SSA_NPPTL_Field Package V3 --- Clean 11.17.22
SSA_NPPTL_Field Package V3 --- Clean 11.17.22.docx
[NIOSH] Assessing Respirator Perceptions, Experiences, and Maintenance
OMB: 0920-1378
Generic Clearance for CDC/NIOSH/NPPTL
Assessing Respirator Perceptions, Experiences, and Maintenance
OMB Control Number: 0920-1378 Expiration Date: 11-30-2025
Supporting Statement A
Jonisha P. Pollard
Supervisory Research General Engineer
Tel. 412.386.5220
August 6, 2025
Table of Contents
Circumstances Making the Collection of Information Necessary
Purpose and Use of the Information Collection
Use of Improved Information Technology and Burden Reduction
Efforts to Identify Duplication and Use of Similar Information
Impact on Small Businesses or Other Small Entities
Consequences of Collecting the Information Less Frequently
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
Explanation of Any Payment or Gift to Respondents
Protection of the Privacy and Confidentiality of Information Provided to Respondents
Institutional Review Board (IRB) and Justification for Sensitive Questions
Estimates of Annualized Burden Hours and Costs
Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
Annualized Cost to the Federal Government
Explanation for Program Changes or Adjustments
Plans for Tabulation and Publication and Project Time Schedule
Reason(s) Display of OMB Expiration Date is Inappropriate
Exceptions to Certification for Paperwork Reduction Act Submissions
List of Attachments
Attachment A – Occupational Safety and Health Act of 1977
Attachment B – 60 Day Federal Register Notice
Attachment C – genIC template
A. JUSTIFICATION
1. Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH), is requesting approval of a new generic umbrella package for a period of three years under the project titled, “Assessing Respirator Perceptions, Experiences, and Maintenance.” This study is being conducted by the National Institute for Occupational Safety and Health (NIOSH). NIOSH, under OSH Act of 1977 (29 USC 669 Section 20(a)(1)) (Attachment A), has the responsibility to conduct research related to innovative methods, techniques, and approaches dealing with occupational safety and health problems.
Further, the National Personal Protective Technology Laboratory (NPPTL) a division of NIOSH, operates within the CDC. NPPTL was established in 2001, at the request of Congress, with the mission of preventing disease, injury, and death for the millions of individuals who rely on personal protective technology (PPT) to protect them on the job. The development of NPPTL filled a need for improved personal protective equipment (PPE) and focused research into PPT. Many of these projects provide the basis for the recommendations and guidelines that NIOSH provides.
According to data from the BLS, from 2011 to 2019 an estimated 52,910 workers experienced injuries or illnesses requiring days away from work and 423 fatal injuries due to inhalation of harmful substances. This results in significant cost to workers, their families, employers, and the U.S. economy. In 2001, NIOSH partnered with BLS to conduct the first voluntary Survey of Respirator Use and Practices. This survey revealed important insights into respiratory use and hazards in the U.S. Using data collected from 40,002 U.S. establishments, the 2001 survey estimated that approximately 281,776 establishments required 3,303,414 U.S. workers to rely on respirators for protection from injuries and illnesses due to respiratory hazards (BLS and NIOSH, 2003). Based on the number of workers who are required to use respiratory protection and the number of inhalation hazards they can help prevent, a mechanism to assess the perceptions and uses around new types of respiratory protection is critical.
A cornerstone of NPPTL is its respirator approval program (NIOSH 42 CFR Part 84) which tests and certifies respirators that provide a specific level of protection for users. Requests for manufacturer evaluation and approval are continuous, with new respirators routinely entering the market and made available. These activities certify respirators that are often assessed in NPPTL research projects. According to NIOSH’s Certified Equipment List (CEL), for Schedule 84A – approvals for air purifying particulate filter respirators – over 8,000 approvals are documented for common types of respiratory protection such as N95 filtering facepiece respirators (FFRs), elastomeric half mask respirators (EHMRs), and full face respirators. These ~8,000 approvals only represent one schedule category. Additional respirator approvals exist for self-contained breathing apparatuses, gas masks, supplied-air respirators, and particulate respirators.
Regardless of the respirator and the protection level it offers, NIOSH research about the design, comfort, user experience, and organizational integration of the respiratory protection into their respiratory protection program (RPP) or another similar framework, remain consistent. This consistency further justifies the need for a generic IC that facilitates the collection of similar data on different respirator types across industries. The information collected from human subjects about their use of respirators is generally consistent across NPPTL studies, with only the use conditions changing (e.g., respirator type or management implementation practices related to cleaning/decontamination, fit testing, and training). Data collection may focus on respirator types ubiquitous to the industry being studied, new to the industry being studied, or novel to any industry.
In addition to the respiratory protection device being provided to workers, federal regulations exist regarding the use of respirators in the workplace (Occupational Safety and Health Standards, 29 CFR 1910.134). The Occupational Safety and Health Administration (OSHA) requires employers whose hazard management includes the required or voluntary use of respirators to have an RPP, which has specified components. Therefore, this generic IC will not only collect information from individuals related to the perceptions and maintenance of respirator use but also the components of their RPPs. The ability to conduct research in this area is imperative as exposure risks can change instantly during public health emergencies (e.g., SARS-CoV-2) and some RPPs may not be comprehensive enough to include the types of respiratory protection needed. In these cases, having standardized measures and frameworks for evaluating respirator use in the context of an RPP or another program is important (Yarbrough et al., 2016).
This data collection activity benefits the Federal Government by providing NIOSH with data to determine how to best manage and improve the maintenance of respirators within a variety of organizational settings. As mentioned earlier, there are over 8,000 approvals for common types of respiratory protection. The same types of data on uses and experiences are collected, most often in different protocols. This generic ICR facilitates consistency in the data that will be collected pertaining to respiratory protection which also supports consistent interpretation and use of such data to better understand barriers and drivers to maintaining respiratory protection programs. Additionally, consistent feedback about different types of respirators used by workers informs what types of respiratory protection gaps that may exist across industry sectors.
Data collection for this project is authorized under the OSH Act of 1977 (29 USC 669 Section 20(a)(1)) (Attachment A).
2. Purpose and Use of Information Collection
NIOSH has a need to collect data to evaluate the use of approved respiratory protective devices under 42 CFR 84 CFR to pinpoint areas where improvements in respirators and complementary RPPs are necessary. Further, recent assessments conducted by the National Academies of Sciences, Engineering, and Medicine (NASEM), have recommended that NIOSH continue to engage in research that informs respiratory protection for workers both with and without RPPs to enhance the understanding of respirator use in the U.S. (2022). To fulfill this recommendation, an integrated research effort is needed to fill in the gaps around respirator use and maintenance as well as organizational programs that reduce barriers surrounding these topics. Similar data collection activities occur across NPPTL research projects to understand barriers to respirator use and integration as well as the development of programs and best practices in the workplace.
