Ss.b 20100127
SS.B 20100127.doc
Longitudinal follow-up of Youth with Attention-Deficit/Hyperactivity Disorder identified in Community Settings: Examining Health Status, Correlates, and Effects associated with treatment for ADHD
OMB: 0920-0747
01/27/10
Supporting Statement – Part B
for
Longitudinal Follow-up of Youth with Attention-Deficit/Hyperactivity Disorder Identified in Community Settings: Examining Health Status, Correlates, and Effects Associated with Treatment for ADHD
Revision of 0920-0747
Primary Contact:
Susanna Visser, M.S.,
Epidemiologist
Division of Human Development and Disability
NCBDDD
CDC
MS E-88
1600 Clifton Road
Atlanta, GA
30333
404-498-3008
404-498-3050 (fax)
B. Collections of Information Employing Statistical Methods
B.1. Respondent Universe and Sampling Methods
The cohort for the PLAY study follow-up will be comprised of the participants of the PLAY follow up study at the University of South Carolina. These youth were originally sampled from the pool of elementary-aged youth screened by their teachers and parents, invited to participate in the baseline interview, and then invited to participate in the follow-up study. Of those with screening information, all children who either screened high on Attention-Deficit/Hyperactivity Disorder (ADHD) risk based on the teacher report, or were identified by parents as being diagnosed and/or medicated for ADHD, were invited for the baseline interview portion of the PLAY study. For a control group, the sample of children at low risk for ADHD was first stratified by gender and then cases were sampled proportional to the gender distribution of the high risk group. Design weights were generated for participants of the interview phase of the PLAY sample such that study findings using this weighted sample may be generalized to the school districts from which the youth were sampled.
There were 481 eligible participants for South Carolina. Of them, 35 declined participation in the follow up study, 335 are currently engaged in the study, and 111 are being tracked and pursued for participation in the study. All participants in Waves 1-3 of the PLAY study follow up are eligible for the present study and will be invited to participate. Of the 260 participants enrolled in Wave 1 whose annual assessments have been due to date, 15 withdrew from Wave 2 data collection, 2 declined Wave 2 only, and 27 additional cases have moved and currently unreachable. This represents a retention rate of 83% from year to year. Of the cases who were lost for Wave 1, 11 were found due to increased tracking activities and became active participants for Wave 2. Based on these retention rates and the experiences of our principal investigator with other longitudinal studies, we expect to retain an average of 85% of the participants throughout the two-year study extension. For the present follow-up study, we will estimate changes in prevalence over time as well as incidence of ADHD and co-morbid conditions within the control group. Participants have been contacted on a quarterly basis. Because baseline data have already been collected for these families, any differences between families who choose to continue participation in the follow up study and those who do not choose to continue will be statistically analyzed.
A detailed break down of respondents by site, including parents, teachers, and children in different age groups can be seen in Attachment F2.
B.2. Procedures for the Collection of Information
Design
The proposed project is a cohort study. The case-status variable (ADHD vs. not ADHD) will be treated as a risk factor for later developmental changes as opposed to a dependent variable modeling approach appropriate for a traditional case-control design. In this situation, case status will be treated as a fixed covariate and the probabilities of interest will involve developmental outcomes conditional on case status (i.e., probability, as well as, magnitude of change in longitudinal outcome given case status). The models proposed allow us to explore multivariate developmental change contingent on case status through the incorporation of bivariate, trivariate, etc., latent growth models or latent dynamic difference models (see McArdle, 2006).
Procedures
Measures:
The parent interviews continue to include assessments for ADHD symptoms and diagnosis, co-morbidities, current treatments and satisfaction and cost associated with treatment, questionnaires about the child’s health risk behaviors, quality of life, functioning and impairment, school performance, as well as questions about the parent’s expectation about their child’s future functioning, their parenting style, the parents’ own mental health history and demographic information. The children will also continue to be included in the annual assessments and will complete assessments for ADHD symptoms and diagnosis, co-morbidities, health risk behaviors, quality of life, functioning and impairment. The child’s current teachers will complete surveys of ADHD symptoms, impairment, and about the child’s school functioning. Assessment instruments are described in detail in Attachment C2.
