Submitter User Guide (Redline)
DTS-ASP-Submitter-UserGuide-20250829-UPDATES (1).docx
[IRA Collection] Manufacturer Submission of Average Sales Price (ASP) Data for Medicare Part B Drugs and Biological and Supporting Regulations (CMS-10110)
Submitter User Guide (Redline)
OMB: 0938-0921

Medicare Part B Average Sales Price (ASP) Module
Submitter User Guide
Version 2.0
3.1.2 Assign by Alternate ID 10
3.1.3 Request New NDC1/ALT ID/Manufacturer/Generic Name 13
3.2.1 Add/Update Product Data 20
3.3.1 Add/Update Financial Data 39
3.3.2 Add/Update Financial Data - Certified Drugs 45
3.3.3 Upload Financial Data 47
3.4 Restating Financial Data 51
3.4.1 Add/Update Restate Financial Data 52
3.4.2 Upload Restate Financial Data 54
3.6 Generate One-Time Password 70
3.7.2 Upload Assumption File 75
4. Technical Support Contact Information 78
Appendix A: Field Definitions 79
The purpose of this user guide is to provide guidance and instructions to representatives of drug manufacturing companies as they submit federally required Medicare Part B drug Average Sales Price (ASP) data to the Centers for Medicare & Medicaid Services (CMS).
CMS uses the Fee-for-Service Data Collection System (FFSDCS) to house various Fee-for-Service modules.
The ASP Data Collection System, referred to within this user guide as the ASP Module, is one of the modules under the FFSDCS system, and offers the following:
Provides users with an online-based software application for automating the collection, editing, and processing of drug product pricing data drug manufacturers submit on a quarterly basis.
Establishes a relationship between the manufacturers’ reported data and the billing codes Medicare providers use to calculate a weighted average sales price for each billing code.
Establishes prices for billing codes to determine payment limits of Part B drugs on certain Medicare claims.
Eliminates data entry errors, data formatting errors, and incomplete submitted data, and greatly reduces the process cycle and resource time needed to provide the pricing to contractors through automation of the manually intensive processes.
Accepts, stores, validates, and calculates drug pricing on Medicare Part B drug data received for the Center for Medicare Management (CMM) stakeholders.
Section 303 (b) and (c) of the Medicare Modernization Act (MMA) of 2003 revised the payment methodology for the majority of Part B covered drugs and biologicals that are not priced on a cost or prospective payment basis (hereafter referred to as drugs).
CMS applies the ASP methodology to the data drug manufacturers have submitted to the ASP Module. Per the MMA, ASP methodology determines the payment limit for these drugs. Local contractors calculate pricing for compounded drugs.
First time users must register and create an account in the CMS Enterprise Portal before logging in to the ASP Module. Refer to the Resource Library on the Education and Outreach page to view the ASP Module Registration User Guide for registration steps.
Once registration is complete, follow these steps to log in to the ASP Module as a Submitter using Multi-Factor Authentication (MFA):
Navigate to the CMS Enterprise Portal main page.
The ASP Module Login Page opens. Refer to Figure 1.
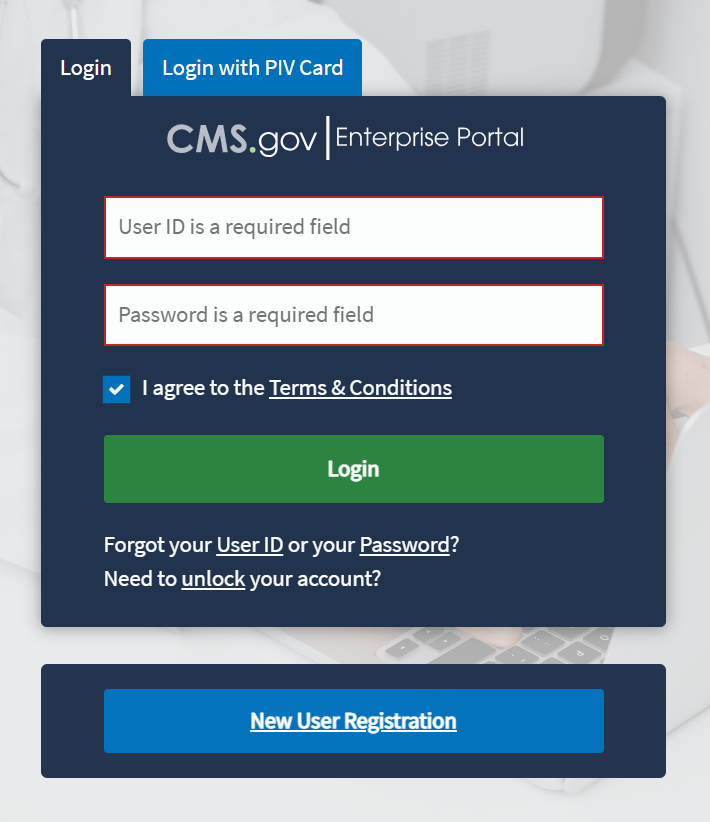
Figure 1: Logging in Using MFA - ASP Module Login
Enter your login information in the required User ID and Password fields.
Click the Terms & Conditions hyperlink and review the text in the pop-up window; close the window to move on to the next step.
Review the terms and conditions and select the I agree to the Terms & Conditions checkbox.
Note: By selecting this checkbox, you certify that you read and consent to monitoring while accessing and using the ASP Module. The terms and conditions link provides additional hyperlinks to the HHS Rules of Behavior and the CMS Privacy Act Statement.
Click Login.
Note: If you forget your user ID or password, click the Forgot your User ID or your Password? hyperlink under the Login button and follow the provided instructions. If you still cannot access your account and need to unlock it, click the Need to unlock your account? hyperlink under Login button.
The Multi-Factor Authentication page opens. Refer to Figure 2.
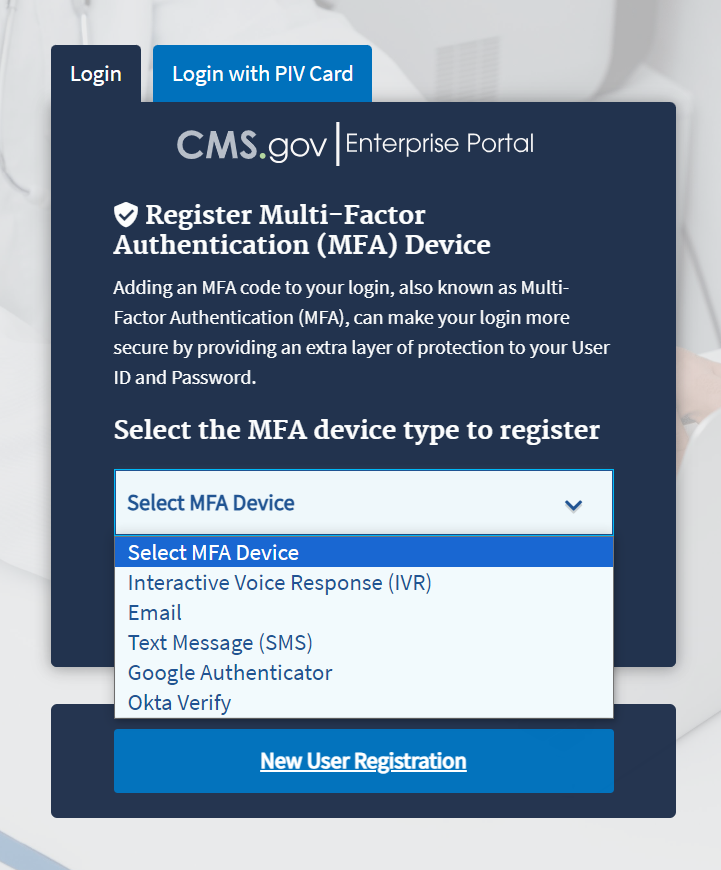
Figure 2: Logging in Using MFA - Select MFA Device Type Drop-Down Menu
To ensure the security of high value data submitted to the ASP Module, you must authenticate your identity using an MFA process. The first time you attempt to log in, you must choose an authentication method. Users have various authentication options, including Interactive Voice Response (IVR), Email, Text Message (Short Message Service (SMS)), Google Authenticator and Okta Verify.
Click the Select MFA Device drop-down menu; select your preferred MFA device type from the list. Whenever you log back into the ASP Module through this process, your preferred method of MFA reloads automatically.
Note: Figure 3 demonstrates MFA registration using IVR as the selected option.
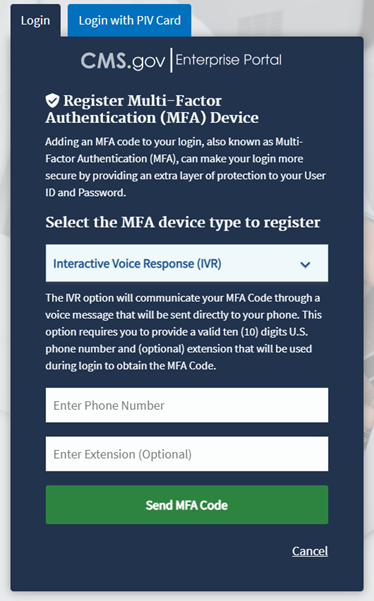
Figure 3: Logging in Using MFA - Multi-Factor Authentication - (IVR) Example
Enter your phone number in the Phone Number field; enter your extension in the Extension field, if necessary.
Click the Send MFA Code button to receive a six-digit code via your chosen contact method.
Record and enter the six-digit code you received into the Enter MFA Code field. Refer to Figure 4.
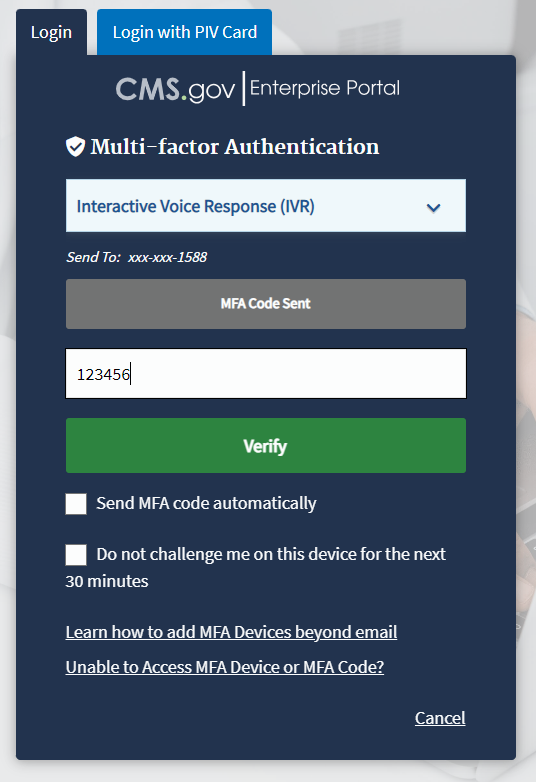
Figure 4: Logging in Using MFA - Multi-Factor Authentication - Verify MFA Code
Check the Send MFA code automatically and Do not challenge me on this device for the next 30 minutes checkboxes depending on your preference.
Note: If you need help, click the Learn how to add MFA Devices beyond email and Unable to Access MFA Devices or MFA Code? hyperlinks.
Click the Verify button to confirm your identity and enter the ASP Module homepage.
The My Portal landing page opens. Refer to Figure 5.
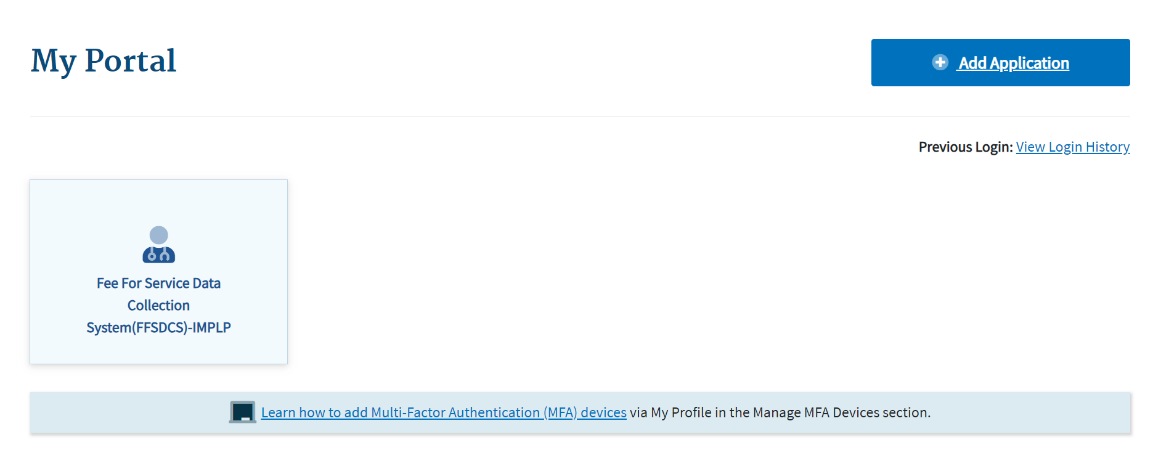
Figure 5: Logging in Using MFA - My Portal Landing Page
Note: Other CMS applications you have access to may display on the My Portal landing page.
Click the Fee For Service Data Collection System (FFSDCS) box.
A Fee for Service Data Collection System (FFSDCS) drop-down menu displays. Refer to Figure 6.
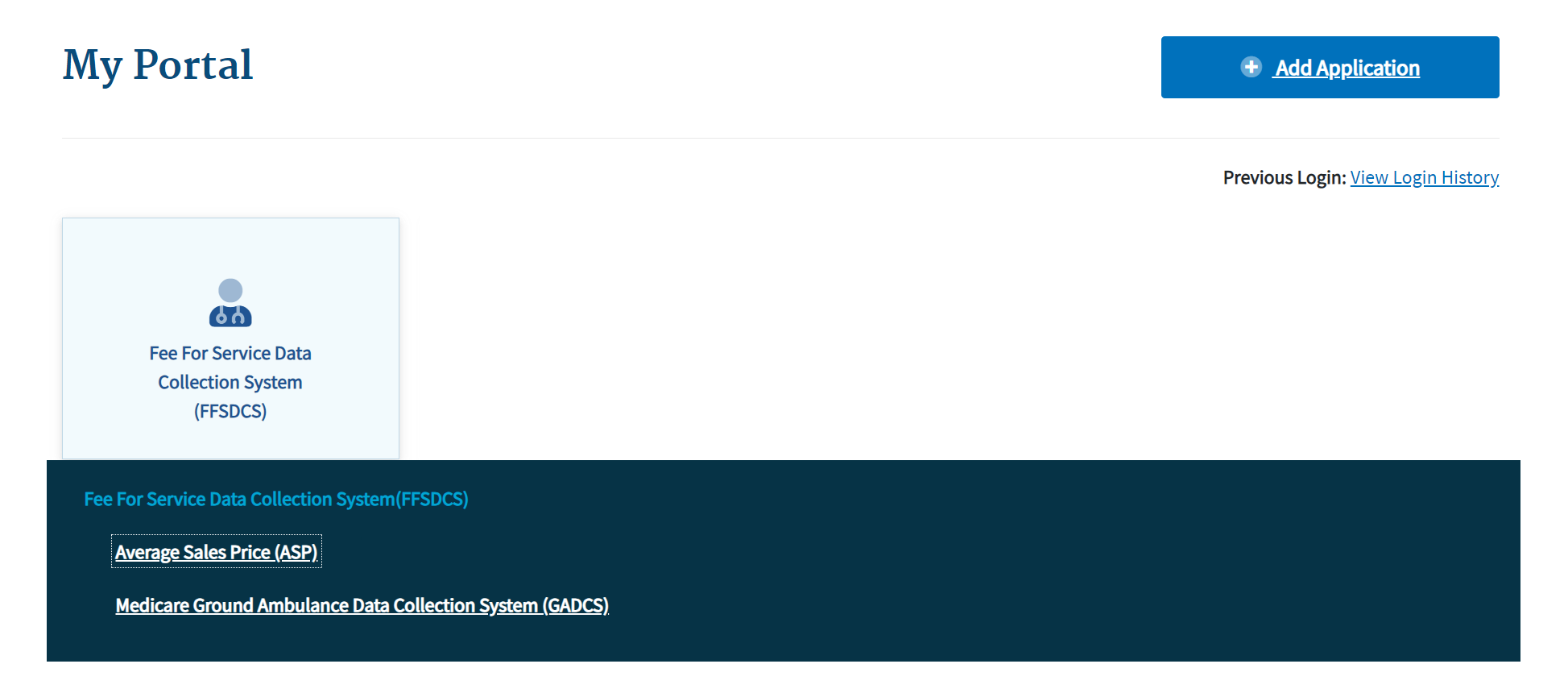
Figure 6: Logging in Using MFA - My Portal Landing Page - FFSDCS Drop-down Menu
Click the Average Sales Price (ASP) hyperlink.
A full-page statement displays, titled ASP Data for Drugs and Biologics Covered Under Medicare Part B. The statement details recent statutory requirements stated in the Social Security Act (the Act), and the Consolidated Appropriations Act (CAA), 2021. These requirements hold that manufacturers must report their ASP data to CMS with precision on a quarterly basis without errors or miscalculations. Refer to Figure 7.
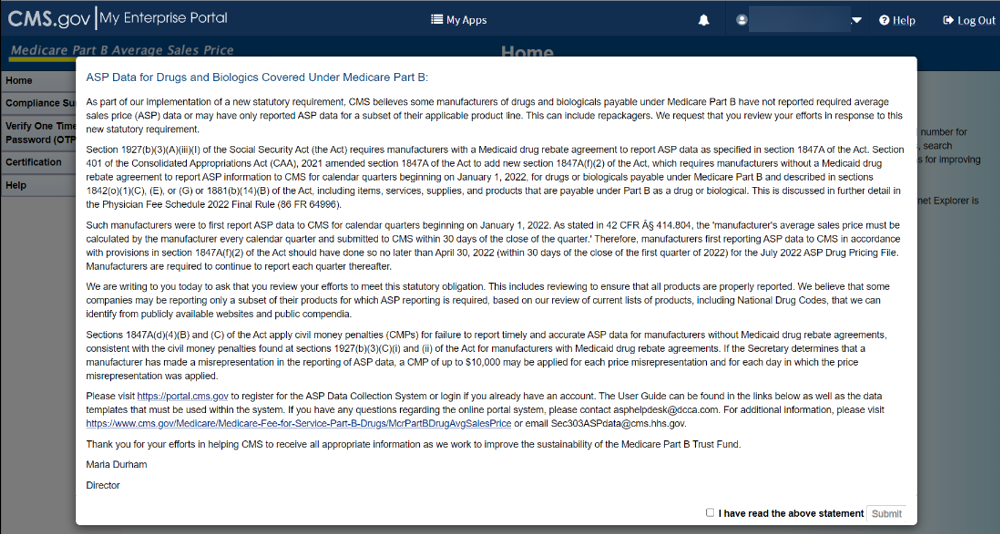
Figure 7: Logging in Using MFA - ASP Data for Drugs and Biologics Under Medicare Part B
Read the statement; select the I have read the above statement checkbox and click Submit.
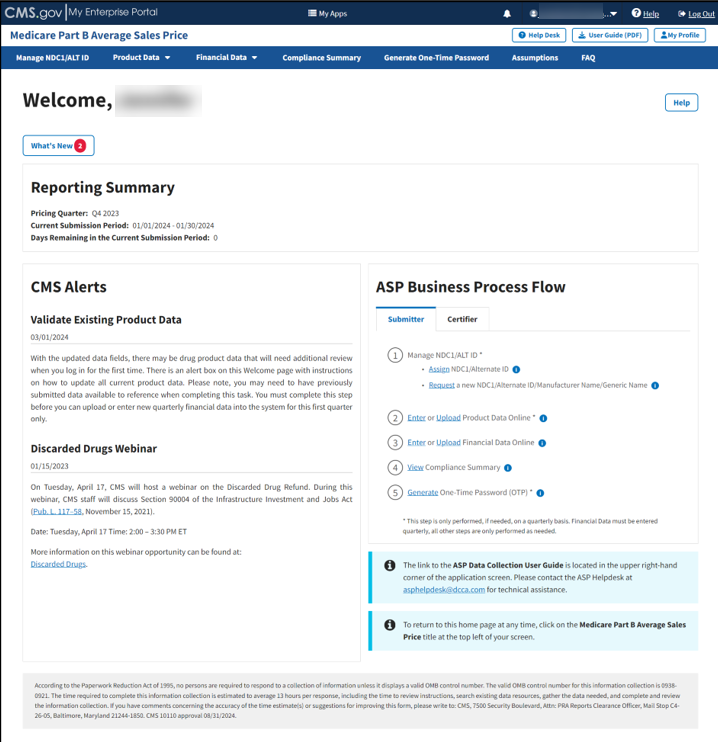
The Medicare Part B Average Sales Price homepage opens. Refer to Figure 8.
Figure 8: Medicare Part B Average Sales Price Homepage
The following sections describe the functionality of each menu tab on the ASP homepage, including Manage NDC1/ALT ID, Product Data, Financial Data, Compliance Summary, Generate One-Time Password, Assumptions and FAQ.
Note: This user guide is written in order of the system menu tabs and the respective tasks completed on that page and not necessarily in chronological order of steps to follow for quarterly data submission.
The following sections describe how to assign NDC1s and Alternate IDs, as well as how to request a new NDC1, Alternate ID, and manufacturer or generic name.
Note: To add a new product, users must first request to add an NDC1/ALT ID and Manufacturer/Generic Name, if needed. (Refer to Section 3.1.3 - Request New NDC1/ALT ID/Manufacturer/Generic Name). Once the new product has been approved into the system, users can establish the relationship between the manufacturer and the product by assigning the NDC1/ALT ID to the manufacturer. (Refer to Section 3.1.1 - Assign by NDC1 and Section 3.1.2 - Assign by Alternate ID.)
Follow these steps to assign NDC1s:
From the Medicare Part B Average Sales Price homepage, click the Manage NDC1/ALT ID tab.
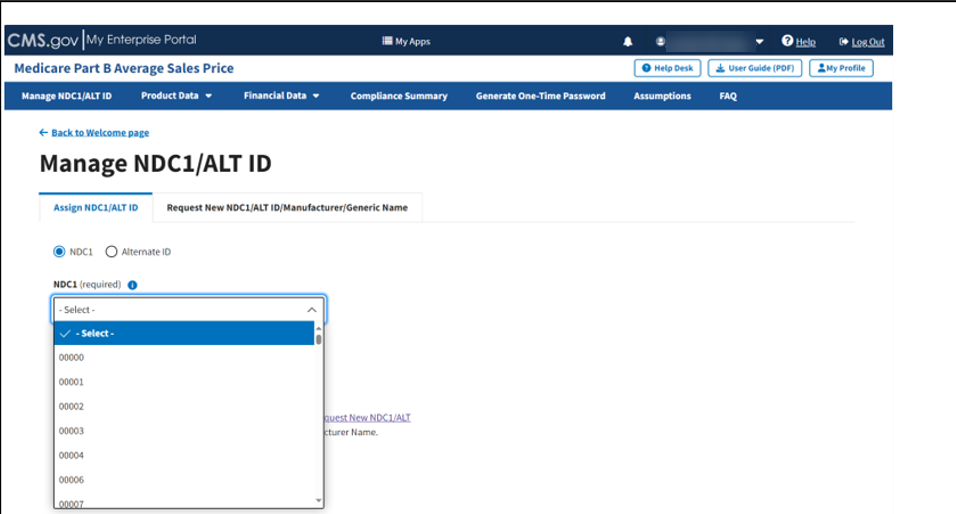
The Manage NDC1/ALT ID page opens and displays the Assign NDC1/ALT ID tab by default. Refer to Figure 9.
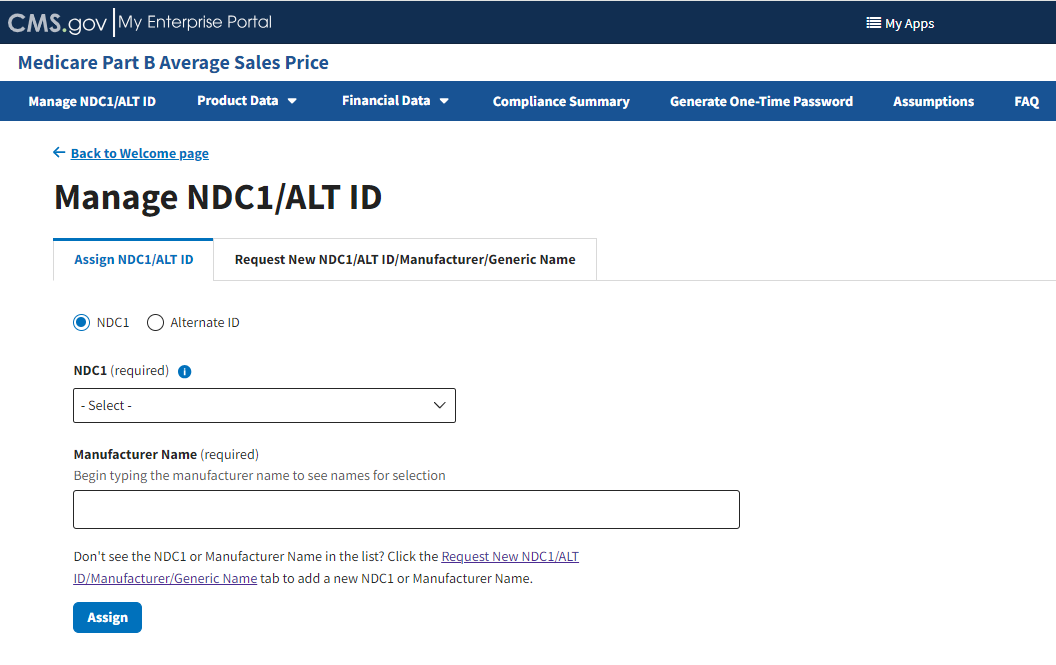
Figure 9: Manage NDC1/ALT ID Page - Assign NDC1
From the Assign NDC1/ALT ID tab, select the NDC1 radio button to specify the product data you need to submit to the Module.
Under NDC1 (required), click the -Select- drop-down menu to expand the list of submitted drugs and additional products in the Module to date; select the appropriate NDC1. Refer to Figure 10.
Figure 10: Manage NDC1/ALT ID Page - Assign NDC1 Drop-down Menu
Under Manufacturer Name (required), type and select the appropriate manufacturer. Refer to Figure 11.
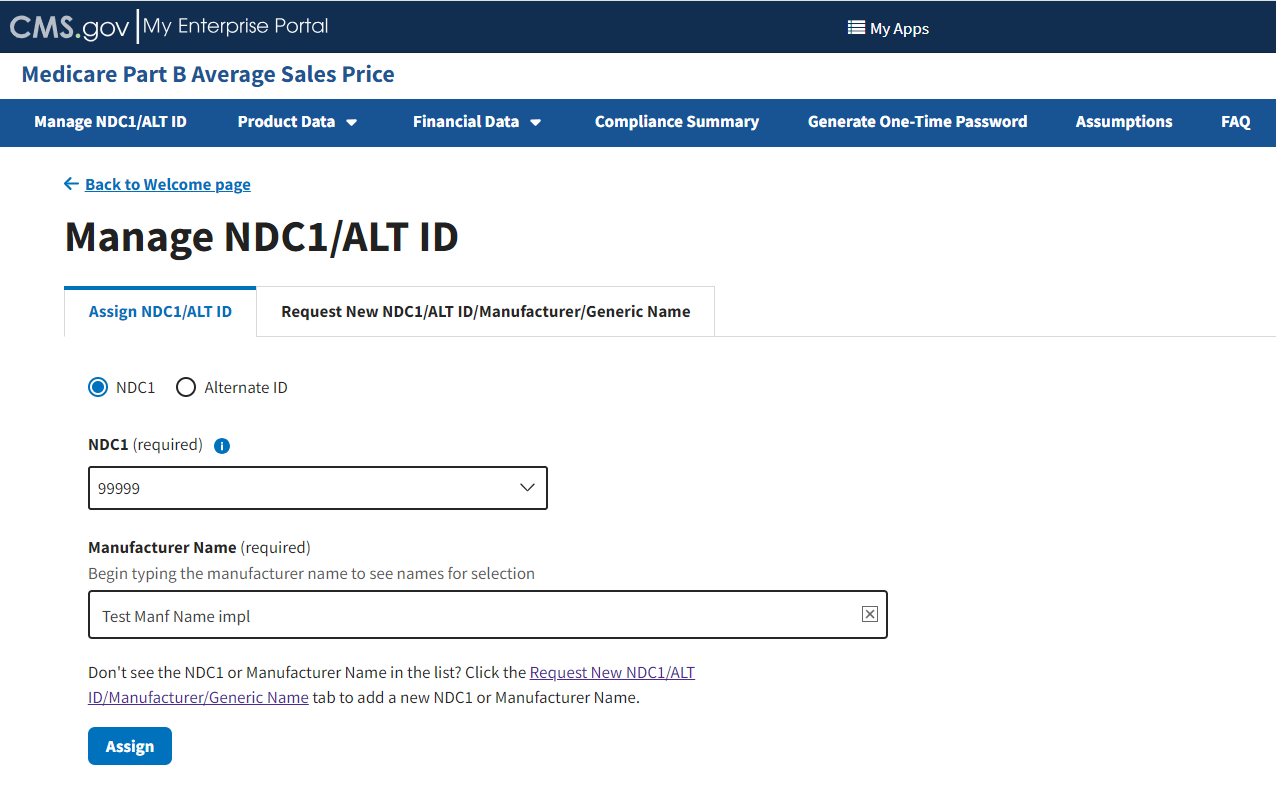
Figure 11: Manage NDC1/ALT ID Page - Enter NDC1 Manufacturer Name
Click Assign to submit your information.
A message displays confirming you have successfully added your selections. Refer to Figure 12.
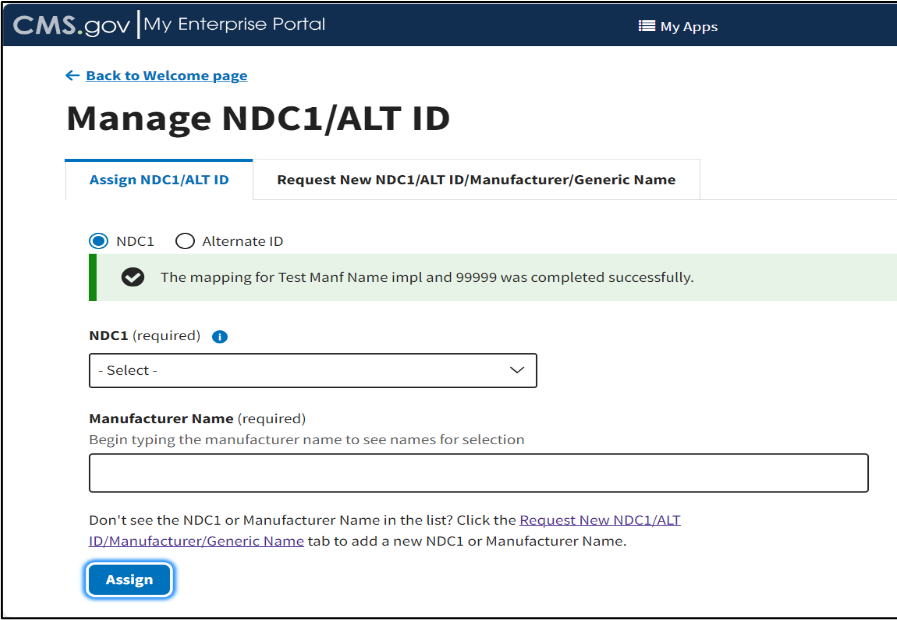
Figure 12: Manage NDC1/ALT ID - NDC1 Assigned Successfully
Follow these steps to assign Alternate IDs:
From the Medicare Part B Average Sales Price homepage, click the Manage NDC1/ALT ID tab.
