Attachment 5B3
MMP Attach 5 B3.doc
Medical Monitoring Project
Attachment 5B3
OMB: 0920-0740
Attachment 5 B3
Toxoplasma Antibody Titer
Indicate whether the patient had a toxoplasma antibody titer during the visit by checking the appropriate box (only one box should be checked). If yes, indicate the results by checking the appropriate box.
Also Note: Alternative names for a toxoplasma antibody titer are:
Toxoplasma antibody IgG
Tuberculin Skin Test
Indicate whether the patient has a tuberculin skin test (TST) during this visit by checking the appropriate box. If Yes, record the result using the boxes provided (only one box should be checked).
Also Note: Alternative names for a tuberculin skin test are:
Mantoux
Purified protein derivative (PPD)
Pap smear
Cervical Pap smears (women only)
This question should be completed only for female patients. In the past traditionally Pap smear information was collected only from women since the test was offered only for women to screen for cervical cancer (HPV). However, this test is now also offered for screening for anorectal cancer both in women and in men who have sex with men.
Anal Pap smears (men and women)
Complete this form both for men and women. If a woman has cervical and anal Pap smear enter yes for both questions and enter the appropriate results for the corresponding tests. Indicate whether the patient received a Pap smear during this visit by checking the appropriate box (only one box should be checked).

Hepatitis A Vaccine
Indicate whether the patient received Hepatitis A vaccine by checking the appropriate box (only one box should be checked). Hepatitis A vaccination is indicated for HIV-infected patients. It is given in two intervals. If the patient received a combination Hepatitis A and B vaccine enter accordingly; do not enter as hepatitis A and hepatitis B separately.
Also Note: Alternative names for a Hepatitis A vaccine are:
Havrix
Vaqta
Hepatitis B Vaccine
Indicate whether the patient received Hepatitis B vaccine by checking the appropriate box (only one box should be checked). This vaccine is administered at 0 month, month 1 and month 6.
Also Note: Alternative names for a Hepatitis B vaccine are:
Comvax
Engerix-B
Recombivax HB
Combination Hepatitis A and B Vaccine
Indicate whether the patient received a combination Hepatitis A and B vaccine by checking the appropriate box (only one box should be checked).
Also Note: An alternative name for combination Hepatitis A and B vaccine is:
Twinrix
Influenza Vaccine
Indicate whether the patient received influenza (flu) vaccine by checking the appropriate box (only one box should be checked). This vaccine is generally administered once every year between September and March. Patients can get this vaccination from places other than where they go for their HIV care and including drug stores, which may be documented in the physicians notes. Particular attention should be given to this possibility of vaccination documentation when vaccine is not given at the care facility.
Please Note: Brand names of flu vaccines include:
Fluogen
Fluzone
Fluviren
Flushield
Pneumococcal Vaccine
Indicate whether the patient received pneumococcal vaccine (Pneumovax 23, Pnu-Immune 23) by checking the appropriate box. This vaccine is administered once every five years for patients whose CD4 count is greater or equal to 200 cells/µl. For patients who received pneumococcal polysaccharide vaccine when their CD4 count was less than 200 cells/µl the guidelines recommend that they receive another vaccination as soon as their CD4 count rebound above 200 cells/µL. Remember that in 2000 the FDA approved a different vaccine which is known as pneumococcal conjugate vaccine (PCV) which is mainly used for prevention of invasive pneumococcal disease in children.
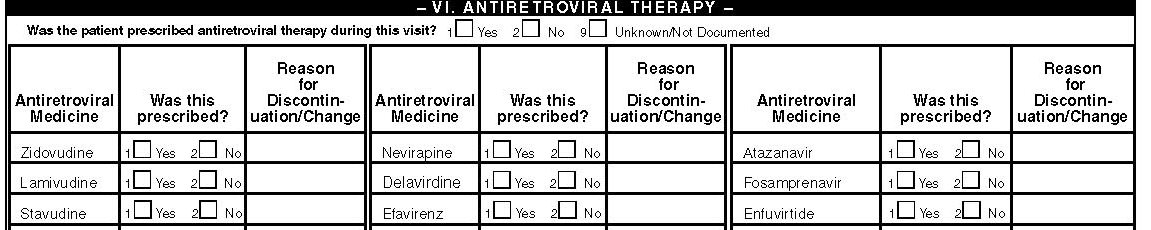
VI. Antiretroviral Therapy
Was the patient prescribed antiretroviral therapy during this visit?
