Facility staff Providing Estimated Patient Loads
Medical Monitoring Project
Attachment7_ Abstraction Manual
Facility staff Providing Estimated Patient Loads
OMB: 0920-0740
Attachment 7
Abstractor
Manual

For Medical Abstraction Forms version 2.0.4

CDC/NCHHSTTP/DHAP-SE/BCSB
Clinical
Outcomes Team
March
2008
TABLE
OF CONTENTS
GENERAL
ISSUES......................................................................................................................1
MEDICAL
HISTORY FORM
(MHF)..............................................................................................7
OPTIONAL
–
FOR LOCAL
USE
ONLY...............................................................................................................7
SECTION
I. ABSTRACTION AND IDENTIFICATION
.............................................................................................7
SECTION
II. PATIENT
DEMOGRAPHICS.........................................................................................................10
SECTION
III. MEDICAL HISTORY
FORM SECTION –
OPTIONAL.......................................................................11
SECTION
IV. AIDS DEFINING OPPORTUNISTIC ILLNESS
(AIDS-OI)...............................................................12
SECTION
V. CONDITIONS OTHER THAN AIDS-OI
.........................................................................................15
SECTION
VI. PROPHYLAXIS
........................................................................................................................17
SECTION
VII. HEPATITIS,TOXOPLASMA, AND TUBERCULOSIS
(TB)SCREENING............................................19
Screening
for Hepatitis
A/B/C..............................................................................................................19
Screening
for
Toxoplasma...................................................................................................................21
Screening
for Tuberculosis
..................................................................................................................22
SECTION
VIII. HEPATITIS AND PNEUMOCOCCAL IMMUNIZATIONS
..................................................................24
Immunizations:
Hepatitis A/B/A &
B.....................................................................................................24
Immunizations:
Pneumococcal
pneumonia.........................................................................................25
SECTION
IX. ANTIRETROVIRAL THERAPY
(ART)..........................................................................................27
SECTION
X. LABORATORY TEST
RESULTS
..................................................................................................29
HIV
Test Results
..................................................................................................................................30
CD4
Counts
.........................................................................................................................................30
HIV
Viral Load
Results.........................................................................................................................31
Liver
Function [AST (SGOT) and ALT (SGPT)] Test
Results..............................................................31
SECTION
XI. HIVART RESISTANCE TESTING
.............................................................................................33
SECTION
XII. SUBSTANCE
ABUSE...............................................................................................................37
SECTION
XIII. MENTAL
HEALTH..................................................................................................................39
SECTION
XIV. FAMILY HISTORY
.................................................................................................................40
SECTION
XV.
REMARKS.............................................................................................................................40
SURVEILLANCE
PERIOD SUMMARY FORM (SPSF)
.............................................................41
OPTIONAL
– FOR LOCAL
USE
ONLY.............................................................................................................41
SECTION
I. ABSTRACTION AND IDENTIFICATION
...........................................................................................41
SECTION
II. PATIENT DEMOGRAPHICS
.........................................................................................................43
SECTION
III. SURVEILLANCE PERIOD SUMMARY FORM SECTIONS –
OPTIONAL..............................................43
SECTION
III. SURVEILLANCE PERIOD SUMMARY FORM SECTIONS –
OPTIONAL..............................................43
SECTION
IV. REIMBURSEMENT
...................................................................................................................44
SECTION
V. OTHER SERVICES
...................................................................................................................46
SECTION
VI. TUBERCULOSIS (TB), CERVICAL AND ANAL CANCER SCREENING
.............................................48
Screening
for Tuberculosis
..................................................................................................................48
Screening
for Cervical and Anal
Cancer..............................................................................................49
SECTION
VII. HEPATITIS, INFLUENZA, AND PNEUMOCOCCAL IMMUNIZATIONS
................................................50
Immunizations:
Hepatitis A/B/A &
B.....................................................................................................50
Immunizations:
Influenza.....................................................................................................................51
Immunizations:
Pneumococcal
pneumonia.........................................................................................52
SECTION
VIII. REFERRALS
.........................................................................................................................53
SECTION
IX. PREGNANCIES AND OUTCOMES (FEMALES ONLY)
....................................................................56
SECTION
X. FAMILY
HISTORY.....................................................................................................................57
SECTION
XI. MORTALITY
DATA...................................................................................................................58
SECTION
XII. OTHER
FACILITIES.................................................................................................................59
SECTION
XIII. REMARKS
............................................................................................................................59
SURVEILLANCE
PERIOD VISIT FORM
(SPVF).......................................................................60
OPTIONAL
– FOR LOCAL
USE
ONLY.............................................................................................................60
SECTION
I. ABSTRACTION AND IDENTIFICATION
...........................................................................................60
SECTION
II. PATIENT
DEMOGRAPHICS.........................................................................................................62
SECTION
III. CHIEF COMPLAINTS
................................................................................................................64
SECTION
IV. SURVEILLANCE PERIOD VISIT FORM SECTIONS –
OPTIONAL.....................................................65
SECTION
V. AIDS DEFINING OPPORTUNISTIC ILLNESSES
(AIDS-OI)............................................................66
SECTION
VI. CONDITIONS OTHER THAN AIDS-OI
.......................................................................................68
SECTION
VII. PROPHYLAXIS
.......................................................................................................................73
SECTION
VIII. SEXUALLY TRANSMITTED
INFECTION (STI)
SCREENING..........................................................75
SECTION
IX. ANTIRETROVIRAL THERAPY
....................................................................................................77
SECTION
X. OTHER
MEDICATIONS..............................................................................................................79
SECTION
XI. LABORATORY TEST
RESULTS
.................................................................................................80
SECTION
XII. SUBSTANCE
ABUSE...............................................................................................................83
SECTION
XIII. REMARK
..............................................................................................................................85
SURVEILLANCE
PERIOD INPATIENT FORM
(SPIF)..............................................................86
OPTIONAL
– FOR LOCAL
USE
ONLY.............................................................................................................86
SECTION
I. ABSTRACTION AND IDENTIFICATION
...........................................................................................86
SECTION
II. CHIEF COMPLAINTS
.................................................................................................................89
SECTION
III. SURVEILLANCE PERIOD INPATIENT FORM SECTION –
OPTIONAL................................................90
SECTION
IV. AIDS DEFINING OPPORTUNISTIC ILLNESS
(AIDS-OI)................................................................91
SECTION
V. CONDITIONS OTHER THAN AIDS-OI
.........................................................................................93
SECTION
VI. HEPATITIS
SCREENING...........................................................................................................98
SECTION
VII. ANTIRETROVIRAL
THERAPY..................................................................................................100
SECTION
VIII. OTHER
MEDICATIONS.........................................................................................................102
SECTION
IX. LABORATORY TEST
RESULTS...............................................................................................103
SECTION
X. REMARKS
.............................................................................................................................104
General Issues
As outlined under “Purpose and Scope” in the Medical Monitoring Project (MMP) protocol, the primary objectives of MMP are to obtain data from a national probability sample of HIV-infected persons receiving care in the United States to
Describe the clinical and virologic status of these persons
Describe the prevalence of co-morbidities related to HIV disease
Describe HIV care and support services received and the quality of such services
Determine prevalence of ongoing risk behaviors and access to, and use of, prevention services among persons living with HIV
Identify met and unmet needs for HIV care and prevention services to inform prevention and care planning groups, health care providers, and other stakeholders
To accomplish these objectives, medical record abstraction should be completed for all eligible sampled participants who have received medical care during the surveillance period. Medical care is defined as a visit to the facility for medical treatment and follow up, laboratory test, or prescription of medications, including refill authorizations.
At which facilities should medical record abstraction be completed?
Whenever possible, medical record information should be obtained from ALL facilities where a participant has received medical care for HIV infection during the Surveillance Period. In addition to the facility where the participant was sampled, there may be others:
Facility log sheet – a list of facilities reported by the participant during the MMP interview
Medical records – during abstraction, there may be references to medical care received at other facilities (e.g., hospital admissions, medical referrals, transfers).
Whenever it is not possible to conduct abstraction at ALL facilities that provided HIV care to a participant during the Surveillance Period, high priority should be placed on completing abstraction at the following places:
Facility where the participant was sampled
Facility reported by participant as being the primary provider of his/her medical care for HIV.
Facilities where the participant received inpatient care during the surveillance period.
2
For which time period should medical record abstraction be done?
The following table summarizes the time period covered by the vast majority of MMP medical record abstraction:
Table 1. Medical History Period and Surveillance Period, for Medical Record Abstraction, Medical Monitoring Project (MMP), 2007
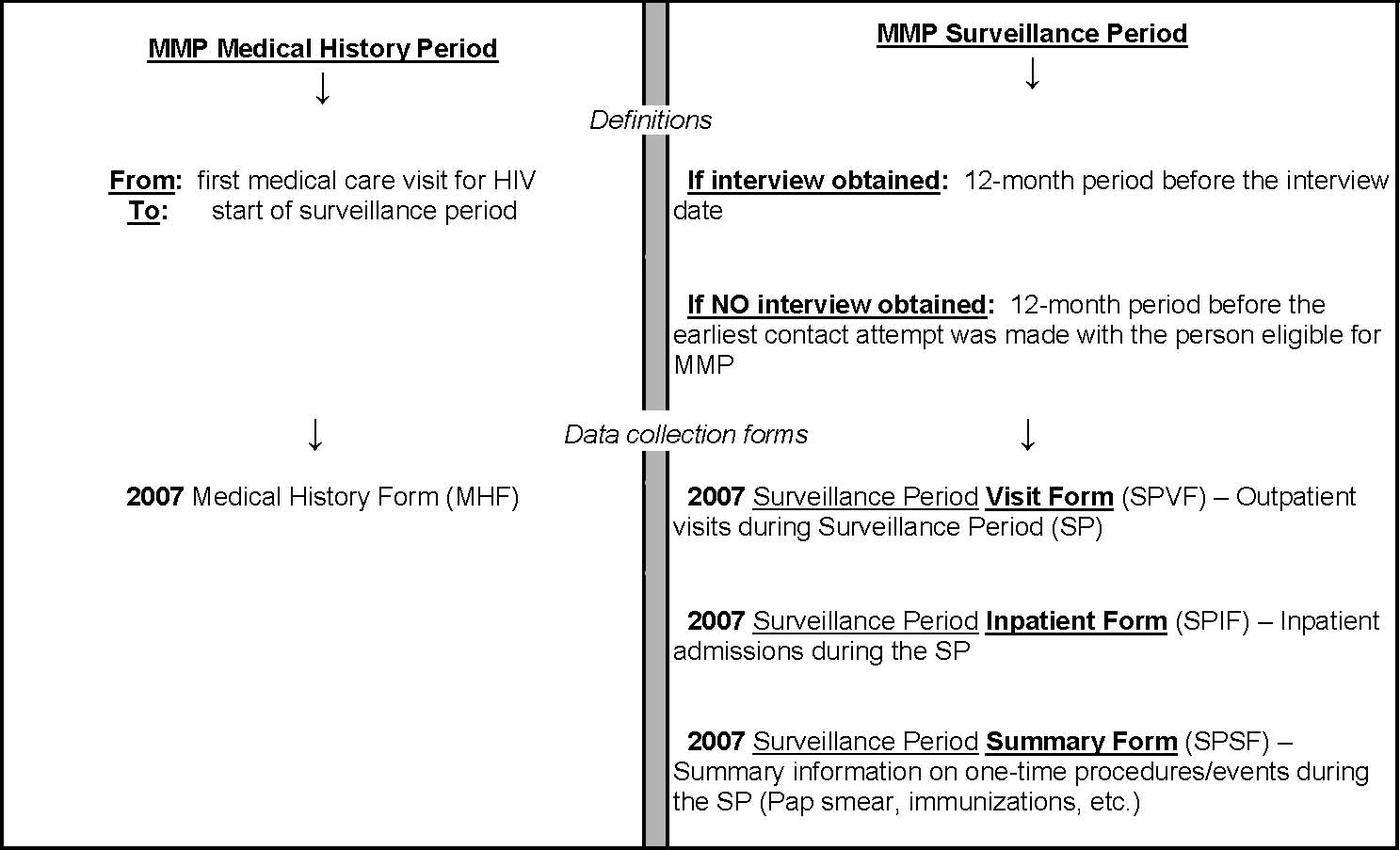
*** EXCEPTION *** Although the vast majority of medical record abstraction will cover the duration from first medical care visit for HIV infection until the date of the interview (or the earliest attempt to contact the patient), the following medical record information from before that time frame should be collected:
Diagnosis of Hepatitis B and/or C
Vaccination against Hepatitis A and/or B
TB screening tests
TB prophylaxis
This information is needed to assess the standard of care, with regards to screening for, vaccinating against, or providing treatment for these illnesses.
For example, a provider might not have vaccinated the patient against hepatitis B during the medical history period or the surveillance period because the patient was known to be immune, based on earlier testing. This may not be known without reviewing medical records from an earlier time period – perhaps even before the patient became HIV-infected.
Note that the end of the medical history period is defined by the beginning of the surveillance period (the 12-month period before the MMP interview date or the earliest contact attempt with the patient).
This definition of the surveillance period is important for relating information from the abstraction component of MMP with information from the patient interview component, in which many questions refer to behaviors or events in the past 12 months.
If a patient is newly diagnosed with HIV or is new to care, it is possible that fewer than 12 months have passed before the MMP interview (or earliest contact attempt) – in which case, there would be no medical history period. In this case, an MHF should still be used to record some limited information, such as date of birth, sex, race/ethnicity. The reason is because the data fields for recording this type of general patient information are only available on the MHF. Thus, an MHF should be completed for every patient.
At any particular facility, whether inpatient or outpatient, be sure to consider all the information that is available for abstraction at that facility for the patient. To be sure that the abstraction is complete, the following questions should be investigated when requesting medical records on a patient at a facility.
Are there records from both the medical history period as well as the surveillance period at that facility?
Are there inpatient medical records as well as outpatient medical records?
Which abstraction forms to use for which purpose?
As shown in Table 1 above, four different forms will be used to collect data in 2007.
• The MHF will be used to abstract information from the “Medical History Period.”
- Before abstracting, the abstractor should determine which facilities provided care to the patient during the Surveillance Period.
-For each of the above facilities (which provided care during the SP), one MHF should be completed.
-If a patient is newly diagnosed with HIV or is new to care, it is possible that fewer than 12 months have passed before the MMP interview (or earliest contact attempt) – in which case, there would be no medical history period. In this case, an MHF should still be used to record some limited information, such as date of birth, sex, race/ethnicity. The reason is because the data fields for recording this type of general patient information are only available on the MHF. Thus, an MHF should be completed for every patient.
The SPVF, as the name implies, will be used to abstract information from each outpatient visit during the Surveillance Period. One SPVF will be used for every qualifying outpatient, non-ER visit (please see below under “What is an outpatient visit, for MMP abstraction purposes?”) to a given facility during the surveillance period.
The SPIF is designed to abstract information about any inpatient care received during the surveillance period. One or more SPIF may be needed to collect information on all hospital stays a patient may have had at a particular hospital.
An SPIF may be used to abstract information directly from the inpatient medical records at the facility where the patient was admitted for inpatient care.
-If the patient was transferred from one hospital to another during the same hospital admission, a second SPIF will be needed to conduct abstraction at the second hospitals.
-When an inpatient discharge summary is found in the outpatient medical record, an SPIF will be needed to abstract the information in that document (if there is sufficient information regarding which specific facility provided the inpatient care). The discharge summary contains a brief description of a patient’s hospital stay. A copy of his summary is usually transmitted to the patient’s primary care provider, who would provide the outpatient follow-up care after the hospital stay.
-If there is no discharge summary found in the outpatient medical records, but there is a clinical note referring to an inpatient stay, the SPIF may also be used to capture this information.
• The SPSF will also be used to abstract information from the Surveillance Period. However, the SPSF will always be used only once for a given participant at that facility, regardless of the number of medical visits or hospital stays there.
-Information collected in the SPSF may be used to capture events that are not likely to recur in the surveillance period (eg., PAP smear, pneumovax, pregnancy) or those that recur very frequently (e.g., 3 visits a week for hemodialysis).
-Note that you an SPSF may need to be completed for a facility where the patient has received inpatient care, as well as for a facility where the patient has received outpatient care.
Table 2. A complete set of abstraction forms (a full complement of forms) that should be associated with each facility that has information available for abstraction on a given patient, Medical Monitoring Project, 2007
Abstraction Form to Be Completed for Each Facility |
Number to be completed |
Comments |
Medical History Form (MHF, yellow) |
1 |
If no Medical History Period, complete only Section I, Section II, and question about first HIV+ result in Section X |
Surveillance Period Summary Form (SPSF, purple) |
+/- 1 |
Depending on the type of information available, an SPSF may or may not be completed. |
Surveillance Period Visit Form (SPVF, green) |
At least 1 |
One form completed per outpatient visit |
Surveillance Period Inpatient Form (SPIF, blue) |
At least 1 |
One form completed per inpatient stay |
What is an outpatient “visit,” for MMP abstraction purposes?
In general, whenever there is documentation of a physical examination by a medical provider, this would be considered a visit.
Abstractors should also look at section IV of the SPVF (“Surveillance Period Visit Form Sections – Optional”) to help determine whether documented information on a particular date can be counted as a “visit.” -For example, if it was documented that the patient came in one day for
phlebotomy (a blood draw), this information should be counted as a visit, since “phlebotomy” is listed in section IV of the SPVF as one type of information that should be captured.
- In addition, providers will sometimes document medical information from a telephone discussion they had with other providers (e.g., medical consultants) or with the patient. Be careful to look at the documentation to see whether there is sufficient information from the phone call for it to count as a visit to be recorded on an SPVF.
• Note that outpatient “visits,” for MMP abstraction purposes, do NOT include any outpatient care that is provided in an ER. MMP was designed to look at HIV care provided in a non-ER setting. It is beyond the scope of the project to assess all outpatient HIV care provided in the ER setting as well.
Should an MHF be completed for an inpatient stay?
It depends – if the inpatient stay occurred at a place which is part of a larger “umbrella” facility that includes another medical practice already known to have provided HIV care to the patient, then only one MHF is needed for the larger “umbrella” facility. Otherwise, one MHF should be completed for each separate facility, whether inpatient or outpatient. For examples:
University A Hospital has an outpatient HIV clinic, and both the outpatient clinic and inpatient components share a common medical record system. If a patient of the outpatient HIV clinic was hospitalized at University A Hospital, only one MHF would be needed for all outpatient visits and inpatient stays that occurred at University A Hospital.
An HIV care provider has a private group practice and has medical privileges at the local community hospital, but the group practice and the community hospital have separate medical record systems. If a patient of the HIV care provider was admitted to this community hospital, then one MHF is needed for abstracting information from the private group practice, and a second MHF is needed for abstracting information from the community hospital.
What information about care provided in the ER should or should not be abstracted?
ER visits that may have been used for outpatient care: Under the current MMP project design, these would only be detected by abstractors if there was some documentation of the ER visits in the medical records of non-ER medical practices where the patient has received care. In other words, it would not be possible to capture ER care sufficiently to be able to make meaningful inferences regarding all ER care provided to HIV patients under the current MMP study design. Therefore, abstractors should exclude from their abstraction any documented information regarding outpatient ER care provided to the MMP participant.
ER visits that are precursor to inpatient admissions: Information on what was done in the ER just before the patient is admitted to the hospital can be seen as part of the inpatient care, and, therefore, can be captured on the same SPIF being completed for that particular hospital stay.
Should abstraction be done for a facility that provides HIV care only as part of a clinical trial?
Yes, consider the clinical trial facility as another MMP facility and assign an MMP ID to it. Also, please indicate that the patient is participating in a clinical trial -- to do that, the abstractor should complete an SPSF for the clinical trial facility. On the SPSF, record the name or number of the clinical trial (if available) or record "Clinical Trial, NOS" (if name and number not available) under Section IV, "Reimbursement" as an entry in "Other, Specify."
How to record dates on abstraction forms?
Also, an abstractor should be familiar with instruction on how to make entries for date fields in different situations. In the MMP abstraction forms, the month and year (or month, day and year) values will be stored in the data as separate fields, and the default values for each of these fields will be blank (i.e., no default value).
-month will be selected from the values 1, 2, ………, 12 or 99 (with 99 indicating the month component of the date is missing).
-day will be selected from the values 1, 2, …….…, 31 or 99 (with 99 indicating the day component of the date is missing).
-year will be selected from values ranging from the minimum possible through the maximum possible (2007 or 2008, depending on the field, since project areas will be collecting data through early 2008). Note that if the year component is not available, the month or month and day are not useful. In this scenario where the year is unknown, an abstractor should select 'Date not documented'.
Don’t forget:
Each of the abstraction forms has local use sections outlined by a dashed line and indicated by the following text “OPTIONAL-FOR LOCAL USE ONLY” or “FOR LOCAL USE ONLY.”
DO NOT send the local sections to CDC.
Medical History Form (MHF)
OPTIONAL
– FOR LOCAL USE ONLY
This is the section at the bottom of the cover page of the form. It can be used to record information like patient name and medical record number, for obtaining the appropriate medical records for abstraction. This section should be separated at the perforations from the cover page and retained for local use only, before the rest of the form is sent to CDC.
![]()
MMP Participant ID
Participants will be identified only by a 12-digit numeric participant ID number. This is a unique identifier that will be associated with that patient throughout the project period. Patients should have been assigned a participant ID when the patient lists are compiled for sampling.
The Participant ID consists of the following:
• The first eight digits designate the facility where a particular patient was sampled, and is called the “facility ID”
- The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
- The next four-digit code is assigned to the selected facility by the MMP project area.
The final four-digits of the Participant ID are assigned through the consecutive numbering of MMP-eligible patients on each participating facility’s edited patient list.
The Participant ID is a mandatory field for each Medical History Form. This 12-digit numeric ID is also the number that will be used to match the interview data with the medical record abstraction data.
When requesting medical records, it is a good idea for abstractors to have this Participant ID number on hand, as well as any other patient identification number(s) (e.g., medical record number) that is/are necessary to obtain medical records on the correct patient.
Surveillance Period (SP)
This is the period from which medical record will be abstracted at each facility using the three surveillance period forms. These dates should be entered in mm/dd/yyyy format:
The SP start date is the date marking the beginning of the 12-month period immediately preceding the date of the MMP interview or of the first attempted contact (if the patient was not interviewed).
The SP end date is the date of the MMP interview or of the first attempted contact if a patient was not interviewed.
