Attachment 5A4
MMP Attach 5A4.doc
Medical Monitoring Project
Attachment 5A4
OMB: 0920-0740
Attachment 5 A 4
Pap smear
Note: This question should be completed for both female and male patients. In the past traditionally this information was collected only from women since this test was offered only for women to screen for cervical cancer (HPV). However, this test is now also offered for screening for anorectal cancer both in women and in men who have sex with men.
Indicate whether the patient received a Pap smear during the period covered by the Medical History Form by checking the appropriate box (only one box should be checked). Enter the site (anal, cervical) from which Pap smear was performed by checking all that apply. Enter the date of the most recent Pap smear irrespective of the site of specimen; this can be anal Pap, cervical Pap, or both. If the patient did not receive Pap smear during the period covered by medical history form but received one after that, do not enter it in this form. This information should be documented on the Surveillance Period (Visit) Form if the patient received a Pap smear during the surveillance period.
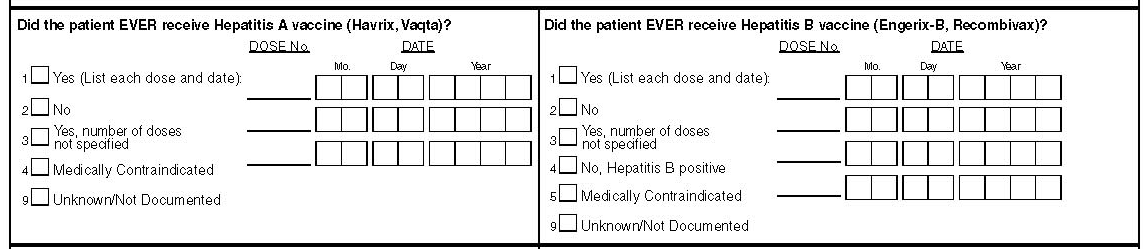
Hepatitis A Vaccine
Indicate whether the patient EVER received a Hepatitis A vaccine by checking the appropriate box (only one box should be checked).
Please Note: While "Yes" should be checked if the patient received their Hepatitis A vaccine during the period covered by the Medical History Form, "No" should be checked if the patient received it after the review period. This information should be documented on the Surveillance Period (visit) Form if the patient received the vaccine during the surveillance period.
For patients who received Hepatitis A vaccine, list each dose and date.
Also Note: Alternative names for a Hepatitis A vaccine are:
Havrix
Vaqta
Hepatitis B Vaccine
Indicate whether the patient EVER received a Hepatitis B vaccine by checking the appropriate box (only one box should be checked).
Please Note: While "Yes" should be checked if the patient received their Hepatitis B vaccine during the period covered by the Medical History Form, "No" should be checked if the patient received it after the review period. This information should be documented on the Surveillance Period (visit) Form if the patient received the vaccine during the surveillance period.
For patients who received Hepatitis B vaccine, list each dose and date.
Also Note: Alternative names for a Hepatitis B vaccine are:
Comvax
Engerix-B
Recombivax HB
Combination Hepatitis A and B Vaccine
Indicate whether the patient received a combination Hepatitis A and B vaccine by checking the appropriate box (only one box should be checked).
Please Note: While "Yes" should be checked if the patient received their combination Hepatitis A and B vaccine during the period covered by the Medical History Form, "No" should be checked if the patient received it after the review period. This information should be documented on the Surveillance Period (visit) Form if the patient received the vaccine during the surveillance period.
For patients who received combination Hepatitis A and B vaccine, list each dose and date.
Also Note: An alternative name for combination Hepatitis A and B vaccine is:
Twinrix
Influenza Vaccine
Indicate whether the patient EVER received an influenza (flu) vaccine by checking the appropriate box (only one box should be checked). For those who have ever received the influenza vaccine, enter the date of the most recent influenza vaccination prior to the surveillance period. Remember, this vaccine should be administered once every year.
Please Note: Brand names of flu vaccines include:
Fluogen
Fluzone
Fluviren
Flushield
Pneumococcal Vaccine
Indicate whether the patient EVER received a pneumococcal vaccine (Pneumovax 23, Pnu-Immune 23) by checking the appropriate box (only one box should be checked).
