Summary and Response to Public Comments
Responses_2007 Part D Reporting Requirements_082006.doc
Medicare Part D Reporting Requirements
Summary and Response to Public Comments
OMB: 0938-0992
Responses to Public Comments on
CMS-10185 - 2007 Part D Reporting Requirements
6/16/06-8/15/06
Background:
Draft 2007 Part D reporting requirements were posted to the CMS website on June 16, 2006 for public comment. The document that follows reflects a summary of comments and questions received by close of business on August 15, 2006, and our responses to these questions and concerns. Comments received after August 15, 2006 were also reviewed, and found to be either duplicative to other comments received, or include requests for changes outside the scope of these reporting requirements. Final reporting requirements will be released by October 2006 pending final OMB approval.
General:
CMS received two requests to make data submitted for these reporting requirements publicly available. One commenter specified that these data should be released within two months of the quarterly due dates. It was urged that these data are publicly released prior to the fall 2007 open enrollment season.
Response: CMS is currently developing Part D performance metrics which will utilize various data sources in an effort to provide consumers more useful information about better quality of care and lower costs. Data may be obtained from sources other than Contract-reported data. Results are expected to be displayed on the Medicare Prescription Drug Plan Finder during open enrollment.
A commenter urged CMS to establish consequences for submission of erroneous data, or the failure to submit data to CMS.
Response: Part D Contracts who fail to satisfy their contractual obligation to provide data per CMS’ timelines presently face repercussions, including corrective action plans and sanctions. CMS agrees the accuracy of these data is critical for Part D monitoring. Evaluations of these data include identifying potential data errors or outliers. Follow-up is conducted with the respective Part D Contracts in order to resolve these data issues.
It was suggested that CMS require non-numerical data in the future, such as which drugs are restricted by utilization management or transition requirements.
Response: CMS appreciates this suggestion, and it will be taken into consideration for 2008 reporting requirements.
A suggestion was made to develop measures to assess the accuracy of information provided by Part D Contracts to beneficiaries.
Response: While this is an important component in determining customer service, these reporting requirements may not provide the best method for collecting this information. CMS believes other systems can help capture this information, such as complaints received by 1-800 Medicare and Regional offices.
CMS received a few requests to modify the level of reporting for these reporting requirements from plan-level to contract-level whenever appropriate in order to allow better reconciliation and comparison detail, as well as improving the usefulness of the data provided. A commenter stated grievance and appeals systems are developed at a contract level, while data are required at plan-level. The commenter also stated the Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions reporting section required plan-level reporting.
Response: The revised 2007 reporting requirements document includes changes to allow submission of data at sponsor, contract or plan (PBP) level depending on the level of detail necessary for program oversight and monitoring. In early August 2006, CMS revised the Medication Therapy Management (MTM) reporting section from plan-level to contract-level reporting. While CMS understands there are contract-level operational systems, there are other factors that may result in differences across plans within the same contract that would be masked if contract-level data are submitted (e.g. formulary). CMS wishes to clarify that the Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions reporting section allows data to be submitted at either the Part D Sponsor (parent organization) or Part D Contract. Lastly, CMS will use the following terminology to ensure consistency in these reporting requirements:
Part D Sponsor –a parent organization which encompasses a group of Part D Contracts.
Part D Contract – an organization contracted with CMS to provide Part D benefits to Medicare beneficiaries (e.g. H#)
Part D Plan – a plan benefit package (PBP) offered within a Part D contract (e.g. Plan ID #)
Commenters urged CMS to remove reporting requirements which appear to provide duplicative information from internal CMS sources.
Response: For CY 2007, CMS will strike the Enrollment/Disenrollment reporting section, as information is available via CMS enrollment systems. The remaining sections collect data necessary for monitoring and oversight, and are not duplicative to other information available. For example, the Appeals reporting section provides data which are not available from the Independent Review Entity (IRE), such as the total number of redeterminations requested. Submission of PDE claims data may vary among Part D Contracts throughout a contract year, and therefore the Generic Dispensing Rate and Drug Benefit Analyses reporting sections provide regular data that are otherwise unavailable.
It was suggested that CMS provides the final CY 2007 reporting requirements document as soon as possible in order to allow time for system modifications and testing. Additionally, it was recommended that a red-line comparison between CY 2006 and CY 2007 is provided to provide a quick overview of changes. Another commenter asked if an additional comment period for these requirements will occur.
