REVISED_Green Housing Study ICR_ Part A_October 20.2011
REVISED_Green Housing Study ICR_ Part A_October 20.2011.doc
The Green Housing Study
OMB: 0920-0906
The Green Housing Study
Supporting Statement
(Part A)
October 20, 2011
Project Official:
Ginger L. Chew, ScD
Principal Investigator
Healthy Homes and Lead Poisoning Prevention Branch
National Center for Environmental Health
U.S. Centers for Disease Control and Prevention (CDC)
4770 Buford Hwy., N.E., MS-F60
Atlanta, GA 30341
Tel: (770) 488-3992
Fax: (770) 488-3635
TABLE OF CONTENTS
Page
A. Justification…………………………………………………………………………….. 3
1. Circumstances Making the Collection of Information Necessary 3
2. Purpose and Use of Information Collection 26
3. Use of Improved Information Technology and Burden Reduction 30
4. Efforts to Identify Duplication and Use of Similar Information 30
5. Impact on Small Businesses or Other Small Entities 30
6. Consequences of Collecting the Information Less Frequently 31
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 31
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 31
9. Explanation of Any Payment or Gift to Respondents 33
10. Assurance of Confidentiality Provided to Respondents 35
11. Justification for Sensitive Questions 38
12. Estimates of Annualized Burden Hours and Costs 38
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 42
14. Annualized Cost to the Government 42
15. Explanation for Program Changes or Adjustments 42
16. Plans for Tabulation and Publication and Project Time Schedule 43
17. Reason(s) Display of OMB Expiration Date is Inappropriate 44
18. Exceptions to Certification for Paperwork Reduction Act Submissions 44
B. Collections of Information Employing Statistical Methods 45
1. Respondent Universe and Sampling Methods 46
2. Procedures for the Collection of Information 53
3. Methods to Maximize Response Rates and Deal with Nonresponse 56
4. Tests of Procedures or Methods to be Undertaken 56
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data 58
References………………………………………………………………………………………………………..........59
List of Appendices
APPENDIX A Authorizing Legislation and Other Relevant Laws
APPENDIX B 60-Day Federal Register Notice (FRN)
APPENDIX C Public Comments received in Response to the 60-Day FRN
APPENDIX D Questionnaires
APPENDIX D1 Baseline Questionnaire (Home Characteristics)
APPENDIX D2 Baseline Questionnaire (Part 2 brief Home Characteristics)
APPENDIX D3 Baseline Questionnaire (Demographics)
APPENDIX D4 Baseline Questionnaire (Children with asthma 7-12 years)
APPENDIX D5 3 and 9-month Phone contact
APPENDIX D6 Text Messages (Children with asthma 7-12 years)
APPENDIX D7 6 and 12-month Follow-up Questionnaire (Environment)
APPENDIX D8 6 and 12-month Follow-up Questionnaire (Children with asthma 7-12 years)
APPENDIX D9 Illness Checklist (Children with asthma 7-12 years)
APPENDIX D10 Time/Activity form (Children with asthma 7-12 years)
APPENDIX D11 Time/Activity form (Mothers/ primary caregivers– including only information pertaining to the impact of behavior on child’s health))
APPENDIX D12 Screening questionnaire
APPENDIX E IRB Approval Letter
APPENDIX F Consent Form
APPENDIX G Assent Form
APPENDIX H Recruitment flyer (prototype)
A. JUSTIFICATION
A.1. Circumstances Making the Collection of Information Necessary
This ICR classification is New. This data collection uses Section 301 of the Public Health Service Act (42 U.S.C. 241) as the authorizing law (Appendix A).
Background
The efficacy of green building design features in reducing allergens and toxic substances within the home has been assumed based on conventional wisdom. A better understanding is needed of the extent to which green-built, low-income housing actually reduces exposures to these compounds when compared to standard-built, low-income housing. In addition, this study may provide insight into how specific green building practices (e.g., use of low chemical-emitting paints and carpets) may influence levels of substances in the home (such as volatile organic compounds (VOCs). A study investigating these topics would provide a solid foundation upon which to explore green affordable housing’s potential to promote healthy homes principles. This investigation is consistent with the Centers for Disease Control and Prevention’s (CDC) health protection research agenda, which calls for research to identify the major environmental causes of disease and disability and related risk factors. In addition, this study directly supports several of the United States Health and Human Services’ (HHS) Healthy People 2010 objectives and the proposed 2020 objectives (proposed objectives available at www.healthypeople.gov/HP2020/Objectives/TopicAreas.aspx ):
Goal: Promote health for all through a healthy environment.
8-16 Indoor allergens
8-24 Exposure to pesticides
8-25 Exposure to heavy metals and other toxic chemicals
8-27 Monitoring environmentally related diseases
Goal: Promote respiratory health through better prevention, detection, treatment, and education efforts.