The Manage NDC1/ALT ID page opens and displays the Assign NDC1/ALT ID tab by default.
From the Assign NDC1/ALT ID tab, select the Alternate ID radio button.
Additional fields specific to assigning an Alternate ID display. Refer to Figure 13.
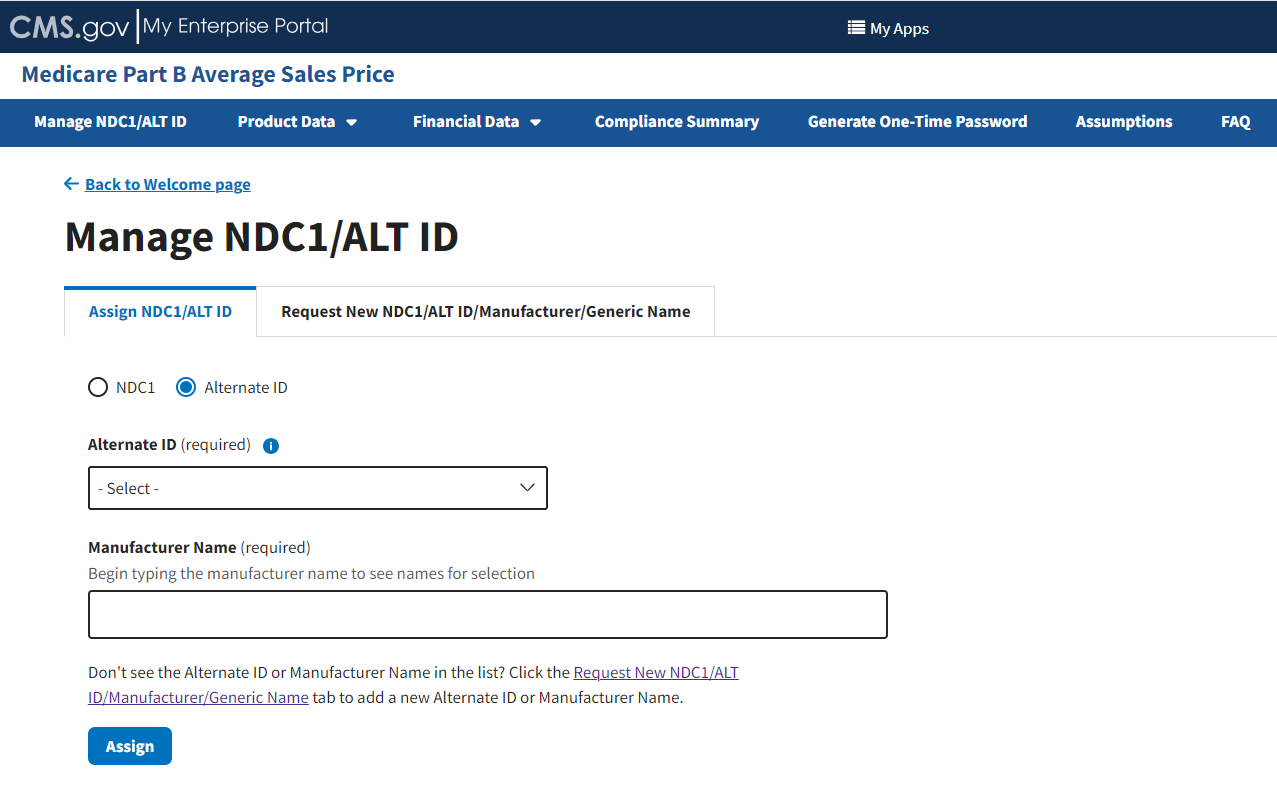
Figure 13: Manage NDC1/ALT ID Page - Assign ALT ID
Under Alternate ID (required), click the -Select- drop-down menu to expand the list of submitted drugs and additional products in the Module to date; select the appropriate alternate ID. Refer to Figure 14.
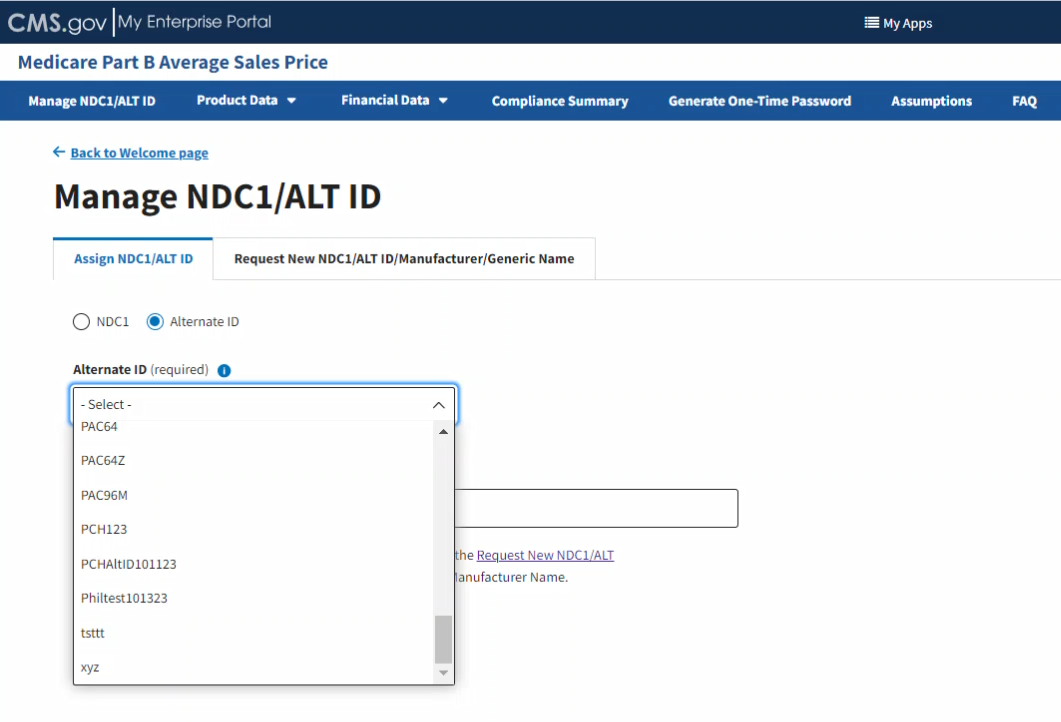
Figure 14: Manage NDC1/ALT ID Page - Assign ALT ID Drop-down Menu
In the Manufacturer Name (required) auto-fill field, begin to type the manufacturer name; select the appropriate manufacturer from the list that generates. Refer to Figure 15.
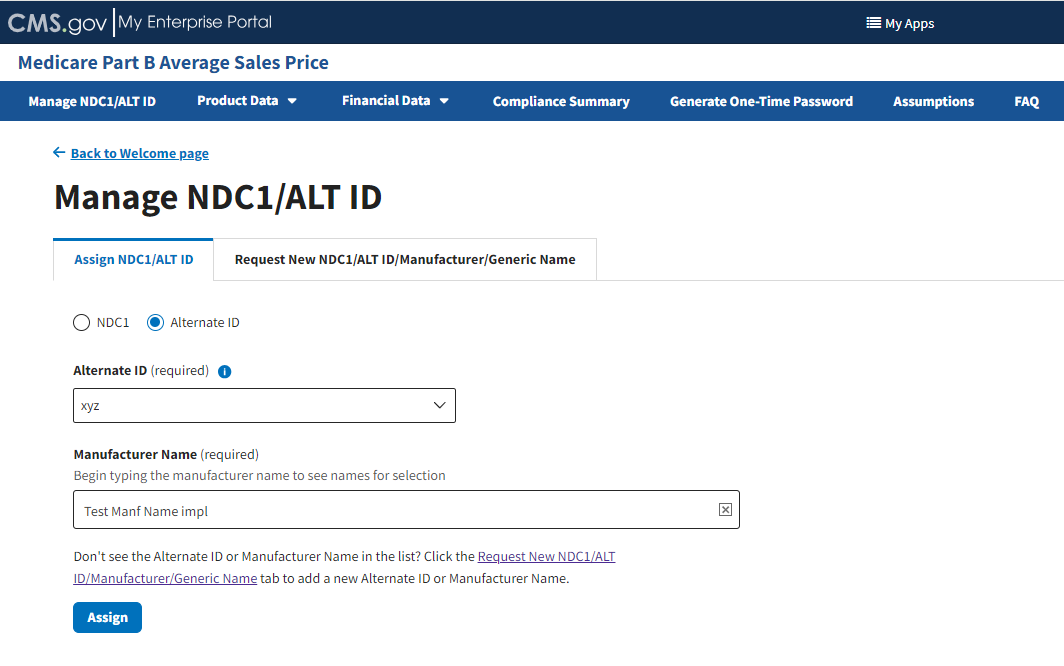
Figure 15: Manage NDC1/ALT ID Page - Enter ALT ID Manufacturer Name
Click Assign to submit your information.
A message displays confirming you have successfully added your selections. Refer to Figure 16.
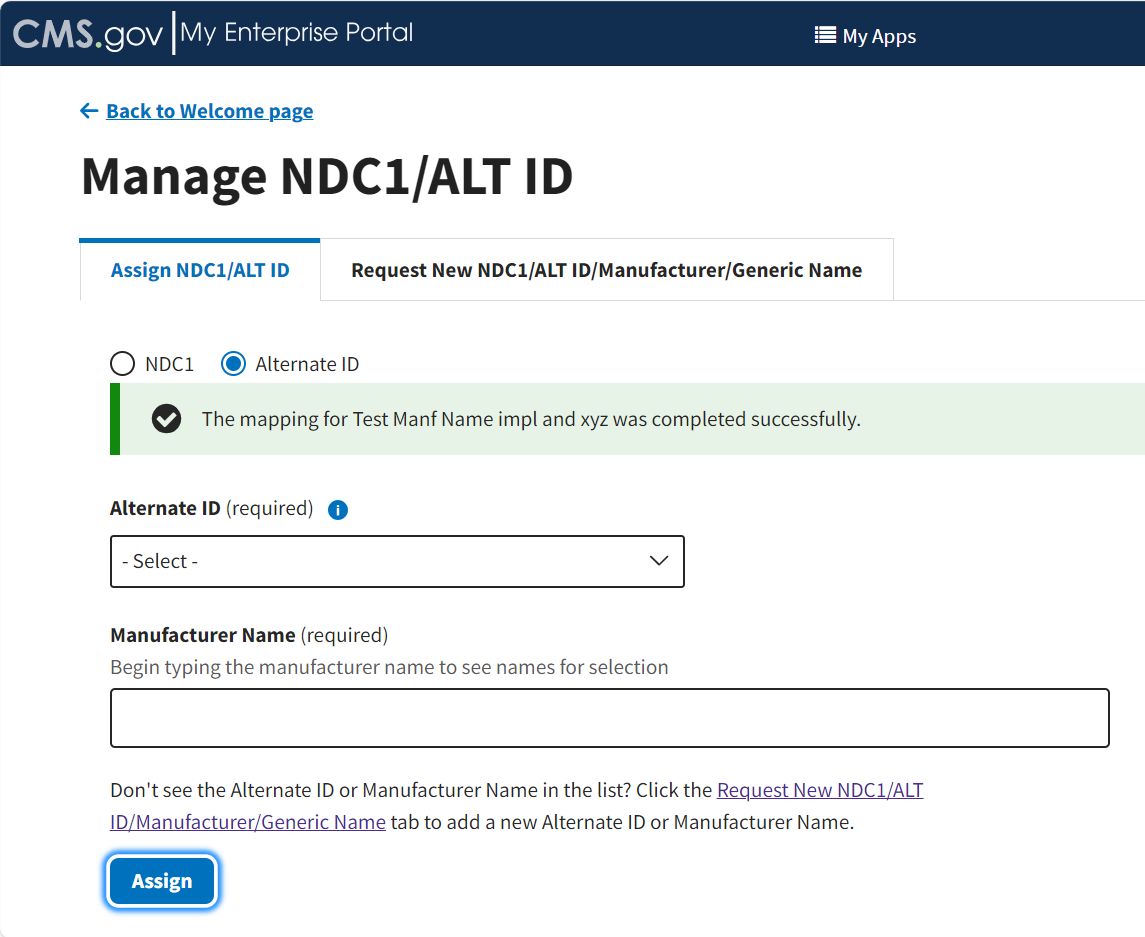
Figure 16: Manage NDC1/ALT ID - ALT ID Assigned Successfully
The following sections describe how to request a new NDC1, ALT ID, manufacturer, and generic name.
Request New NDC1
Follow these steps to request a new NDC1:
Navigate to the Manage NDC1/ALT ID page, which automatically opens on the Assign NDC1/ALT ID tab.
Click the Request New NDC1/ALT ID/Manufacturer/Generic Name tab.
The Request New NDC1/ALT ID/Manufacturer/Generic Name page opens, showing the status (Pending, Approved, or Rejected) for each submitted request. The Module organizes data by Request Type, Requested Value as well as Request Date and Status (Pending, Approved, or Rejected). Refer to Figure 17.
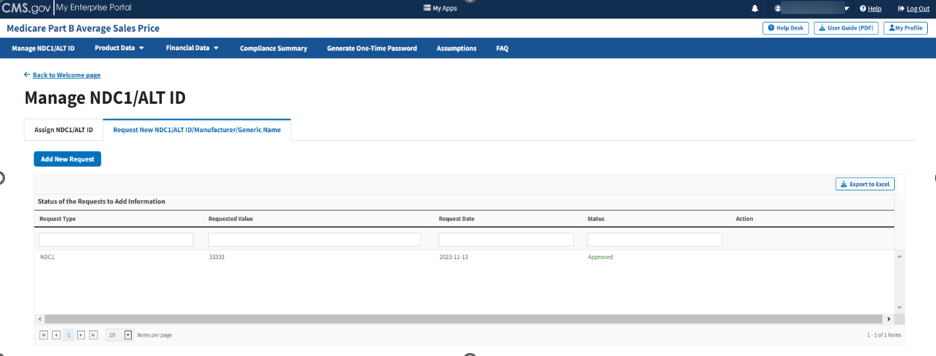
Figure 17: Request New NDC1/ALT ID/Manufacturer/Generic Name Page
Click the Add New Request button.
An Add New NDC1/ALT ID/Manufacturer/Generic Name window opens. Refer to Figure 18.
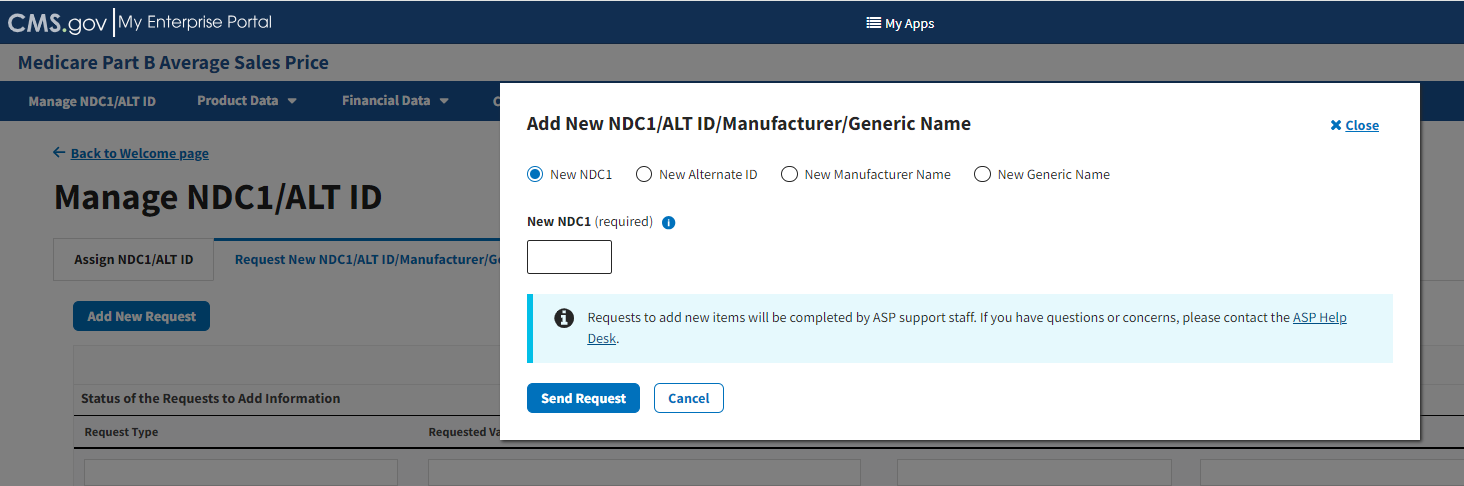
Figure 18: Request New NDC1/ALT ID/Manufacturer/Generic Name Page - Add New NDC1
Select the New NDC1 radio button to specify the product data you need to submit to the Module. Note that the Module automatically defaults to select the New NDC1.
Under New NDC1 (required), enter the appropriate NDC for the data product you are requesting to add to the Module. Refer to Figure 19.
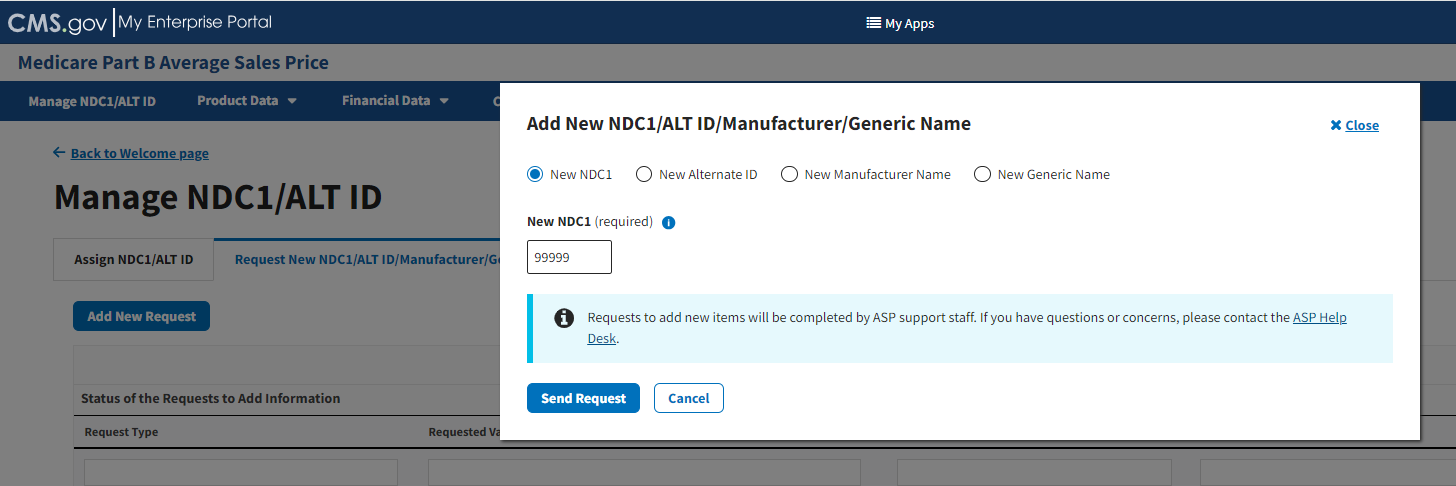
Figure 19: Request New NDC1 - Field Filled
Click Send Request to submit your information.
A message displays confirming you have successfully added your selections. Refer to Figure 20.
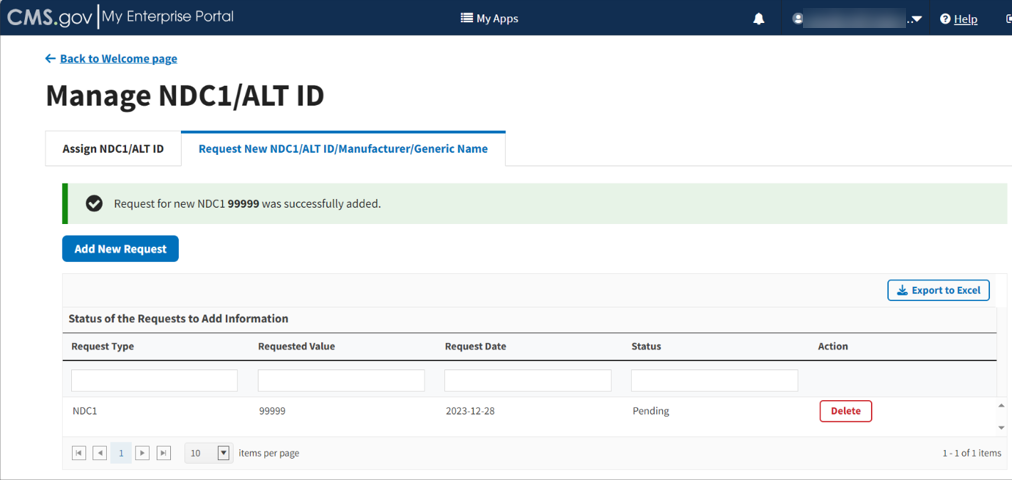
Figure 20: Request New NDC1 - NDC1 Successfully Added
Follow these steps to request a new Alternate ID:
Click the Add New Request button. Refer to Figure 20.
An Add New NDC1/ALT ID/Manufacturer/Generic Name window opens. Note that the Module automatically defaults to the New NDC1 tab.
Select the New Alternate ID radio button to specify the product data you need to submit to the Module. Refer to Figure 21.
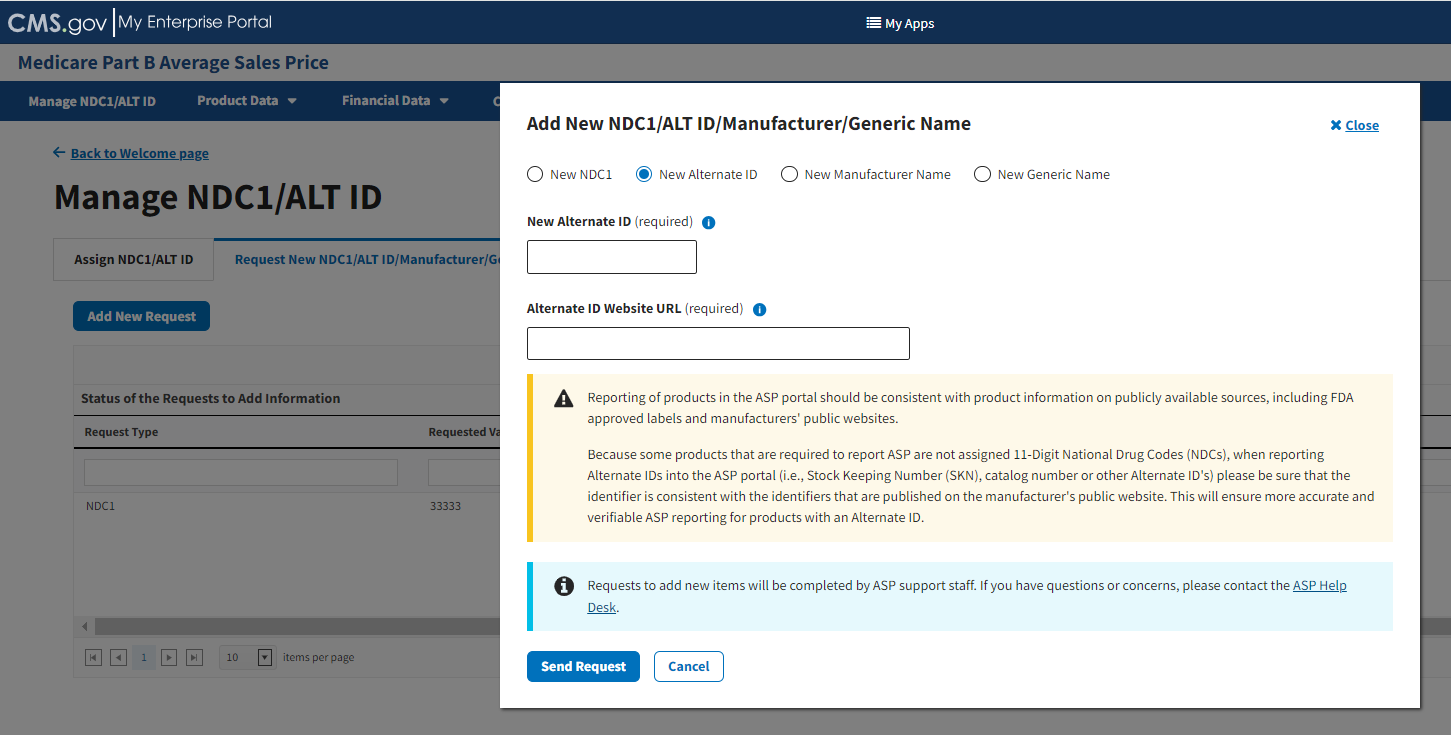
Figure 21: Request New NDC1/ALT ID/Manufacturer/Generic Name Page - Add New ALT ID
Under New Alternate ID (required), enter the appropriate alternate ID for the product you want to add to the Module.
Note: An Alternate ID is a manufacturer-selected product identifier that can be any combination of letters or numbers unique to the product (i.e., Stock Keeping Number (SKN) or product number). The New Alternate ID field allows up to a maximum of 23 characters and special characters (colon, dash, or period).
Under Alternate ID Website URL (required), enter the hyperlink for the drug manufacturer’s website. Refer to Figure 22.
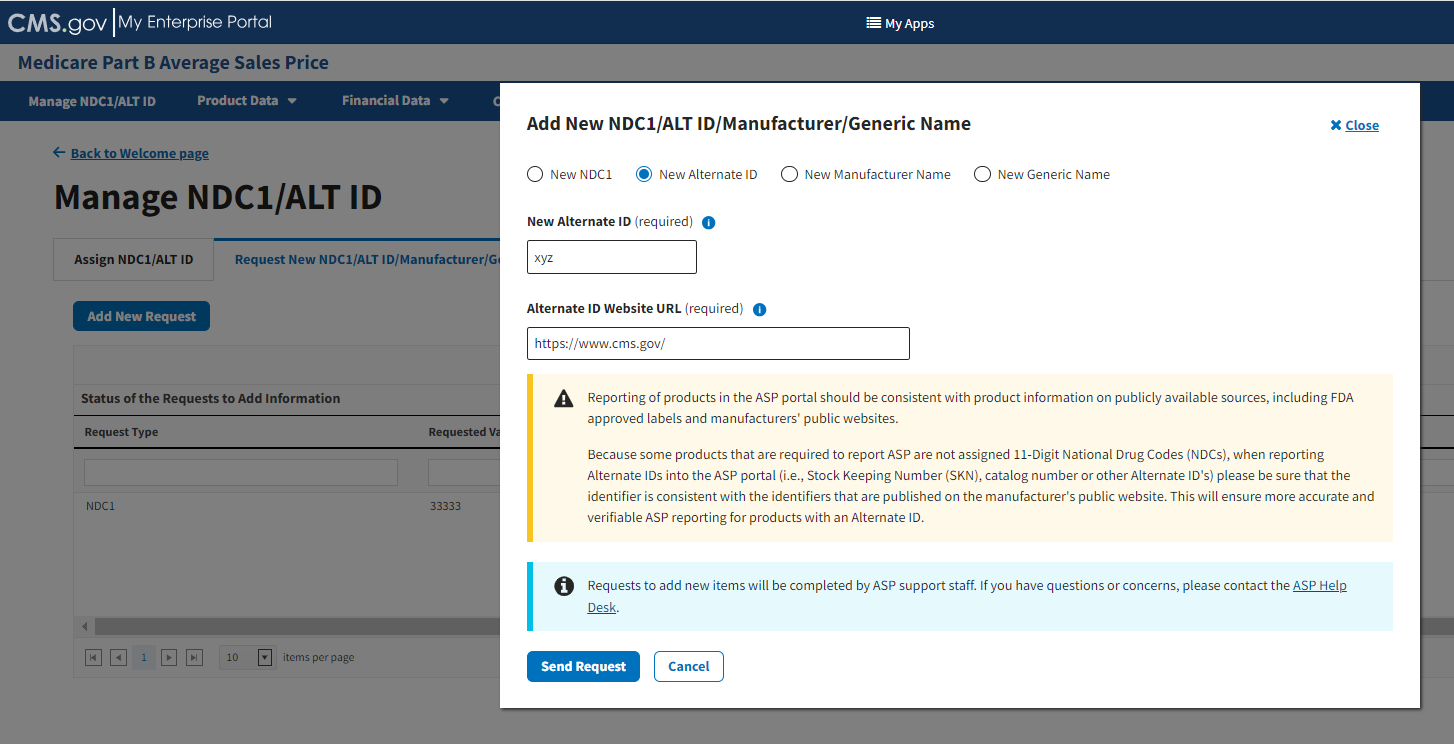
Figure 22: Request New Alternate ID - ALT ID Field Filled
Click the Send Request button to submit your information.
A message displays confirming you have successfully added your selections. Refer to Figure 23.
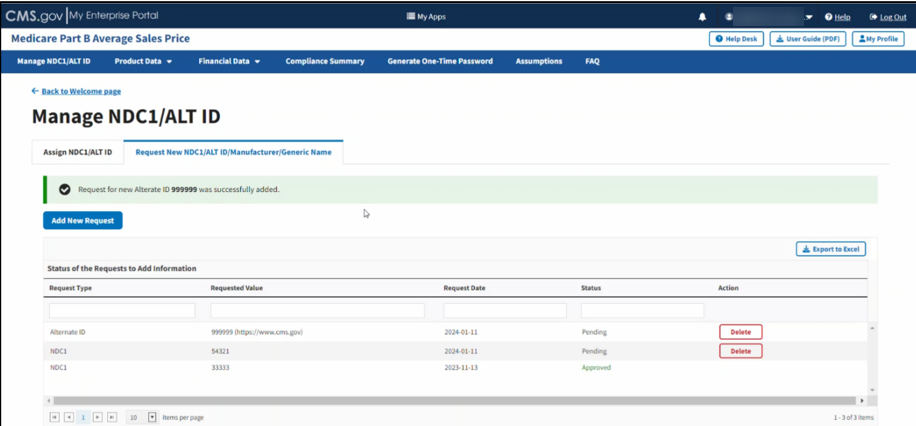
Figure 23: Request New Alternate ID - ALT ID Successfully Added
Request New Manufacturer Name or New Generic Name
Follow these steps to request a New Manufacturer or New Generic Name:
Click the Add New Request button. Refer to Figure 23.
An Add New NDC1/ALT ID/Manufacturer/Generic Name window opens. Note that the Module automatically defaults to the New NDC1 tab.
Select either the New Manufacturer Name or the New Generic Name radio button to specify the product data you need to submit to the Module.
Additional fields display as the next page opens for either selection. Refer to Figure 24 and Figure 25.