Response: Final 2007 Part D reporting requirements will be distributed in October pending OMB final approval. Part of OMB’s approval process includes an additional 30 day public comment period. A summary table listing all changes made for CY 2007 will be included in the revised reporting requirements.
It was suggested that CMS further explores opportunities for automating the data submission process, including the ability to upload files (e.g. flat files, Excel files) instead of manual entry into HPMS.
Response: CMS is investigating the automation of data entry for Part D reporting requirements, and will incorporate this change for as many reporting sections as possible for CY 2007 reporting.
A commenter suggested that CMS require Contracts to submit the name of the PBM that serves as the claims processor for the Part D Contracts. Retail pharmacies feel that this is critical information necessary for the claims reconciliation process.
Response: CMS has added a reporting section to allow Part D Contracts to update information pertaining to companies performing Part D functions and activities, including claim processing. At this time, this information will not be released publicly.
Enrollment/Disenrollment:
CMS received many comments regarding this section. A commenter noted some data elements are primarily obtained by Contracts from CMS data, and therefore recommended these data elements are removed. Several comments were related to the additional reporting for low-income subsidy (LIS) and non-low-income-subsidy (non-LIS) data. Some suggested due to potential changes in LIS status, Contracts should report data as of the close of the reporting period for each enrolled beneficiary. Others noted separation of LIS versus non-LIS data increased the reporting burden without providing additional value to the data collected. Other commenters requested CMS collect LTC residents’ enrollment and disenrollment data in addition to the current data elements.
Response: Based on these comments and other considerations, CMS will strike the Enrollment/Disenrollment reporting section for CY 2007, as information is available via CMS enrollment systems.
Reversals
One commenter suggested that CMS require Contracts to report the number of claim reversals related to inappropriately-assigned co-pays for residents of long-term care facilities.
Response: CMS appreciates this suggestion, and will consider it in the future. At this time, the information collected from Part D Contracts relates to those claims reversed outside of the Contract’s billing cycle. No information is currently collected regarding the reason for the reversal, e.g. inappropriate assignment of co-pays for LTC residents.
Generic Dispensing Rate
A commenter suggested CMS collect the rate of generic dispensing in retail settings as well as long-term care settings.
Response: This suggestion will be considered in the future. As this information is collected from claims data, Part D Contracts may not be able to accurately map data to either the type of dispensing pharmacy (as retail pharmacies can provide prescriptions to long-term care facilities) or the long-term care status of an enrollee. CMS will continue investigating if this type of information can be obtained from other sources.
Medication Therapy Management Programs
CMS received comments about data element A regarding the MTM enrollment method, and a request to clarify the combination of opt-in and opt-out.
Response: Part D Contracts provided their MTM enrollment method as part of their 2007 MTM application. Method of enrollment may be opt-in, opt-out, a combination of opt-in and opt-out, or other, since the combination of opt-in and opt-out was listed in some MTM applications. Contracts should report this information as submitted in their CMS-approved MTM application.
CMS was asked how plan to plan (P2P) transfers may affect this reporting section, and whether TDS transferred from other plans should be included when targeting members for MTMP.
Response: For plan to plan transfers within the same Part D contract, Contracts should not report duplicative information (e.g. double-count the same beneficiary as being offered MTM) as data are reported at contract level. In general, Contracts are responsible for reporting to CMS information pertaining to their identification and offer of MTM to eligible enrollees during each reporting period. For this reporting section, the second reporting period encompasses the full calendar year. If a beneficiary enrolled in Period 1 is not identified as MTM eligible until Period 2, this data should be reported in Period 2.
A commenter noted that data element E may include death data available from CMS, and should be removed. Two other commenters stated that data elements E and F related to discontinuation from MTMP would be difficult to report.
Response: The Social Security Administration provides death notification. Additionally, CMS does not have beneficiary specific information about MTM programs, such as enrolled beneficiaries, in order to match death data to participants. Discontinuation due to death provides a more comprehensive picture to the overall discontinuation rates. These data should be available to Part D Contracts, and are necessary as part of CMS’ monitoring of MTMPs.
Another commenter asked if data element E is a subset of data element D.
