Attachment 5A3
MMP Attach 5A3.doc
Medical Monitoring Project
Attachment 5A3
OMB: 0920-0740
Attachment 5 A 3
III. Insurance Status
Type of insurance

Indicate the type of health insurance that the patient had during the visit prior to the surveillance period by checking the appropriate boxes. If the patient had more than one form of insurance, multiple boxes may be checked. If the patient had a type of health insurance not listed on the form, check "Other" and record the type of insurance in the adjacent field.
If the patient was enrolled in the state AIDS Drug Assistance Program (ADAP) during the visit prior to the surveillance period, check the appropriate box. It is not necessary to see documentation of specific drugs received through the program if enrollment is documented in the medical record.
Please Note: Ryan White and support from service providers are not types of insurance.
IV. Diseases Indicative of AIDS

In this section, mark an X in the appropriate box to indicate a diagnosis of AIDS-defining opportunistic illness (definitive or presumptive). Definitions for definitive and presumptive diagnoses are available in MMWR 1992; 41[no RR-17]: 1-19 and MMWR 1987; 36 [suppl. No.1S]:1S-15S or can be accessed online at http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.html. This is also attached as an appendix to this manual.
In this section, record all AIDS-defining opportunistic illnesses that occurred at any time in the past and during the visit prior to the surveillance period. If there is no diagnosis of a disease indicative of AIDS during this period, check the box at the top of the section. Indicate the month and year of the first known diagnosis in the corresponding boxes and the number of episodes during this period. Multiple illnesses may be indicated in this section.
Many diseases indicative of AIDS are typically episodic, and in most instances, the number of episodes is relevant (e.g., candidiasis, coccidioidomycosis, herpes simplex, histoplasmosis, tuberculosis, Pneumocystis carninii pneumonia [PCP], salmonella). Count recurrences as separate episodes. Number of episodes refers to the total number of episodes during an interval. Write the number of episodes in the box or put a “C” in the box if the disease was chronic for all diseases indicative of AIDS.
Please note: recurrent pneumonia is defined as more than 1 episode in a one-year period, acute (new X-ray evidence not present earlier) pneumonia diagnosed by both (1) culture (or other organism-specific diagnostic method) of material obtained from a clinically reliable source of a pathogen that typically causes pneumonia (other than Pneumocystis carninii or Mycobacterium tuberculosis), and (2) radiologic evidence of pneumonia make a definitive diagnosis. Cases that do not have laboratory confirmation of a causative organism for one of the episodes of pneumonia can be presumptively diagnosed. A presumptive criterion of recurrent pneumonia is pneumonia diagnosed on clinical or radiological grounds by the patient’s physician.
Also note: Use the date of the earlier episode as the first date. Do NOT use this guideline for recurrent pneumonia; enter date of the second episode within 12 months of the first diagnosis. A single episode of pneumonia will not be entered as a disease indicative of AIDS.
![]()
Dates of medical record information abstracted:
This box appears on top of each page to remind you of the date from which you will abstract medical records of visits using the MHF. It is recommended that you enter the dates to the boxes on top of each page before you start abstraction.
V. Prophylaxis
Was the patient ever prescribed prophylaxis for the following conditions?
Prescription of prophylaxis
The USPHS/IDSA guidelines for prevention of opportunistic infections among HIV-infected persons recommend prophylactic drug treatment to prevent either primary or secondary opportunistic infections when the patient’s CD4 count falls below critical levels (<200 cells/L for most conditions) or following a diagnosis and successful treatment of an opportunistic infection . The conditions for which the guidelines recommend primary or secondary prophylaxis include: Pneumocystis carinii pneumonia, cytomegalovirus disease, extrapulmonary cryptococcosis, latent TB infection, Myocbacterium avium complex, and toxoplasmosis. Check the corresponding box for each condition if any medication was prescribed during the visit prior to the surveillance period. Note that some physicians could refer to PCP as pneumocystis jirovecii and this name can be found instead of PCP in some medical records following this new nomenclature in 2002. In this manual we will refer to PCP both to include physicans still using the term Pneumocystis carinii pneumonia and for those who use Pneumocystis jirovecii. The other term that can be used to refer to PCP can be pneumocystosis.