• Examples
- A patient was interviewed on 8/20/2007:
SP start date for this patient – 8/20/2006
SP end date for this patient – 8/20/2007
- A patient was discovered to be too ill to be interviewed. The initial attempt to contact the patient was a telephone call to the patient’s provider on 8/31/2007, and the provider responded to the call on 9/4/2007:
SP start date for this patient – 8/31/2006
SP end date for this patient – 8/31/2007
Medical History Period (MHP)
This is the period from which medical records will be abstracted using the Medical History Form. Medical record data from the medical history period will be used to determine how long the patient had been in care for HIV. The dates describing the MHP should be entered in mm/dd/yyyy format.
• The MHP start date is the date of the patients’ first visit to any facility for the medical care of their HIV infection.
-Note that the medical records at any particular facility on a patient may contain photocopies of records or documents from other facilities where the patient may have also received care for HIV infection (e.g., records from hospital admissions or medical referrals). Therefore, when determining the MHP start date, be careful to consider care received for HIV infection at any facility, based on all available information in the medical records (including information about care received at other facilities).
-Also, note that the MHP start date may vary from one facility to the next, depending on what information is available in the medical records at each facility.
-It is possible for patients to have visits for care before being diagnosed with HIV infection, and continue receiving care in the same facility after the HIV diagnosis. Be careful when determining the “MHP start date,” which should fall on a date after HIV diagnosis in medical records.
The first visit to this facility refers to the earliest documented visit for HIV infection at a particular facility. This date may differ from the MHP start date, as explained above.
The MHP end date is the date immediately preceding the surveillance period start date.
Example: Medical record abstraction is being conducted on a patient at facility A, an outpatient clinic. The patient was diagnosed with HIV in April 2004, and was first seen at facility A on Jan 30, 2005. In the medical records at facility A, there is a copy of a discharge summary from a local hospital where the patient was admitted on December 30, 2004 for inpatient detoxification. During that hospital admission, the patient was also found to have pulmonary TB. The patient was detoxified, had labs drawn – including CD4 counts and HIV viral load tests, and was started on treatment for pulmonary TB until AFB sputum smears were negative. At discharge – January 25, 2005, the patient was given a referral to facility A for follow-up care. The patient was interviewed for MMP on 8/29/2007.
-MHP
start
date: 12/30/2004
-MHP
end
date: 8/28/2006
-SP
start
date: 8/29/2006
-SP
end
date: 8/29/2007
If a patient is newly diagnosed with HIV or is new to care for HIV infection at a particular facility, it is possible not to have a medical history period for that patient.
This can occur if there have only been 12 months or less between first visit for HIV and the interview date.
In such instances
-Select the check box “No visit prior to the SP start date” and proceed to complete section I, section II and the question regarding documentation of the first positive HIV test result (Section X) of the MHF, before moving on to complete the surveillance period forms.
-No date information should be recorded for the MHP start date and MHP end date.
Facility ID
This is an 8-digit number
The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
The next four-digit code was one that was assigned to the selected facility during the facility sampling frame construction. If additional HIV care facilities not already in the facility sampling frame were identified during patient interview or medical record abstraction, the project coordinator or data manager should assign a new code to each of these facilities.
Date of abstraction Enter the Date of abstraction at a particular facility in mm/dd/yyyy format. The earliest date of abstraction at a particular facility should be recorded, if repeated visits to the facility are needed to complete abstraction on any individual patient.
Abstractor ID Enter the preset 3 digit Abstractor ID, as assigned by the project area, for the person completing the abstraction forms.
Is the medical record complete for this abstraction?
Depending on local practices, some facilities may archive records frequently, and retrieval may not be possible or too costly. It is also possible that patients have large records due to having received care for a long period at a facility, having had complex medical problems, or having participated in a clinical trial. Sometimes, records may be lost or misplaced due to storage and paper record tracking issues.
During abstraction, it may be apparent that there is missing information in the medical records.
• If there is missing information in the medical records at the facility where abstraction is conducted: -Select ‘No’ for “Is the medical record complete for this abstraction?” and -Document the dates of missing records: First missing record date and Last
missing record date. If either of these dates are unknown, select “Date not documented”.
- Briefly describe the missing part in the space provided following “Describe the missing sections”. This question is relevant only to the MHF, not collected in the SPFs.
• If there is no apparent missing information in the medical records during abstraction, then please select ‘Yes’ to the question above.
![]()
Most of the patient demographic information will be collected using the MHF. Thus, without completing the MHF, it will be extremely difficult to get the required demographic information particularly with regards to age, sex, race, ethnicity, and country of birth as documented in the medical records. Even for patients with no visit prior to the surveillance period, this information should be completed on the MHF.
Date of Birth
This space is to be used to enter the patients’ month, day, and year of birth from the medical records. The Date of Birth should be entered as mm/dd/yyyy.
If the date of birth is missing
• Select “Date not documented”
• Complete an entry for the “If date of birth is not documented, enter documented age prior to the SP start date” field and the “date of this documented age” field, if known.
-If there is no documented age before the surveillance period, select “Age not
documented.”
-If the date of the documented age is unknown, select “Date not documented.”
• Recording incomplete date information
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
Most recent height (ft/in) prior to the SP start date
Most patients are assumed to have achieved their adult height by the time they are eligible for MMP participation, but because heights are not as routinely measured or documented as weight is, the information is being captured from both the Medical History Period and the Surveillance Period.
If there is more than one height documented during the Medical History Period, enter the most recent measurement in the MHF.
Select “Height not documented” if the height information is missing.
Sex at Birth
Fill in the appropriate choice for the patient's sex at birth. If the sex at birth is not documented, select “Not documented.” Often, it is not explicitly stated whether the documented sex in the medical record was the patient’s sex “at birth.” In these cases, do not assume that the documented sex is the patient’s sex at birth; instead, record the information as the patient’s “gender during the MHP.”
Gender during the MHP
Select the patient's gender during the Medical History Period.
Note whether there were references to a different gender during different time periods in the medical records. If there was discrepant information about the patient’s gender during different time periods, determine whether this was due to errors or due to the patient having had gender reassignment.
If there were indications that the patient was in the process of gender reassignment such as treatment with hormones and scheduled surgery, look for information about the type of reassignment – whether “Male to Female” or “Female to Male.”
If the patient’s gender during the MHP is not documented select “Not documented” for this information.
Hispanic/Latino Ethnicity
Indicate if the patient is of Hispanic/Latino ethnicity by selecting the appropriate choice. Select “Not documented” if this information is not found in the medical records.
Race
Indicate the patient's race by selecting from the appropriate text on the form.
Select all choices that apply
Enter as many as three choices that are not already listed by selecting “Other, Specify” and entering the race in the space provided.
If no information with regards to race is recorded select “Not documented” for this
information.
Country of Birth
If the documented country of birth is different from the choices given, select “Other, Specify” and enter the country in the space provided. If the country of birth is not documented anywhere in the patient medical record, select “Not documented.”
![]()
Is there documentation of any of the following prior to the SP start date?
This section is available as an optional tool to help guide the abstraction process; abstractors are not required to complete this section.
If “Yes” is selected for the above question, select all the appropriate choices below the question in this section of the form, and follow the instructions that indicate which section to complete, for each choice selected.
![]()
The following stem question is used to determine whether AIDS-OI(s) was/were diagnosed in the Medical History Period (MHP): “Is there documentation that any AIDS defining opportunistic illnesses (AIDS OI), and /or AIDS with AIDS OI not specified, were diagnosed prior to the SP start date?”
If no,
Select “No” if there is no evidence in the medical records that the patient was diagnosed with an AIDS OI at any time in the medical history period.
In this situation, no further data collection for Section IV is necessary.
If yes,
• Select “Yes” if at any time during the medical history period, the medical records indicate that the patient was diagnosed with
-Any AIDS OI or
-AIDS, but no OI specified [any evidence of immunologic AIDS (i.e., CD4 cell count <200 cells/µl) is captured later in Section X, under “Laboratory Test Results”.]
Complete the requested information on each AIDS OI that was documented in the medical records.
Record all documented diagnoses – whether presumptive or definitive.
Select from the list of AIDS OIs, which are listed in alphabetical order.
• In general, no documented laboratory confirmation is necessary for abstracting AIDS OIs.
-Accept an AIDS OI diagnosis if it is documented in the clinical notes as the medical provider’s assessment of the patient’s condition, or if the diagnosis is found in a hospital discharge summary, a transfer note (transferring the patient’s care from one facility to another), or a medical consultation summary – even if these documents do not demonstrate complete information on how the diagnosis was made.
-See additional guidance on some AIDS OIs later in this section.
• Whenever possible, record both the date of first diagnosis and the date of most recent diagnosis prior to the surveillance period start date for these conditions. -Record the available date information.
-If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
-If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
The date of first diagnosis refers to the date that the diagnosis of an AIDS OI was first documented by provider report. Enter the date in mm/yyyy format.
The date of the most recent diagnosis should be different from the date of first diagnosis.
-It is possible that the date of first diagnosis is earlier than the date of first visit at a particular facility, which may have photocopies of documentation from another facility in the medical records showing that the patient was diagnosed with an AIDS-OI elsewhere in the past.
-For the “most recent diagnosis,” be careful that the most recent documentation of the AIDS OI is a specific reference to a separate new episode, rather than to a history of the patient having had that AIDS OI before.
-On the MHF, the date of the most recent diagnosis should NOT be on or later than the start date of the Surveillance Period.
• If an AIDS OI was diagnosed only once during the MHP, record the date of diagnosis under “Date of first diagnosis,” and leave the field blank under “Date of most recent diagnosis” for that particular AIDS OI.
Notes on conditions with criteria for extrapulmonary (outside the lungs) involvement
The following illnesses may present in a variety of ways, but can only be considered as AIDS OIs if they affect organs/tissues in addition to the lungs (as in disseminated disease) or as a disease of organs/tissues other than the lungs. When abstracting, be careful that a documentation of one of the following conditions refers to extrapulmonary involvement, as described above.
Coccidioidomycosis, disseminated or extrapulmonary
Cryptococcosis, extrapulmonary
Histoplasmosis, disseminated or extrapulmonary
Mycobacterium avium complex or M. kansasii, disseminated or extrapulmonary
Notes on conditions with criteria for chronicity
To qualify as an AIDS OI, the following illnesses should be mentioned as being “chronic” in the medical records, whether or not a specific time frame was mentioned.
Cryptosporidiosis, chronic intestinal (>1 month duration)
Herpes simplex: chronic ulcer (>1 month duration) or bronchitis, pneumonitis, or esophagitis
Isosporiasis, chronic intestinal (>1 month duration)
Notes on Carcinoma, invasive cervical
There should be documentation that the cancer is “invasive,” or corresponds to stage 1A or higher, based on the International Federation of Gynecologists and Obstetrician (FIGO) clinical staging system.
Stage 1A indicates microinvasive disease, which means that there is microscopic evidence of invasiveness, but there is not yet any evidence that the cancer has spread beyond the cervix.
The following terms are NOT indicative of invasive cervical carcinoma:
-Atypical cells of undetermined significance (ASCUS)
-Intraepithelial neoplasia or Cervical intraepithelial neoplasia (CIN)
-Squamous Intraepithelial Neoplasia (SIL) or dysplasia
-Low-grade SIL (LSIL)
-High-grade SIL (HSIL)
-Carcinoma in situ
Notes on Cytomegalovirus (CMV)
Note the criteria that should be met for a CMV-related condition to qualify as an AIDS OI.
• To qualify as CMV disease -The condition should affect areas of the body in addition to, or other than, the liver, spleen, or lymph nodes
- Examples: CMV colitis, esophagitis, pneumonitis, or CMV neurologic disease such as dementia, ventriculoencephalitis, or ascending polyradiculomyelopathy (spinal cord disease affecting multiple nerve roots)
To qualify as CMV retinitis -There should be documented loss of vision as a result of the CMV retinitis. -“Peripheral retinitis” may be asymptomatic or with mild visual disturbances, and
therefore, does not qualify as an AIDS OI.
Please refer to Appendix B, “AIDS Defining Opportunistic Illnesses” for additonal information on specific AIDS OIs.
![]()
In the MHF, the following stem question is used to determine whether conditions other than AIDS-OI(s) was/were diagnosed in the Medical History Period (MHP): “Is there documentation that any of the following conditions other than AIDS OI were diagnosed prior to the SP start date?”
If no,
Select “No” if there is no evidence in the medical records that the patient was diagnosed with one of the conditions listed in the MHF.
In this situation, no further data collection for Section V is necessary.
If yes,
Select “Yes” if at any time in the medical history period the medical records indicate that the patient was diagnosed with any of the listed conditions.
Complete the information on the listed condition(s) that was/were documented in the medical records during the medical history period.
***NOTE***
Be
careful not to confuse the diagnoses that should be recorded in
Section IV, “AIDS
Defining Opportunistic
Illnesses
(AIDS OI)” with
those that should be recorded in this section.
This
section includes some clinical conditions that may occur as a result
of HIV disease, but are NOT AIDS-defining.
What evidence of a “diagnosis” to accept?
In general, for MMP, the providers’ diagnosis of the conditions listed in this section should be accepted – whether with or without documented laboratory confirmation. Similarly, when the health care provider makes references to medical conditions that have been diagnosed elsewhere, these should be recorded if it could be determined from documented information that the conditions remain active health problems for the patient.
When there is documentation that a patient is concerned about a particular condition (e.g., “patient noticed oral thrush this week” or “patient thinks she has a vaginal yeast infection”), without documented evidence of a physician diagnosis, do not report as an established diagnosis.
Diagnoses with qualifying terms: Often in medical records, qualifying words are used with diagnoses to denote the degree of certainty surrounding the diagnosis.
-As a general rule of thumb, diagnoses described with the following commonly used “qualifying words” should be counted in MMP:
“diagnostic
procedure results consistent with. . .”
“presumptive
. . .”
“responded
to treatment for . . .”
-On the other hand, diagnoses described by the following qualifiers should not be considered established diagnoses, and should generally not be recorded as a diagnosis in the medical records:
“questionable
diagnosis of . . .”
“Diagnosis
A vs. Diagnosis B . . .”
“conceivable.
. .”
“differential
diagnosis includes X, Y, and Z. . .”
“symptoms
of . . .”
“iffy. ..”
“plausible
. . .”
“probable
. . .”
“possible
. . .”
“potential
. . .”
“questionable
. . .”
“rule
out (abbreviated R/O) . . .”
• If uncertain, reviewing additional information about the clinical course of the patient and other documented impressions of the medical providers over time may assist in determining whether this is an established diagnosis. If possible, consultation with the health care provider would also be helpful.
Selecting conditions other than AIDS OI on the MHF
There are 17 items listed on the MHF as “Conditions other than AIDS OI.” When considering selecting one of these conditions, look for terms in the medical records that correspond to those listed on the MHF. For guidance on terms that correspond to each condition on the MHF, see Appendix C.
Conditions that may be related to Each Other
Be aware that some conditions listed as “Conditions other than AIDS OI” may be related to each other, such that the selection of one may indicate the need to select another.
• Chronic kidney disease: Select both “Chronic kidney disease” AND “Renal failure” in either of the following situations:
-A patient has documentation of chronic kidney disease that has progressed to renal failure. -A patient has renal failure that is documented as “chronic” or due to “end-stage renal disease.”
Lipoatrophy: Record this condition as both “Lipodystrophy” AND “Lipoatrophy.”
Drug-induced hepatitis: Select both “Drug-induced hepatitis” AND “Non-alcoholic fatty liver disease” if both of the following criteria are met:
-The diagnosis is either steatohepatitis (fatty liver with inflammation) or steatonecrosis (fatty necrosis) that is specifically documented as being drug-induced.
AND -There is no documentation that the steatohepatitis or steatonecrosis is related to alcohol use.
![]()
This section collects prophylaxis information for two conditions: 1) Pneumocystis jiroveci (PCP); and 2) Mycobacterium avium complex (MAC). PCP and MAC prophylaxis are recommended to prevent either primary (first occurrence of) or secondary (recurrence of) PCP or MAC opportunistic infection when the patient’s CD4 T-lymphocyte count falls below 200 cells/μL (for PCP) and 50 cells/μL (for MAC).
Two stem questions are used to determine whether PCP or MAC prophylaxis were prescribed:
“Is there documentation of prescription for prophylaxis of Pneumocystis jirovecii pneumonia (PCP) prior to the SP start date?”
“Is there documentation of prescription for prophylaxis of Mycobacterium avium complex (MAC) prior to the SP start date?”
If no (to both questions),
• Select “No” if there is no documentation of prophylaxis being prescribed before the SP start date.
• No further data collection for Section VI is necessary. If yes (to either question),
• Select “Yes” if before the SP start date, -There was documentation that “prophylaxis” for PCP and/or MAC was initiated or -There was documentation that the patient was either prescribed or continued on
medical regimens typically provided for prophylaxis (please see Table 1 below).
***NOTE***
Some physicians refer to the following terms interchangeably, but all refer to the same condition:
Pneumocystis
carinii
pneumonia (PCP)
Pneumocystis
jiroveci
(P.
jiroveci)
pneumonia (PCP)
Pneumocystosis
Table 1. Medications used in primary or secondary prophylaxis of Pneumocystis jirovecii pneumonia (PCP) and Mycobacterium avium complex (MAC) disease among HIV-infected patients, Medical Monitoring Project, 2007 data collection cycle.
Medication |
Other Names |
Prophylaxis use |
atovaquone |
Mepron |
PCP prophylaxis if atovaquone 1,500mg by mouth (po) once daily (qd) |
azithromycin |
Zithromax |
MAC prophylaxis if azithromycin 1,200mg by mouth (po) weekly alone or azithromycin 1,200mg plus rifabutin 300mg, po daily (qd) or azithromycin 500mg plus ethambutol 15mg/kg body weight, with or without rifabutin 300mg, po qd |
clarithromycin |
Biaxin |
MAC prophylaxis if clarithromycin 500mg by mouth (po) twice daily(bid) alone or clarithromycin 500mg po twice daily (bid) plus ethambutol 15mg/kg body weight, with or without rifabutin 300mg, po daily (qd) |
dapsone |
DDS |
PCP prophylaxis if dapsone 100mg by mouth (po) daily (qd) or dapsone 50mg po twice daily (bid) or dapsone 50mg po daily (qd), along with pyrimethamine 50mg and leucovorin 25mg, po weekly or dapsone 200mg plus pyrimethamine 75mg plus leucovorin 25mg, po weekly |
ethambutol |
Myambutol |
MAC prophylaxis if ethambutol 15mg/kg body weight, by mouth (po) daily (qd), along with clarithromycin or azithromycin (please see details above for azithromycin and clarithromycin) |
leucovorin |
Folinic acid, calcium folinate, calcium levofolinate, sodium folinate, Sodiofolin, Wellcovorin, Isovorin |
See details above for dapsone |
pentamidine |
Pentam 300 |
PCP prophylaxis if given as aerosolized medication monthly |
pyrimethamine |
Daraprim |
See comments above for dapsone |
rifabutin |
Ansamycin, Mycobutin, RBU |
MAC prophylaxis if rifabutin 300mg by mouth (po) daily (qd) alone or rifabutin 300mg po qd, along with azithromycin (sometimes also with ethambutol) – please see details above for azithromycin and ethambutol |
trimethoprimsulfamethoxazole |
TMP/SMX, TMP/SMZ, Bactrim, Septra, Co-trimoxazole, Sulfatrim, Cotrim, Cotrima |
PCP prophylaxis if TMP/SMX, 1 double strength (DS) tablet or 1 single strength (SS) tablet given by mouth (po) daily (qd) or TMP/SMX, 1 DS tablet po three times weekly |
For
additional information, please refer to the following guidelines
available online at http://www.aidsinfo.nih.gov/guidelines/
1)
“Guidelines for the Prevention of Opportunistic Infections
among HIV-Infected Persons – 2002”
2)
“Treating Opportunistic Infections among HIV-Infected Adults
and Adolescents”
![]()
In the MHF, the following stem question is used to determine whether screening for hepatitis A, B, and C, Toxoplasma, or tuberculosis (TB) occurred prior to the SP start date: “Is there documentation of screening for hepatitis A, B, or C, Toxoplasma, or tuberculosis (TB) prior to the SP start date?”
If no,
Select “No” if there is no evidence in the medical records that the patient was ever screened for any of the listed infections.
No further data collection for Section VII is necessary
If yes,
Select “Yes” if the medical record indicates that the patient was screened for any of the listed infections.
Continue to each of the successive sections and sub-sections to complete the information documented screening for each of the following: hepatitis, Toxoplasma, and TB.
Hepatitis A/B/C The following guidance is given for the three hepatitis sub-sections on the MHF: “Hepatitis A,” “Hepatitis B,” and “Hepatitis C.”
The HIV Medicine Association of IDSA recommends serologic screening for viral hepatitis infections as part of the baseline evaluation of individuals initially presenting for care for their HIV infection. The primary method for detecting these infections is by demonstrating the presence of antigens and/or antibodies.
In the MHF, the following stem question is used to determine whether screening for each hepatitis A, B, and C occurred prior to the SP start date: “Was hepatitis A/B/C screening performed prior to the SP start date?”
If no,
Select “No” if the record specifically states that the patient was NOT tested for hepatitis A, B, and/or C
Record the reason(s) why screening test(s) was/were not performed, if the reason(s) is/are documented in the medical record.
-If the documented reason is other than prior immunization or prior infection, record the other reason stated in the “Other, Specify” area provided.
-If the medical record specifically states that hepatitis A, B, and/or C screening test(s) was/were not performed but does not state the reason why screening test(s) was/were not performed, indicate this by selecting “Not documented.”
If yes,
Select “Yes” if the record indicates that the patient was ever tested for hepatitis A, B, and/or C (whether or not the testing was done to address signs/symptoms of hepatitis), and
Indicate which tests was done, the date of testing, and the results of each test. See below for the different types of test used to screen for hepatitis A, B, or C.
-Remember that the hepatitis testing date may be earlier than the date of HIV diagnosis and the medical history period start date. (Patients may have been tested for hepatitis before becoming HIV infected).
-If both negative and positive screening test results are available in the record, record the date of the first positive result for the positive test(s) and the date of the last negative result for the negative test(s).
. Record the available date information.
. If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
. If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
If the medical record makes no specific mention of whether hepatitis A, B, or C test(s) was/were performed, indicate this by selecting “not documented” in each of the hepatitis sub-sections.
Interpretation of serologic test results is beyond the scope of the abstraction forms or abstractors manual. These results will be coded and analyzed to arrive at a conclusion regarding hepatitis infection and/or immunity after data collection is completed.
