Medicare Part D Reporting Requirements (CMS-10185)
Medicare Part D Reporting Requirements
CMS-10185 Final 2007 Medicare Part D Reporting Requirements_revised082006.DOC
Medicare Part D Reporting Requirements (CMS-10185)
OMB: 0938-0992
MEDICARE PART D
REPORTING REQUIREMENTS
Contract Year 2007
Updated: 9/11/06
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-00992. The time required to complete this information collection is estimated to average 32 hours annually per respondent, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850.
Table of Contents
Section II. Medication Therapy Management Programs 6
Section III. Generic Dispensing Rate 8
Section V. Pharmacy & Therapeutics (P&T) Committees 11
Section IX. Call Center Measures: Beneficiary Service line and Pharmacy Support line 16
Section XI. Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions 19
Section XII. Pharmaceutical Manufacturer Access/Performance Rebates Received by LTC Pharmacies 21
Section XIII. Licensure and Solvency, Business Transactions and Financial Requirements 22
Section XIV. Drug benefit analyses 25
Table 1. Summary of Reporting Elements 26
Table 2: Changes made from CY 2006 Reporting Requirements 31
Introduction
In December 2003, Congress passed the Medicare Prescription Drug Benefit, Improvement and Modernization Act (MMA), allowing coverage of outpatient prescription drugs under the new Medicare Part D benefit. In accordance with Title I, Part 423, Subpart K (§ 423.514), the Act requires each Part D Contract to have an effective procedure to provide statistics indicating:
the cost of its operations
the patterns of utilization of its services
the availability, accessibility, and acceptability of its services
information demonstrating it has a fiscally sound operation
other matters as required by CMS
The purpose of this document is to assure a common understanding of reporting requirements and how these data will be used to monitor the prescription drug benefit provided to Medicare beneficiaries. CMS will use the following terminology to ensure consistency in these reporting requirements:
Part D Sponsor –a parent organization which encompasses a group of Part D Contracts.
Part D Contract – an organization contracted with CMS to provide Part D benefits to Medicare beneficiaries (e.g. H#)
Part D Plan – a plan benefit package (PBP) offered within a Part D contract (e.g. Plan ID #)
This document represents current expectations of data elements to be reported by Part D Contracts at the Part D Sponsor (parent organization), Contract, or Plan (PBP) level, reporting timeframes, and monitoring of Part D contracts. These requirements will be in effect for Contract Year 2007 and are subject to change at the discretion of CMS. According to Subpart O, sanctions may be imposed on Part D Contracts who fail to comply with these reporting requirements.
The following criteria were used in selecting reporting requirements:
Minimal administrative burden on Part D Sponsors
Legislative and regulatory authority
Validity, reliability, and utility of data elements requested
Wide acceptance and current utilization within the Industry
Reporting requirements are described in this document for the following areas: Reversals, Medication Therapy Management Programs, Generic Dispensing Rate, Grievances, Pharmacy & Therapeutics (P&T) Committees, Transition, Exceptions, Appeals, Call Center Measures – Beneficiary Service line and Pharmacy Support line, Overpayment, Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions, Pharmaceutical Manufacturer Access/Performance Rebates Received by LTC Pharmacies, Licensure and Solvency, Business Transactions and Financial Requirements, and Drug Benefit Analyses.
Each Part D Contract shall provide necessary data to CMS to support payment, program integrity, program management, and quality improvement activities. Specifically, additional reporting requirements are identified in separate guidance documents for the following areas: formulary, TrOOP, coordination of benefits, payment and 1/3 audit, and low income subsidy.
Part D Contracts may also be required to submit other information as defined by requirements in the application, guidances, or other documents (e.g. pharmacy access and formularies) during the annual contract bidding, application, or renewal process. Information is also required to be submitted throughout the contract year as allowable changes are made (e.g. formulary changes).
Part D Contract Reporting Requirements
In each of the sections that follow, the method of submission (e.g. entered into or uploaded via the Health Plan Management System (HPMS)) and the level of reporting are specified following the reporting timeline. Sections that refer to prescriptions should encompass all Part D drugs, including compounded drugs.
For PACE Organizations offering Part D coverage, reporting requirements will be limited to: Section III. Generic Dispensing Rate; Section V. Pharmacy & Therapeutics (P&T) Committees (for PACE Organizations utilizing formularies); Section VI. Transition (for PACE Organizations utilizing formularies); Section VII. Exceptions (for PACE Organizations utilizing formularies); Section X. Overpayment; Section XI. Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions; and Section XII. Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions for Long Term Pharmacies.
MA Organizations and Medicare Cost Plans that offer Part D benefits will be required to comply with all reporting requirements contained herein, with the exception of subsections 1, 2 and 3 of Section XIII. Licensure and Solvency, Business Transactions and Financial Requirements.
Part D Contracts will be responsible for reporting data elements related to claim reversals. Information on claim reversals will serve as a component in the monitoring of Part D operational functions
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at either the Contract or Plan (PBP) level:
Provide the total number of out-of-cycle pharmacy transactions with reversal as the final disposition, which were adjudicated during the time period specified above. This should be a numeric field.
Note: Reversed claim records must be maintained (the number of elements retained per record should at a minimum be equivalent to those of the prescription drug event record), and upon request, submitted to CMS.
The requirements stipulating that Part D Contracts provide Medication Therapy Management Programs (MTMP) are described in Title I, Part 423, Subpart D, § 423.153. For monitoring purposes, Part D Contracts will be responsible for reporting several data elements related to their MTMP.
Data related to the identification and participation in the MTMP will be submitted according to the following timeline (note: Period 2 encompasses one full year):
|
Period 1 |
Period 2 |
Reporting Period |
January 1 - June 30 |
January 1 - December 31 |
Data due to CMS/HPMS |
August 31 |
February 29 |
Data elements to be entered into the HPMS at the Contract level.
The method used to enroll beneficiaries into the MTMP. Method of enrollment may be opt-in, opt-out, a combination of opt-in and opt-out, or other. This should be a text field.
The number of beneficiaries who met the eligibility criteria for the MTMP in the specified time period above. This should be a numeric field.
The total number of beneficiaries who participated in the MTMP at any point during the time period specified above. This should be a longitudinally cumulative total. This should be a numeric field.
The total number of beneficiaries who discontinued participation from the MTMP at any time during the specified time period above. This should be a numeric field.
The number of beneficiaries who discontinued participation from the MTMP due to death at any time during the specified time period above. This should be a numeric field.
The number of beneficiaries who discontinued participation from the MTMP due to disenrollment from the Plan at any time during the specified time period above. This should be a numeric field.
The number of beneficiaries who discontinued participation from the MTMP at their request at any time during the specified time period above. This should be a numeric field.
The number of beneficiaries who declined to participate in the MTMP during the specified time period above. This should be a numeric field.