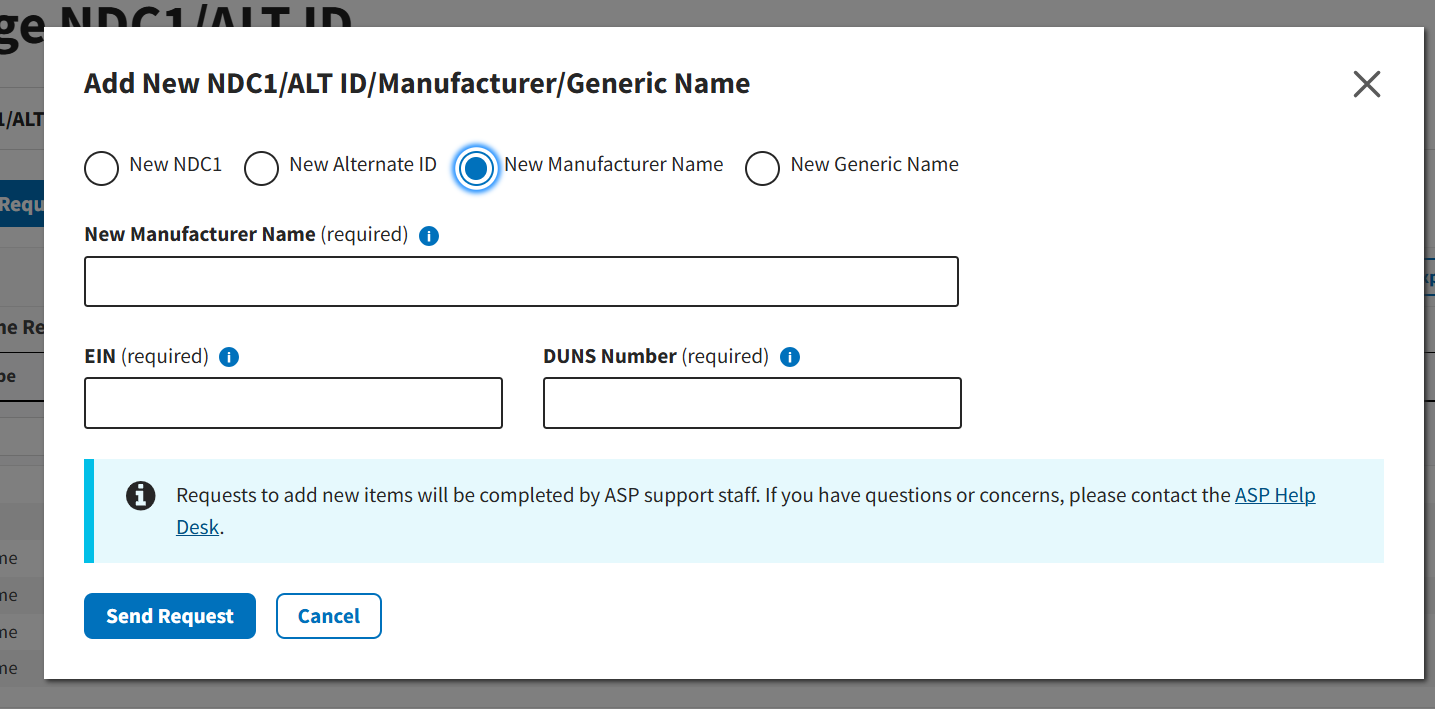
Figure 24: Request New Manufacturer Name
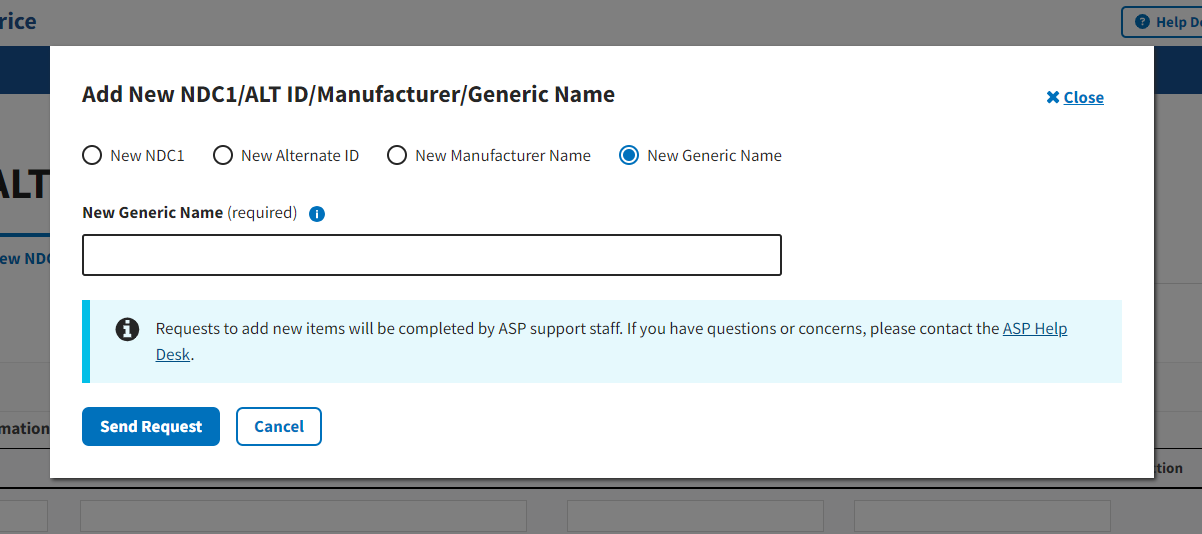
Figure 25: Request New Generic Name
Under New Manufacturer Name (required) or New Generic Name (required), enter the appropriate information for the data product you want to add to the Module. Refer to Figure 26 and Figure 27.
Note: For New Manufacturer Name requests, users will need to submit business identification information including the manufacturer’s Employer Identification Number (EIN) and Data Universal Numbering System (DUNS) number.
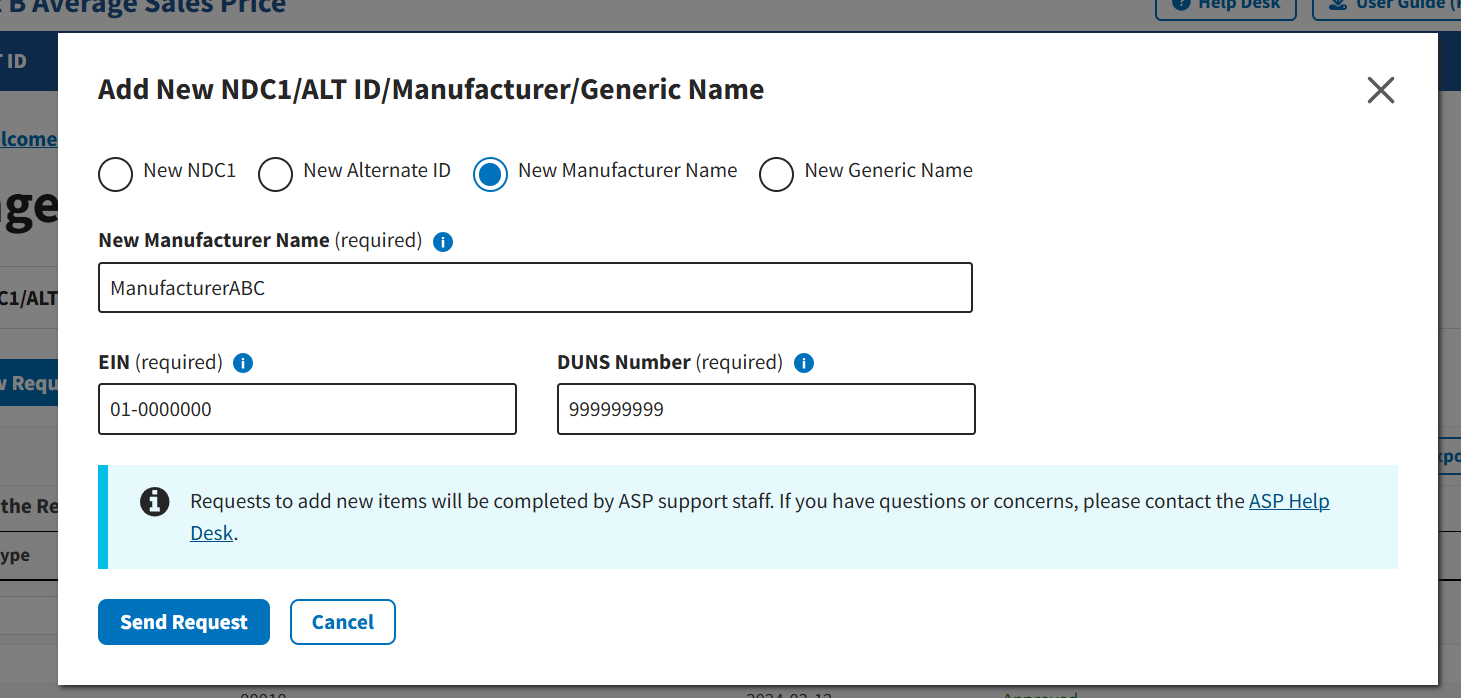
Figure 26: Request New Manufacturer Name - Field Populated
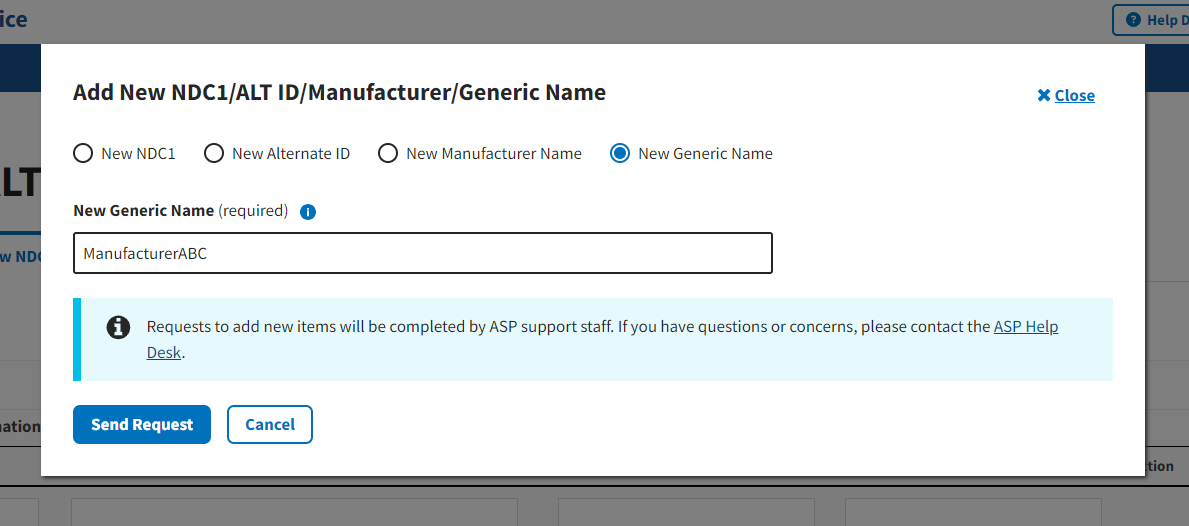
Figure 27: Request New Generic Name - Field Populated
Click Send Request to submit your information for either selection.
A message displays confirming you have successfully added your selections. Refer to Figure 28 and Figure 29.
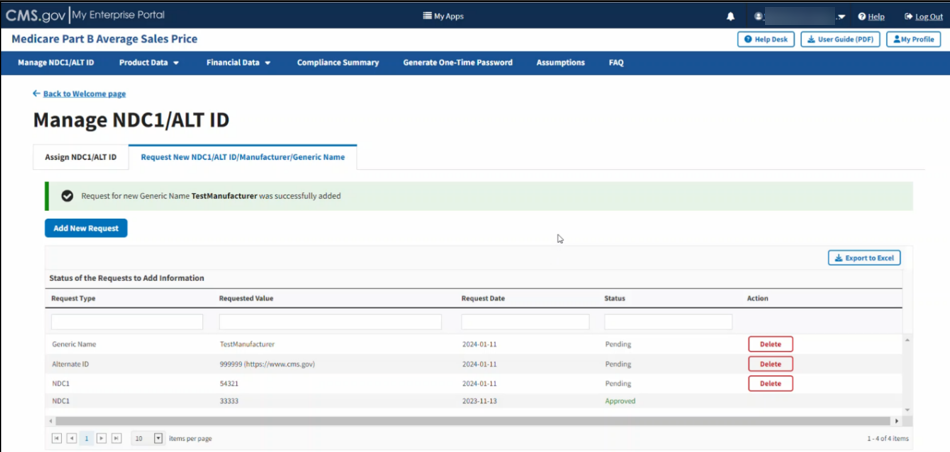
Figure 28: Request New Manufacturer Name - Successfully Added
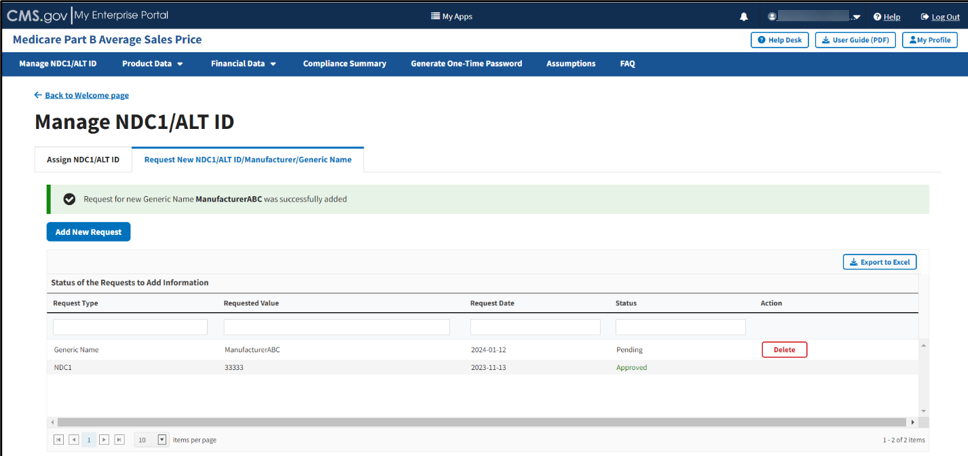
Figure 29: Request New Generic Name - Successfully Added
Note: ASP support staff complete requests to add new items. Contact [email protected] for further questions or concerns about the process.
Drug manufacturers must submit quarterly drug pricing data using a file transfer process, or through online data entry in the ASP module. Drug data consists of product and financial data. Click the Product Data tab on the Medicare Part B Average Sales Price homepage to view the drop-down menu for the Add/Update Product Data, Upload Product Data, and View Drugs tabs. Refer to Figure 30.
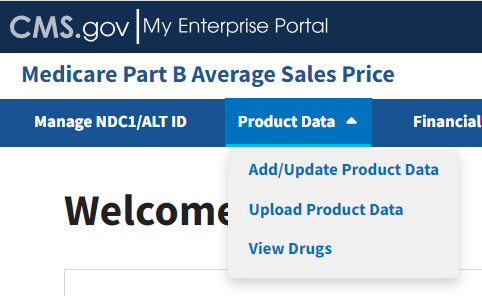
Figure 30: Product Data - Main Drop-down Menu
The following sections describe how to add/update, upload product data, and view drugs.
Follow these steps to add and/or update product data:
From the Medicare Part B Average Sales Price homepage, click the Product Data tab; then select the Add/Update Product Data tab.
The Add/Update Product Data page opens with default selections. Refer to Figure 31.
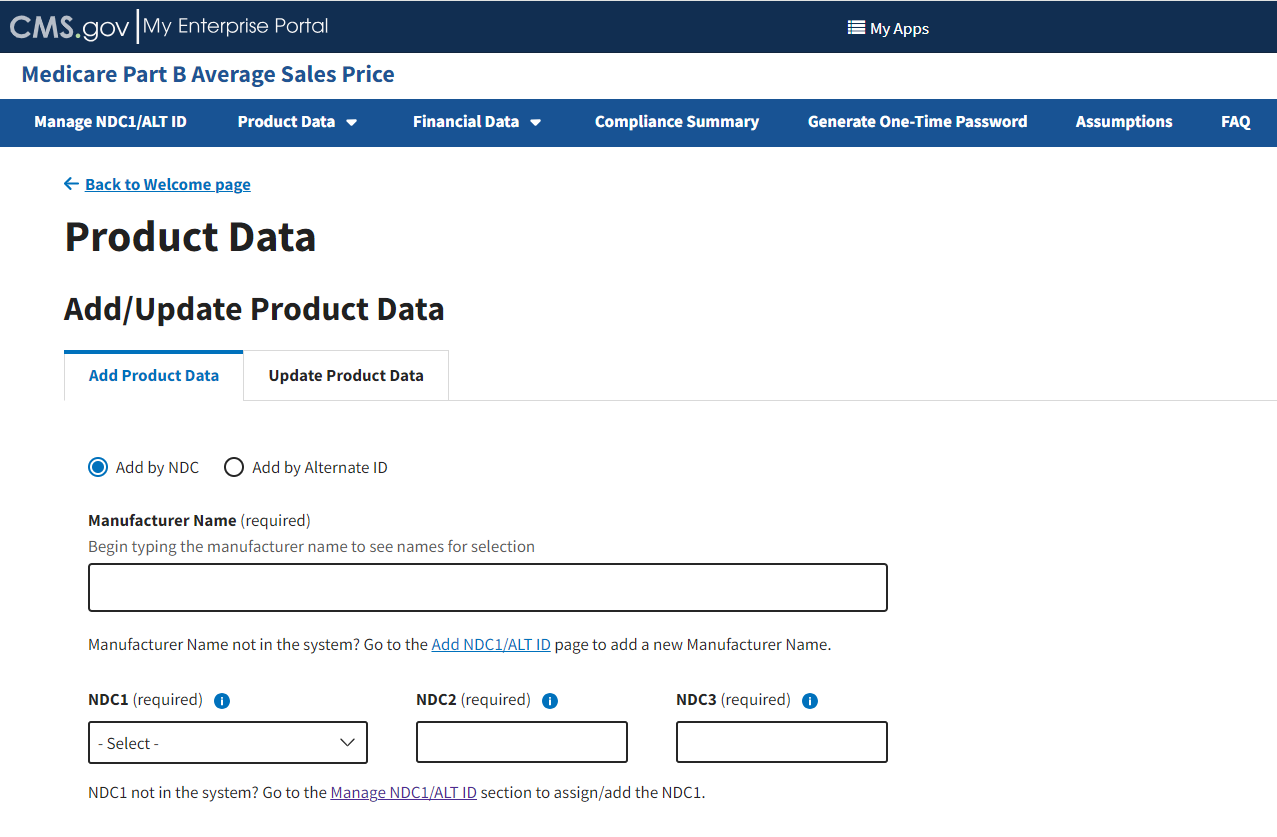
Figure 31: Add/Update Product Data
Note: It is imperative that the spelling matches each time you enter product data for the same drug manufacturer. The spelling must also match when entering data under the Upload Product Data tab.
Follow these steps to add product data by NDC:
From the Add Product Data page, select the Add by NDC radio button if it is not already selected when the page opens.
In the Manufacturer Name (required) field, begin to type and then select the appropriate manufacturer.
Under NDC1 (required), click the -Select- drop-down menu to expand the list of submitted drugs and additional products in the Module to date; select your required NDC1* code.
Enter your 4-digit number in the NDC2* (required) field.
Enter your 2-digit number in the NDC3* (required) field.
As you complete the NDC3* (required) field, the Add Product Data page expands to display multiple drop-down menus and empty fields.
Enter or select the required information as follows:
Select the Drug has brand name checkbox if the product you are submitting has a brand name. (If so, an empty field displays to submit the brand name; type information here as needed.)
Click the Generic Name (required) drop-down menu; select the generic name you need to enter for your product.
Note: Return to the Manage NDC1/ALT ID page if you cannot find the appropriate generic name in the system. Refer to Section 3.1 - Manage NDC1/ALT ID for guidance.
Enter the volume per item in the Volume Per Item (required) field.
Click the Unit for Volume Per Item (required) drop-down; select the appropriate option for your product.
Enter the appropriate number in the Number of Items per NDC (required) field.
Click the Package Type (required) drop-down; select the appropriate package type.
Enter the strength in the Strength (required) field.
Click the Unit for Strength (required) drop-down; select the appropriate unit.
Enter the FDA application number in the FDA Application Number (required) field.
Enter the FDA application supplement number in the FDA Application Supplement Number field, if applicable.
Note: Click the Add Additional FDA Application Numbers button if applicable for the drug, and repeat steps i and j.
Enter the FDA approval date in the FDA Approval Date (required) field.
Click the FDA Approval Type (required) drop-down; select the appropriate approval type.
Enter the first marketing date in the First Marketing Date (required) field.
Enter the date of first sale in the Date of First Sale (required) field.
Enter the WAC in the Wholesale Acquisition Cost (required) field.
Note: The Wholesale Acquisition Cost (required) field is required and displays when the First Marketing Date occurs after the current reporting period.
Note: The date of first sale cannot occur before the FDA approval date and must occur prior to the current reporting period start date.
Confirm your selections. Refer to Figure 32.
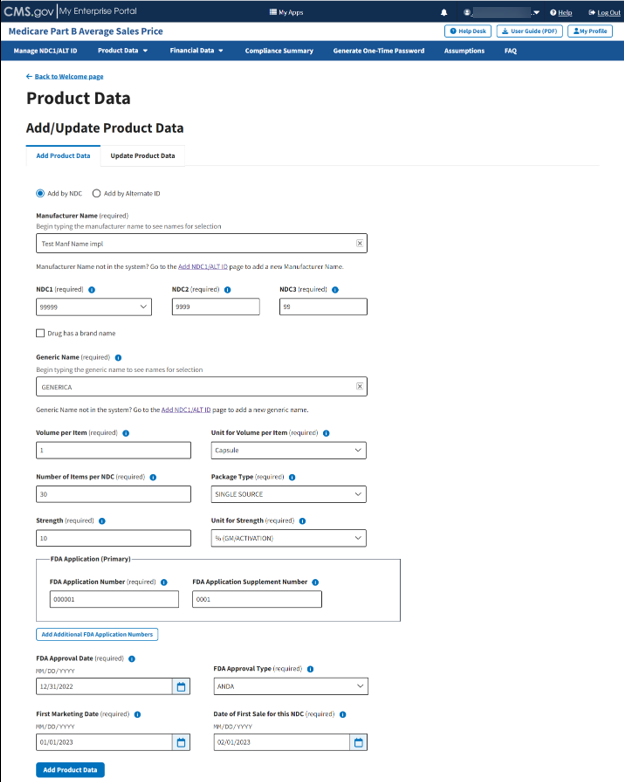
Figure 32: Add/Update Product Data Fields Populated
Click Add Product Data to submit your information.
A message displays confirming you have successfully added your selections. Refer to Figure 33.
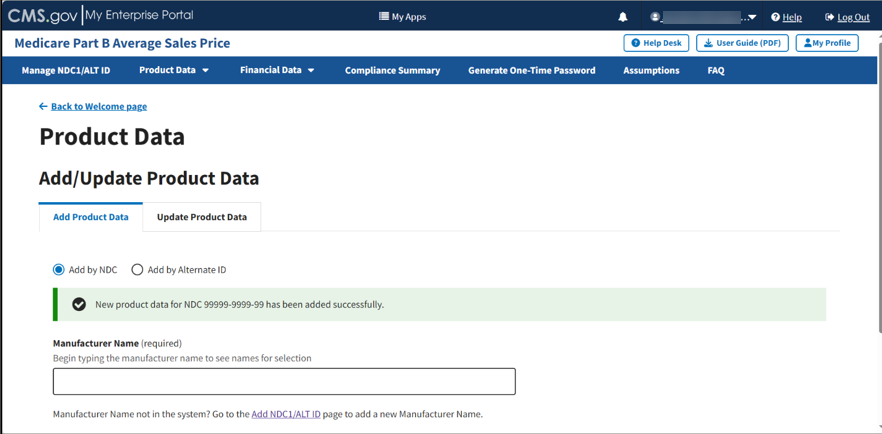
Figure 33: Add/Update Product Data Successfully Added
Note: It is imperative that the spelling matches each time you enter product data for the same drug manufacturer. The spelling must also match when entering data under the Upload Product Data tab.
Add Product Data by Alternate ID
Follow these steps to add product data by alternate ID:
From the Add/Update Product Data page, select the Add by Alternate ID radio button.
The Add Product Data page expands to display additional empty fields. Refer to Figure 34.
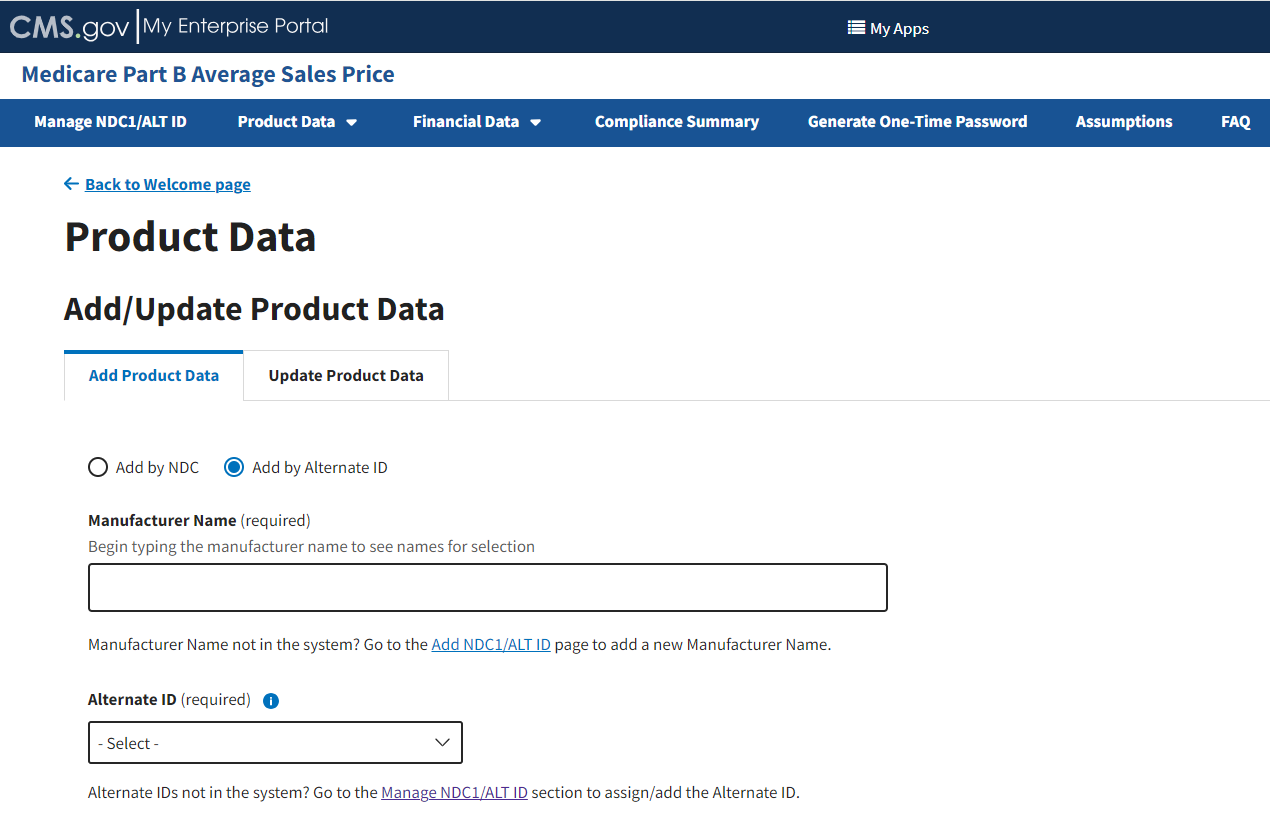
Figure 34: Add Product Data by Alternate ID
Under Manufacturer Name (required), begin to type and then select the appropriate manufacturer.
Under Alternate ID (required), click the -Select- drop-down to expand the list. Select the required alternate ID code. Refer to Figure 35.
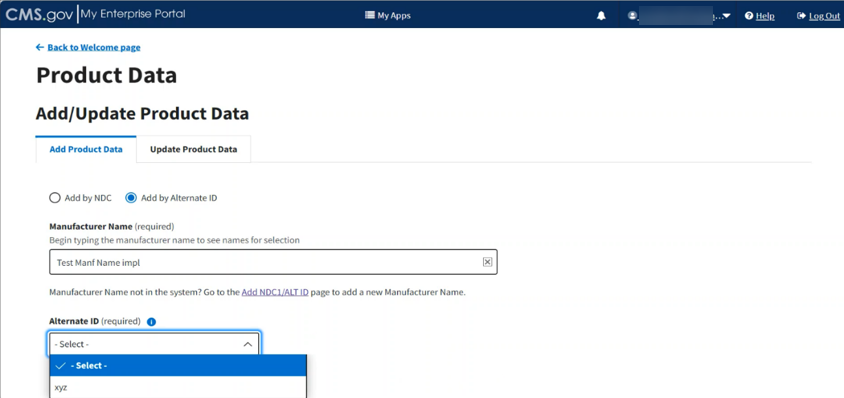
Figure 35: Add Product Data by Alternate ID - Fields Populated
As you complete the Alternate ID (required) field, the Add Product Data page expands to show multiple drop-down menus and empty fields.
Enter or select the required information as follows:
Enter the uniform resource locator (URL) to the manufacturer website in the Manufacturer’s Website URL (required) field for verification purposes.
Select the Drug has a brand name checkbox if the product you are submitting has a brand name. (If so, an empty field displays in which to enter the brand name; type information here as needed.)
Click the Generic Name (required) drop-down; select the generic name you need to enter for your product.
Note: Return to the Manage NDC1/ALT ID page if you cannot find the appropriate generic name in the system. Refer to Section 3.1 - Manage NDC1/ALT ID for guidance.
Enter the volume per item in the Volume Per Item (required) field.
Click the Unit for Volume Per Item (required) drop-down; select the appropriate option for your product.
Note: For skin substitute products such as powders, sheets or discs, enter “One” for Volume and “Each” for Unit for Volume.
Enter the appropriate number in the Number of Items per Alternate ID (required) field.
Click the Package Type (required) drop-down; select the appropriate package type. Package Type is not applicable to skin substitute sheets.
Enter the strength in the Strength (required) field.
Note: For skin substitute products, strength is determined by calculating the area of the product.
Click the Unit for Strength (required) drop-down; select the appropriate unit.
Enter the FDA registration number in the FDA Registration Number (required) field.
Enter the FDA approval date in the FDA Approval Date (required) field.
Enter the FDA approval type in the FDA Approval Type (required) field.
Enter the first marketing date in the First Marketing Date (required) field.
Enter the date of first sale in the Date of First Sale for this ALT ID (required) field.
Note: The date of first sale cannot occur before the FDA approval date and must occur prior to the current reporting period start date.
Confirm your selections; click Add Product Data to submit your information. Refer to Figure 36.
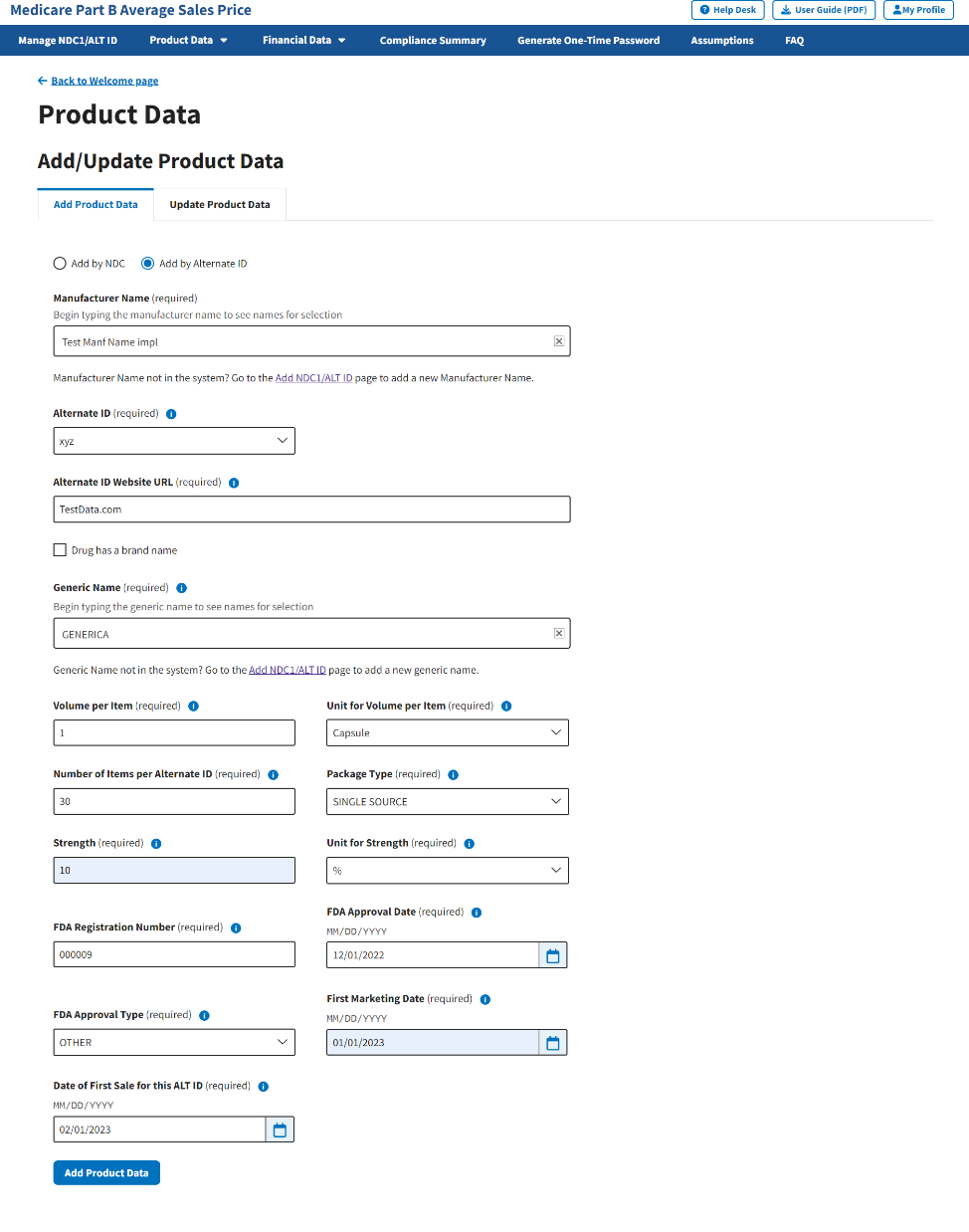
Figure 36: Add Product Data by Alternate ID - Additional Fields
A message displays confirming you have successfully added your product data. Refer to Figure 37.