Indicate whether the patient has a history of antiretroviral therapy at any time during this visit by checking the appropriate box. This includes antiretroviral therapy that the patient is taking currently or just started.
Antiretroviral Regimens
Collect only the name of antiretroviral drug that was prescribed in the chart. No information on dosage and duration of treatment will be collected. For any prescription made during this visit check the box under the column titled “Was this prescribed?” next to the prescribed medication. For medicines that were discontinued or changed search for the reasons in the medical record and enter the applicable code from the list of codes following the table.
Also enter the name of medication for which there is documentation of receipt in the medical records by patient history, referral notes or doctor’s report. For patients prescribed combination pills enter as prescribed, not as a separate pills (if Combivir is prescribed enter accordingly; not as Zidovudine and Lamivudine). If patient is not receiving antiretroviral therapy skip to the next section. It is possible that a patient can be on and off treatment during the surveillance period. Therefore, it is possible that the patient have a visit with antiretroviral therapy and another visit without therapy. Under this table there is a code for reason for discontinuation/change of antiretroviral medication at this visit. Please enter the code closest to the words of the physician in the space next to the medicine that was discontinued or changed. In this form we are not collecting reasons for prescribing the new regimen. If the reason for discontinuation/change is not documented enter ”not indicated in medical record.”
VII. Other Treatments
In order to understand the problem of drug interactions, effect on quality of care and adherence to other treatments and pill burden, and to get an indirect assessment of the spectrum of conditions for which patients with HIV/AIDS require medical treatment; information will be collected on treatments other than antiretroviral medications. The list of medicines used by a patient can range from just a few to too much to enter on the available space in the surveillance period form. All treatments (other than antiretroviral) will be entered into this part of the surveillance period form. Since this form is designed to collect the name of the medicines as documented by the physician you may encounter difficulty in understanding what is written in the charts. It is important to exercise caution in transcribing this information to the abstraction form. When there are difficulties in understanding the information on prescriptions, it should be clarified before you leave the facility or within a short interval thereafter.
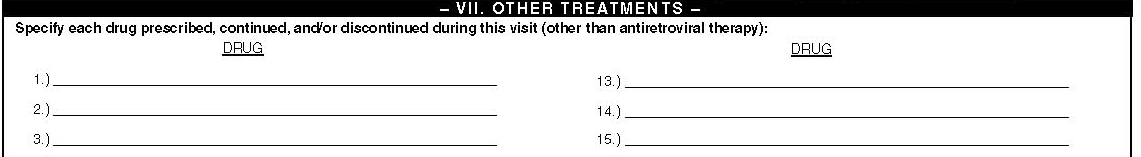
VIII. Other diagnoses

Opportunistic illnesses included in the AIDS case definition should not be recorded in the Other Diagnoses section. This section included other infections, not AIDS, other conditions, or other primary or secondary neoplasm excluding the AIDS-defining or AIDS-related cancers. The Other Diagnoses section of the Surveillance Period (Visit) Form will collect all diagnoses present, active, and requiring treatment during this visit. Note: do not enter any diagnoses from the period earlier than surveillance period if it does not extend to the surveillance period. If the condition is completely treated and cured and does not require further follow up (regular lab follow up or treatment), then do not document the diagnosis in this section.
In this section there is a list of common clinical conditions, diagnoses, and adverse events that can occur in patients with HIV infection as a consequence of HIV disease or due to antiretroviral therapy. This is followed by a blank space to enter diagnoses or ICD codes when given in the medical records. For any diagnoses present, active, and requiring treatment during this visit determine whether this diagnoses was new during this visit, or is an existing diagnosis (from previous visits), or an adverse event of antiretroviral therapy. For each diagnosis listed in this section enter the diagnosis status code in to the box associated with each diagnosis. If the condition’s diagnosis status code is 3 “adverse event” re-enter the data into the space following the table listing the diagnoses.