24-2 Hospitalizations for asthma
24-3 Hospital emergency department visits for asthma
24-4 Activity limitations
24-5 School or work days lost
Prior to this proposed study, there have been no multi-site studies of how green housing factors are associated with health effects such as asthma. Two main goals of this study are: 1) to compare levels of certain environmental chemical and biological agents in green vs. comparison, multi-family, low-income housing; and 2) to ascertain differences in the health of the residents in these homes. These goals will be accomplished in an ongoing building renovation programs including but not limited to public housing and “Mark-to-Market” (M2M), sponsored by United States Department of Housing and Urban Development (HUD). Thus, the residents of these homes are similar in terms of socioeconomic status. Briefly, the M2M program is a nationwide initiative that encourages landlords of multi-family properties to use green building principles. In partnership with HUD, CDC will leverage this opportunity to collect survey and biomarker data from residents and to take environmental measurements in their homes. The results of this study will provide data that will allow CDC and HUD to identify housing factors that are not only energy-efficient, but have the potential to improve the health outcomes of one of the most sensitive populations, low-income children with asthma.
Many studies exist that examine the indoor environment in relation to health outcomes such as asthma. Table 1 lists contaminants in homes that have been shown to exacerbate respiratory symptoms.
Table 1. Contaminants in homes that are known to exacerbate respiratory symptoms.
Factor |
References |
Moisture |
Bornehag 2004, Franchi 2006, Gunnbjörnsdóttir 2006, Savilahti 2000, Skorge 2005 |
Poor ventilation and heating |
Franchi 2006 |
Environmental tobacco smoke |
Franchi 2006 |
Wall-to-wall carpeting |
Franchi 2006 |
Pet allergens |
Custovic 2003, Munir 2003, Skorge 2005 |
Dust mites |
Gotzsche 2004 |
Cockroach allergens |
Rosenstreich 1997 |
Rodent allergens |
Matsui 2006, Phipatanakul 2002 |
Pesticides |
Senthilselvan 1992 |
Plastic materials |
Jaakkola 2000 |
Nitrogen dioxide |
Zota 2005 |
Combinations of the above |
Salam 2004, Platts-Mills 2000, Sobottka 1996, Spengler 2004 |
Green building principles and indoor air quality:
Few studies have explored how green building practices affect indoor air quality(IAQ) and even fewer have examined how the health of occupants changed as a result of these practices. In Finland, IAQ and resident health were assessed in two buildings situated next to each other. One building had improved ventilation and policies against smoking and furred pets; the other had no intervention and served as the comparison. After one year, total VOCs were lower in the intervention vs. the comparison homes, and asthmatics in the intervention building reported improvements in respiratory symptoms (Tuomainen, Tuomainen, Liesivuori, & Pasanen, 2003). In a more recent study in the US, children who moved into asthma-friendly homes (e.g, improved ventilation, low VOC paint and cabinetry, improved insulation) and asthma education were compared to those who had received asthma education alone (Takaro et al., 2011). Exposures to mold, rodents, and moisture were reduced significantly in the intervention group and night-time awakening due to asthma was significantly different between the intervention and comparison group.
HUD Guidelines for Green Housing: In the HUD green renovation projects, several rehabilitation components could affect health. Some of these components are listed below. CDC and HUD will work together to document which of these occurred in the individual study homes.
-Window replacement
-Integrated pest management (IPM)* vs. traditional pest management
-Insulation
-Individual water heaters
-Heating and cooling equipment (appropriately sized)
-Central heating and cooling systems (appropriately sized and joints sealed in air distribution system)
-Cleaning products and materials
-Kitchen and bath exhaust fan
-Carbon monoxide alarms
-Smooth-surfaced floors
-Low VOC carpet
-Low or no VOC paint, primers, adhesives, caulk, and sealants
-Rubber walk-off mats
-Rubber stair tread
-Cementitious siding
-Changes to facilitate household waste recycling
-Green management of construction/rehabilitation debris
-Combined heat and power system
-Roofing replacement
-Landscaping replacement/modification
-Thermostat
-Air and thermal barriers
*Integrated Pest Management (IPM) – Comprehensive IPM involves reducing a variety of pests (e.g., rodents, cockroaches, termites, ants). Some IPM strategies are relatively easy to implement, while others are more difficult. For example, rodent- and cockroach-focused IPM can involve sealing food in containers, decreasing access to pet food sources, caulking cracks, and repairing holes in floors and walls. On the other hand, termite treatments can be more extensive. Optimally, IPM measures should be implemented with the advice of a professional trained in IPM. IPM has been shown to reduce cockroach and mouse allergen levels in homes (Arbes, Sever et al., 2003; Phipatanakul et al., 2004; Sever et al., 2007). The energy efficient design of green housing may incorporate many IPM principles, reducing the need for pesticides in these homes (Williams et al., 2006).