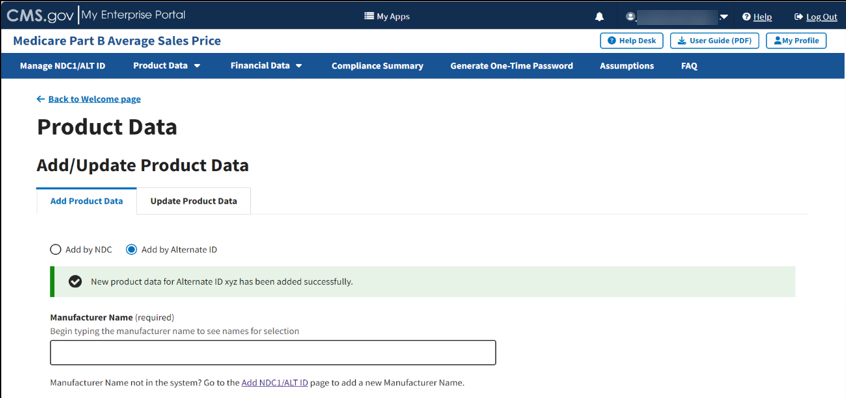
Figure 37: Product Data by Alternate ID Added Successfully
Note: It is imperative that the spelling matches each time you enter product data for the same drug manufacturer. The spelling must also match when entering data under the Upload Product Data tab.
Update Product Data by NDC
Follow these steps to update product data by NDC:
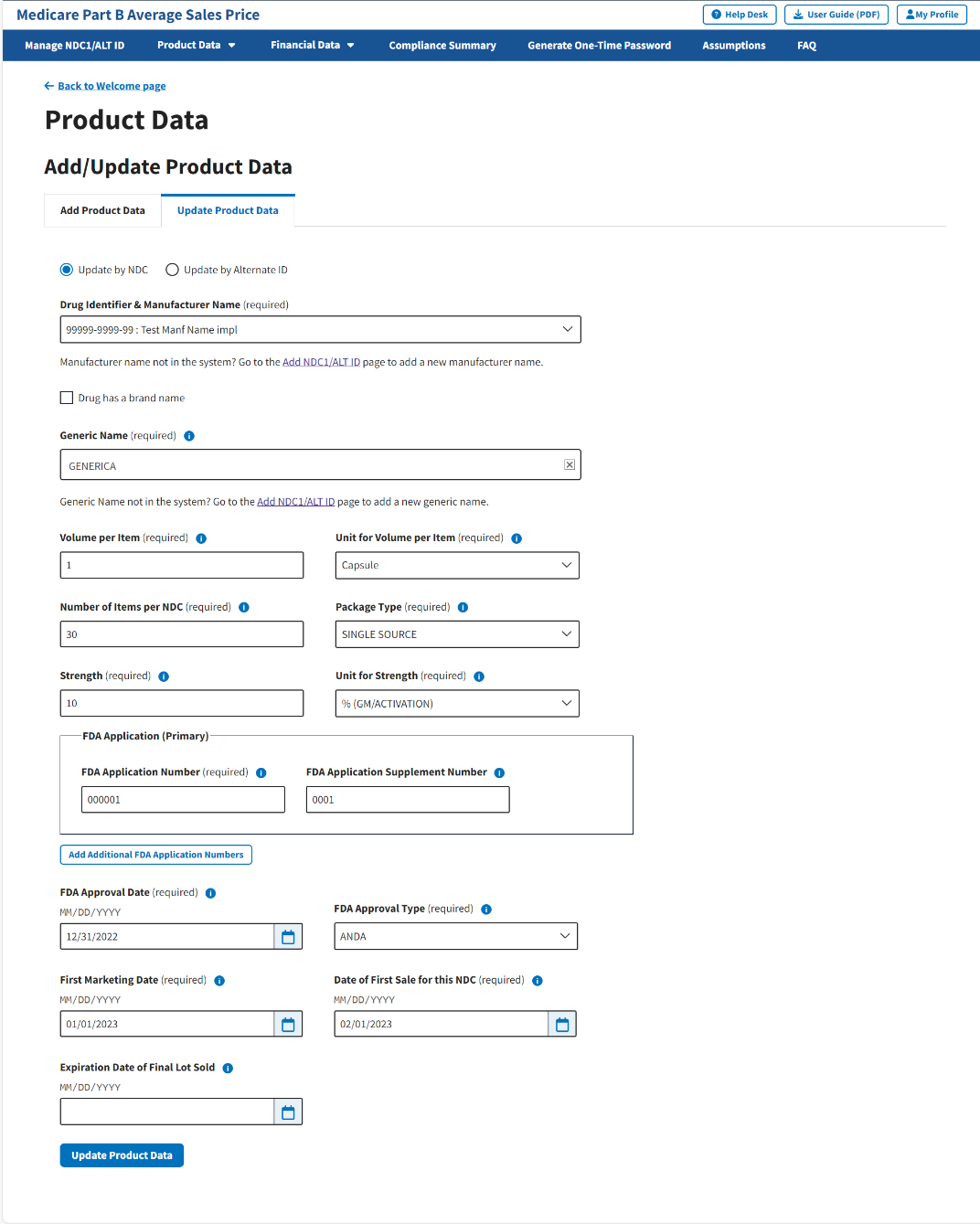
From the Add/Update Product Data page, select the Update Product Data tab; then, select the Update by NDC radio button if it is not already selected when the page opens. Refer to Figure 38.
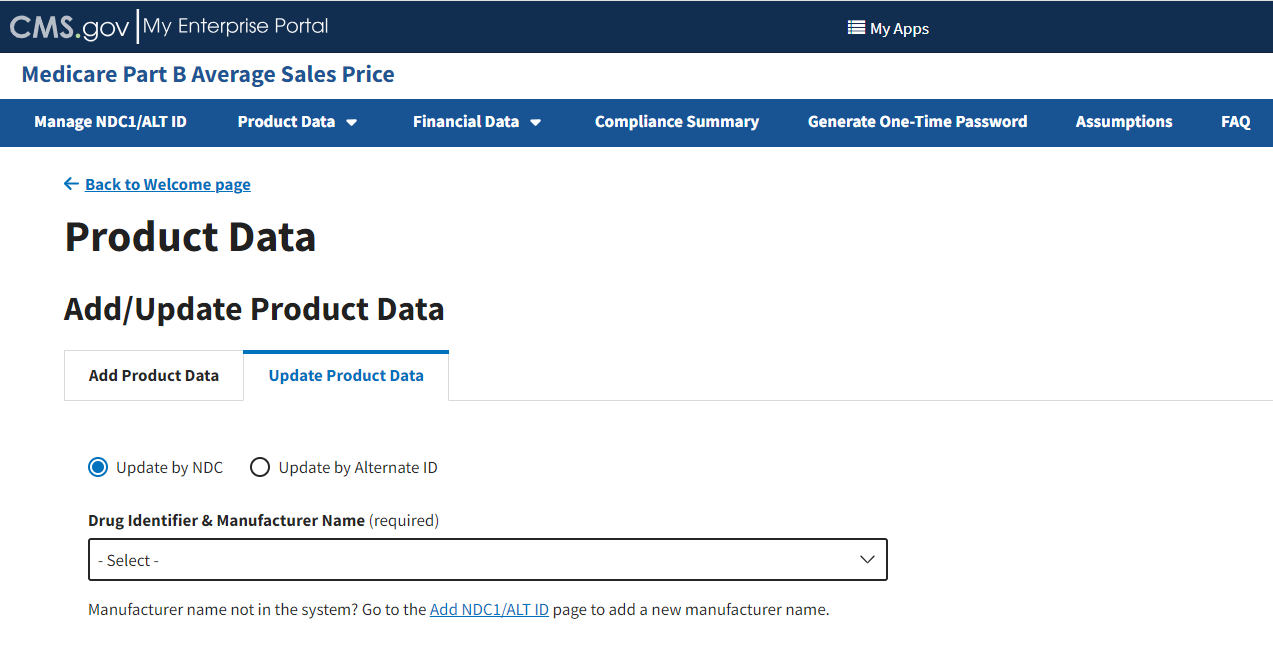
Figure 38: Update Product Data - Drug Identifier & Manufacturer Name
In the Drug Identifier & Manufacturer Name (required) drop-down menu, click -Select- to expand the list of submitted drugs and additional products in the Module to date; select the appropriate drug identifier.
The page automatically loads the product data for that specific drug. Refer to Figure 39.
Figure 39: Update Product Data by NDC
Review all your information in the appropriate boxes previously submitted in Section 3.2 - Product Data.
Confirm your selections; click Update Product Data to submit any changes in your drug product data.
A message displays confirming you have successfully updated your product data. Refer to Figure 40.
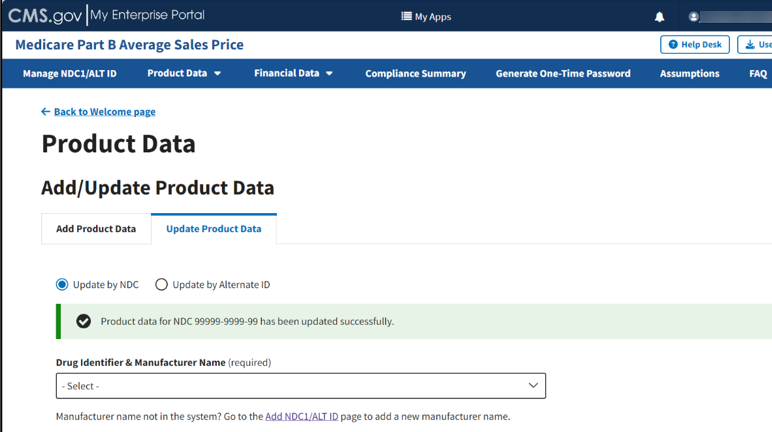
Figure 40: Update Product Data by NDC - Data Updated Successfully
Update Product Data by Alternate ID
Follow these steps to update product data by Alternate ID:
From the Add/Update Product Data page, select the Update Product Data tab; then, select the Update by Alternate ID radio button. Refer to Figure 41.
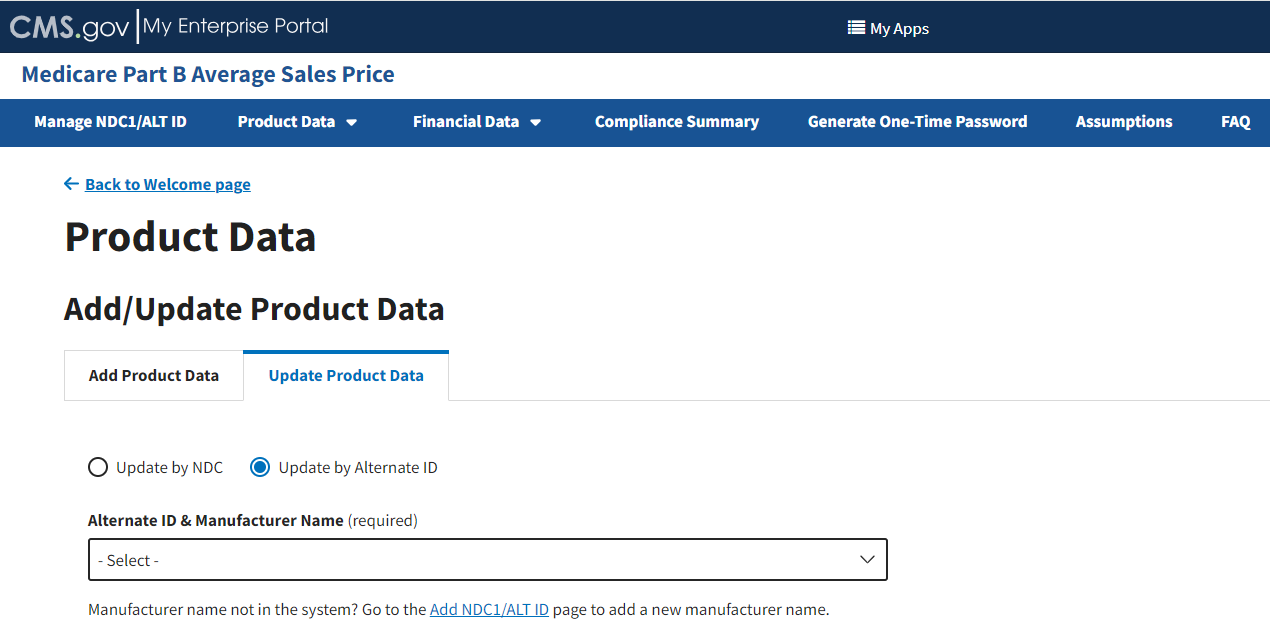
Figure 41: Update Product Data by Alternate ID
Under the Alternate ID & Manufacturer Name (required) drop-down; click the -Select- drop-down to expand the list; select the appropriate information.
The page automatically loads the product data for that specific drug. Refer to Figure 42.
Note: Additional fields display on the next page. Ensure that you complete all required fields, and that all added financial information is accurate.
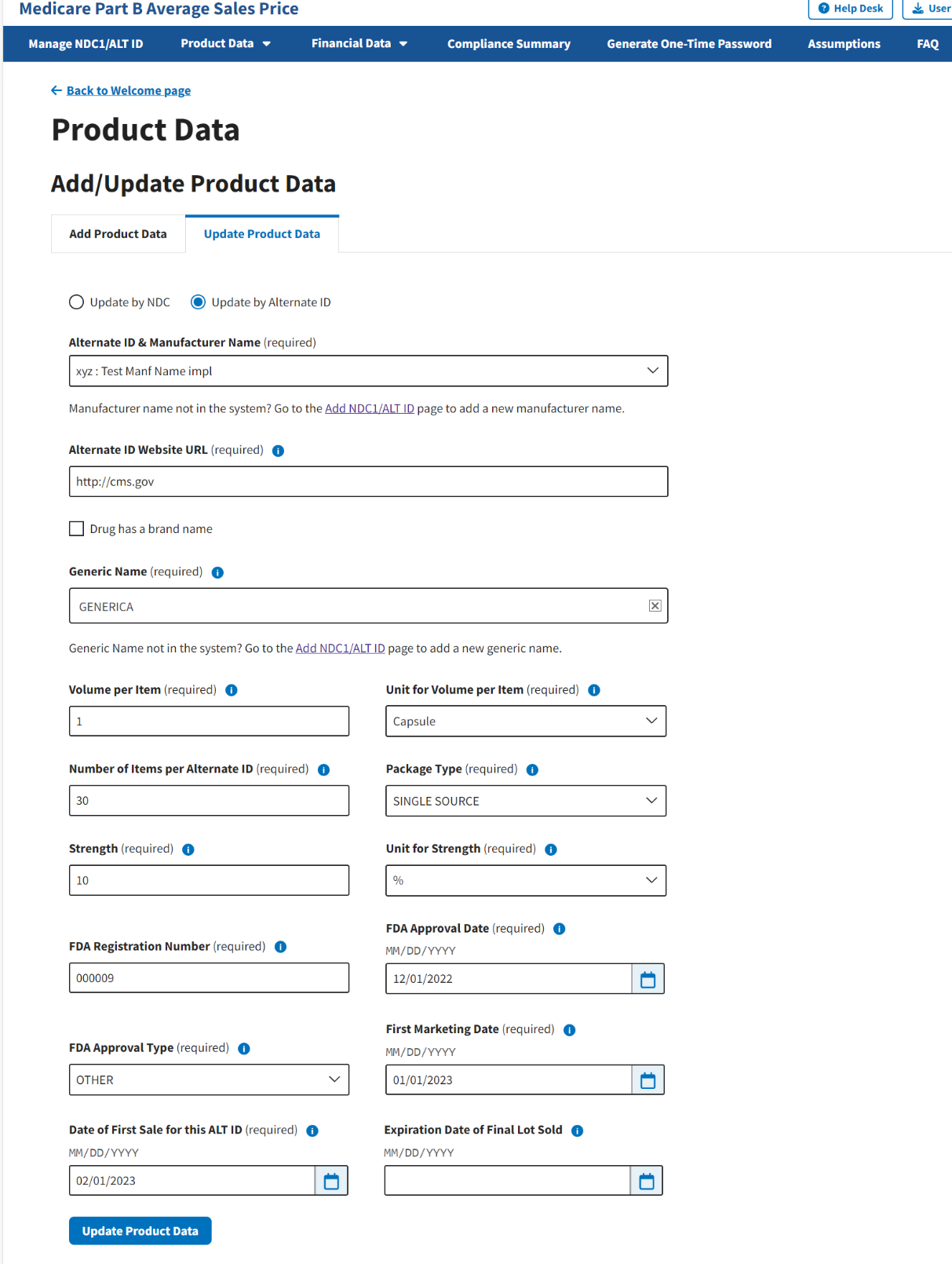
Figure 42: Update Product Data by Alternate ID - Drug Identifier Drop-down Menu
Review all your information in the appropriate boxes previously submitted in Section 3.2 - Product Data.
Confirm your selections; click Update Product Data to submit any changes in your drug product data.
A message displays confirming you have successfully updated your product data. Refer to Figure 43.
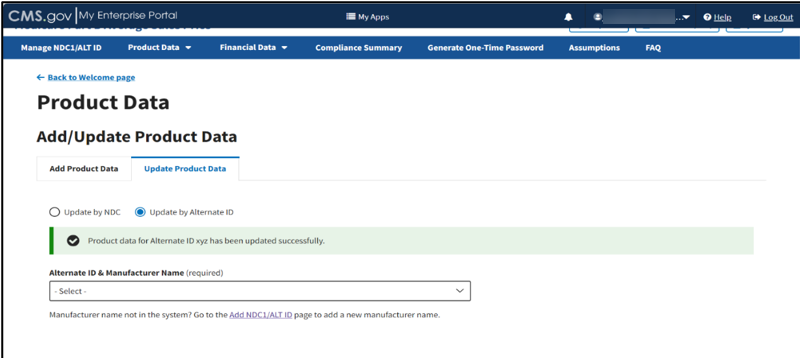
Figure 43: Update Product Data by Alternate ID - Updated Successfully
Follow these steps to upload product data:
From the Medicare Part B Average Sales Price homepage, click the Product Data tab; then select the Upload Product Data tab.
The Upload Product Data page opens, listing the financial quarter and year for the upcoming reporting period. Refer to Figure 44.
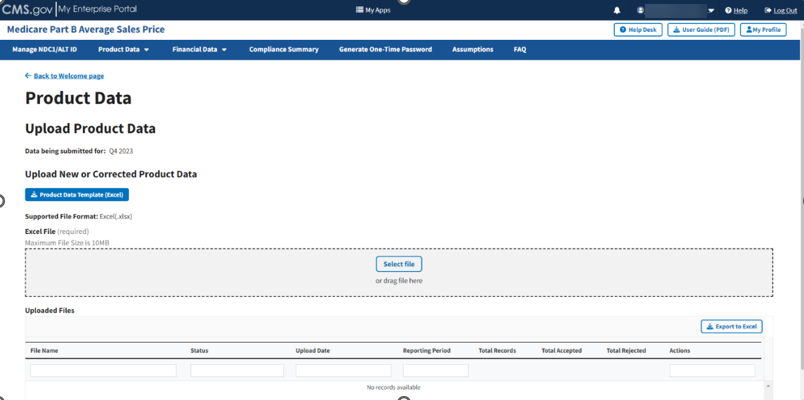
Figure 44: Upload Product Data - New or Corrected
Note: Click the Product Data Template (Excel) box to download a copy of the product data template.
Upon preparing your .xlsx file (required) and verifying your information for accuracy, click Select File; then select the Excel file in the dialog box. You may also drag the file into the Select File box. Refer to Figure 45.
Figure 45: Upload Product Data - Uploading Files from Desktop
A download bar displays as your file uploads. A message displays confirming you have successfully uploaded your .xlsx file. Refer to Figure 46.
Note: If the Module cannot process your file, an error message displays, and a New Report generates under Uploaded Files.
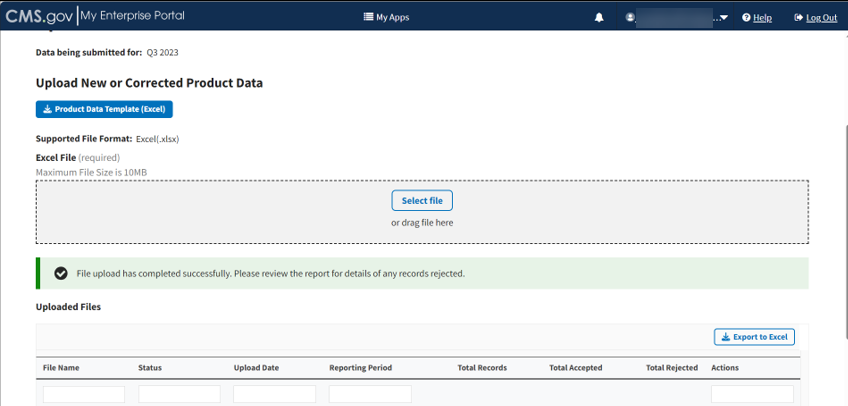
Figure 46: Upload Product Data - New File Successfully Uploaded
Refresh your browser to allow the system to update and display your new file.
The Uploaded Files section displays files you uploaded recently as well as previous files still in the Module. Refer to Figure 47.
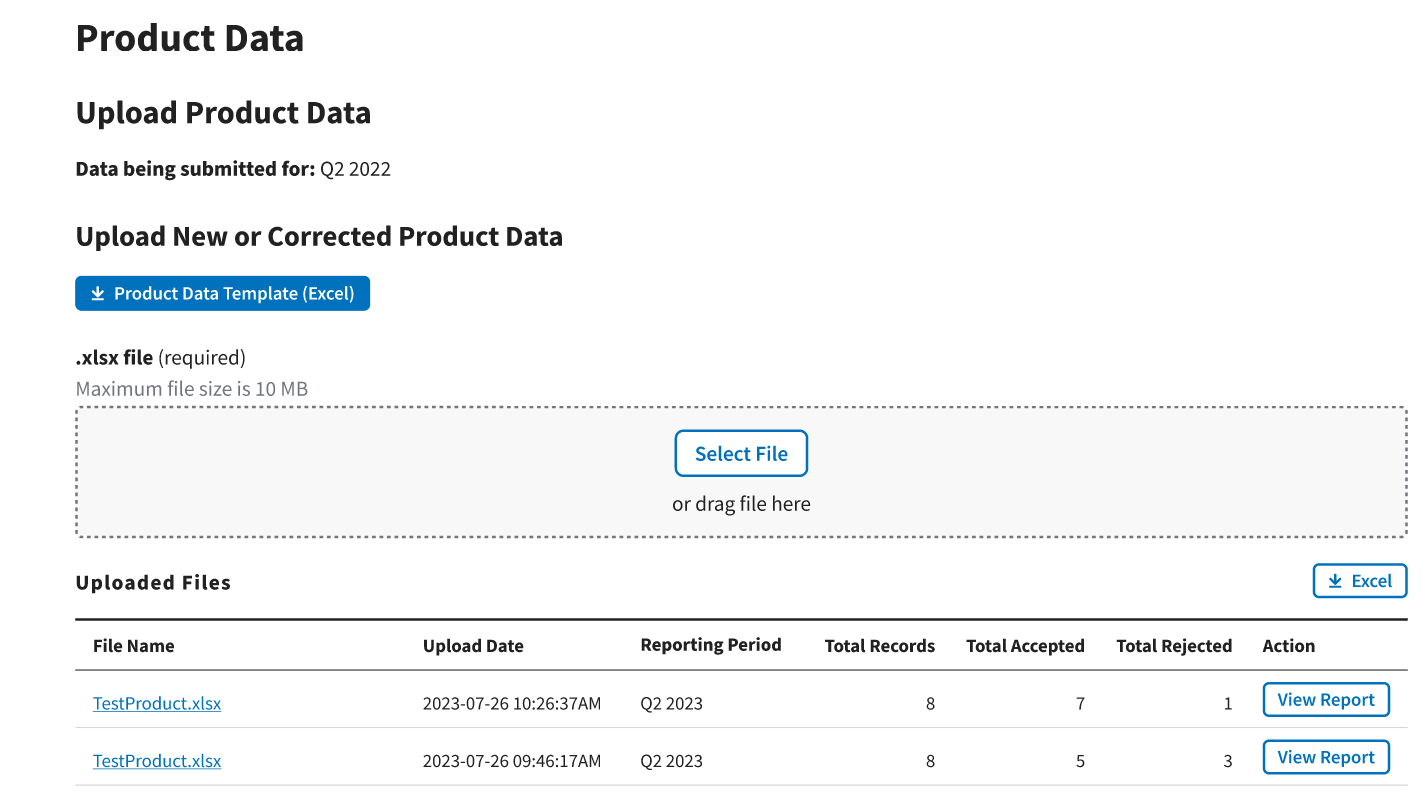
Figure 47: Upload Product Data - Uploaded Files
Each uploaded file displays the File Name, Upload Date, Reporting Period, Total Records, Total Accepted, Total Rejected, and Action categories submitted to the Module.
Click View Report under Action in the Uploaded Files section to view the full report for a submitted file.
The report opens on the next page. Refer to Figure 48.
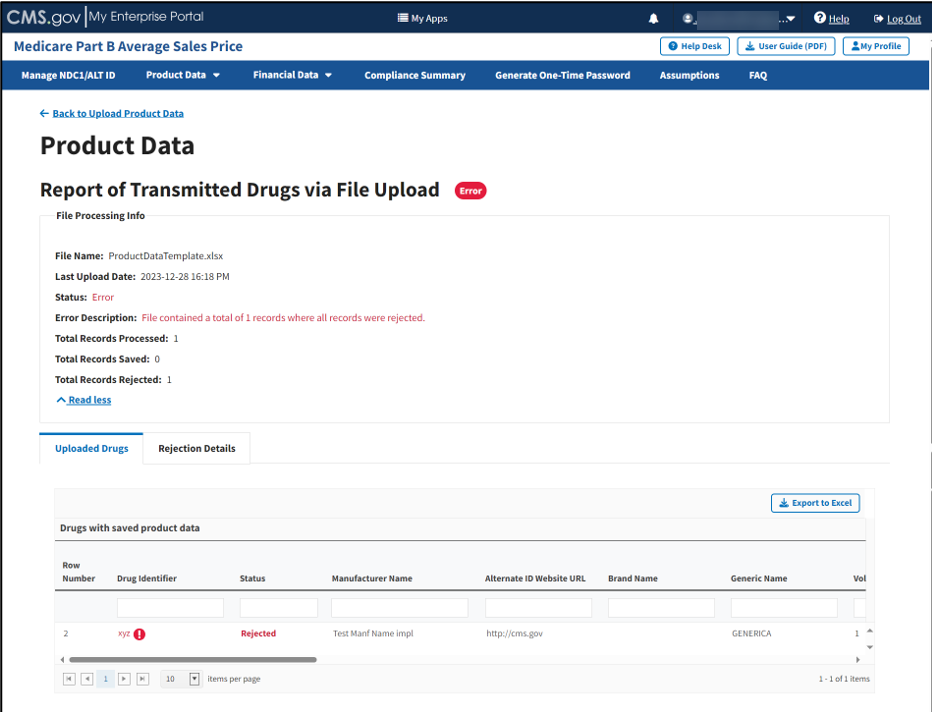
Figure 48: Upload Product Data - Full Report of Transmitted Drugs via File Upload
Click the Read More tab under the Report of Transmitted Drugs via File Upload to view all File Processing Information related to this report.
The report lists all uploaded drugs with saved product data in the ASP system. The Module organizes the full list by row number and includes each drug identifier, status, and all previously submitted information from the Add Product Data sections.
Note: The Module highlights errors in red. Hover over the red text to display information about the specific error.
Click the Rejection Details tab.
A listing of drugs with rejected product data displays. Refer to Figure 49.
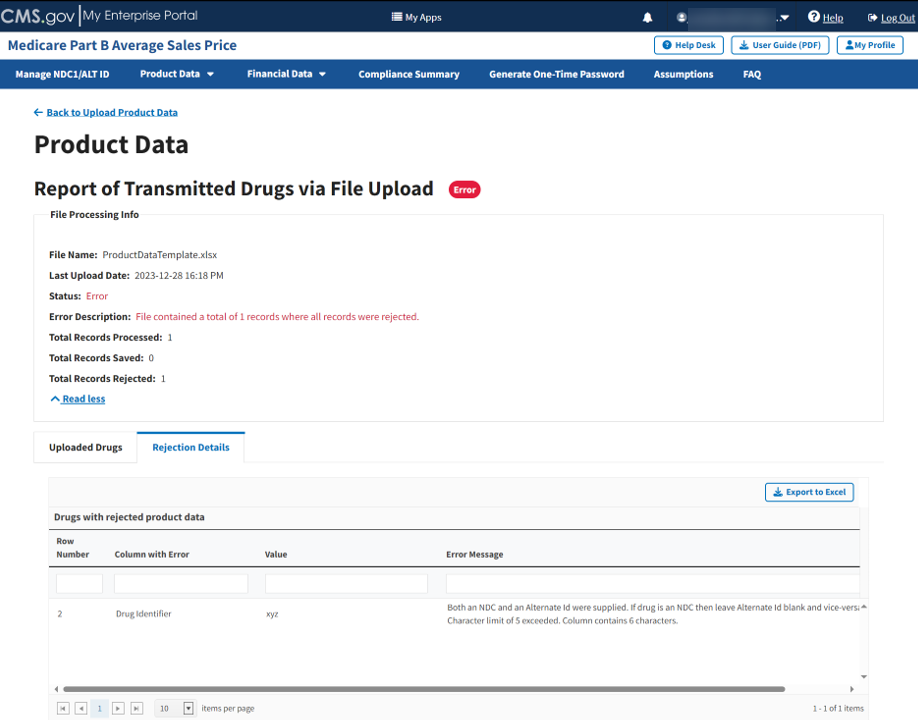
Figure 49: Upload Product Data - Reported Rejection Details
The Module lists all errors found in submitted data by Row Number, Column with Error, and Error Message under Drugs with Rejected Product Data.
Return to the Add/Update Product Data section of the Module to request any changes to your product data.
Drug manufacturers can use the ASP module to view drug data submitted during the current reporting period. However, manufacturers cannot update or edit drug data using this feature. From the Medicare Part B Average Sales Price homepage, click the Product Data tab; then select the View Drugs tab to view the View Drugs page.
The following sections describe how to view active and expired drugs.