Please Note: While "Yes" should be checked if the patient received their pneumococcal polysaccharide vaccine during the period covered by the Medical History Form, "No" should be checked if the patient received it after the review period, i.e., during the surveillance period. For those who have received a pneumococcal polysaccharide vaccine, enter the date of the most recent pneumococcal polysaccharide vaccine prior to the surveillance period. Remember, this vaccine is administered once every five years for patients whose CD4 count is greater or equal to 200 cells/µl, but for patients who received the pneumococcal polysaccharide vaccine when their CD4 count was less than 200 cells/µL,the guidelines recommend that they receive another vaccination as soon as their CD4 count rebounds above 200 cells/µL. Remember that in 2000 the FDA approved a different vaccine whch is known as pneumococcal conjugate vaccine (PCV) which is mainly used for prevention of invasive pneumococcal disease in children.
VII. Antiretroviral Therapy
Did the patient ever have a history of antiretroviral therapy?
Indicate whether the patient has a history of antiretroviral therapy at any time before or during the visit prior to the surveillance period by checking the appropriate box. This includes antiretroviral therapy that the patient is taking currently or just started.
Antiretroviral Regimens
Indicate all antiretroviral medications that the patient was EVER prescribed at this facility and/or documented in the records from referral letters or patient interview by checking the appropriate boxes (multiple boxes may be checked). If the patient was prescribed an antiretroviral medication not listed on the questionnaire, record its name under "Other"). In this section of the Medical History Form we will collect only the name of antiretroviral drug that was prescribed. No information on dosage and duration of treatment will be collected. However, if the prescription is continued during the visit prior to the surveillance period, check the box under the column titled “check if patient is prescribed this medicine during the visit prior to the surveillance
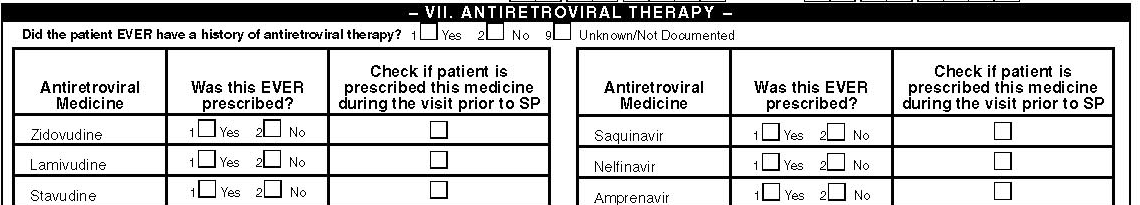
period” for the respective antiretroviral medicine. Enter the name of the medication that was prescribed in the medical records or any medication about which there is documentation of receipt in the medical records by patient history, referral notes or doctor’s report.
It will be helpful to learn the different names of the antiretroviral medications. In practice some providers use different combination of names (generic, brand) simultaneously. Knowing these medicines by class is also very helpful. Currently there are 4 classes of antiretroviral medications that are licensed by FDA with a total of 24 single and combined formulations. This abstraction form allows you to enter all currently licensed medicines. When a prescription is given for combined pills (Combivir; Trizivir) enter the exact prescription, not by the constituents of the combined pill (zidovudine/lamivudine; abacavir/lamivudine/zidovudine). This is very important in the understanding of the role of these combination in adherence to treatment and outcomes of therapy. Please look at the attached list of currently licensed antiretroviral drugs on the last page of this manual. You can also carry this page with you when you go to facilities to abstract records.
VIII. Other Treatments
In order to be able to understand the problem of drug interactions, effect on quality of care and adherence to other treatments, pill burden, and to obtain an indirect assessment of the spectrum of conditions for which patients with HIV/AIDS require medical treatment, it is necessary to collect information on treatments other than antiretroviral medications. Since the number of other treatments that a patient may be prescribed can be difficult to follow, this section of the Medical History Form will concentrate on the visit prior to the surveillance period. The following treatments will be collected; antihypertensive medications, treatment of HBV and HCV, treatment for diabetes mellitus, treatment for lipid disorders including treatment of lipoatrophy/lipodystrophy, preventive/curative treatment for conditions listed under Section V (Prophylaxis), antineoplastic treatment, and treatment for mental health conditions. Note: do not abstract other treatments from other time period except for the visit prior to the surveillance period.