Cockroach allergens: Low-income inner city homes often have high levels of cockroach infestation. Both home and building-level characteristics can be related to high pest exposure (Chew et al., 2006; Rauh, Chew, & Garfinkel, 2002). Inner-city children were more likely to be allergic and exposed to high levels cockroach allergen than to dust mite or cat allergen (Rosenstreich et al., 1997). The children in the study who were allergic to cockroach allergen had three times the rate of hospitalizations and nearly twice as many unscheduled medical visits compared to non-allergic children or those allergic to dust mites or cat dander. Asthma severity has been linked to cockroach specific immunoglobulin E (IgE) in the sera of patients with mild, moderate, and severe asthma (Henderson, Ownby, Trumble, DerSimonian, & Kellner, 2000).
In 2000, the Institute of Medicine (IOM) concluded that:
1) There is sufficient evidence of a causal relationship between cockroach allergen exposure and exacerbation of asthma in sensitized individuals.
2) There is suggestive evidence of an association between cockroach allergen exposure and the development of asthma in preschool-age children.
3) There is insufficient information to determine whether or not associations exist between cockroach reduction, symptom improvement, and lung function in sensitized asthmatics (IOM, 2000).
Rodent allergens: The National Survey of Lead and Allergens in Housing estimated that detectable levels of mouse allergen existed in 82% of the nation’s homes, and homes with low-income residents and older homes were likely to have increased concentrations of this allergen (Cohn, Arbes, Yin, Jaramillo, & Zeldin, 2004). Ninety-five percent of homes in the National Cooperative Inner-City Asthma Study contained Mus m 1 allergen in the settled dust (W. Phipatanakul, Eggleston, Wright, Wood, & Study, 2000a). The mouse allergen concentrations in many of these inner-city homes were similar to those found in animal facilities and were sufficiently high to elicit symptoms in sensitized individuals. However, the true source of a biologically relevant exposure in the home environment remains unknown. Many researchers have assumed that the bedroom would be the most significant source of exposure for many indoor allergens (Phipatanakul 2006). In New York, the mouse allergen levels in beds and kitchens were significantly correlated ( r= 0.63, p < 0.001); however, kitchen levels tended to be higher (p < 0.001) and more variable (Chew, Perzanowski et al. 2003). Less is known about residential rat allergen exposure, although 33% of the homes of inner city children had detectable rat allergen, Rat n 1 (Perry, Matsui, Merriman, Duong, & Eggleston, 2003). The number of hospitalizations and unscheduled medical visits because of asthma were significantly higher in those children who were both exposed and sensitive to rat allergen.
Dust mite allergens: Most houses in temperate climates have several characteristics necessary for maintaining populations of mites. These include multiple nest sites for mites (e.g., carpets, upholstered furniture, and bedding); a food supply in the form of human skin scales; and temperature and humidity levels that are optimal for mite growth (IOM, 2000). Dust mites can produce an array of proteins, many of which have been shown to be allergenic to humans. Some of the most common taxa of dust mites include Dermatophagoides farinae, D. pteronyssinus, Euroglyphus maynei (Platts-Mills, Vervloet, Thomas, Aalberse, & Chapman, 1997; Voorhorst & Spieksma, 1969). In sensitized individuals, inhalation of Der p 1, an allergen from the dust mite Dermatophagoides pteronyssinus, causes an immediate drop in forced expiratory volume and may produce asthma-related late responses that persist for up to 2 weeks. In a study of 4 year olds, an independent effect of allergen sensitization on asthma was observed only with house dust mites, odds ratio 8.07 (95% CI 4.60–14.14) (Arshad, Tariq, Matthews, & Hakim, 2001). Other studies have demonstrated that moving asthmatic children and adults into mite-free environments was associated with improvement of asthma symptoms (Platts-Mills, Vaughan, Carter, & Woodfolk, 2000).
Allergens in the urban environment: At least two studies found that low-income African American children were neither sensitized nor exposed to high levels of cat allergen (Call, Smith, Morris, Chapman, & Platts-Mills, 1992; Huss et al., 2001). Several studies have demonstrated that in homes where exposure to multiple allergens is likely, exposure to cockroach allergen or exposure to the combination of cockroach and dust mite allergen is the most significant predictor of sensitization and that these exposures are major risk factors for asthma (Alp, Yu, Grant, Rao, & Moy, 2001; Call et al., 1992; Gruchalla et al., 2005; Huss et al., 2001; Rosenstreich et al., 1997; Turyk et al., 2006). Dust mite concentrations greater than 2 μg/g have been associated with a greater risk of allergic sensitization (Sporik, Holgate, Platts-Mills, & Cogswell, 1990). Indoor allergen concentrations in excess of 8 U/g (cockroach) and 1.6 μg/g (mouse) have been associated with higher frequencies of medication use and medical provider visits (W. Phipatanakul, Eggleston, Wright, Wood, & Study, 2000b; Rosenstreich et al., 1997). Dust sample concentrations for rat allergen between 4 to 1413 ng/g were noted to be significantly higher in sensitized asthmatic children versus those without asthma (Perry et al., 2003). Average levels of allergens in the National Survey of Lead and Allergens in Housing were: 1.40 μg/g (dust mite), 0.292 – 1.376 U/g (cockroach), and 0.38 – 0.52 μg/g (mouse) (Arbes, Cohn et al., 2003; Cohn, Arbes, Jaramillo, Reid, & Zeldin, 2006; Cohn et al., 2004) . Simultaneous exposure to fungi, indoor allergens (e.g., from cats, dogs, dust mites, cockroaches, mice and rats), and outdoor allergens (e.g., from grass, tree, and weed pollens) is common. Exacerbation of asthma in low-income populations is likely to be multifactorial, and no single exposure dominates (Brugge et al., 2003).