View Active Drugs
From the View Drugs page, the Active Drugs tab displays by default. Refer to Figure 50.
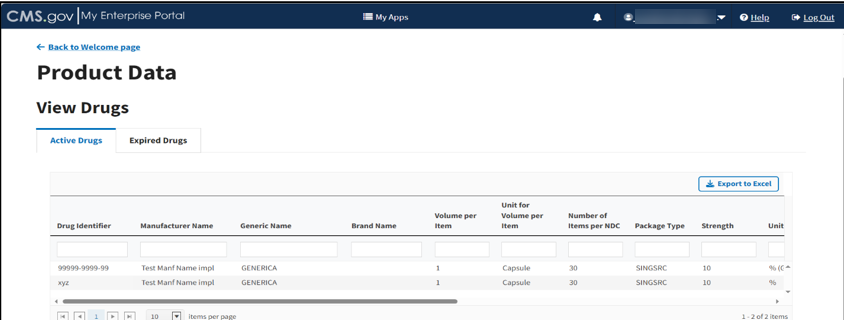
Figure 50: Product Data - View Active Drugs
Follow these steps to view submitted drug data for Active Drugs from the View Drugs page:
Scroll through the list of active drugs to view submitted data and status.
The Module organizes all active drugs by Drug Identifier, Manufacturer Name, Generic Name, Brand Name, Volume per Item, Unit for Volume per Item, Number of Items per NDC, Package Type, and Strength categories, and previously submitted information from the Add Product Data sections.
Click the arrows on the bottom left to scroll through all submitted drugs by page. View, filter, and sort active drugs by clicking on the category name.
Note: Click the Export to Excel button to download all products under the Compliance Summary.
View Expired Drugs
Follow these steps to view submitted drug data for Expired Drugs:
From the View Drugs page, select the Expired Drugs tab.
The Expired Drugs page opens. Refer to Figure 51.
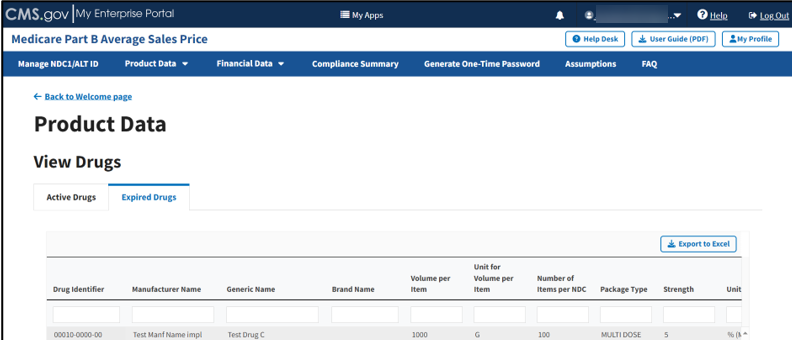
Figure 51: Product Data - View Expired Drugs
Scroll through the list of expired drugs to view submitted data and status.
The Module organizes expired drugs by Drug Identifier, Manufacturer Name, Generic Name, Brand Name, Volume per Item, Unit for Volume per Item, Number of Items per NDC, Package Type, and Strength categories, and previously submitted information from the Add Product Data sections.
Click the arrows on the bottom left to scroll through all submitted drugs by page. View, filter, and sort active drugs by clicking on the category name.
Click the Export to Excel button to download all expired drug products.
Click the Financial Data tab on the Medicare Part B Average Sales Price homepage to view the drop-down menu tabs, Add/Update Financial Data for Current Quarter, Upload Financial Data for Current Quarter, Restate Financial Data or Add for Prior Quarters, and Upload Financial Data for Prior Quarters. Refer to Figure 52.
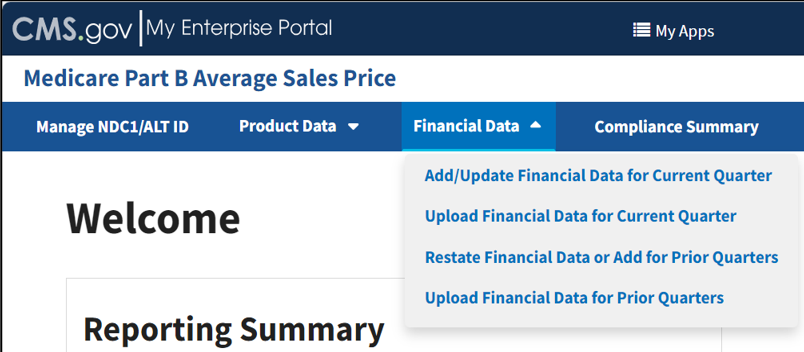
Figure 52: Financial Data - Main Drop-down
The following sections describe how to add/update and upload financial data.
To add or update financial data, click the Add/Update Financial Data for Current Quarter tab.
Note: If you are a manufacturer of certain drugs that contain variable amounts of product, such as radiopharmaceuticals and blood clotting factors, your data should be reported to CMS at the HCPCS level rather than the NDC level. CMS maintains and publishes a list of these drugs on a quarterly basis on the ASP Reporting page under the Reporting Resources section. If you are a manufacturer of a drug that contains variable amounts of product, please check the “ASP Report in Units Other than NDC” document prior to submitting your financial data for the quarter. Should you have any questions, please contact [email protected].
The Add/Update Financial Data page opens with default selections. Refer to Figure 53.
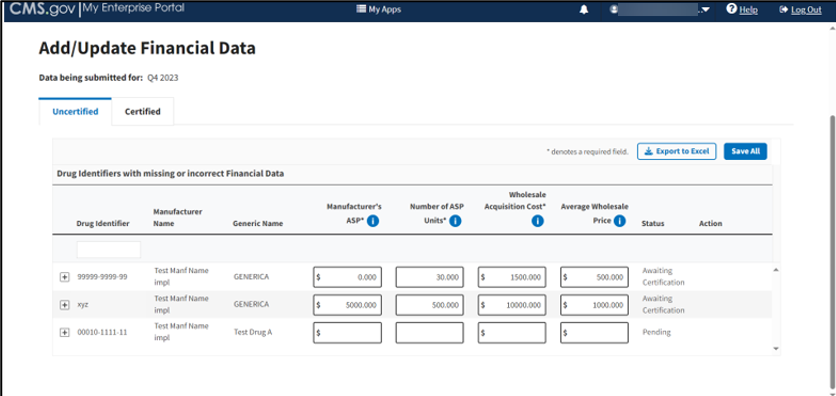
Figure 53: Add/Update Financial Data
Note: The Module collects data submissions for the upcoming financial quarter. As an example, figures in this section feature data submitted for Q1 2023.
The next section describes steps to indicate if your 505(b)(2) products have any therapeutic equivalent changes. If this is not applicable to you, you may skip ahead to the next section, Section 3.3.1.2 - Add/Update Financial Data for Uncertified Drugs.
If you are associated with any 505(b)(2) products, the system displays a prompt to review the list of products and indicate if any of those products have therapeutic equivalent changes. Refer to Figure 54.
Note: If you are associated with any 505(b)(2) products, you must complete these steps before proceeding with adding or editing data. If you are not associated with 505(b)(2) products or are a new user, skip ahead to Section 3.3.1.2 - Add/Update Financial Data for Uncertified Drugs.
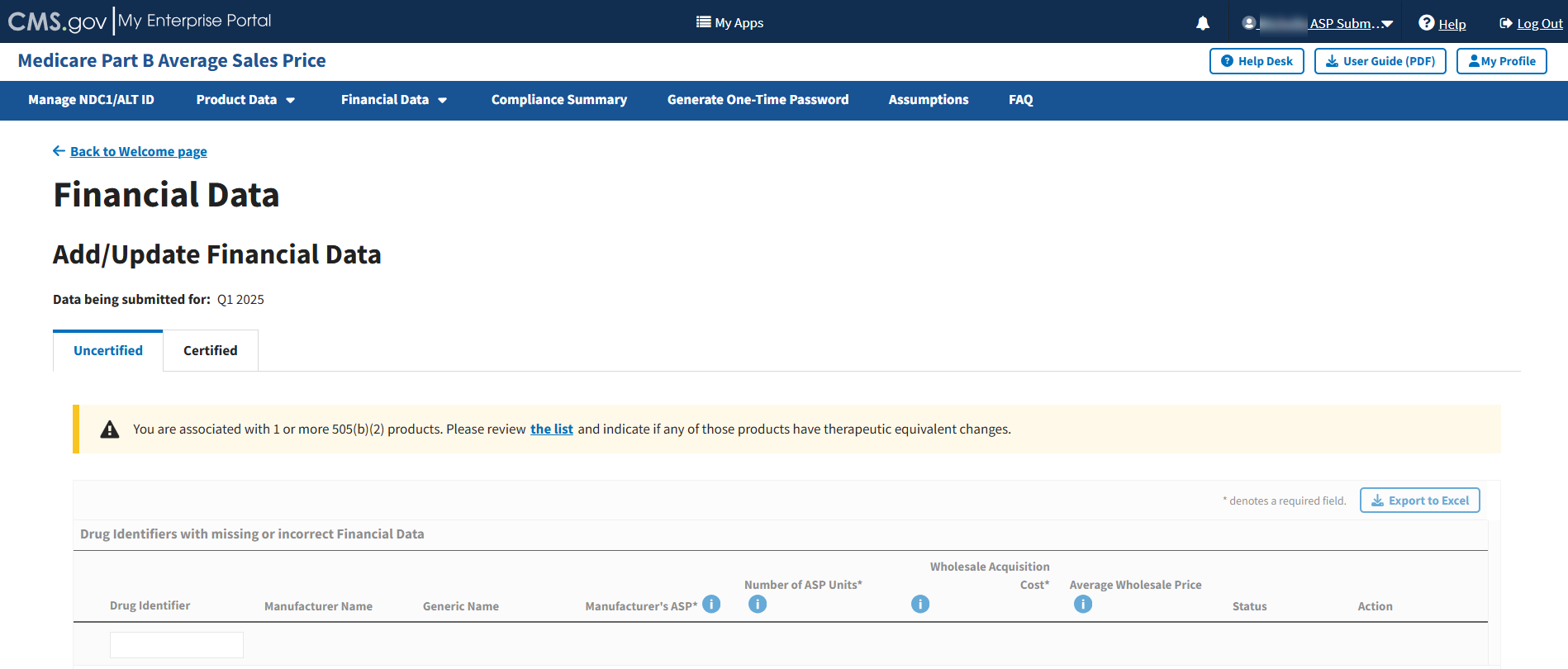
Figure 54: Add/Update Financial Data 505(b)(2)
Follow these steps to review your list of products and indicate any therapeutic equivalent changes:
Click the list hyperlink in the prompt. Refer to Figure 54.The list of products displays.
Select Yes or No in the drop-down menu for each product to indicate whether your product has a therapeutic equivalent. Refer to Figure 55 and Figure 56.
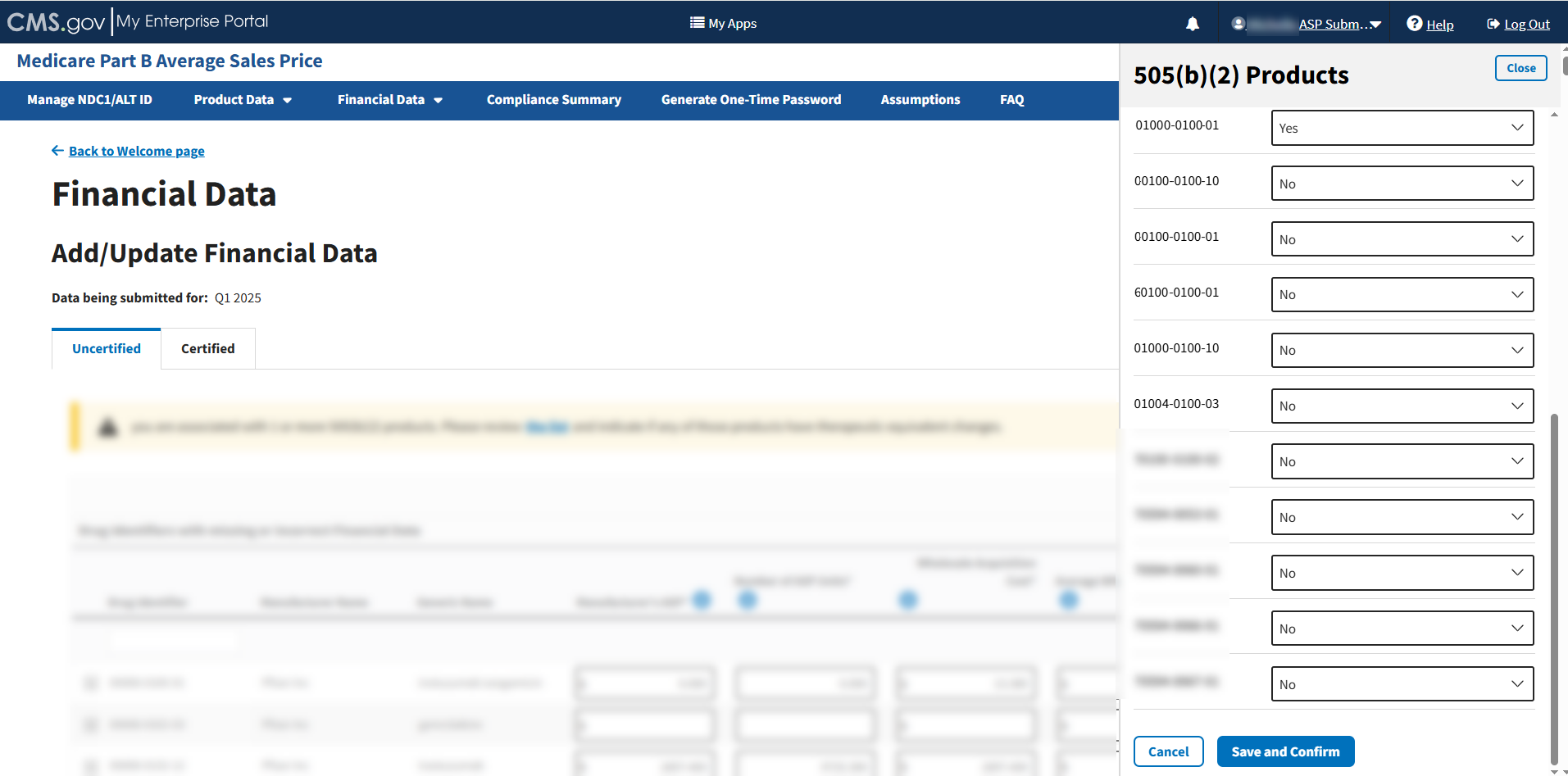
Figure 55: Add/Update Financial Data 505(b)(2) Products List
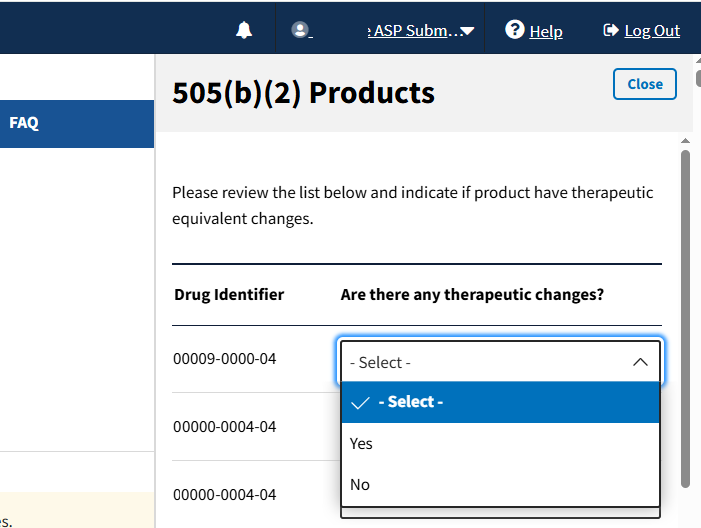
Figure 56: Add/Update Financial Data 505(b)(2) Products List
Once you have reviewed the list and made your selections, click Save and Confirm.
A confirmation message displays asking if you have reviewed all of your products.
If you have finished reviewing your products, click Confirm. Refer to Figure 57.
Figure 57: 505(b)(2) Confirmation
A message displays confirming you have successfully updated your therapeutic changes. Refer Figure 58.
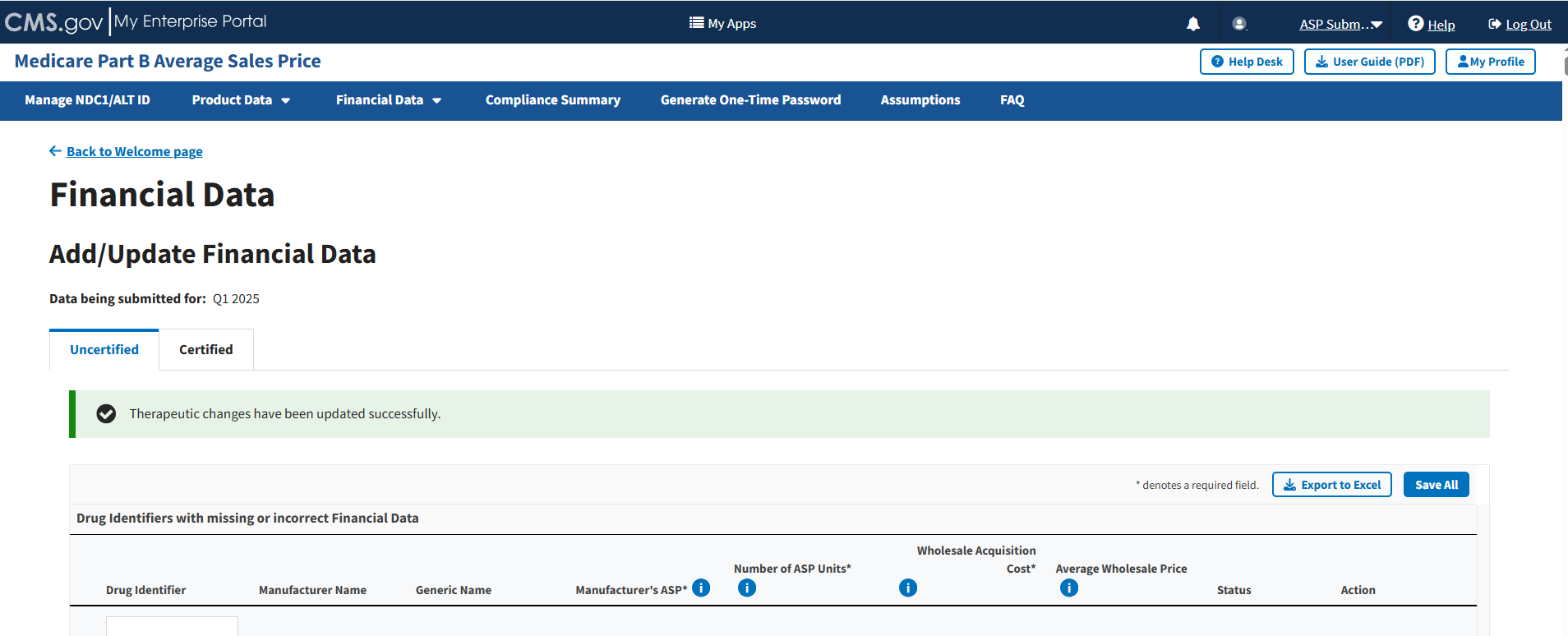
Figure 58: Add/Update Financial Data 505(b)(2) Successfully Updated
Follow these steps to add/update financial data for uncertified drugs:
From the Add/Update Financial Data page, select the Uncertified Drugs tab if it is not already selected.
Note: The Module denotes the Manufacturer’s ASP, Number of ASP Units, and Wholesale Acquisition Cost fields with an (*) to indicate that each field is required.
Enter or edit any missing or inaccurate financial data for your submitted drug products.
As you add or update information onto the page, click the Save All button to save your changes in the Module.
Note: As an alternative to entering data directly into the Module, under Drug Identifiers with Missing or Incorrect Financial Data, you can click the Excel box on the right side to convert all information on this page into an Excel file. You can upload the Excel file after making your updates. Refer to Section 3.3.3 - Upload Financial Data.
Scroll through the list of submitted drugs and products on the page. Filter through all the information by clicking on the category name.
Click the plus symbol on each row of the table to expand each product’s information and view additional categories, including Brand Name, FDA Approval and all other information previously submitted or acknowledged in the Product Data section. Refer to Figure 59.
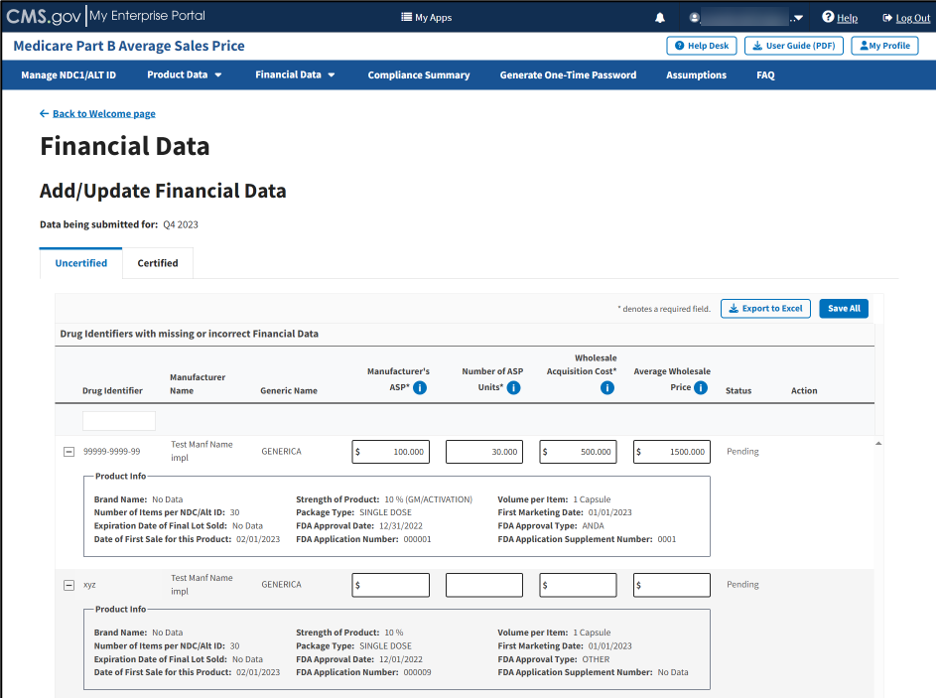
Figure 59: Add/Update Financial Data - Drug Identifiers With Missing or Incorrect Data
Enter and review your information to ensure the highest level of accuracy in data reporting.
Click the Save All button to submit your information to the Module.
A message displays confirming you have successfully updated your financial data. Refer to Figure 60.
Figure 60: Add/Update Financial Data Successfully Added
Note: When there is an error in the submitted data or a missing field, the page highlights each box in yellow to flag an error.
Each row with errors displays a View Alerts button. Refer to Figure 61.
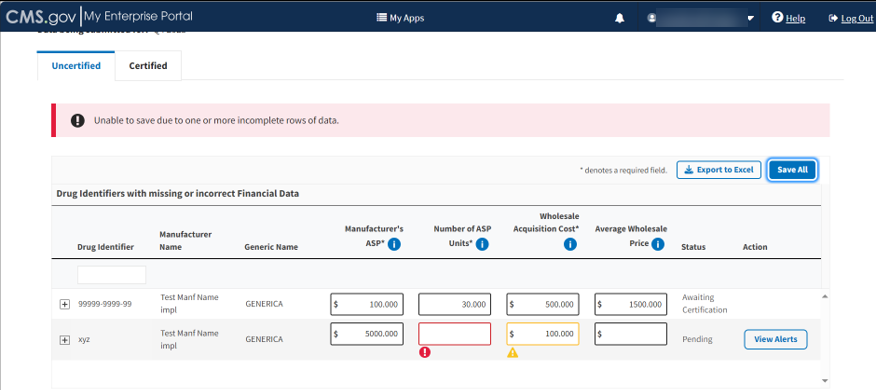
Figure 61: Add/Update Financial Data - Error Menu
Click the View Alerts button for more information regarding the data reporting errors in your submitted financial data.
A side panel opens and displays a listing with descriptions of various errors and warnings. Refer to Figure 62.
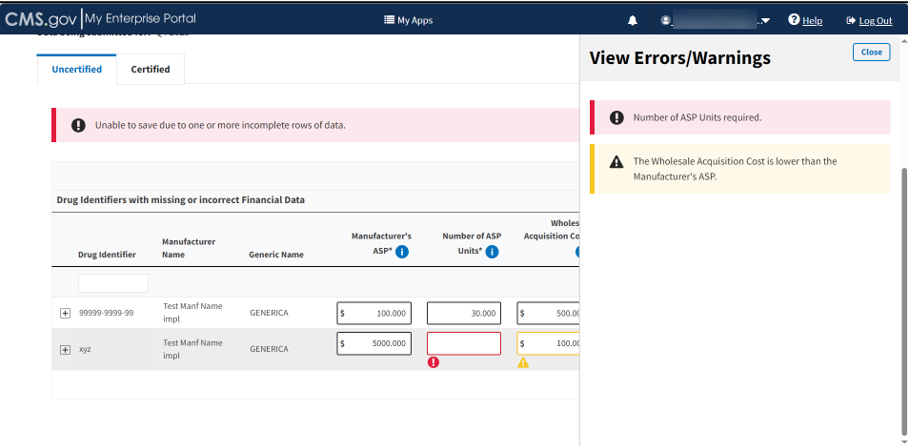
Figure 62: Add/Update Financial Data - View Errors/Warnings Page
Click Save Changes once you address any errors and confirm your product data is accurate.
A message displays confirming that you have successfully added your data. Refer to Figure 63.
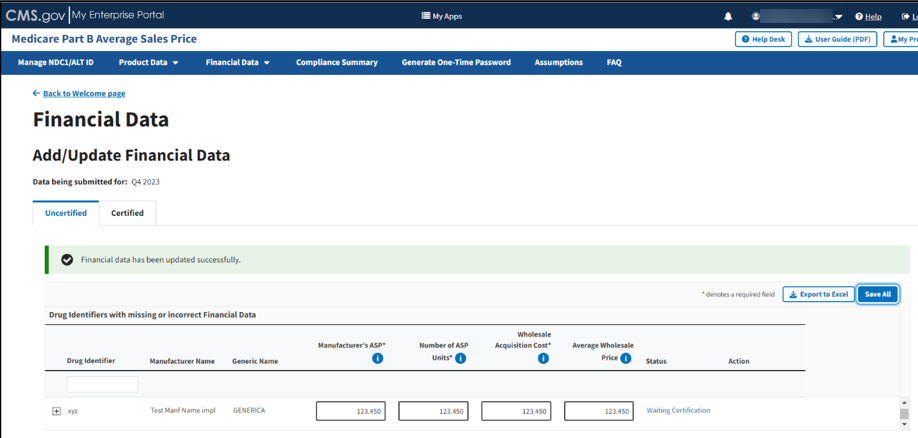
Figure 63: Add/Update Financial Data - Successfully Updated
The Module collects data submissions for the upcoming financial quarter. Follow these steps to view submitted data for certified drugs:
From the Add/Update Financial Data page, select the Certified Drugs tab.
The Certified Drugs page opens. Refer to Figure 64.
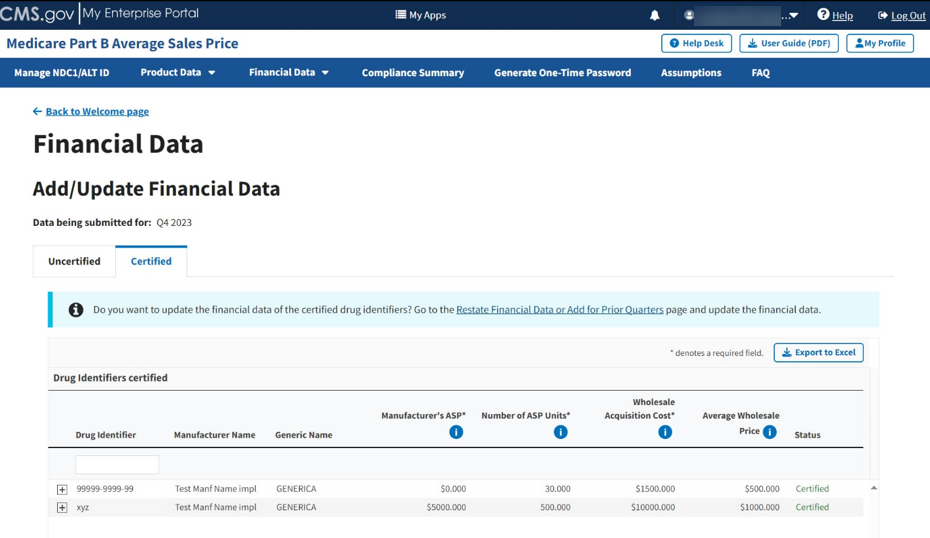
Figure 64: Add/Update Financial Data - Certified Drugs
Note: To update financial data for Certified drug identifiers, refer to the steps in Section 3.4.1- Add/Update Restate Financial Data.
Under Drug Identifiers certified, click the Export to Excel button to convert all information on this page into an Excel file.
Note: The Module denotes the Manufacturer’s ASP, Number of ASP Units, and Wholesale Acquisition Cost fields with an (*) to indicate that each field is required.
Scroll through the list of certified drugs and products on the page. Filter through all the information by clicking on your preferred category name.
Click the arrows on the bottom left to scroll through all submitted drugs by page.
Click on the plus symbol on each row of the table to expand each product’s information and view additional categories, including Brand Name, FDA Approval and all other information previously submitted or acknowledged in the Product Data section. Refer to Figure 65.
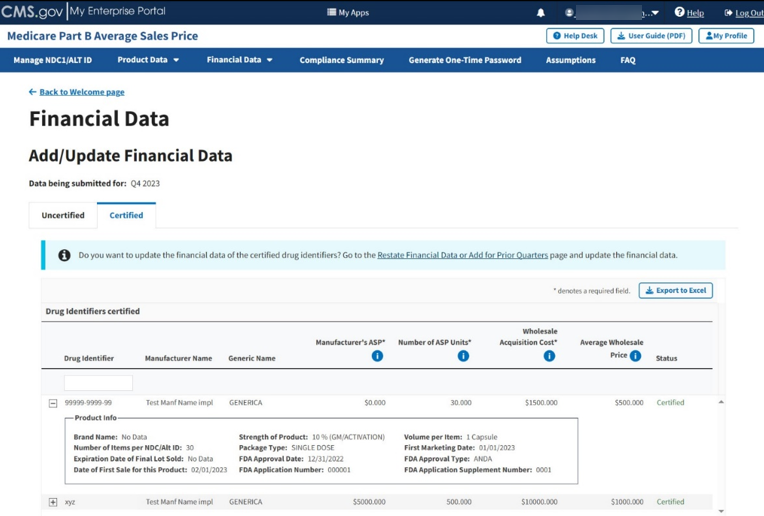
Figure 65: Add/Update Financial Data - Certified Drugs More Information
Follow these steps to upload financial data:
From the Medicare Part B Average Sales Price homepage, click the Financial Data tab; then select the Upload Financial Data for Current Quarter tab.
The Upload New or Corrected Financial Data page opens, listing the financial quarter and year for the upcoming reporting period. Refer to Figure 66.