For beneficiaries participating in the MTMP as of the last day of the reporting period specified, provide the prescription cost of all covered Part D medications on a per MTMP beneficiary per month basis. This should be a currency field, rounded to the nearest dollar. The total prescription cost should be limited to covered Part D medications and be calculated using gross drug cost as follows: (Ingredient Cost Paid + Dispensing Fee + Sales Tax). This amount should be summed for all covered Part D prescriptions that were dispensed within the reporting period specified for each beneficiary enrolling in the MTMP as of the last day of the reporting period specified. Finally, this sum should be divided by the total number of member months for the included beneficiaries. These member months should include all months enrolled in the Part D Contract during the reporting period specified, not only the months that the beneficiary enrolled in the MTMP.
The following equation also describes this calculation
![]()
For beneficiaries participating in the MTMP as of the last day of the reporting period specified, provide the number of covered Part D 30-day equivalent prescriptions on a per MTMP beneficiary per month basis. This should be a numeric field.
This amount should be calculated by first summing days supply of all covered Part D prescriptions dispensed for beneficiaries participating in MTMP as of the last day of the reporting period, and dividing by 30 to determine the number of 30 day equivalent prescriptions dispensed. This number is then divided by the total number of member months for the included beneficiaries. These member months should include all months enrolled in the Part D Plan during the reporting period specified, not only the months that the beneficiary enrolled in the MTMP.
The following equation also describes this calculation:
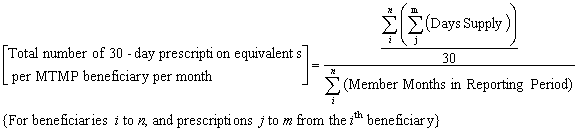
Cost control requirements for Part D Contracts are presented in Title I, Part 423, Subpart D. Accordingly, Part D Contracts will be responsible for reporting data elements needed to monitor utilization of generic drugs (defined by Title I, Part 423, Sub-Part A, § 423.4).
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at the Plan (PBP) level:
Number of paid claims for generic drugs (regardless of days supply) with dates of service during the specified reporting period identified above. First DataBank or Medispan generic drug classifications will be used to identify generic drugs. This should be a numeric field.
Total number of paid claims (regardless of days supply) with dates of service during the specified reporting period identified above. This should be a numeric field.
Title I, Part 423, Subpart M of the regulation includes regulations that require Part D Contracts to maintain grievance information. All plans (PBPs) will be responsible for reporting data related to grievances received.
A grievance is defined as any complaint or dispute, other than one that involves a coverage determination, expressing dissatisfaction with any aspect of the operations, activities, or behavior of a Part D organization, regardless of whether remedial action is requested. Examples of subjects of a grievance provided in the solicitation for applications include, but are not limited to, timeliness, appropriateness, access to, and/or setting of services provided by the PDP, concerns about waiting times, demeanor of pharmacy or customer service staff, a dispute concerning the timeliness of filling a prescription or the accuracy of filling the prescription.
Part D Contracts are required by the regulations to track and maintain records on all grievances received orally and in writing. Grievance data, requested herein by CMS, should be reported based on the date the grievance was received by the Plan (PBP), not the date the event or incident that precipitated the grievance occurred. Multiple grievances by a single complainant should be tracked and followed as separate grievances. Plans may report grievances in the categories as determined by the Plans after initial investigation. Plans should not dismiss or exclude any grievances filed by beneficiaries from this reporting section.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at the Plan (PBP) level:
For the time period identified above, provide the number of fraud and abuse grievances received related to Part D. A fraud grievance is a statement, oral or written, alleging that a provider, pharmacy, pharmacist, PBM, Plan, or beneficiary engaged in the intentional deception or misrepresentation that the individual knows to be false or does not believe to be true, and the individual makes knowing that the deception could result in some unauthorized benefit to himself/herself or some other person. An abuse grievance is a statement, oral or written, alleging that a provider, pharmacy, pharmacist, PBM, Plan, or beneficiary engaged in behavior that the individual should have known to be false, and the individual should have known that the deception could result in some unauthorized benefit to himself/herself or some other person. This should be a numeric field.
For the time period identified above, provide the number of enrollment/disenrollment grievances received related to Part D. Examples include, but are not limited to, discrimination in the enrollment process, enrollment information and/or identification cards not being received by beneficiaries in a timely manner, and disenrollment requests not being processed in a timely manner. This should be a numeric field.
For the time period identified above, provide the number of benefit package grievances received related to Part D. Examples include, but are not limited to, beneficiary cost sharing, pricing co-insurance issues and issues related to coverage during the coverage gap period. This should be a numeric field.
For the time period identified above, provide the number of pharmacy access/network grievances received related to Part D. Examples include, but are not limited to, network pharmacy refusing to accept a beneficiary’s card and network/non-network pharmacy concerns. This should be a numeric field.
For the time period identified above, provide the number of marketing grievances received related to Part D. Examples include, but are not limited to, marketing materials or promotional messages by sales representatives that include misrepresentations or false/misleading information about plans and benefits, and discriminatory practices identified in marketing materials or through oral/written promotional messages. This should be a numeric field.
For the time period identified above, provide the number of customer service grievances received related to Part D. Examples include, but are not limited to, grievances regarding services provided by the pharmacist/pharmacy staff, plan or subcontractor representatives, or customer service representatives. This should be a numeric field.
For the time period identified above, provide the number of confidentiality/privacy grievances received related to Part D. Examples include, but are not limited to, potential violations of medical information privacy standards by the plan or pharmacy. This should be a numeric field.
For the time period identified above, provide the number of quality of care grievances received related to Part D. Examples include, but are not limited to, grievances received from beneficiaries or Quality Improvement Organizations (QIOs) regarding quality of care. This should be a numeric field.
For the time period identified above, provide the number of exception grievances received related to Part D. An example of an exception grievance is one which is filed because an enrollee’s request to have their coverage determination expedited was denied. This should be a numeric field.
For the time period identified above, provide the number of appeal grievances received related to Part D. An example of an appeal grievance is one which is filed because an enrollee’s request to have a redetermination expedited was denied. This should be a numeric field.
For the time period identified above, provide the number of other grievances received related to Part D not falling into one of the categories described above. This should be a numeric field.
For the time period identified above, provide the total number of grievances received related to Part D. This should be a numeric field.
In addition to satisfying and maintaining P&T committee requirements described in §423.120, Part D Contracts will be responsible for providing information to CMS relating to changes made during a contract year to their P&T committees on a periodic basis. CMS recognizes the importance of maintaining confidentiality of these records. CMS will do everything within its authority to limit access to those who have appropriate use or oversight role and will track those who have accessed these records.
Additionally, CMS will provide methods other than HPMS data submission for those Part D Contracts with contractual limitations in providing these data.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at the Contract level:
For each new P&T committee member added since the previous reporting period, provide the following:
First name. This should be a text field.
Middle name. This should be a text field.
Last name. This should be a text field.
Name suffix (e.g. Sr, Jr). This should be a text field.
Date of birth. This should be a date field.
Credential (e.g. MD, PharmD, RN, etc). This should be a text field.
Effective start date of P&T membership. This should be a date field.
If applicable, effective termination date of P&T membership. This should be a date field.