Recruitment:
All participants of the interview phase of the PLAY study are contacted on a quarterly basis. This contact will continue for Wave 4 and 5 and families will be invited to participate in the follow-up assessments for two additional years. The assessment schedule will continue to include annual assessment visits, semi-annual surveys, and brief quarterly contacts. During the initial visit for Wave 1 parents were asked consent to participate, consent for their child to participate, and consent for teachers to complete questionnaires. After parents consented for their children to participate, the children were asked for their assent to participate. For Wave 4 and 5, those children who reach 18 years of age will be asked to provide consent. See Attachments D1-3.
All questionnaires are in English. For families where Spanish is the primary language, Spanish Consent forms are available, and assessments will be conducted by bilingual English/Spanish interviewers, who are able to provide translations and explanations in Spanish if needed.
Assessment Schedule:
Teacher assessments: Teachers will continue to be contacted between December and March to complete the rating scales. This has been determined in prior studies as the most optimal time period for teachers to complete surveys.
Annual visits: The annual assessments will continue to involve an in-person interview, conducted at the assessment sites or at meeting rooms in local schools, at the convenience of the participants. All surveys and interviews will be administered during this visit. The selected modules from the DISC-IV interview will be administered via computer assisted interview. To limit the length of the assessment visit, parents will be mailed the self-report surveys to complete prior to the assessment visit. However, interviewers will also provide assistance with completing the forms during the visit, as necessary.
For the children and teens, assessments may be conducted by self-report with an interviewer available for assistance and questions. Interviewers will ensure that children’s responses will be kept confidential and not be reviewed by the parents.
Semi-annual assessments: Parents will continue to be contacted by phone or mail to complete an updated contact information sheet, the treatment portion of the ADHD Treatment, Cost, and Client Satisfaction Questionnaire, and the Critical School Events form. Parents who need assistance with the surveys but cannot be interviewed by phone will be assessed in person.
The Strengths and Difficulties Questionnaire and the Vanderbilt Rating Scale will also be conducted again during the semi-annual assessment, if the annual assessment was conducted during the summer months, to ensure that the teacher and parent ratings on these two instruments are conducted no more than 3 months apart.
Quarterly contact: Parents will continue to be contacted by phone or mail to verify contact information, to review any changes the treatment portion of the ADHD Treatment, Cost, and Client Satisfaction Questionnaire, and to assess recent school disciplinary actions. This information is gathered quarterly to maximize accuracy of recall.
Training of interviewers
The Principal Investigators as well as the Project Managers at the site have been conducting the baseline and follow-up PLAY study and thus are experienced with the participants and well trained on the study procedures and methods, including enrolment, assessment, data entry and processing. They have completed and are maintaining ethics and human subject certifications. The overall procedures and methods proposed for the current longitudinal study, e.g., in-person interviews, mail, and phone contact with participants, have been extensively tested in the initial PLAY study.
All additional study staff at both sites have completed mandatory ethics and human subjects training provided through the universities. In addition, all additional study staff have received in-house training by the Principal Investigators as well as the Project Managers on all procedures and methods. Detailed manuals of operation have been created to document all procedures for contacting participants, setting up interviews, conducting phone or in person interviews, and data handling and storage. In order to train staff who will be conducting the interviews, mock interviews were conducted and staff are observed during the initial assessments of study participants. If additional staff are hired during Wave 4 and 5, they will receive the same training as the existing study staff.
Quality control of data collection
Immediately upon hire, all study staff are trained for human subject’s research via the CITI Program, and, upon completion, certification lasts 3 years. The training program includes the following modules: Basic Courses in the Protection of Human Research Subjects; Biomedical Focus; Social and Behavioral Focus; Health Information Privacy and Security Course (HIPS); and, Responsible Conduct of Research (RCR).
