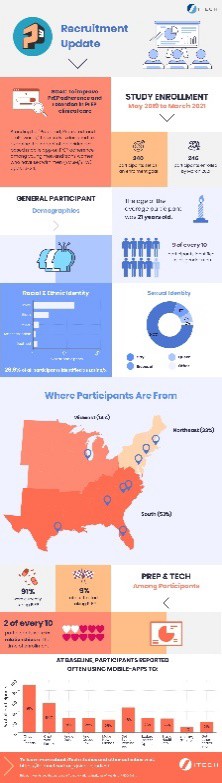
Example of research findings: [this can be embedded as a thumbnail that links to full-size]:
Please click here to continue.
Thank you so much for answering all of the questions! We really appreciate your time. Unfortunately, you are not eligible for this particular research study.
FOR THOSE INELIGIBLE
[Required question] Would you like to receive email updates in the future about other research study opportunities and/or research findings?
Yes, I want to receive email updates about opportunities to enroll in other research studies
Yes, I want to receive email updates about research findings
No, I do not want to sign up for this
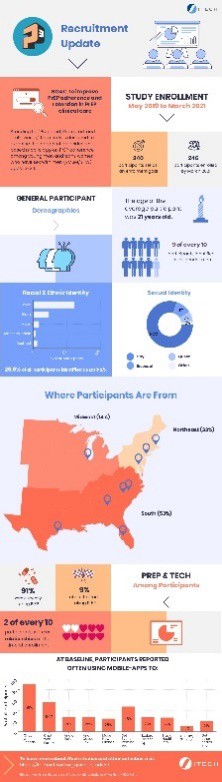
Example of research findings: [this can be embedded as a thumbnail that links to full-size]:
Please click here to continue.
If you are interested in receiving updates, please enter your contact information below so that we may contact you.
Name: Phone:
Email:

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Aim 3 Cohort Screener |
| Author | Qualtrics |
| File Modified | 0000-00-00 |
| File Created | 2024-12-24 |
© 2026 OMB.report | Privacy Policy