In addition, the U.S. economy has seen significant changes in recent years, necessitating additional research studies around respirator use and practices. For example, in 2017, the healthcare and social assistance industry sector employed more U.S. workers than the manufacturing sector (Thompson, 2018). This is in stark contrast to 2000, where there were 7 million more workers in the manufacturing sector and 2.4 million more in the retail sector than in healthcare (Thompson, 2018). An increase in alternative or non-traditional work arrangements such as the increase of gig employees, traveling nurses, and contractor work is also raising challenges in the management of respiratory protection for workers. Finally, the employment of young adults or children in industries such as agriculture and those who work for family businesses who have fewer employees, also creates a new space where the use and oversight of respiratory protection has not received enough attention (OSHA, 2014; NASEM, 2022). Overall, the combination of these major shifts in the U.S. economy represents the potential for a drastic change in how respirators are used and managed in the workplace.
This package will allow for the emergence of patterns in respiratory protection use, as well as health and safety programs including practices and directives that are missing in RPPs. This includes workplaces whose use of an RPP framework is not under OSHA for enforcement purposes so it can include workers in nontraditional work arrangements such as contractors. None of the studies proposed under the auspices of this generic IC intend to produce results that can be generalized beyond the scope of each study. The objective of this request is to enable NIOSH to engage in information collection activities that are focused on assessment and will result in the development of interventions (e.g., frameworks and materials for implementing and communicating about new respiratory protection in the workplace based on industry type, size, or location), new or improved respirator designs and adoption of respiratory protection/best practices, and concept development and/or product development and testing and decrease burden to the public. Outcomes will inform new content for improved respirator training, management of respiratory protection in the workplace, and employee adherence of respirators and complementary practices (i.e., cleaning, disinfection, and storage). The types of information collection activities included in this generic package include qualitative interviewing or focus groups for exploratory and formative research purposes, perception and knowledge-based surveys, qualitative fit testing, and physiological monitoring while wearing a respirator. See Appendix A for a sample instruments that may be used for these various information collection activities to accomplish the general goals of this ICR.
This information is not intended to be used for wider policy development, budget formulation, or other public-facing purposes not described in the paragraph above.
The information collected for each project will be maintained or stored locally under strict access controls limited to the local project leader/manager or his/her designate. Individual projects will not collect sensitive personally identifiable information (PII) but will likely collect derived PII (e.g., job, age). If a project under this generic IC is going to collect any form of PII, it will be kept in a separate location and accessible only to the project-specific research staff. This information will be destroyed when the project has ended. Under no circumstances will an individual be identified using a combination of variables such as sex, race, birth date, and/or other descriptors.
3. Use of Improved Information Technology and Burden Reduction
All data collected will require respondents to answer questions during a survey, interview, focus group, or laboratory monitoring that may be completed in-person, over the phone, or electronically. To reduce burden to the respondents and comply with the Government Paperwork Elimination Act, Public Law 105-277, title XVII, signed into law on October 21, 1998, data collection will occur electronically when possible. In situations where an electronic survey can be used, projects will use this mechanism to reduce burden because this approach ensures data quality but decreases respondent burden with built-in skip logic. Most often electronic platforms such as CDC’s Research Electronic Data Capture (REDCap), an approved IT platform, will be used.
Where possible, qualitative data collection can occur over the phone using a secure government license such as Teams or Zoom. The use of data collection using virtual platforms reduces burden on the respondent in multiple ways. For instance, by not traveling to the site or organization, employers do not have to provide CDC NIOSH employees with site-specific health or safety training or review emergency evacuation procedures. Additionally, electronic participations allow respondents more flexibility in their schedule, and, if time is limited at least some information can be collected. These calls may or may not be recorded, depending on the individual project.
Though electronic technologies will be used by many of the individual projects in this data collection, the nature of some proposed activities requires direct interaction between respondents and project staff, especially in the case of in-depth focus groups and psychological observation and monitoring.
4. Efforts to Identify Duplication and Use of Similar Information
As the nation’s respirator approver for all workplaces (42 CFR Part 84), NPPTL conducts respiratory protection research to examine exposures to inhalation hazards, dermal hazards, and any other hazardous environmental threats within an occupational setting. The agency collaborates with other federal agencies, academic institutions, and contracting mechanisms to advance its mission in PPT. These extensive collaborations serve as mechanisms to ensure research efforts are necessary and filling a critical gap in respiratory protection research, design, and implementation. For example, NPPTL established a Memorandum of Understanding with the Food and Drug Administration (FDA) to ensure research and development efforts around respirators are not only warranted but coordinated. Similar coordination is occuring with OSHA.
Also, in 2005, NPPTL requested that the National Academies of Sciences, Engineering, and Medicine (NASEM) form a standing Committee on Personal Protective Equipment (COPPE) for Workplace Safety and Health. The committee provides a forum for discussion of scientific and technical issues relevant to the development, deployment, and use of PPE, standards, and related systems to ensure workplace safety and health. The COPPE coordinates and meets with NPPTL multiple times per year to become informed of existing conditions and emerging issues related to respirators, to discuss studies around respiratory protection. This generic package will allow such recommendations to be coordinated and addressed holistically and systematically across industry sectors and respiratory hazards.
5. Impact on Small Businesses or Other Small Entities
Some surveillance or research activities involve data collection from small business (e.g., medical offices) or small governmental entities; therefore, methods and instrument development activities may also be conducted with these groups. If such activities are conducted, these businesses will be approached in the same manner as the individuals we normally recruit, we will ask the organization to identify the appropriate staff members with whom to conduct the activities. In some studies, no small businesses will be involved in the data collection activities. The methods used to minimize burden on small businesses or other small entities will be explained in each study submitted under this generic.
6. Consequences of Collecting the Information Less Frequently
As more occupational sectors continue to implement new types of respirators, hire new workers, and implement updates to their RPPs, it is critical that research be conducted to assess and update best practices. If this research is not conducted, assessments and subsequent recommendations about how to safely integrate new types of respirators will not be disseminated to industry personnel.
As an example, although N95 filtering facepiece respirators (FFRs) have been traditionally used in healthcare, there has been a surge in interest in reusable respirators such as elastomeric half mask respirators (EHMRs) and powered air purifying respirators (PAPRs). When there was a shortage in traditionally used N95 FFRs, healthcare organizations were confronted with first, the use of unfamiliar types of respiratory protection and second, questions in how to best support its implementation. This information collection would support and be able to address problems like this in a timely manner. Similarly, as temporary rules (e.g., the Food and Drug Administration (FDA) issued an emergency use authorization (EUA), allowing the use of all NIOSH Approved respiratory protective devices in healthcare settings during extreme shortages (85 FR 17335, March 27, 2020), NIOSH needs to be poised to respond to the massive ebbs and flows of interest in some of these types of respiratory protection.
Because this generic clearance covers a wide range of studies, each individual project submitted under this generic IC will clearly define the specific data collection methods and procedures. Individual data collections will be time-limited and generally conducted only once, except in the cases of the pilot testing of interventions where respondents may have to be approached several times on the same or similar topic under evaluation. There are no legal obstacles to reducing the burden.
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
The Federal Register notice was published for this collection on May 6, 2022, Vol. 87, No. 88, pg. 27152-27154. (See Attachment 2) No substantive public comments were received.
No other public contacts and opportunities for public comments were received.
Representatives from CDC NIOSH were engaged to develop this request. The names and representatives are available upon request.