Response: Yes, data element D is the total number of beneficiaries discontinuing their participation from the MTMP. Data elements E, F and G are subsets of this total, as they specify reasons for this discontinuation (e.g. death, disenrollment from the Contract). A beneficiary discontinuing participation from the MTMP due to death is captured separately from beneficiaries discontinuing due to their disenrollment from the Contract. It is possible that data elements E, F and G do not account for all beneficiaries discontinuing their participation from the MTMP.
A commenter requested clarification on the difference between data elements G and H.
Response: Data element G is the number of beneficiaries that request to be discontinued from the MTMP after participating in the MTMP. Data element H is the number of beneficiaries who decline the opportunity to participate in the MTMP.
CMS received a question about the value of data element I, the total prescription cost of all medications on a per MTMP beneficiary per month basis.
Response: This data element helps to generally characterize participation along with the other MTM data elements. Since one of the MTM eligibility criteria is "likely to incur annual costs of at least $4000 for all covered Part D drugs", this information may be helpful to assess adherence to this criteria. These data could also be useful in the setting future thresholds by the Secretary. Overall, these data provide valuable insight on potential effects of MTM services to a MTMP beneficiary’s prescription costs. It should be clarified that the denominator of this calculation is the total number of member months for the MTM participating beneficiaries. Member months should include all months enrolled in the Part D Contract during the reporting period specified, not only the months that the beneficiary enrolled in the MTMP.
Two commenters requested a formula for data element J. A suggestion for an algorithm was provided by one commenter. It was also asked how Part D Contracts should account for 10-day prescriptions (e.g. medications). Another commenter asked if this data element should capture all Part D medications or just medications related to the MTM program.
Response: CMS appreciates the suggestion to include an algorithm, and will include the following description and formula in the revised reporting requirements document.
For beneficiaries participating in the MTMP as of the last day of the reporting period specified, provide the number of covered Part D 30-day equivalent prescriptions on a per MTMP beneficiary per month basis. This should be a numeric field.
This amount should be calculated by first summing days supply of all covered Part D prescriptions dispensed for beneficiaries participating in MTMP as of the last day of the reporting period, and dividing by 30 to determine the number of 30 day equivalent prescriptions dispensed. This number is then divided by the total number of member months for the included beneficiaries. These member months should include all months enrolled in the Part D Contract during the reporting period specified, not only the months that the beneficiary enrolled in the MTMP.
The following equation also describes this calculation:
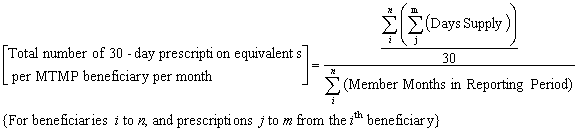
Using this formula, a 10 day supply is counted as a 0.30 30-day equivalent. All covered Part D medications dispensed to MTM participating beneficiaries should be included in this calculation.
A commenter asked if data elements I and J should be limited to only Part D drugs.
Response: Yes, both data elements I and J should be limited to covered Part D drugs. CMS will make this clarification in the revised reporting requirements document.
It was suggested that Part D Contracts report to CMS the participation of beneficiaries residing in long-term care facilities.
Response: CMS will consider this suggestion as a future reporting requirement. At this point, however it is not clear what data are available to Contracts for timely and accurate reporting of LTC status.
Commenters recommended CMS expand this reporting section in order to collect more robust data set for MTMP outcome evaluations. One commenter requested reporting of the number and types of providers under each Part D Contract offering MTMP, the amount of payments being made for each service being provided by category of service and provider, and the scope and number of services offered by each provider type. A commenter suggested that CMS consider the Ambulatory Quality Alliance (AQA) starter set of metrics for measures of medication adherence and persistence. Other suggested measures included patient safety and improved quality. Another commenter specifically recommended that data are collected in the following domains:
MTMP design including criteria for inclusion and exclusion
Population level summaries of MTM participants’ demographics, medication risk assessments, medication related problem assessments
Average MTM participant medication costs and number of medications at program enrollment and each subsequent reporting period
Adverse events and medication errors among MTM participants
Reduction in treatment costs based on medical claims among MTM participants
Response: CMS supports the evolution of MTMP as best practices are identified past the initial few years of the Medicare Part D benefit. Overall, the Part D reporting requirements may not be the best method to collect this information. These reporting requirements are limited by the wide range of MTMPs currently offered by Part D Contracts since data required by CMS must be available by all Part D Contracts. Suggestions for data elements that involve utilization of medical claims can therefore not be added to these reporting requirements, as PDPs are unable to access these data. Some of the suggestions also would include information duplicative to that already included in Part D Contracts’ MTM applications. Further, CMS cannot require Part D Contract to report data for outcomes that were not mandatory for all MTMPs’ designs, e.g. reduction of adverse events and medication errors. The 2007 Reporting requirements already include data elements to collect average medication cost and number of medications of MTM participants during each reporting period. CMS believes this longitudinal data are more valuable than collecting drug utilization information at the time of initial program participation. Lastly, it should be noted that CMS helped found a Pharmacy Quality Assurance (PQA) in April 2006, which is modeled after AQA. This stakeholder-led group is intended to promote high-value pharmacy services, including measurement approaches. PQA is helping to standardize measures that could help inform MTM processes in the future.