The Yes check box should be marked if there is either a documentation of prophylaxis in the medical record or if medicines indicated for prophylaxis were prescribed for the length of time recommended for prophylaxis, which is significantly longer than that recommended for treatment of diseases caused by the above conditions (except TB). It is not necessary to determine whether this was a primary or secondary prophylaxis, nor is it necessary to enter dates or duration of prophylactic treatment for these conditions. The only information necessary is whether the prophylaxis was either EVER prescribed prior to the surveillance period. Note: Only treatment and prophylaxis following HIV diagnosis should be documented. Please refer to the USPHS/IDSA guidelines for prophylaxis and treatment of opportunistic infections among persons infected with HIV available online at http://www.aidsinfo.nih.gov/guidelines/op_infections%5COI_112801.html
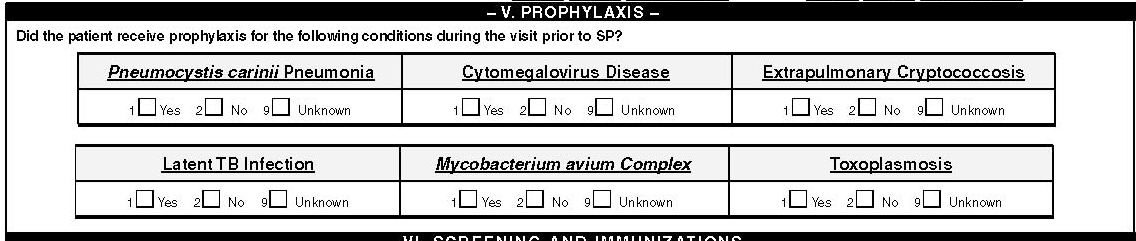
Pneumocystis carinii pneumonia
Medicines used for PCP prophylaxis include Trimethoprim Sulfamethoxazole (TMP-SMZ), Dapsone, Pyrimethamine, Leucovorin, aerosolized Pentamidine, and Atovaquone. These medicines are used for three or more months and until the CD4 has increased to above 200 cells/µL.
Cytomegalovirus disease
Medicines that are recommended for cytomegalovirus disease prophylaxis include Ganciclovir (tablets and/or implants), Foscarnet, Cidofovir, and Valganciclovir. These agents are also used for the treatment of cytomegoalovirus infection of the retina but a different formulation of these agents is used for retinal administration.
Extrapulmonary cryptococcosis
Medicines that are recommended for extrapulmonary cryptococcosis prophylaxis include Fluconazole, Amphotericin B, and Itraconazole.
Latent TB infection
Medicines that are recommended for latent TB infection prophylaxis include Isoniazid (INH), Pyrazinamide, Rifampicin, and Rifabutin. Some physicians refer to latent TB infection (LTBI) as positive tuberculosis skin test (TST), positive purified protein derivative (PPD) test, or simply as abnormal skin test. It is important to pay attention for conditions other than TB where patients will be given skin tests to test for anergy or allergy and not to confuse them with a TB test.
Mycobacterium avium complex
Medicines that are recommended for Mycobacterium avium complex (MAC) infection prophylaxis include Clarithromycin, Azithromycin, and Rifabutin. These medicines can be given for prohylaxis of either primary or secondary infection with the MAC organisms which can include or be specified as M. kansasii or M. intracellulare.
Toxoplasmosis
Medicines that are recommended for Toxoplasmosis prophylaxis include Sulfadiazine, Pyrimethamine, Clindamycin, Atovaquone, and Leucovorin. Toxoplasmosis prophylaxis can be either for primary or secondary infection with the organism Toxoplasma gondii.
VI. Screening and Immunizations
Did the patient ever receive screening for the following conditions?
Understanding if a provider has evaluated a patient for co-morbid infections, and knowing the result of this evaluation is a vital aspect of determining quality of care. However collecting this information is challenging because of the different tests required for screening of certain conditions and the varying immunization schedules for the different conditions. Some conditions require repeated screenings as they can occur repeatedly whereas others might not require repeated screening because once infection is detected the person is protected for the rest of his/her life.