Screening Tests: Hepatitis A
There are two serologic tests that are performed to screen or diagnose infection with hepatitis A virus (HAV): 1) Anti-HAV Total and 2) Anti-HAV IgM.
• “Anti-HAV Total” is a general test for the presence of any type of antibody to the Hepatitis A virus (includes both IgM IgG); in general,
-The presence of IgM antibodies (whether to HAV or to another infectious agent) indicates acute infection or recent immunization.
-The presence of IgG antibodies indicates long-term immunity (whether from natural infection or vaccination) acquired in the more remote past.
• “Anti-HAV
IgM”
– antibodies specific to HAV indicating acute/recent infection
with or
immunity
against HAV.
Screening Tests: Hepatitis B
The MHF is concerned with the following tests for hepatitis B: 1) Anti-HBc Total (or HBcAb); 2) Anti-HBc IgM; 3) Anti-HBs (or HBsAb); and 4) HBsAg.
The first two (Anti-HBc Total and Anti-HBc IgM) test for antibodies produced against the core of the hepatitis B virus and are found only after a true hepatitis B infection.
The third test (Anti-HBs) detects antibodies developed against hepatitis B surface proteins and is not diagnostic of natural infection as those who have been vaccinated against hepatitis B will also produce Anti-HBs. The Anti-HBs test is usually used to test for immunity to hepatitis B in patients who have been immunized previously.
The fourth test (HBsAg) detects a protein from the virus that is generated if the virus is actively replicating.
Screening Tests: Hepatitis C
The main serologic test to screen for hepatitis C virus infection is Anti-HCV (enzyme linked immunoassays [EIA] or radio immunoblot assay [RIBA]). Anti-HCV may also be recorded in the medical record as Hepatitis C antibody, anti-Hep C or HCV Ab.
After a patient receives a positive serologic test for hepatitis C, hepatitis C viral load testing – HCV RNA Quantitative PCR may be performed.
Even if a patient tests negative for anti-HCV antibodies, a hepatitis C viral RNA test (qualitative or quantitative) may also be performed if there is unexplained evidence of liver damage. Some patients co-infected with HIV and HCV may test negative for anti-HCV antibodies.
The tests for hepatitis C are known by different names in different labs. Abstractors should familiarize themselves with the exact naming of hepatitis C tests used in their jurisdiction.
Toxoplasma
The USPHS guidelines recommend serologic screening of all HIV-infected persons for Toxoplasma as part of the initial evaluation on presentation for HIV care. If the patient initially tests negative, the screening should be repeated when the CD4 lymphocyte count decreases to 100/mm3. The primary method for diagnosing these infections is by demonstrating the presence of antibodies.
Screening Tests: Toxoplasma
In the MHF, the following stem question is used to determine whether screening for Toxoplasma occurred prior to the SP start date: “Was Toxoplasma screening performed prior to the SP start date?” Patients are screened for Toxoplasma my measuring the amount of antibody (called antibody titer) in the blood.
If no,
Select “No” if the record specifically states that the patient was NOT screened for Toxoplasma.
No further information needs to be recorded about Toxoplasma screening.
If not documented,
Select “Toxoplasma screening not documented.” If the medical record makes no specific mention of whether Toxoplasma screening was performed.
No further information needs to be recorded about Toxoplasma screening.
If yes,
Select “Yes” if the record indicates that the patient was screened for Toxoplasma during the medical history period.
Continue to complete the information in this section
The next question follows the Toxoplasma section’s stem question and should be completed only if the answer to the stem question (above) was “Yes.”
“Was there a positive result for the most recent Toxoplasma antibody titer prior to the SP start date?”
If no
Select “No” if the documented result for the most recent test for Toxoplasma antibody titer from before the SP start date that was NOT “positive.”
No further information needs to be recorded about Toxoplasma screening.
If the result of the most recent Toxoplasma antibody titer was not available,
Select “Result of most recent test not documented.”
No further information needs to be recorded about Toxoplasma screening.
If yes,
Select “Yes” and
Record the date of the positive test result in the area provided.
-Record the available date information.
-If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
-If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
Tuberculosis
The USPHS recommends screening of HIV-infected persons annually for infection with Mycobacterium tuberculosis (TB). When a patient tests positive for latent tuberculous infection, this means that the patient has the TB germ in the body and is at increased risk for getting active TB disease, but is not ill with the disease yet. Once an HIV-infected patient tests positive for latent tuberculous infection or is diagnosed with active TB, he/she will no longer be screened.
In the MHF, the following stem question is used to determine whether screening for latent tuberculous infection occurred prior to the SP start date: “Was screening for tuberculosis (TB) performed prior to the SP start date?”
If no,
Select “No” ONLY if the medical record specifically states that the patient was NOT screened for latent tuberculous infection prior to the SP start date.
No further information needs to be recorded about tuberculosis screening.
If not documented,
Select “TB screening not documented” if the medical record has no specific mention of whether screening for latent tuberculous infection was performed prior to the surveillance period start date.
No further information needs to be recorded about tuberculosis screening.
If yes,
Select “Yes” if the medical record indicates that the patient was screened for latent tuberculous infection in the medical history period.
Continue to complete the requested information on screening tests for latent tuberculous infection.
Screening Tests: Latent TB infection
The Tuberculin skin test (TST) is the standard test used to screen for tuberculous infection in persons with HIV and is administered by an intradermal injection on the forearm. The TST is also called the Mantoux test or purified protein derivative (PPD) test.
A new test called the QuantiFERON test (QFT) – approved by the Food and Drug Administration in 2005 – may also be used to screen for TB using a blood sample, though the performance characteristics of QFT have not been fully evaluated in HIV-infected patients.
The next data field follows the TB section’s stem question and should be completed only if the answer to the stem question was “Yes.” “Date of the most recent tuberculin skin test (TST/PPD/Mantoux) or QuantiFERON test (QFT) prior to the SP start date.”
• Record the date of the most recent TST/PPD/Mantoux/QFT prior to the surveillance period start date, if documented in the medical records.
-Record the available date information.
-If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
-If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
• “Result of the most recent TST/PPD/Mantoux/QFT prior to the SP start date.”
-If TST/PPD/Mantoux was the most recent TB screening test prior to the surveillance period start date,
. Record the size of the skin reaction to this test in millimeters, if documented, OR
. Select one of the other options for a result, as appropriate.
-If the QFT was the most recent TB screening test prior to the SP start date, choose from the list of options to record the QFT results.
• “If TST/PPD/Mantoux positive (result > 5mm) or QFT positive, is there documentation of prescription for treatment of latent TB infection (LTBI), i.e., with Isoniazid, Rifampin, Pyrazinamide, or any combination of these, prior to the SP start date?”
-Note that this question applies only if the documented TST/PPD/Mantoux result was > 5 mm or “Positive,” or if the QFT result was “Positive.”
-Look for documentation of prescribed treatment for latent TB infection, and select “Yes” or “No” to this question, as appropriate.
• “Is there documentation that the patient completed treatment for LTBI?
-If treatment was prescribed for latent TB infection (LTBI) – when a patient had a positive screening test – there may be documentation regarding the patient successfully completing the treatment.
-Select “Yes” or “No” to this question, as appropriate.
![]()
In the MHF, the following stem question is used to determine whether immunization(s) for hepatitis A and/or B and pneumococcal pneumonia were given prior to the SP start date: “Is there documentation of whether or not hepatitis A, B, A and B, or pneumoccoal immunizations were given prior to the SP start date?”
If no,
Select “No” if there is no evidence in the medical records that the patient was vaccinated for hepatitis A or B or pneumococcal pneumonia.
No further data collection for Section VIII of the MHF is necessary.
If yes,
Select “Yes” if the medical record indicates that the patient was immunized against hepatitis A or B or pneumococcal pneumonia in the MHP and
Complete the sub-sections under this question for each type of vaccine administered.
Hepatitis A/B/A & B
The USPHS recommends vaccinating HIV-infected persons against hepatitis A unless the patient has been previously diagnosed with hepatitis A; the same holds true for hepatitis B.
There is no vaccination for hepatitis C, D, or E. Nevertheless, HIV patients who have hepatitis C are at a particularly high risk of serious illness if they become infected with hepatitis A or B – making vaccination for hepatitis A and B especially important in these patients.
Hepatitis A Vaccine - Alternative names are Havrix and Vaqta.
Hepatitis B Vaccine -Alternative names are Comvax, Engerix-B, and Recombivax HB
Hepatitis A & B Vaccine -Alternative name for combined hepatitis A and B vaccine is Twinrix.
On the MHF, the following stem questions are used to determine whether immunization for hepatitis A, B, or A and B occurred prior to the SP start date:
“Was hepatitis A vaccine given (Havrix, Vaqta) given prior to the SP start date?” “Was hepatitis B vaccine (Energix B, Recombivax) given prior to the SP start date?” “Was hepatitis A and B vaccine (Twinrix) given prior to the SP start date?”
If no (to any of the three hepatitis vaccine stem questions above),
Select “No” ONLY IF the medical record specifically states that the patient was NOT given that particular hepatitis vaccine.
Specify the reason why the particular hepatitis vaccine was not given, or indicate that a reason was “Not documented.”
If no specific mention of whether a particular hepatitis vaccination was administered,
Select “Not documented.”
No further data collection is needed for this sub-section.
If yes (to any of the three hepatitis vaccine stem questions above),
Select “Yes” for each type of hepatitis vaccination that was given (hepatitis A, hepatitis B, or combined hepatitis A and B)
For each type of hepatitis vaccine given,
-Indicate the number of vaccine doses given.
-Record the date each vaccine dose was given, or select “Date not documented” for any dose with missing date information.
. Record the available date information.
. If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
. If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
-Select “Yes, but number of doses not documented” for each type of hepatitis vaccine that did not have documented information on the number of doses given.
Pneumococcal pneumonia
The USPHS Guidelines recommend vaccination of HIV-infected persons against pneumococcal pneumonia when the CD4 T-lymphocyte count is > 200 cells/mm3. A single dose of 12-valent polysaccharide pneumococcal vaccine should be given if the patient has not received this vaccine in the previous five years. The vaccine should also be considered for patients whose CD4 T-lymphocyte count is <200 cells/ mm3, though the efficacy in these patients is not known.
In the MHF, the following stem question is used to determine whether immunization for pneumococcal pneumonia occurred prior to the SP start date: “Was pneumococcal vaccine (Pneumovax 23, Pneu-Immune 23) given prior to the SP start date?”
If no,
Select “No” ONLY IF there is specific documentation that the patient was NOT vaccinated with a pneumococcal vaccine, and
Specify the reason why pneumococcal vaccine was not given, or indicate that a reason was “Not documented.”
If no specific mention of whether pneumoccal vaccination was given,
Select “Pneumococcal vaccination not documented.”
No further data collection is needed for this section.
If yes,
• Record “1” for the number of vaccination doses given (before the surveillance period start date). -The pneumococcal vaccine is usually given as a single dose, rather than as a
series over several months. The current recommendation is to give a single dose if the patient has not had one in the previous 5 years.
-In a future revision, this section will be revised to better reflect the current recommendation for pneumococcal immunization..
• Specify the date the vaccine dose was given or select “Date not documented” if there is no documented date of administration.
- Record the available date information.
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
![]()
Information on prescribed medications (including ART), may be found in various places in medical records, including the initial history & physical form or summary, referral forms, consultation summary, facility transfer notes, physician’s order sheets, physician’s notes, nurse’s notes, or a pharmacy-generated list of medications for the patient.
On the MHF, the following stem question is used to determine whether there was documentation that ART was prescribed before the surveillance period start date. “Is there documentation of antiretroviral therapy (ART) prior to the SP start date?”
If no,
Select “No” if there is no documented evidence that medications other than ART was
prescribed at any time before the SP start date.
No further data collection is needed on Section IX of the MHF.
If yes,
Select “Yes” if the medical records indicate that ART was prescribed before the surveillance period start date. Please include medications that were mentioned as having been prescribed for the patient by other provider(s) at other facilities before the SP start date.
Complete the requested information in this section of the MHF by selecting all ART medications documented as having been prescribed for the patient at any time before the SP start date.
The different formulations of current FDA-approved ARTs comprise individual drugs that are organized into several categories – depending on the phase in the HIV infection cycle that the drug disrupts. Some illustrations and explanations of the HIV cycle can be found in Appendix D and at the following web sites:
http://www.hopkins-hivguide.org/tutorial/launch.html (animated presentation) http://www.wellcome.ac.uk/en/labnotes5/animation_popups/hiv.html (animated presentation) http://aidsinfo.nih.gov/contentfiles/HIVLifeCycle_FS_en.pdf http://www.aidsinfonet.org/factsheet_detail.php?fsnumber=106
The MMP abstraction forms include all FDA-approved ART formulations and several ART medications not yet approved by the FDA.
On the MHF, the ART medications are listed in alphabetical order by a common name, and other known names of each medication are included in parentheses.
Because providers may refer to these medications using a number of different names in the medical records, it would be good to become familiar with the different names of the drugs and to know in which ART class/category the different drugs belong.
Whenever
a combined ART formulation is found in the medical records, please
indicate on the
abstraction
form the exact formulation that was prescribed, rather than the
constituents of the formulation,
for
example:
If
Combivir was a documented prescription,
Select only “Combivir (AZT/3TC)”
Do NOT select the two drugs in the formulation separately:
-“Zidovudine (AZT, Retrovir)” and
-“Lamivudine (3TC, Epivir)”
If Trizivir was a documented prescription,
Select only “Trizivir (ABC/3TC/AZT)”
Do NOT select the three drugs in the formulation separately:
-“Abacavir (ABC, Ziagen)” and
-“Lamivudine (3TC, Epivir)” and
-“Zidovudine (AZT, Retrovir)”
If the patient was prescribed an antiretroviral medication not listed on the abstraction form, record its name at the end of this section, under “Other, Specify.”
![]()
On the MHF, the following stem question is used to determine whether there was documentation of the first positive HIV test result, laboratory test results for CD4 cell count, HIV viral load, or abnormal liver enzyme levels before the surveillance period start date. “Is there documentation of the first positive HIV test result, or laboratory test results for CD4 cell count, HIV viral load, or abnormal ALT (SGPT) or AST (SGOT), prior to the start date?”
If no,
Select “No”
No further data collection is needed for Section X.
If yes,
Select “Yes” if there is/are documented results for any of the laboratory tests listed in the stem question above.
Complete the relevant information in this section on the MHF.
For explanations of the laboratory tests in the stem question above, please see Appendices E, F, and G.
***
NOTE
***
Be
careful to look in the correct time frame when answering this
section’s stem question – the correct time
frame
for the MHF is the period from the first visit for medical care after
HIV infection until the surveillance
period
start date.
Looking for laboratory results
Although many laboratory results can be found in the “Laboratory” section of a facility’s medical records, it is always a good idea to look for this information throughout other parts of the medical records as well, because formal laboratory reports may not always be available in a patient’s chart, for many different reasons.
Medical providers routinely follow-up the results of tests that they order, sometimes obtaining these results before laboratory reports are printed out. Therefore, the physician’s notes and/or nurse’s notes (care plans, clinic notes, progress notes, history & physical forms, etc.) are also good places to look for laboratory results, as are transfer notes (if patients are transferred from one facility to another) and summaries on referral forms or from medical consultations. In addition, the physician’s order sheets will show when specific tests were ordered – which would provide a clue as to approximately when specimens were obtained for testing.
Date of test result = Date specimen collected
When abstracting information on the “date of laboratory test result,” keep in mind that this refers to the date when the specimen was collected from the patient – rather than the date when the final result was reported by the laboratory – for a particular test. This is because laboratory report print-outs may show both the date test results were reported as well as the date specimens were collected (Appendix E).
Verify the units used with test results
Laboratory reports will show the units associated with the values of test results. Whenever possible, it’s a good idea to verify what units are used with the documented test results in medical records. Although most laboratory results will be reported in a conventional way across facilities, it is good practice to always check and confirm that the laboratory values being abstracted are compatible with what is requested on the abstraction form.
HIV Test Results
The next question is a follow-up to the main stem question in Section X and should be completed only if the response to the stem question was “Yes.” “Is there documentation of the first positive HIV test result?”
If no,
If there is no documentation of a positive HIV test result, verify that patient is eligible for participation in MMP (i.e., that the patient is truly HIV-infected).
No further data collection is needed in this sub-section on HIV diagnostic testing.
If yes,
Information on the patient’s earliest HIV-positive test result should be obtained from medical records only and NOT from the HIV/AIDS Reporting System (HARS) database. In determining the earliest date of HIV-positive test result, any documentation on the first HIV+ test result should be considered, whether it is patient self-reported information, by laboratory report, or other sources of information.
• Record the date of the earliest HIV positive test result:
- If a specific HIV test date (month/year or year) is mentioned: Enter the earliest date when the specimen was submitted for HIV testing, regardless of the type of test used (e.g., screening versus confirmatory) to diagnose HIV infection.
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
CD4 Counts
CD4 cells (CD4 T-lymphocytes, T-helper cells) are a type of white blood cells that help to fight infections in the body. HIV specifically targets these white blood cells; thus, the CD4 cell count is a measure of how severely the immune system is affected by the HIV infection. Generally, the lower the CD4 cell count, the more severely affected, and the greater is the risk for acquiring an opportunistic infection.
CD4 cell count is typically reported as number of cells per microliter (μl, cubic millimeter, or mm3) of blood. Normal CD4 counts in adults range from approximately 500 to 1,500 cells/ μl. For an example of a laboratory report on CD4 lymphocyte count, see Appendix E.
This sub-section is for recording information about any laboratory test results on CD4 lymphocyte counts, and begins with the following question. “Is there documentation of CD4 cell count test results prior to the SP start date?”
If no,
Select “No” if there is no documented CD4 lymphocyte count result before the SP start date.
No further data collection is needed for this sub-section on CD4 lymphocyte count.
If yes,
Record the value of the lowest CD4 count that occurred at any time before the SP start date.
Record the date of the lowest CD4 count documented before the SP start date.
- Record the available date information.
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
HIV Viral Load Results
An HIV viral load test is a measure of the burden of HIV in the blood. It is another way of gauging the severity of HIV infection in patients. Generally, the higher the viral load, the higher is the HIV burden and the more severe the infection. If the patient is on antiretroviral therapy, the HIV VL test helps to assess the effectiveness of that therapy.
There are several types of tests for measuring the HIV VL; these tests are different methods of measuring the concentration of HIV RNA (the genetic material inside the virus) in the blood. When the HIV VL is so low that a test cannot detect any RNA, the test result is reported as “undetectable.” For an example of a report on an HIV viral load test, see Appendix E.
This sub-section is for recording information about HIV viral load test results before the SP start date, and begins with the following question. “Is there documentation of HIV viral load (VL) test results prior to the SP start date?”
If no,
Select “No” if there is no documented HIV VL test results before the SP start date.
No further data collection is needed in this sub-section on HIV VL test results.
If yes,
Select “Yes” if there is a documented HIV VL test results before the SP start date.
Respond to the next question in this sub-section, as appropriate, “Is there documentation of an undetectable VL?
If there is an undetectable VL test before the SP start date,
- Record the available date information on the most recent undetectable VL test result.
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
Liver Function [AST (SGOT) and ALT (SGPT)] Test Results
The AST (SGOT) and ALT (SGPT) are acronyms for the names of two enzymes that are normally located inside the cells of the liver. When the blood levels of these liver enzymes are abnormally high, this indicates that liver cells have been damaged.
The AST (SGOT) and ALT (SGPT) levels are components of one of several larger set or panel of lab tests. Thus, expect to find AST (SGOT) and ALT (SGPT) values as part of the test results when any of the following test panels is ordered by the provider (i.e., on the physician’s order sheets):
Liver function tests (LFTs)
Comprehensive metabolic panel (CMP) or metabolic panel
Chem 12 or Chem 20
SMA 12 or SMA 20
SMAC
For an example of a report on comprehensive metabolic panel results, please see Appendix E.
This sub-section is for recording information about abnormal liver function tests before the SP start date, and begins with the following question. “Is there documentation of abnormal ALT (SGPT) or AST (SGOT) test results prior to the SP start date?”
If no,
Select “No” if there is no documented abnormal ALT (SGPT) or AST (SGOT) test results before the SP start date.
Note that if there are documented ALT (SGPT) and/or AST (SGOT) results before the SP start date, but none is abnormal, then the appropriate response to the above stem question is “No.”
Each laboratory has a “reference range” for each type of laboratory test performed. To determine whether a test result is “abnormal,” compare the result to the normal reference range on the laboratory report.
No further data collection is needed in this sub-section on liver function testing.
If yes,
Select “Yes” if there are documented results from before the SP start date, showing abnormal levels of ALT (SGPT) or AST (SGOT).
If there are abnormal test results on two or more different dates, enter the date of the earliest abnormal test result before the surveillance period – but no earlier than the date of the first positive HIV test.
- Record the available date information.
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
![]()
HIV ART resistance testing is done to help determine which antiretroviral regimen(s) is/are most likely to be effective for the patient. It is a way of obtaining information about the capacity of the virus to tolerate or resist the effect of specific antiretroviral drugs on its ability to replicate.
On the MHF, the following stem question is used to determine whether there was documentation of HIV ART resistance testing before the surveillance period start date. “Is there documentation of HIV ART resistance testing prior to the SP start date?”
If no,
Select “No”
No further data collection is needed for Section XI.
If yes,
Select “Yes” to this question if any result from HIV ART resistance testing at anytime before the surveillance period was documented, including any mention of results done at another facility.
Complete the relevant information in this section on the MHF.
Notes on Resistance Testing
Currently, there are two basic laboratory methods for determining HIV ART resistance:
Genotypic testing involves detecting certain genetic mutation patterns that may confer an ability in the virus to tolerate specific antiretroviral drugs.
Phenotypic testing involves measuring the actual drug concentration that is needed to suppress HIV growth (production) by a specified amount; the higher the drug concentration that is needed, the more resistant the virus. The term “decreased susceptibility” may also be used to refer to drug resistance.
Results from ART resistance testing may also be reported as “virtual phenotypes.” This refers to a way of using the results from genotypic testing to predict the resistance patterns of the virus, without actually conducting phenotypic testing.
It is an enhanced analysis or interpretation of information obtained from genotypic testing.
Results from genotypic testing are used to predict the type of resistance profile that would normally be obtained from phenotypic testing.
The laboratory report of virtual phenotypes would show not only the actual mutations identified through genotypic testing, but also a “virtual” or predicted profile of drug resistance (that may resemble phenotypic testing results).
Be careful not to confuse results on a combined report (results from both genotypic and phenotypic testing) with a report on virtual phenotypes – please see explanations of the different types of laboratory reports below.