Because of different housing stock across the country, some home characteristics are not consistently associated with dust mite, mouse or cockroach allergen (Chew, Burge et al. 1998; Chew, Higgins et al. 1999; Phipatanakul, Eggleston et al. 2000; Rauh, Chew et al. 2002; Chew, Perzanowski et al. 2003; Cohn, Arbes et al. 2004; Matsui, Simons et al. 2005; Cho, Reponen et al. 2006). For example, the U.S. national housing survey which included information from buildings in 75 locations found that mouse allergen was higher in high-rise buildings (≥ 5 floors) compared to low-rise apartments (1-4 floors) (Cohn et al. 2004). This finding is not directly applicable to some cities such as New York where a majority of the housing in low-income neighborhoods is greater than 5 floors. In fact, shorter apartment buildings (i.e., fewer than 8 stories in New York) had 10-fold and 6.25-fold greater odds (compared with taller high-rise buildings) of having high mouse allergen levels in the kitchen and bed, respectively (Chew et al. 2003). This highlights the importance of considering the geographic factors that influence allergen levels within the home.
Fungi: There has been a substantial amount of research examining the impact of fungi and moisture on occupant health. Up to 40% of United States homes are reported to have problems with fungi (Brunekreef et al., 1989). Skin test results indicate that between 3 and 10% of persons worldwide demonstrate hypersensitivity to common airborne fungi (Horner, Helbling, Salvaggio, & Lehrer, 1995). Sensitization to allergens early in life increases the risk of developing asthma (Peat, Salome, & Woolcock, 1990). Specifically, sensitization to fungi is associated with the existence and severity of asthma (Bush & Prochnau, 2004; Jaakkola, Hwang, & Jaakkola, 2005; Maurya, Gugnani, Sarma, Madan, & Shah, 2005); inner-city children are especially affected (Crain et al., 2002; Kattan et al., 1997). Infants with a maternal history of asthma were significantly more likely to exhibit persistent cough and wheeze when exposed to increased concentrations of indoor fungi (Belanger et al., 2003; Gent et al., 2002). Furthermore, a Boston prospective birth cohort study found a significantly increased risk of developing lower respiratory tract illness among infants exposed to high indoor fungi levels (Stark, Burge, Ryan, Milton, & Gold, 2003) and a greater risk of allergic sensitization by age 5 (Stark et al., 2005). The presence of a “mold odor” in a home, while controlling for confounding variables, has been shown to be an independent risk factor for the development of asthma with an incidence rate ratio of 2.4 (95% CI 1.1–5.6) (Jaakkola et al., 2005).
Homes with damp indoor spaces and high concentrations of fungi can aggravate pre-existing respiratory conditions such as asthma (IOM, 2004). The Inner-City Asthma study looked at homes demonstrating an increased concentration of fungi in the home compared to the outdoor air concentration measured on the same day (O'Connor et al., 2004). Residents of homes with higher concentrations of airborne fungi indoors than outdoors were significantly more likely to report dampness or leaks in any room, evidence of moisture and leaks, musty smell, and evidence of cockroaches. Modern building practices, such as increased use of synthetic building materials and inadequate ventilation or drainage, can promote fungal growth (NIH, 2005). Further research is needed regarding the efficacy of green building practices in preventing the growth of, or reducing the burden of, indoor fungi.
Volatile Organic Compounds (VOCs): A number of VOCs that can cause adverse respiratory effects are commonly found in the home environment. These include formaldehyde, benzene, toluene, xylene, ethylbenzene, styrene among others (IOM, 2000; Sunesson, Rosen, Stenberg, & Sjostrom, 2006). In 2000, the IOM concluded that there was insufficient evidence to determine whether or not an association exists between indoor residential VOC exposures and the development or exacerbation of asthma. The report recommends that indoor exposures to VOCs be limited where practical by source removal, source avoidance and increased ventilation. The IOM called for prospective cohort studies to characterize exposure (IOM, 2000).