An exception to this will be infection with Hepatitis C. Hepatitis C is emerging as a major comorbid infection for patients with HIV. To better assess the quality of care delivered to patients coinfected with Hepatitis C and HIV it is important to determine if patients who had Hepatitis C before or during the interval had any Hepatitis C testing or treatment at any time before or during the interval. Enter a diagnosis of Hepatitis C at any time in to the Other Diagnoses section (write in under “Other”) of this form.
Enter all treatments that were given to patient co-infected with HIV and HCV in Section VIII, Other Treatments. Treatments for Hepatitis C include the following: various forms of interferon (e.g., Interferon alpha-1, Interferon alpha-2a, Interferon alpha-2b, Roferon, Infergen, PEG-Intron, pegylated interferon), Ribavirin, Rebetron (a single prescribed kit that combines interferon with ribavirin), and certain experimental agents such as amantadine, CellCept (mycophenolate mofetil), Levovirin, Pegasys, and Heptazyme (ribozymes), and Viramadine.
Please refer to the attached list of medications that we are collecting if these medications were ever prescribed after the patient was diagnosed with HIV.
Drug classes used for the treatment of Diabetes Mellitus
Biguanides
Sulfonylurea
Insulin
Thiazolidinediones
Alpha-glucosidase inhibitors
Drug classes used for the treatment of HPN, CHD, and Peripheral vascular disorders Diuretics
Beta blockers
ACE inhibitors
Calcium channel blockers
Alpha blockers
Nitrites
Antiarrhythmics
Cholesterol lowering agents
Statins
Niacin
Gemfibrozil
Clofibrates
colestipol
IX. Other Diagnoses

Opportunistic illnesses included in the AIDS case definition should not be coded in the Other Diagnoses section. This section includes other infections, not AIDS, other conditions, or other primary, metastasis, or secondary neoplasm excluding the AIDS-defining or AIDS-related cancers. The purpose of the Other Diagnoses section of the Medical History Form is to collect all diagnoses present, active, and requiring treatment during the visit prior to the surveillance period. Note: do not enter any diagnoses before the visit prior to the surveillance period unless the condition extends to the visit prior to the surveillance period. If the condition is completely treated and cured and does not require further follow up (e.g., regular lab follow up or treatment), then do not document the diagnosis in this section.
In this section there is a list of common clinical conditions, diagnoses, and adverse events that can occur in patients with HIV infection as a consequence of HIV disease or due to antiretroviral therapy. This is followed by a blank space to enter diagnoses or ICD codes when given in the medical records. For any diagnoses present, active, and requiring treatment during the visit prior to the surveillance period, determine whether this diagnoses was new during this period, or is an existing diagnosis (from previous visits), or an adverse event of antiretroviral therapy. For each diagnosis listed in this section enter the diagnosis status code in the box associated with each diagnosis. If the condition’s diagnosis status code is 3 “adverse event” re-enter the data in the space following the table listing the diagnosis.
To be entered as an adverse event there needs to be a clear note by the treating physician stating that this is an adverse event. Search and enter the name of the offending agent if clearly stated by the treating physician. If no offending agent is suggested, leave the space blank or enter “unknown”. If there is a diagnosis of a primary or secondary neoplasm in the medical record, check the box corresponding to the list on the table and enter the diagnosis with site codes on the space provided. For other diagnosis not listed in Section IX, enter the diagnosis or ICD code and other related information in to the blank space in the section. In a situation that there are more than 21 active diagnoses during this period write the additional diagnosis in the notes page on the last page of the form.
Please refer to the attached list of other diagnosis that we are collecting if these diagnoses were ever made or the patient was treated for after the patient was diagnosed with HIV. This will help us in understanding the prevalence of certain chronic conditions that we probably would have missed because of the design of the abstraction form.
![]()
Diagnoses with qualifying terms: Often in medical records, qualifying words are used with diagnoses to denote the degree of certainty surrounding the diagnosis. As a general rule of thumb, diagnoses described with the following commonly used “qualifying words” should be counted in MMP:
“diagnostic procedure results consistent with. . .”
“presumptive . . .”