To be entered as an adverse event there needs to be a clear note by the treating physician stating that this is an adverse event. Search and enter the name of the offending agent if clearly stated by the treating physician. If no offending agent is suggested, leave the space blank or enter “unknown”. If there is a diagnosis of a primary or secondary neoplasm in the medical record, check the box corresponding to the list on the table and enter the diagnosis with site codes on the space provided. For other diagnosis not listed in Section VIII, enter the diagnosis or ICD-9 code and other related information in to the blank space in the section. In a situation that there are more than 10 active diagnoses during this period write the additional diagnosis in the notes page on the last page of the form.
Diagnoses with qualifying terms: Often in medical records, qualifying words are used with diagnoses to denote the degree of certainty surrounding the diagnosis. As a general rule of thumb, diagnoses described with the following commonly used “qualifying words” should be counted in MMP:
“diagnostic procedure results consistent with. . .”
“ presumptive . . .”
“probable . . .”
“likely . . .” (this one is rather soft--consider cautiously)
“responded to treatment for . . .”
On the other hand, diagnoses described by the following qualifiers should not be considered established diagnoses, and should generally not be coded in MMP:
“treat as. . .” (e.g., if a severe diagnosis such as sepsis is suspected, treatment may be initiated pending further diagnostic tests). Reviewing the clinical course and the results of diagnostic tests may assist in determining whether this is an established diagnosis. Alternatively, consultation with the health care provider may be necessary.
“questionable diagnosis of . . .”
“Diagnosis A vs. Diagnosis B . . .”
“conceivable. . .”
“differential diagnosis includes X, Y, and Z. . .”
“symptoms of . . .”
“iffy. ..”
“plausible . . .”
“possible . . .”
“potential . . .”
“questionable . . .”
“rule out (abbreviated R/O) . . .”
“suspect(ed) . . .”
Accepting physicians’ diagnosis: In general, for MMP, physicians’ diagnoses for Other Infections, Not AIDS and Other Conditions should be accepted. However, specific organisms should not be recorded with the diagnosis unless a culture result can be found or unless the medical record mentions a culture in which an organism was isolated. For example, if a physician makes a diagnosis of acute staphylococcal impetigo based on the appearance of skin lesions, but no cultures were performed, the diagnosis of impetigo should be reported, but the organism Staphylococcus should not be included in the diagnosis.
Historical information: When a patient states that he or she has had a history of diagnosis X,Y,or Z during a visit in the surveillance period, accept and record these diagnoses if treatment is continued or the condition is chronic and warrants further follow up. Similarly, when the health care provider makes reference to previous medical conditions, perhaps diagnosed elsewhere, these should be counted for MMP.
Patients’ complaints: For situations in which a patient is concerned about a particular condition (e.g., “patient noticed oral thrush this week” or “patient thinks she has a vaginal yeast infection”), but there is no evidence of a physician diagnosis in the physical exam, the assessment, or the plan, do not report as an established diagnosis for MMP.
Other infections, not AIDS: Specify all non AIDS-defining infection diagnoses if present, active, or requiring treatment during this visit.
Other primary neoplasms, not AIDS-defining
Enter in this section all malignant neoplasms that are present, active, or requiring treatment during this visit.
A neoplasm diagnosed before this interval should be coded unless there is evidence that it has been cured (e.g., hysterectomy for cervical cancer).
Important Note: Do not code a malignant neoplasm included in the AIDS Case Definition in this section. AIDS-defining malignant neoplasms should be coded in the Diseases Indicative of AIDS section.
Benign neoplasms and neoplasms of unspecified behavior should be coded; metastatic sites should not be recorded. For example, adenocarcinoma of the lung with metastases to brain and liver should be coded as “adenocarcinoma of the lung.” This means that both primary and metastasis will be checked, however, site code will be entered only for the primary site.
Each diagnosis should have the corresponding ICD-9-CM code entered if it is available in the medical record; if only ICD code is available the code should be entered.