Indication if each new P&T committee member is a practicing physician independent and free of conflict from the Part D contracted organization, and Pharmaceutical manufacturers, and is an expert in the care of elderly or disabled individuals
Indication if each new P&T committee member is a practicing pharmacist independent and free of conflict from the Part D contracted organization, and Pharmaceutical manufacturers, and is an expert in the care of elderly or disabled individuals
For each P&T committee member terminating P&T membership since the previous reporting period, provide the following:
First name. This should be a text field.
Middle name. This should be a text field.
Last name. This should be a text field.
Name suffix (e.g. Sr, Jr) . This should be a text field.
Date of birth. This should be a date field.
Credential (e.g. MD, PharmD, RN, etc). This should be a text field.
Effective termination date of P&T membership. This should be a date field.
As described in §423.120 and other guidance issued by CMS, Part D Contracts must maintain and implement an effective transition process to ensure that beneficiaries transitioning into a Plan are provided a smooth transition to drugs on the formulary. Plans (PBPs) will be responsible for reporting various data elements related to prescriptions dispensed during newly enrolled beneficiaries’ transition periods for CMS oversight.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at the Plan (PBP) level:
Total number of newly enrolled beneficiaries during the reporting time period. This should be a numeric field.
Number of prescriptions authorized during transition periods within the reporting time period. This should be a numeric field.
Number of enrollees receiving one or more prescriptions authorized during transition periods within the reporting time period. This should be a numeric field.
Title I, Part 423, Subpart D includes regulations regarding formulary and tier exceptions, and exceptions to established drug utilization management programs. Plans (PBPs) that utilize prior authorization or step therapy edits as utilization management tools (including for non-formulary exceptions) will be responsible for reporting several data elements related to these activities.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at the Plan (PBP) level:
Number of pharmacy transactions rejected due to failure to complete step therapy edit requirements in the time period specified above. This should be a numeric field.
Number of pharmacy transactions rejected due to need for prior authorization (not including first pass step therapy edits or early refills) in the time period specified above. This should be a numeric field.
Number of pharmacy transactions rejected due to quantity limits in the time period specified above. This should be a numeric field.
Number of prior authorizations requested for formulary medications in the time period specified above (not including first pass step therapy edits or early refills). This should be a numeric field.
Number of prior authorizations approved for formulary medications in the time period specified above (not including first pass step therapy edits or early refills). This should be a numeric field.
Number of exceptions requested for non-formulary medications in the time period specified above (not including early refills). This should be a numeric field.
Number of exceptions approved for non-formulary medications in the time period specified above (not including early refills). This should be a numeric field.
Number of tier exceptions requested in the time period specified above (not including first pass step therapy edits or early refills). This should be a numeric field.
Number of tier exceptions approved in the time period specified above (not including first pass step therapy edits or early refills). This should be a numeric field.
Number of quantity limit exceptions requested in the time period specified above (not including early refills). This should be a numeric field.
Number of quantity limit exceptions approved in the time period specified above (not including early refills). This should be a numeric field.
Title I, Part 423, Subpart M includes regulations regarding coverage determinations and appeals under Part D. As defined in §423.560, an appeal is any of the procedures that deal with the review of adverse coverage determinations made by the Plan on the benefits the enrollee believes he or she is entitled to receive, including a delay in providing or approving the drug coverage (when a delay would adversely affect the health of the enrollee), or on any amounts the enrollee must pay for the drug coverage. These procedures include redeterminations by the Plan and reconsiderations by the independent review entity (IRE). Redeterminations or reconsiderations may result in reversal or partial reversal of the original decision.
Example of a reversal of an original decision: Non-formulary exception request approved upon redetermination for drug and quantity prescribed.
Example of a partial reversal of an original decision: Non-formulary exception request approved upon redetermination for drug, but full quantity prescribed is not approved.
CMS will request appeal data as part of the monitoring of a Plan’s availability, accessibility, and acceptability of its services.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
Data elements to be entered into the HPMS at the Plan (PBP) level:
Number of appeals submitted for standard redetermination in the time period specified above. This should be a numeric field.
Number of appeals submitted for expedited redetermination in the time period specified above. This should be a numeric field.
Number of appeals submitted for expedited redetermination that were granted expedited status in the time period specified above. This should be a numeric field.
Number of appeals submitted for standard redetermination withdrawn by the enrollee in the time period specified above. This should be a numeric field.
Number of appeals submitted for expedited redetermination withdrawn by the enrollee in the time period specified above. This should be a numeric field.
Number of redeterminations in the time period specified above resulting in reversal of original decision. This should be a numeric field.
Number of redeterminations in the time period specified above resulting in partial reversal of original decision. This should be a numeric field.
Number of adverse redeterminations in the time period specified above due to insufficient evidence of medical necessity from enrollee’s prescribing physician. Examples of insufficient evidence of medical necessity may include, but are not limited to, when the plan does not receive the information, or the information received does not support medical necessity. This should be a numeric field.
Number of appeals submitted for IRE reconsideration in the time period specified above due to inability to meet timeframe for coverage determination. This should be a numeric field.
Number of appeals submitted for IRE reconsideration in the time period specified above due to inability to meet timeframe for redetermination. This should be a numeric field.
Number of IRE decisions for standard reconsideration in the time period specified above resulting in reversal of original coverage determination or redetermination. This should be a numeric field.
Number of IRE decisions for standard reconsideration in the time period specified above resulting in partial reversal of original coverage determination or redetermination. This should be a numeric field.
Number of IRE decisions for expedited reconsideration in the time period specified above resulting in reversal of original coverage determination or redetermination. This should be a numeric field.
Number of IRE decisions for expedited reconsideration in the time period specified above resulting in partial reversal of original coverage determination or redetermination. This should be a numeric field.
Number of IRE decisions for standard reconsideration in the time period specified above resulting in upholding of original coverage determination or redetermination. This should be a numeric field.
Number of IRE decisions for expedited reconsideration in the time period specified above resulting in upholding of original coverage determination or redetermination. This should be a numeric field.
CMS will suspend reporting by Part D Contracts for the Call center reporting section through 3rd quarter 2007 due to CMS’ direct monitoring of Part D call centers. All Part D Contracts are required to continue collection of these data, in the event CMS reinstitutes call center data submission via HPMS.
Part D Contracts will report several data elements related to customer service center calls related to Part D. This information will be utilized to monitor plan performance. These reporting requirements were designed to provide flexibility around each Part D call center structure. Part D Contracts may choose to submit data at the Part D Sponsor level, Contract level, or Plan (PBP) level. Part D Contracts must record in HPMS the level of data provided. It should be noted that the level of data reported will be used for performance monitoring and reporting as submitted to CMS. Part D Contracts, therefore, who submit data at the Part D Sponsor level will be considered as providing the equivalent level of call center service as all other Part D Contracts associated with that Sponsor. Also, while call centers may track other metrics such as calls related to medical care, calls related in any matter to Part D should be tracked separately for inclusion in this reporting requirement.
Reporting timeline: Part D Contracts will provide monthly data on a quarterly basis to CMS.
