Form No number No number Healthcare provider survey
Evaluation of Genomic Applications in Practice and Prevention (EGAPP)
Healthcare Provider Survey Revised 2-6-09
(EGAPP) - Health Care Providers (att D1)
OMB: 0920-0751
Attachment
D1 - Healthcare Provider Survey
This survey is intended for health care providers accessed through: 1) professional organizations; 2) health plans or HMOs; and 3) health care payers/insurers.
Note: Skip patterns will be programmed into the online form, making a streamlined survey for respondents.
Objectives – Types of information to be collected include:
Identify general descriptive characteristics of respondents (e.g., type of practitioner, medical specialty, practice setting).
Understand respondents’ awareness of the EGAPP project and products (e.g., evidence reports, EGAPP Working Group recommendations).
Determine if the respondent has read any specific EGAPP products (e.g., published or web-posted evidence reports, published recommendations).
Get feedback on whether specific products address their needs.
Identify any perceived impact on the respondents’ clinical practice (e.g., decision to offer testing).
Form
Approved
OMB
No.: 0920-0751
Exp.
Date: 8/31/2010
Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Survey
Introduction to the EGAPP Survey
Evaluation of Genomic Applications in Practice and Prevention (EGAPP) is an initiative launched in 2004 by the National Office of Public Health Genomics (NOPHG) at the Centers for Disease Control and Prevention (CDC). The efforts of EGAPP are focused around an independent, non-federal, multidisciplinary EGAPP Working Group. The goal of EGAPP is to establish a systematic, evidence-based process to assess the effectiveness of selected genetic tests that are in transition from research to clinical and public health practice.
Products of the EGAPP project include evidence reports on selected genetic tests and published EGAPP Working Group recommendations on the appropriate use of the tests based on the evidence collected. Some evidence reports sponsored by the EGAPP project are conducted and released by Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers.
To evaluate the value and impact of the EGAPP products, an independent consultant has been contracted to survey key stakeholder groups, including healthcare providers, healthcare payers and purchasers, certain policy organizations, targeted consumer groups, and website visitors. Response to these surveys is very important to inform the EGAPP Working Group and CDC about the best methods and approaches for future review of the effectiveness of emerging genetic tests, and about the potential impact of accurate and timely information on genetic tests on current healthcare practices.
Your feedback will provide important information about the relevance of EGAPP products to your practice. The questions relate only to EGAPP-supported evidence reports and EGAPP Working Group Recommendations. Thank you for your time and assistance.
Public reporting burden of this collection of information is estimated to range between 5 and 10 minutes with an average of 8 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-0751).
Please note: While taking the survey, please do not use your browser's back and/or forward buttons; please only use the next and previous buttons within the survey. Thank you.
1. What is your role as a healthcare provider?
___ Physician
___ Laboratory Director
___ Physician Assistant
___ Genetic Counselor
___ Nurse Practitioner
___ Nurse
___ Other (please specify): ________________________________________
___ I am no longer in practice
SUBMIT
2. What is your primary specialty?
___ Family Medicine
___ Internal Medicine
___ Obstetrics/Gynecology
___ Oncology
___ Pathology
___ Psychiatry
___ Clinical Genetics
___ Other (please specify): ________________________________________
3. Which best describes the setting in which you practice? (Please check only one)
___ Hospital
___ Group practice
___ Solo practice
___ HMO
___ Academic medical center
___ Independent laboratory
___ Other (please specify): ________________________________________
In what country do you practice?
___ United States
___ Other (please specify): _________Exit survey with note SUBMIT
5. What is your sex?
___ Male
___ Female
6a. What is your ethnicity?
___ Hispanic or Latino
___ Not Hispanic or Latino
6b. What is your race (Select one or more)?
___ American Indian or Alaska Native
___ Asian
___ Black or African American
___ Native Hawaiian or Other Pacific Islander
___ White
7. How many years of professional experience do you have?
___ 0 - 4 years
___ 5 - 9 years
___ 10 - 14 years
___ 15 -19 years
___ 20 or more years
8. Prior to this survey, had you read or heard about EGAPP?
___ yes
___ no Skip to question 10a (first survey distribution) or 11a (second survey distribution)
___ unsure Skip to question 10a (first survey distribution) or 11a (second survey distribution)
9. Where have you read or heard about EGAPP activities? (Please check all that apply)
____ I read about EGAPP on the CDC or www.egappreviews.org website.
____ I heard about EGAPP through a professional journal/newsletter.
____ A colleague told me about EGAPP
____ I learned about EGAPP at a meeting.
____ Other (please describe) _______________________________________
If you checked the "I heard about EGAPP through a professional journal/newsletter" response above, please specify that journal/newsletter title here: _______________
If you checked the "I learned about EGAPP at a meeting" response above, please specify that meeting here: _______________
If this is the second survey distribution, respondents will be skipped to question 11a.