IX. Laboratory Data

In this section of the form we will abstract most laboratory tests that were performed during each visit. The laboratory data section is divided into four sub-sections. The first sub-section contains a list of test codes that will be used to enter data for tests and results in the space, below the list (second sub-section). The third sub-section is used to collect information on resistance to antiretroviral medicines and the fourth sub-section will collect information on antimicrobial resistance.
Enter the lab tests code in the column under the heading “lab test number”. If there is any indications of whether the test was done for screening or diagnosis enter a mark in either one or both boxes as applicable. Leave these boxes blank if there is no information on the purpose for testing. It is only necessary to enter a date in the column for Lab order date if that date is different from the date of the visit. In addition, if the date the lab test was ordered does not differ from the report date by more than 10 days, it is not necessary to enter a date in the column for Lab report date. If there is a difference of more than 10 days then enter the date of lab report. The lab results can be entered either qualitatively or quantitatively as available. Enter the limits of normal for the tests when given because these may differ from lab to lab.
It is possible that some records will contain so many results that it is not possible to enter all of them in the space provided. In this case give priority to tests specifically used to evaluate the progression of HIV disease (like CD4 and viral load) followed by blood chemistry, other tests with abnormal test results and finally blood cell counts.
Phenotypic or Genotypic Resistance Testing
Indicate whether phenotypic and/or genotypic or virtual phenotypic resistance testing was performed (only one box should be checked). Genotypic and phenotypic resistance testing are FDA licensed procedures to determine whether HIV is resistant to one or more antiretroviral agents. These tests may appear under the following names:
Antivirogram
Vircogen
HIV-GenotypR Plus
HIV sequencing
Recombinant phenotyping
Results of genotypic testing are usually reported as genetic mutations (X###Y, or ###Y), for example:
L90M
90M
M184V
184V
Results of genotypic testing may be referred to as “virtual phenotype”. “Virtual phenotype” results should be recorded as genotypic results, NOT as phenotypic results. However, if phenotypic results are provided along with “virtual phenotype” results, box 4 should be checked. In this form this part is used to enter all detected genotypic resistance mutations to the different genes tested. Enter all resistant mutations detected from a genotypic or virtual resistance testing done during this visit.
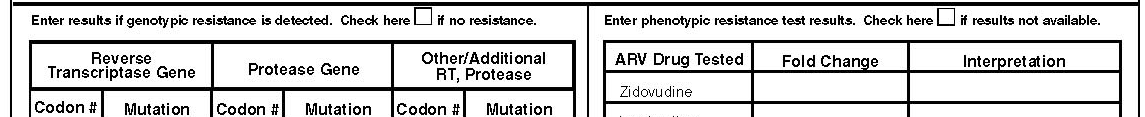
Results of phenotypic testing may be represented as MIC values, expressed as “X-fold resistance,” or expressed as “sensitive,” “mild (moderate, intermediate) resistance,” or “resistance (resistant).” Since only a few labs use to specialize on phenotypic and particularly virtual phenotypic testing we would like to get information on which labs performed the test. The interpretation of the results may also vary by lab performing the test. In the last column of the phenotype table enter the lab code. The lab code will be attached to the abstractors’ manual. This code will be a three digit number for the specified lab. Since each state can have its own home brew labs a blank space will be left for you to enter the lab names. Final coding will be performed when data collection is completed.
This is the list of laboratories that test for antiretroviral resistance. This list might not be complete or might not include labs that perform resistance testing in your jurisdiction. You can enter the name of the lab as other if not found on this list
001 Applied Sciences
002 Dynacare
003 LabCorps
004 Microbiology Reference Laboratory
005 Specialty Laboratories
006 Quest Diagnostics
007 Rheumatology Diagnostics Laboratory (RDL)
008 University of Washington
009 Virco
010 ViroLogic
011 Viromed
012 Visible Genetics
013 American medical laboratories (AML)
014 Other
Antimicrobial resistance testing
Was antimicrobial culture and sensitivity test ordered during this visit?