“probable . . .”
“likely . . .” (this one is rather soft--consider cautiously)
“responded to treatment for . . .”
On the other hand, diagnoses described by the following qualifiers should not be considered established diagnoses, and should generally not be coded in MMP:
“treat as. . .” (e.g., if a severe diagnosis such as sepsis is suspected, treatment may be initiated pending further diagnostic tests). Reviewing the clinical course and the results of diagnostic tests may assist in determining whether this is an established diagnosis. Alternatively, consultation with the health care provider may be necessary.
“questionable diagnosis of . . .”
“Diagnosis A vs. Diagnosis B . . .”
“conceivable. . .”
“differential diagnosis includes X, Y, and Z. . .”
“symptoms of . . .”
“iffy. ..”
“plausible . . .”
“possible . . .”
“potential . . .”
“questionable . . .”
“rule out (abbreviated R/O) . . .”
“suspect(ed) . . .”
Accepting physicians’ diagnosis: In general, for MMP, physicians’ diagnoses for Other Infections, Not AIDS and Other Conditions should be accepted. However, specific organisms should not be recorded with the diagnosis unless a culture result can be found or unless the medical record mentions a culture in which an organism was isolated. For example, if a physician makes a diagnosis of acute staphylococcal impetigo based on the appearance of skin lesions, but no cultures were performed, the diagnosis of impetigo should be reported, but the organism Staphylococcus should not be included in the diagnosis.
Historical information: When a patient states that he or she has had a history of diagnosis X,Y,or Z during the visit prior to the surveillance period, accept and record these diagnoses if treatment is continued during the visit prior to the surveillance period or the condition is chronic and warrants further follow up or is one of the conditions in the list of chronic co-morbid conditions MMP will collect. Similarly, when the health care provider makes reference to previous medical conditions, perhaps diagnosed elsewhere, these should be counted for MMP.
Patients’ complaints: For situations in which a patient is concerned about a particular condition (e.g., “patient noticed oral thrush this week” or “patient thinks she has a vaginal yeast infection”), but there is no evidence of a physician diagnosis in the physical exam, the assessment, or the plan, do not report as an established diagnosis for MMP.
Other infections, not AIDS
Specify all non AIDS-defining infection diagnoses if present, active, or requiring treatment during this abstraction period.
Other primary neoplasms, not AIDS-defining
Specify all malignant neoplasms that are present, active, or requiring treatment during the visit prior to the surveillance period. A neoplasm diagnosed before this interval should be coded unless there is evidence that it has been cured (e.g., hysterectomy for cervical cancer).
Important Note: Do not code malignant neoplasm included in the AIDS Case Definition in this section. AIDS-defining malignant neoplasms should be coded in the Diseases Indicative of AIDS section.
Benign neoplasms and neoplasms of unspecified behavior should be coded; metastatic sites should not be recorded. For example, adenocarcinoma of the lung with metastases to brain and liver should be coded as “adenocarcinoma of the lung.” This means that both primary and metastasis will be checked, however, site code will be entered only for the primary site.
Each diagnosis should have the corresponding ICD code entered if it is available in the medical record; if only ICD code is available the code should be entered.
X. Laboratory Data

Date of Earliest HIV-Positive Test
Record the month and the year of the patient's earliest HIV-positive test in the appropriate spaces provided on the form. If the date of the earliest HIV-positive test is not known, check "Date Unknown/Not documented" and leave the month and year fields blank.
Please Note: The patient's earliest HIV-positive test should not be obtained from the HARS database, but left blank if this information is not in the patient's medical records. See Documentation of Earliest HIV-Positive Test below.
The names of months should be converted to numeric representations and years should be recorded in full. Single digit months should be preceded by a zero.
For example, February 1986 would be recorded as "02 / 1986."
Documentation of Earliest HIV-Positive Test
Indicate how the patient's earliest HIV-positive test is documented by checking the appropriate box (only one box should be checked). If the earliest positive test is documented by several means, check the first that is listed on the data collection form (the choices are in order of importance, starting with a laboratory report). For example, if the patient's earliest positive test is documented by a physician's report and a laboratory report, only check "Laboratory report" since this appears first in the hierarchy of importance.