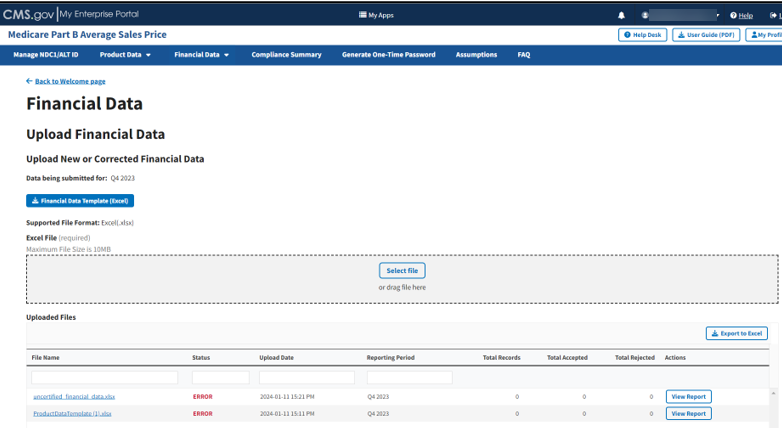
Figure 66: Upload Product Data - New or Corrected
Note: Under Data being submitted for: (current quarter), click Financial Data Template (Excel) to download a financial data template.
If you are associated with any 505(b)(2) products, you may be prompted to indicate if your products have any therapeutic equivalent changes before you can proceed. Refer to Section 3.3.1.1 - Add Therapeutic Equivalent Changes for 505(b)(2) Drugs for more information.
Upon preparing your .xlsx file (required) and verifying your information for accuracy, click Select File to browse your desktop and upload the file to the Module. You may also drag the file into the Select File box. Refer to Figure 67.
Figure 67: Upload Financial Data - Uploading Files From Desktop
A download bar displays as your file uploads. A message displays confirming you have successfully uploaded your .xlsx file. Refer to Figure 68.
Note: If the Module cannot process your file, an error message displays, and a New Report generates under Uploaded Files.
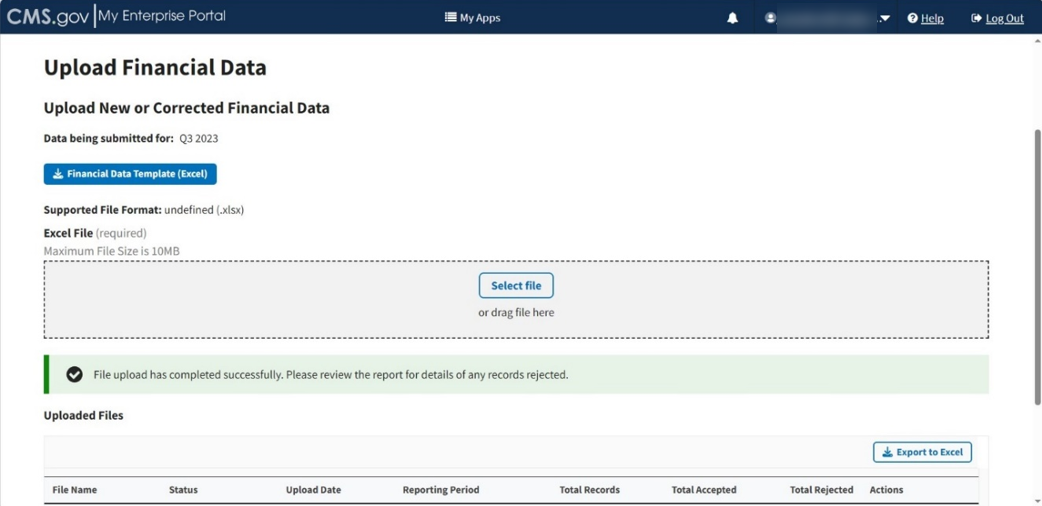
Figure 68: Upload Financial Data Page - New File Successfully Uploaded
The Uploaded Files section displays files you uploaded recently as well as previous files still in the Module. Refer to Figure 69.
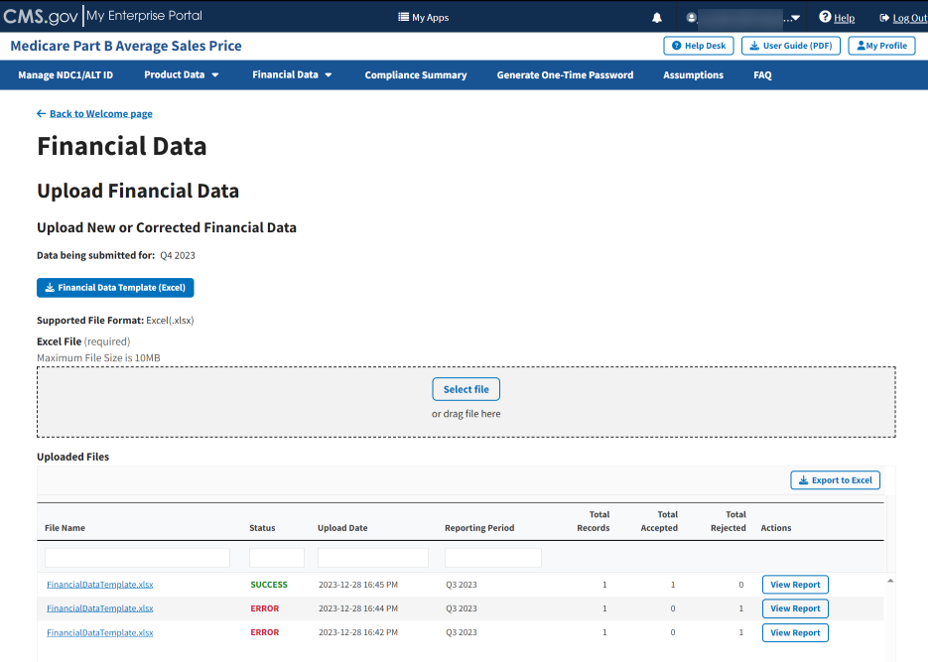
Figure 69: Upload Financial Data - Uploaded Files
Each uploaded file displays the File Name, Status, Upload Date, Reporting Period, Total Records, Total Accepted, Total Rejected, and Actions categories submitted to the Module.
Click View Report under Actions in the Uploaded Files section to view the full report for a submitted file.
The report opens on the next page. Refer to Figure 70.
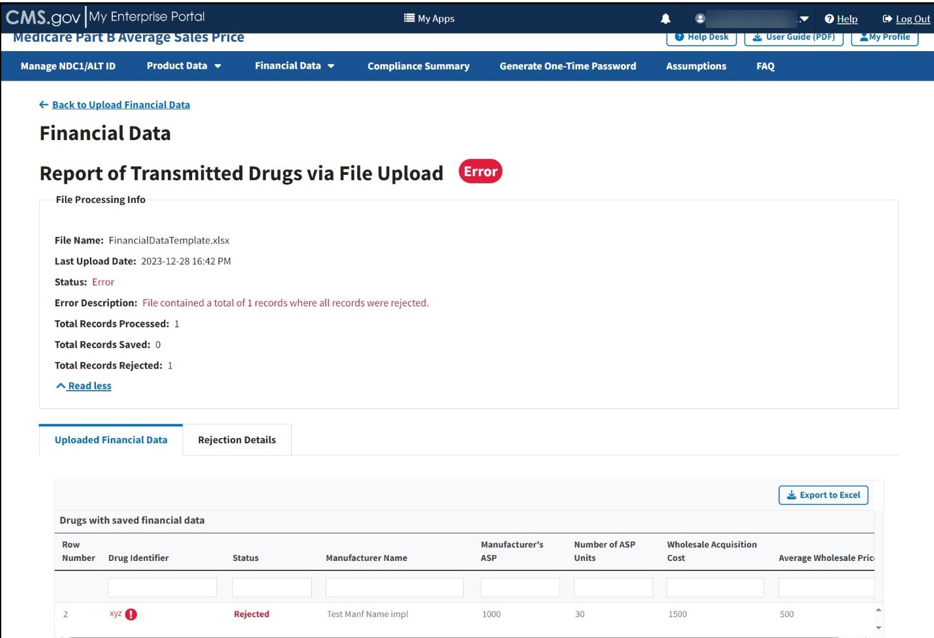
Figure 70: Upload Financial Data - Report of Transmitted Drugs via File Upload
The report lists all drugs with saved product data in the ASP system. The Module organizes the full list by row number and includes each drug identifier, status, and all previously submitted information from the Add Product Data sections.
Note: The Module highlights errors in red. Hover over the red text to display information about the specific error.
Click the Rejection Details tab.
A listing of drug identifiers with rejected financial data displays. Refer to Figure 71.
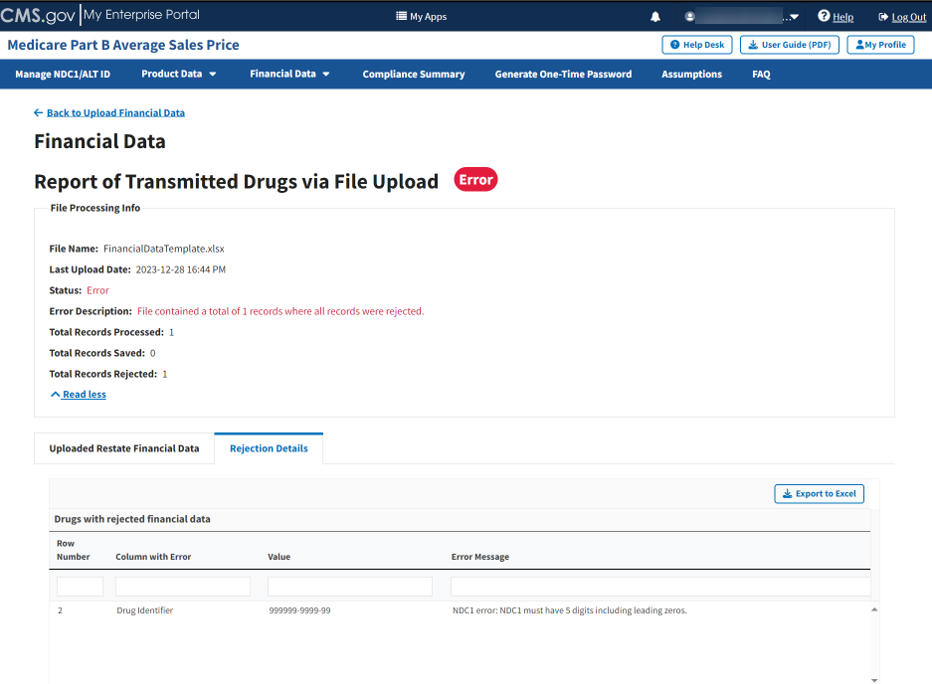
Figure 71: Upload Financial Data - Reported Rejection Details
The Module lists all errors found in submitted data by Row Number, Column with Error, Value and Error Message under Drugs with rejected financial data.
Return to the Add/Update Financial Data for Current Quarter section of the Module to request any changes to your product data.
Manufacturers of drugs and biologicals payable under Medicare Part B have an obligation to report accurate ASP data to CMS, including addressing data miscalculations and other errors in previously submitted data. Upon identifying an error, manufacturers must submit corrected data through the ASP Module. Additionally, CMS may identify an error and contact the manufacturer to request corrected data for prior quarters.
CMS evaluates resubmitted data and decides whether to issue a restatement of the payment limit. Criteria evaluated includes, but is not limited to, timing of the corrected data, changes to the payment limit, and/or administrative burden.
The following sections describe how to add/update or upload restate financial data using the online data entry process.
Follow these steps to add/update restate financial data:
Click the Financial Data tab; select Restate Financial Data or Add for Prior Quarters. Refer to Figure 72.
Figure 72: Financial Data - Main Dropdown
The Restate Financial Data or Add for Prior Quarters page opens, listing the financial quarter and year for the upcoming reporting period. Refer to Figure 73.
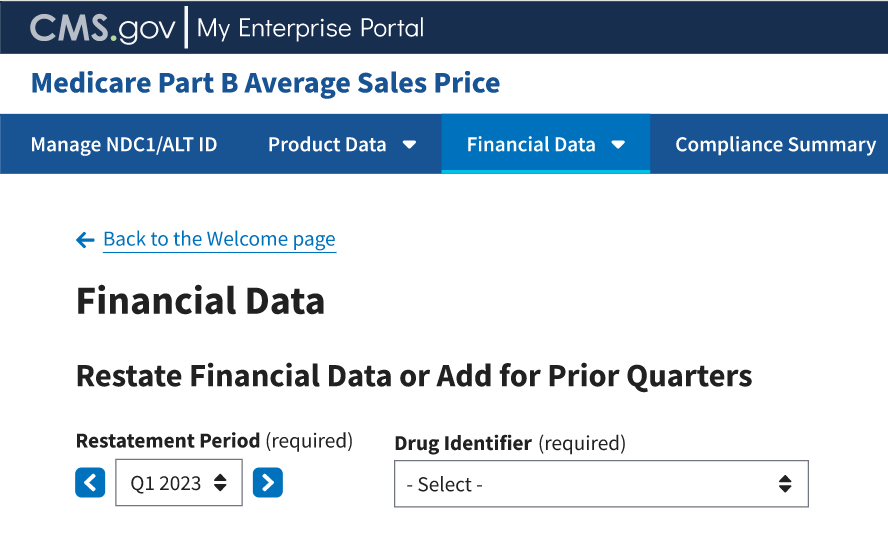
Figure 73: Financial Data - Add/Update Restate Financial Data
Note: Click the Restatement Period (required) drop-down in the top left to scroll through previous quarters. Click the blue arrows to navigate to a previous quarter starting with the most recent or next quarter.
Click the -Select- box under Drug Identifier (required) to expand the list of submitted drugs in the Module. Refer to Figure 74.
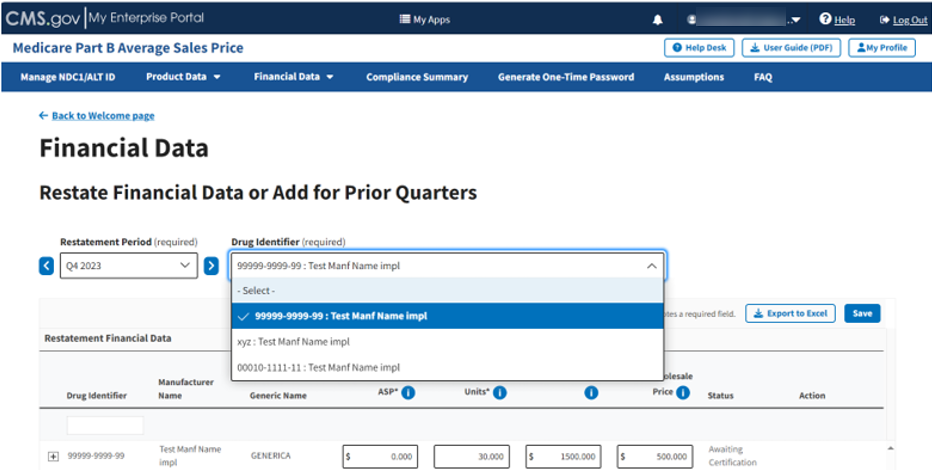
Figure 74: Add/Update Restate Financial Data - Drug Identifier Drop-down
Select the Drug Identifier you need to close the drop-down. Once you click a product, the Review Restatement List expands to show the selected restatement. Refer to Figure 75.
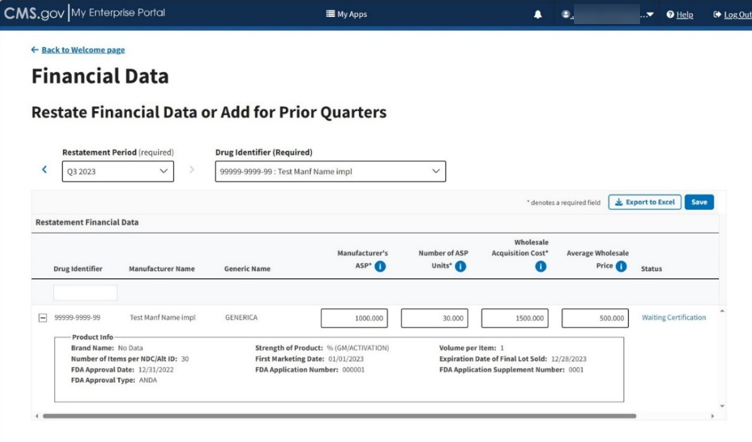
Figure 75: Add/Update Restate Page - Review Restatement List
Review and make any corrections necessary for the drug to the Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost (all required) and Average Wholesale Price fields.
Click the plus symbol on each row of the table to expand each product’s information and view additional categories previously submitted or acknowledged in the Product Data section.
Click the Save button to submit your data.
A message displays confirming you have successfully updated your Restate Financial Data. Refer to Figure 76.
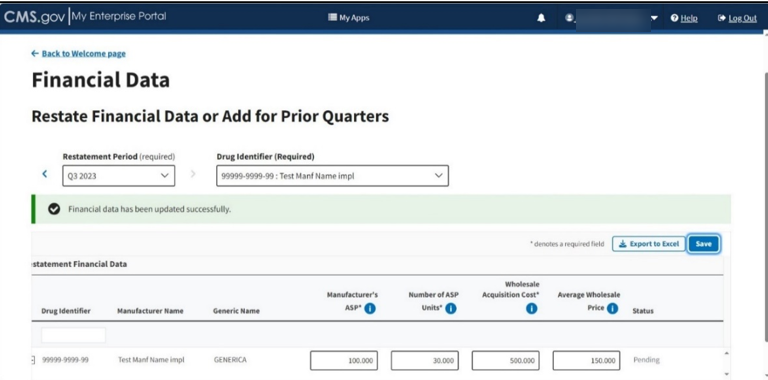
Figure 76: Add/Update Restate Page - Restate Data Successfully Saved
Contact your Certifier to recertify the corrected data you submitted to the Module.
Follow these steps to upload restate financial data:
From the Medicare Part B Average Sales Price homepage, click the Financial Data tab; then select Upload Financial Data for Prior Quarters. Refer to Figure 77.

Figure 77: Financial Data - Main Drop-down
The Upload Financial Data for Prior Quarters page opens, listing the financial quarter and year for the upcoming reporting period. Refer to Figure 78.
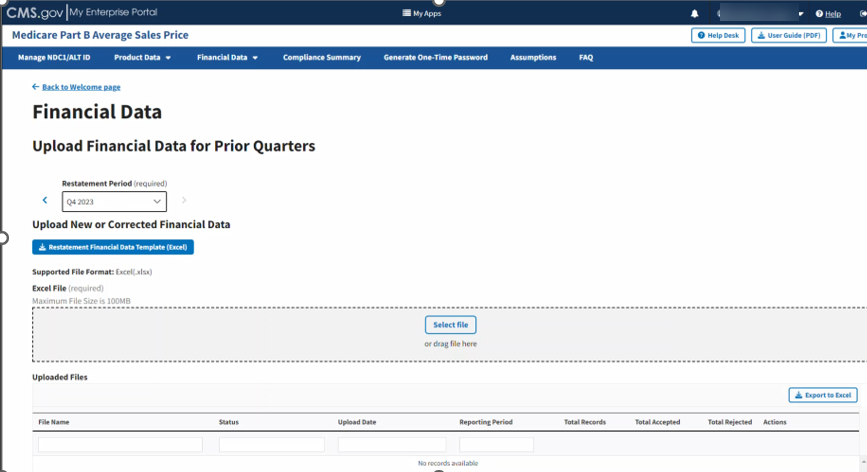
Figure 78: Upload Financial Data for Prior Quarters Restate Financial Data
Note: Under Upload New or Corrected Financial Data, there is a Restatement Financial Data Template (Excel) available for download. Click the button to download a desktop copy.
Upon preparing your .xlsx file (required) and verifying your information for accuracy, click Select File to browse your desktop and upload the file to the Module. You may also drag the file into the Select File box. Refer to Figure 79.
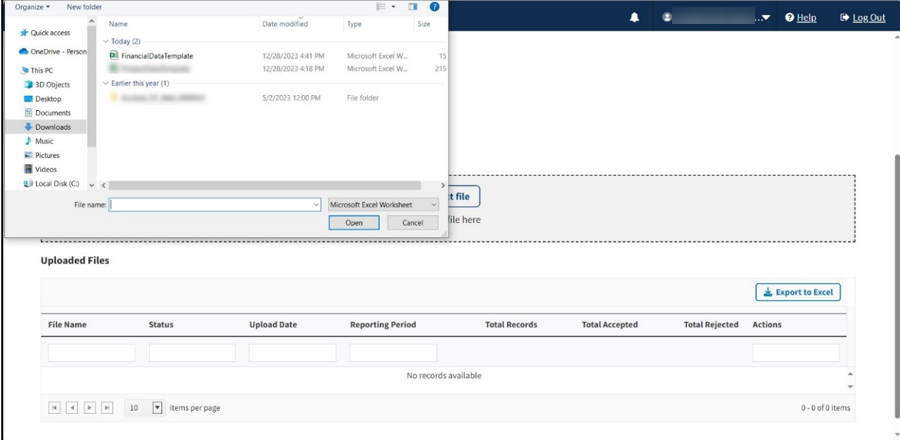
Figure 79: Upload Financial Data for Prior Quarters - Uploading Files From Desktop
A download bar displays as your file uploads. A message displays confirming you have successfully uploaded your .xlsx file. Refer to Figure 80.
Note: If the Module cannot process your file, an error message displays, and a New Report generates under Uploaded Files.
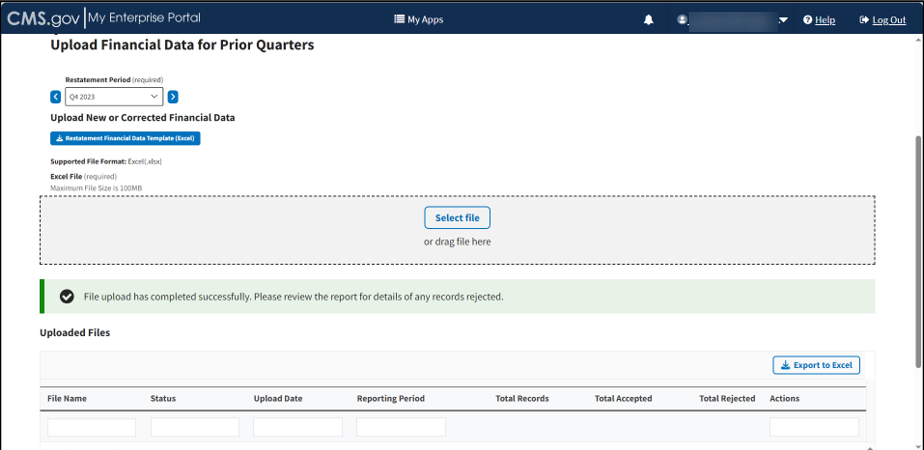
Figure 80: Upload Financial Data for Prior Quarters - New File Successfully Uploaded
The Uploaded Files section displays files you uploaded recently as well as previous files still in the Module. Refer to Figure 81.
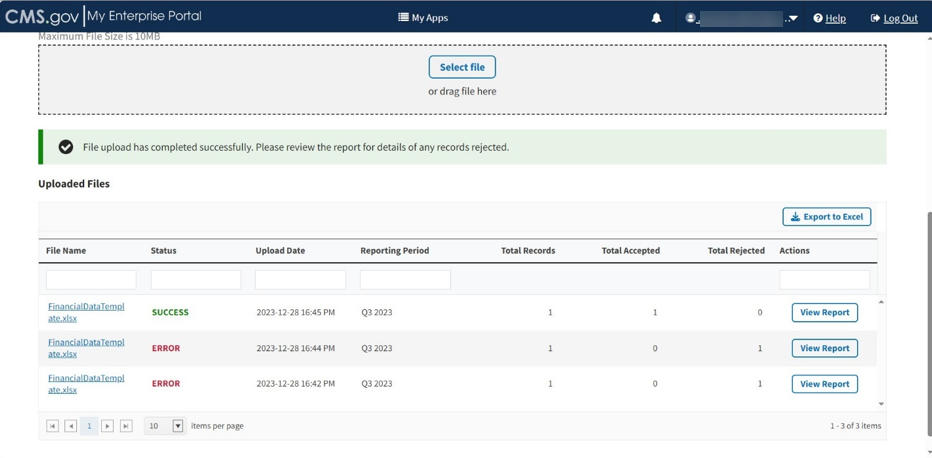
Figure 81: Upload Financial Data for Prior Quarters - Uploaded Files
Each uploaded file displays the File Name, Status, Upload Date, Reporting Period, Total Records, Total Accepted, Total Rejected, and Actions categories submitted to the Module.
Click View Report under Actions in the Uploaded Files section to view the full report for a submitted file.
The report opens on the next page. Refer to Figure 82.
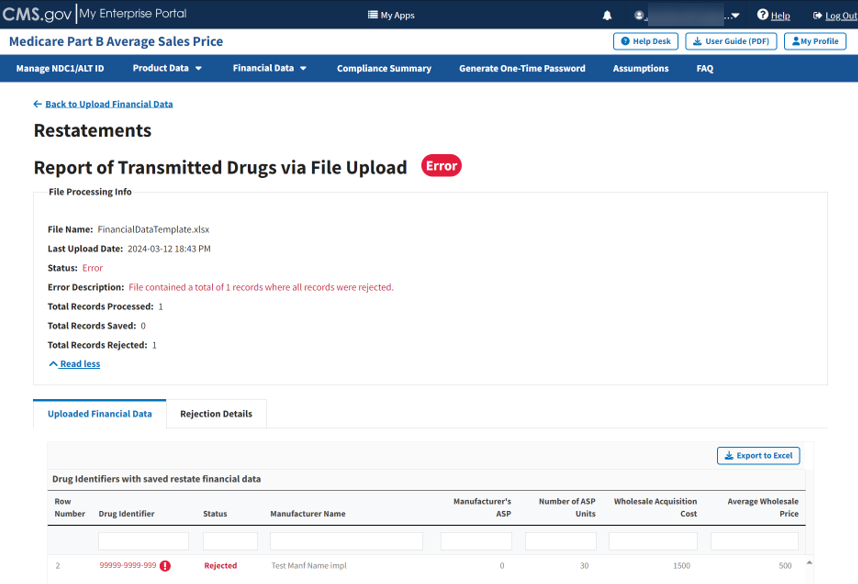
Figure 82: Upload Financial Data for Prior Quarters - Report of Transmitted Drugs
The report lists all drug identifiers with saved restate financial data in the ASP system. The Module organizes the full list by row number and includes each drug identifier, status, and other previously submitted information from the Add Product Data sections.
Note: The Module highlights errors in red. Hover over the red text to display information about the specific error.
Click the Rejection Details tab.
A listing of drug identifiers with rejected restate financial data displays. Refer to Figure 83.
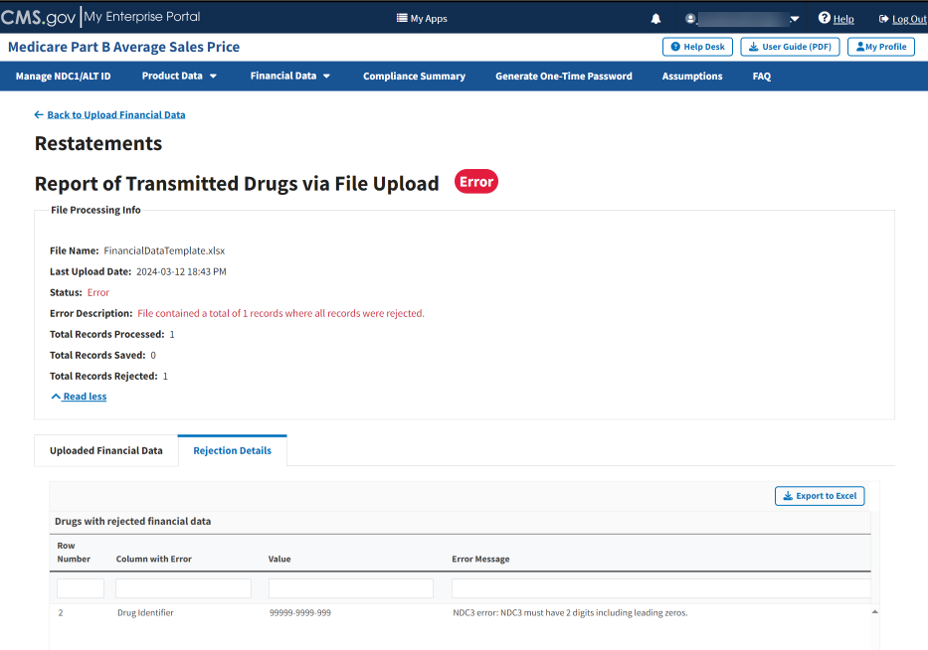
Figure 83: Upload Financial Data for Prior Quarters - Reported Rejection Details
The Module lists all errors found in submitted data by Row Number, Column with Error, Value and Error Message under Drugs with rejected financial data.
Return to the Add/Update Financial Data section of the Module to request any changes to your product data.
Contact your Certifier to recertify the corrected data you submitted to the Module.
The features in the Compliance Summary section allow drug manufacturers to determine if their products meet the current submission reporting requirements.
The Compliance Summary consists of the following sections:
Missing: Displays drug products that are missing financial data for the selected reporting period.
Pending: Displays drug products that are both pending certification and pending restatement certification, combined under one tab.
Certified: Displays previously certified drug products for the selected reporting period.
Note: Financial data will be suppressed for prior quarters.
New: Displays drug products with a first marketing date in the same reporting period.
Off Cycle: Displays drug products added on or after the first day of the submission window of the current quarter.
Expired: Displays drug products that have an expired date of final lot sold. A drug product that expired in an earlier quarter will continue to show in subsequent quarters.
Follow these steps to navigate the Compliance Summary section:
From the Medicare Part B Average Sales Price homepage, click the Compliance Summary tab.
The Compliance Summary page opens. The page displays the status for each submitted drug product regarding the drug manufacturer’s compliance for the selected reporting period. The page automatically defaults to the Missing tab. Refer to Figure 79.
Note: Figure 84 shows an alert message under Reporting Period stating that there are drug products in need of attention.
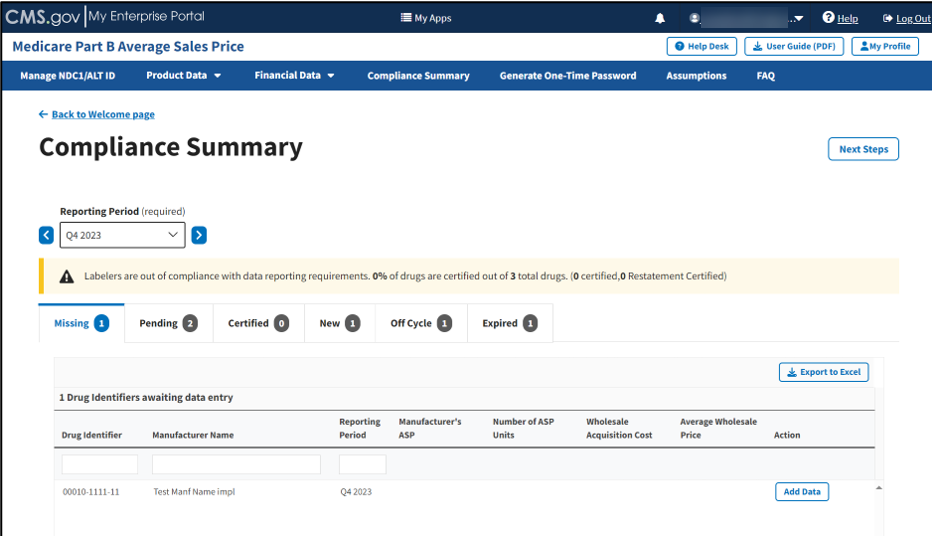
Note: Click the Reporting Period (required) tab in the top left to scroll through previous quarters. Click the blue arrows to navigate to a previous quarter starting with the most recent or next quarter.
Follow these steps to add data in the Missing tab of the Compliance Summary:
Under Drug Identifiers waiting for data entry, review and identify the missing fields or incorrect financial information to address; confirm the accuracy of all the necessary financial information listed on the page.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, and Average Wholesale Price fields.
Note: Click the Export to Excel button to download all products under the Missing tab.
Click the Add Data tab next to the appropriate drug product.
An Add Financial Data window opens. Refer to Figure 85.