Grievances
CMS received a comment that grievance data are available through the IRE, and therefore this section should be removed.
Response: This comment is inaccurate. The IRE does not receive grievance data from Contracts.
A commenter requested clarification if a grievance should be categorized after investigation to confirm the nature of the grievance.
Response: As stated in the FAQ released mid-August, CMS clarified that Contracts may report grievances in the categories as determined by the Contracts after initial investigation. Contracts should not dismiss or exclude any grievances filed by beneficiaries from this reporting section.
Several comments were received regarding data element I, the number of transition grievances received related to Part D. One commenter stated that most transition grievances are related to formulary exceptions, and could therefore be duplicative to data element J. A commenter stated that appeals and grievances data do not identify beneficiaries who are in the transition period versus regular beneficiaries. In addition, members may not know they are in the transition period to be able to self-identify themselves.
Response: From these comments, CMS has determined this data element will be removed from the revised reporting requirements document.
A commenter stated that data elements J and K are invalid since Contracts do not deny a request for an expedited exception or appeal.
Response: MMA allows Contracts to deny requests for expedited exceptions or appeals. Contracts who have not denied any expedited requests during a reporting period should report zero for these two data elements.
A few commenters stated that data element H is duplicative as these data are reported by the QIOs directly to CMS.
Response: Grievances received by QIOs regarding quality of care is only one example. Contracts may receive these grievances directly from beneficiaries.
A commenter noted that data element J is not tracked or recorded.
Response: As these data are necessary for oversight and monitoring of the Part D Program, CMS requests Part D Contracts develop systems in which to report these data if none exist currently. The subcategory of exception grievances was added in order to allow more accurate reporting of all grievances received (these previously would have fallen into the other grievance subcategory).
Many comments were received regarding data elements N, O, and P. Some stated these data were currently not collected or reported at the PBP level in the appeals and grievance database. One commenter asked if data element N should be equivalent to the total number of grievances in compliance with CMS guidance. Another requested clarification for data element P regarding the method of inputting the average number of hours. It was suggested that CMS provides a description for data element P, the average number of hours for the Contract to complete disposition and notification of all expedited grievances. Another commenter requested this data element is revised to be the number of days, instead of hours.
Response: From these comments, CMS has determined that data elements N, O, and P will be removed from the revised reporting requirements document.
CMS received a suggestion to add a subcategory of grievances submitted by residents of LTC facilities, separate from the current subcategories of grievances which relate to the area of the grievances submitted.
Response: CMS appreciates this suggestion, and will consider incorporating this in the future. Currently, the intent of this reporting section is to monitor the areas of grievances submitted to Contracts, as well as monitor Contracts’ compliance to MMA timeframes for responses to grievances. Cross-cutting these data based on beneficiaries’ LTC status may not provide additional benefit for Part D oversight.
Pharmacy & Therapeutics Committee
CMS was asked why P&T members’ date of birth is a necessary data element for reporting.
Response: This information is needed for comprehensive monitoring and oversight of P&T committee members.
One commenter stated information from this reporting section is already included in the contract’s bid, and suggested this section is deleted. The commenter suggested instead that CMS require any Part D Contracts with P&T changes to notify CMS.
Response: This reporting section does not require Part D Contracts to report duplicate information from the bids. Only changes to P&T committees (e.g. additions or deletions of members) will be reported. Part D Contracts who have no changes will be able to indicate this fact.
One commenter suggested CMS solicit Part D Contracts to identify specialized geriatric certifications of P&T Committee members.