“Physician report" should only be checked if the patient's medical record contains a letter or report from another physician that documents the earliest positive test. If the earliest positive test is mentioned in a physician's note without any formal confirmation or was provided by the patient, "Patient self-report" should be checked. If the earliest positive test is documented by a means not listed on the questionnaire, check "Other" and record how the test is documented in the adjacent field.
Please Note: Since a laboratory report is the preferred means of documenting the patient's earliest HIV-positive test, always search the available medical records for a laboratory report even if a physician report or patient self-report is already available.
Other laboratory data
CD4+ Results
Record the First Documented CD4 count at this facility, the Lowest Ever CD4, and Highest Ever CD4, the Most Recent CD4 result the patient had ever including the visit just prior to the earliest visit in the surveillance period and enter the dates of these tests in the appropriate fields. CD4 results may be obtained from both inpatient and outpatient reports and reports from the patient or other physician.
Enter the documentation method of each result. Check only one method but if more than one method is available for the same result check in the following hierarchical order (lab report > physician report> patient-self report > other). Record the CD4 percent, even if the count is available.
Viral Load Results
Record the First Documented Viral Load test at this facility, the Lowest Ever, and Highest Ever, the Most Recent Viral Load test the patient had ever including the visit just prior to the earliest visit in the surveillance period and enter the dates of these tests in the appropriate fields. Viral load test results may be obtained from both inpatient and outpatient reports and reports from the patient or other physician. Enter the documentation method of each result. Check only one method but if more than one method is available for the same result check in the following hierarchical order (lab report > physician report> patient-self report > other), and enter the method in the blank space.
Please Note: The viral load results should be recorded exactly as they are documented in the patient's medical record.
For example, if a result was reported as <500 copies/ml, then the viral load result would be recorded as "<500," not "500" or "499."
As another example, if a result was reported as undetected, then the viral load result would be recorded as "Undetected," not "0" or "<###."
Exponential numbers and logarithmic (log) values should not be converted to whole numbers.
For example, if a result was reported as 8.65 X 10E3 copies/ml, then the viral load result would be recorded as "8.65 X 10E3," not "8.65" or "8,650."
As another example, if a result was reported as log 4.7 copies/ml, then the viral load result would be recorded as "log 4.7," not "4.7" or "50,000."
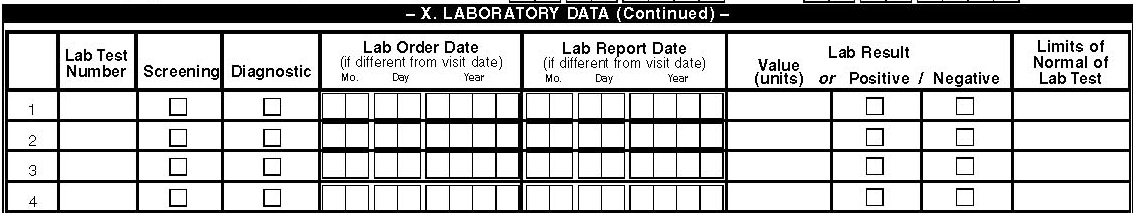
The Laboratory Data section will be used to enter laboratory tests that were done during the visit prior to the surveillance period. Abnormal test results for the following tests, ALT, AST, Creatinine, FBS, TC, LDLc, anti HAV, anti HBV, anti HCV, HCV genotype, HBV genotype, hematocrit and TG) will also be entered. For HCV related tests determine if the test was done any time in the past and enter the date of test for the first positive test if any. Please enter the test number from the list of codes on page 4 (e.g., #1 = anti HAV) to the column titled lab test number if this test was done. If in the part of the medical record where this test was requested there is a mention of screen for Hepatitis A then check screening. If the test was requested and the comment was to rule out hepatitis or abnormal LFT then check diagnosis. When the laboratory report date is different from the laboratory order date by more than 2 weeks then enter both dates. There is no need to enter the date of the order date for most tests as we are looking for tests during the period prior to the surveillance period only.
The laboratory results can be quantitative, e.g., CD4 count = 444) or qualitative (VDRL = positive/negative/indeterminate). When the lab tests have quantitative results there are both upper and lower limits of normal and units of tests depending on the type of test. This limit of normal also varies from lab to lab and this is the reason why we need to enter the limits of normal as per the reporting laboratory. This is especially important for chemistry tests and for hormone assays.