Periodic call backs for a random selection of participants will continue to be used to monitor data collection procedures and quality. Supervising project staff, including investigators, will observe selected interviews for quality assurance throughout the study duration. All staff members, who conduct interviews, are responsible for storing paper and electronic data appropriately and checkpoints are set up to ensure this. Interviewers are responsible for completing a quality control check for each interview to ensure that the questions were filled out properly and completely and to make sure that the post-interview back-up of the electronic data is completed. A checklist is filled out for each child and parent annual interview by the assigned interviewer. The checklist is designed to ensure interview consistency and to make sure that study protocol is followed and that all of the onsite data checks are completed.
Quality control of data entry
Initial training for new staff and periodic training for all staff on IT security procedures is conducted by the principal investigator and the project manager. All data are collected on scanable forms to minimize data entry errors. The scanner and database servers at the study site is programmed to reject out-of-range data, and database queries were written to identify key missing data or inconsistencies. After data have been entered and preliminary edits completed, data on the variables are screened. The screening is used to identify extreme and outlier data points and to provide a preliminary indication of variable distribution shape prior to conducting formal analyses. Outlier data points will be manually reexamined against the raw data record to ensure that they are accurate. A random selection of 10% of all forms are entered a second time and compared for accuracy. All data in the database marked as missing will be re-inspected to verify that data are not available.
Statistical power
Statistical power was assessed using the known completion rate of the first completed follow-up wave. Relevant contrasts for this power analysis were those related to the primary research questions of interest of the project, specifically contrasting rates of health risk behavior engagement and rates of co-occurring conditions among youth with and without ADHD.
Currently available sample size for both baseline and year 1 follow-up data sets is 293. The responses of interest are DISC major depression definition (pmdy), conduct disorder diagnosis (pcdy, i.e. cd), oppositional defiant disorder diagnosis (pody, i.e. odd), sleep disturbance (slpdisturb), sleepy during the day (slpslpydy), composite score of the questions that refer to types of injury causes (injury) and how many times injured in the past 12 months (hrbinj12). We assume that repeated measures will be collected over time at three time points. We consider testing the null hypothesis that there is no time-averaged difference (i.e. incorporating longitudinal information of the data) between the two groups in terms of certain responses of interest while accounting for the potential intra-class correlation (rho) at the two-sided 5% level. The intra-class correlation represents the correlation among the repeated measurements within each child. We assume compound symmetry for the correlation matrix of the repeated measurements.
To calculate the power for testing the difference in responses between two groups, we first fit the linear mixed model (sometimes also named as the latent growth model) for the binary outcomes. The effect size can be calculated based on the intercepts and slopes in the mixed model. The effect size (delta) here is defined as the probability difference across two groups over time. We assume that the sample size remains 293 across three time points. We inspect six intra-class correlations, 0.05, 0.1, 0.2, 0.3, 0.4 and 0.5. (Liu and Liang, 1997; Diggle et al., 2005). The power for each response is then calculated in Table 1.