Laboratory reports on HIV ART resistance testing
For any method of HIV ART resistance testing, identify the class(es) of antiretroviral drug for which evidence of viral resistance (or decreased susceptibility) was detected.
For examples of laboratory reports on HIV ART resistance testing, please see Appendix G.
Report on genotypic testing only
- This type of report lists specific mutations detected in the sample and the antiretroviral drugs (and antiretroviral drug class) associated with the different mutations.
- The mutations detected may be specified using one of the following naming conventions:
Alphabet # # # Alphabet for example, M184V represents a mutation associated with resistance to Epivir, a nucleoside reverse transcriptase inhibitor (NRTI)
# # # Alphabet for example, 184V refers to the same mutation as above
• Report on phenotypic testing only
- For each antiretroviral drug involved in the testing, the report shows the ratio of the drug concentration that was required to suppress viral growth in the patient’s blood to the concentration that was required to suppress viral growth of an HIV strain known to be sensitive to the drug.
- For example, the ratio of the concentrations of Nelfinavir required to suppress HIV growth by 50% (IC50):
Concentration in patient’s blood being tested = 80 Concentration in sample of known sensitive HIV strain
This indicates an 80-fold resistance (or reduced susceptibility) to Nelfinavir.
• Combined report for genotypic and phenotypic test results – will show both
- Mutations identified through genotypic testing
- Inhibitory concentration (e.g., IC50) of each antiretroviral drug and the amount of fold change in concentration (e.g., 80-fold resistance), based on comparison with a known sensitive strain, from phenotypic testing.
Report on virtual phenotypes – will show the following information
- Mutations identified through genotypic testing
- Results from a comparison of the mutation patterns with those of other previously tested samples (from a large database) on which both the mutations and actual antiretroviral drug resistance profiles have been determined.
- Predicted susceptibility to specific antiretroviral drugs, based on the enhanced analysis explained above.
Genotypic Resistance Testing
This sub-section is for recording information on any results of genotypic resistance testing before the SP start date, and begins with the following question. “Was genotypic ART resistance testing performed prior to the SP start date?”
If no,
Select “No, documented that resistance testing was not done” only if there is specific documentation from before the SP start date indicating that genotypic resistance testing was NOT done.
No further data collection is needed for this sub-section.
If not documented,
Select “Genotypic resistance testing not documented” if there is no documentation to indicate whether this type of resistance testing was done before the SP start date.
No further data collection is needed for this sub-section.
If yes,
• Select “Yes, resistance reported” if the documented results indicate that there is evidence of ART resistance from the genotypic testing before the SP start date.
- Select the classes of ART for which there is documented resistance, based on the genotypic test results.
- Select “ART classes not specified” if the documentation of the genotypic test results did not specify the ART classes to which resistance occurred.
Select “Yes, no resistance reported” if the documented results specifically indicate that there is NO evidence of ART resistance from genotypic testing done before the SP start date.
Select “Yes, but test results not documented” if there is documentation that genotypic
resistance testing was ordered, but no result is documented.
Phenotypic Resistance Testing
This sub-section is for recording information on any results of phenotypic resistance testing before the SP start date, and begins with the following question. “Was phenotypic ART resistance testing performed prior to the SP start date?”
If no,
Select “No, documented that resistance testing was not done” only if there is specific documentation from before the SP start date indicating that phenotypic resistance testing was NOT done.
No further data collection is needed for this sub-section.
If not documented,
Select “Phenotypic resistance testing not documented” if there is no documentation to indicate whether this type of resistance testing was done before the SP start date.
No further data collection is needed for this sub-section.
If yes,
• Select “Yes, resistance reported” if the documented results indicate that there is evidence of ART resistance from the phenotypic testing before the SP start date.
- Select the classes of ART for which there is documented resistance, based on the phenotypic test results.
- Select “ART classes not specified” if the documentation of the phenotypic test results did not specify the ART classes to which resistance occurred.
Select “Yes, no resistance reported” if the documented results specifically indicate that there is NO evidence of ART resistance from phenotypic testing done before the SP start date.
Select “Yes, but test results not documented” if there is documentation that phenotypic resistance testing was ordered, but no result is documented.
Virtual Phenotypic Resistance Testing
This sub-section is for recording information on any results from virtual phenotypic resistance testing before the SP start date, and begins with the following question. “Was virtual phenotypic ART resistance testing performed prior to the SP start date?”
If no,
Select “No, documented that resistance testing was not done” only if there is specific documentation from before the SP start date indicating that virtual phenotypic testing was NOT done.
No further data collection is needed for this sub-section.
If not documented,
Select “Virtual phenotypic resistance testing not documented” if there is no documentation to indicate whether this type of resistance testing was done before the SP start date.
No further data collection is needed for this sub-section.
If yes,
• Select “Yes, resistance reported” if the documented results indicate that there is evidence of ART resistance from the virtual phenotypic testing before the SP start date.
- Select the classes of ART for which there is documented resistance, based on the virtual phenotype test results.
- Select “ART classes not specified” if the documentation of the virtual phenotypic test results did not specify the ART classes to which resistance occurred.
Select “Yes, no resistance reported” if the documented results specifically indicate that there is NO evidence of ART resistance from virtual phenotypic testing done before the SP start date.
Select “Yes, but test results not documented” if there is documentation that virtual phenotypic resistance testing was ordered, but no result is documented.
![]()
In this section the following stem question is used to determine whether substance abuse was diagnosed in the Medical History Period (MHP): “Is there documentation of reported or suspected substance abuse, including substance abuse counseling or treatment, prior to the SP start date?”
If no,
Select “No” if there is no documentation in the medical records that the patient had substance abuse at any time during the medical history period.
No further data collection for Section XIII is necessary.
If yes,
Select “Yes” if, at any time during the MHP, the medical records indicate that the patient had any substance abuse whether it was by patient self-report (e.g., medical history) or provider diagnosis (e.g., assessment).
• To meet the definition of drug abuse, the patient's medical record should have documentation of one of the following
- Report of injection of illicit drugs or of drugs obtained without a prescription or used contrary to medical indication.
- Report of treatment (including documentation of referrals or consultations) for abuse of injection drugs.
Proceed with completing the relevant information in this section.
Select a response for the question, “Is evidence of any injection substance abuse documented prior to the SP start date?”
Select “No” if there is no documentation indicating injection drug abuse before the SP start date.
Select “Yes” if there is documentation indicating injection drug abuse before the SP start date. For example, terms such as “skin popping” or “track marks” are possible references to injection drug abuse.
Abused Substances
If substance abuse occurred, (non-prescription or prescription) indicate which substance(s) was/were abused prior to the surveillance period start date.
Multiple substances may be selected, but select only those that are documented in the medical records.
• For each substance selected, indicate the type of abuse (injection or non-injection) as indicated in the medical record (both injection and non-injection abuse may be selected if the documented information suggests both types of abuse occurred).
- If the documented abused substance(s) is/are not listed on the abstraction form, up to three “other” substances in this section may be entered under “Other, Specify.”
- If there is evidence of substance abuse, but the abused substance was not specified in the medical records, select “Substance not specified.”
Type of Abuse
Indicate the type of abuse that occurred with each abused substance that was documented in the medical records, by selecting “Injection,” “Non-injection,” or “Not documented.”
• Type of Abuse: Injection
- Examples of some substances commonly injected include:
Methamphetamine
Amphetamines (speed) and other stimulants
Cocaine
Heroin and other opiates
Speedball (heroin and cocaine)
Steroids
- Select “Injection” ONLY if there is specific documentation that the patient injected the substance. If the patient used a substance that is commonly injected, such as heroin, but there is no specific documentation that it was injected, select "Not documented" instead.
• Type of Abuse: Non-injection
- Examples of some common non-injection drugs include:
Amphetamines (speed) and other stimulants
Barbiturates
Cocaine (including crack)
Heroin and other opiates
Marijuana and hashish
Nitrites, poppers, and other inhalants
PCP, LSD, and other hallucinogens
Steroids
Valium and other benzodiazepines
- If the medical record documentation indicates that marijuana was used only for medical purposes, do not select “Non-injection drug Use.”
- Select “Non-injection” ONLY if there is specific documentation that the substance was administered in a way other than through injection.
Type of Abuse: Not documented
Select “Not documented” if there is no specific documentation of how the abused substance was administered.
Date of Last Use
For each selected substance, enter the date of the last use documented in the medical record by entering the 2-digit month and 4-digit year (i.e. 04/2006). If the date of last documented use is not specified in the medical records, select “Date not documented” and follow the instructions below for entering missing date values.
Entering incomplete date information:
If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
If the year is missing, or both year and month are not documented, select “Date not
documented and enter “99/9999.”
![]()
In this section the following stem question is used to determine whether mental illnesses were diagnosed during the MHP: “Is there documentation of any of the following mental illnesses prior to the SP start date?”
If no,
Select “No” if there is no evidence that the patient was diagnosed with any of the listed mental illnesses before the SP start date.
No further data collection is necessary for Section XIII.
If yes,
Select “Yes” if there is documentation that the patient was diagnosed with one or more of the listed mental illnesses (select all diagnoses that apply) before the SP start date.
Indicate which mental illness(es) was/were diagnosed before the SP start date.
Mental illness diagnosis
There should be specific documentation that one or more of the mental illnesses listed on the MHF was/were diagnosed.
There should also be documentation that referral(s) was/were made for specialized care (e.g., psychotherapy, inpatient mental health care) or that the patient is receiving such care for a mental illness.
![]()
On the MHF, the following stem question is used to determine whether there is documentation, before the surveillance period start date, of the patient’s family history of one or more of four common chronic conditions: “Is there documentation of family history of any of the following conditions prior to the SP start date?”
If no,
Select “No” if there is no documented family history of any of the listed conditions: Diabetes mellitus, hypertension, hypercholesterolemia, ischemic heart disease, including myocardial infarction.
No further data collection is needed for section XIV.
If yes,
Select “Yes” if, before the surveillance period start date, there is documentation of a positive family history of at least one of the listed conditions (i.e., at least one of the listed conditions has previously affected one or more of the patient’s family members).
Indicate any of the listed conditions for which there is a family history.
![]()
Enter the MMP Participant ID number in the text box provided. Also, enter the Facility ID number in the text box.
This space can be used to document information that is conflicting, requires discussion with the project coordinator and/or the CDC project officer, or if there is additional information to enter from any section in the MHF.
This section should not be sent to the CDC.
Surveillance Period Summary Form (SPSF)
OPTIONAL
– FOR LOCAL USE ONLY
This is the section at the bottom of the cover page of the form. It can be used to collect information like patient name and medical record number, for obtaining the appropriate medical records for abstraction. This section should be separated at the perforations from the cover page and retained for local use only, before the rest of the form is sent to CDC.
![]()
MMP Participant ID
Participants will be identified only by a 12-digit numeric participant ID number. This is a unique identifier that will be associated with that patient throughout the project period. Patients should have been assigned a participant ID when the patient lists are compiled for sampling.
The Participant ID consists of the following:
• The first eight digits designate the facility where a particular patient was sampled, and is called the “facility ID”
- The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
- The next four-digit code is assigned to the selected facility by the MMP project area.
The final four-digits of the Participant ID are assigned to each person eligible for participation in MMP. This ID is usually assigned through the consecutive numbering of MMP-eligible patients on each participating facility’s edited patient list.
The Participant ID is a mandatory field for each SPSF. This 12-digit numeric ID is also the number that will be used to match the interview data with the medical record abstraction data.
When requesting medical records, it is a good idea for abstractors to have this patient ID number on hand, as well as any other patient identification number(s) (e.g., medical record number) that is/are necessary to obtain medical records on the correct patient.
Surveillance Period (SP)
This is the period from which medical record will be abstracted at each facility using the three surveillance period forms. The SP start date is the 12-month period immediately preceding the date of interview or the first attempted contact (if the patient was not interviewed). Therefore, the SP end date will be the date of interview or first attempted contact (if a patient was not interviewed). The dates should be entered in mm/dd/yyyy format.
Facility ID
This is an 8-digit number
The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
The next four-digit code was one that was assigned to the selected facility during the facility sampling frame construction. If additional HIV care facilities not already in the facility sampling frame were identified during patient interview or medical record abstraction, the project coordinator or data manager should assign a new code to each of these facilities.
Date
of Abstraction
Enter
the Date
of Abstraction
in mm/dd/yyyy format.
Abstractor
ID
Enter
the preset 3 digit Abstractor
ID
in the appropriate place on the SPSF.
![]()
Most recent height (ft/in) during the SP:
Most patients are assumed to have achieved their adult height by the time they are eligible for MMP participation, but because heights are not as routinely measured or documented as weight is, the information is being captured from both the Surveillance Period and the Medical History Period.
If there is more than one height documented during the Surveillance Period, enter the most recent measurement in the SPSF.
Select “Height not documented” if the height information is missing.
![]()
Is there documentation of any of the following during the SP?
This section is available as an optional tool to help guide the abstraction process; abstractors are not required to complete this section.
If “Yes” is selected for the above question, select all the appropriate choices below the question in this section of the form, and follow the instructions that indicate which section to complete, for each choice selected.
![]()
Look for documented evidence regarding reimbursement for care (e.g., health care coverage or insurance) received during the SP in the medical records. Much of this information may be found in the administrative/accounting section of the medical records; sometimes, there may also be documentation in the “progress notes” section. Documented patient-reported information on reimbursement or health care coverage should be accepted in this section.
In the SPSF the following stem question is used to determine whether any reimbursement was provided for the patient’s medical care during the surveillance period at the facility. Is there documentation of the type of reimbursement for medical care or other services at this facility during the SP?
If no,
Select “No”
No further data collection is needed for Section IV
If yes,
Select “Yes” if there is documented evidence of the type of reimbursement for medical care during the surveillance period.
Proceed by indicating the type of reimbursement (explained below).
Types of reimbursement for medical care
Select all choices that apply
AIDS Drug Assistance Program – Also known as “ADAP,” provides medications for the treatment of HIV disease. Program funds may also be used to purchase health insurance for eligible clients. Amendments to the Ryan White CARE Act in October 2000 added additional language allowing ADAP funds to be used to pay for services that enhance access, adherence, and monitoring of drug treatments. The program is funded through Title II of the CARE Act, which provides grants to States and Territories. (See http://hab.hrsa.gov/programs/factsheets/adap1.htm). Also, be aware of local branding (names) for ADAP, e.g., Texas HIV Medication Program (THMP).
CHAMPUS/Tricare – Health care insurance for uniformed services personnel and their families/dependents. Formerly known as “CHAMPUS,” it has been replaced by “Tricare.”
Clinical Trial/Clinical Study – Patients may receive medical care and/or medications, free of charge, as part of a clinical trial or study at the facility.
Medicaid/Medicare – Be aware of any local branding (names) of government insurance programs like Medicare and Medicaid, e.g., MediCal in California.
None/Self-pay (during all or part of the SP) – select this choice ONLY if there is a specific mention that the patient had no insurance or other type of reimbursement for care or if the patient paid out-of-pocket for care.
Private (including HMO/PPO) – It would be a good idea to become familiar with some of the common types of private insurance in the local area. Remember that private insurance coverage can be in the patient’s name, or the patient may be listed on another individual’s (e.g., spouse, relative) policy.
Prison/Jail – Some MMP participants may be inmates in a correctional facility that provides medical care.
Ryan White (excluding ADAP) – Indicate if there is documentation that any of the patient’s medical care is being supported by Ryan White Care Act (RWCA) funds.
Veterans Administration – Veterans of the United States armed forces may be eligible for a broad range of programs and services provided by the federal Department of Veterans Affairs (VA). Eligibility for most VA benefits is based upon discharge from active military service under other than dishonorable conditions.
Other public insurance, Specify – Enter up to two other types of public insurance (provided by the government) not already listed as a choice on the form. Be aware of local branding (names) of public assistance programs.
Other insurance, Specify – Enter any other type of insurance not already listed on the form.
Other, Specify – Enter any other type of health care coverage not already listed on the form.
![]()
The following stem question is used to determine whether certain medical and/or ancillary service(s) was/were provided to the patient at the facility during the surveillance period. Is there documentation that other services were provided at this facility during the SP?
If no,
Select “No” if none of the listed services was provided at the facility during the surveillance period.
No further data collection is needed for Section V.
If yes,
Select “Yes” if, at any time during the surveillance period, there was documentation that one or more of the listed services was/were provided to the patient.
Only include services that were provided at the facility where abstraction is being conducted. If there was documentation that one of the services was provided at another facility, then complete a separate SPSF for the information from that facility.
Proceed by indicating all listed services that was/were provided.
Services
Select all of the listed services that apply
Case management – Case management is a collaborative approach to providing and coordinating health care services. Patients with multiple and/or complex health issues would often need the services of a case manager, who are usually social workers or registered nurses with certification in case management.
Chemotherapy – This refers to medical therapy given as treatment for cancer. This does not include radiation therapy, which may also be given as treatment for cancer in some situations.
Dental care – This would include routine preventive check-ups as well as treatment for dental problems.
Dialysis – This is usually provided to patients with kidney failure. Include any type of dialysis – hemodialysis, which is usually provided at a medical facility, and peritoneal dialysis, which can be performed at home with the help of family members or home health care professional.
Education session – This should be a session specifically designed to educate the patient about particular behavioral and/or health issues. The health issues being addressed do not have to be HIV-related, and it can be either an individual or group session. However, it should involve more than a mention in the medical records that certain topics were briefly discussed with the patient during a medical visit.
Hospice care – This refers to palliative care provided to patients who are in the final stages of a terminal illness.
Mental health counseling or treatment – This refers to counseling or psychotherapy provided by a psychiatrist, psychologist, or other individuals who are professionally certified to provide mental health counseling. The “treatment” portion of this term refers to comprehensive treatment programs for mental health conditions, for example – substance abuse or eating disorders. Do not select this choice if the provider only provided a prescription for an anti-depressant or anti-anxiety medication.
Nursing home care – A Nursing Home, also known as a “Skilled Nursing Facility or SNF,” provides care 24-hours a day to people who cannot care for themselves due to physical, emotional, or mental conditions. A licensed physician supervises each patient’s care and a nurse or other medical professional is almost always on the premises. Most nursing homes have two basic types of services: skilled medical care and custodial care.
Skilled medical care includes services of trained professionals that are needed for a limited period of time following an injury or illness (e.g., physical therapy, speech therapy, wound care). However, skilled medical care may also be provided on a long-term basis (e.g., mechanical ventilation).
Custodial care refers to assistance with activities of daily living (e.g., bathing, dressing, eating).
Nutritional counseling – This should be counseling that is provided by a registered dietitian or nutritionist. This type of counseling is usually formally requested by the medical provider as a consultation.
Physical therapy – This refers to any type of physical therapy provided by a licensed physical therapist. This is usually requested by the medical provider as a consultation.
Prenatal care – This refers to any type of prenatal care, provided during facility visits or inpatient stays that involved an assessment of the patient’s pregnancy through physical examination, laboratory testing, ultrasonography, adjustments of antiretroviral medications.
Receipt of equipment or supplies – Some facilities may provide, loan, or rent certain types of equipment or supplies for the patient’s use.
Substance abuse counseling or treatment – This should be counseling or treatment that is provided as part of a comprehensive substance abuse treatment program.
Support group – Some facilities may have ongoing support groups, organized around common medical issues among patients. For example, an HIV patient support group may focus on helping patients to improve adherence to antiretroviral therapy. The support group may be formal (with a facilitator) or informal. Select this choice if there is documentation in medical records that the patient has participated in a support group during the surveillance period.
Pharmacist consultation – This refers to a formal consultation with a pharmacist, to provide education about medications or to improve adherence to antiretroviral therapy. This type of consultation is usually requested by a medical provider.
Other, Specify – Record up to six other services (other than medical care for HIV infection) in “Other, Specify” fields in this section.
![]()
The following stem question is used to determine whether screening for tuberculosis, cervical cancer, and anal cancer occurred during the SP: “Is there documentation of screening for tuberculosis(TB), or cervical or anal cancer, during the SP?”
If no,
Select “No” if there is no evidence of screening for these conditions during the surveillance period.
No further data collection is needed for Section VI.
If yes,
Select “Yes” if the medical record indicates that the patient was screened for any of these conditions during the surveillance period.
Continue to each of the successive sub-sections on screening for TB and for cervical and anal cancer
Tuberculosis
The USPHS recommends screening of HIV-infected persons annually for infection with Mycobacterium tuberculosis (TB). When an HIV-infected patient tests positive for TB or is diagnosed with active TB, this means that the patient has the TB germ in the body and is at increased risk for getting active TB disease, but is not ill with the disease yet. Once an HIV-infected patient tests positive for latent tuberculous infection or is diagnosed with active TB, he/she will no longer be screened.
The following stem question is used to determine whether screening for latent tuberculous infection occurred during the SP start date: “Was screening for tuberculosis (TB) performed during the SP?”
If no,
Select “No” ONLY if the medical record specifically states that the patient was NOT screened for latent tuberculous infection during the surveillance period.
No further information needs to be recorded about tuberculosis screening.
If not documented,
Select “TB screening not documented” if the medical record has no specific mention of whether screening for latent tuberculous infection was performed during the surveillance period.
No further information needs to be recorded about tuberculosis screening.
If yes,
Select “Yes” if the medical record indicates that the patient was screened for latent tuberculous infection during the surveillance period.
Continue to the requested information on screening tests for latent tuberculous infection.
Screening Tests: Latent TB infection
The Tuberculin skin test (TST) is the standard test used to screen for latent tuberculous infection in persons with HIV and is administered by an intradermal injection on the forearm. The TST is also called the Mantoux test or purified protein derivative (PPD) test.
A new test called the QuantiFERON test (QFT) – approved by the Food and Drug Administration in 2005 – may also be used to screen for TB using a blood sample, though the performance characteristics of QFT have not been fully evaluated in HIV-infected patients.
The next data field follows the TB section’s stem question and should be completed only if the answer to the stem question was “Yes.” “Date of the most recent tuberculin skin test (TST/PPD/Mantoux) or QuantiFERON test (QFT) during the SP.”
• Record the date of the most recent TST/PPD/Mantoux/QFT during the surveillance period. -Record the available date information.
-If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
-If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
• “Result of the most recent TST/PPD/Mantoux/QFT during the SP”
-If TST/PPD/Mantoux was the most recent screening test for latent tuberculous infection during the surveillance period,
. Record the size of the skin reaction to this test in millimeters, if documented, or
. Select one of the other options for a result, as appropriate.
-If the QFT was the most recent screening test for latent tuberculous infection prior to the SP start date, choose from the list of options to record the QFT results.