Associations between VOCs and asthma: Following the IOM report, a few studies have provided preliminary evidence for an association between elevated VOC levels and adverse health effects, including asthma. Young Australian children with asthma were exposed to significantly higher VOC levels than controls (Rumchev, Spickett, Bulsara, Phillips, & Stick, 2004). Among the VOCs observed in this study; benzene, ethylbenzene, and toluene were most strongly associated with a primary diagnosis of asthma. The study also found that for each 10 μg/m3 increase in concentration, the risk of having asthma increased by nearly two and three times for toluene and benzene respectively. In one study of asthmatic children living in public housing, 32% of samples collected hadbenzene levels that exceeded the cancer risk level , and 38% of samples had chloroform levels that exceeded the cancer risk level. Of all VOCs measured, toluene and 1,4-dichlorobenzene had the overall highest mean and maximum levels (Brugge et al., 2003) . A recent review article noted that although observational studies have identified an association between VOC and asthma indicators, further studies are needed to confirm this finding, characterize effect size, and determine the biologically relevant duration of exposure (Dales & Raizenne, 2004).
Pesticides: While health effects associated with pesticide exposure are myriad and range from mucus membrane irritation to neuropathies, cancer, and death (Amdur et al., 1991), we will focus on one main health outcome, asthma exacerbation. Similar to the case of VOCs, assessment of the biologically relevant time period of exposure can be difficult for pesticides. For example, a population-based school study in California found that children with pesticide exposure in the first year of life were more likely to have early persistent wheezing than those not exposed during the first year of life (OR=3.6, 95%CI (1.6-8.1) (Salam, Li, Langholz, & Gilliland, 2004). In the same study, pesticide exposure at any other time (other than the first year of life) was negatively associated with early persistent wheezing (OR=0.7, 95%CI (0.3-2.0), but this did not reach statistical significance.
In the past, organochlorine, organophosphate, carbamate and pyrethroid pesticides could be found in most U. S. homes (Quandt et al., 2004). However, recent bans on residential use of chlorpyrifos (2002) and diazinon (2004) have led to lower exposures of these pesticides in the homes, particularly of inner-city apartments (Whyatt et al., 2004). Several housing characteristics have been found to predict indoor pesticide levels. For example, housing dilapidation has been associated with cockroach infestation, cockroach allergen and multiple pest eradication efforts (including use of pesticides) (Rauh et al., 2002). Many pesticides have low volatility and if not exposed to UV light, they can persist in indoor environments at high concentrations, although levels vary substantially depending on use level (Rudel, Camann, Spengler, Korn, & Brody, 2003). For these reasons, researchers who have studied pesticide exposure in children’s homes, have concluded that household pesticides are best measured via dust sampling (Bradman et al., 2005).
Pesticides and asthma: There are considerable data indicating that dysregulation of both parasympathetic (cholinergic) and sympathetic autonomic control of airways, such as by pesticide exposure, may be important in the occurrence of asthma and its severity (P. J. Barnes, 1995). Dysregulation of parasympathetic function predicts the onset of wheezing in adults. (Sparrow, O'Connor, Basner, Rosner, & Weiss, 1993) Although there are few direct studies of the effects of organophosphate and carbamate pesticide exposure on asthma risk, farm workers' exposure to carbamate pesticides has been associated with the occurrence of asthma after adjustment for other relevant factors (Senthilselvan, McDuffie, & Dosman, 1992). Professional fumigators have an increased occurrence of allergy and asthma in parallel with a greater than 20% decrease in red blood cell levels of acetylcholinesterase (Garry, Kelly, Sprafka, Edwards, & Griffith, 1994). Exposure to chlorpyrifos has also been associated with an increase in the occurrence of atopic conditions (Thrasher, Madison, & Broughton, 1993). These studies suggest that pesticide exposures could be important etiologic and morbidity-modifying factors in the occurrence of childhood asthma. Nonetheless, only two major studies of childhood exposures (not exclusively set in an agricultural environment) have shown associations between pesticides and asthma prevalence (Salam et al., 2004; Sunyer et al., 2006). In the school-based California study, exposure to herbicides or pesticides in the home during the first year of life was associated with a greater odds of children presenting with early persistent wheeze (OR=3.8, (1.7-8.40)) (Salam et al., 2004). In the Spanish study, diagnosed asthma and persistent wheezing were associated with the organochlorine and DDE at birth (for each 1 ng/ml increase, OR=1.18 [1.01-1.39] and OR=1.13 [0.98-1.30], respectively), but not with DDE at age 4 years (Sunyer et al., 2006).
New methodologies for exposure assessment: In 2006, the NIH established the Genes, Environment and Health Initiative (GEI) with the long range goal of providing a foundation of technology and knowledge to enable population scale studies on the interaction of genetic and environmental factors in human disease. At the outset of the GEI, it was determined that large scale, broadly focused Gene-Environment interaction studies would require an improved capacity in exposure assessment. Specifically two aspects were identified, the first being the need for improved definition of exposure at the level of the individual and the second being a comprehensive view of the environment integrating an assessment of exposures and lifestyle factors.