9. Explanation of Any Payment or Gift to Respondents
Per OMB guidance, incentives are generally not appropriate for contractors, cooperators, grantees or program participants because they already have a pre-existing relationship with the agency. Incentives are most appropriate where participants are being asked to travel to a site to participate in a focus group or cognitive interview. Incentives are generally not appropriate for questionnaires/surveys.
If an incentive is proposed, a detailed justification based on the type of collection, population of respondents, and other circumstances will be provided in the individual information collection request. Per the Office of Information and Regulatory Affairs, Office of Management and Budget guidance document Questions and Answers when Designing Surveys for Information Collections (Updated Oct. 2016) , justifications will focus on data quality, burden on the respondent, past experience, improved coverage of specialized respondents, rare groups, or minority populations; and/or reduced survey costs.
Each justification will cite the research literature that demonstrates significant improvements in response rates and non-response bias when applied to similar participants, data collection methods, and data collection contexts. OMB does not consider it appropriate to use private sector market rates as a justification for incentives in government information collections. Where no evidence is available, ACF may propose a field test or experiment to evaluate the effects of the incentive.
The following includes expected ceiling amounts for different types of collections:
Focus groups where participates are expected to travel to a central site: Up to $75
Cognitive Interviews or similar exercises (intensive one-on-one probing of basis for thoughts) in which participants are expected to travel to a central site: Up to $40
Questionnaires/Surveys: TBD, under special circumstances
For any collection over 90 minutes, participants may be offered an incentive to account for incidental expenses (transportation, child care, lost wages, etc.).
10. Protection of the Privacy and Confidentiality of Information Provided by Respondents.
Information collected will be kept private to the extent permitted by law. Respondents will be informed of all planned uses of data, that their participation is voluntary, and that their information will be kept private to the extent permitted by law.
Depending on the specifics of the individual data collection project, the Privacy Act may or may not apply to an information collection. Depending on the specifics of the individual investigation (i.e., data collection project), the Privacy Act may or may not apply to an information collection. The appropriate CDC NIOSH contacts are consulted for an official Privacy Act determination for each individual investigation.
11. Institutional Review Board (IRB) and Justification for Sensitive Questions
IRB Approval
IRB requirements are investigation specific. Some investigations require IRB approval while others fall within the IRB exemption criteria (45 CFR Part 46.104) or are considered a non-research, public health surveillance activity (45 CFR Part 46.102(l)(2). For individual investigations, the appropriate CDC NIOSH contacts are consulted for an official research determination.
Projects that need IRB approval will be submitted with a copy of the approval document. If the study has been determined to be exempt from IRB, a copy of the exemption determination will be attached. If the appropriate CDC official has determined that the data/ information collection is not research involving human subjects, the information collection submitted under this generic clearance will state that IRB approval is not required.
Sensitive Questions
At times the diseases that will be covered by these information collections may involve non-compliant attitudes and practices that may be considered private. Race and ethnicity data, as well as diagnoses of medical conditions that may affect employability or insurability may also be viewed as sensitive or even threatening by a portion of respondents. The reasons for collection of sensitive information and their application for the improvement of CDC’s prevention efforts for the specific population sub-group will be addressed in specific requests. The procedures used to obtain consent and the content of the consent form will also be explained and justified.
12. Estimates of Annualized Burden Hours and Costs
The annualized response burden is estimated at 214,542 hours.
We anticipate approximately 6 information collections per year. These may include surveys, interviews, focus groups, or physiological monitoring in response to routine and emergency/extended use of respirators during different job activities.
Exhibit A.12. Annualized Burden Hours
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Hours Per Response |
Total Response Burden (Hours) |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Informed consent |
110,000 |
1 |
5/60 |
9,167 |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Demographics standardized survey with decision logic allowing some questions to be omitted |
110,000 |
1 |
15/60 |
27,500 |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Qualitative fit testing survey measurements |
675 |
20 |
15/60 |
3,375 |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Perceptions-based survey instrument |
105,000 |
2 |
15/60 |
52,500 |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Knowledge-based survey instrument |
105,000 |
2 |
30/60 |
105,000 |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Interview/Focus group
|
4,000 |
2 |
1 |
8,000 |
Individuals who wear respirators in any occupational setting or oversee/advise on respirator use |
Physiological Monitoring: Heart rate, blood pressure, blood oxygen saturation, breathing rate, etc. |
1,000 |
1 |
9 |
9,000 |
Total |
|
|
|
|
Estimated Annualized Burden Costs
Collections by health jurisdictions are generally funded through cooperative grants and these will be noted in the specific collection requests. The annualized cost to the respondent is segmented accordingly in Exhibit A.12.B.
The United States Department of Labor, Bureau of Labor Statistics, May 2021 http://www.bls.gov/oes/current/oes_nat.htm.) data were used to estimate the hourly wage rate for the general public and for private providers for the purpose of this generic request. Each project will have cost specific to the category of the respondents. Because it is not known what the wage rate category will be appropriate for the specific projects (or even whether they will be employed at all), the figure of $25.00 per hour was used as an estimate of average hourly wage across the country.
Exhibit A.12.B. Annualized Cost to Respondents
Activity |
Total Burden Hours |
Hourly Wage Rate |
Total Respondent Cost |
Data collection |
214,542 |
$25.00 |
$5,363,550 |
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
NIOSH does not anticipate providing start up or other related costs to private entities.
14. Annualized Costs to the Government
Actual annualized costs to the government will vary depending on the specific needs of the individual information collection activity. Generally, each development activity will involve participation of at least one NIOSH project officer (GS-12, 13, 14, or 15 levels) who will be responsible for the project design, obtaining IRB approvals, providing project oversight, and analysis and dissemination of the results. The NIOSH project officer will provide remote and onsite technical assistance to the local areas implementing the data collection. Travel may be required to provide this technical assistance. In most cases, a NIOSH data manager’s (typically equivalent to GS-9, 11 or 12) time will also be required. An estimated average cost per individual activity is listed below, but detailed costs will be submitted with each individual collection request.
Expense Type |
Expense Explanation |
Annual Costs (dollars) |
Direct Costs to the Federal Government |
|
|
|
NIOSH Project Officer (GS-13/14, 0.5 FTE) |
$40,641 |
|
NIOSH Data Manager (GS-9/11, 0.5 FTE) |
$13,450 |
|
CDC NIOSH IT Security Compliance |
$100,000 |
|
NIOSH Travel (10 trips) |
$20,000 |
|
Subtotal, Direct costs |
$174,091 |
Cooperative Agreement or Contract |
Cooperative Agreements, Task orders, or Contracts for implementation or information management |
$400,000 |
|
TOTAL COST TO THE GOVERNMENT |
$574,091 |
15. Explanation for Program Changes or Adjustments
This is an extension to a previously approved data/information collection.
16. Plans for Tabulation and Publication and Project Time Schedule
Individual data collections under this generic approval will be time-limited and generally conducted only once, except in the cases of individual interviews conducted during pilot testing of interventions where respondents may have to be approached several times on the same or similar topic under evaluation. No single data collection activity will take longer than 2 years to complete from inception of information collection to the first report of findings. Proposed timelines will be submitted for each individual data collection activity. Only in rare cases would data that is collected not be published and made publicly available in aggregate form. At the time of this submission, NIOSH has not identified any such cases. It is expected that each data collection would result in at least one journal article publication. In addition, during the preliminarly phase of data analysis and interpretation, each individual data collection may also be published in a proceedings for a conference administered by a professional society where experts in the domain of interest would be permitted to engage and provide feedback about the interpretation of analyses prior to final publication.Finally, findings from these information collections may be used to develop NIOSH-numbered publications such as fact sheets or infographics to ensure members of the public such as workers benefit from these information collections. In general, publication of findings is expected to occur anywhere from 6-12 months after the completion of information collection.