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
||||||||
Reporting Period |
1/1 – 1/31 |
2/1 – 2/28 |
3/1 – 3/31 |
4/1 – 4/30 |
5/1 – 5/31 |
6/1 – 6/30 |
7/1 – 7/31 |
8/1 – 8/31 |
9/1 – 9/30 |
10/1 – 10/31 |
11/1 – 11/30 |
12/1 – 12/31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
||||||||
Data elements to be entered into the HPMS at the Part D Sponsor, Contract, or Plan (PBP) level:
For the time period specified above, provide the total number of inbound Part D connections abandoned to the Beneficiary Service line. This should be a numeric field. For call centers that cannot separate abandoned Part D calls from other calls, the total number of inbound connections abandoned will be reported and also the total number of inbound calls for the customer service center, during the reporting period specified.
For the time period specified above, provide the total number of inbound Part D connections abandoned to the Pharmacy Support line. This should be a numeric field. For call centers that cannot separate abandoned Part D calls from other calls, the total number of inbound connections abandoned will be reported and also the total number of inbound calls for the customer service center, during the reporting period specified.
For the time period specified above, provide the total number of inbound Part D calls to the Beneficiary Service line. This should be a numeric field.
For the time period specified above, provide the total number of inbound Part D calls to the Pharmacy Support line. This should be a numeric field.
For the time period specified above, provide the average hold time for Part D calls to the Beneficiary Service line. This is defined as the average time spent on hold following the IVR system and before reaching a customer service representative. All calls, including abandoned calls, should be included in this calculation. This should be a numeric field (mm:ss).
For the time period specified above, provide the average hold time for Part D calls to the Pharmacy Support line. This is defined as the average time spent on hold following the IVR system and before reaching a customer service representative. All calls, including abandoned calls, should be included in this calculation. This should be a numeric field (mm:ss).
For the time period specified above, provide the number of Part D calls to the Beneficiary Service line answered in ≤30 seconds. This should be a numeric field.
For the time period specified above, provide the number of Part D calls to the Pharmacy Support line answered in ≤30 seconds. This should be a numeric field.
For the time period specified above, provide the average length of calls to the Beneficiary Support line. Length of call is defined as the period of time between call connection and disconnection. All increments of the call should be included, such as time spent navigating the IVR. This should be a numeric field (mm:ss).
For the time period specified above, provide the average length of calls to the Pharmacy Support line. Length of call is defined as the period of time between call connection and disconnection. All increments of the call should be included, such as time spent navigating the IVR. This should be a numeric field (mm:ss).
Part D Contracts will be responsible for reporting data related to overpayments associated with Part D benefits. An overpayment occurs when a Part D Contract erroneously makes a payment in excess of the amount due and payable under the Part D drug benefit. Examples would include overpayments a plan makes to pharmacies, sub-contractors, or PBMs for claims payment. This information is necessary to ensure that overpayments are being identified and recouped appropriately.
Reporting timeline:
|
Period 1 |
Period 2 |
Reporting Period |
January 1 - June 30 |
July 1 – December 31 |
Data due to CMS/HPMS |
August 31 |
February 29 |
Data elements to be entered into the HPMS at the Contract level:
For the time period identified above, provide the total overpayment dollars identified to be recouped by the Contract (i.e., any funds recovered from any entity it has overpaid, including, pharmacies, providers, Pharmaceutical Benefit Managers, etc.) This should be a currency field.
For the time period identified above, provide the total overpayment dollars recouped by the Contract. This should be a currency field.
Part D Contracts will be responsible for reporting multiple data elements related to rebates. These data will be monitored as components of a Part D Contract’s operational costs. CMS recognizes the importance of maintaining confidentiality of these records. CMS will do everything within its authority to limit access to those who have appropriate use or oversight role and will track those who have accessed these records.
Rebates, discounts, and other price concessions will be reported at either the CMS Part D Sponsor or Contract level. Reporting will not be combined by the subcontractor PBM to include multiple Part D Sponsors’ data. For example: (1) national Part D sponsors with multiple regional plans contracting independently or through a PBM will report rebates from the level of the national Part D sponsor; (2) regional or local Part D sponsor whether utilizing subcontractor PBM or not report at the Part D sponsor specific level; (3) PBM providing Part D coverage outside of a subcontractor role will report rebates at the PBM level. Rebate information should be summarized for each drug, rolled up to include multiple strengths, package sizes, dosage formulations, or combinations. The quarterly reported totals are not cumulative YTD totals.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
September 30 |
December 31 |
March 31 |
June 30 |
Data files to be uploaded through the HPMS at the CMS Part D Sponsor or Contract level as specified above:
Part D Sponsors/Contracts will provide an Excel file (filename=REBATES_(SPONSORNAME)_(2007Q#).XLS, replacing ‘(SPONSORNAME)’ following the below file layout.
-
Pharmaceutical Manufacturer Rebate File Record Layout
Field Name
Field Type
Field Length
Field Description
Manufacturer Name
CHAR REQUIRED
100
For each rebate, provide the contracting manufacturer name. This should be a
character field.
Drug Name
CHAR REQUIRED
100
For each rebate, provide the brand name.
Rebates Received
NUM (CUR) REQUIRED
12
For each unique manufacturer/brand name combination, provide the rebate amount received in the reporting period specified.
- Limit to 999,999,999,999, no decimals, can be a negative number.
- Zero should be entered in the currency fields if no data are available
Pending Rebates
NUM (CUR) REQUIRED
12
For each unique manufacturer/brand name combination, provide the rebate amount requested for the reporting period specified but not yet received (if applicable).
- Limit to 999,999,999,999, no decimals, can be a negative number
- Zero should be entered in the currency fields if no data are available
Prior Rebates
NUM (CUR) REQUIRED
12
For each unique manufacturer/brand name combination, provide the rebate amount received that is associated with a prior reporting period (if applicable).
- Limit to 999,999,999,999, no decimals, can be a negative number
- Zero should be entered in the currency fields if no data are available
It is expected that the file specified above will summarize most rebate information. However, for all non-rebate discounts, price concessions, or other value adds such as gift-in-kind or other programs (e.g., coupons or disease management programs specific to a Part D Sponsor), Part D Sponsors will provide an additional Excel file (filename=DISCOUNTS_( SPONSORNAME)_(2007Q#).XLS, replacing ‘(SPONSORNAME)’ with the Part D Sponsor’s name and ‘(2007Q#)’ with the year and quarter number) following the below file layout.
Discounts and Other Price Concessions File Record Layout
|
|||
Field Name |
Field Type |
Field Length |
Field Description |
Manufacturer/ Company Name |
CHAR REQUIRED |
100 |
List the name of each manufacturer for whom there is an associated discount, price concession, or other value add. |
Description
|
CHAR REQUIRED |
250 |
Describe the discount, price concession, or other value add. |
Value
|
NUM (CUR) REQUIRED |
12 |
Provide the value of the discount, price concession, or other value add.
|
Justification
|
CHAR OPTIONAL |
4000 |
For each discount, price concession, or value add, provide a justification for receipt. |
As
described in the CMS 2007 Call Letters, Part D Contracts
must require disclosure of access/performance rebates or other price
concessions received by their LTC network pharmacies designed to or
likely to influence or impact utilization of Part D drugs. The term
“access/performance rebates” refers to rebates
manufacturers provide to pharmacies that are designed to prefer,
protect, or maintain that manufacturer’s product selection by
the pharmacy or to increase the volume of that manufacturer’s
products that are dispensed by the pharmacy under its formulary
(referred to as “moving market share”). As evidence that
they are managing and monitoring drug utilization, Part D Contracts
must report these data to CMS for oversight. CMS
recognizes the importance of maintaining confidentiality of these
records. CMS will do everything within its authority to limit
access to those who have appropriate use or oversight role and will
track those who have accessed these records.