The Exposure Biology Program is divided into four component areas: sensors for assessment of chemical exposures (SACE), diet and physical activity, psychosocial stress and addictive substances, and biological response indicators to each of these environmental agents. Each of these programs is working individually, with opportunities for cross-program collaboration, to develop a new set of tools which will address the most common limitations of the current technologies used for exposure assessment: indirect measurement, lack of temporal or spatial resolution, limitation to single endpoints and a high degree of obtrusiveness. Each of the programs is product oriented with a goal of delivering prototype devices and biomarker panels for field testing and validation at the end of the four year granting period. The Sensors for Analysis of Chemical Exposures (SACE) program within the Exposure Biology Program of GEI was developed to build a next generation of sensors for defining real-time exposure with the expectation that this will increase the power of environmental epidemiology and gene-environment interaction studies.
Through SACE, the NIEHS and NIH have funded eight projects to develop integrated sensor devices which include not only the capability to detect multiple analytes of interest in a highly time resolved manner, but also integrate on board data handling, GPS based localization and in a few cases activity pattern analysis as well. The projects are detecting a wide range of analytes including particulate matter (PM 10, 2.5 and 1), allergens (dust mite, cat, cockroach and more), pesticides, oxidants, molecular gases (O3, COx, SOx, NOx), and volatile organic compounds (benzene, toluene, xylene, and high priority industrial pollutants). In summer 2010, CDC established an interagency agreement with NIEHS to use three types of these devices in each of the home visits in order to improve exposure assessment in the Green Housing Study and also validate their use. The details of the devices are described later in this section (section A1).
Outdoor air pollution: In laboratory studies, investigators often have the ability to carefully control exposures that might be related to health effects. Because this study is tethered to HUD’s green renovations programs, randomization is not a feasible option for study site selection. Nonetheless, there are some factors such as outdoor air pollution which we can control by using GIS to match green buildings to comparison buildings. The greenest building located in a heavily polluted neighborhood (i.e., proximity to major roadways, airports, and bus depots) might have outdoor exposures that overwhelm any potential health benefit of the green attributes. Proximity to major roadways has been associated with high concentrations of particulate matter (PM) less than 10µm (PM10) which is from coarse grinding activities and also with high concentrations of particles less than 2.5 µm (PM2.5) which is associated with combustion sources (Liao et al., 2006). Moreover, proximity to major roadways is associated with emergency department (ED) visits (Tolbert, Klein, Peel, Sarnat, & Sarnat, 2007; Tolbert et al., 2000), asthma prevalence (van Vliet et al., 1997) and morbidity (e.g, lung function and bronchial hyperreactivity) (Brunekreef et al., 1997; Janssen et al., 2003), and allergy (Morgenstern et al., 2008). Specifically, the diesel exhaust particulates within the PM2.5 fraction augment the allergenicity of the particles (Diaz-Sanchez, 1997). This indicates the importance of GIS to match proximity to sources of PM for both site selection and statistical analysis.
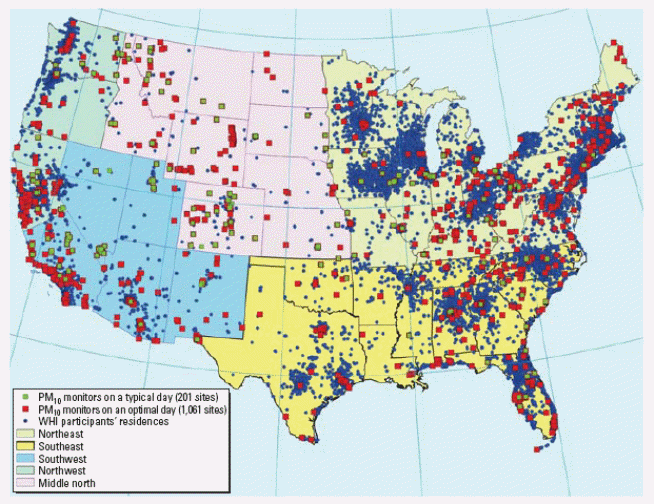
Figure 1. Spatial relationships between residential locations in a study by Liao et al (2006) and EPA monitoring sites for PM2.5 and PM10.
The proposed study (The Green Housing Study) will address several of the research gaps that were mentioned above. The study participants are children with asthma (age 7-12 years). Comparison homes are those not currently receiving a green housing renovation (see inclusion criteria in Table 2 later in this section). The specific aims of this study are as follows:
1. To conduct an exposure assessment of chemical and biological contaminants, pesticides, volatile organic compounds (VOCs), fungi, indoor allergens (in terms of variety and concentration) in green vs. comparison housing.
a. We will measure interior levels of pesticides in surface wipe samples; fungi and indoor allergens in dust samples; and VOCs in air samples.
b. We will also compare levels of biomarkers of VOCs and pesticides (in terms of variety and concentration) from the participating residents of green and comparison housing.