17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is not inappropriate.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
29 C.F.R. § 1910.134. Final rule. Fed. Reg. 63:1152–1300 Respiratory Protection. See also Appendix A to § 1910.134 Fit Testing Procedures (Mandatory).
42 C.F.R. § 84.2 (2020) (“NPPTL administers the NIOSH conformity assessment program for respiratory protective devices, replacing the former Certification and Quality Assurance Branch”).
BLS and NIOSH (2003). Respirator usage in private sector firms, 2001. U.S. Department of Labor, U.S. Department of Health and Human Services. Available at: https://www.cdc.gov/niosh/docs/respsurv/pdfs/respsurv2001.pdf
BLS (2021). May 2021 national occupational employment and wage estimates. Available at: https://www.bls.gov/oes/current/oes_nat.htm.
National Academies of Sciences, Engineering, and Medicine 2022. Frameworks for Protecting Workers and the Public from Inhalation Hazards. Washington, DC: The National Academies Press. https://doi.org/10.17226/26372.
OSHA (2014). Policy clarification on OSHA’s enforcement authority at small farms. Available at: https:// www.osha.gov/memos/2014-07-29/policy-clarification-oshas-enforcement-authority small-farms
Thompson D (2018). Health care just became the U.S.’s largest employer. The Atlantic. Published January 18, 2028. Available at: https://www.theatlantic.com/business/archive/2018/01/health-care-america-jobs/550079/
Yarbrough, M. I., M. E. Ficken, C. U. Lehmann, T. R. Talbot, M. D. Swift, P. W. McGown, R. F. Wheaton, M. Bruer, S. W. Little, and C. A. Oke (2016). Respirator use in a hospital setting: Establishing surveillance metrics. Journal of the International Society for Respiratory Protection 33(1):1-11.
Appendix A
Example Data Collection Instruments Sorted by Information Activity
Example 1: Quantitative Respirator Use Survey
This survey will cover the use of the following devices (images and definitions below):
NIOSH Approved respirators, which we’ll refer to as “respirators,” include:
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100)
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators
Powered Air-Purifying Respirators (PAPRs)
Supplied-Air Respirators
Self-Contained Breathing Apparatus (SCBAs)
NIOSH Approved Respirators (referred to as “Respirators”) |
|
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100)
FFRs are considered disposable half facepiece respirators that filter out particles such as dusts, mists, and fumes. They do NOT provide protection against gases and vapors.
Includes a NIOSH Approved label and has two head straps |
|
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators
Elastomeric respirators have reusable facepieces and replaceable cartridges or filters. They provide protection against particles, gases, or vapors when equipped with the appropriate cartridge or filter. |
|
Powered Air-Purifying Respirators (PAPRs)
PAPRs have a battery-powered blower that pulls air through attached filters, canisters, or cartridges. They provide protection against gases, vapors, or particles, when equipped with the appropriate cartridge, canister, or filter. Loose-fitting PAPRs do not require fit testing and can be used with facial hair. |
|
Supplied-Air Respirators
Supplied-air respirators are connected to a separate source that supplies clean compressed air through a hose. They can be lightweight and used while working for long hours in environments. They are used not used for entry into an atmosphere immediately dangerous to life and health (IDLH). |
|
Self-Contained Breathing Apparatus (SCBAs)
SCBA’s are used for entry into, work in, or escape from environments considered to be IDLH. They contain their own breathing air supply and can be either open circuit or closed circuit. |
|
This survey does not cover other devices (Non-NIOSH approved respirators and other face coverings), which include:
Non-NIOSH Approved Emergency Use International Respirators (e.g., KN95)
Surgical Masks (medical grade, Food and Drug Administration (FDA) cleared)
Dust Masks (non-medical, not FDA cleared)
All other non-medical masks or face coverings including cloth masks, disposable masks, gaiters, etc.
What information will I need?
The survey will cover the use of NIOSH Approved respirators at your business. We are asking about the number of employees, number using respirators, and topics related to your respiratory protection program (RPP). It may be helpful to have all the information gathered before you start the survey. You can find a PDF version of the survey on the respondent website. Once you start, if you find you need more information, your data can be saved and you cand complete the survey later.
Instructions: Complete this survey about the use of respiratory devices at the location(s) listed above. Only answer the questions below about the location(s) listed above.
2. How many people are currently employed at this location?
Include:
Full-time and Part-time employees
Employees who are contracted to work at this location and other locations for other companies
Temporary and Seasonal employees
Do not include:
Transition screen: The next few questions will ask about the availability and use of respirators during the past 12 months, and current use at this location.
Include all devices that employees have been qualified to wear (i.e., fit tested and/or trained). For FFRs (such as N95s and P100s), include all employees who have worn a device.
|
Yes, used during the past 12 months |
No, not used during the past 12 months |
Don’t Know |
Respirators |
|
|
|
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100) |
|
|
|
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators |
|
|
|
Powered Air-Purifying Respirators (PAPRs) |
|
|
|
Supplied-Air Respirators |
|
|
|
Self-Contained Breathing Apparatus (SCBAs) |
|
|
|
Other: |
|
|
|
[Note: going forward for all questions that have the grid with respirators, only respirators that are selected ‘Yes’ should be displayed for each question. Question 4 is designed to be a screener question and reduce burden if the respondent has not used certain respirators in the past 12 months.]
If at least one row in this had a “Yes” response, continue to the next question.
If a respondent answers No or Don’t know for all rows in the question, then they go to the Data Review screen and submit the survey.
4. Do employees at this location currently use respirators? (Select all that apply)
Voluntary Use: Employee decides to use the respirator or mask (with the employer’s approval) for personal reasons (e.g., allergy, desire to reduce exposure beyond that required by regulation, etc.).
Required Non-Emergency Use: Respirator or mask use for exposure to known substances which is required by regulation or by the employer.
Required Emergency Use: Respirator or mask use as a result of an unplanned situation, including escape from or entry into a potentially hazardous environment.
-
Voluntary Use
Required Non-Emergency Use
Required Emergency Use
Don’t know
Respirators
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100)
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators
Powered Air-Purifying Respirators (PAPRs)
Supplied-Air Respirators
Self-Contained Breathing Apparatus (SCBAs)
Other:
5. Have you experienced a shortage of respirators (provide timeframe?) at this location? A shortage is defined as a difficulty in obtaining respirators when and where they were needed or an actual shortage of supply.
All rows will be displayed in the chart below regardless of the responses provided in the screener question 4.
-
Yes
No
Don’t Know
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100)
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators
Powered Air-Purifying Respirators (PAPRs)
Supplied-Air Respirators
Self-Contained Breathing Apparatus (SCBAs)
Filters, cartridges, and other respirator supplies
12. How many employees for the occupation <fill> are currently using the following types of respirators at this location?