For this section of laboratory data, first determine if the physician has ordered an antimicrobial culture and sensitivity testing during the visit. If testing was ordered and results are available, enter the diagnosis that prompted the culture and sensitivity testing, the code for the site of specimen, the organisms identified and the antibiotics to which the bacterium is resistant. Enter the scientific name (eg. Nisseria meningitides or N. meningitides) instead of the morphology (eg. Gram negative diplococcus).
The specimen site code for the site of specimen collection is listed below. It is advisable to carry a copy of this code whenever you go out for abstraction. Please be aware that it is possible to have specimen taken from many sites for a single diagnosis.
001 Blood
002 CSF
003 Pleura
004 Peritoneum
005 Pericardium
006 Urine
007 Stool
008 Urethra
009 Cervix
010 Skin
011 Nares
012 Infected wound
013 Other
X. Substance Abuse/Mental Health

Substance Abuse
Indicate whether the patient was newly diagnosed with use or continued (previously diagnosed) substance use during this visit. If yes, determine whether there was documentation of any of the types of substance abuse listed during this visit and check the corresponding box (multiple boxes may be checked). If the patient's medical record documents that they have not abused any substances, or if there is no information on substance abuse in the medical record, check the No Abuse box. Either patient self-report or provider diagnosis may be used to document a history of substance abuse. This information will also be simultaneously collected in the Other Diagnoses section (Section VIII) if patient required treatment or follow up for such conditions during this visit. This section will provide information about ongoing use in contrast to prior use requiring current treatment.
Injection Drug Use
To meet the definition of injection drug use, the patient's medical record must document that they (1) injected illicit drugs (including references to “skin popping”), (2) injected drugs obtained without a prescription or used contrary to medical indication, and (3) required treatment for injection drug abuse.
Please Note: Examples of some drugs commonly injected include:
Amphetamines (speed) and other stimulants
Cocaine
Heroin and other opiates
Speedball (heroin and cocaine)
Steroids
Also Note: If the patient used a drug that is commonly injected, such as heroin, but there is no documentation that the patient injected the drug, check "Non-injection drug use." The patient's medical record must document that they injected the drug to check "Injection drug use."
Non-injection Drug use
To meet the definition of non-injection drug abuse, the patient's medical record must document that they (1) used illicit non-injection drugs; (2) used non-injection drugs obtained without a prescription or used contrary to medical indication, and (3) required treatment for non-injection drug abuse.
Also Note: Examples of some common non-injection drugs include:
Amphetamines (speed) and other stimulants
Barbiturates
Cocaine (including crack)
Heroin and other opiates
Marijuana and hashish
Nitrites, poppers, and other inhalants
PCP, LSD, and other hallucinogens
Steroids
Valium and other benzodiazepines
Also Note: Marijuana used for medical purposes does not constitute non-injection drug use.
Abused Substances
If substance abuse occurred, indicate which substances were abused by checking the appropriate box (multiple boxes may be checked). If club drugs were abused, write the specific drug in the blank provided.
Please Note: Examples of club drugs include:
Ecstasy, MDMA, X, XTC
Fentanyl, Actiq
GHB, Gamma-hydroxybutyrate
Ketamine, Special-K, Vitamin K, cat valiums
LSD, lysergic acid diethylamide, acid
Rohypnol, Roofies
Cigarette smoking
If it is documented in the medical record that the patient is a current smoker, enter Yes for “Is the patient currently a smoker?” and enter the number of cigarettes smoked per day and the length of time for which the patient has been a smoker to the nearest whole year. If the number of cigarettes smoked is recorded in pack-years in the medical record enter this number and clearly state the unit of measure.
Alcohol abuse
To meet the definition of alcohol use for Section X, the patient's medical record must document that they abused alcohol, were treated for alcohol abuse, or reported heavy alcohol use. In this section enter Yes only if there is documentation of alcohol abuse. This section is to determine the prevalence of such behaviors currently, therefore, if the patient is on treatment for chronic alcoholism and not currently using alcohol enter No. Enter any quantifying information on current use of alcohol in the space provided.