Following is a set of questions about a genetic test for which EGAPP has completed an evidence review and a recommendation. The test is described briefly before the questions.
Cytochrome P450 (CYP450) genotyping is a genetic test proposed for use in patients treated for depression with selective serotonin reuptake inhibitors (SSRIs) to help in selection of drug and dosage.
10a. Are you aware of the CYP450 genetic test?
___ yes
___ no Skip to question 13
___ unsure Skip to question 13
10b. From what source(s) have you heard about the CYP450 genetic test? (check all that apply)
___ An EGAPP-sponsored evidence report or published summary
___ An EGAPP Working Group recommendation
___ Primary research/review article
___ Professional organization
___ Colleague
___ Meeting/conference
___ News media
___ Other (please specify)
The following questions refer specifically to the evidence report/published summary and EGAPP Working Group recommendation on CYP450 testing
10c. Have you read the EGAPP-sponsored evidence report on CYP450 testing, or a published summary of the evidence report?
____ yes ____ no ____unsure
If no or unsure, respondent skips to item 10f
10d. How understandable did you find the evidence report/published summary to be?
___very understandable ____somewhat understandable ___not understandable
10e. Will the evidence on CYP450 testing influence your decision about whether to offer the test to your patients with depression treated with SSRIs?
___ yes ___ no ___ unsure
If yes, please explain: _____________________________________
10f. Have you read the EGAPP Working Group recommendation about the use of
CYP450 testing in patients with depression treated with SSRIs?
___ yes ___ no ___unsure
If no or unsure, respondent skips to item 10j
10g. How understandable did you find the EGAPP recommendation to be?
___very understandable ____somewhat understandable ___not understandable
10h. Will the EGAPP recommendation on the use of CYP450 testing influence your decision about whether to offer the test to your patients with depression treated with SSRIs?
___ yes ___ no ___ unsure
If yes, please explain: _____________________________________
10i. Which will be more useful to you in your practice? (Please check one)
___evidence report/published summary ___EGAPP recommendation ___Not applicable
10j. Are you currently offering CYP450 testing to your patients with depression treated with SSRIs?
___ yes ___ in some cases ___ no ___ unsure
10k. Please provide any comments about the evidence report/published summary or EGAPP recommendations on the use of CYP450 testing that you feel would improve the information for health care providers.
-
Comment box here
If this is the first survey distribution, respondents will be skipped to question 13.
Testing for Hereditary Non-Polyposis Colorectal Cancer (HNPCC or Lynch Syndrome) in newly diagnosed colorectal cancer patients and their families may be may be offered to a selected subset of high risk patients to detect a heritable form of colorectal cancer.
11a . Are you aware of genetic testing for HNPCC (Lynch Syndrome) in patients with newly diagnosed colorectal cancer?
___ yes
___ no Skip to question 12a
___ unsure Skip to question 12a
11b. From what source(s) have you heard about genetic testing for HNPCC (Lynch Syndrome)? (Please check all that apply)
___ An EGAPP-sponsored evidence report or published summary
___ An EGAPP Working Group recommendation
___ Primary research/review article
___ Professional organization
___ Colleague
___ Meeting/conference
___ News media
___ Other (please specify)
The following questions refer specifically to the evidence report/published summary and EGAPP recommendation on HNPCC (Lynch Syndrome) testing
11c. Have you read the EGAPP-sponsored evidence report on genetic testing for HNPCC (Lynch Syndrome), or a published summary of the evidence report?
____ yes ____ no ____unsure
If no or unsure, respondent skips to item 11f
11d. How understandable did you find the evidence report/published summary to be?
___very understandable ____ somewhat understandable ___ not understandable
11e. Will this information on genetic testing for HNPCC (Lynch Syndrome) influence your decision on whether to offer genetic testing for HNPCC (Lynch Syndrome) to patients with newly diagnosed colorectal cancer?