Response: As specialized geriatric certification is not a MMA requirement of P&T committee members, this information cannot be collected within these Part D reporting requirements.
It was requested that CMS allow submission of hard copies for P&T reporting as opposed to using the HPMS upload process to protect confidentiality.
Response: CMS recognizes the importance of maintaining confidentiality of these records. Electronic submission of these data will help CMS limit access to those who have appropriate use or oversight role and to track those who have accessed these records. Additionally, CMS will provide methods other than HPMS data submission for those Part D Contracts restricted by contractual limitations from providing these data.
Transition
A request was made to require reporting of Part D Contracts’ transition policies. This request was based on interest in having Contracts’ transition policies publicly available.
Response: CMS appreciates this comment however this is out of the scope of Part D reporting requirements. This information is provided to CMS directly as part of the Part D application, and requiring re-submission would be duplicative. Further, these policies are proprietary information, and at this time, CMS has no plans to release this information.
It was requested that CMS define newly enrolled members.
Response: A newly enrolled member is a member whose starting effective date is within the time period reported.
CMS received a suggested revision for data element C.
Response: CMS will revise this data element to be: Number of enrollees receiving one or more prescriptions authorized during transition periods within the reporting time period. This should be a numeric field.
A commenter stated data elements B and C are only possible if the claim receives an edit or override code indicating the drug was issued as part of the transition policy. The commenter also the level of difficulty required by data element C will be greater than the benefit of the data. A commenter suggested that CMS clarifies its expectations of health plans if inaccurate data are reported for data elements B and C. It was asked if data elements B and C should include all Part D drugs.
Response: CMS feels Part D Contracts are able to provide these data with currently available systems. Merging more than one data set may be required, for example claims records with enrollment data. CMS believes this information is extremely important to ensure Medicare beneficiaries are receiving appropriate support during their transition into Part D. Sanctions may be imposed on Part D Contracts who fail to comply with these reporting requirements. Noncompliance includes the failure to submit data, and the submission of inaccurate data. Data elements B and C should include all Part D drugs dispensed during transition periods.
CMS received a suggestion to require Contracts report transition data for ambulatory beneficiaries and long-term care residents separately.
Response: This suggestion will be considered in the future. As this information may be collected from claims data, Part D Contracts may not be able to accurately map data to either the type of dispensing pharmacy (as retail pharmacies can provide prescriptions to long-term care facilities) or the long-term care status of a member. CMS will continue investigating if this type of information can be obtained from other sources.
A commenter suggested these additional data elements related to the continued coverage of prescriptions after the transition period. Specifically, it was recommended that data on exceptions filed after transition periods, and rates of therapeutic substitution rates are collected.
Response: CMS agrees additional details about new enrollees transitioning into Part D would be useful; however collection at this time would be burdensome for Part D Contracts. CMS will consider other methods of obtaining this information in the future.
Clarification was requested if these data elements are intended to capture prescriptions filled due to a transition policy, or filled during a transition period.
Response: Part D Contracts should report the number of prescriptions filled during all newly enrolled beneficiaries’ transition periods. This clarification will be made in the revised reporting requirements document.
It was asked what value was provided by this reporting section, as transition periods were more important in early 2006. The commenter recommended due to the questionable consistency and accuracy of data, this section was removed.
Response: CMS continues to be very interested in transition of Medicare beneficiaries into Part D plans beyond 2006. For example, dual-eligible beneficiaries may elect to change Part D plans as often as every 30 days. These data are necessary to evaluate the consistency of care provided.
Prior Authorization, Step Edits, Non-formulary Exceptions and Tier Exceptions
CMS received a suggestion to require Contracts report exceptions data for ambulatory beneficiaries and long-term care residents separately.
Response: This suggestion will be considered in the future. As this information is collected from claims data, Part D Contracts may not be able to accurately map data to either the type of dispensing pharmacy (as retail pharmacies can provide prescriptions to long-term care facilities) or the long-term care status of a member. CMS will continue investigating if this type of information can be obtained from other sources.
A commenter recommended that CMS collect data on quantity limits, in addition to the current utilization management tools.
Response: CMS agrees data about the utilization of quantity limits would be useful in monitoring Part D Contracts’ formulary management. The revised reporting requirements document reflects the addition of these data elements, and the renaming of this reporting section as Exceptions.