Record whether there is any evidence in the chart that a provider evaluated the patient in any way for the condition. Such evidence would include notation that the patient had reported or denied the infection, documentation in correspondence to the same effect, or testing for the specific condition. Check “No” only if there is no evidence in the chart that evaluation for the condition of any kind was conducted.
Following a screening, if evidence in the chart is sufficient to determine whether the patient did or did not have the condition, check the corresponding boxes for “Yes” or “No”. This can be based on a lab result or documentation by the physician of personal communication with the patient or the patient’s other care providers. Check “Unknown” if the evidence provided in the chart is equivocal. For example, a Hepatitis A diagnosis would be equivocal if i) there is a note that the patient denied having hepatitis A but ii) another note records that the patient did have hepatitis A and iii) there is not sufficient laboratory evidence (such as serology) and other history (such as vaccinations) to determine if the patient did or did not have the infection. In other words the evidence for screening is not available in the records.
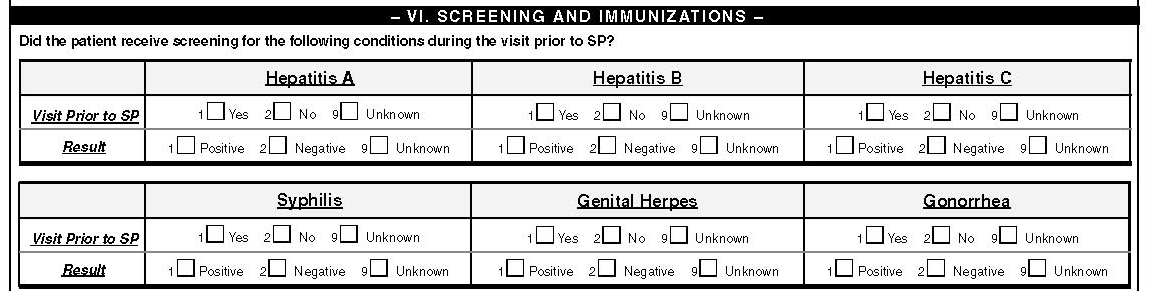
Viral Hepatitis
Infections with the Hepatitis A, B and C viruses can cause substantial, sometimes life-threatening illness in HIV-infected persons. The primary method for diagnosing these infections is by demonstrating the presence of antibodies. The USPHS guidelines recommend serologic screening of all HIV-infected persons for hepatitis. As part of the assessment of quality of care, it is important to document if, and how, patients were evaluated for viral hepatitis. Since viral hepatitis is one of the serious co-morbid conditions among HIV infected persons it will be important to look through the available medical record to capture screening and if screened the date of the first positive test. Enter the date of the first positive result for all hepatitis (A,B, and C) in the space provided if there is a documented positive test. If the patient was screened but never had a positive result check “negative” next to the date of first positive test. Please remember this is one of the few situations that we will look for screening data from the time beyond the visit prior to the surveillance period using the Medical History Form. Serologic testing can also help differentiate a true infection with Hepatitis A or B virus from the protective immune response generated by vaccination for these infections.
Hepatitis A
There are two serologic tests that can be performed to help diagnose Hepatitis A infection:
anti-HAV (total) a general test for the presence of any type of antibody to the Hepatitis A virus
anti-HAV IgM a test for the specific antibodies that provide long-lasting immunity after a true infection or after vaccination.
Check the box for “Hepatitis A” as Yes if:
a note in the chart indicates to “screen patient for Hepatitis A” or there is a lab request for anti HAV antibody (IgM, total)
Hepatitis B
There are two tests that can be performed to help diagnose acute Hepatitis B infection:
Anti-HBc also recorded as “HbcAb”, “Anti-HBc IgM” and “Anti-HBc total”, tests for antibodies patients produce against the core of the virus; these antibodies arise only after a true infection. Patients who become immune due to natural infection may also show positive test result for anti-HBc.
HbsAg a protein from the virus that is generated if the virus is actively reproducing.
Other serologic tests diagnostic of Hepatitis B include:
HbeAg another protein (E-antigen) that is generated if the virus is actively reproducing and which can be present during either acute or chronic active Hepatitis and is an indicator of infectiousness.
Presence of anti-HBs (antibodies that develop against surface proteins of Hepatitis B) is not diagnostic of a Hepatitis B infection, since the vaccine induces the same antibody response. However, tests for this antibody need to be considered as screening tests as this is usually offered to patients to find out if the patients are immune from vaccination.