___ yes ___ no ___ unsure
If yes, please explain: _____________________________________
11f. Have you read the EGAPP Working Group recommendation about the use of genetic testing for HNPCC (Lynch Syndrome) in patients with newly diagnosed colorectal cancer?
___ yes ___ no ___unsure
If no or unsure, respondent skips to item 11j
11g. How understandable did you find the EGAPP recommendation to be?
___very understandable ____ somewhat understandable ___ not understandable
11h. Will this recommendation on the use of genetic testing for HNPCC (Lynch Syndrome) influence your decision on whether to offer genetic testing for HNPCC (Lynch Syndrome) to patients with newly diagnosed colorectal cancer?
___ yes ___ no ___ unsure
If yes, please explain: _____________________________________
11i. Which will be more useful to you in your practice? (Please check one.)
___evidence report/published summary ___ EGAPP recommendation ____ not applicable
11j. Are you currently using genetic testing for HNPCC (Lynch Syndrome) in patients with newly diagnosed colorectal cancer?
___ yes ___ in some cases ___ no ___ unsure
11k. Please provide any comments about the EGAPP sponsored evidence report/published summary or EGAPP recommendation on genetic testing for HNPCC (Lynch Syndrome) that you feel would improve the information for providers.
-
Comment box here
UGT1A1 testing is a pharmacogenetic test for colorectal cancer patients treated with irinotecan.
12a. Are you aware of genetic testing for UGT1A1 in colorectal cancer patients treated with irinotecan?
___ yes
___ no Skip to question 13
___ unsure Skip to question 13
12b. From what source(s) have you heard about genetic testing for UGT1A1? (Please check all that apply)
___ An EGAPP-sponsored evidence report or published summary
___ An EGAPP Working Group recommendation
___ Primary research/review article
___ Professional organization
___ Colleague
___ Meeting/conference
___ News media
___ Other (please specify)
The following questions refer specifically to the evidence report/published summary and EGAPP recommendation on UGT1A1 testing
12c. Have you read the EGAPP-sponsored evidence report on genetic testing for UGT1A1, or a published summary of the evidence report?
____ yes ____ no ____unsure
If no or unsure, respondent skips to item 12f
12d. How understandable did you find the evidence report/published summary to be?
___very understandable ____ somewhat understandable ___ not understandable
12e. Will this information on genetic testing for UGT1A1 influence your decision on whether to offer genetic testing for UGT1A1 to colorectal cancer patients treated with irinotecan?
___ yes ___ no ___ unsure
If yes, please explain: _____________________________________
12f. Have you read the EGAPP Working Group recommendation about the use of genetic testing for UGT1A1 in colorectal cancer patients treated with irinotecan?
___ yes ___ no ___unsure
If no or unsure, respondent skips to item 12j
12g. How understandable did you find the EGAPP recommendation to be?
___very understandable ____ somewhat understandable ___ not understandable
12h. Will this recommendation on the use of genetic testing for UGT1A1 influence your decision on whether to offer genetic testing for UGT1A1 to colorectal cancer patients treated with irinotecan?
___ yes ___ no ___ unsure
If yes, please explain: _____________________________________
12i. Which will be more useful to you in your practice? (Please check one.)
___evidence report/published summary ___ EGAPP recommendation
____ not applicable
12j. Are you currently using genetic testing for UGT1A1 for colorectal cancer patients treated with irinotecan?
___ yes ___ in some cases ___ no ___ unsure
12k. Please provide any comments about the EGAPP sponsored evidence report/published summary or EGAPP recommendation on genetic testing for UGT1A1 that you feel would improve the information for providers.
-
Comment box here
13. Have you read any EGAPP sponsored evidence reports/published summaries or EGAPP recommendations other than the one/those mentioned in this survey?
___ yes ___ no ___unsure
Respondent directed to 14 if no or unsure to:
10a and 13 (first survey distribution)
11a and 12a and 13 (second survey distribution)
Respondent directed to question 15 if yes to 13 (first and second survey distribution)
14. If an evidence-based report on a specific test and a recommendation from a credible expert panel were available, please indicate your response to the statements below based on the scale given:
.