Cervical and Anal Cancer
Screening for both cervical and anal cancers is performed with the Pap smear test. Although, traditionally, the Pap smear has been used to screen for cervical cancer only, it is now also offered as a screening test for anorectal cancer both in women and in men who have sex with men. However, note that the use of Pap smear as a screening test for anal cancer (whether in women or men) is not an official USPHS guideline, and anal cancer screening by Pap smear will likely not be as common as screening for cervical cancer. Published guidelines recommend screening schedules that are based on age and risk behaviors, but most sexually active women should undergo Pap smear screening for cervical cancer annually.
The following stem question is used to determine whether screening for cervical and/or anal cancer occurred during the surveillance period: “Was screening for cervical or anal cancer performed during the SP?”
If no,
Select “No, documented that screening was not done” if the medical record specifically states that the patient was NOT screened for cervical and/or anal cancer during the surveillance period
No further information needs to be recorded about cervical or anal cancer screening.
If not documented,
Select “Cervical and anal cancer screening not documented” if the medical record makes no specific mention of whether cervical and/or anal cancer screening was performed.
No further information needs to be recorded about cervical or anal cancer screening.
If yes,
Select “Yes” if the medical record indicates that the patient was screened for cervical and/or anal cancer during the SP.
Indicate the results of the testing that was done during the SP.
-Specify the Site (cervical, anal, or unspecified)
-Record the results for each site selected from the options provided.
![]()
The following stem question is used to determine whether immunization(s) for hepatitis A and/or B, influenza and/or pneumococcal pneumonia were given at any time during the surveillance period (SP):
“Is there documentation of whether or not hepatitis A, B, A and B, Influenza or pneumococcal immunizations were given during the SP?”
If no,
Select “No” if there is no evidence in the medical records that the patient was vaccinated for hepatitis A or B, influenza, and pneumococcal pneumonia during the surveillance period.
No further data collection for Section VII.
If yes,
Select “Yes” if the medical record indicates that the patient was immunized against hepatitis A or B, influenza and/or pneumococcal pneumonia in the surveillance period.
Complete the sub-sections under this question for each type of vaccine administered.
Hepatitis A/B/A & B
The USPHS recommends vaccinating HIV-infected persons against hepatitis A unless the patient has been previously diagnosed with hepatitis A; this recommendation also applies to hepatitis B vaccination.
There is no vaccination for hepatitis C, D, or E. Nevertheless, HIV patients who have hepatitis C are at a particularly high risk of serious illness if they become infected with hepatitis A or B – making vaccination for hepatitis A and B especially important in these patients.
The following stem questions are used to determine whether immunization for hepatitis A, B, or A and B occurred during the surveillance period (SP):
“Was hepatitis A vaccine given (Havrix, Vaqta) given during the SP?” “Was hepatitis B vaccine (Energix B, Recombivax) given during the SP?” “Was hepatitis A and B vaccine (Twinrix) given during the SP?”
If no (to any of the three hepatitis vaccine stem questions above),
Select “No” ONLY IF the medical record specifically states that the patient was NOT given that particular hepatitis vaccine during the surveillance period.
Specify the reason why the particular hepatitis vaccine was not given, or indicate that a reason was “Not documented.”
If not documented,
• If the medical record makes no specific mention of whether a particular hepatitis vaccination was administered, indicate that the particular hepatitis vaccination was “not documented.”
If yes (to any of the three hepatitis vaccine stem questions above),
Select “Yes” for each type of hepatitis vaccination that was given (hepatitis A, hepatitis B, or combined hepatitis A and B) during the surveillance period.
For each type of hepatitis vaccine given,
-Indicate the number of vaccine doses given.
-Record the date each vaccine dose was given, or select “Date not documented” for any dose with missing date information.
. Record the available date information.
. If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
. If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
-Select “Yes, but number of doses not documented” for each type of hepatitis vaccine that did not have documented information on the number of doses given.
Influenza
The USPHS Guidelines recommend vaccination of all HIV-infected persons against influenza annually, before the start of the influenza season. Because of a theoretical concern that increases in HIV plasma RNA following vaccination during pregnancy might increase the risk of perinatal transmission of HIV, the guidelines also include a statement that providers may wish to defer vaccination for such patients until after HAART is initiated.
The following stem question is used to determine whether vaccination against influenza occurred during the surveillance period (SP): “Was influenza vaccine (flushield, fluzone) given during the SP?”
If no,
Select “No” if there was specific documentation during the surveillance period that that influenza vaccination was NOT administered to the patient at the facility where abstraction is being conducted.
Select the reason why vaccination was not performed, or if a reason was not documented, select “Not documented.”
If not documented,
• If the medical record makes no specific mention of whether influenza vaccination was given, indicate this by selecting “Influenza vaccination not documented.”
If yes,
Select “Yes” if, at any time during the surveillance period, the medical records at the facility indicate that the patient was vaccinated against influenza.
• Enter the date of the most recent dose given during the surveillance period and which dose number (e.g., dose number 1 or number 2) that the most recent dose represents. Alternatively, select “Date not documented” if the date of the most recent dose of influenza vaccine was not documented.
. Record the available date information.
. If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
. If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
Pneumococcal pneumonia
The USPHS Guidelines recommend vaccination of HIV-infected persons against pneumococcal pneumonia when the CD4 T-lymphocyte count is > 200 cells/mm3. A single dose of 12-valent polysaccharide pneumococcal vaccine should be given if the patient has not received this vaccine in the previous five years. The vaccine should also be considered for patients whose CD4 T-lymphocyte count is <200 cells/ mm3, though the efficacy in these patients is not known.
The following stem question is used to determine whether immunization for pneumococcal pneumonia occurred during the surveillance period (SP): “Was pneumococcal vaccine (Pneumovax 23, Pneu-Immune 23) given during the SP?”
If no,
Select “No” ONLY IF there is specific documentation that the patient was NOT vaccinated with a pneumococcal vaccine.
Specify the reason why pneumococcal vaccine was not given, or indicate that a reason was “Not documented.”
If not documented,
• If there is no specific mention of whether pneumococcal vaccination was given during the surveillance period, select “Pneumococcal vaccination not documented.”
If yes,
• Record “1” for the number of vaccination doses given (during the surveillance period) or select “Yes, but number of doses not documented,” as appropriate. -The pneumococcal vaccine is usually given as a single dose, rather than as a
series over several months. The current recommendation is to give a single dose if the patient has not had one in the previous 5 years.
-In a future revision, this section will be revised to better reflect the current recommendation for pneumococcal immunization..
• Specify the date the vaccine dose was given or select “Date not documented,” if there is no documented date of administration.
. Record the available date information.
. If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
. If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
![]()
The following stem question is used to determine whether there is documentation that formal referrals were made by the medical providers for certain services during the surveillance period. “Is there documentation of any of the following referrals during the SP?”
If no,
Select “No” if no documentation that any referrals were made by the providers at the facility for any of the listed services during the surveillance period.
No further data collection is needed for Section VIII.
If yes,
Select “Yes” if, at any time during the surveillance period, there was documentation that one or more formal referrals for the listed services was/were requested by the medical provider(s) at the facility where abstraction is being conducted.
Only include referrals that were initiated at the specific facility. If there was mention in of referrals made by providers at another facility, then complete a separate SPSF for the information on that facility.
Proceed by indicating all referrals made for the listed services in this section of the form.
Referrals for services during the surveillance period
This section is very similar to a previous section, Section V (“Other Services”) on the SPSF. However, the basic difference is that this section emphasizes the initiation of a request or recommendation for services by the facility; whereas the earlier Section V focuses on whether certain services were actually provided to the patient at the facility during the surveillance period.
Referrals usually involve the completion of a formal written request. There may be a specific section for referrals or consults in the medical records, which would contain the original written request for referrals. Documentation that certain referrals were made may also be found in the provider’s “progress notes” or “clinic notes,” in the “nurse’s notes,” or the physician’s orders section of the medical records. In addition, once the patient receives the services for which a referral was made, a note or summary may be written by the party that provided the services. A note may be written in the “progress notes” or “clinic notes” section, if the services was delivered at the same facility, or a summary from an external source of services may be placed in the patient’s medical records, documenting that services were provided.
In this section, record information on referrals only if they were initiated at the facility where abstraction is being conducted; however, the actual services (for which referrals were made) may be delivered inside or outside of this facility.
Adherence support – This should be a formal referral, with a specific documentation that the referral is to address the issue of the patient’s adherence to medications. Providers of adherence support services may be a pharmacist, a nurse educator, or a multidisciplinary team providing the services as part of a specific adherence support program.
Case manager services – Case management is a collaborative approach to providing and coordinating health care services. Case managers assess the needs of the patient, and assist with identifying resources and connecting the patient to resources to provide for those needs. Patients with multiple and/or complex health issues would often need the services of a case manager, who are usually social workers or registered nurses with specific certification in case management. The referral for this type of services should mention that the purpose is for case management, as described above.
Financial assistance – This may be a referral to a social worker/case manager, a community-based organization, or to a specific patient financial assistance program. Documentation related to the referral should include some indication that the purpose is to provide assistance with paying for the patient’s medical care. There may be mention of helping the patient with regards to applying for public assistance (e.g., Social Security, Medicaid, Medicare) or to other sources of financial assistance.
Food and housing support services – This may be a referral to a social worker/case manager, a community-based organization, or to a specific food and housing support program. Documentation related to the referral should include some indication that the purpose is to provide for food and housing needs/issues of the patient. Referrals made to a program like the Women’s, Infants, and Children’s (WIC) Program would qualify as food/nutrition support services if the HIV patient (not only the patient’s children) is a direct recipient of the services.
HIV prevention counseling services – This may be a referral to an individual with professional qualifications in HIV prevention counseling (e.g., HIV counselor, nurse educator), a community-based organization, or a specific HIV prevention counseling program. Documentation related to the referral should include some indication that the purpose is to provide HIV prevention counseling or to address behavioral risk reduction issues with the patient.
Home-based care services – This may include referrals for home health care services or personal assistance with daily activities (e.g., bathing, dressing, cooking). The referral may be made to a community-based service organization, to a home health care agency, or to a specific home-based care program provided by the medical facility.
Intimate partner violence services – This may include referrals for victim’s support services or batterer’s treatment (for the offending partner). Victim’s support services may include emergency shelter, counseling (for individuals, couples, families), and/or support group, and services that address reducing the risk of violence or improving the safety of the battered partner. A police report alone does not qualify as a referral, but a referral for legal counseling services would qualify, if it is specifically for the intimate partner violence situation.
Mental health services – Referrals should be for mental health assessment or mental health counseling that is delivered in an individual or group setting. The services should be provided by mental health professionals, like psychiatrists, psychologists, or designated “mental health counselors.” The referrals may be specifically for counseling, or it may be for a treatment program designed to address certain mental health issues. The services may address any type of mental health issue, including specific mental illnesses, substance abuse, bereavement or other coping/adjustment issues. A support group (e.g., Alcoholics Anonymous), by itself, does not qualify as “mental health services.”
Partner counseling and referral services – Also known as “PCRS,” this refers to services that involve notifying the HIV patients’ sex or drug injection partners regarding their possible exposure to HIV and referring them to HIV counseling and testing services. These services are usually provided by public health professionals in state or local health departments. Documentation related to the referral should include some indication that the purpose is to notify and refer partners of the HIV patients, as explained above.
Reproductive health services – Referrals should be to a professional provider of reproductive health care, such as an obstetrics/gynecology (OB/GYN) specialist, a fertility specialist, a family planning services provider, a community-based organization (e.g., Planned Parenthood) that provides reproductive health services, a men’s health facility or women’s health facility. The referrals may be for any type of reproductive health issues, including sexual health, family planning, fertility treatment, prenatal care. However, a referral to a sexually transmitted disease (STD) clinic does not qualify.
Social worker services – Referrals should be specifically to a social worker or to “social services.” However, the referral may be for any issue that is handled by a social worker, including case management, financial assistance, housing or food assistance, etc.
Substance abuse prevention services – The referral may be to a community-based organization or a program specifically addressing substance abuse prevention, or it may be to a mental health care provider. However, documentation related to the referral should include some mention of substance abuse prevention.
TB treatment services – Referrals may be made to a medical provider specializing in TB, a “TB clinic,” a TB treatment program, or Directly Observed Therapy (DOT). Patients may be referred for conventional TB treatment or for a special problem with TB treatment – for example, multi-drug resistant TB or health issues that may complicate treatment for TB (e.g., liver disease, drug interactions). Documentation related to the referrals should include some mention of treatment or therapy for TB (tuberculosis, Mycobacterium tuberculosis, M. tuberculosis, M. tb). The treatment/therapy may be for either latent infection or for active TB disease.
![]()
IX. Pregnancies and Outcomes (Females only) – SPSF
In this section the following stem question is used to determine if the patient was pregnant during the SP:
“Is there documentation that the patient was pregnant during the SP?”
If no,
Select “No” if there was no documentation that the patient was pregnant during the
surveillance period.
No further data collection is needed for Section IX.
If yes,
• Select “Yes” if there is documentation that the patient was pregnant during the surveillance period. -There may be documentation in the medical provider’s notes or the nurse’s notes
about the patient being pregnant.
-There also may be a documented positive laboratory result on a pregnancy test (urine hCG).
• Complete all relevant information in this section regarding the number of pregnancies and the outcome of each pregnancy during the surveillance period.
-Select the number of times the patient was pregnant, as documented in the medical records, during the surveillance period.
-
-Select
only one outcome for each pregnancy
. Indicate the date of each outcome (or select “Date not documented,” if appropriate) that occurred during the surveillance period.
. If the outcome for a pregnancy was either “Intrauterine fetal death” or “Live birth,” select the method of delivery.
. However, if the pregnancy has continued past the surveillance period, select “Still pregnant” for the outcome, (regardless of what the pregnancy outcome was after the surveillance period). In this situation, enter the end date of the surveillance period for the date of outcome.
![]()
The following stem question is used to determine whether there is documentation, during the surveillance period, of the patient’s family history of one or more of four common chronic conditions: “Is there documentation of family history of any of the following conditions during the SP?”
If no,
Select “No” if there is no documented family history of any of the listed conditions: Diabetes mellitus, hypertension, hypercholesterolemia, ischemic heart disease, including myocardial infarction.
No further data collection is needed for Section X.
If yes,
Select “Yes” if, during the surveillance period, there is documentation of a positive family history of at least one of the listed conditions (i.e., at least one of the listed conditions has previously affected one or more of the patient’s family members).
Indicate all of the listed conditions for which there is a family history.
![]()
On the SPSF, the following stem question is used to record the mortality status of the patient during the surveillance period: “Is there documentation that the patient died during the SP?”
If no,
• Select “No” if there is no documentation that the patient had died during the surveillance period.
• No further data collection is needed for Section XI. If yes,
Select “Yes” if there is documentation that the patient had died during the surveillance period.
Enter the date of death, using the mm/dd/yyyy format. If the date of death is missing, select “Date not documented.”
- Record the available date information.
-If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
- If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
Select the appropriate “Cause of death,” as documented in the medical records at the specific facility.
Enter all diagnoses at death – exactly as documented (and in the same order) in the medical records. If diagnoses are not documented then select “Diagnosis not documented.”
![]()
Enter the MMP Participant ID number and the Facility ID number in the appropriate text boxes.
This section of the SPSF is similar to the “Facility Log Sheet” used with the patient interview portion of MMP. The purpose is to identify, during abstraction, any other facilities where the patient may have visited for medical care for HIV infection during the surveillance period.
The following stem question is used to determine whether there is documentation in the medical records that the patient had received medical care for HIV infection at one or more other facilities: “Is there documentation that the patient visited other facilities for HIV care during the SP?”
If no,
Select “No” if there is no documentation that the patient had made visits to any other facility for medical care during the surveillance period.
No further entry is needed in this section.
If yes,
Select “Yes” if, while abstracting medical record information at a specific facility, there was documentation that the patient had made visits to one or more other facilities for medical care for HIV infection during the surveillance period.
Record the names of all facilities/providers that were found during abstraction in the boxes provided in this section. For each facility/provider that is recorded, include any available contact information – street, city, state, zip code, and telephone number.
Note that the information collected in this section is “for local use only” and should NOT be sent to the CDC.
![]()
Enter the MMP Participant ID number in the text box provided. Also, enter the Facility ID number in the text box.
This space can be used to document information that is conflicting, requires discussion with the project coordinator and/or the CDC project officer, or if there is additional information to enter from any section in the SPSF.
This section should not be sent to the CDC.
Surveillance Period Visit Form (SPVF)
OPTIONAL
– FOR LOCAL USE ONLY
This is the section at the bottom of the cover page of the form. It can be used to collect information like patient name and medical record number, for obtaining the appropriate medical records for abstraction. This section should be separated at the perforations from the cover page and retained for local use only, before the rest of the form is sent to CDC.
![]()
MMP Participant ID
Participants will be identified only by a 12-digit numeric participant ID number. This is a unique identifier that will be associated with that patient throughout the project period. Patients should have been assigned a participant ID when the patient lists are compiled for sampling.
The Participant ID consists of the following:
• The first eight digits designate the facility where a particular patient was sampled, and is called the “facility ID”
- The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
- The next four-digit code is assigned to the selected facility by the MMP project area.
The final four-digits of the Participant ID are assigned through the consecutive numbering of MMP-eligible patients on each participating facility’s edited patient list.
The Participant ID is a mandatory field for each Surveillance Period Visit Form (SPVF). This 12-digit numeric ID is also the number that will be used to match the interview data with the medical record abstraction data.
When requesting medical records, it is a good idea for abstractors to have this patient ID number on hand, as well as any other patient identification number(s) (e.g., medical record number) that is/are necessary to obtain medical records on the correct patient.
Surveillance Period (SP)
This is the period from which medical record will be abstracted at each facility using the three surveillance period forms. The SP start date is the 12-month period immediately preceding the date of interview or the first attempted contact (if the patient was not interviewed). Therefore, the SP end date will be the date of interview or first attempted contact (if a patient was not interviewed). The dates should be entered in mm/dd/yyyy format.
Facility ID
This is an 8-digit number
The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
The next four-digit code was one that was assigned to the selected facility during the facility sampling frame construction. If additional HIV care facilities not already in the facility sampling frame were identified during patient interview or medical record abstraction, the project coordinator or data manager should assign a new code to each of these facilities.
Date of Visit
This refers to the date of the documented medical care visit from which information is being abstracted at a facility.
For example, let’s assume that a surveillance period covers August 31, 2006 to August 31, 2007. If a patient had 2 visits during this period at the facility from which abstraction is performed and the corresponding dates were 02/01/07 and 07/01/07, then the “Date of visit” will be 02/01/07 for the first SPVF and 07/01/07 for the second SPVF.
The number of SPVF completed at a facility should correspond to the number of visits that were made by the patient to that facility, with each visit having a unique date of visit.
The date of visit should be within the SP start date and SP end date. The date of visit is relevant only for the SPVF.
Date
of Abstraction
Enter
the Date
of Abstraction
in mm/dd/yyyy format.
Abstractor
ID
Enter
the preset 3 digit Abstractor
ID
in the appropriate place on the SPVF.
![]()
Weight during this visit (lbs)
Information on weight is collected only from the Surveillance Period using the SPVF. In some instances weight may be given in kilograms; change this to pounds by multiplying by 2.2. If no weight is documented during the visit, select “Weight not documented.”
Is there documentation of the patient being homeless or living in a shelter during this visit?
Indicate if there is any documentation regarding the patient being homeless or living in a shelter during this visit by selecting “Yes” or “No,” as appropriate.
Is this the first SP visit?
If no (regarding the first SP visit to a facility),
Select “No if the information being abstracted on this SPVF is NOT from the first visit to the facility during the surveillance period.
Select an answer to the question, “Is the patient’s residence during this visit is the same as the previous SP visit?” (Information on the patient’s residence can usually be found in the administrative/accounting section of the medical records.)
If no,
-Select “No” if it could be determined from available documentation that the patient’s address of residence was NOT the same as from the previous visit during the Surveillance Period.
-Indicate the country of residence by selecting one of the listed choices on the SPVF under “Patient’s country of residence during this visit.” The “country of residence” should come from the address of residence that applies to the patient at the time of the visit. This information is usually found in the administrative/accounting section of the medical records.
-Enter patient’s new residence address in tear-off section on the front cover of the SPVF, if this information is being collected for local purposes.
If not documented,
-Select “Residence not documented” if there was insufficient information in the medical records to determine whether the patient’s address has changed from the previous visit during the surveillance period.
-If there is an address associated with the particular visit, enter the address in the tear-off section on the front cover of the SPVF, if this information is being collected for local purposes. Do NOT transmit the tear-off section or any other document with potentially identifying information to the CDC.
If yes,
-Select “Yes” if available documentation indicates that the patient’s address of residence was the same as from the previous visit during the surveillance period.
-Proceed to section III- Chief Complaints.
If yes (regarding the first visit to the facility),
Select “Yes” if the information that is being abstracted on this SPVF is from the first visit to the facility during the surveillance period.
Indicate the country of residence by selecting one of the listed choices on the SPVF under “Patient’s country of residence during this visit.” The “country of residence” should come from the address of residence that applies to the patient at the time of the visit. This information is usually found in the administrative/accounting section of the medical records.
Enter the patient’s residence address in tear-off section on the front cover of the SPVF, if this information is being collected for local purposes.
![]()
The following stem question is used to determine if there were chief complaints at each visit during the surveillance period: “Is there documentation of any chief complaints during this visit?”
If no,
Select “No” if there is no documentation of any “chief complaints” and no documented reason for the visit in the medical records.
No further data collection is needed for this section.
If yes,
Select “Yes” if there is documentation of any “chief complaints” or reason for the visit.
Indicate what the complaint(s) or reason was/were for this visit by selecting all applicable choices in this section on the SPVF.
Notes on chief complaints
At the beginning of each medical visit with a physician, nurse practitioner, or physician’s assistant, the patient is usually asked if there are any specific health complaints that should be addressed during that visit. This is done even if the reason for the visit is a routine follow-up. These complaints are commonly called “chief complaints.” Sometimes, the complaints are included in a more comprehensive list called a “problem list,” which refers to the patient’s ongoing problems as well as any new ones.
Look for the chief complaints specific to each visit. This information may be found at the beginning of the documentation for a visit on a particular date, in the progress notes (clinic notes) section (written by the medical provider) and in the nurse’s notes section of the medical records.
Chief complaints are usually recorded using the same or nearly the same wording used by the patient, but sometimes, they also may be reworded into medical terms when documented by the provider.