Check the box for result as “positive” if:
notes in the chart indicate that the patient experienced, was diagnosed with, or was treated for Hepatitis B;
OR
Laboratory testing indicates that the patient had a serologic testing for anti-HBc, HbsAg or HbeAg that was positive.
Hepatitis C
There is one main serologic test for screening or diagnosing Hepatitis C. It may be variably recorded as anti-HCV, Hepatitis C antibody, anti-Hep C or HCVAb. The methods used for serologic testing include three generations of enzyme linked immunoassays (EIA) and more recently RIBA (radio immunoblot assay).
Additional tests for Hepatitis C include Hepatitis C viral load testing and Hepatitis C genotyping. But these tests are offered after the patient’s serologic test result was positive. The serologic tests for hepatitis C are known with different names in different labs. Abstractors should familiarize themselves with the tests that are used in their jurisdictions.
Check the box for result as “positive” if:
notes in the chart indicate that the patient experienced, was diagnosed with, or was treated for Hepatitis C;
OR
testing for anti-HCV was positive.
Did the patient receive screening for the following conditions during the visit prior to SP?
Sexually Transmitted Diseases
Syphilis
Syphilis is caused by the bacteria Treponema pallidum. Any part of the body can be affected and a person can be infected with syphilis unknowingly for many years. Multiple stages of syphilis infection have been defined. We are interested in determining if the patient had syphilis diagnosed at any stage.
Syphilis can be diagnosed by directly examining patient specimens (e.g., scraping of skin lesions, cerebrospinal fluid) by dark-field microscopy for the bacteria. There are also multiple serologic tests used to diagnose syphilis, including VDRL (Venereal Diseases Research Laboratory), RPR (rapid plasma reagin), FTA-ABS (fluorescent treponemal antibody absorbed), or MHA-TP (microhemagglutination assay for antibody to T. pallidum. Check the box for screening for syphilis in the respective interval as Yes if note(s) in the chart indicate that the patient experienced or was treated for syphilis, or serologic testing or other relevant laboratory testing was performed. Enter result as positive if serologic testing was reactive or T. pallidum bacteria were observed in patient specimens. A single serologic test from the tests listed previously may be adequate for screening but when the result of VDRL is positive it requires that one other test or a strong clinical information (like genital or perineal or perianal hard chancre or condylomata lata) be present as a requirement to enter as positive.
Genital Herpes
Genital herpes is caused by the Herpes simplex virus (HSV). After primary infection, patients may experience recurrent outbreaks. Although the diagnosis of genital herpes is most commonly made by history and examination, it also possible to confirm the presence of the virus by swabbing the characteristic lesions and performing immunofluorescence assays or viral culture.
Check the box for genital herpes as Yes if:
notes in the chart indicate that the patient either experienced, was diagnosed with, or was treated for genital herpes, either as a first diagnosis or as a recurrent outbreak;
OR
testing indicates that a patient’s genital lesions were caused by HSV.
Herpes involving the perineum or ano-rectal area should be considered as genital herpes.
Prescription of prophylactic medication to prevent genital herpes recurrences may be used to diagnose genital herpes unless specifically stated otherwise. Examples of medications used to treat or suppress episodes of genital herpes include: Zovirax (acyclovir), Valtrex (Valacyclovir), Famvir (Famciclovir), and Denavir (Penciclovir).
Gonorrhea
Gonorrhea is caused by infection with Neisseria gonorrhea, a Gram-negative bacterium that forms characteristic diplococci (pairs of bacteria). Gonorrhea can infect any part of the genital tract, the ano-rectum, the throat, and in the advanced untreated form can infect the skin (forming characteristic lesions), joints, CNS and rarely other organs from which the bacteria can be recovered.
Diagnosis of gonorrhea requires:
testing (e.g., bacterial culture, observation of the characteristic organism - Gram-negative diplococci - on microscopic examination of a patient specimen, detection of antigen or nucleic acid) indicating that the patient was infected with gonorrhea;
OR
a note(s) in the chart indicating the patient was tested for gonorrhea and that the test was positive.