Access/performance
rebates received and reported by pharmacies will be reported at
either the CMS Part D Sponsor or Contract level. Data should
include rebates received for all Part D drugs, not limited to
formulary/covered drugs. Rebate information should be summarized
for each drug, rolled up to include multiple strengths, package
sizes, dosage formulations, or combinations. The
quarterly reported totals are not cumulative YTD totals.
Reporting timeline:
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
Reporting Period |
January 1 - March 31 |
April 1 - June 30 |
July 1 - September 30 |
October 1 - December 31 |
Data due to CMS/HPMS |
September 30 |
December 31 |
March 31 |
June 30 |
Data
files to be uploaded through the HPMS at the Part D Sponsor or
Contract level as specified above:
Part D
Sponsors/Contracts will provide an Excel file (filename=REBATES_LTC
PHARMACIES_(CONTRACTNAME)_(2007Q#).XLS, replacing ‘(CONTRACTNAME)’
with the Part D Sponsor’s name and ‘(2007Q#)’ with
the year and quarter number) containing the following fields.
LTC Pharmacy Name: Provide the name of the LTC pharmacy for which the listed rebates apply.
LTC Pharmacy NABP Number: Indicate the contracted LTC pharmacy NABP number for which the listed rebates apply. This should be a numeric field.
Manufacturer name: Provide the contracting manufacturer name. This should be a character field.
Brand name: Provide the brand name. This should be a character field.
Gross LTC rebate: Provide the gross Part D rebates received by the LTC pharmacy for all Part D drugs (including non-formulary drugs)
Per unit rebate: Provide the per unit rebates received, associated with the gross Part D rebate
Technical notes: Provide any technical notes regarding the LTC pharmacy rebate calculations
-
LTC Pharmacy Name
LTC Pharmacy NABP Number
Manufacturer name
Brand name
Gross Part D rebate
Rebate $ per unit
Technical notes
Text
Numeric
Text
Text
Currency
Currency
Text
Title I, Part 423, Subpart I includes regulations regarding Licensure and Solvency. Part D Contracts and will be responsible for reporting multiple data elements and documentation related to their licensure and solvency and other financial requirements. Direct Contract Employer Group Waiver Plans (Direct EGWPs) will be responsible for reporting multiple data elements and documentation related to their solvency and other financial requirements. Some data will be entered into the HPMS and other information will be mailed directly to CMS. Documentation requirements are listed separately for Part D PDP Contracts and Direct EGWPs. These data will be used to ensure Part D PDP Contracts and Direct EGWPs continue to be fiscally solvent entities.
Additionally, all Part D Contracts will enter PBM information into the HPMS on a quarterly basis.
Subsection 1. Financial and Solvency Requirements Documentation - Part D PDPs
Subsection 2. Financial and Solvency Requirements Documentation – Direct EGWPs
Subsection 3. Financial and Solvency Requirements HPMS data– Part D PDPs and Direct EGWPs
Subsection 4. Performance of Part D Activities HPMS data – MA-PDs, PDPs, and Direct EGWPs
Reporting timeline:
|
Quarter 1 YTD |
Quarter 2 YTD
|
Quarter 3 YTD |
Annual |
Reporting Period |
January 1 - March 31 |
January 1 - June 30 |
January 1 - September 30 |
January 1 - December 31 |
Data due to CMS/HPMS |
May 15 |
August 15 |
November 15 |
120 days after the end of the calendar year or within 10 days of the receipt of the Annual Audited F/S whichever is earlier. |
I. Financial and Solvency Requirements Documentation for Part D PDP Contracts:
According to the quarterly time periods specified above, Part D PDP Contracts that are licensed will mail the following completed Health Blank form pages directly to CMS:
Jurat
Assets
Liabilities, Capital and Surplus
Statement of Revenue and Expenses
Capital and Surplus Account
Cash Flow
Note: CMS will accept a copy of the Health Blank form submitted to the state in its entirety.
According to the quarterly time periods specified above, non-licensed Part D PDP Contracts will mail un-audited financial statements, which convey the same information contained in the Health Blank form, directly to CMS. An alternative for non-licensed Part D PDP Contracts would be to complete the Health Blank pages as prescribed in A. above.
According to the quarterly time periods specified above, non-licensed Part D PDP Contracts will mail documentation showing that an insolvency deposit of $100,000 is being held in accordance with CMS requirements by a qualified financial institution.
According to the quarterly time periods specified above, Part D PDP Contracts not licensed in any state must submit documentation that demonstrates they possess the allowable sources of funding to cover projected losses for the greater of 7.5% of the aggregated projected target amount for a given year or resources to cover 100% of any projected losses in a given year. This documentation should include a worksheet indicating how they arrived at the aggregated projected target amount and a pro-forma income statement that includes enrollment projections through the remainder of the period. Guarantees, letters of credit and other documents essential to demonstrating that the funding for projected losses requirement has been met must also be included.
All Part D PDP Contracts will mail a copy of their independently audited financial statements (which are statutory based or GAAP based) with a management letter within one hundred twenty days following their fiscal year end or within 10 days of receipt of those statements, whichever is earlier directly to CMS.
All Part D PDP Contracts will mail a copy of an Actuarial Opinion by a qualified actuary within one hundred twenty days following their fiscal year end directly to CMS. The opinion should address the assumptions and methods used in determining loss revenues, actuarial liabilities, and related items.
Part D PDP Contracts with any state licensure waivers, must submit an update on the status of obtaining licensure for each waived state.
Part D PDP Contracts’ Documentation should be mailed to the following address:
Centers for Medicare & Medicaid Services
Attn: Part D Licensure & Solvency
Mail Stop C1-25-04
7500 Security Boulevard
Windsor Mill, Maryland 21244
II. Financial and Solvency Requirements Documentation for Direct EGWPs:
According to the quarterly time periods specified above, Direct EGWPs will mail un-audited financial statements directly to CMS.
According to the quarterly time periods specified above, Direct EGWPs will mail documentation showing that an insolvency deposit of $100,000 is being held in accordance with CMS requirements by a qualified financial institution (unless CMS waived this requirement in writing with respect to the sponsor).
Direct EGWPs will mail a copy of their independently audited financial with a management letter within one hundred twenty days following their fiscal year end or within 10 days of receipt of those statements, whichever is earlier directly to CMS.