Look for the same wording as listed on the SPVF. If the wording in the medical records does not match any of the terms listed on the SPVF, enter the chief complaints, exactly as documented in the medical records, into the “Other, Specify” fields.
If there is no “chief complaint” documented for a visit, record the documented reason for the visit, for example: scheduled follow-up visit, lab draw, med refill, etc.
![]()
Is there documentation of any of the following during this visit?
This section is available as an optional tool to help guide the abstraction process; it is not required to complete this section.
If “Yes” is selected to the above question, select all the appropriate choices below the question in this section of the form. In addition, follow the appropriate instructions that indicate which section to complete, for each choice selected.
![]()
The following stem question is used to determine whether AIDS-OI(s) was/were diagnosed during a specific visit during the Surveillance Period: “Is there documentation that any AIDS defining opportunistic illnesses (AIDS OI) were diagnosed during this visit?”
If no,
Select “No” if there is no evidence in the medical records that the patient was diagnosed with an AIDS OI during this particular visit during the SP.
No further data collection for Section V is necessary.
If yes,
• Select “Yes” if the medical records indicate that, at the particular visit during the SP, the patient was diagnosed with
-Any AIDS OI or
-AIDS, but no OI specified [any evidence of immunologic AIDS (i.e., CD4 cell count <200 cells/µl) is captured later in Section XI, under “Laboratory Test Results”.]
Complete the requested information on each AIDS OI that was documented in the medical records.
Record all documented diagnoses – whether presumptive or definitive.
Select from the list of AIDS OIs, which are listed in alphabetical order.
In general, no documented laboratory confirmation is necessary for abstracting AIDS OIs.
Accept an AIDS OI diagnosis if it is documented in the clinical notes as the medical provider’s assessment of the patient’s condition, or if the diagnosis is found in a hospital discharge summary, a transfer note (transferring the patient’s care from one facility to another), or a medical consultation summary – even if these documents do not demonstrate complete information on how the diagnosis was made.
See additional guidance on some conditions later in this section.
Notes on conditions with criteria for extrapulmonary (outside the lungs) involvement
The following illnesses may present in a variety of ways, but can only be considered as AIDS OIs if they affect organs/tissues in addition to the lungs (as in disseminated disease) or as a disease of organs/tissues other than the lungs. When abstracting, be careful that a documentation of one of the following conditions refers to extrapulmonary involvement, as described above.
Coccidioidomycosis, disseminated or extrapulmonary
Cryptococcosis, extrapulmonary
Histoplasmosis, disseminated or extrapulmonary
Mycobacterium avium complex or M. kansasii, disseminated or extrapulmonary
Notes on conditions with criteria for chronicity
To qualify as an AIDS OI, the following illnesses should be mentioned as being “chronic” in the medical records, whether or not a specific time frame was mentioned.
Cryptosporidiosis, chronic intestinal (>1 month duration)
Herpes simplex: chronic ulcer (>1 month duration) or bronchitis, pneumonitis, or esophagitis
Isosporiasis, chronic intestinal (>1 month duration)
Notes on Carcinoma, invasive cervical
There should be documentation that the cancer is “invasive” or corresponds to stages 1A or higher, based on the International Federation of Gynecologists and Obstetrician (FIGO) clinical staging system.
Stage 1A indicates microinvasive disease, which means that there is microscopic evidence of invasiveness, but there is not yet any evidence that the cancer has spread beyond the cervix.
The following terms are NOT indicative of invasive cervical carcinoma:
-Atypical cells of undetermined significance (ASCUS)
-Intraepithelial neoplasia or Cervical intraepithelial neoplasia (CIN)
-Squamous Intraepithelial Neoplasia (SIL) or dysplasia
-Low-grade SIL (LSIL)
-High-grade SIL (HSIL)
-Carcinoma in situ
Notes on Cytomegalovirus (CMV)
Note the criteria that should be met for a CMV-related condition to qualify as an AIDS OI.
• To qualify as CMV disease -The condition should affect areas of the body, in addition to, or other than, the liver, spleen, or lymph nodes
- Examples: CMV colitis, esophagitis, pneumonitis, or CMV neurologic disease such as dementia, ventriculoencephalitis, or ascending polyradiculomyelopathy (spinal cord disease affecting multiple nerve roots)
To qualify as CMV retinitis -There should be documented loss of vision as a result of the CMV retinitis. -“Peripheral retinitis” may be asymptomatic or with mild visual disturbances, and
therefore, does not qualify as an AIDS OI.
Please refer to Appendix B, “AIDS Defining Opportunistic Illnesses” for additonal information on specific AIDS OIs.
![]()
The following stem question is used to determine whether conditions other than AIDS-OI(s) was/were diagnosed or present in the patient at a specific visit during the surveillance period: “Is there documentation that any of the following conditions other than AIDS OI were diagnosed during this visit?”
If no,
Select “No” if there is no evidence in the medical records that the patient had any of the listed conditions in this section of the SPVF at the particular visit in the surveillance period.
No further data collection for Section VI is necessary.
If yes,
Select “Yes” if the medical records indicate that the patient was diagnosed with any of the listed conditions at the particular visit being abstracted.
This would include all conditions that are present, active, or requiring treatment.
Select any listed condition(s) that was/were documented in the medical records at the particular visit during the surveillance period.
***NOTE***
Be
careful not to confuse the diagnoses that should be recorded in
Section IV, “AIDS
Defining Opportunistic
Illnesses
(AIDS OI)” with
those that should be recorded in this section.
This
section includes some clinical conditions that may occur as a result
of HIV disease, but are NOT AIDS-defining.
What evidence of a “diagnosis” to accept?
In general, for MMP, the providers’ diagnoses of the conditions listed in this section should be accepted – whether with or without documented laboratory confirmation. Similarly, when the health care provider makes references to medical conditions that have been diagnosed elsewhere, these should be recorded if it could be determined from documented information that the conditions remain active health problems for the patient.
When there is documentation that a patient is concerned about a particular condition (e.g., “patient noticed oral thrush this week” or “patient thinks she has a vaginal yeast infection”), without documented evidence of a physician diagnosis, do not report as an established diagnosis.
Diagnoses with qualifying terms: Often in medical records, qualifying words are used with diagnoses to denote the degree of certainty surrounding the diagnosis.
-As a general rule of thumb, diagnoses described with the following commonly used “qualifying words” should be counted in MMP:
“diagnostic
procedure results consistent with. . .”
“presumptive
. . .”
“responded
to treatment for . . .”
-On the other hand, diagnoses described by the following qualifiers should not be considered established diagnoses, and should generally not be recorded as a diagnosis in the medical records:
“questionable
diagnosis of . . .”
“Diagnosis
A vs. Diagnosis B . . .”
“conceivable.
. .”
“differential
diagnosis includes X, Y, and Z. . .”
“symptoms
of . . .”
“iffy. ..”
“plausible
. . .”
“probable…”
“possible
. . .”
“potential
. . .”
“questionable
. . .”
“rule
out (abbreviated R/O) . . .”
• If uncertain, reviewing additional information about the clinical course of the patient and other documented impressions of the medical providers over time may assist in determining whether this is an established diagnosis. If possible, consultation with the health care provider would also be helpful.
Selecting conditions other than AIDS OI on the SPVF
The list of conditions in this section of the SPVF was created to capture conditions that are recognized as being associated with either HIV disease or the consequences of prolonged immune suppression or the treatment of HIV infection.
When considering selecting a condition on the list of conditions in this section of the SPVF, look for terms in the medical records that correspond to those listed on the SPVF. For guidance on terms that correspond to each condition on the SPVF, see Appendix C.
Terms with similar spelling
Be careful when abstracting any of the following conditions, which are represented by terms with very similar spelling:
Myelopathy & Myopathy:
-Myelopathy = condition affecting spinal cord
-Myopathy = condition affecting muscles
Nephropathy & Neuropathy:
-Nephropathy = condition affecting the kidneys
-Neuropathy = condition affecting the nerves
Conditions that are physical signs/symptoms, rather than diagnoses
The following conditions are more likely documented in medical records as the patient’s complaints or as physical exam findings than as diagnoses:
Buffalo hump
Constipation
Hypersomnolence
Conditions other than AIDS OI that require entries in “Other, Specify”
For the following 6 conditions, use the “Other, Specify” fields to record additional information:
Abcess: If selected, record the site of the abcess in “Other, Specify.” For examples: “ABCESS LIVER” “ABCESS BRAIN” “ABCESS NOS” (if location unknown)
2. Diarrhea, infectious: If a specific infectious agent is documented (either in the clinical notes or in a laboratory report) as the cause of the infectious diarrhea, enter this information in an “Other, Specify” field as illustrated in the following examples:
“DIAR E COLI”
“DIAR GIARDIA”
“DIAR INFECT NOS” (if diarrhea documented as infectious, but pathogen unknown)
Ischemic heart disease: If a myocardial infarction (MI) (or any of the terms indicating a heart attack) is
documented, record the MI it in an “Other, Specify” field in the following way: “HEART MI”
Other terms indicating an MI:
Myocardial infarction (MI),
Acute MI (AMI),
Heart attack,
Myocardial necrosis,
ST-elevation MI (STEMI),
non-ST- elevation MI (NSTEMI)
4. Neoplasm: Select “Neoplasm” if there is documentation of a diagnosis of cancer or malignancy other than an AIDS-defining cancer. For patients with metastatic cancer (affecting multiple organs), record only the primary cancer diagnosis.
In addition, specify the type of cancer under “Other, specify.” For examples: “NEO LUNGS” “NEO MELANOMA”
Nephropathy: Record any kidney disease documented as a diagnosis by the medical provider during the Surveillance Period as “Nephropathy.”
In
addition, record the specific nephropathy diagnosis (or diagnoses) in
“Other, Specify.”
For
example, if a patient has diabetic nephropathy with chronic renal
insufficiency (CRI), record these
diagnoses
in the following way:
“NEPH
DIABETIC”
“NEPH CRI”
If the diagnosis is any type of renal failure or end-stage renal disease, select both “Nephropathy” AND “Renal failure” (see explanations for “Renal failure” in Appendix C):
For example, if a patient has diabetic nephropathy with chronic renal failure:
Select both “Nephropathy” AND “Renal failure” AND
Enter “NEPH DIABETIC” in an “Other, Specify” field
6. Psychosis: Select “psychosis,” whether it is documented as a diagnosis by itself (e.g., first episode psychosis) or as a component of another illness (e.g., schizophrenia, depression with psychotic features).
If “Psychosis” is selected, indicate under “Other, Specify” the specific psychosis diagnosis or the condition associated with psychosis, for examples:
“PSY FIRST EPISODE”
“PSY SCHIZOPHRENIA”
“PSY HIV DEMENTIA” “PSY NOS”
7. Fatty liver: Select “Fatty liver” AND indicate under “Other, Specify“ any documented cause of the fatty liver condition, for examples:
“FATLIVER
ALCOHOL”
“FATLIVER DRUG-IND” (for fatty liver specified as being
drug-induced)
“FATLIVER NON-ALC” (for fatty liver without detail other
than non-alcohol-related)
“FATLIVER NOS” (if no documented cause for fatty liver)
If the condition is specified as fatty liver caused by alcoholism, select both “Fatty liver” AND “Alcoholism.”
Conditions that may be related to Each Other
Be aware that some conditions listed as “Conditions other than AIDS OI” may be related to each other, such that the selection of one may indicate the need to select another.
• |
Alcoholism: If any of the following liver conditions is specifically documented as being induced by alcohol use, record the condition as both “Alcoholism” as well as the liver condition: -Alcoholic liver failure – select both “Alcoholism” AND “Hepatic (liver) failure” -Alcoholic fatty liver, alcoholic steatohepatitis (fatty liver with inflammation), alcoholic steatonecrosis (fatty necrosis) – select both “Alcoholism” AND “Fatty liver” |
• |
Bronchitis: Record this condition as both “Bronchitis” AND “Respiratory infection, lower.” |
• |
Guillain-Barré syndrome: record this condition as both “Guillain-Barré syndrome” AND “Neuropathy, peripheral.” |
• |
Fatty liver -If specified as alcohol-induced: select both “Fatty liver” AND “Alcoholism” AND enter “FATLIVER ALCOHOL” under “Other, specify” |
|
-If specified as NOT alcohol induced: select “Fatty liver” AND enter “FATLIVER NONALC” under “Other, specify” |
|
-If the documented information is insufficient to determine the cause of the fatty liver condition, select “Fatty liver” AND enter “FATLIVER NOS” under “Other, specify” |
|
-If a diagnosis of “steatohepatitis” or “steatonecrosis” is specified as being related to the use of drugs or medications (see explanations for “Hepatitis, drug-induced” later in this table), record the condition as both “Fatty liver” and as “Hepatitis, drug-induced” AND enter “FATLIVER DRUG-IND” under “Other, specify” |
• |
Hepatic (liver) failure: An underlying liver disease may be documented as the cause of the liver failure. Record the condition as both “Hepatic (liver) failure” AND the underlying liver disease – if it is among those listed as “Conditions other than AIDS OI” on the SPVF or SPIF: |
-Fatty
liver
-Non-alcoholic
fatty liver disease
- Hepatitis, infectious
- Hepatitis, not infectious, drug-induced
Lipodystrophy: -Buffalo hump – record as both “Lipodystrophy” AND “Buffalo hump” -Lipoatrophy – record as both “Lipodystrophy” AND “Lipoatrophy”
Nephropathy: If a diagnosis of diabetic nephropathy is documented in medical records, record it as both “Nephropathy” AND diabetes mellitus (type 1, type2, or NOS).
Neuropathy, cranial: If the cranial neuropathy diagnosis is auditory neuropathy (a neuropathy affecting cranial nerve VIII, which affects hearing), record this condition as both “Neuropathy, cranial” AND “Hearing loss, acquired.”
• Non-infectious hepatitis: If a diagnosis of steatohepatitis (fatty liver with inflammation) or steatonecrosis (fatty necrosis with inflammation) is specifically documented as being drug-induced and not related to alcohol use, record the condition as all of the following: -“Hepatitis, non-infectious, drug-induced”
- “Fatty liver”
-“Non-alcoholic fatty liver disease”
• Rash, drug-related – If any of the following skin conditions is specifically documented as being a drug reaction, record the condition as both “Rash, drug-related” as well as the specific skin condition:
- Erythema Multiforme
- Erythroderma
- Sebborrheic Dermatitis
- Stevens-Johnson’s Syndrome
Renal failure: Record any renal failure diagnosis as both “Renal failure” AND “Nephropathy.”
Sinusitis: Record this condition as both “Sinusitis” AND “Respiratory infection, upper”
![]()
This section collects prophylaxis information for two conditions: 1) Pneumocystis jiroveci (PCP); and 2) Mycobacterium avium complex (MAC). PCP and MAC prophylaxis are recommended to prevent either primary (first occurrence of) or secondary (recurrence of) PCP or MAC opportunistic infection when the patient’s CD4 T-lymphocyte count falls below 200 cells/μL (for PCP) and 50 cells/μL (for MAC).
Two stem questions are used to determine whether PCP or MAC prophylaxis were prescribed:
“Is there documentation of prescription for prophylaxis of Pneumocystis jirovecii pneumonia (PCP) during this visit?”
“Is there documentation of prescription for prophylaxis of Mycobacterium avium complex (MAC) during this visit?”
If no (to both questions),
• Select “No” if there is no documentation of prophylaxis being prescribed during or continued through this visit.
• No further data collection for Section VII is necessary. If yes (to either question),
• Select “Yes” if, during this visit, -There was documentation that “prophylaxis” for PCP and/or MAC was initiated or -There was documentation that the patient was either prescribed or continued on
medical regimens typically provided for prophylaxis (please see Table 1 below).
***NOTE***
Some physicians refer to the following terms interchangeably, but all refer to the same condition:
Pneumocystis
carinii pneumonia (PCP)
Pneumocystis jiroveci
(P. jiroveci)
pneumonia (PCP)
Pneumocystosis
Table 1. Medications used in primary or secondary prophylaxis of Pneumocystis jirovecii pneumonia (PCP) and Mycobacterium avium complex (MAC) disease among HIV-infected patients, Medical Monitoring Project, 2007 data collection cycle.
Medication |
Other Names |
Prophylaxis use |
atovaquone |
Mepron |
PCP prophylaxis if atovaquone 1,500mg by mouth (po) once daily (qd) |
azithromycin |
Zithromax |
MAC prophylaxis if azithromycin 1,200mg by mouth (po) weekly alone or azithromycin 1,200mg plus rifabutin 300mg, po daily (qd) or azithromycin 500mg plus ethambutol 15mg/kg body weight, with or without rifabutin 300mg, po qd |
clarithromycin |
Biaxin |
MAC prophylaxis if clarithromycin 500mg by mouth (po) twice daily(bid) alone or clarithromycin 500mg po twice daily (bid) plus ethambutol 15mg/kg body weight, with or without rifabutin 300mg, po daily (qd) |
dapsone |
DDS |
PCP prophylaxis if dapsone 100mg by mouth (po) daily (qd) or dapsone 50mg po twice daily (bid) or dapsone 50mg po daily (qd), along with pyrimethamine 50mg and leucovorin 25mg, po weekly or dapsone 200mg plus pyrimethamine 75mg plus leucovorin 25mg, po weekly |
ethambutol |
Myambutol |
MAC prophylaxis if ethambutol 15mg/kg body weight, by mouth (po) daily (qd), along with clarithromycin or azithromycin (please see details above for azithromycin and clarithromycin) |
leucovorin |
folinic acid, calcium folinate, calcium levofolinate, sodium folinate, Sodiofolin, Wellcovorin, Isovorin |
See details above for dapsone |
pentamidine |
Pentam 300 |
PCP prophylaxis if given as aerosolized medication monthly |
pyrimethamine |
Daraprim |
See comments above for dapsone |
rifabutin |
Ansamycin, Mycobutin, RBU |
MAC prophylaxis if rifabutin 300mg by mouth (po) daily (qd) alone or rifabutin 300mg po qd, along with azithromycin (sometimes also with ethambutol) – please see details above for azithromycin and ethambutol |
trimethoprimsulfamethoxazole |
TMP/SMX, TMP/SMZ, Bactrim, Septra, Co-trimoxazole, Sulfatrim, Cotrim, Cotrima |
PCP prophylaxis if TMP/SMX, 1 double strength (DS) tablet or 1 single strength (SS) tablet given by mouth (po) daily (qd) or TMP/SMX, 1 DS tablet po three times weekly |
For
additional information, please refer to the following guidelines
available online at http://www.aidsinfo.nih.gov/guidelines/
1)
“Guidelines for the Prevention of Opportunistic Infections
among HIV-Infected Persons – 2002”
2)
“Treating Opportunistic Infections among HIV-Infected Adults
and Adolescents”
![]()
The following stem question is used to determine whether screening by physical examination for certain sexually transmitted infections (STI) occurred: “Is there documentation of physical screening for any sexually transmitted infections (STI) during this visit?”
If no,
Select “No” if there is no documented evidence that an assessment for the presence of STI(s) was performed as part of the physical examination during the visit.
No further data collection for Section VIII is necessary.
If not specified,
• Select “STI not specified” if
-There is documentation that an assessment for STI(s) was performed through physical examination during the visit, but the specific STI(s) being investigated cannot be determined from the available information in the medical records.
-No further data collection for Section VIII is necessary.
If yes,
Select “Yes’ to the stem question if there is documented evidence that the physical examination performed during the visit included an assessment for the presence of STI(s) other than HIV.
Proceed to indicate all of the STI(s) for which there is documentation that an assessment by physical examination was performed during the specific visit.
Clinical Assessment for STIs
Please note that in this section of the SPVF, the requested information is regarding physical examination for STIs, rather than laboratory testing for such conditions – which will be captured in Section XI, “Laboratory Test Results (including ART resistance testing).” Medical providers may sometimes make a decision about treatment for STIs based on findings from the history and physical examination alone – without laboratory test results.
Among HIV patients, early detection and treatment are crucial in reducing the risk of further transmission of HIV as well as the other STIs. Thus, providers may actively look for the presence of STIs as part of a routine comprehensive assessment – whether or not the patient complains about signs and symptoms of STIs. For MMP purposes, both of the aforementioned scenarios are considered “screening,” (regardless of whether the patient had symptoms of an STI).
The clinical assessment for STIs includes the following components
It usually begins with the “history of present illness” or “review of systems” in which certain questions are posed to the patient regarding possible signs and symptoms of STIs, such as unusual discharges or pain or itching in the genital and/or anal area.
Another component is the physical examination.
-The providers would look for any obvious lesions, discharges, and signs of inflammation (swelling, redness, tenderness) of the skin on or around the genitalia. Documentation of physical findings may include references to the presence or absence of
. Urethral or cervical discharges
. Redness (erythema), pain/tenderness
. Genital warts, ulcers, and/or lesions
-An inspection for enlarged lymph nodes called “inguinal nodes” in the groin area may be documented.
-In both men and women, the anal area may be inspected for lesions and signs of inflammation.
-In women, a pelvic examination may be performed to assess the vagina and cervix for signs of STIs. In the medical records, there may be notes about whether any discharges or lesions were seen, and whether there was “cervical motion tenderness” or “CMT.”
• As previously mentioned, medical providers may decide on a treatment plan for STIs, based ONLY on findings from the history and physical examination – without laboratory testing. Alternatively, providers may provide treatment empirically, while awaiting test results. Specimens may be collected from the genital and/or anal areas during the physical examination for laboratory testing. Blood and urine specimens may also be collected for some tests for STIs.
To qualify as “physical screening” for STIs,
• There must be documented evidence suggesting that the provider had physically inspected or examined the genital area (as explained above), whether or not any abnormality was found. -This documentation may be found in the “progress notes” section or the “nurse’s
notes section.
-Because specimens may be collected during the physical examination for STIs, the physician’s orders for certain laboratory tests may indicate that a physical examination for STIs was performed – for example, “send cervical swab for Chlamydia and gonorrhea culture,” or “send urethral swab for gonorrhea culture.”
• Documentation of the patient reported signs and symptoms (from the history of present illness or review of systems) alone does not constitute “physical screening” for STIs.
When conducting a physical examination for STIs, medical providers may not be targeting specific types of STIs. Thus, to determine which type of STIs for which the providers may be generally investigating,
It may be necessary to look in the portion of the clinical notes called the “assessment” or “impression,” which usually follows the documentation on the physical examination. There may be a list of differential diagnosis mentioning specific STIs or terms such as “rule out syphilis, Chlamydia, etc.”
The types of laboratory tests ordered by the provider would also suggest which STI(s) was/were of greatest concern.