Phenotypic or Genotypic Resistance Testing
Indicate whether phenotypic and/or genotypic or virtual phenotypic resistance testing was EVER performed (only one box should be checked). Genotypic and phenotypic resistance testing are FDA licensed procedures to determine whether HIV is resistant to one or more antiretroviral agents. These tests may appear under the following names:
Antivirogram
Vircogen
HIV-GenotypR Plus
HIV sequencing
Recombinant phenotyping
Results of phenotypic testing may be represented as MIC values, expressed as “X-fold resistance,” or expressed as “sensitive,” “mild (moderate, intermediate) resistance,” or “resistance (resistant).” Since only a few labs use to specialize on phenotypic and particularly virtual phenotypic testing we would like to get information on which labs performed the test. The interpretation of the results may also vary by lab performing the test. In the last column of the phenotype table enter the lab code. The lab code will be attached to the abstractors’ manual. This code will be a three digit number for the specified lab. Since each state can have it’s own home brew labs a blank space will be left for you to enter the lab names. Final coding will be performed when data collection is completed.
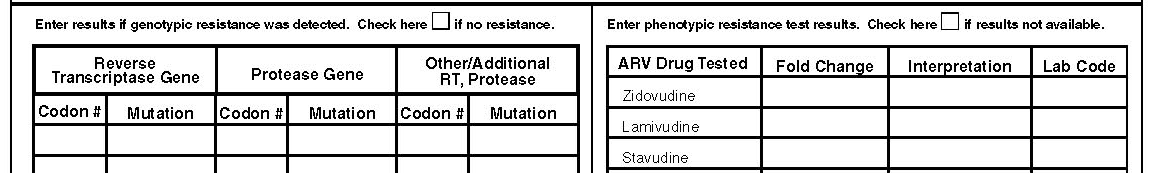
Results of genotypic testing are usually reported as genetic mutations (X###Y, or ###Y), for example:
L90M
90M
M184V
184V
Results of genotypic testing may be referred to as a “virtual phenotype”. “Virtual phenotype” results should be recorded as genotypic results, NOT as phenotypic results. However, if phenotypic results are provided along with “virtual phenotype” results, box 4 should be checked. In this form this part is used to enter all detected genotypic resistance mutations to the different genes tested. Enter all resistant mutations from as many tests as are available during this period. If the same mutation occurs more than once in different tests enter it only once. You can choose to enter the codon number as (184) or as (M184) and the mutation as (V). The purpose of this part of the abstraction is to find out the pattern of prevalence of mutations that patients harbor as they come into the surveillance period. What is entered here may be very different from what the patient harbors in one resistance test but it is clearly known that patients who have some form of mutation usually will have them either circulating in their blood if there is constant selection by drugs or will have the mutation archived for expression in later days.
This is the list of laboratories that test for antiretroviral resistance. This list might not be complete or might not include labs that perform resistance testing in your jurisdiction. You can enter the three digit code for the lab which performed the phenotypic test in the space under lab code. Enter the name of the lab as other if not found on this list or enter the name of the lab if it is common lab for your site.