Table 1 : Power (%) for testing effect sizes for different responses with different intra-class correlations |
||||||
|
Intra-class Correlation |
|||||
Response |
0.05 |
0.10 |
0.20 |
0.30 |
0.40 |
0.50 |
pcdy |
99.9 |
99.8 |
99.5 |
99.0 |
98.3 |
97.2 |
pmdy |
96.9 |
95.6 |
92.8 |
89.6 |
86.3 |
83.0 |
pody |
100 |
100 |
99.9 |
99.9 |
99.9 |
99.9 |
slpdisturb |
100 |
100 |
100 |
99.8 |
99.5 |
99.1 |
slpslpydy |
85.5 |
82.7 |
77.4 |
72.5 |
68.0 |
64.1 |
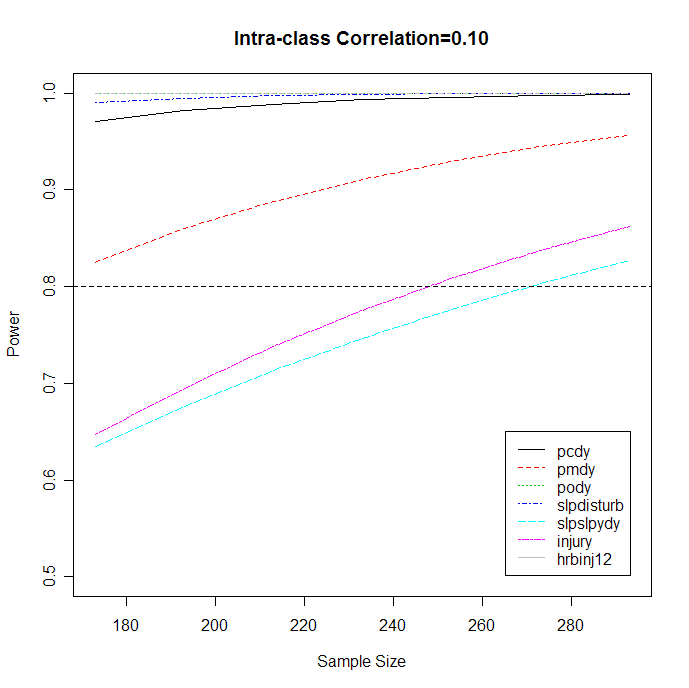
Table 1 shows the estimated power regarding to the estimated smallest meaningful difference of response probabilities between two groups (i.e. delta) at different intra-class correlation (rho) levels. It is shown that with the sample size of 293, an acceptable power can detect the difference of all response probabilities between two groups at intra-class correlation of 0.1. Figure 1 depicts the power curves for detecting the current effect sizes of the responses at intra-class correlation of 0.1 with different sample sizes. It shows that, to retain the power to be above 80% (i.e. the horizontal line in the graph), the sample size should be retained at least 250.
B. 3. Methods to Maximize Response Rates and Deal with Nonresponse
There were 481 completed baseline interviews. All 481 participant families were eligible for the follow-up study. Of these, 35 have declined participation and 335 are engaged participants. 293 of the 335 engaged participants have completed assessments to date. Therefore, of the original baseline sample, 70% were retained in the follow-up study and 61% were assessed with the Wave 1 follow-up assessment battery. The largest gap in contact among these participants was 3 years from Baseline to Wave 1 assessment. To date we have retained 83% of the Wave 1 participants in Wave 2. The measures taken to maximize response rates are described below and will be extended into future waves of the study.
First, to increase participation, monetary incentives will be offered to the families who agree to participate. Please refer to section A9, “Explanation of Any Payment or Gift to Respondents” for level of incentives.
Second, frequent contacts with families will continue to ensure that contact is maintained with participants and to limit the extent of attrition. Instrument, scanning equipment, data management program, and statistical programs have been developed to streamline data collection and analysis. The process enables researchers to have a ready count of response rates and generate lists for further mailings to increase participation rates. A variety of tracking methods will be used to maintain contact with the PLAY cohort, keep addresses and contact information updated, and encourage continuing participation and include the following:
(1) Quarterly contact will be made with each family, reminding them of their participation in the study, indicating our desire to maintain contact, and asking again for verification of address and contact information. We will also ask for the name and contact information of two persons outside the immediate family who would know how to contact them. A random prize drawing will be used as an incentive for the parents to participate in the quarterly contact.
(2) Birthday greetings cards will be mailed each month to participants who have a birthday that month for every year of the study. The cards will be signed from the PLAY project to remind them of our continued interest in their growth and development. Each card will include a postage paid return envelop with a brief form to verify address, phone number, and guardian status. Participants will be informed that all returned forms will be placed in a drawing for a cash prize to be held that year.