Appeals
Three commenters requested CMS provide an example of a partial reversal for data element G.
Response: CMS has provided the following description of a partial reversal in the introductory paragraph of the Appeals reporting section.
Example of a reversal of an original decision: Non-formulary exception request approved upon redetermination for drug and quantity prescribed.
Example of a partial reversal of an original decision: Non-formulary exception request approved upon redetermination for drug, but full quantity prescribed is not approved.
A commenter requested clarification to data element H, and also suggested this type of information is more applicable for coverage determinations, and not redeterminations.
Response: CMS will revise this data element to include this statement: Examples of insufficient evidence of medical necessity may include, but are not limited to, when the Contract does not receive the information, or the information received does not support medical necessity. CMS agrees this information may also be useful in the future for monitoring Contract’s exceptions processes, and consider it as an additional data element for the Exceptions reporting section.
Several commenters noted that data elements Q and R should be revised to be reported as the average numbers of days for redeterminations. Another commenter recommended these data are reported in hours and days, rounded to the nearest hour. It was asked if data elements Q and R refer to the Contract as the Contract, PBM, or IRE. Another commenter requested these data elements are removed as CMS already captures these data, and because reporting would be labor intensive and onerous when compared against the analytical value.
Response: After considering all comments, CMS will strike data elements Q and R from the revised reporting requirements document.
A commenter stated that they do not track adverse redeterminations due to insufficient evidence of medical necessity.
Response: As these data are necessary for oversight and monitoring of the Part D Program, CMS requests Part D Contracts develop systems in which to report these data if none exist currently. This data element was added to allow more accurate reporting of Part D Contracts’ redeterminations. Once these reporting requirements become effective for CY 2007, sanctions may be imposed on Part D Contracts who fail to comply with these reporting requirements.
It was suggested that CMS request information about the number of appeals completed by Part D Contracts outside of the MMA mandatory timeframes. This is based on concerns that some Part D Contracts are not forwarding these cases to the Independent Review Entity (IRE) as required.
Response: Data collected for this type of reporting requirement may not be reliable or accurate. Moreover, including this as a reporting requirement may incorrectly give an impression that it is an acceptable practice for Contracts to routinely make decisions outside of the MMA timeframes. Instead, CMS believes this information may be more accurately obtained via audit, and will incorporate this evaluation into the Part D Audit process.
Call Center
CMS will suspend reporting by Part D Contracts for the Call center reporting section through 3rd quarter 2007 due to CMS’ direct monitoring of Part D call centers. All Part D Contracts are required to continue collection of these data, in the event CMS reinstitutes call center data submission via HPMS. Additionally, all comments received regarding this section were considered and necessary changes were made to the Call center reporting section. These changes will be effective if HPMS reporting resumes.
A few commenters noted differences between MA-PD and PDP call centers. Two commenters suggested MA-PD Contracts should be waived from reporting Part D calls separately. One commenter noted that PDP and MA-PD call centers are different, and therefore standardized reporting is not appropriate.
Response: The 2006 call center reporting section states all Part D Contracts should track and report calls related to Part D separately. CMS, however recognized that this separation may not be available in some current call center systems. HPMS therefore allows Contracts to indicate if data are reported based on a dedicated Part D line, or based on a line receiving a combination of Part D and non-Part D calls. MA-PDs unable to separate MA and Part D calls may indicate this fact.
A commenter requested that Call center data are reported at the Sponsor, or parent organization name, instead of the current level of reporting at Part D Contract or PBP.
Response: CMS agrees that this additional level of reporting should be available to provide flexibility around each Part D Contract’s call center structure. If a group of Part D Contracts are being serviced by one main telephone number at a large call center, the statistics of that call center’s performance should be measured instead of extrapolation to each Part D Contract’s call volume. It should be noted that the level of data reported will be used for performance monitoring and reporting as submitted to CMS. Part D Contracts, therefore, who submit data at the Part D Sponsor level will be considered as providing the equivalent level of call center service as all other Part D Contracts associated with that Sponsor.
Commenters voiced concerns around the collection of statistics for the Pharmacy support lines. One commenter was not aware that all Part D Contracts are required to provide Pharmacy support lines. Another asked if the definitions for the data elements for the Pharmacy support lines were identical to those for the Beneficiary support line. One commenter had concerns about potential operational changes necessary to separate Part D from non-Part D callers, and to provide plan-level data. Lastly, a commenter stated that collection of Pharmacy call center statistics is burdensome for smaller MA-PD Contracts.