![]()
The following stem question is used to determine whether there was documentation that ART was newly prescribed during or continued through a particular visit (from an earlier prescription). “Is there documentation of prescription or continuation of antiretroviral therapy (ART) during this visit?” ”
If no,
Select “No” if there is no documented evidence that ART was prescribed during or continued through the visit.
No further data collection is needed on Section IX.
If yes,
• Select “Yes” if the medical records indicate that ART was prescribed during or continued through the particular visit.
- Please include medications that were mentioned as having been prescribed for the patient by other provider(s) at other facilities during the surveillance period.
- Please also include a medication if it was documented as having been prescribed before a visit but was continued through the visit (i.e., a previously prescribed medication was not discontinued at the visit).
Complete the requested information in this section of the SPVF by selecting all ART
medications documented as having been prescribed during or continued through the
particular visit for the patient.
Notes on antiretroviral drugs on the SPVF
Information on prescribed medications (including ART), may be found in various places in medical records, including the initial history & physical form or summary, referral forms, consultation summary, facility transfer notes, physician’s order sheets, physician’s notes, nurse’s notes, or a pharmacy-generated list of medications for the patient.
The different formulations of current FDA-approved ARTs comprise individual drugs that are organized into several classes – depending on the phase in the HIV infection cycle that the drug disrupts. Some illustrations and explanations of the HIV cycle can be found in Appendix D and at the following web sites:
http://www.hopkins-hivguide.org/tutorial/launch.html (animated presentation) http://www.wellcome.ac.uk/en/labnotes5/animation_popups/hiv.html (animated presentation) http://aidsinfo.nih.gov/contentfiles/HIVLifeCycle_FS_en.pdf http://www.aidsinfonet.org/factsheet_detail.php?fsnumber=106
The MMP abstraction forms include all FDA-approved ART formulations and several ART medications not yet approved by the FDA.
In this section of the SPVF, the ART medications are listed in alphabetical order by a common name, and other known names of each medication are included in parentheses.
Because providers may refer to these medications using a number of different names in the medical records, it would be good to become familiar with the different names of the drugs and to know in which ART classes the different drugs belong.
A “cheat sheet” to help look up information on the ART medications is provided in Appendix
D.
Whenever
a combined ART formulation is found in the medical records, please
indicate on the
abstraction
form the exact formulation that was prescribed, rather than the
constituents of the formulation,
for
example:
If
Combivir was a documented prescription,
Select only “Combivir (AZT/3TC)”
Do NOT select the two drugs in the formulation separately:
-“Zidovudine (AZT, Retrovir)” and
-“Lamivudine (3TC, Epivir)”
If Trizivir was a documented prescription,
Select only “Trizivir (ABC/3TC/AZT)”
Do NOT select the three drugs in the formulation separately:
-“Abacavir (ABC, Ziagen)” and
-“Lamivudine (3TC, Epivir)” and
-“Zidovudine (AZT, Retrovir)”
If the patient was prescribed an antiretroviral medication not listed on the abstraction form, record its name at the end of this section, under “Other, Specify.”
![]()
The purpose of this section is to collect data on selected “other medications” – medications other than antiretroviral drugs. These data will help to assess issues regarding overall standard of care for and treatment burden on patients.
On the SPVF, the following stem question is used to determine whether or not there is documentation that medications other than antiretroviral drugs were prescribed during or continued through a particular visit. “Is there documentation of prescription or continuation of medications other than ART during this visit?”
If no,
Select “No” if there is no documented evidence that medications other than ART was prescribed during or continued through the visit.
No further data collection is needed on Section X of the SPVF.
If yes,
• Select “Yes” if there was documentation that medications other than antiretroviral drugs were prescribed during or continued through the particular visit. A list (“cheat sheet”) of medications will be provided to help determine whether a particular drug name found in the medical records is associated with a medication on the form.
- Please include medications that were mentioned as having been prescribed for the patient by other provider(s) at other facilities.
- Please also include a medication if it was documented as having been prescribed before a visit but was continued through the visit (i.e., a previously prescribed medication was not discontinued at the visit).
Complete this section by selecting any of the medications that pertains to the visit, as explained above.
Notes on “other medications” on the SPVF
Information on prescribed medications may be found in various places in medical records, including the initial history & physical form or summary, referral forms, consultation summaries, physician’s order sheets, physician’s clinic notes, nurse’s notes, or a pharmacy-generated list of medications for the patient.
The medications that should be abstracted in this section of the SPVF are listed in alphabetical order of one of their common names. However, please note that many of these medications may also be mentioned by one or several different names in the medical records. A “cheat sheet” will be provided to help with selecting the appropriate medications, based on a number of different names for each medication. However, it would be a good idea to become familiar with the different names of the medications on this list.
Remember that for the SPVF, information is collected on medications provided as part of treatment at each outpatient visit. An SPVF should be completed for every outpatient visit during the surveillance period.
![]()
On the SPVF, the following stem question is used to determine whether there is documentation of laboratory test results (including ART resistance testing) at a particular visit during the SP, “Is there documentation of any of the following laboratory test results, including HIV ART resistance tests, for this visit?”
If no,
Select “No” if there was no documented laboratory test result for one of the laboratory tests listed in this section of the SPVF.
No further data collection is needed for Section XI.
If yes,
• Select “Yes” if there is at least one result documented at the particular visit for at least one of the laboratory tests listed in this section of the SPVF.
- Please do NOT include laboratory test results performed at other facilities – record laboratory results from each facility during the surveillance period on a separate SPVF, specific to the facility.
- If the date of a test result (i.e., the date the specimen was obtained for the laboratory test) is NOT the same as the date of the particular visit being abstracted, please complete a separate SPVF for the laboratory test result.
- For explanations of the laboratory tests, please see Appendices E and F.
Complete the rest of the section by indicating which laboratory test result(s) was/were documented at the particular visit.
Looking for laboratory results
Although many laboratory results can be found in the “Laboratory” section of a facility’s medical records, it is always a good idea to look for this information throughout other parts of the medical records as well, because formal laboratory reports may not always be available in a patient’s chart, for many different reasons.
Medical providers routinely follow-up the results of tests that they order, sometimes obtaining these results before laboratory reports are printed out. Therefore, the physician’s notes and/or nurse’s notes (care plans, clinic notes, progress notes, history & physical forms, etc.) are also good places to look for laboratory results, as are transfer notes (if patients are transferred from one facility to another) and summaries on referral forms or from medical consultations. In addition, the physician’s order sheets will show when specific tests were ordered – which would provide a clue as to approximately when specimens were obtained for testing.
Date of test result = Date specimen collected
When abstracting information on the “date of laboratory test result,” keep in mind that this refers to the date when the specimen was collected from the patient – rather than the date when the final result was reported by the laboratory – for a particular test. This is because laboratory report print-outs may show both the date test results were reported as well as the date specimens were collected (Appendix E).
Verify the units used with test results
Laboratory reports will show the units associated with the values of test results. Whenever possible, it’s a good idea to verify what units are used with the documented test results in medical records. Although most laboratory results will be reported in a conventional way across facilities, it is good practice to always check and confirm that the laboratory values being abstracted are compatible with what is requested on the abstraction form.
Notes on Resistance Testing
Currently, there are two basic laboratory methods for determining HIV ART resistance:
Genotypic testing involves detecting certain genetic mutation patterns that may confer an ability in the virus to tolerate specific antiretroviral drugs.
Phenotypic testing involves measuring the actual drug concentration that is needed to suppress HIV production by a specified amount; the higher the drug concentration that is needed, the more resistant the virus. The term “decreased susceptibility” may also be used to refer to drug resistance.
Results from ART resistance testing may also be reported as “virtual phenotypes.” This refers to a way of using the results from genotypic testing to predict the resistance patterns of the virus, without actually conducting phenotypic testing.
It is an enhanced analysis or interpretation of information obtained from genotypic testing.
Results from genotypic testing are used to predict the type of resistance profile that would normally be obtained from phenotypic testing.
The laboratory report of virtual phenotypes would show not only the actual mutations identified through genotypic testing, but also a “virtual” or predicted profile of drug resistance (that may resemble phenotypic testing results).
Be careful not to confuse results on a combined report (with results from both genotypic and phenotypic testing) with a report on virtual phenotypes – please see explanations of the different types of laboratory reports below.
Laboratory reports on HIV ART resistance testing
For any method of HIV ART resistance testing, identify the class(es) of antiretroviral drug for which evidence of viral resistance (or decreased susceptibility) was detected.
For examples of laboratory reports on HIV ART resistance testing, please see Appendix G.
Report on genotypic testing only
- This type of report lists specific mutations detected in the sample and the antiretroviral drugs (and antiretroviral drug class) associated with the different mutations.
- The mutations detected may be specified using one of the following naming conventions:
Alphabet # # # Alphabet for example, M184V represents a mutation associated with resistance to Epivir, a nucleoside reverse transcriptase inhibitor (NRTI)
# # # Alphabet for example, 184V refers to the same mutation as above
• Report on phenotypic testing only
- For each antiretroviral drug involved in the testing, the report shows the ratio of the drug concentration that was required to suppress viral growth in the patient’s blood to the concentration that was required to suppress viral growth of an HIV strain known to be sensitive to the drug.
- For example, the ratio of the concentrations of Nelfinavir required to suppress HIV growth by 50% (IC50):
Concentration
in patient’s blood being tested
=
80
Concentration
in sample of known sensitive HIV strain
This indicates an 80-fold resistance (or reduced susceptibility) to Nelfinavir.
• Combined report for genotypic and phenotypic test results – will show both
- Mutations identified through genotypic testing
- Inhibitory concentration (e.g., IC50) of each antiretroviral drug and the amount of fold change in concentration (e.g., 80-fold resistance), based on comparison with a known sensitive strain, from phenotypic testing.
Report on virtual phenotypes – will show the following information
- Mutations identified through genotypic testing
- Results from a comparison of the mutation patterns with those of other previously tested samples (from a large database) on which both the mutations and actual antiretroviral drug resistance profiles have been determined.
- Predicted susceptibility to specific antiretroviral drugs, based on the enhanced analysis explained above.
![]()
In this section the following stem question is used to determine whether substance abuse was diagnosed during the visit that is being abstracted: “Is there documentation of reported or suspected substance abuse, including substance abuse counseling or treatment, during this visit?”
If no,
Select “No” if there is no documentation in the medical records that the patient was
diagnosed with substance abuse during the visit being abstracted.
No further data collection for Section XII is necessary.
If yes,
Select “Yes” if there was documentation that during the visit, the patient was diagnosed with any substance abuse whether it was patient self-report or provider diagnosis.
• To meet the definition of drug abuse, the patient's medical record must have documentation of one of the following
- Report of illicit drugs or of drugs obtained without a prescription or used contrary to medical indication.
- Documented treatment (including referrals) for abuse of injection drugs.
Proceed with completing the relevant information in this section.
Select a response for the question, “Is evidence of any injection substance abuse documented during this visit?”
Select “No” if there is no documentation from the visit indicating current injection drug abuse by the patient.
Select “Yes” if there is documentation from the visit indicating current injection drug abuse by the patient. For example, terms such as “skin popping” or “track marks” are possible references to injection drug abuse.
Abused Substances
If substance abuse occurred, (non-prescription or prescription) indicate which substance(s) was/were abused during the Surveillance Period.
Multiple substances may be selected, but select only those that are documented in the medical records.
• For each substance selected, indicate the type of abuse (injection or non-injection) as indicated in the medical record (both injection and non-injection abuse may be selected if the documented information suggests both types of abuse occurred).
- If the documented abused substance(s) is/are not listed on the abstraction form, up to three “other” substances in this section may be entered under “Other, Specify.”
- If there is evidence of substance abuse, but the abused substance was not specified in the medical records, select “Substance not specified.”
Type of Abuse
Indicate the type of abuse that occurred with each abused substance that was documented in the medical records, by selecting “Injection,” “Non-injection,” or “Not documented.”
• Type of Abuse: Injection
- Examples of some substances commonly injected include:
Methamphetamine
Amphetamines (speed) and other stimulants
Cocaine
Heroin and other opiates
Speedball (heroin and cocaine)
Steroids
- Select “Injection” ONLY if there is specific documentation that the patient injected the substance. If the patient used a substance that is commonly injected, such as heroin, but there is no specific documentation that it was injected, select "Not documented" instead.
• Type of Abuse: Non-injection
- Examples of some common non-injection drugs include:
Amphetamines (speed) and other stimulants
Barbiturates
Cocaine (including crack)
Heroin and other opiates
Marijuana and hashish
Nitrites, poppers, and other inhalants
PCP, LSD, and other hallucinogens
Steroids
Valium and other benzodiazepines
- If the medical record documentation indicates that marijuana was used only for medical purposes, do not select “Non-injection drug Use.”
- Select “Non-injection” ONLY if there is specific documentation that the substance was administered in a way other than through injection.
Type of Abuse: Not documented
Select “Not documented” if there is no specific documentation of how the abused substance was administered.
![]()
Enter the MMP Participant ID number in the text box provided. Also, enter the Facility ID number in the text box.
This space can be used to document information that is conflicting, requires discussion with your project coordinator and/or the CDC project officer, or if there is additional information to enter from any section on the SPVF.
This section should not be sent to the CDC.
Surveillance Period Inpatient Form (SPIF)
OPTIONAL
– FOR LOCAL USE ONLY
This is the section at the bottom of the cover page of the form. It can be used to record information like patient name and medical record number, for obtaining the appropriate medical records for abstraction. This section should be separated at the perforations from the cover page and retained for local use only, before the rest of the form is sent to CDC.
![]()
MMP Participant ID
Participants will be identified only by a 12-digit numeric participant ID number. This is a unique identifier that will be associated with that patient throughout the project period. Patients should have been assigned a participant ID when the patient lists are compiled for sampling.
The Participant ID consists of the following:
• The first eight digits designate the facility where a particular patient was sampled, and is called the “facility ID”
- The first four digits of a particular facility ID represent the “Site ID” or the code for a particular project area (Appendix A of this document).
- The next four-digit code is assigned to the selected facility by the MMP project area.
The final four-digits of the Participant ID are assigned through the consecutive numbering of MMP-eligible patients on each participating facility’s edited patient list.
The Participant ID is a mandatory field for each Surveillance Period Inpatient Form (SPIF). This 12-digit numeric ID is also the number that will be used to match the interview data with the medical record abstraction data.
When requesting medical records, it is a good idea for abstractors to have this patient ID number on hand, as well as any other patient identification number(s) (e.g., medical record number) that is/are necessary to obtain medical records on the correct patient.
Surveillance Period (SP)
This is the period from which medical record will be abstracted at each facility using the three surveillance period forms. The SP start date is the 12-month period immediately preceding the date of interview or the first attempted contact (if the patient was not interviewed). Therefore, the SP end date will be the date of interview or first attempted contact (if a patient was not interviewed). The dates should be entered in mm/dd/yyyy format.
Facility ID
This is an 8-digit number representing an MMP facility that provided outpatient care (or both outpatient and inpatient care) to an MMP participant during the Surveillance Period.
• If, during abstraction at a facility that provided outpatient care to the MMP participant, documentation of an inpatient stay during the SP at another facility is found (e.g., discharge summary or a clinical note indicating the patient was hospitalized at another facility):
-The “Facility ID” of the outpatient facility (where abstraction is being conducted) should be recorded in the “Facility ID” field on the SPIF. This should be done even if the inpatient stay did not occur at the facility where abstraction is being conducted.
-The “Inpatient Facility ID” should also be recorded on the SPIF (see explanation below under “Inpatient Facility ID”).
• As with any MMP facility ID -The first four digit code represents the project area code, which is included in Appendix A.
- The next four-digit code was one that was assigned to the selected facility during the facility sampling frame construction. If additional HIV care facilities not already in the facility sampling frame were identified during patient interview or medical record abstraction, the project coordinator or data manager should assign a new code to each of these facilities.
Inpatient Facility ID
This is an 8-digit number representing a facility that provided inpatient care to an MMP participant during the Surveillance Period.
Information about a particular hospital stay (inpatient stay) may be captured in two different ways,
• If captured by abstracting information from a copy of a “discharge summary” (or from a clinical note indicating the patient was hospitalized) found in the medical records at the facility where the patient received outpatient care,
-In this case, complete both the “Facility ID” and the “Inpatient Facility ID” fields. -The “Facility ID” would represent the facility where the discharge summary is found -The “Inpatient Facility ID” would represent the facility where the inpatient stay actually occurred.
If captured by abstracting information directly from medical records at the facility where the patient was admitted for inpatient care. In this case, complete only the “Inpatient Facility ID” field.
As with any MMP facility ID -The first four digits code represents the project area code, which is included in Appendix A.
- The next four-digit code was one that was assigned to the selected facility during the facility sampling frame construction. If additional HIV care facilities not already in the facility sampling frame were identified during patient interview or medical record abstraction, the project coordinator or data manager should assign a new code to each of these facilities.
Date
of Abstraction
Enter
the Date
of Abstraction
in mm/dd/yyyy format.
Abstractor
ID
Enter
the preset 3 digit Abstractor
ID
in the appropriate place on the SPIF.
Date of admission
This refers to the beginning date of a hospitalization – the first date of the hospital stay. For purposes of abstracting information for the Surveillance Period using the SPIF, the date of admission should fall either within the Surveillance Period or before the Surveillance Period start date, as long as the date of discharge for the same inpatient stay is within the Surveillance Period. However, the date of admission should not fall after the Surveillance period end date.
For example, let’s assume that a Surveillance Period covers August 31, 2006 to August 31, 2007. If a patient had 2 hospitalizations during this period at the facility where the hospital stays occurred, and the corresponding dates of admissions were 02/01/07 and 07/01/07, then
Two separate SPIF should be completed
-The date of admission would be 02/01/07 for the first SPIF and
-The date of admission would be 07/01/07 for the second SPIF.
Enter the dates in mm/dd/yyyy format.
- If only the month is not documented, enter “99 for the 2-digit month field and enter the year.
-If the year is missing, or both year and month are not documented, select “Date not documented and enter “99/9999.”
Date of discharge
The date of discharge refers to the date of release from hospitalization – the last date of a hospital stay. For purposes of abstracting information for the Surveillance Period using the SPIF, the date of discharge may fall within the Surveillance Period or after the Surveillance Period, as long as the date of admission for the same inpatient stay falls within the Surveillance Period. However, the date of discharge should not fall in the Medical History Period.
As with the “Date of Admission” described above, the number of SPIF completed should correspond to the number of hospitalizations and discharges that occurred during the SP, with each hospitalization having a unique date of discharge, as well as a unique date of admission. Enter the date in mm/dd/yyyy format. Enter incomplete date information as shown above.
Patient’s country of residence during this inpatient stay
Record the patients’ residence during each inpatient stay. This is minimal information about residence of the patient which is not likely to be identifying and will be transferred to CDC.
This information is usually found in the administrative/accounting section of the medical records.
Select “Other, Specify” if the documented country of residence is not one of three listed (U.S., Canada, Mexico).
If country of residence is not documented, select “Not documented”.
Remember that a patient’s country of residence during this inpatient stay may change during subsequent hospitalizations.
This information is collected only using the SPIF.
![]()
Patients may be admitted to the hospital because of an acute medical problem or for a special procedure (e.g., chemotherapy, elective surgery).
The following stem question is used to determine if, for a particular hospital stay, the patient was admitted to the hospital for an acute medical problem (which is usually associated with some chief complaints): “Is there documentation of any chief complaints during this inpatient stay?”
If no,
Select “No” if there is no documentation of any “chief complaints” by the patient and no documented reason for the hospital admission that is being abstracted.
No further data collection is needed for this section.
If yes,
Select “Yes” if there is documentation of any “chief complaints” or reason for the hospital admission.
Indicate what the complaint(s) or reason for the hospital admission was/were by selecting all applicable choices in this section on the SPIF.
Notes on chief complaints
Information on the reason for the patient to be admitted to the hospital, including any chief complaints that led the patient to seek care, is usually collected at the time of admission and summarized in a report called the “admission note.” A new admission note is written each time the patient is admitted to the hospital. In the admission note, the complaints may be listed as “chief complaints” or included in a more comprehensive list called a “problem list,” which refers to the patient’s ongoing problems as well as any new ones.
Chief complaints are usually recorded using the same or nearly the same wording used by the patient, but sometimes, they also may be reworded into medical terms when documented by the provider.
Look for the same wording as listed on the SPIF. If the wording in the medical records does not match any of the terms listed on the SPIF, enter the chief complaints, exactly as documented in the medical records, into the “Other, Specify” fields.
If there is no “chief complaint” documented for the inpatient admission, record the reason for the admission, for example: chemotherapy, transfusion, elective surgery, etc.
![]()
Is there documentation of any of the following during this inpatient stay?
This section is available as an optional tool to help guide the abstraction process; it is not a required section to complete.
If “Yes” is selected to the above question, select all the appropriate choices below the question in this section of the form. In addition, follow the appropriate instructions that indicate which section to complete, for each choice selected.
![]()
The following stem question is used to determine whether AIDS-OI(s) was/were diagnosed during a specific inpatient hospital stay during the SP: “Is there documentation that any AIDS defining opportunistic illnesses (AIDS OI) were diagnosed during this inpatient stay?”
If no,
Select “No” if there is no evidence in the medical records that the patient was diagnosed with an AIDS OI during the hospital stay.
No further data collection for Section IV is necessary.
If yes,
Select “Yes” if the medical records indicate that the patient was diagnosed with any AIDS OI during the inpatient stay.
This section covers only clinical AIDS. Any evidence of immunologic AIDS (i.e. CD4 cell count <200 cells/µl) is captured in Section XI, “Laboratory Test Results.”
Indicate which AIDS OIs was/were diagnosed by selecting from the list in this section on the SPIF.
Select from the list of AIDS OIs, which are listed in alphabetical order.
In general, no documented laboratory confirmation is necessary for abstracting AIDS OIs.
Accept an AIDS OI diagnosis if it is documented in the clinical notes as the medical provider’s assessment of the patient’s condition, or if the diagnosis is found in a hospital discharge summary, a transfer note (transferring the patient’s care from one facility to another), or a medical consultation summary – even if these documents do not demonstrate complete information on how the diagnosis was made.
See additional guidance on some conditions later in this section.
Notes on conditions with criteria for extrapulmonary (outside the lungs) involvement
The following illnesses may present in a variety of ways, but can only be considered as AIDS OIs if they affect organs/tissues in addition to the lungs (as in disseminated disease) or as a disease of organs/tissues other than the lungs. When abstracting, be careful that a documentation of one of the following conditions refers to extrapulmonary involvement, as described above.