(3) All mailings will be marked “address correction requested” to obtain forwarding addresses from the post office rather than having letters forwarded immediately to addressees. Though adding to the cost of mailing, address correction allows us to be notified of address changes on record with the postal service. This method is extremely helpful when participants are highly mobile.
(4) For those participants have e-mail accounts, email addresses are collected. This no-cost correspondence has been helpful to the project for reminding participants of upcoming interviews, arranging interview times, or even rescheduling interviews. E-mail, of course, is not available to all participants. The site also has a toll-free number for the project so participants are able to call in with address or phone number updates.
(5) Phone calls to the last listed phone number will be made to determine if the participant still has the same number or if a number change is provided for those who had moved recently.
(6) If mailings are not deliverable, and correct phone numbers unobtainable, PLAY team members will write or call the backup contacts who had been supplied by the parents to obtain a current participant address and phone number.
(7) Telephone directory searches (including city directories, telephone information services, and internet searches) will also be used to locate new phone numbers and addresses of participants.
(8) For those not located by other methods, reverse directories for the area will be used to locate neighbors of participants at the last known address. Neighbors will be asked if they have any information about the family, including forwarding address and names of anyone who may know how to contact them. In addition, tracking services will be employed as needed. For Wave 1-3, Lexis Nexis have been and will be used to update addresses and telephone numbers.
(9) If participants are either unable or unwilling to attend in-person interviews, or move too far to travel to the assessment sites, they will still be able to participate in data collection. Parent data will be collected over the phone or by mail, and data on children older than 12 years of age will also be collected over the phone or by mail. The children will not be administered sensitive questions on the Health Risk Behavior Survey (i.e., drug use, sexuality) if not assessed in person, because confidentiality of answers cannot be ascertained.
(10) In cases where children’s custody status changes, the new guardian/parent will be invited to participate in the study. In cases where children enter state custody, consent from the appropriate agency will be sought to maintain the child’s participation in the study.
If non-response becomes an issue, all sites will be generating and imposing statistical weights for non-response in additional to those generated for their sampling frame. These corrections will adjust the prevalence rates for biases due to non-response.
B. 4. Tests of Procedures or Methods to be Undertaken
The overall procedures and methods proposed in the current longitudinal study have been extensively tested in the current PLAY study. For study procedures, sites utilized in-person interviews, mail, and phone contact with participants which allowed them to determine the overall utility of the proposed procedures as well as to specify which survey instruments may be suitable for participants to complete as self-report (e.g., Health Risk Behavior Survey) and which instruments must be administered by phone or in-person interview (i.e., ADHD treatment, cost, and satisfaction questionnaire). No changes to the assessment protocol were made for Waves 1-3. The proposed changes for the Wave 4 and 5 in the assessment protocol are minor and do not change the nature of the assessment. The survey items to be added were taken from nationally administered surveys or from psychometrically tested instruments from studies of similar populations. Thus, the procedures and methods have been tested with the same participants as will be invited for Wave 4 and 5 of the longitudinal study.
B. 5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
All of the following CDC and site investigators or representatives have been part of the process of considering the statistical issues and analysis potential of the proposed data collection. Additionally, these represent a wide variety of disciplines and expertise in analysis and study design; most of the following individuals will be continuously involved in the analysis and reporting of the data collected in these studies.