2. To examine the relationship between living in green vs. comparison housing and asthma morbidity (e.g., symptoms, ED visits, use of medications, lost school/work days) of children with doctor-diagnosed asthma (ages 7-12 years). We will adjust for allergic sensitization and ETS.
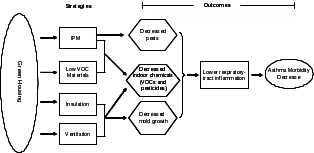
Figure 2. Hypothesized relationships among green housing rehabilitation strategies, environmental exposures, and asthma-related health outcomes.
The hypotheses of this study are as follows:
Green housing utilizes different strategies to reduce environmental contaminants. We hypothesize that these strategies will lead to 1) lower levels of environmental contaminants compared with those of comparison housing, and 2) lower levels of related biomarkers in the residents of green vs. comparison housing.
Integrated pest management (IPM) is a method to reduce pests such as cockroaches and mice by eliminating entry points in the home and harborage areas.
We hypothesize that IPM will result in lower cockroach and mouse allergen levels while at the same time lowering the concentrations and array of pesticides in the green vs. comparison homes.
We hypothesize that concentrations of pesticide metabolites in urine of children living in green housing will be lower than those living in comparison homes.
The use of low VOC paints, carpeting, and other building materials contain lower concentrations of aldehydes, ketones, and alcohols.
We hypothesize that the levels of VOCs will be lower at baseline in green-renovated vs. comparison homes.
We hypothesize that concentrations of VOCs in urine of children with asthma (ages 7-12 years) living in green housing will be lower than those living in comparison homes.
Insulation can reduce sources of moisture, specifically condensation. We hypothesize that green housing will have more and possibly better insulation (e.g., higher R-value) than comparison housing. We hypothesize that insulation (e.g., dual-paned windows, insulated cold water pipes, and rigid insulation above concrete floors and in exterior walls) will result in lower concentrations of dust mite (and therefore their allergens) and fungi.
Another aspect of green housing is improved ventilation which can reduce moisture and decrease indoor concentration of VOCs. For example, improved exterior wall insulation can reduce condensation and a properly-sized and maintained central heating, ventilating, and air-conditioning unit (HVAC) can help buildings keep dry and at the same time, exhaust environmental contaminants to the outside.
We hypothesize that green housing will have a higher percentage of units with the recommended air exchange rates than comparison housing.
We hypothesize that green housing units will have lower VOCs than comparison homes.
We hypothesize that green housing units will have lower levels of fungi and dust mite allergen than comparison homes.
If irritants and allergens are lower in green vs. comparison housing, residents of green housing should experience decreased asthma morbidity. Specifically, we hypothesize that children with asthma (ages 7-12 years) in green housing will have lower asthma morbidity, adjusting for environmental tobacco smoke (ETS) exposure.
Privacy Impact Assessment
Below, we discuss three aspects of privacy impact assessment: (i) an overview of the data collection system, (ii) a delineation or listing of the items of information to be collected, and (iii) an indication of whether the system hosts a website.
Overview of the Data Collection System
The United States Department of Housing and Urban Development (HUD) subsidizes both publicly- and privately-owned housing across the country, notably in urban areas. HUD requires that these subsidized properties be rehabilitated to maintain a certain level of habitability. CDC will leverage the opportunity to study rehabilitated properties in thirteen (13) study locations (large metropolitan areas that are located in different climactic regions of the United States). The selection criteria are described in Part B. From each of these geographically-stratified study sites, 32 green intervention homes and 32 comparison homes (total = 832) will be included. Within each study site (i.e., city), both the green-renovated and comparison homes will be from the same housing development or neighborhoods to ensure homogeneity with regard to housing type and other socioeconomic factors. Changes in environmental measurements (pesticides, VOCs, particulate matter (i.e., PM 2.5 and 1.0), indoor allergens, and fungi) over the 1-year follow-up in both types of housing (green intervention and comparison) will be compared, thus each home’s follow-up measurements will be compared with its own baseline exposure level. This two-group pre-post within-group and between-group comparison will increase ability to detect differences in exposure levels and asthma outcomes that might result from the green renovations in our study. At this time, these sites have not been determined by HUD and CDC. When the study sites are selected, the data collection partners will include: 1) CDC; 2) HUD, and 3) contracted research institutions (to be determined).
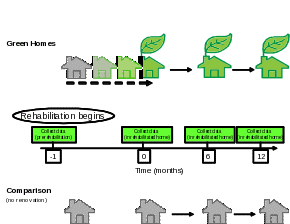
In Figure 3, we describe a scenario of how measurements collected in green-renovated homes would be compared to: 1) those of the baseline, 2) those of homes without any renovation at all. Residents will participate for 1 month prior to rehabilitation, the time required for rehabilitation of their home (usually just a few days), and 12 months after completion of the rehabilitation. The duration of the participation for the residents of comparison homes is the same except no renovation will occur. More details of the study design are provided in Part B of this information request.