Coccidioidomycosis, disseminated or extrapulmonary
Cryptococcosis, extrapulmonary
Histoplasmosis, disseminated or extrapulmonary
Mycobacterium avium complex or M. kansasii, disseminated or extrapulmonary
Notes on conditions with criteria for chronicity
To qualify as an AIDS OI, the following illnesses should be mentioned as being “chronic” in the medical records, whether or not a specific time frame was mentioned
Cryptosporidiosis, chronic intestinal (>1 month duration)
Herpes simplex: chronic ulcer (>1 month duration) or bronchitis, pneumonitis, or esophagitis
Isosporiasis, chronic intestinal (>1 month duration)
Notes on Carcinoma, invasive cervical
There should be documentation that the cancer is “invasive” or corresponds to stages 1A or higher, based on the International Federation of Gynecologists and Obstetrician (FIGO) clinical staging system.
Stage 1A indicates microinvasive disease, which means that there is microscopic evidence of invasiveness, but there is not yet any evidence that the cancer has spread beyond the cervix.
The following terms are NOT indicative of invasive cervical carcinoma:
-Atypical cells of undetermined significance (ASCUS)
-Intraepithelial neoplasia or Cervical intraepithelial neoplasia (CIN)
-Squamous Intraepithelial Neoplasia (SIL) or dysplasia
-Low-grade SIL (LSIL)
-High-grade SIL (HSIL)
-Carcinoma in situ
Notes on Cytomegalovirus (CMV)
Note the criteria that should be met for a CMV-related condition to qualify as an AIDS OI.
• To qualify as CMV disease -The condition should affect areas of the body in addition to or other than the liver, spleen, or lymph nodes
- Examples: CMV colitis, esophagitis, pneumonitis, or CMV neurologic disease such as dementia, ventriculoencephalitis, or ascending polyradiculomyelopathy (spinal cord disease affecting multiple nerve roots)
To qualify as CMV retinitis -There should be documented loss of vision as a result of the CMV retinitis. -“Peripheral retinitis” may be asymptomatic or with mild visual disturbances, and
therefore, does not qualify as an AIDS OI.
Please refer to Appendix B, “AIDS Defining Opportunistic Illnesses” for additonal information on specific AIDS OIs.
![]()
The following stem question is used to determine whether conditions other than AIDS-OI(s) was/were diagnosed during a specific inpatient stay: “Is there documentation that any conditions other than AIDS OI were diagnosed during this inpatient stay?”
If no,
Select “No” if there is no evidence in the medical records that the patient had any of the listed conditions in this section of the SPIF during the particular hospital stay in the surveillance period.
No further data collection for Section V is necessary.
If yes,
Select “Yes” if the medical records indicate that the patient was diagnosed with any of the listed conditions during the particular hospital stay being abstracted.
This would include all conditions that are present, active, or requiring treatment.
Select any listed condition(s) that was/were documented in the medical records during the particular hospital stay in the surveillance period.
***NOTE***
Be
careful not to confuse the diagnoses that should be recorded in
Section IV, “AIDS
Defining Opportunistic
Illnesses
(AIDS OI)” with
those that should be recorded in this section.
This
section includes some clinical conditions that may occur as a result
of HIV disease, but are NOT AIDS-defining.
What evidence of a “diagnosis” to accept?
In general, for MMP, the providers’ diagnoses of the conditions listed in this section should be accepted – whether with or without documented laboratory confirmation. Similarly, when the health care provider makes references to medical conditions that have been diagnosed elsewhere, these should be recorded if it could be determined from documented information that the conditions remain active health problems for the patient.
When there is documentation that a patient is concerned about a particular condition (e.g., “patient noticed oral thrush this week” or “patient thinks she has a vaginal yeast infection”), without documented evidence of a physician diagnosis, do not report as an established diagnosis.
Diagnoses with qualifying terms: Often in medical records, qualifying words are used with diagnoses to denote the degree of certainty surrounding the diagnosis.
-As a general rule of thumb, diagnoses described with the following commonly used “qualifying words” should be counted in MMP:
“diagnostic procedure results consistent with. . .” “presumptive . . .” “responded to treatment for . . .”
-On the other hand, diagnoses described by the following qualifiers should not be
considered established diagnoses, and should generally not be recorded as a diagnosis
in the medical records:
“questionable
diagnosis of . . .”
“Diagnosis
A vs. Diagnosis B . . .”
“conceivable.
. .”
“differential
diagnosis includes X, Y, and Z. . .”
“symptoms
of . . .”
“iffy. ..”
“plausible
. . .”
“probable
. . .”
“possible
. . .”
“potential
. . .”
“questionable
. . .”
“rule
out (abbreviated R/O) . . .”
• If uncertain, reviewing additional information about the clinical course of the patient and other documented impressions of the medical providers over time may assist in determining whether this is an established diagnosis. If possible, consultation with the health care provider would also be helpful.
Selecting conditions other than AIDS OI on the SPVF
The list of conditions in this section was created to capture conditions that are recognized as being associated with either HIV disease or the consequences of prolonged immune suppression or the treatment of HIV infection.
When considering selecting a condition on the list of conditions in this section of the SPIF, look for terms in the medical records that correspond to those listed on the SPIF. For guidance on terms that correspond to each condition on the SPIF, see Appendix C.
Terms with similar spelling
Be careful when abstracting any of the following conditions, which are represented by terms with very similar spelling:
Myelopathy & Myopathy:
-Myelopathy = condition affecting spinal cord
-Myopathy = condition affecting muscles
Nephropathy & Neuropathy:
-Nephropathy = condition affecting kidneys
-Neuropathy = condition affecting nerves
Conditions that are physical signs/symptoms, rather than diagnoses
The following conditions are more likely documented in medical records as the patient’s complaints or as physical exam findings than as diagnoses:
Buffalo hump
Constipation
Hypersomnolence
Conditions other than AIDS OI that require entries in “Other, Specify”
For the following 6 conditions, use the “Other, Specify” fields to record additional information:
Abcess: If selected, record the site of the abcess in “Other, Specify.” For examples: “ABCESS LIVER” “ABCESS BRAIN” “ABCESS NOS” (if location unknown)
2. Diarrhea, infectious: If a specific infectious agent is documented (either in the clinical notes or in a laboratory report) as the cause of the infectious diarrhea, enter this information in an “Other, Specify” field as illustrated in the following examples:
“DIAR E COLI”
“DIAR GIARDIA”
“DIAR INFECT NOS” (if diarrhea documented as infectious, but pathogen unknown)
Ischemic heart disease: If a myocardial infarction (MI) (or any of the terms indicating a heart attack) is
documented, record the MI it in an “Other, Specify” field in the following way: “HEART MI”
Other terms indicating an MI:
Myocardial infarction (MI),
Acute MI (AMI),
Heart attack,
Myocardial necrosis,
ST-elevation MI (STEMI),
non-ST- elevation MI (NSTEMI)
4. Neoplasm: Select “Neoplasm” if there is documentation of a diagnosis of cancer or malignancy other than an AIDS-defining cancer. For patients with metastatic cancer (affecting multiple organs), record only the primary cancer diagnosis.
In addition, specify the type of cancer under “Other, specify.” For examples: “NEO LUNGS” “NEO MELANOMA” “NEO HODGKIN”
Nephropathy: Record any kidney disease documented as a diagnosis by the medical provider during the Surveillance Period as “Nephropathy.”
In
addition, record the specific nephropathy diagnosis (or diagnoses) in
“Other, Specify.”
For
example, if a patient has diabetic nephropathy with chronic renal
insufficiency (CRI), record these
diagnoses
in the following way:
“NEPH
DIABETIC”
“NEPH CRI”
If the diagnosis is any type of renal failure or end-stage renal disease, select both “Nephropathy” AND “Renal failure” (see explanations for “Renal failure” in Appendix C):
For example, if a patient has diabetic nephropathy with chronic renal failure:
Select both “Nephropathy” AND “Renal failure” AND
Enter “NEPH DIABETIC” in an “Other, Specify” field
6. Psychosis: Select “psychosis,” whether it is documented as a diagnosis by itself (e.g., first episode psychosis) or as a component of another illness (e.g., schizophrenia, depression with psychotic features).
If “Psychosis” is selected, indicate under “Other, Specify” the specific psychosis diagnosis or the condition associated with psychosis, for examples:
“PSY FIRST EPISODE”
“PSY SCHIZOPHRENIA”
“PSY HIV DEMENTIA” “PSY NOS”
7. Fatty liver: Select “Fatty liver” AND indicate under “Other, Specify“ any documented cause of the fatty liver condition, for examples:
“FATLIVER
ALCOHOL”
“FATLIVER DRUG-IND” (for fatty liver specified as being
drug-induced)
“FATLIVER NON-ALC” (for fatty liver without detail other
than non-alcohol-related)
“FATLIVER NOS” (if no documented cause for fatty liver)
If the condition is specified as fatty liver caused by alcoholism, select both “Fatty liver” AND “Alcoholism.”
Conditions that may be related to Each Other
Be aware that some conditions listed as “Conditions other than AIDS OI” may be related to each other, such that the selection of one may indicate the need to select another.
• |
Alcoholism: If any of the following liver conditions is specifically documented as being induced by alcohol use, record the condition as both “Alcoholism” as well as the liver condition: -Alcoholic liver failure – select both “Alcoholism” AND “Hepatic (liver) failure” -Alcoholic fatty liver, alcoholic steatohepatitis (fatty liver with inflammation), alcoholic steatonecrosis (fatty necrosis with inflammation) – select both “Alcoholism” AND “Fatty liver” |
• |
Bronchitis: Record this condition as both “Bronchitis” AND “Respiratory infection, lower.” |
• |
Guillain-Barré syndrome: record this condition as both “Guillain-Barré syndrome” AND “Neuropathy, peripheral.” |
• |
Fatty liver -If specified as alcohol-induced: select both “Fatty liver” AND “Alcoholism” AND enter “FATLIVER ALCOHOL” under “Other, specify” |
|
-If specified as NOT alcohol induced: select “Fatty liver” AND enter “FATLIVER NONALC” under “Other, specify” |
|
-If the documented information is insufficient to determine the cause of the fatty liver condition, select “Fatty liver” AND enter “FATLIVER NOS” under “Other, specify” |
|
-If a diagnosis of “steatohepatitis” or “steatonecrosis” (of the liver) is specified as being related to the use of drugs or medications (see explanations for “Hepatitis, drug-induced” later in this table), record the condition as both “Fatty liver” and as “Hepatitis, drug-induced” AND enter “FATLIVER DRUG-IND” under “Other, specify” |
• |
Hepatic (liver) failure: An underlying liver disease may be documented as the cause of the liver failure. Record the condition as both “Hepatic (liver) failure” AND the underlying liver disease – if it is among those listed as “Conditions other than AIDS OI” on the SPVF or SPIF: |
-Fatty
liver
-Non-alcoholic
fatty liver disease
- Hepatitis, infectious
- Hepatitis, not infectious, drug-induced
Lipodystrophy: -Buffalo hump – record as both “Lipodystrophy” AND “Buffalo hump” -Lipoatrophy – record as both “Lipodystrophy” AND “Lipoatrophy”
Nephropathy: If a diagnosis of diabetic nephropathy is documented in medical records, record it as both “Nephropathy” AND diabetes mellitus (type 1, type2, or NOS).
Neuropathy, cranial: If the cranial neuropathy diagnosis is auditory neuropathy (a neuropathy affecting cranial nerve VIII, which affects hearing), record this condition as both “Neuropathy, cranial” AND “Hearing loss, acquired.”
• Non-infectious hepatitis: If a diagnosis of steatohepatitis (fatty liver with inflammation) or steatonecrosis (fatty necrosis with inflammation) is specifically documented as being drug-induced and not related to alcohol use, record the condition as all of the following: -“Hepatitis, non-infectious, drug-induced”
- “Fatty liver”
-“Non-alcoholic fatty liver disease”
• Rash, drug-related – If any of the following skin conditions is specifically documented as being a drug reaction, record the condition as both “Rash, drug-related” as well as the specific skin condition:
- Erythema Multiforme
- Erythroderma
- Sebborrheic Dermatitis
- Stevens-Johnson’s Syndrome
Renal failure: Record any renal failure diagnosis as both “Renal failure” AND “Nephropathy.”
Sinusitis: Record this condition as both “Sinusitis” AND “Respiratory infection, upper”
![]()
On the SPIF, the following stem question is used to determine whether screening for each hepatitis A, B, and C occurred during a specific hospital stay: “Is there documentation of screening for hepatitis A, B, or C during this inpatient stay?”
If no,
Select “No” if there is no evidence in the medical record that the patient was tested for hepatitis A, B, or C during the specific inpatient stay.
No further data collection is needed for Section VI.
If yes,
Select “Yes” if there was documentation that laboratory testing for hepatitis A, B, or C was conducted (whether or not the patient had signs and/or symptoms of hepatitis) during the specific inpatient stay in the surveillance period.
Indicate for which type(s) of hepatitis the patient was tested during the hospital stay. (Please see the explanations below for the different tests used to detect infection with hepatitis A, B, or C).
Screening Tests: Hepatitis A
There are two serologic tests that are performed to screen or diagnose infection with hepatitis A virus (HAV): 1) Anti-HAV Total and 2) Anti-HAV IgM.
• “Anti-HAV Total” is a general test for the presence of any type of antibody to the Hepatitis A virus (includes both IgM IgG); in general,
-The presence of IgM antibodies (whether to HAV or to another infectious agent) indicates acute infection or recent immunization.
-The presence of IgG antibodies indicates long-term immunity (whether from natural infection or vaccination) acquired in the more remote past.
• “Anti-HAV
IgM” –
antibodies specific to HAV indicating acute/recent infection with or
immunity against HAV.
Screening Tests: Hepatitis B
Look for the following tests for hepatitis B: 1) Anti-HBc Total (or HBcAb); 2) Anti-HBc IgM; 3) Anti-HBs (or HBsAb); and 4) HBsAg.
The first two (Anti-HBc Total and Anti-HBc IgM) test for antibodies produced against the core of the hepatitis B virus and are found only after a true hepatitis B infection.
The third test (Anti-HBs) detects antibodies developed against hepatitis B surface proteins and is not diagnostic of natural infection as those who have been vaccinated against hepatitis B will also produce Anti-HBs. The Anti-HBs test is usually used to test for immunity to hepatitis B in patients who have been immunized previously.
The fourth test (HBsAg) detects a protein from the virus that is generated if viral particles are being produced.
Screening Tests: Hepatitis C
The main serologic test to screen for hepatitis C virus infection is Anti-HCV (enzyme linked immunoassays [EIA] or radio immunoblot assay [RIBA]). Anti-HCV may also be recorded in the medical record as Hepatitis C antibody, anti-Hep C or HCV Ab.
After a patient receives a positive serologic test for hepatitis C, hepatitis C viral load testing – HCV RNA Quantitative PCR may be performed.
Even if a patient tests negative for anti-HCV antibodies, a hepatitis C viral RNA test (qualitative or quantitative) may also be performed if there is unexplained evidence of liver damage. Some patients co-infected with HIV and HCV may test negative for anti-HCV antibodies.
The tests for hepatitis C are known by different names in different labs. Abstractors should familiarize themselves with the exact naming of hepatitis C tests used in their jurisdiction.
![]()
On the SPIF, the following stem question is used to determine whether there was documentation that ART was newly prescribed during or continued (from an earlier prescription) through the specific inpatient stay that is being abstracted. “Is there documentation of prescription of antiretroviral therapy (ART) during this inpatient stay?”
If no,
Select “No” if there is no documented evidence that ART was prescribed during or continued through the hospital stay.
No further data collection is needed for Section VII.
If yes,
Select “Yes” if the medical records indicate that ART was prescribed during or continued through the particular hospital (inpatient) stay.
Complete the requested information in this section of the SPIF by selecting all ART
medications documented as having been prescribed during or continued through the
particular hospital stay.
Notes on antiretroviral drugs on the SPIF
Information on prescribed medications (including ARTs), may be found in various places in medical records, including the initial history & physical form or summary (admission notes), referral forms, consultation summary, facility transfer notes, physician’s order sheets, physician’s notes, nurse’s notes, or a pharmacy-generated list of medications for the patient.
The different formulations of current FDA-approved ARTs comprise individual drugs that are organized into several classes – depending on the phase in the HIV infection cycle that the drug disrupts. Some illustrations and explanations of the HIV cycle can be found in Appendix D and at the following web sites:
http://www.hopkins-hivguide.org/tutorial/launch.html (animated presentation) http://www.wellcome.ac.uk/en/labnotes5/animation_popups/hiv.html (animated presentation) http://aidsinfo.nih.gov/contentfiles/HIVLifeCycle_FS_en.pdf http://www.aidsinfonet.org/factsheet_detail.php?fsnumber=106
The MMP abstraction forms include all FDA-approved ART formulations and several investigational ART medications not yet approved by the FDA.
On the SPIF, the ART medications are listed in alphabetical order by a common name, and other known names of each medication are included in parentheses.
Because providers may refer to these medications using a number of different names in the medical records, it would be good to become familiar with the different names of the drugs and to know in which ART classes the different drugs belong.
Whenever
a combined ART formulation is found in the medical records, please
indicate on the
abstraction
form the exact formulation that was prescribed, rather than the
constituents of the formulation,
for
example:
If Combivir was a documented prescription,
Select only “Combivir (AZT/3TC)”
Do NOT select the two drugs in the formulation separately:
-“Zidovudine (AZT, Retrovir)” and
-“Lamivudine (3TC, Epivir)”
If Trizivir was a documented prescription,
Select only “Trizivir (ABC/3TC/AZT)”
Do NOT select the three drugs in the formulation separately:
-“Abacavir (ABC, Ziagen)” and
-“Lamivudine (3TC, Epivir)” and
-“Zidovudine (AZT, Retrovir)”
Selecting the exact ART formulation prescribed is very important in understanding the role of these combined formulations in adherence to and outcome of treatment.
If the patient was prescribed an antiretroviral medication not listed on the abstraction form, record its name at the end of this section, under “Other, Specify.”
![]()
The purpose of this section is to collect data on selected “other medications” – medications other than antiretroviral drugs. These data will help to assess issues regarding overall standard of care for and treatment burden on patients.
On the SPIF, the following stem question is used to determine whether or not there was documentation that medications other than antiretroviral drugs were provided during the specific hospital (inpatient) stay.
“Is there documentation of prescription of medications other than ART during this inpatient stay?”
If no,
Select “No” if there is no documented evidence that medication(s) other than ART was/were provided during the hospital stay.
No further data collection is needed on Section VIII.
If yes,
Select “Yes” if there was documentation that medications other than antiretroviral drugs were provided for the patient during the hospital stay. A list (“cheat sheet”) of medications will be provided to help determine whether a particular drug name found in the medical records is associated with a medication on the form.
Complete this section by selecting any of the medications that pertains to the visit, as
explained above.
Notes on “other medications” on the SPIF
Information on prescribed medications may be found in various places in the inpatient medical records, including the initial history & physical form/summary (or admission notes), referral forms, consultation summaries, physician’s order sheets, physician’s clinic notes, nurse’s notes, or a pharmacy-generated list of medications for the patient.
The medications that should be abstracted in this section of the SPIF are listed in alphabetical order of their generic names. However, note that many of these medications may also be mentioned by one or several different names in the medical records. A “cheat sheet” will be provided to help with selecting the appropriate medications, based on a number of different names for each medication. In addition, it would be a good idea to become familiar with the different names of the medications on this list.
Remember that for the SPIF, information is collected on medications provided as part of treatment during a particular hospital (inpatient) stay. An SPIF should be completed for every hospital stay during the surveillance period.
![]()
The following stem question is used to determine whether there is documentation of certain laboratory test results during the specific hospital (inpatient) stay, “Is there documentation of laboratory test results for CD4 cell count or percentage, HIV viral load, creatinine, or abnormal ALT (SGPT) or AST (SGOT), for this inpatient stay?”
If no,
Select “No” if there was no documented laboratory test result for one of the laboratory tests listed in this section of the SPIF.
No further data collection is needed for this section.
If yes,
• Select “Yes” if there is at least one result documented during the specific hospital stay for at least one of the laboratory tests listed in this section of the SPIF.
- For CD4 cell count or percentage, HIV viral load, and creatinine – include any results (whether normal or abnormal)
- For the ALT (SGPT) and AST (SGOT) – include only results that are ABNORMAL during the hospital stay.
- For brief explanations of the laboratory tests, please see Appendices E and F.
Complete the rest of the section by indicating which of the listed laboratory tests were documented and the specific results of those tests during the hospital stay.
Looking for laboratory results
Although many laboratory results can be found in the “Laboratory” section of a facility’s medical records, it is always a good idea to look for this information throughout other parts of the medical records as well, because formal laboratory reports may not always be available in a patient’s chart, for many different reasons.
Medical providers routinely follow-up the results of tests that they order, sometimes obtaining these results before laboratory reports are printed out. Therefore, the physician’s notes and/or nurse’s notes (care plans, clinic notes, progress notes, history & physical forms, etc.) are also good places to look for laboratory results, as are transfer notes (if patients are transferred from one facility to another) and summaries on referral forms or from medical consultations. In addition, the physician’s order sheets will show when specific tests were ordered – which would provide a clue as to approximately when specimens were obtained for testing.
Date of test result = Date specimen collected
When abstracting information on the “date of laboratory test result,” keep in mind that this refers to the date when the specimen was collected from the patient – rather than the date when the final result was reported by the laboratory – for a particular test. This is because laboratory report print-outs may show both the date test results were reported as well as the date specimens were collected (Appendix E).
Verify the units used with test results
Laboratory reports will show the units associated with the values of test results. Whenever possible, it’s a good idea to verify what units are used with the documented test results in medical records. Although most laboratory results will be reported in a conventional way across facilities, it is good practice to always check and confirm that the laboratory values being abstracted are compatible with what is requested on the abstraction form.
![]()
Enter the MMP Participant ID number in the text box provided. Also, enter the Facility ID number (if abstraction conducted using a discharge summary found in an outpatient MMP facility) in the text box, and record the Inpatient facility ID (facility where patient was hospitalized) in the available space in this section.
This space can be used to document information that is conflicting, requires discussion with the project coordinator and/or the CDC project officer, or if there is additional information to enter from any section on the SPIF.
This section should not be sent to the CDC.
| File Type | application/msword |
| File Title | Instructions for Completing the Medical Monitoring Project (MMP) |
| Author | aad9 |
| Last Modified By | ziy6 |
| File Modified | 2009-02-26 |
| File Created | 2009-02-26 |
© 2026 OMB.report | Privacy Policy