If employees wore multiple types of devices, include them in the count for each kind they wore.
For respirators, include all employees who are qualified to wear them (fit tested and/or trained). For FFRs (such as N95s and P100s), include all employees who have worn a device.
For masks, include all employees who wear or wore them.
-
Number of Employees in this occupation who currently use the device
Respirators
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100)
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators
Powered Air-Purifying Respirators (PAPRs)
Supplied-Air Respirators
Self-Contained Breathing Apparatus (SCBAs)
13. Why do employees in this occupation <fill> currently use respirators at this location? (Select all that apply)
Only include the rows that have a positive number of employees entered for question 12 in the chart below.
|
Used to protect against work-related hazards |
Used to protect against coronavirus |
Don’t know |
Respirators |
|
|
|
NIOSH Approved Filtering Facepiece Respirators (FFRs) (e.g., N95, P100) |
|
|
|
Elastomeric Half-Mask or Full Facepiece Air-Purifying Respirators |
|
|
|
Powered Air-Purifying Respirators (PAPRs) |
|
|
|
Supplied-Air Respirators |
|
|
|
Self-Contained Breathing Apparatus (SCBAs) |
|
|
|
Transition screen: The next sections will address questions regarding your respirator program for ALL employees at this location.
Respiratory Protection Program (RPP)
This location has a qualified Program Administrator who is dedicated to our Respiratory Protection Program (RPP)
Yes, it is me
Yes, it is someone else
No
Unsure
The written RPP is reviewed and evaluated at this location:
Monthly
Quarterly
Biannually
Annually
Unsure
Fit Testing
16. Are employees who wear respirators at this location fit tested prior to using respirators?
Yes, all are fit tested (continue to question 17)
Yes, some are fit tested (continue to question 17)
No (skip to question 19)
Don’t know (skip to question 19)
17. Which of the following methods do you currently use to assess your employees’ medical fitness to wear respirators at this location?
No medical fitness assessment
Questionnaire only
Questionnaire with follow-up exam required
Questionnaire with follow-up exam as needed
Medical exam only
Other—please list:
Don’t know
18. Who currently conducts the fit testing of the respirators at this location? (Select all that apply)
The respirator program manager or other health and safety expert
Medical personnel
A supervisor
A designated employee other than those listed above.
A respirator manufacturer sales/technical representative
Another external consultant or third-party company
Other—please list:
19. During the past 12 months, which of the following fit testing methods were used at this location?
• If only ONE fit testing method was used, enter 100% in the percentage column.
• If more than one fit testing method was used, indicate the percentage of times each method was used.
• The percentage column should total up to 100%.
Fit testing method |
Percentage |
Check here if you don’t know the percentage |
Saccharin |
|
|
Bitrex |
|
|
Irritant smoke |
|
|
Isoamyl acetate |
|
|
Generated-aerosol (corn oil, salt, etc.) |
|
|
Ambient aerosol (PortaCount) |
|
|
Pressure (controlled negative pressure) |
|
|
Other |
|
|
Training
19. Is respirator training currently provided to your employees who wear respirators at this location?
Yes (continue to question 20)
No (skip to question 23)
Don’t know (skip to question 23)
20. When is respirator training currently provided to employees who wear respirators at this location? (Select all that apply)
When the employee starts at the company
Annually
Other frequency (please specify):
Don’t know
21. How is respirator training currently provided to employees at this location? (Select all that apply)
Classroom training (real time, in-person or remote)
One-on-one training (real time, in-person or remote)
Online training (pre-recorded videos and materials)
Other, please specify:
Don’t know
22. Who currently provides the respirator training at this location? (Select all that apply)
The respirator program manager or other health and safety expert
Medical personnel
A supervisor
A designated employee other than those listed above.
A respirator manufacturer sales/technical representative
Another external consultant or third-party company
Other—please list:
Example 2: Quantitative Respirator Perceptions Survey
The purpose of these questions is to understand what you think about respiratory protection in the workplace, with a focus on Elastomeric Half Mask Respirators (EHMRs) as well as what has the biggest impact on health and safety at your organization. Please think about a current, typical work week when responding.
1. What is your current job title? [Open text]
Length of time you have been in your current job:
0–3 months |
4 – 6 months |
7 – 11 months |
1 – 2 years |
3 – 5 years |
6 – 10 years |
11 – 15 years |
16 – 20 years |
21+ years |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
What is the type of unit or area where you work? [Open text]
Length of time you have been with this organization:
0–3 months |
4 – 6 months |
7 – 11 months |
1 – 2 years |
3 – 5 years |
6 – 10 years |
11 – 15 years |
16 – 20 years |
21+ years |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
How many hours is your usual work shift (not including overtime):
8 hours
9 – 10 hours
11 – 12 hours
More than 12 hours
What is your height? _____ feet ______inches
What is your current weight? _______ pounds
Does your current job require you to wear respiratory protection at some point throughout the day?
Yes/No
If so, what manufacturer, model, and level of protection (e.g., N95, P100?)
During a typical shift on a workday, what is the total length of time you wear respiratory protection?
Respiratory protection is typically used only when there is a concern for airborne transmission of disease. Cloth masks and surgical masks are not considered respiratory protection.
-
0 hours
Less than 1 hour
1 – 2 hours
3 – 4 hours
5 – 6 hours
More than 6 hours
During a typical shift on a workday, what is average length of time you wear respiratory protection before doffing?
Respiratory protection is typically used only when there is a concern for airborne transmission of disease. Cloth masks and surgical masks are not considered respiratory protection.
-
1 – 15 minutes
16 – 30 minutes
31 – 45 minutes
46 – 60 minutes
More than 1 hour
More than 2 hours
Have you used an EHMR as a part of any job in the past?
Yes / No
Briefly describe the job in which you were required to use an EHMR.
Currently, what procedures/tasks do you do that require wearing respiratory protection? Please describe. [Open text]
At the time of completing this survey, have you received your new EHMR for work use as a part of this project?
Yes/No
Baseline Questions (some to be included and/or reworded for the post-survey)
Section 2: Respiratory Protection Baseline Use (Questions adapted from EHMR contract survey)
Currently, which type of respirator do you prefer?
N95 Filtering Facepiece Respirator |
Elastomeric Half Mask Respirator (EHMR) |
Powered Air-Purifying Respirator (PAPR) |
No basis to judge |
1 |
2 |
3 |
4 |
Why do you prefer this type of respirator over either of the others? [Open text]
What is your current familiarity with EHMRs on a scale from 0 to 100, with 0 being not at all familiar and 100 being extremely familiar?
0 |
10 |
20 |
30 |
40 |
50 |
60 |
70 |
80 |
90 |
100 |
Not at all familiar |
|
|
|
|
Limited/Somewhat familiar |
|
|
|
|
Extremely familiar |
Have you been fit tested to wear an Elastomeric Half Mask Respirator (EHMR) respirator?
Yes
No
No, but fit testing is scheduled
Don’t know
Have you received training on how to use an EHMR?
Yes
No
No, but EHMR training is scheduled
Don’t know
Section 3: EHMR Baseline Efficacy, Perceptions, and Risk Assessment (Questions adapted from EHMR contract survey, and the Precaution Adoption Process Model framework)
Which of the following best describes your thoughts about using an EHMR as a method of respiratory protection at work?