Response: Early 2006, CMS established the requirement for all Part D Contracts to have Pharmacy support lines. The data elements associated with Pharmacy support lines are the same as those associated with Beneficiary support lines. CMS will allow Contracts to indicate if data are reported based on a dedicated Part D line, or based on a line receiving a combination of Part D and non-Part D calls. Contracts therefore unable to separate calls on a shared Pharmacy support line may indicate this fact. Further, this reporting section will allow submission of data at the Part D Sponsor (parent organization), Contract, or Plan (PBP) level. Plan-level data are not required. CMS believes this flexibility can accommodate current call center systems without introducing too great of a burden.
It was requested that CMS provides a definition for call abandonment.
Response: Contracts must report the total # of inbound Part D connections abandoned. The definition of a call abandoned: a call in which the person originating the call disconnects or cancels the call after a connection has been made, but before a live agent has answered.
Commenters requested that data elements E and F are clarified, due to similarities between average speed of answer and average hold time.
Response: In response to these comments, CMS will revise these data elements to collect average hold time for the Beneficiary Service and Pharmacy Support lines. The average speed of answer will not be collected for CY 2007.
Many commenters requested data elements I and J are removed as they require additional system enhancements for accurate tracking. At a minimum, a revision was suggested for data elements I and J in order to clarify the definition of a resolved call. One commenter requested these data are limited to cases where a CSR is required to perform the call back. One commenter noted that they would require additional sophisticated software in order to be able to report information for data element I.
Response: CMS acknowledges the concerns about these two data elements, and has removed them from the revised 2007 Reporting requirements document.
Two commenters asked if data elements K and L, average lengths of calls to Beneficiary and Pharmacy support lines, can be clarified to exclude the welcome message in an ACD, time to navigate IVR, or hold time prior to connection to a CSR. Other commenters suggested these are equivalent to average talk time.
Response: This clarification has been added to the revised reporting requirements document: Length of call is defined as the period of time between call connection and disconnection. All increments of the call should be included, such as time spent navigating the IVR.
A commenter asked for clarification to the due date table, and the statement that Part D Contracts will provide monthly data on a quarterly basis to CMS.
Response: CMS expects Part D Contracts measure these data elements on a monthly basis, and report three sets of monthly data on a quarterly basis.
It was asked if presale/prospective call are to be included in the call center metrics.
Response: The objective of this reporting requirement section is to evaluate customer service for the beneficiary. All customer service lines, whether for existing or prospective beneficiaries, should be summarized and included in Contracts’ reports to CMS.
Overpayments
One commenter requested clarification to the term overpayment.
Response: An overpayment occurs anytime Medicare directly, or through one of its contractors, erroneously makes a payment. The actual overpayment amount is the amount of money received in excess of the amount due and payable under the Part D drug benefit. Examples would include overpayments made to pharmacies, overpayments a Contract makes to a PBM for claims payment, and findings from pharmacy audits. This means any funds the Contract recovers from any entity it has overpaid. The term overpayment does not include premium overpayments. CMS has not made a policy decision regarding beneficiary liability generally or related to LIS determinations, therefore at this time Contracts should not include money related to beneficiary liability and debt collection.
Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions
One commenter requested the capability to upload a spreadsheet file of these data, due to the volume of data that may be required.
Response: HPMS already provides the functionality to upload these data via Excel files for CY 2006.
A commenter noted the CY 2007 Call letter indicated 100% of rebates and admin fees should be reported, regardless of the share retained by the PBM or other entities.
Response: CMS agrees that 100% of rebates and admin fees should be reported in this reporting section.
Pharmaceutical Manufacturer Access/Performance Rebates Received by LTC Pharmacies
CMS received many comments and concerns regarding the provision of these data. Concerns included LTC pharmacies’ difficulty in separating Part D rebates from other lines of business; clarification around access/performance rebates and CMS’ understanding of LTC pharmacy rebates being volume based as opposed to utilization based; rebate data being limited to Part D Contracts’ formulary drugs; potential inaccuracies if rebates are reported by drug name, versus drug units; potential violation of manufacturer rebate agreement confidentiality clauses; and potential risk to LTC pharmacy access if non-compliant LTC pharmacies are terminated. Other commenters requested the purpose of this reporting section, and information regarding how these data may be used. Another commenter asked why these data are not required for all dispensing pharmacies, e.g. retail and mail order. One commenter requested a consistent definition of “other price concessions”.