001 Applied Sciences
002 Dynacare
003 Lab Corp
004 Microbiology Reference Laboratory
005 Specialty Laboratories
006 Quest Diagnostics
007 Rheumatology Diagnostics Laboratory (RDL)
008 University of Washington
009 Virco
010 ViroLogic
011 Viromed
012 Visible Genetics
013 American medical laboratories (AML)
014 Other
XI. Substance Abuse/Mental Health
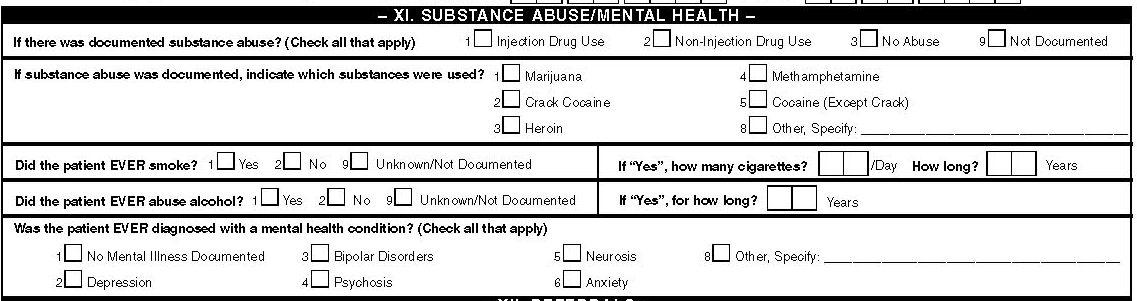
Substance Abuse
Indicate whether the patient was diagnosed with substance abuse and there was a documentation of any of the types of substance abuse listed during the visit prior to the surveillance period (multiple boxes may be checked). If the patient's medical record documents that they have not abused any substances, or if there is no information on substance abuse in the medical record, check the No Abuse or Abuse Not Documented box. Either patient self-report or provider diagnosis may be used to document a history of substance abuse. This information will also be simultaneously collected in the Other Diagnoses section (Section V) if patient required treatment or follow up for such conditions during the visit prior to the surveillance period. This section will inform of ongoing abuse in contrast to prior use/abuse requiring current treatment.
Injection Drug Use
To meet the definition of injection drug use, the patient's medical record must document that they (1) injected illicit drugs (including references to “skin popping”), (2) injected drugs obtained without a prescription or used contrary to medical indication, or (3) required treatment for injection drug abuse.
Please Note: Examples of some drugs commonly injected include:
Amphetamines (speed) and other stimulants
Cocaine
Heroin and other opiates
Speedball (heroin and cocaine)
Steroids
Also Note: If the patient used a drug that is commonly injected, such as heroin, but there is no documentation that the patient injected the drug, check "Non-injection Drug Use." The patient's medical record must document that they injected the drug to check "Injection drug use."
Non-injection Drug Use
To meet the definition of non-injection drug abuse, the patient's medical record must document that they (1) used illicit non-injection drugs; (2) used non-injection drugs obtained without a prescription or used contrary to medical indication, or (3) required treatment for non-injection drug abuse.
Also Note: Examples of some common non-injection drugs include:
Amphetamines (speed) and other stimulants
Barbiturates
Cocaine (including crack)
Heroin and other opiates
Marijuana and hashish
Nitrites, poppers, and other inhalants
PCP, LSD, and other hallucinogens
Steroids
Valium and other benzodiazepines
Also Note: Marijuana used for medical purposes does not constitute substance abuse. Do not check non-injection drug use.
Abused Substances
If substance abuse occurred, indicate which substances were abused by checking the appropriate box (multiple boxes may be checked). If club drugs were abused, write the specific drug in the blank provided.
Please Note: Examples of club drugs include:
Ecstasy, MDMA, X, XTC
Fentanyl, Actiq
GHB, Gamma-hydroxybutyrate
Ketamine, Special-K, Vitamin K, cat valiums
LSD, lysergic acid diethylamide, acid
Rohypnol, Roofies
Cigarette smoking
If the patient is currently or has ever been a smoker and there is documentation in the medical record, enter Yes for “Did the patient ever smoke?” and record information on the number of cigarettes per day and for length of time smoked to the nearest year. If the number of cigarettes smoked is recorded in pack-years in the medical record, enter this number and clearly state the unit of measure.
Alcohol Abuse
To meet the definition of alcohol abuse for Section V, the patient's medical record must document that they abused alcohol, were treated for alcohol abuse, or reported heavy alcohol use. In this section enter Yes only for alcohol abuse during the interval before the visit prior to the surveillance period. This section is to determine the prevalence of such behaviors currently, therefore, if the patient is on treatment for chronic alcoholism and not currently using alcohol enter No.
Mental Health Conditions
Indicate whether the patient’s medical record documented the following conditions (multiple boxes may be checked):
Severe Mental Illness
Diagnosis of the following mental illnesses in the past should be recorded (multiple boxes may be checked):
Affective disorder (bipolar disorder, severe depression, mania, manic-depression, severe mood disorder, dysthymia, anxiety, and neurosis)
Post-traumatic stress disorder (PTSD)
Schizophrenia (psychotic disorder, schizo-affective, other psychoses)
Please Note: The above diagnoses may occur as mild, moderate, or severe manifestations; only severe manifestations that required treatment for or referral to a mental health specialist should be documented.