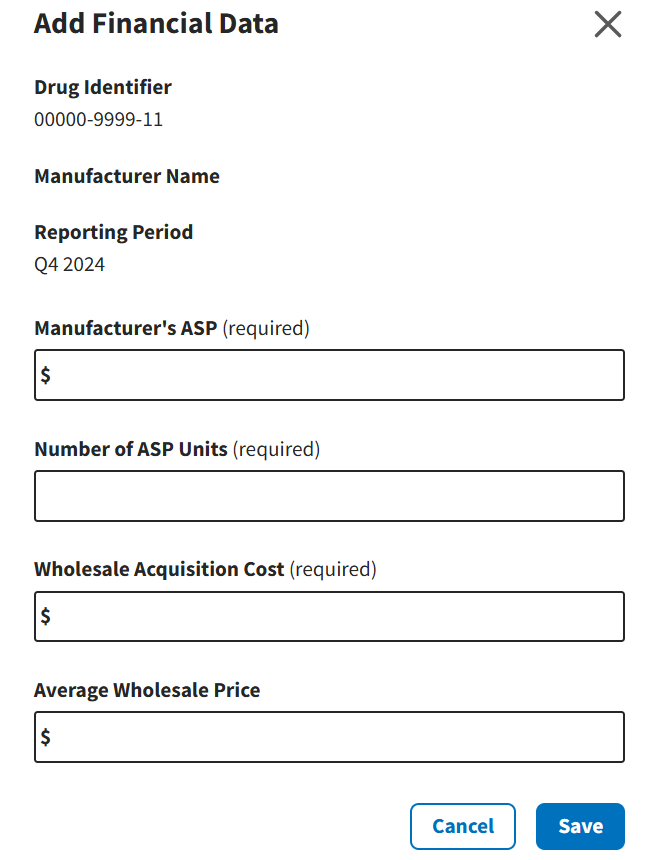
Figure 85: Compliance Summary - Add Data Screen
Type the requested information in the empty Manufacturer’s ASP (required), Number of ASP Units (required), Wholesale Acquisition Cost (required), and Average Wholesale Price (required) fields.
Click Save to submit your information to the Module.
A message displays confirming you have successfully added your data, and that your product is now pending certification. Refer to Figure 86.
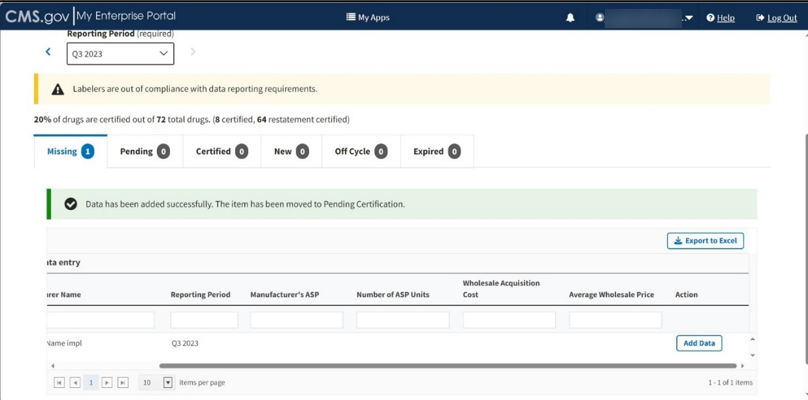
Figure 86: Compliance Summary - Successfully Saved
Follow these steps to review your data in the Pending tab of the Compliance Summary:
From the default Compliance Summary page, click the Pending tab.
The Pending tab displays. Refer to Figure 87.
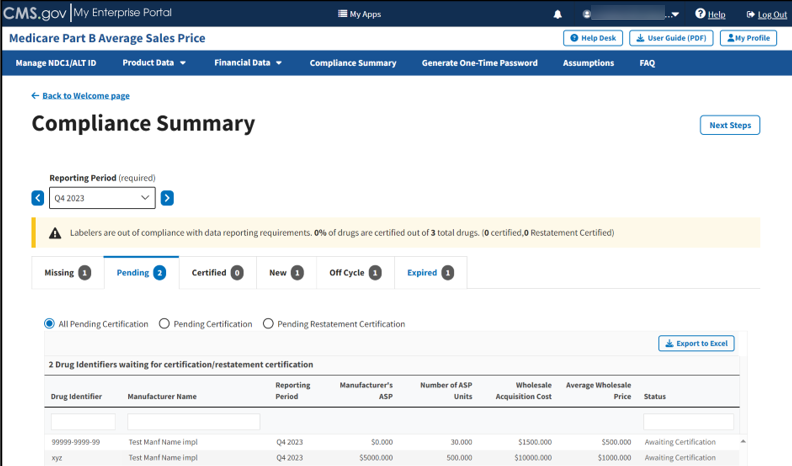
Figure 87: Compliance Summary - All Pending Certification
The Module automatically selects the All Pending Certification radio button, and the page displays the drug identifiers waiting for certification/restatement certification.
Note: Click the Export to Excel button to download all products under the Pending tab.
Under Drug Identifiers Waiting for Certification/Restatement Certification, review your information in the appropriate boxes previously submitted in Section 3.2 - Product Data and Section 3.3 - Financial Data.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, Average Wholesale Price, and Status fields.
Click the Pending Certification radio button to filter only for drugs pending certification. Refer to Figure 88.
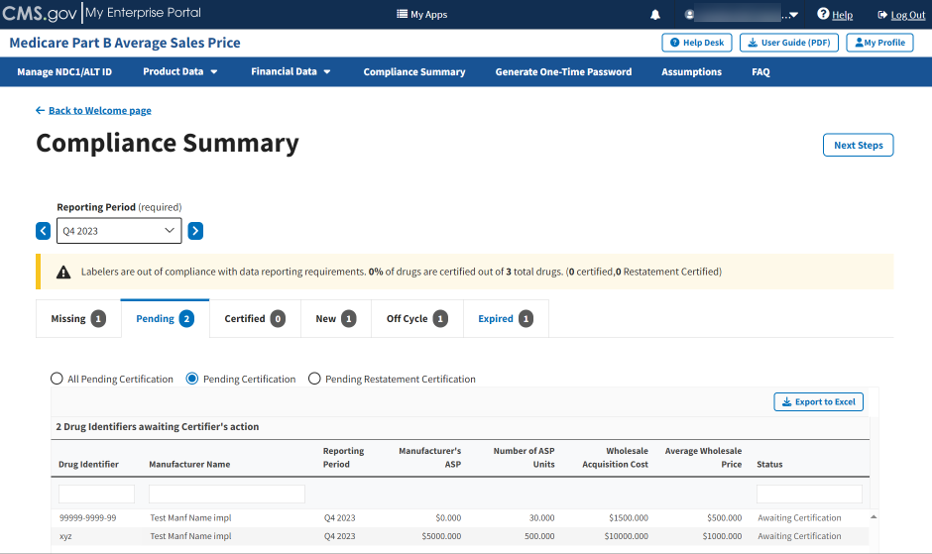
Figure 88: Compliance Summary - Pending Certification
Note: Click the Export to Excel box to download all products under the Pending tab.
Review the information previously submitted in Section 3.2 - Product Data.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, Average Wholesale Price, and Status fields.
Click the Pending Restatement Certification radio button to filter only for drugs that are pending restatement certification. Refer to Figure 89.
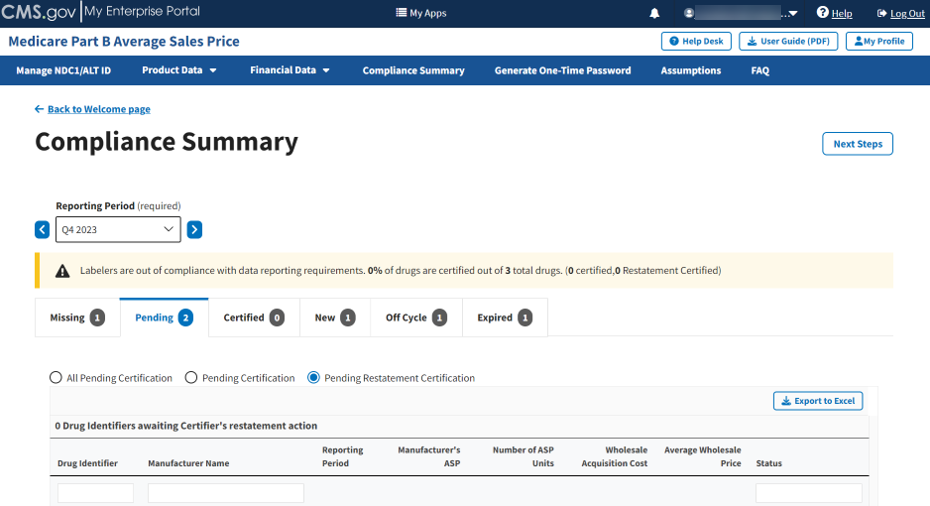
Figure 89: Compliance Summary - Pending Restatement Certification
Note: Click the Export to Excel button to download all products under the Pending tab.
Review all your information in the appropriate boxes previously submitted in Section 3.2 - Product Data and Section 3.3 - Financial Data.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, and Average Wholesale Price, and Status fields.
Follow these steps to review your data in the Certified tab of the Compliance Summary:
From the default Compliance Summary page, click the Certified tab.
The Certified page displays. The Module automatically selects the All Certified radio button. Refer to Figure 90.
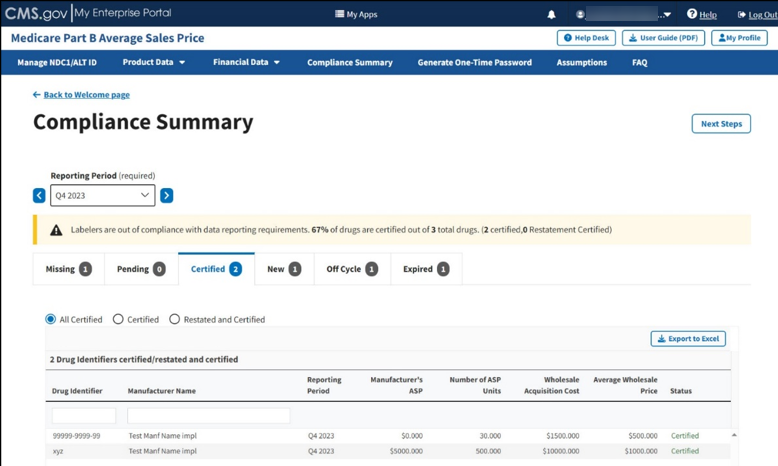
Figure 90: Compliance Summary - All Certified
Note: Click the Export to Excel button to download all products under the Certified tab.
Review all your information in the appropriate boxes previously submitted in Section 3.2 - Product Data and Section 3.3 - Financial Data.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, and Average Wholesale Price, and Status fields.
Click the Certified radio button to filter only for certified drugs. Refer to Figure 91.
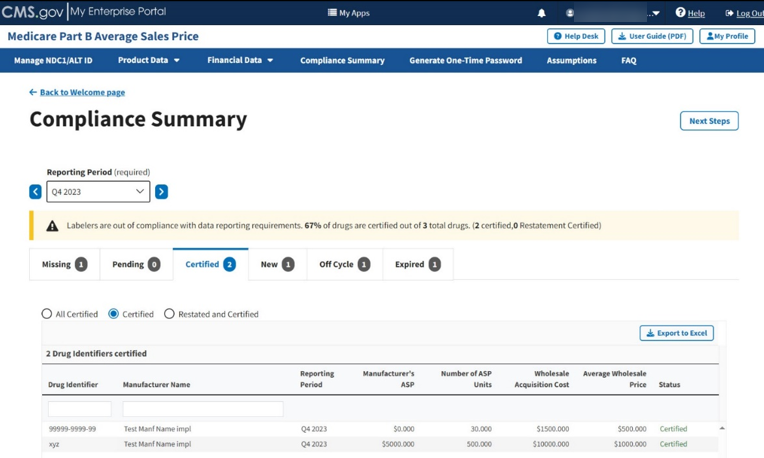
Figure 91: Compliance Summary - Certified
Note: Click the Export to Excel button to download all products under the Certified tab.
Review your information for accuracy.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, and Average Wholesale Price, and Status fields.
Click the Restated and Certified radio button to filter only for restated and certified drugs. Refer to Figure 92.
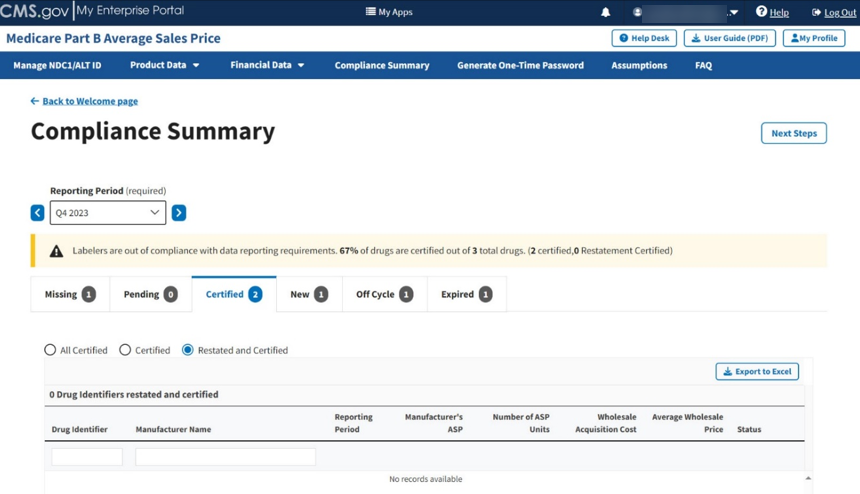
Figure 92: Compliance Summary - Restated and Certified
Note: Click the Export to Excel box if you need to download all products under the Certified tab.
Review your information for accuracy.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, and Average Wholesale Price, and Status fields.
Follow these steps to review your data in the New tab of the Compliance Summary:
From the default Compliance Summary page, click the New tab.
The New page displays. Refer to Figure 93.
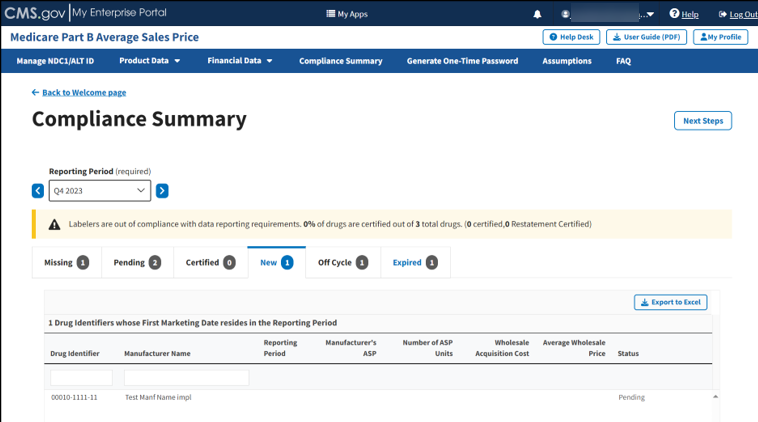
Figure 93: Compliance Summary - New
Note: Click the Export to Excel button to download all products under the New tab.
Review your information for accuracy.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Manufacturer’s ASP, Number of ASP Units, Wholesale Acquisition Cost, and Average Wholesale Price, and Status fields.
Follow these steps to review your data in the Off Cycle tab of the Compliance Summary:
From the default Compliance Summary page, click the Off Cycle tab.
The Off Cycle page displays. Refer to Figure 94.
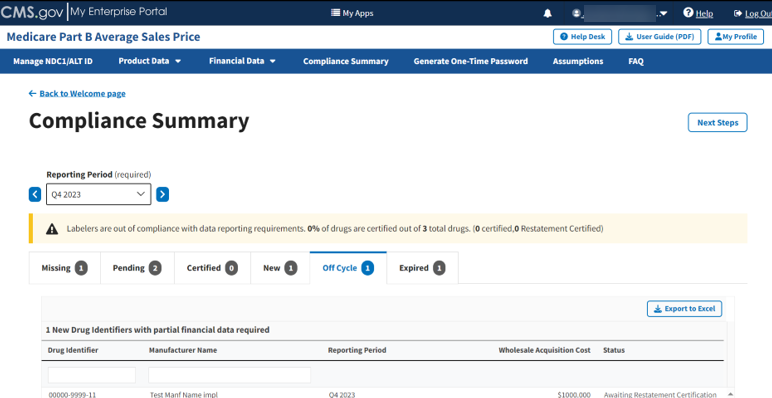
Figure 94: Compliance Summary - Off Cycle
Note: Click the Export to Excel button to download all products under the Off Cycle tab.
Review your information for accuracy.
The Module organizes the full list by Drug Identifier and Manufacturer Name, and includes Reporting Period, Wholesale Acquisition Cost, and Status fields.
Follow these steps to review your data in the Expired tab of the Compliance Summary:
From the default Compliance Summary page, click the Expired tab.
The Expired page displays. Refer to Figure 95.
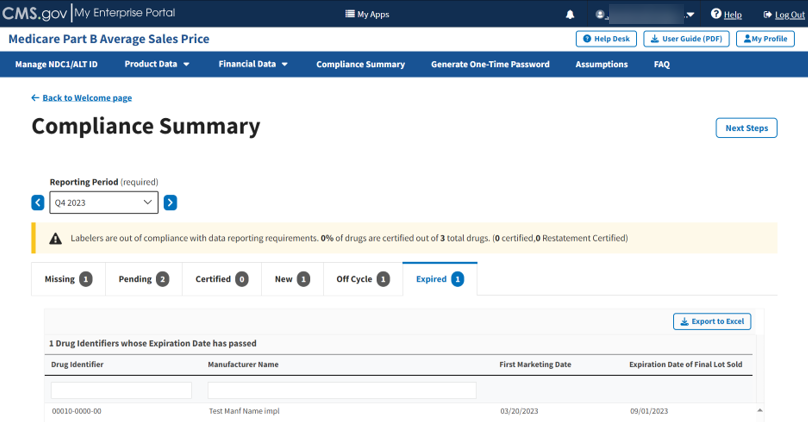
Figure 95: Compliance Summary - Expired
Note: Click the Export to Excel box if you need to download all products under the Expired tab.
Review your information for accuracy.
The Module organizes the full list by Drug Identifier and Manufacturer Name and includes First Marketing Date and Expiration Date of Final Lot Sold fields.
Once you successfully enter product and financial data in the ASP Module, you can generate a one-time password (OTP) for each manufacturer name. Note the following about OTPs:
OTPs protect sensitive information and product specific drug-data from tampering or alterations by others outside of the Submitter or Certifier.
The OTP is a one-time authentication step to link a Submitter to a Certifier within the system. This step does not need to take place during every submission. There can only be one active Certifier per manufacturer. If the Certifier changes, the Submitter must create and share a new OTP with the new Certifier.
The Submitter and Certifier cannot be the same person within your organization.
You can share the OTP with the Certifier. This passcode will remain the same for as long as the Certifier is the same person in your organization who uses the ASP Module.
If the OTP expires, you can generate another OTP and provide it to the Certifier again.
Note: Refer to the Certifier User Guide for more information about the Certifier role.
Follow these steps to generate an OTP:
From the Medicare Part B ASP Homepage, click the Generate One-Time Password tab.
The Generate One-Time Password page opens. Refer to Figure 96.
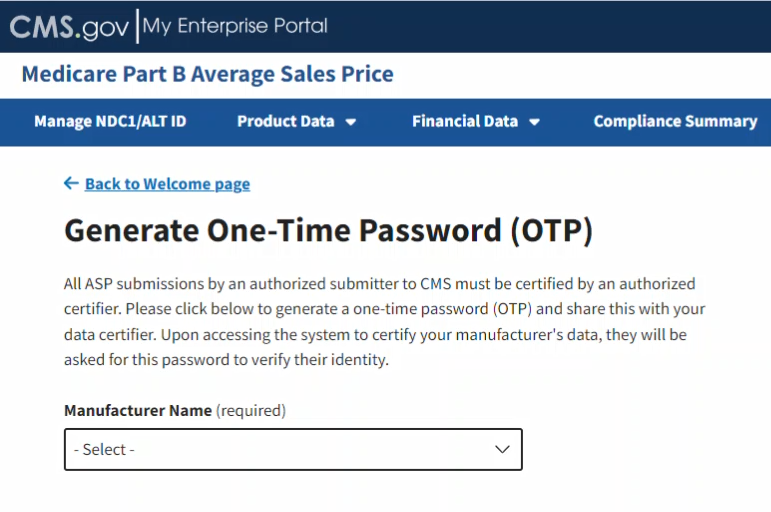
Figure 96: Generate One-Time Password
Click the -Select- box under Manufacturer Name (required) to expand the list. Refer to Figure 97.
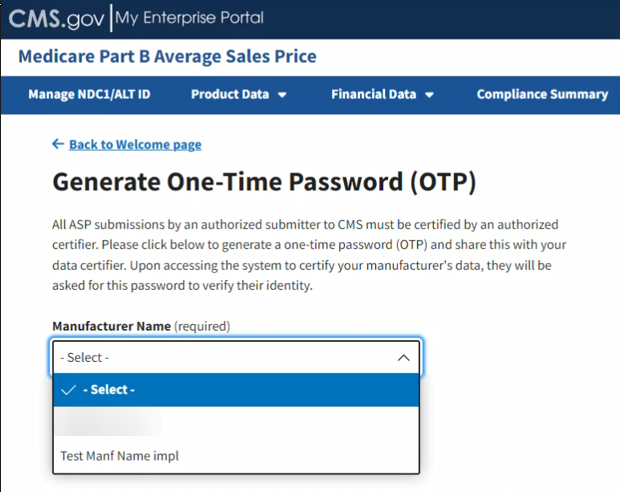
Figure 97: Generate One-Time Password - Manufacturer Name
Select the appropriate manufacturer name.
A new OTP displays. Refer to Figure 98.
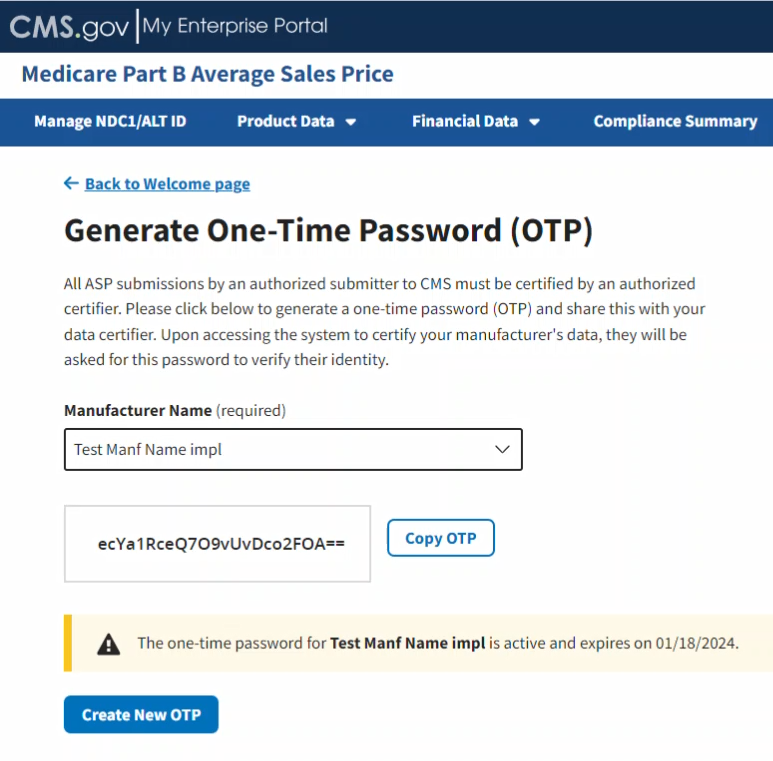
Figure 98: Generate One-Time Password - Password Created
Click Copy OTP to copy your OTP.
Hover text indicates that you have successfully copied the new password. Refer to Figure 99.
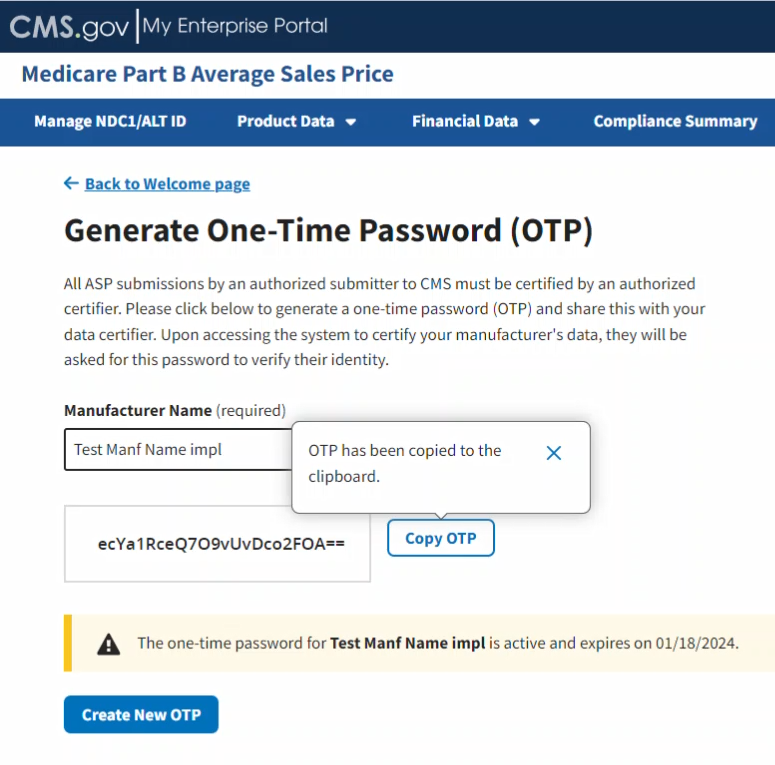
Figure 99: Generate One-Time Password - Password Copied
Copy the OTP and send it to your Certifier. You must recreate the OTP if the Certifier cannot confirm the OTP on the Module, or if it expires.
Note: A message displays at the bottom of the window noting the expiration date for your new password. The Certifier must log in to the ASP Module to use that OTP before the noted expiration date.
Note: An OTP is only valid for seven days. After seven days, you must generate a new OTP.
Drug manufacturers can submit comments regarding their certifications to CMS. Manufacturers may submit these comments for either the current or prior reporting periods. Each quarter, manufacturers will submit these comments for the current reporting period, or they may submit assumptions for any previous quarters they are restating and resubmitting. Submitters can enter assumptions, but certifiers must complete the assumptions form before certification.
Follow these steps to submit certification assumptions to CMS:
From the Medicare Part B Average Sales Price homepage, click the Assumptions tab.
The Assumptions page opens, and defaults to the current quarter and year. Select the appropriate reporting period before clicking the Reasonable Assumptions tab. Refer to Figure 100.


Note: Click the Reporting Period (Required) tab in the top left to scroll through previous quarters.
Click the Reasonable Assumptions Form button.
The Reasonable Assumption Form window displays. The Module automatically defaults to the Reporting Period selected on the Assumptions default page with a Manufacturer Name (required) drop-down menu and empty required response fields.
Refer to Figure 101.
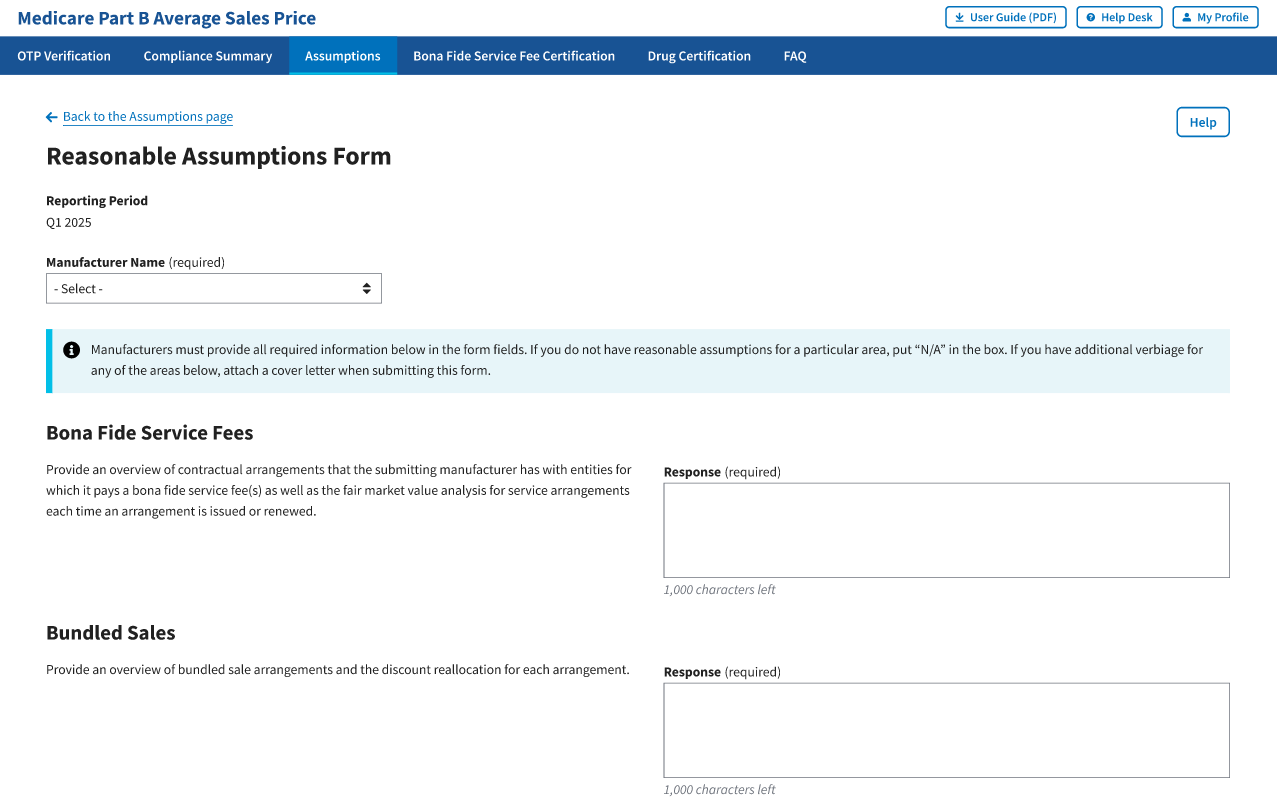
Figure 101: Reasonable Assumptions Form
From the Manufacturer Name (required) drop-down menu, click the -Select- drop-down menu to expand the list and select the manufacturer name.
Click “View All” to view all the required response fields. Refer to Figure 102.
Bona Fide Service Fees
Bundled Sales
Price Concessions and Discounts
Reporting of Products with Zero, Negative, or False Positive ASPs
Sales Excluded from Best Price
Sales to U.S. Territories
Time Value of Money
Free Goods Not Contingent on a Purchase Requirement
Value-Based Purchasing Agreements
Sales to 340B Covered Entities
Returned Goods
Billing Corrections
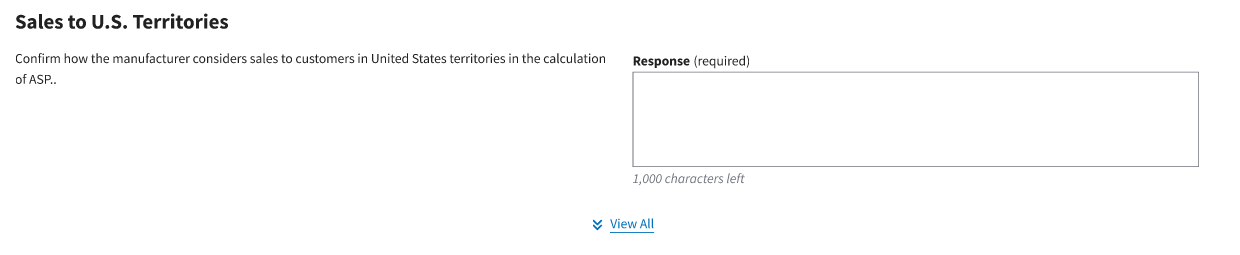
Figure 102: “View All” Required Response Fields
Complete all the response fields. Enter “N/A” if reasonable assumptions are not available for a particular field.