Mental Health Conditions
Indicate whether the patient’s medical record documented the following conditions (multiple boxes may be checked):
Severe Mental Illness
Diagnosis of the following mental illnesses in the past should be recorded (multiple boxes may be checked):
Affective disorder (bipolar disorder, severe depression, mania, manic-depression, severe mood disorder, dysthymia, anxiety, and neurosis)
Post-traumatic stress disorder (PTSD)
Schizophrenia (psychotic disorder, schizo-affective, other psychoses)
Please Note: The above diagnoses may occur as mild, moderate, or severe manifestations; all levels of severity and manifestations that required treatment for or referral to mental health specialist should be included for the purposes of completing these check boxes. This part will capture both the incident mental health conditions and prevalent conditions that are requiring follow up and/or treatment.
XI. Referrals
![]()
Document if the patient was referred to any sources of additional care at or around this visit. Information collected in this block will also help to understand the services that are frequently required by patients. Enter Yes if there is documentation of referral, No if it is clearly indicated that patient was not referred, and Unknown/Not Documented if there is no information. If the patient was advised of the availability and usefulness of these services but refused to be referred enter Yes. If patients have requested for any of the specified services and there is a note documenting this communication enter Yes. Search for this information not only in the physicians’ notes but also in the nurses’, case managers’, and social workers’ notes, too.
In addition, document discussions on smoking cessation, condom use and sex safe, HIV disclosure to other partners, domestic violence prevention and adherence to therapy. The documentation of the discussion is what is important, not who the patient had the discussion with.
XII. Reproductive Health
This section is completed only for persons who have documented pregnancy during the surveillance period. Pregnancy can be documented in the medical history by a health care provider or there can be a positive pregnancy test in the laboratory section. It is preferable to enter this information on reproductive health after the outcome of pregnancy has been recorded, i.e., after delivery or some form of termination of the pregnancy if this outcome of pregnancy occurred earlier in the surveillance period. If the pregnancy continues past the surveillance period, enter continuing pregnancy and enter the gestational age at the last visit.
We will be collecting information on maternal services and outcomes. Information on the neonate including prenatal treatment and postnatal outcomes will not be collected. This information may be available both at the primary physician and the Ob-Gyn specialist. Information will be collected from Ob-Gyn specialists for women who indicated they have received care from these specialists even if the specialty clinic was not included in the sample. The abstraction for the prenatal care received from the Ob-Gyn clinic will be completed only once either at the end of the surveillance period or at the visit the outcome occurred.

XIII. Mortality data
As explained earlier, this section is completed only for patients who died during the period between interview and medical record abstraction. This is one of the few pieces of information that is collected for a time that is beyond the end of the surveillance period.
Vital status at the end of this visit
Since the eligibility for medical record abstraction depends on consent obtained during interview and the abstraction period ends on the date of interview, it is not possible that we will have death as a vital status for most patients. In the rare event that a patient dies on the day of interview or the patient who dies during the time between the interview and abstraction, it will be useful to capture information on death and events related to death. The names of months should be converted to numeric representations and years should be recorded using 4 digits. Single digit months or days should be preceded by a zero.
For example, if the patient died on February 15, 2006, the date of death would be recorded as "02 / 15 / 2006."
Please Note: If any part of the date of death is unknown, the space(s) corresponding to the missing information should contain a decimal point(s). However, we think this date will be available as the time that this event can occur is a defined interval well demarcated by the end of PDP and date of interview.
Enter any cause of death if death was documented in the medical record. In the first part of this section enter the immediate cause of death on the first line (a) and sequentially list causes (if any) leading to the immediate cause of death. Here is an example of how this form will be completed. Enter ventricular fibrillation as immediate cause of death in (a), and then enter left sided massive transmural myocardial infarction in (b) where it says due to (as a consequence of), then enter atherosclerotic heart disease in (c) and finally enter due to diabetes mellitus in (d). The interval between onset and death will be entered as 4 minutes for immediate cause, may be 1 day or 24 hours for myocardial infarction, 5 years for atherosclerotic heart disease, and finally 20 years for diabetes mellitus.