Evidence-based information/guidelines on genetic tests are useful in my practice to:
![]()
understand what uses of the test are supported by
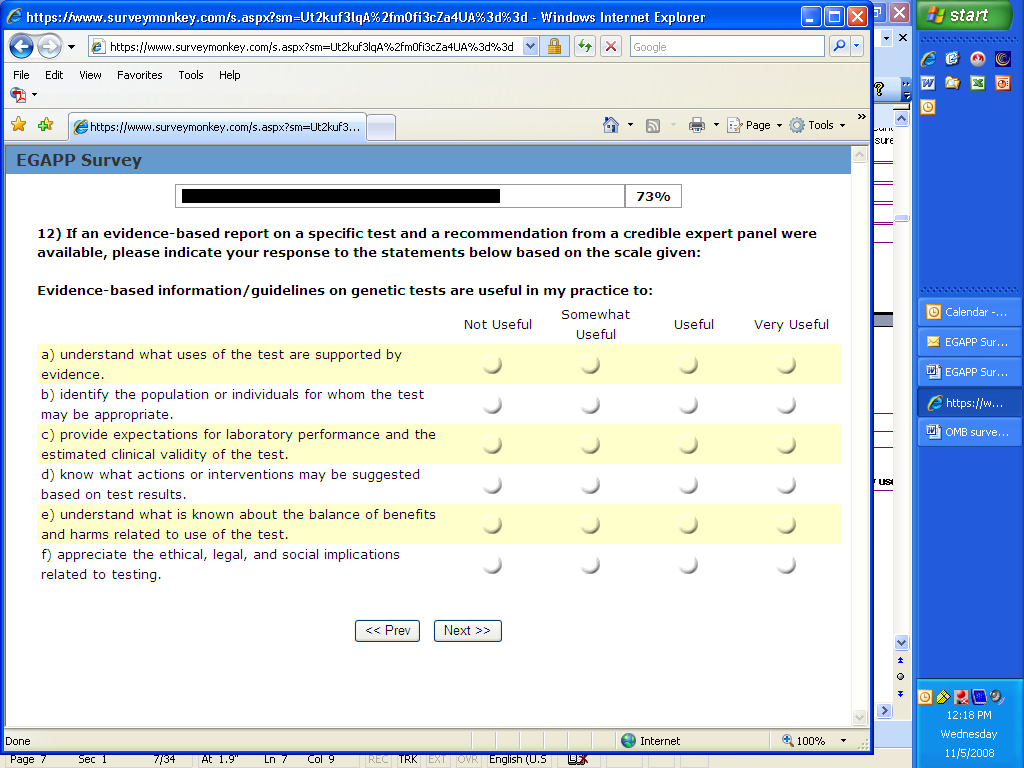
evidence.
identify the population or individuals for
whom the test may be appropriate.
provide expectations for laboratory performance
and the estimated clinical validity of the test.
know what actions or interventions may be
suggested based on test results.
understand what is known about the balance
of benefits and harms related to use of the test.
appreciate the ethical, legal, and social
implications related to testing.
Respondents completing 14 are skipped to 18
Question 15 will only be asked of those respondents who have read at least one EGAPP-sponsored evidence review/summary or recommendations.
15. Based on your experience with EGAPP sponsored evidence reports and/or Working Group recommendations, please indicate your response to the statements below based on the scale given:
Evidence-based information/guidelines on genetic tests are useful in my practice to:
![]()
understand what uses of the test are supported by

evidence.
identify the population or individuals for
whom the test may be appropriate.
provide expectations for laboratory performance
and the estimated clinical validity of the test.
d) know what actions or interventions may be
suggested based on test results.
understand what is known about the balance
of benefits and harms related to use of the test.
appreciate the ethical, legal, and social
implications related to testing.
16. Have you visited the EGAPP website: www.egappreviews.org?
___ yes
___ no Skip to question 18
17. How useful did you find the EGAPP website?
___very useful ____ somewhat useful ___ not useful
18. If you have other comments you would like to make please do so in the box below.
-
COMMENT BOX HERE
This is the end of the survey, thank you for your feedback. Click the "Submit" button below to submit your responses.--------→ SUBMIT
| File Type | application/msword |
| File Title | DETS Project Evaluation Interviews |
| Author | Lynn M. Short, PhD, MPH |
| Last Modified By | shari steinberg |
| File Modified | 2009-02-09 |
| File Created | 2009-02-05 |
© 2026 OMB.report | Privacy Policy