All Direct EGWPs will mail a copy of their credit rating (or, if they have no credit rating, a Dun & Bradstreet report) on a quarterly basis directly to CMS as follows:
For Quarter 1: May 15th
For Quarter 2: Aug. 15th
For Quarter 3: Nov. 15th
For Quarter 4: Feb. 15th
All Direct EGWPs will mail an ERISA Sec. 411(a) attestation directly to CMS by February 15th. See 2007 Solicitation for Application for Employer/Union Direct Contract PDPs, Appendix VI, Sec. E.4 for explanation of this attestation.
Direct EGWPs’ Documentation should be mailed to the following address:
Centers for Medicare & Medicaid Services
Attn: Financial Solvency Reporting
Mail Stop C1-22-06
7500 Security Boulevard
Windsor Mill, Maryland 21244
III. Financial and Solvency Requirements data elements to be entered into HPMS – For Part D PDP Contracts / Direct EGWP Sponsors:
Data to be entered at the Part D Contract level:
Total assets as of the end of the quarterly reporting period identified above. This should be a currency field.
Total liabilities as of the end of the quarterly reporting period identified above. This should be a currency field.
Total cash as of the end of the quarterly reporting period identified above. This should be a currency field.
Total cash equivalents as of the end of the reporting period identified above. This should be a currency field.
Total current assets as of the end of the quarterly reporting period identified above. This should be a currency field.
Total current liabilities as of the end of the quarterly reporting period identified above. This should be a currency field.
Total revenue as of the end of the quarterly reporting period identified above. This should be a currency field.
Total expenses as of the end of the quarterly reporting period identified above. This should be a currency field.
Total administrative expense as of the end of the quarterly reporting period identified above. This should be a currency field. NOTE: Direct EGWPs are waived from this element
Total net income as of the end of the quarterly reporting period identified above. This should be a currency field.
Drug benefit expenses (excluding administrative expenses) as of the end of the quarterly reporting time period. Drug benefit expenses are paid claims costs which would be comprised of negotiated costs and dispensing fees less member share. This should be a currency field.
Drug benefit revenues as of the end of the quarterly reporting period. Drug benefit revenues would include premiums, CMS subsidies, rebates and other reinsurance. This should be a currency field.
IV. Performance of Part D Activities data elements to be entered into HPMS – For All Part D Contracts (including MA-PDs, PDPs, and Direct EGWPs)
Data to be entered at the Part D Contract level: (For reporting periods following Quarter 1, Contracts will either indicate changes to previously submitted data, or indicate no changes have occurred.)
Provide the legal entity name(s) of companies responsible for Part D functions. This should be a text field.
From the above list, identify the company performing the following functions:
Performs adjudication and processing of pharmacy claims at the point of sale.
Performs negotiation with prescription drug manufacturers and others for rebates, discounts, or other price concessions on prescription drugs
Performs administration and tracking of enrollees’ drug benefits in real time.
Performs coordination with other drug benefit programs, including, for example, Medicaid, state pharmaceutical assistance programs, or other insurance.
Develops and maintains a pharmacy network.
Operates an enrollee grievance and appeals process.
Performs customer service functionality that includes serving seniors and persons with a disability.
Performs pharmacy technical assistance service functionality.
Maintains a pharmaceutical and therapeutic committee.
Other (User will be requested to enter additional information)
Provide the effective date of each company’s service period. This should be a date field (mm/dd/yy).
If an individual Plan (PBP) within a Part D contract utilizes different companies, data may be submitted for that individual PBP.
Part D Contracts must provide enrollees with coverage of benefits as described within §423.104. For the purposes of CMS review, Plans (PBPs) will be required to report multiple data elements related to their provision of Part D benefits. Data reported in this section are based upon the specific benefit design offered under each plan (e.g. standard, enhanced, etc.)
Reporting timeline: Part D Contracts will provide monthly data on a quarterly basis to CMS.
|
Quarter 1 |
Quarter 2 |
Quarter 3 |
Quarter 4 |
||||||||
Reporting Period |
1/1 – 1/31 |
2/1 – 2/28 |
3/1 – 3/31 |
4/1 – 4/30 |
5/1 – 5/31 |
6/1 – 6/30 |
7/1 – 7/31 |
8/1 – 8/31 |
9/1 – 9/30 |
10/1 – 10/31 |
11/1 – 11/30 |
12/1 – 12/31 |
Data due to CMS/HPMS |
May 31 |
August 31 |
November 30 |
February 29 |
||||||||
Data elements to be entered into the HPMS at the Plan (PBP) level:
From the drop-down menu provided, select the benefit type of the Plan (e.g. defined standard, actuarially equivalent, basic alternative, enhanced alternative).
Provide the total number of enrollees in the pre-initial coverage limit phase as of the last day of the month. This should be a numeric field.
Provide the total number of enrollees in the coverage gap as of the last day of the month. This should be a numeric field.
Provide the total number of enrollees in the catastrophic coverage level as of the last day of the month. This should be a numeric field.
Appendix
Table 1. Summary of Reporting Elements
Note: this summary table is for quick reference use only. Please refer to the respective detailed sections for full definitions, timelines, reporting level, and submission procedures.