Response: The purpose of reporting these data to CMS was described in the CMS 2007 Call Letters. Submission of these data provides evidence that Part D Contracts are managing and monitoring drug utilization. These data will not be used for reconciliation purposes. In order to avoid adverse impact to LTC pharmacy access, CMS recommends that disclosure of these data is a contractual obligation between the LTC pharmacy and the Part D Contract. This recommendation was also included in the 2007 Call Letters. CMS expects LTC pharmacies will make appropriate allocations to separate Part D rebates and business from commercial and other lines of business. These allocations also provide CMS authority to receive Part D information. CMS recognizes the importance of maintaining confidentiality of these records, and will do everything within its authority to limit access to those who have appropriate use or oversight role. These data should include all Part D rebates, and not be limited to rebates received for formulary drugs. Changes have been made to the revised reporting requirements document to reflect reporting of rebates per unit. At this time, CMS is not collecting data related to other price concessions, which are discounts provided to the Part D Contract other than those offered via rebates.
Concerns were voiced regarding the frequency of LTC rebate reporting. The commenter recommended reducing the reporting to annually following 60-90 days after the close of the calendar year.
Response: Annual reporting of these data may not be adequate for CMS to determine that Part D Contracts are effectively managing and monitoring drug utilization by LTC beneficiaries. CMS believes, therefore, quarterly submission of data is necessary.
A commenter asked if long-term care included nursing homes and skilled care facilities.
Response: Yes, all Part D prescriptions dispensed to enrollees residing in nursing homes and skilled care facilities should be included.
It was requested that the level of reporting is at the Part D Contract level to allow submission of data for more than one Part D Contract level.
Response: Part D Contracts will be able to provide these data at either the Part D Sponsor (parent organization) or Contract level. This change is reflected in the revised reporting requirements document.
Licensure and Solvency, Business Transactions and Financial Requirements
A commenter asked if this reporting section still only applies to PDPs.
Response: For 2007, this reporting section has been expanded to include four subsections. The first three subsections apply to PDPs and Direct Contract Employer Group Waiver Plans (Direct EGWPs). The last subsection requires all Part D Contracts to provide PBM information.
It was noted that Medicare cost plans choosing to offer Part D benefits are exempt from the Licensure and Solvency, Business Transactions and Financial Requirements reporting section.
Response: This is correct, and will be reflected in the revised reporting requirements document.
A commenter asked if this section applies only to employer groups contracting directly with CMS.
Response: Yes, this section only applies to employers that enter into direct contracts with CMS to become PDPs for their own Medicare beneficiaries.
A comment was received that indicated the method of submission relating the Licensure and Solvency is not included in the Part D reporting requirements.
Response: CMS disagrees that the method of submission is unclear in the reporting requirements. The introductory paragraph of this section describes the types of data to be submitted as well as the methods for providing them.
Drug Benefit Analyses
It was recommended that CMS clarify data reported in this section are based upon the specific benefit design offered under each plan (e.g. defined standard, actuarially equivalent, basic alternative, enhanced alternative).
Response: All Part D Contracts will complete this reporting section, for each PBP offered. The HPMS module for this reporting section will allow each Plan to indicate the specific benefit design in addition to the data elements listed.
One commenter stated this reporting section would require plans to review utilization of ever member, every quarter.
Response: CMS feel Part D Contracts are able to provide these data with currently available systems. CMS requests Part D Contracts develop systems in which to report these data if none exist currently.
A commenter suggested that the level of reporting for this section is changed the Part D Contract or Sponsor (parent organization) level.
Response: CMS believes that reporting the Drug Benefit Analyses at the plan level is an important tool in the oversight and monitoring of each coverage level for each Part D plan.
Responses to Public Comments on CY 2007 Reporting Requirements – 8/2006
Page
| File Type | application/msword |
| File Title | Part D Sponsor Reporting Requirements |
| Author | CMS |
| Last Modified By | CMS |
| File Modified | 2006-09-11 |
| File Created | 2006-09-06 |
© 2026 OMB.report | Privacy Policy