XII. Referrals

Document if the patient was referred to any sources of additional care at any time from first HIV-related visit to the visit prior to the surveillance period. Information collected in this block will also help to understand the services that are frequently required by patients. Enter Yes if there is documentation of referral, No if it is clearly indicated that patient was not referred, and Unknown/Not Documented if there is no information. If the patient was advised of the availability and usefulness of these services but refused to be referred enter Yes. If patients have requested for any of the specified services and there is a note documenting this communication enter Yes. Search for this information not only in the physicians’ notes but also in the nurses’, case managers’, and social workers’ notes, too.
XIII. Other information
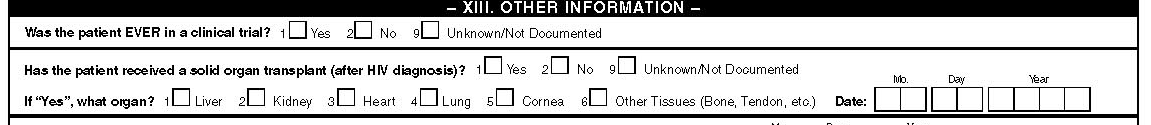
Clinical trial
Indicate whether the patient participated in a clinical trial involving medications to treat HIV-related conditions during the interval. Specific information about the type of trial or the medication involved in the clinical trial is not documented. There is no need to enter the number of different clinical trials the patient has participated in during this time. The period covered by this abstraction form is the time from the first HIV related visit to this facility to the visit prior to the surveillance period.
Solid organ transplant
As patients with HIV are surviving longer, organ transplantation is becoming more frequent. Information on the prevalence of organ transplantation and the type of organ or tissue that is transplanted will be collected. Enter Yes if organ transplantation was done and enter the organ transplanted. Check all appropriate boxes if more than one organ was transplanted and enter in the blank space if not on the list. The period covered by this abstraction form is the time from the first HIV related visit to this facility to the visit prior to the surveillance period.
In this part we will also like to capture information on other tissues (in addition to solid organs) including cornea, bone, and pancreas. Since there is no way that we can capture transfusion please enter any type of transfusion under others in this section and specify (like RBC, platelets, FFP, etc).
Facial implants and liposuction
Facial implants and liposuction are treatment modalities offered to patients who develop the specific side effects of antiretroviral therapy, namely facial lipoatrophy (wasting of the buccal area due to selective loss of fat) and lipodystrophy (abnormal deposition of fat on the trunk).
Check Yes if the patient received facial implants and document the date received.
Check Yes if the patient received liposuction and document the date this procedure was performed. If the patient has received liposuction more than once, document the most recent date of liposuction during this period. Also enter other treatments targeting removal of fat as it is possible that fat removal can also be done without liposuction. Information on the site of liposuction or fat removal is not collected.
Dialysis
Patients could be receiving chronic courses of either peritoneal or hemodialysis due to chronic renal failure pending other treatments. This is a significant cause of morbidity and can complicate the course of antiretroviral therapy and adherence to therapy. If the patient has required dialysis enter Yes. Information on renal failure and blood chemistry supporting the indications for dialysis will also be collected.
Family history
In this section the information to be entered is family history of the conditions on the list. Most of this information will be available on the intake form completed by the patient or the medical history in the chart.
XIV. Remarks
This space can be used to document information that is conflicting, requires discussion with your project coordinator and/or the CDC project officer, or if there is additional information to enter from any section.
Thank you very much for going over this manual. Remember this is a manual that will help you as you abstract information using the Medical History Form. The manual for the Surveillance Period (visit) Form will follow. Please do not hesitate to communicate your comments, questions, and concerns about this abstractor’s manual. We hope this will make your abstraction easier and enjoyable. Good luck!
| File Type | application/msword |
| File Title | Attachment 5 |
| Author | evu0 |
| Last Modified By | USER |
| File Modified | 2007-06-07 |
| File Created | 2007-06-07 |
© 2026 OMB.report | Privacy Policy