Receiving medication to prevent gonorrhea after a possible exposure without laboratory evidence (or reference to laboratory evidence) of active infection does not qualify as a diagnosis of gonorrhea.
Chlamydia
Chlamydia is caused by the bacteria Chlamydia trachomatis. The bacterium is difficult to culture because it can only grow inside human cells. Tests for Chlamydia therefore rely on detecting characteristic antigens or nucleic acids (e.g., DNA, RNA) that the bacterium produces.
Diagnosis of Chlamydia requires:
testing (e.g., culture, detection of antigen or nucleic acid) indicating that the patient experienced an episode of chlamydia;
OR
a note(s) in the chart indicating the patient was tested for chlamydia and that the test was positive.
Receiving medication to prevent chlamydia after a possible exposure without laboratory evidence (or reference to laboratory evidence) of active infection does not qualify a diagnosis of Chlamydia.
Chlamydia is a common cause of NGU (see below). Clinical symptoms and/or the presence of urethral discharge without laboratory testing that demonstrates the presence of C. trachomatis does not qualify as a diagnosis of Chlamydia, and the diagnosis of NGU should be considered (see definition below).
Non-Gonococcal Urethritis (NGU)
NGU refers to the condition of urethritis where infection with gonorrhea or chlamydia cannot be demonstrated. Urethritis results from inflammation of the urethra as evidenced by pain on urination and/or the presence of a urethral discharge (e.g., pus, or fluid with white cells visible on microscopic examination).
Diagnosis of NGU requires:
a note(s) in the chart reporting diagnosis of NGU;
OR
a note(s) or lab reports in the chart indicating the patient was tested for gonorrhea and chlamydia and that these evaluations were negative.
Human Papillomavirus (HPV)
Human Papillomavirus (HPV) refers to a family of viruses that cause warts. Particular types of HPV cause genital warts. The genital warts caused by HPV are highly characteristic of infection with this virus. Presence of the virus can be demonstrated by a test which detects characteristic antigens or nucleic acids (e.g., DNA, RNA) that HPV produces. HPV infection can also be diagnosed by special examination of pathologic specimens (e.g., cervical cuttings).
Diagnosis of HPV requires:
a note(s) in the chart reports diagnosis of HPV infection based on the presence of characteristic lesions;
OR
testing (e.g., detection of antigen or nucleic acid, review of pathology specimens) indicates that the patient was infected with HPV;
OR
a note(s) in the chart indicating the patient was tested for HPV and that the test was positive.
Did the patient ever receive a Toxoplasma Antibody Titer?
Indicate whether the patient EVER receive a toxoplasma antibody titer during the review period by checking the appropriate box (only one box should be checked).
Please Note: While "Yes" should be checked if the patient received their toxoplasma antibody titer before or during the period covered by the Medical History Form, "No" should be checked if the patient received it after the review period. We are able to capture this screening using the surveillance period (visit) (SP) form. This information should be documented on the Surveillance Period (visit) Form if the patient received a toxoplasma antibody titer during the surveillance period.
Also Note: Alternative names for a toxoplasma antibody titer are:
Toxoplasma antibody IgG
If the patient received a toxoplasma antibody titer, indicate the results by checking the appropriate box (only one box should be checked). It is possible that patients could receive more than one toxoplasma antibody titer. We will collect only the date of the first positive toxoplasma antibody titer result; priority should be given to the first positive test result.
Tuberculin Skin Test
Indicate whether the patient received a tuberculin skin test (TST) during the period by checking the appropriate box (only one box should be checked). If Yes, record the result using the boxes provided (only one box should be checked). Enter the result of the first positive result and the corresponding date for the result.
Please Note: While "Yes" should be checked if the patient received their tuberculin skin test during the review period, "No" should be checked if the patient received it after the period covered by the Medical History Form. This information should be documented on the Surveillance Period (visit) Form if the patient received a TST during the surveillance period.
Also Note: Alternative names for a tuberculin skin test are:
Mantoux
Purified protein derivative (PPD)
| File Type | application/msword |
| File Title | Attachment 5 |
| Author | evu0 |
| Last Modified By | ziy6 |
| File Modified | 2006-11-09 |
| File Created | 2006-11-09 |
© 2026 OMB.report | Privacy Policy