Section |
Element |
Format |
Frequency |
HPMS |
Reversals
|
Total number of out-of-cycle pharmacy transactions with reversal as the final disposition |
Numeric |
Quarterly |
Yes |
Medication Therapy Management Programs (MTMP) |
The method used to enroll beneficiaries into the MTMP |
Text |
Semi-annually |
Yes |
Number of beneficiaries who met the eligibility criteria for the MTMP |
Numeric |
Semi-annually |
Yes |
|
Number of beneficiaries who participated in the MTMP |
Numeric |
Semi-annually |
Yes |
|
Number of beneficiaries who discontinued participation from the MTMP |
Numeric |
Semi-annually |
Yes |
|
Number of beneficiaries who discontinued participation from the MTMP due to death |
Numeric |
Semi-annually |
Yes |
|
Number of beneficiaries who discontinued participation from the MTMP due to disenrollment from the Contract |
Numeric |
Semi-annually |
Yes |
|
Number of beneficiaries who discontinued participation from the MTMP at their request |
Numeric |
Semi-annually |
Yes |
|
Number of beneficiaries who declined to participate in the MTMP |
Numeric |
Semi-annually |
Yes |
|
Prescription cost of all medications for all beneficiaries participating in the MTMP (as of the last day of the reporting period specified) on a per MTMP beneficiary per month basis |
Currency |
Semi-annually |
Yes |
|
Number of covered Part D 30-day equivalent prescriptions on a per MTMP beneficiary per month basis |
Numeric |
Semi-annually |
Yes |
|
Generic Dispensing Rate |
Number of paid claims for generic drugs |
Numeric |
Quarterly |
Yes |
Total number of paid claims |
Numeric |
Quarterly |
Yes |
|
Grievances
|
Number of fraud and abuse grievances received |
Numeric |
Quarterly |
Yes |
Number of enrollment/disenrollment grievances received |
Numeric |
Quarterly |
Yes |
|
Number of benefit package grievances received |
Numeric |
Quarterly |
Yes |
|
Number of pharmacy access/network grievances received |
Numeric |
Quarterly |
Yes |
|
Number of marketing grievances received |
Numeric |
Quarterly |
Yes |
|
Number of customer service grievances received |
Numeric |
Quarterly |
Yes |
|
Number of confidentiality/privacy grievances received |
Numeric |
Quarterly |
Yes |
|
Number of quality of care grievances received |
Numeric |
Quarterly |
Yes |
|
Number of exception grievances received |
Numeric |
Quarterly |
Yes |
|
Number of appeal grievances received |
Numeric |
Quarterly |
Yes |
|
Number of other grievances received |
Numeric |
Quarterly |
Yes |
|
Total number of grievances |
Numeric |
Quarterly |
Yes |
|
Pharmacy & Therapeutics (P&T) Committees
|
First name of each new P&T committee member |
Text |
Quarterly |
Yes |
Middle name of each new P&T committee member |
Text |
Quarterly |
Yes |
|
Last name of each new P&T committee member |
Text |
Quarterly |
Yes |
|
Name suffix (e.g. Sr, Jr) of each new P&T committee member |
Text |
Quarterly |
Yes |
|
Date of birth of each new P&T committee member |
Date |
Quarterly |
Yes |
|
Credential (e.g. MD, PharmD, RN, etc) of each new P&T committee member |
Text |
Quarterly |
Yes |
|
Effective start date of each new P&T committee member |
Date |
Quarterly |
Yes |
|
Effective termination date of each new P&T committee member (if applicable) |
Date |
Quarterly |
Yes |
|
Indication if each new P&T committee member is a practicing physician independent and free of conflict from the Part D Sponsor, Part D plan, and Pharmaceutical manufacturers, and is an expert in the care of elderly or disabled individuals |
Text |
Quarterly |
Yes |
|
Indication if each new P&T committee member is a practicing pharmacist independent and free of conflict from the Part D Sponsor, Part D plan, and Pharmaceutical manufacturers, and is an expert in the care of elderly or disabled individuals |
Text |
Quarterly |
Yes |
|
First name of each terminating P&T committee member |
Text |
Quarterly |
Yes |
|
Middle name of each terminating P&T committee member |
Text |
Quarterly |
Yes |
|
Last name of each terminating P&T committee member |
Text |
Quarterly |
Yes |
|
Name suffix (e.g. Sr, Jr) of each terminating P&T committee member |
Text |
Quarterly |
Yes |
|
Date of birth of each terminating P&T committee member |
Date |
Quarterly |
Yes |
|
Credential (e.g. MD, PharmD, RN, etc) of each terminating P&T committee member |
Text |
Quarterly |
Yes |
|
Effective termination date of each terminating P&T committee member |
Date |
Quarterly |
Yes |
|
Transition
|
Number of newly enrolled beneficiaries |
Numeric |
Quarterly |
Yes |
Number of prescriptions authorized during transition periods |
Numeric |
Quarterly |
Yes |
|
Number of enrollees receiving one or more prescriptions authorized during transition periods |
Numeric |
Quarterly |
Yes |
|
Exceptions |
Number of pharmacy transactions rejected due to failure to complete step edit requirements |
Numeric |
Quarterly |
Yes |
Number of pharmacy transactions rejected due to need for prior authorization (not including first pass step therapy edits or early refills) |
Numeric |
Quarterly |
Yes |
|
Number of pharmacy transactions rejected due to quantity limits in the time period specified above. |
Numeric |
Quarterly |
Yes |
|
Number of prior authorizations requested for formulary medications (not including first pass step therapy edits or early refills) |
Numeric |
Quarterly |
Yes |
|
Number of prior authorizations approved for formulary medications (not including first pass step therapy edits or early refills) |
Numeric |
Quarterly |
Yes |
|
Number of exceptions requested for non-formulary medications (not including early refills) |
Numeric |
Quarterly |
Yes |
|
Number of exceptions approved for non-formulary medications (not including early refills) |
Numeric |
Quarterly |
Yes |
|
Number of exceptions requested for tier exceptions (not including first pass step therapy edits or early refills) |
Numeric |
Quarterly |
Yes |
|
Number of exceptions approved for tier exceptions (not including first pass step therapy edits or early refills) |
Numeric |
Quarterly |
Yes |
|
Number of exceptions requested for quantity limits (not including early refills) |
Numeric |
Quarterly |
Yes |
|
Number of exceptions approved for quantity limits (not including early refills) |
Numeric |
Quarterly |
Yes |
|
Appeals
|
Number of appeals submitted for standard redetermination |
Numeric |
Quarterly |
Yes |
Number of appeals submitted for expedited redetermination |
Numeric |
Quarterly |
Yes |
|
Number of appeals submitted for expedited redetermination that were granted expedited status |
Numeric |
Quarterly |
Yes |
|
Number of appeals submitted for standard redetermination withdrawn by the enrollee |
Numeric |
Quarterly |
Yes |
|
Number of appeals submitted for expedited redetermination withdrawn by the enrollee |
Numeric |
Quarterly |
Yes |
|
Number of redeterminations resulting in reversal of original decision |
Numeric |
Quarterly |
Yes |
|
Number of redeterminations resulting in partial reversal of original decision |
Numeric |
Quarterly |
Yes |
|
Number of adverse redeterminations due to insufficient evidence of medical necessity from enrollee’s prescribing physician |
Numeric |
Quarterly |
Yes |
|
Number of appeals submitted for IRE reconsideration due to inability to meet timeframe for coverage determination |
Numeric |
Quarterly |
Yes |
|
Number of appeals submitted for IRE reconsideration due to inability to meet timeframe for redetermination |
Numeric |
Quarterly |
Yes |
|
Number of IRE decisions for standard reconsideration resulting in reversal of original coverage determination or redetermination |
Numeric |
Quarterly |
Yes |
|
Number of IRE decisions for standard reconsideration resulting in partial reversal of original coverage determination or redetermination |