_____ I’ve never thought about this issue
_____ I’m undecided about EHMRs in general
_____ I don’t want to use an EHMR
_____ I do want to use an EHMR
How confident are you in the personal protection of an EHMR to you without an exhalation valve with 0 being not at all confident in to 100 being fully confident? (if not applicable skip)
This type of EHMR is a non-powered, NIOSH Approved respirator that has a tight-fitting facepiece that covers the nose and mouth, where the exhalation valve is absent from the facepiece and exhaled air is filtered
-
0
10
20
30
40
50
60
70
80
90
100
Not at all confident
Moderately confident
Fully confident
How confident are you in the personal protection provided by an EHMR to you with an exhalation valve with 0 being not at all confident in to 100 being fully confident? (if not applicable skip)
An EHMR is a non-powered, NIOSH Approved respirator that has a tight-fitting facepiece that covers the nose and mouth where an exhalation valve is present on the facepiece to reduce exhalation resistance
-
0
10
20
30
40
50
60
70
80
90
100
Not at all confident
Moderately confident
Fully confident
How confident are you in the source control effectiveness provided to others by an EHMR with an exhalation valve with 0 being not at all confident in to 100 being fully confident? (if not applicable skip)
Source control effectiveness refers to the ability of a respirator or mask to help reduce the spread of large respiratory droplets to others when the person talks, sneezes, or coughs. Respiratory protection protects the wearer, source control protects others.
-
0
10
20
30
40
50
60
70
80
90
100
Not at all confident
Moderately confident
Fully confident
How confident are you in the protection provided to others by an EHMR that has an exhalation valve with an additional mask/covering over the valve with 0 being not at all confident in to 100 being fully confident? (if not applicable skip)
How confident are you with donning and doffing the EHMR with 0 being not at all confident in to 100 being fully confident?
Donning is the term used to described putting on personal protective equipment. Doffing is the term used to described taking off personal protective equipment
-
0
10
20
30
40
50
60
70
80
90
100
Not at all confident
Moderately confident
Fully confident
List any benefits you anticipate to using your EHMR respirator. [Open text]
List any challenges/problems you anticipate with using your EHMR respirator. [Open text]
Which of the following are problems that bother you when you wear an EHMR respirator? (select N/A if you do not have experience wearing an EHMR yet or a specific question is not relevant to you)
|
Never |
Rarely |
Sometimes |
Usually |
Always |
N/A |
It is difficult to breathe |
1 |
2 |
3 |
4 |
5 |
0 |
Reduced visual perception |
1 |
2 |
3 |
4 |
5 |
0 |
I am bothered by moisture build-up |
1 |
2 |
3 |
4 |
5 |
0 |
The EHMR interferes with my other PPE, such as my face shield or goggles |
1 |
2 |
3 |
4 |
5 |
0 |
The EHMR interferes with the eyeglasses I normally wear |
1 |
2 |
3 |
4 |
5 |
0 |
I have difficulty speaking to others (e.g., patients, colleagues) |
1 |
2 |
3 |
4 |
5 |
0 |
I have difficulty being understood by others (e.g., patients, colleagues) |
1 |
2 |
3 |
4 |
5 |
0 |
It is uncomfortable to wear an EHMR for less than one hour |
1 |
2 |
3 |
4 |
5 |
0 |
It is uncomfortable to wear an EHMR for more than one hour |
1 |
2 |
3 |
4 |
5 |
0 |
I feel uncomfortably warm |
1 |
2 |
3 |
4 |
5 |
0 |
I have facial irritation/discomfort |
1 |
2 |
3 |
4 |
5 |
0 |
I feel claustrophobic |
1 |
2 |
3 |
4 |
5 |
0 |
Negative/frightened reaction from patient |
1 |
2 |
3 |
4 |
5 |
Section 5: Workplace Safety Climate (adapted from EHMR contract questions and standard safety climate scale questions from NIOSH)
45. Please indicate the degree to which you agree or disagree with these statements:
|
Never |
Rarely |
Sometimes |
Often |
Always |
A properly fitted respirator can protect workers from on-the-job exposure to airborne infectious diseases |
1 |
2 |
3 |
4 |
5 |
An EHMR respirator is more effective than a N95 FFR at protecting workers from airborne infectious diseases |
1 |
2 |
3 |
4 |
5 |
Workers at my workplace use respirators when they are required |
1 |
2 |
3 |
4 |
5 |
Supervisors correct workers if they do not wear a respirator when required |
1 |
2 |
3 |
4 |
5 |
Supervisors correct workers if they do not wear a respirator properly (for example, if only one strap was used) |
1 |
2 |
3 |
4 |
5 |
At my workplace, safety hazards are quickly corrected |
1 |
2 |
3 |
4 |
5 |
At my workplace, all reasonable steps are taken to minimize workers' risk of exposure to airborne infectious diseases |
1 |
2 |
3 |
4 |
5 |
The health and safety of workers is a high priority with management where I work |
1 |
2 |
3 |
4 |
5 |
The health and safety of workers is a high priority with coworkers where I work |
1 |
2 |
3 |
4 |
5 |
Workers are provided adequate training about proper use of respiratory protection |
1 |
2 |
3 |
4 |
5 |
Management communicates information about safety and health |
1 |
2 |
3 |
4 |
5 |
Management seeks feedback from workers about health and safety issues |
1 |
2 |
3 |
4 |
5 |
My input is solicited (either by surveys or during meetings) on the selection of respirators |
1 |
2 |
3 |
4 |
5 |
My organization has been doing everything it can to protect the workforce from respiratory hazards |
1 |
2 |
3 |
4 |
5 |
My coworkers have been doing everything they can to protect themselves from respiratory hazards |
1 |
2 |
3 |
4 |
5 |
My coworkers have been taking extra precautions to protect the workforce from respiratory hazards |
1 |
2 |
3 |
4 |
5 |
Psychological well-being of staff is a priority for this organization |
1 |
2 |
3 |
4 |
5 |
There is good communication here about safety issues which affect me |
1 |
2 |
3 |
4 |
5 |
Employees are encouraged to become involved in psychological health and safety issues |
1 |
2 |
3 |
4 |
5 |
Employees receive resources and/or support that assist in managing job demands |
1 |
2 |
3 |
4 |
5 |
Section 6: Perception of Organization’s Respiratory Protection Program (RPP) (adapted from OSHA’s RPP checklist)
Do you know what a respiratory protection program is?
Yes/No
Have you seen or reviewed your written RPP at any point in time?
Yes/No
Were any changes made to your written RPP (indicate timeframe)? [Open text]
Regarding your RPP, select whether each area is covered appropriately or if improvements are needed.