Note: Each required field allows for 1,000 characters of text to provide a summary of the assumption. If a response exceeds the character limit, please submit or upload the additional verbiage on the Other Assumptions tab. Refer to Section 3.7.2 for instructions.
Click the Save Form button located at the bottom of the form. Refer to Figure 103.

Figure 103: Save Reasonable Assumptions Form
A message displays confirming you have successfully created your Reasonable Assumptions. The Module lists saved forms under Added Forms. Refer to Figure 104.


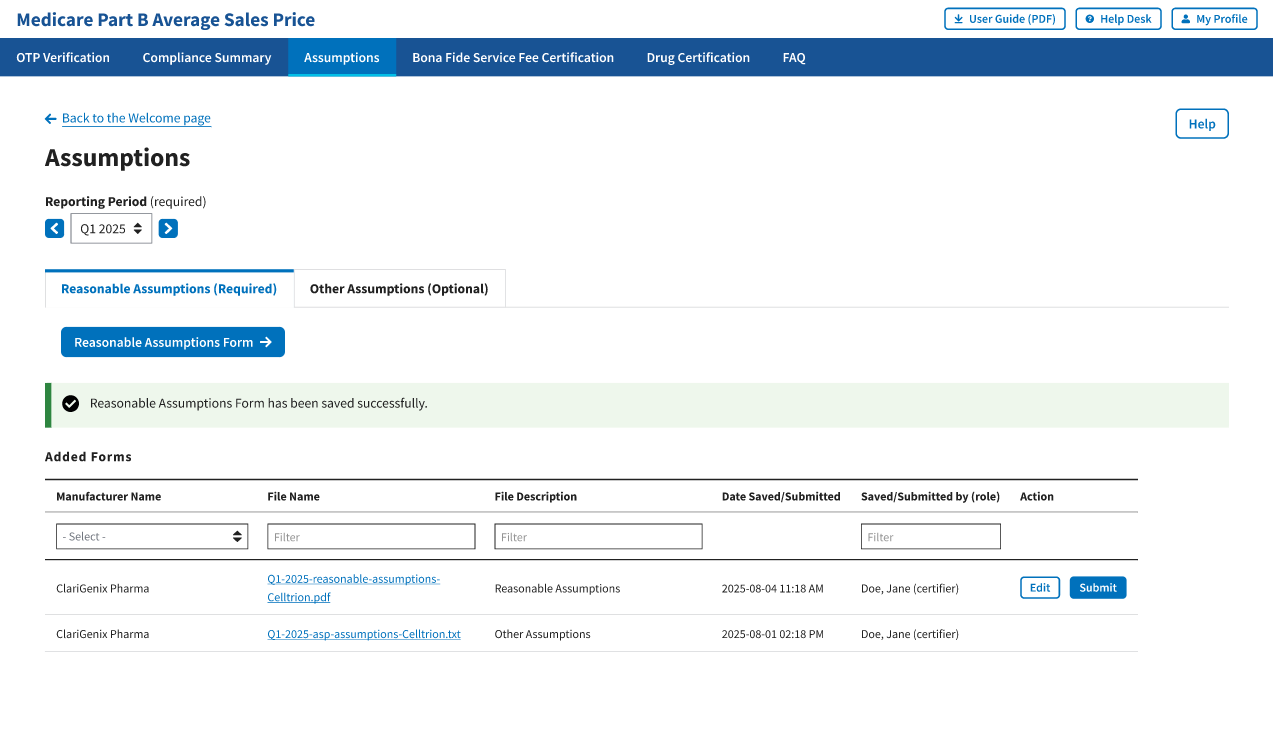
Figure 104: New Assumption Successfully Saved
To make any necessary revisions before submitting, click the Edit button.
If the submission does not require additional revisions, click the Submit button.
A message displays confirming you have successfully submitted your Reasonable Assumptions. Refer to Figure 105.


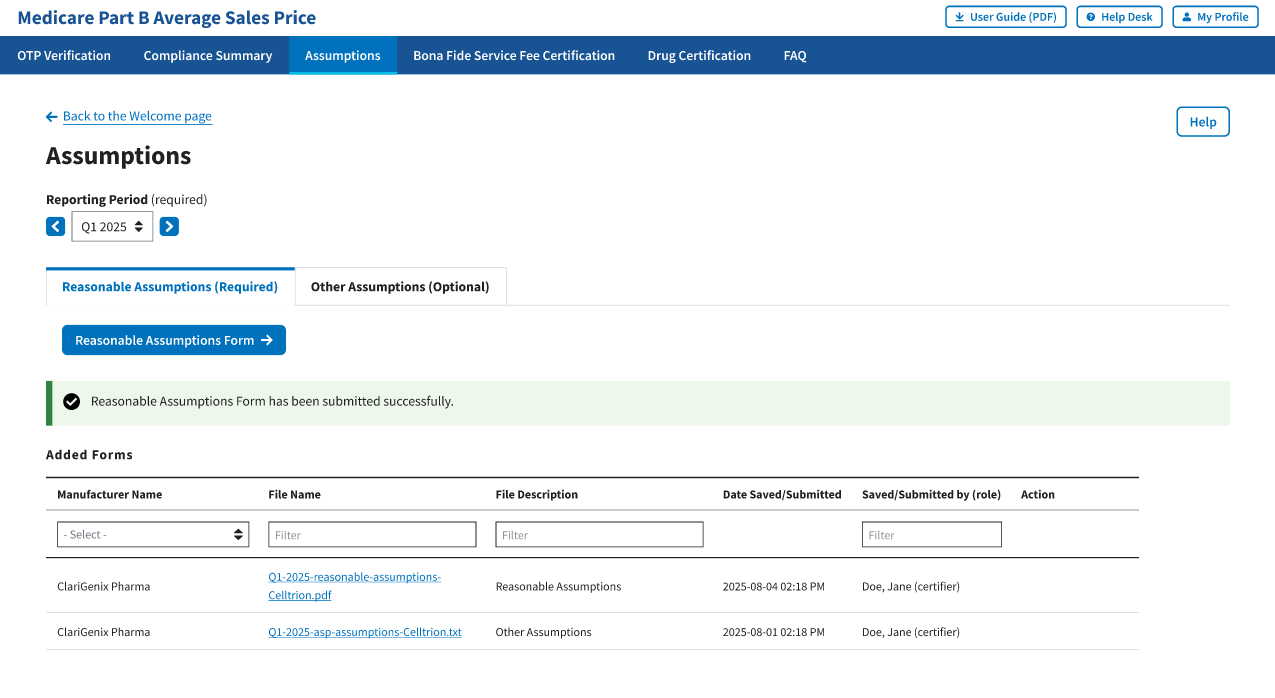
Figure 105: Reasonable Assumptions Successfully Submitted
This section provides instructions on how drug manufacturers can submit comments regarding their certifications to CMS via Create Assumptions or Upload Assumptions.
Create Assumptions
Follow these steps to create an assumption:
From the Medicare Part B Average Sales Price homepage, click the Assumptions tab. The Module automatically defaults to the Reasonable Assumptions tab. Click the Other Assumptions tab. Refer to Figure 106.


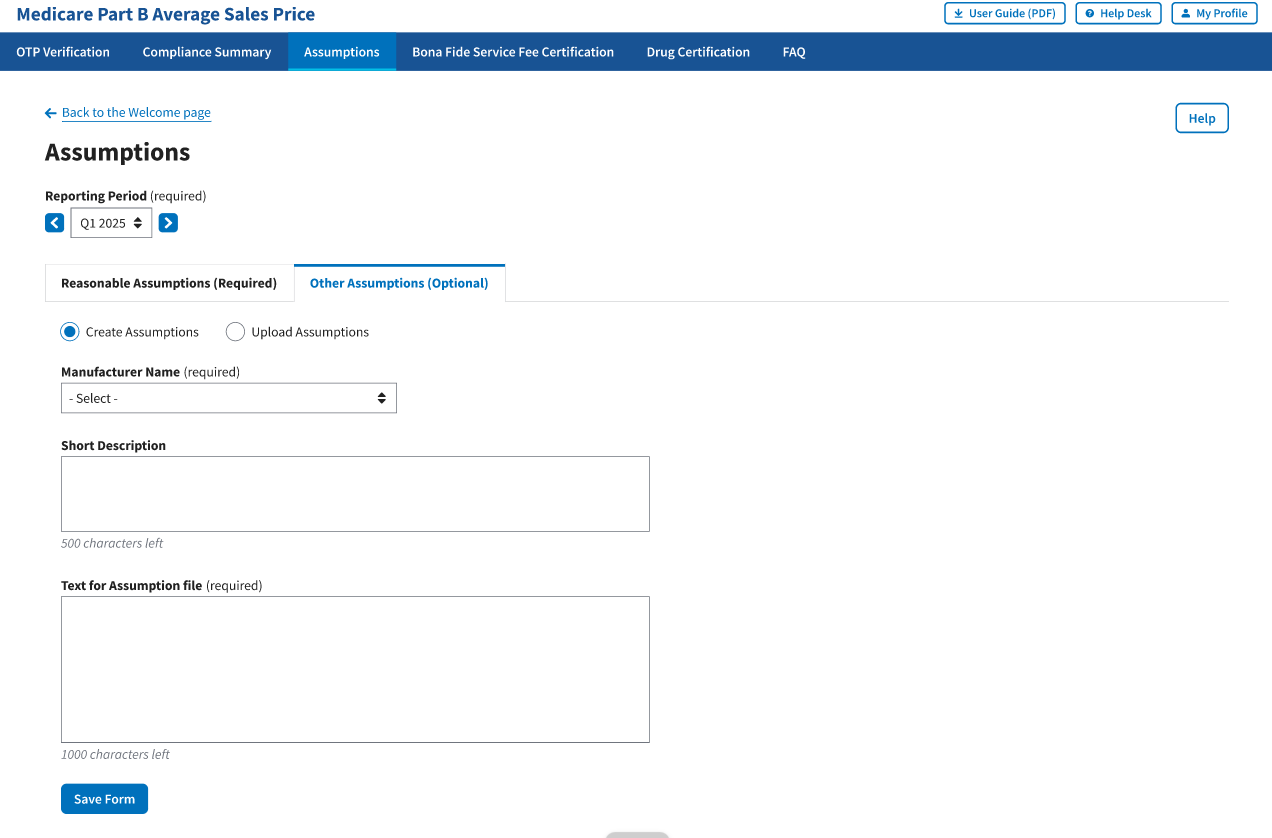
Figure 106: Create Other Assumptions
Note: Click the Reporting Period tab in the top left to view previous quarters. Use the drop-down menu to navigate to select the appropriate quarter.
Click the Other Assumptions file button.
. The Module automatically defaults to the Create Assumption radio button with a Manufacturer Name (required) drop-down menu and empty Short Description and Text for Assumption file fields. Refer to Figure 106.
From the Manufacturer Name (required) drop-down menu, click the -Select- drop-down menu to expand the list and select the manufacturer name.
Complete the Short Description and Text for Assumption file fields.
Note: The Short Description field is optional and allows for 500 characters of text to provide a summary of the complete assumption you are submitting to CMS. The Text for Assumption file field is required and allows for 1,000 characters to provide as much detail as possible related to the selected period’s financial submission.
Click the Save Form button.
A message displays confirming you have successfully created your Assumption. Refer to Figure 107.
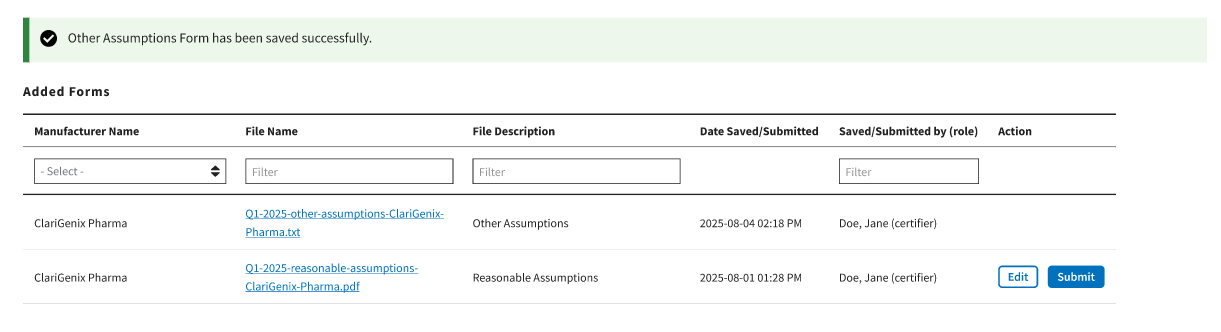
Figure 107: Other Assumptions Saved Successfully
Follow these steps to upload an assumption file to the Module:
Click the Other Assumptions file tab.
The Create Assumption or Upload Assumption File window displays. The Module automatically defaults to the Create Assumption radio button.
Click the Upload Assumption File radio button.
A Manufacturer Name (required) drop-down menu and empty File Description (required) field display. Refer to Figure 108.


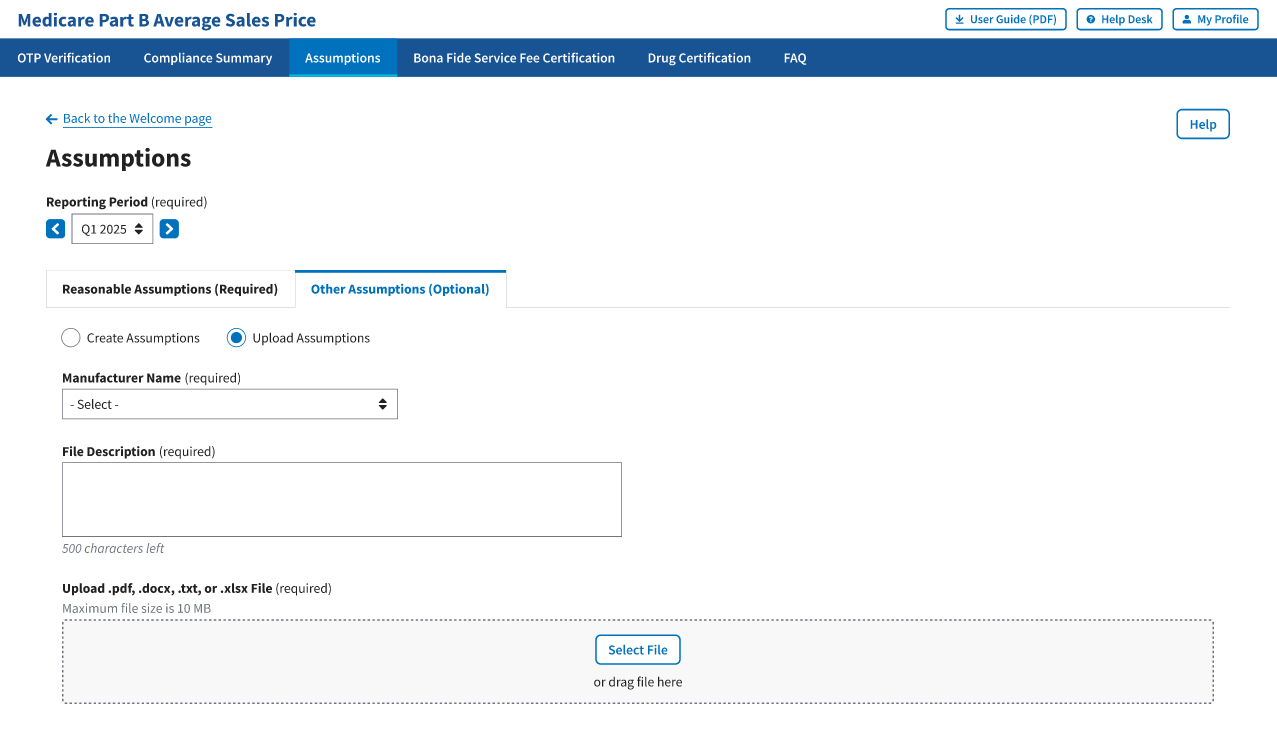
Figure 108: Upload Assumptions
From the Manufacturer Name (required) drop-down menu, click the -Select- drop-down menu to expand the list and select the manufacturer name.
In the File Description field, enter your assumption about a data submission. You have 500 characters of total text to comment about your submission in this section.
Click Select File to browse your desktop and upload your Assumption File to the Module. You may also drag your Assumption File into the Select File box.
A message opens to confirm you have successfully uploaded your Assumption File. Refer to
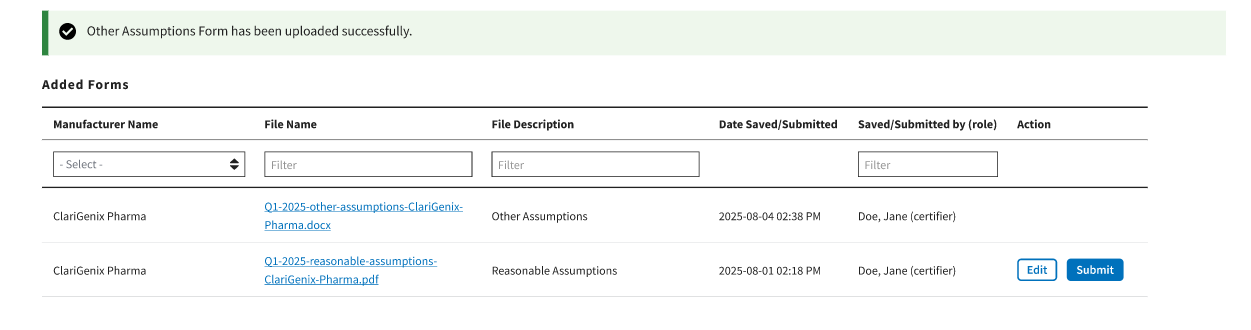
Figure 109: Upload Assumption File – Successfully Added
Contact the FFSDCS (ASP) Application Helpdesk for issues such as:
Account unlock
Password reset
Registration process questions
System availability escalations
Table 1 provides contact information for technical support.
Table 1: Technical Support Contacts
Email Address |
Phone Number |
Hours |
1-844-876-0765 |
9:00 a.m. to 6:00 p.m. Eastern Standard Time (EST), Monday through Friday |
Table 2 provides an overview of field definitions for this document.
Column/Field Name |
Format |
Allowed/Sample Values |
Required/Optional |
Notes |
Manufacturer Name |
Alphanumeric |
Maximum of 250 characters |
Required |
|
NDC1 |
5-digit number |
e.g., 12345 |
Required |
|
NDC2 |
4-digit number |
e.g., 1234 |
Required |
|
NDC3 |
2-digit number |
e.g., 12 |
Required |
|
Alternate ID |
alphanumeric |
maximum of 23 characters |
Required |
|
Alternate ID Website URL |
NA |
e.g., http://www.medicare.gov |
NA |
Must have http:// or https:// prefix. |
Brand Name |
Alphanumeric |
Maximum of 250 characters |
Optional |
Enter strength and package size in their respective fields unless it is a part of the registered brand name. |
Generic Name |
Alphanumeric |
Maximum of 250 characters |
Required |
Refer to valid values in Generic Name. |
Volume Per Item |
Numeric |
NA |
Required |
For Alternate ID, report the volume amount in one item. (For instance, enter 10 for 10 ml in one vial, and enter 1 for powders, sheets, or patches.) |
Unit for Volume per Item |
NA |
NA |
NA |
See valid value in Unit of Volume per Item. For example, for Alternate ID, select EACH for powders, sheets, or patches. |
Number of Items Per NDC or Alternate ID |
Numeric |
Maximum of 9 digits and 2 decimal places |
Required |
|
Package Type |
Alphanumeric |
2 characters |
Required |
Enter SD, MD, or NA. (SD = Single dose, MD = Multi dose, NA = Not Applicable) |
Strength |
Numeric |
e.g., 300 |
Required |
NA |
Unit for Strength |
NA |
NA |
NA |
See valid values in Unit for Strength |
FDA Application Number/Registration Number |
Alphanumeric |
Maximum of 6 characters |
Required |
|
FDA Application Supplement Number |
Alphanumeric |
Maximum of 9 characters |
Optional |
NA |
Additional FDA Application Number #1 |
Alphanumeric |
Maximum of 6 characters |
Optional |
NA |
Additional FDA Application Supplement Number #1 |
Alphanumeric |
Maximum of 9 characters |
Optional |
NA |
Additional FDA Application Number #2 |
Alphanumeric |
Maximum of 6 characters |
Optional |
NA |
Additional FDA Application Supplement Number #2 |
Alphanumeric |
Maximum of 9 characters |
Optional |
NA |
FDA Approval/Registration Date |
MM/DD/YYYY |
e.g., 01/01/2023 |
Required |
Must be prior to the current submission period start date. |
FDA Approval Type |
NA |
NA |
Required |
Refer to valid values in FDA Approval Type. |
First Marketing Date |
MM/DD/YYYY |
e.g., 01/01/2023 |
Required |
|
First Marketing Date (continued) |
MM/DD/YYYY |
e.g., 01/01/2023 |
Required |
|
Date of First Sale for this Product |
MM/DD/YYYY |
e.g., 01/01/2023 |
Required |
|
Table 3 provides a revision history for this document.
Version Number |
Date |
Author/Editor |
Description of Change |
1.0 |
03/15/2024 |
Index Analytics/DCCA |
Initial version of ASP Data Collection System Submitter User Guide |
2.0 |
07/11/2025 |
Index Analytics/DCCA |
|
Table 4 provides a list of terms, acronyms, and definitions in this document.
Expanded Form |
Acronym/Term |
Definition |
510(k) |
NA |
A 510(k) submission is the mechanism through which the majority of medical devices obtain U.S. marketing clearance. Such devices include catheters, contact lenses, and absorbable sutures. |
Abbreviated New Drug Application |
ANDA |
An ANDA is an application for a U.S. generic drug approval for an existing licensed medication or approved drug. Authorized generics do not require ANDAs. |
Average Sales Price |
ASP |
ASP refers to the price at which an organization typically sells a certain class of good or service. CMS uses manufacturer-reported ASPs, based on manufacturers’ actual quarterly drug sales, to calculate provider payment amounts for these drugs. Federal law defines the price. |
Biologics License Application |
BLA |
A BLA is used to request permission to introduce or deliver a biologic product into interstate commerce. |
Center for Medicare Management |
CMM |
The CMM oversees the fee-for-service Medicare program. |
Centers for Medicare & Medicaid Services |
CMS |
CMS is a federal agency within the U.S. Department of Health and Human Services that administers the Medicare program and works in partnership with state governments to administer Medicaid, the State Children’s Health Insurance Program, and health insurance portability standards. |
Consolidated Appropriations Act, 2021 |
CAA |
The CAA establishes protections for consumers related to surprise billing and transparency in health care. The No Surprises Act (NSA) is part of the CAA. |
Eastern Standard Time |
EST |
EST is the standard time in the 5th time zone west of Greenwich, reckoned at the 75th meridian. This time zone is in the eastern part of the United States. |
Fee-for-Service Data Collection System |
FFSDCS |
The FFSDCS is an instrument to collect cost, revenue, utilization, and other information for FFS claims. |
Human Cells, Tissues, and Cellular Products |
HCT/P |
HCT/Ps include human cells or tissue intended for implantation, transplantation, infusion, or transfer into a human recipient. The FDA Center for Biologics Evaluation and Research (CBER) regulates HCT/Ps. |
Interactive Voice Response |
IVR |
IVR is a technology that allows a computer to detect voice and DTMF keypad inputs. |
Medicare |
NA |
Medicare is the federal system of health insurance for people over 65 years of age and for certain younger people with disabilities. |
Medicare Part B |
NA |
Medicare Part B is the part of Medicare that covers doctor services, outpatient hospital care, and other medical services that Part A does not cover such as physical and occupational therapy, X-rays, medical equipment, or limited ambulance service. |
New Drug Application |
NDA |
An NDA is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical drug for sale and marketing. |
Okta |
NA |
Okta is an enterprise-grade, identity management service, built for the cloud, but compatible with many on-premises applications. |
One-Time Password |
OTP |
An OTP is a password that is valid for only one login session or transaction. |
Premarket Approval |
PMA |
PMA is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Such devices include implants, ventilators, and pacemakers. |
Short Message Service |
SMS |
SMS is a text messaging service component of phone, web, or mobile communication systems. It uses standardized communication protocols to allow fixed-line or mobile phone devices to exchange short text messages. |
Social Security Act |
SSA |
The SSA is a law that provides income to retired workers aged 65 or older. |
Uniform Resource Locator |
URL |
The URL is a global address of documents and other resources on the World Wide Web. |
Appendix D: Figures and Tables
Figure 1: Logging in Using MFA - ASP Module Login 2
Figure 2: Logging in Using MFA - Select MFA Device Type Drop-Down Menu 3
Figure 3: Logging in Using MFA - Multi-Factor Authentication - (IVR) Example 4
Figure 4: Logging in Using MFA - Multi-Factor Authentication - Verify MFA Code 5
Figure 5: Logging in Using MFA - My Portal Landing Page 5
Figure 6: Logging in Using MFA - My Portal Landing Page - FFSDCS Drop-down Menu 6
Figure 7: Logging in Using MFA - ASP Data for Drugs and Biologics Under Medicare Part B 6
Figure 8: Medicare Part B Average Sales Price Homepage 7
Figure 9: Manage NDC1/ALT ID Page - Assign NDC1 8
Figure 10: Manage NDC1/ALT ID Page - Assign NDC1 Drop-down Menu 9
Figure 11: Manage NDC1/ALT ID Page - Enter NDC1 Manufacturer Name 9
Figure 12: Manage NDC1/ALT ID - NDC1 Assigned Successfully 10
Figure 13: Manage NDC1/ALT ID Page - Assign ALT ID 11
Figure 14: Manage NDC1/ALT ID Page - Assign ALT ID Drop-down Menu 11
Figure 15: Manage NDC1/ALT ID Page - Enter ALT ID Manufacturer Name 12
Figure 16: Manage NDC1/ALT ID - ALT ID Assigned Successfully 12
Figure 17: Request New NDC1/ALT ID/Manufacturer/Generic Name Page 13
Figure 18: Request New NDC1/ALT ID/Manufacturer/Generic Name Page - Add New NDC1 13
Figure 19: Request New NDC1 - Field Filled 14
Figure 20: Request New NDC1 - NDC1 Successfully Added 14
Figure 21: Request New NDC1/ALT ID/Manufacturer/Generic Name Page - Add New ALT ID 15
Figure 22: Request New Alternate ID - ALT ID Field Filled 16
Figure 23: Request New Alternate ID - ALT ID Successfully Added 16
Figure 24: Request New Manufacturer Name 17
Figure 25: Request New Generic Name 17
Figure 26: Request New Manufacturer Name - Field Populated 18
Figure 27: Request New Generic Name - Field Populated 18
Figure 28: Request New Manufacturer Name - Successfully Added 19
Figure 29: Request New Generic Name - Successfully Added 19
Figure 30: Product Data - Main Drop-down Menu 20
Figure 31: Add/Update Product Data 20
Figure 32: Add/Update Product Data Fields Populated 23
Figure 33: Add/Update Product Data Successfully Added 24
Figure 34: Add Product Data by Alternate ID 25
Figure 35: Add Product Data by Alternate ID - Fields Populated 25
Figure 36: Add Product Data by Alternate ID - Additional Fields 27
Figure 37: Product Data by Alternate ID Added Successfully 28
Figure 38: Update Product Data - Drug Identifier & Manufacturer Name 28
Figure 39: Update Product Data by NDC 29
Figure 40: Update Product Data by NDC - Data Updated Successfully 30
Figure 41: Update Product Data by Alternate ID 30
Figure 42: Update Product Data by Alternate ID - Drug Identifier Drop-down Menu 31
Figure 43: Update Product Data by Alternate ID - Updated Successfully 32
Figure 44: Upload Product Data - New or Corrected 33
Figure 45: Upload Product Data - Uploading Files from Desktop 33
Figure 46: Upload Product Data - New File Successfully Uploaded 34
Figure 47: Upload Product Data - Uploaded Files 34
Figure 48: Upload Product Data - Full Report of Transmitted Drugs via File Upload 35
Figure 49: Upload Product Data - Reported Rejection Details 36
Figure 50: Product Data - View Active Drugs 37
Figure 51: Product Data - View Expired Drugs 38
Figure 52: Financial Data - Main Drop-down 38
Figure 53: Add/Update Financial Data 39
Figure 54: Add/Update Financial Data 505(b)(2) 40
Figure 55: Add/Update Financial Data 505(b)(2) Products List 40
Figure 56: Add/Update Financial Data 505(b)(2) Products List 41
Figure 57: 505(b)(2) Confirmation 41
Figure 58: Add/Update Financial Data 505(b)(2) Successfully Updated 42
Figure 59: Add/Update Financial Data - Drug Identifiers With Missing or Incorrect Data 43
Figure 60: Add/Update Financial Data Successfully Added 44
Figure 61: Add/Update Financial Data - Error Menu 44
Figure 62: Add/Update Financial Data - View Errors/Warnings Page 45
Figure 63: Add/Update Financial Data - Successfully Updated 45
Figure 64: Add/Update Financial Data - Certified Drugs 46
Figure 65: Add/Update Financial Data - Certified Drugs More Information 47
Figure 66: Upload Product Data - New or Corrected 47
Figure 67: Upload Financial Data - Uploading Files From Desktop 48
Figure 68: Upload Financial Data Page - New File Successfully Uploaded 49
Figure 69: Upload Financial Data - Uploaded Files 49
Figure 70: Upload Financial Data - Report of Transmitted Drugs via File Upload 50
Figure 71: Upload Financial Data - Reported Rejection Details 51
Figure 72: Financial Data - Main Dropdown 52
Figure 73: Financial Data - Add/Update Restate Financial Data 52
Figure 74: Add/Update Restate Financial Data - Drug Identifier Drop-down 53
Figure 75: Add/Update Restate Page - Review Restatement List 53
Figure 76: Add/Update Restate Page - Restate Data Successfully Saved 54
Figure 77: Financial Data - Main Drop-down 55
Figure 78: Upload Financial Data for Prior Quarters Restate Financial Data 55
Figure 79: Upload Financial Data for Prior Quarters - Uploading Files From Desktop 56
Figure 80: Upload Financial Data for Prior Quarters - New File Successfully Uploaded 56
Figure 81: Upload Financial Data for Prior Quarters - Uploaded Files 57
Figure 82: Upload Financial Data for Prior Quarters - Report of Transmitted Drugs 58
Figure 83: Upload Financial Data for Prior Quarters - Reported Rejection Details 59
Figure 84: Compliance Summary 60
Figure 85: Compliance Summary - Add Data Screen 61
Figure 86: Compliance Summary - Successfully Saved 62
Figure 87: Compliance Summary - All Pending Certification 62
Figure 88: Compliance Summary - Pending Certification 63
Figure 89: Compliance Summary - Pending Restatement Certification 64
Figure 90: Compliance Summary - All Certified 65
Figure 91: Compliance Summary - Certified 66
Figure 92: Compliance Summary - Restated and Certified 67
Figure 93: Compliance Summary - New 68
Figure 94: Compliance Summary - Off Cycle 69
Figure 95: Compliance Summary - Expired 70
Figure 96: Generate One-Time Password 71
Figure 97: Generate One-Time Password - Manufacturer Name 71
Figure 98: Generate One-Time Password - Password Created 72
Figure 99: Generate One-Time Password - Password Copied 72
Figure 101: Assumptions - Create Assumption or Upload Assumption File 74
Figure 102: New Assumption Successfully Created 75
Figure 103: Upload Assumption File 75
Figure 104: Upload Assumption File - Expanded Fields 76
Figure 105: Upload Assumption File - Uploading Files from Desktop 77
List of Tables
CMS Proprietary
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Average Sales Price (ASP) Submitter User Guide |
| Subject | Submitter User Guide for the ASP Module |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2026-01-09 |
© 2026 OMB.report | Privacy Policy