Numeric |
Quarterly |
Yes |
|
Number of IRE decisions for expedited reconsideration resulting in reversal of original coverage determination or redetermination |
Numeric |
Quarterly |
Yes |
|
Number of IRE decisions for expedited reconsideration resulting in partial reversal of original coverage determination or redetermination |
Numeric |
Quarterly |
Yes |
|
Number of IRE decisions for standard reconsideration resulting in upholding of original coverage determination or redetermination |
Numeric |
Quarterly |
Yes |
|
Number of IRE decisions for expedited reconsideration resulting in upholding of original coverage determination or redetermination |
Numeric |
Quarterly |
Yes |
|
Call Center Measures: Beneficiary Service line and Pharmacy Support line
|
Total number of inbound Part D connections abandoned to the Beneficiary Service line |
Numeric |
Quarterly |
Yes |
Total number of inbound Part D connections abandoned to the Pharmacy Support line |
Numeric |
Quarterly |
Yes |
|
Total number of inbound Part D calls to the Beneficiary Service line |
Numeric |
Quarterly |
Yes |
|
Total number of inbound Part D calls to the Pharmacy Support line |
Numeric |
Quarterly |
Yes |
|
Average hold time for Part D calls to the Beneficiary Service line |
Numeric |
Quarterly |
Yes |
|
Average hold time for Part D calls to the Pharmacy Support line |
Numeric |
Quarterly |
Yes |
|
Number of Part D calls to the Beneficiary Service line answered in ≤30 seconds |
Numeric |
Quarterly |
Yes |
|
Number of Part D calls to the Pharmacy Support line answered in ≤30 seconds |
Numeric |
Quarterly |
Yes |
|
Average length of calls to the Beneficiary Service line |
Numeric |
Quarterly |
Yes |
|
Average length of calls to the Pharmacy Support line |
Numeric |
Quarterly |
Yes |
|
Overpayment |
Total overpayment dollars identified to be recouped |
Currency |
Semi-Annually |
Yes |
Total overpayment dollars recouped |
Currency |
Semi-Annually |
Yes |
|
Pharmaceutical Rebates, Discounts, and Other Price Concessions |
REBATES_(SPONSORNAME)_(2007Q#).XLS |
MS Excel |
Quarterly |
Yes |
DISCOUNTS_(SPONSORNAME)_(2007Q#).XLS |
MS Excel |
Quarterly |
Yes |
|
Pharmaceutical Manufacturer Access/Performance Rebates Received by LTC Pharmacies |
REBATES_LTCPHARMACIES_(SPONSORNAME)_(2007Q#).XLS |
MS Excel |
Quarterly |
Yes |
Licensure and Solvency, Business Transactions and Financial Requirements
|
Licensed Part D PDP
Contracts will submit Completed Health Blank form pages: Jurat,
Assets, Liabilities, Capital and Surplus, Statement of Revenue
and Expenses, Capital and Surplus Account, and Cash Flow
|
Mailed to CMS |
Quarterly |
No |
Documentation showing that an insolvency deposit of $100,000 is being held (for non-licensed Part D PDP Contracts and Direct EGWPs) |
Mailed to CMS |
Quarterly |
No |
|
Funding for projected losses worksheet (for non-licensed Part D PDP Contracts only) |
Mailed to CMS |
Quarterly |
No |
|
Independently audited financial statement with a management letter for Part D PDPs and Direct EGWPs |
Mailed to CMS |
Yearly (fiscal) |
No |
|
Copy of an Actuarial Opinion by a qualified actuary for the Part D PDP |
Mailed to CMS |
Yearly (fiscal) |
No |
|
Documentation on the status of obtaining licensure for each waived state (for Part D PDP Contracts with any state licensure waivers only) |
Mailed to CMS |
Quarterly |
No |
|
Un-audited financial statements for Direct EGWPs |
Mailed to CMS |
Quarterly |
No |
|
Copy of credit rating for Direct EGWPs |
Mailed to CMS |
Quarterly |
No |
|
ERISA Sec. 411(a) attestation for Direct EGWPs |
Mailed to CMS |
Yearly
|
No |
|
Total assets |
Currency |
Quarterly |
Yes |
|
Total liabilities |
Currency |
Quarterly |
Yes |
|
Total cash |
Currency |
Quarterly |
Yes |
|
Total cash equivalents |
Currency |
Quarterly |
Yes |
|
Total current assets |
Currency |
Quarterly |
Yes |
|
Total current liabilities |
Currency |
Quarterly |
Yes |
|
Total revenue |
Currency |
Quarterly |
Yes |
|
Total expenses |
Currency |
Quarterly |
Yes |
|
Total administrative expense |
Currency |
Quarterly |
Yes |
|
Total net income |
Currency |
Quarterly |
Yes |
|
Drug benefit expenses (excluding administrative expenses) |
Currency |
Quarterly |
Yes |
|
Drug benefit revenues |
Currency |
Quarterly |
Yes |
|
Legal entity name(s) of companies responsible for Part D functions |
Text |
Quarterly |
Yes |
|
The company that performs adjudication and processing of pharmacy claims at the point of sale |
Text |
Quarterly |
Yes |
|
The company that performs negotiation with prescription drug manufacturers and others for rebates, discounts, or other price concessions on prescription drugs |
Text |
Quarterly |
Yes |
|
The company that performs administration and tracking of enrollees’ drug benefits in real time. |
Text |
Quarterly |
Yes |
|
The company that performs coordination with other drug benefit programs, including, for example, Medicaid, state pharmaceutical assistance programs, or other insurance. |
Text |
Quarterly |
Yes |
|
The company that develops and maintains a pharmacy network. |
Text |
Quarterly |
Yes |
|
The company that operates an enrollee grievance and appeals process. |
Text |
Quarterly |
Yes |
|
The company that performs customer service functionality that includes serving seniors and persons with a disability. |
Text |
Quarterly |
Yes |
|
The company that performs pharmacy technical assistance service functionality. |
Text |
Quarterly |
Yes |
|
The company that maintains a pharmaceutical and therapeutic committee. |
Text |
Quarterly |
Yes |
|
Other |
Text |
Quarterly |
Yes |
|
Effective date of each company’s service period |
Date |
Quarterly |
Yes |
|
Part D Benefit Analyses |
The benefit type of the Plan (e.g. defined standard, actuarially equivalent, basic alternative, enhanced alternative) |
Selection |
Quarterly |
Yes |
Number of enrollees in the pre-initial coverage limit phase |
Numeric |
Quarterly |
Yes |
|
Number of enrollees in the coverage gap |
Numeric |
Quarterly |
Yes |
|
Number of enrollees in the catastrophic coverage level |
Numeric |
Quarterly |
Yes |
Table 2: Changes made from CY 2006 Reporting Requirements
Reporting Requirements Section |
Changes
|
Enrollment/Disenrollment |
This section has been deleted. |
Reversals |
This section can now be reported at the Part D Contract or Plan (PBP) level |
Medication Therapy Management Programs |
Data element revised:
Data elements added:
|
Generic Dispensing Rate |
No changes made to this reporting section |
Grievances |
Data elements added:
|
Pharmacy & Therapeutics (P&T) Committees |
New reporting section added for 2007 |
Transition |
New reporting section for 2007 |
Exceptions
|
This reporting section was renamed Exceptions, previously titled as Prior Authorization, Step Edits, Non-Formulary Exceptions, and Tier Exceptions
Data elements added:
|
Appeals |
Data elements added:
|
Call Center Measures: Beneficiary Service line and Pharmacy Support line
|
Data elements removed from 2006
Data elements added:
|
Overpayment
|
This section can now be reported at the Contract level.
|
Pharmaceutical Manufacturer Rebates, Discounts, and Other Price Concessions |
This section can now be reported at the Contract level as well as the Part D Sponsor
|
Pharmaceutical Manufacturer Access/Performance Rebates Received by LTC Pharmacies |
New Reporting Section for 2007 |
Licensure and Solvency, Business Transactions and Financial Requirements |
|
Drug benefit analyses
|
New Reporting Section for 2007 |
| File Type | application/msword |
| File Title | Part D Plan Reporting Requirements |
| Author | Christopher A. Powers |
| Last Modified By | CMS |
| File Modified | 2006-09-12 |
| File Created | 2006-09-12 |
© 2026 OMB.report | Privacy Policy