Susanna Visser, M.S., Epidemiologist, Child Development Studies Team, CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333, 404-498-3008, [email protected]
Ruth Perou, Ph.D., Team Leader, Research Psychologist, Child Development Studies Team, CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333, 404-498-3005, [email protected]
Angelika H. Claussen, Ph.D., Research Psychologist, Child Development Studies Team, CDC, 404-498-3557, [email protected]
Melissa Danielson, M.S.PH., Health Scientist, Child Development Studies Team, CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333, 404-498-3016, [email protected]
Rebecca Bitsko, Ph.D., Health Scientist, Child Development Studies Team, CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333, 404-498-3556, [email protected]
Camille Smith, M.S., Ed.S., Behavioral Scientist, Child Development Studies Team, CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333, 404-498-3007, [email protected]
Jeannette Bloomfield, M.S., Public Health Analyst, Child Development Studies Team, CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333 404-498-3003, [email protected]
Owen Devine, Ph.D., Statistician, NCBDDD/CDC, 1600 Clifton Road, MS E-88, Atlanta, GA 30333, 404-498-3073, [email protected]
Ann Abramowitz, Ph.D., Department of Psychology, Emory University, 532 North Kilgo Circle, Atlanta, GA 30322, 404-712-9513, [email protected]
Robert E. McKeown, Ph.D., Associate Dean for Research, Professor of Epidemiology, Department of Epidemiology and Biostatistics, Norman J. Arnold School of Public Health, University of South Carolina, Columbia, SC 29208, 803-777-6220, [email protected]
Lorie James, M.PH., Project Manager, Research Associate at University of South Carolina, Norman J. Arnold SPH, University of South Carolina,800 Sumter Street, Columbia, SC 29208, 803-777-1124, [email protected]
Matteo Bottai, Sc.D., Lead Statistician, Department of Epidemiology and Biostatistics, University of South Carolina, 803-777-6653, [email protected]
Bo Cai, Ph.D., Statistician, Department of Epidemiology and Biostatistics, University of South Carolina, 803-777-5053, [email protected]
Joe Holbrook, M.PH., Graduate Assistant, Department of Epidemiology and Biostatistics, University of South Carolina, 803-777-7492, [email protected]
Charity G. Moore, M.S.PH., Ph.D., Research Assistant Professor, Department of Epidemiology and Biostatistics, SPH, University of South Carolina, Columbia, SC 29208, 803-777-2524, [email protected]
Lareissa Stumm, M.PH., Graduate Assistant, 803-777-7492, University of South Carolina, Norman J. Arnold SPH, University of South Carolina,800 Sumter Street, Columbia, SC 29208 [email protected]
Mark L. Wolraich, M.D., CMRI/Shaun Walters Professor of Pediatrics, Child Study Center, 1100 NE 13th Street, Oklahoma City, OK 73117, 405-271-6824 ext. 45124, [email protected]
Laoma Beck, Ph.D., Project Manager, Child Study Center, 1100 NE 13th Street, Oklahoma City, OK 73117, 405-271-6824 ext. 42117, [email protected]
David Bard, M.S., Statistician, University of Oklahoma Health Sciences Center, 1100 NE 13th Street, Oklahoma City, OK 73117, 405-271-6824 ext. 45141, [email protected]
Melissa Doffing, M.S., Project Director, Child Study Center, 1100 NE 13th Street, Oklahoma City, OK 73117, 405-271-6824 ext. 42117, [email protected]
Barbara Neas, Ph.D., Epidemiologist, Oklahoma University Health Sciences Center, 1100 NE 13th Street, Oklahoma City, OK 73117, 405-271-2229 ext. 48067, [email protected]
Jessica Son, B. S., Research Assistant, University of Oklahoma Health Sciences Center, 1100 NE 13th Street, Oklahoma City, OK 73117, (405) 271-5700 x45139, [email protected]
Donna
Wells, M.S., Data Manager, University of Oklahoma Health Sciences
Center, 1100 NE 13th Street, Oklahoma City, OK 73117, (405)271-5700,
[email protected]
Data collection will be completed at the University of South Carolina, with Robert McKeown as the Principal Investigator, Lorie James as the Project Manager, and Lareissa Stumm as the primary research assistant. Additional graduate student assistants are hired on a yearly basis to assist with data collection.
| File Type | application/msword |
| Author | Angelika Claussen |
| Last Modified By | Angelika Claussen |
| File Modified | 2010-01-27 |
| File Created | 2009-11-23 |
© 2026 OMB.report | Privacy Policy