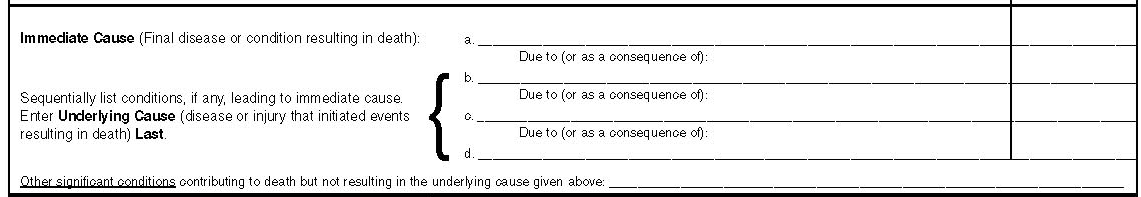
Enter Yes if an autopsy was performed and enter available information on the manner of death. This information will be readily available when it is documented in the death certificate and can be directly copied into the abstraction form.
If this information is not available in the death certificate or if the death certificate is not yet available, it will be worthwhile to complete the second part of the mortality data section. Most of the information here will be diagnosis at death rather than cause of death. It is not necessarily true that the diagnosis at death is the cause of death. However, this information is also very helpful in understanding the circumstances around death including the prevalent diagnoses around time of death.
XIV. Other information
Clinical trial
Indicate whether the patient participated in a clinical trial involving medications to treat HIV-related conditions at the time of this visit. Specific information about the type of trial or the medication involved in this clinical trial is not collected. There is no need to enter the number of different clinical trials the patient participated at this time.
Solid organ transplant
As patients with HIV are surviving longer, organ transplantation is becoming more frequent. Information on the prevalence of organ transplantation and the type of organ or tissue that is transplanted will be collected. Enter Yes if organ transplantation was done and enter the organ transplanted. Check all appropriate boxes if more than one organ was transplanted and enter in the blank space if not on the list.
In this part we will also like to capture information on other tissues (in addition to solid organs) including cornea, bone, and pancreas. Since there is no way that we can capture transfusion please enter any type of transfusion under others in this section and specify (like RBC, platelets, FFP, etc). Blood and blood transfusion can also be entered in the blank space following Other, Specify.
Facial implant and Liposuction
Facial implants and liposuction are treatment modalities offered to patients who develop the specific side effects of antiretroviral therapy, namely facial lipoatrophy (wasting of the buccal area due to selective loss of fat) and lipodystrophy (abnormal deposition of fat on the trunk).
Check Yes if the patient received facial implants during this visit.
Check Yes if the patient received liposuction during this visit. Also enter other treatments targeting removal of fat as it is possible that fat removal can also be done without liposuction. Information on the site of liposuction or fat removal is not collected.
Dialysis
Patients could be receiving chronic courses of either peritoneal or hemodialysis due to chronic renal failure pending other treatments. This is a significant cause of morbidity and can complicate the course of antiretroviral therapy and adherence to therapy. If the patient required dialysis at any time between visits enter Yes. Information on renal failure and blood chemistry supporting the indications for dialysis will also be collected.
.
Nutritional support, Alternative therapy, and Potency enhancing drugs
Enter information on nutritional support between visits and during the visit including parenteral feeding and enterostomy. If the patient has volunteered information about use of alternative therapy, enter this accordingly. Alternative therapy includes herbal treatment, acupuncture and others. Also search the records for prescription of potency enhancing drugs during this visit.
XV. Remarks
This space can be used to document information that is conflicting, requires discussion with your project coordinator and/or the CDC project officer, or if there is additional information to enter from any section.
Thank you very much for going over this manual. Remember this is a manual that will help you as you abstract information using the Surveillance Period (Visit) Form. Also note that there are additional attachments explaining the approaches to abstracting medical records of patients selected using real time sampling and first list of two list pickup. Refer to the manual for the Medical History Form for competing that form. Please do not hesitate to communicate your comments, questions, and concerns about this abstractor’s manual. We hope this will make your abstraction easier and enjoyable. Good luck!
| File Type | application/msword |
| File Title | Surveillance Period (SP) (abstract events occurring during this period) |
| Author | evu0 |
| Last Modified By | ziy6 |
| File Modified | 2006-11-09 |
| File Created | 2006-11-09 |
© 2026 OMB.report | Privacy Policy