Regarding your RPP, select whether each area is comprehensive or if improvements are needed. |
Not Included |
Included but Needs Improvement |
Covered Appropriately |
Not Applicable |
Selecting respirators |
|
|
|
|
Medical evaluations of employees required to wear respirators |
|
|
|
|
Fit testing |
|
|
|
|
Routine and emergency respirator use |
|
|
|
|
Schedules for cleaning, disinfecting, storing, repairing, maintaining, etc. respirators |
|
|
|
|
Training in respiratory hazards |
|
|
|
|
Training in proper use and maintenance of respirators |
|
|
|
|
Program evaluation |
|
|
|
|
Ensuring employees who voluntarily wear respirators comply with all associated standards |
|
|
|
|
a designated program administrator who is qualified to administer the program |
|
|
|
|
updating the written program as necessary to account for changes in the workplace affecting respirator use |
|
|
|
|
providing equipment, training, and medical evaluations at no cost to employees |
|
|
|
|
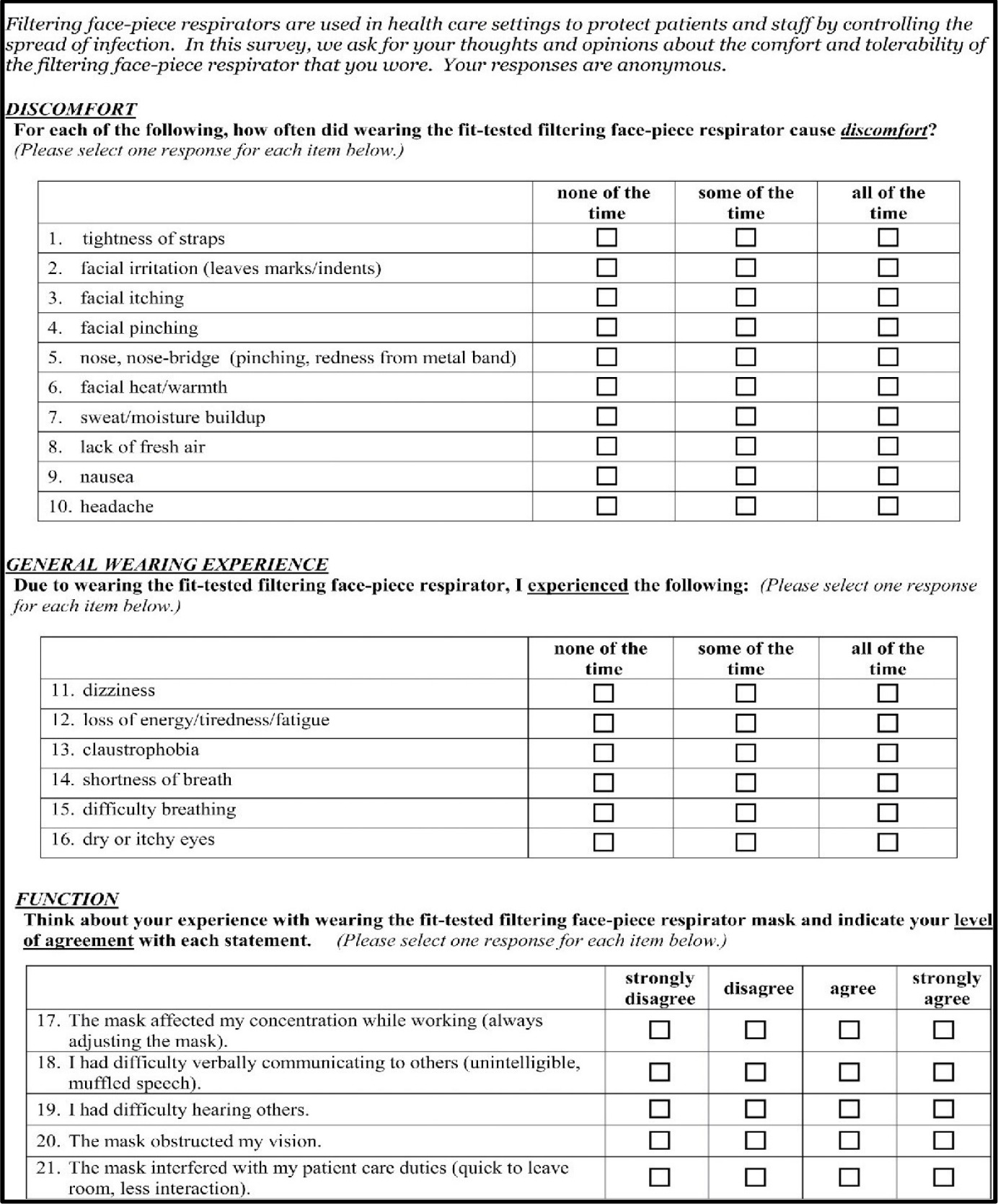
Example 2b: Respirator Experience Survey
Example 3: Qualitative Interview Guide – Implementation and Management of Respirators in the Workplace
What is your current job title and length of time you have been in this current job:
What is the type of unit or area where you work?
Length of time you have been with this organization:
During a typical shift, what is the total length of time your employees wear respiratory protection?
During a typical shift, what is average length of time your employees wear respiratory protection before doffing?*
Please share some common scenarios or procedures where employees may use respiratory protection on the job.
In what scenarios may source control be an issue for your employees wearing an EHMR?
Source control effectiveness refers to the ability of a respirator or mask to help reduce the spread of large respiratory droplets to others when the person talks, sneezes, or coughs. Respiratory protection protects the wearer, source control protects others.
Please describe the experience that your organization has with EHMRs.
How would you rate your overall workforces’ current familiarity with EHMRs?
Discuss any benefits you anticipate to your employees using EHMR respirators in the workplace.
In your opinion, what are advantages to using an EHMR as a source of respiratory protection?
Please discuss any challenges/problems you anticipate with implementing EHMRs in the workplace.
For your organization, who is responsible for monitoring/observing employees for compliance in properly wearing the EHMR?
What criteria did you/are you using to assign EHMR use in your organization? (may be NA if assigning to all).
Approximately, what percentage of your workforce is going to receive EHMRs as a part of this project?
Reflecting on your current RPP, what changes, if any, were made to your written RPP during (provide timeframe)?
[If applicable] What changes do you anticipate permanently remaining in your program?
There are many aspects of an RPP. We are going to discuss some general sections. Please provide what aspects you think need improvement or to be updated based on incorporating EHMRs as well as other general input provision.
Involving employees in the selection of respirators
Medical evaluations of employees required to wear respirators
Fit testing
Routine and emergency respirator use
Schedules for cleaning, disinfecting, storing, repairing, maintaining, etc. respirators
Training in respiratory hazards
Training in proper use and maintenance of respirators
Updating the written program as necessary to account for changes in the workplace affecting respirator use
Providing equipment, training, and medical evaluations at no cost to employees
Example 4: Pre-fit testing Questionnaires
1) Last fit test date (auto populate from previous testing via REDCap)
________________
2) Length of your shift in hours:
8
10
12
Other : ________
3) Estimate the number of total hours this N95 was worn this shift:
<1
1-2
2-4
5-6
7-8
8-10
10-12
4) Have you discarded your N95 since the last fit test? y/n
If yes,
4b) why?
Comfort?
Soiled?
Damaged?
If Yes, STOP. If this is the first mask, this completes the 1st half of the study. Move to mask 2 and start new form. If this is mask 2, this completes study participation.
If no,
5) Since the last fit test, approximately how many times have you donned and doffed your N95? Enter number.
6) Since the last fit test, have you noticed your N95 has been deformed or misshapen at any point? y/n
7) Since the last fit test, has the N95 been soiled or gotten wet? Y/n
8) Since the last fit test, has the N95 produced noticeable humidity from talking or performing tasks that produced facial sweating while wearing it? y/n
9) Since the last fit test, have you worn the N95 improperly (around neck, on arm, etc.)? y/n
10) Are you using skin protectant? Y/N if yes 9b
10b) what kind of skin protectant?
Band-Aid
Cream
Ointment
Other
11) are you wearing makeup that crosses the skin seal surface? y/n
12) Do you have facial hair or facial jewelry: y/n, if yes go to 7b and 7c
12b) does facial hair cross respirator skin seal surface? if yes either remove or shave.
13) Did you cover your N95 with a surgical mask? Y/n
14) Did you cover your N95 with a face shield? y/n
Are you between the ages of 18-59? o Yes o No
What is your job title? ____________________________
Note: Participant must be a physician’s assistant (PA), registered nurse (RN), licensed practical nurse (LPN), patient care technician, nurse practitioner (NP), or respiratory therapist.
Where do you work for your primary employment? _________________________________________
Note: Participants must work in a hospital or inpatient care setting
On average, how many hours do you work each week? ___________ hours (<30 hours is exclusionary)
Are you currently required to wear a NIOSH Approved respirator such as a disposable N95 filtering facepiece respirator continuously at work? o Yes o No
Have you been fit-tested for wearing a disposable N95 filtering facepiece respirator in the past year?
o Yes o No
If yes, please record the model and size that the participant is fit-tested for.
Model: ____________________________________________ Size: ____________________
Did you have any significant weight gain or loss or new facial scarring since being fit tested?
o Yes o No
Are you able and willing to shave your face during the study participation across two working shifts?
o Yes o No
Are you currently pregnant or plan on becoming pregnant in the next month? o Yes o No
Are you able to walk 2 city blocks and climb 2 flights of stairs safely? o Yes o No
Are you currently being treated for any serious medical conditions such as kidney disease, liver disease, cancer, or heart disease? o Yes o No
16. Please describe the thermal sensation of your face while wearing this N95.
4 |
Very Hot |
¨ |
3 |
Hot |
¨ |
2 |
Warm |
¨ |
1 |
Slightly Warm |
¨ |
-1 |
Slightly Cool |
¨ |
-2 |
Cool |
¨ |
-3 |
Cold |
¨ |
-4 |
Very Cold |
¨ |
Please describe the thermal sensation of your whole body while wearing this N95.
4 |
Very Hot |
¨ |
3 |
Hot |
¨ |
2 |
Warm |
¨ |
1 |
Slightly Warm |
¨ |
-1 |
Slightly Cool |
¨ |
-2 |
Cool |
¨ |
-3 |
Cold |
¨ |
-4 |
Very Cold |
¨ |
Fit testing
Staff note: Solution taste threshold (how many sprays, 10, 20, 30)
Passed fit test: y/n.
Example 5: Physciological Questionnaire
------------------------------------------------------------------------------------------------------
STEP 1: DON RESPIRATOR or CLOTH MASK
--------------------------------------------------------------------------------------------------------
Instructions: Please don the N95 filtering facepiece respirator (FFR) or cloth mask that was provided to you by the study as you would for your normal daily work activities. Record the donning time in your respirator or face covering wear log.
What is today’s date? _______________________
Are you wearing an N95 FFR or cloth mask (cloth mask) today?
□ N95 FFR □ Cloth mask
-------------------------------------------------------------------------------------------------------
STEP 2: PUT ON ALL MONITORS
--------------------------------------------------------------------------------------------------------
Instructions: Using the video and/or paper instructions provided in the bag, please put the monitors on your body.
--------------------------------------------------------------------------------------------------------
STEP 3: COMPLETE THE RESPIRATOR/CLOTH MASK COMFORT QUESTIONNAIRE
--------------------------------------------------------------------------------------------------------
DISCOMFORT
For each of the following, please rate your current level of discomfort described by each category (Please select one response for each item below.)
|
No discomfort at all |
|
|
|
|
Very Uncomfortable |
|
0 |
1 |
2 |
3 |
4 |
5 |
1. tightness of straps |
□ |
□ |
□ |
□ |
□ |
□ |
2. facial irritation (leaves marks/indents) |
□ |
□ |
□ |
□ |
□ |
□ |
3. facial itching |
□ |
□ |
□ |
□ |
□ |
□ |
4. facial pinching |
□ |
□ |
□ |
□ |
□ |
□ |
5. facial pain |
□ |
□ |
□ |
□ |
□ |
□ |
6. facial bruising |
□ |
□ |
□ |
□ |
□ |
□ |
7. facial rash |
□ |
□ |
□ |
□ |
□ |
□ |
8. nose, nose-bridge (pinching, redness from the metal band) |
□ |
□ |
□ |
□ |
□ |
□ |
9. facial heat/warmth |
□ |
□ |
□ |
□ |
□ |
□ |
10. sweat/moisture buildup |
□ |
□ |
□ |
□ |
□ |
□ |
11. lack of fresh air |
□ |
□ |
□ |
□ |
□ |
□ |
12. nausea |
□ |
□ |
□ |
□ |
□ |
□ |
13. headache |
□ |
□ |
□ |
□ |
□ |
□ |
GENERAL WEARING EXPERIENCE
Please rate the level at which you are currently experiencing the following symptoms: (Please select one response for each item below.)
|
Not experiencing at all |
|
|
|
|
Experiencing to a very high degree |
|
0 |
1 |
2 |
3 |
4 |
5 |
1. dizziness |
□ |
□ |
□ |
□ |
□ |
□ |
2. loss of energy/tiredness/fatigue |
□ |
□ |
□ |
□ |
□ |
□ |
3. claustrophobia |
□ |
□ |
□ |
□ |
□ |
□ |
4. shortness of breath |
□ |
□ |
□ |
□ |
□ |
□ |
5. difficulty breathing |
□ |
□ |
□ |
□ |
□ |
□ |
6. dry or itchy eyes |
□ |
□ |
□ |
□ |
□ |
□ |
THERMAL SENSATION
Please rate the thermal sensation that you are experiencing right now for your face and for your whole body: (Please select one response for each item below.)
|
Very Cold |
Cold |
Cool |
Slightly Cool |
Slightly Warm |
Warm |
Hot |
Very Hot |
|
-4 |
-3 |
-2 |
-1 |
1 |
2 |
3 |
4 |
1. Face |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
2. Whole body |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
□ |
--------------------------------------------------------------------------------------------------------
STEP 4: SIT QUIETLY FOR 10 MINUTES
--------------------------------------------------------------------------------------------------------
Instructions: Please sit in a quiet, distraction-free place for 10-minutes. Please sit with your feet flat on the ground and your arms supported in your lap or in in the arm of a chair.
Record the start time of the seated rest period: : am/pm
Within the last minute of the 10-minute rest period, push the large (play/stop) button in the middle of the blood pressure monitor to initiate a blood pressure measurement.
Record the end time of the seated rest period: : am/pm
--------------------------------------------------------------------------------------------------------
THE STUDY IS COMPLETE. THANK YOU FOR PARTICIPATING.
--------------------------------------------------------------------------------------------------------
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2025-11-25 |
© 2026 OMB.report | Privacy Policy