Figure 3. Diagram of renovation schedule (green intervention vs. comparison)
Eligible participants will be limited to children with doctor-diagnosed asthma (ages 7-12 years). Health information for eligible children will be reported by the mother/primary caregiver living in HUD-subsidized housing that either received a green renovation (i.e., green intervention) or living in HUD-subsidized housing that received no renovation at all (i.e., comparison). Details of the eligibility criteria are listed in Table 2.
Table 2. The Green Housing Study’s inclusion and exclusion criteria
Inclusion Criteria |
Exclusion Criteria |
- Mother/ primary caregiver reports that child has ever been diagnosed with asthma by a physician and child has experienced asthma-related symptoms (wheezing, slow play or night awakening) during the past 6 months. 2. Mothers/primary caregivers of the children listed above. - No clinical markers will be collected, but we will ask questions regarding their home environment that might be related to child’s health outcomes of interest. 3. Green homes will be renovated using low VOC materials and integrated pest management (IPM) principles.
|
|
Residents who express interest in the study can contact the site projector coordinator by telephone or e-mail. Subsequently, subcontracted staff (trained by the CDC study investigators) will schedule a home visit with the residents. During this home visit each resident’s eligibility will be assessed (i.e. the Screening Form will be filled out by the aforementioned staff based on responses from the mother/ primary caregiver). If a child is eligible, then the study will be explained to the mother/ primary caregiver, and if they are willing to participate, individual participant consent will be obtained from the mother/ primary caregiver. Child assent will be obtained from all children 7-12. The children ages 7-12 will be assenting to provide blood and urine samples for the study; they will not be asked to respond to survey questions—their mothers/ primary caregivers will be providing that information. Consent and Assent forms are in Appendices F and G. After consent and assent as appropriate is obtained, the technicians will collect all of the study baseline information during the initial visit. Participants will receive monetary compensation for participation as outlined in section A.9 (Explanation of Any Payment or Gift to Respondents).
The methods of data collection will include written survey data collected through personal telephone, and text messaging interviews of enrolled mothers/ primary caregivers (Table 8). Trained staff will visit each enrolled child’s home four times (including the initial visit to obtain consent and baseline measurements) during a 1-year period to administer a battery of questionnaires. Each of the surveys will be administered in-person to the enrollee’s mother/ primary caregiver in the study by bilingual (English and Spanish or English and Chinese) interviewers. In addition, brief text messages to inquire about respiratory infections will be sent at the end of months 1, 2, 4, 5, 7, 8, 10, and 11. The enrollee’s mother/ primary caregiver will also be contacted by phone at two time points during the same 1-year period just to update contact information and inquire about respiratory morbidity. Enrolled children (ages 7-12 years) will not be interviewed; however, their mothers/ primary caregivers will provide information about their children’s exposures and health outcomes.
Table 3. Surveys administered during a 1-year period
Type of Survey/ Form |
Responses of the Mother/ Primary caregiver (regarding the participating child with asthma age 7-12 years)
|
|
Screening |
10 minutes |
|
Baseline Questionnaire |
Home Characteristics |
15 minutes |
Demographics |
15 minutes |
|
Children with asthma 7-12 years |
15 minutes |
|
Monthly Texts about child’s respiratory symptoms (occurs during months when phone or home visit not conducted) |
1 minute (eight timepoints) = 8 minutes |
|
3 and 9-month Phone contact |
5 minutes (two timepoints) = 10 minutes |
|
6 and 12-month Follow-up Questionnaire |
Environment |
10 minutes (two timepoints) = 20 minutes |
Mother/ primary caregiver |
10 minutes (two timepoints) = 20 minutes |
|
Children with asthma 7-12 years |
10 minutes (two timepoints) = 20 minutes |
|
Time/Activity |
Mother/ primary caregiver |
5 minutes (four timepoints) = 20 minutes |
Children with asthma 7-12 years |
5 minutes (four timepoints) = 20 minutes |
|
|
|
|
Total Number of surveys |
|
27
|
Estimated response time during a 1-yr period |
|
163 minutes |
All paper copies of consent forms and questionnaires will be scanned into electronic files. The paper copies of the data will be maintained at each study site’s contracted research institution (to be determined) for a period of 5 years beyond the last peer-reviewed publication of the results. At that time, paper copies will be shredded and then recycled. The electronic files will be shared with CDC, and CDC will keep the electronic files in accordance with approved record control schedules.
Health and Environmental Assessments:
For Intervention Homes: Summaries of the clinical and environmental measurements are shown in Tables 4 and 5. The baseline measurement will occur up to one (1) month prior to commencement of rehabilitation activities. Baseline part 2 will be collected in the home one (1) week after completion of rehabilitation activities. Total time of study participation is approximately 1 year, although the exact time will vary depending upon the rehabilitation scenario. Residents will participate for 1 month prior to rehabilitation, the time required for rehabilitation of their home, and 12 months after completion of the rehabilitation. Estimated time for rehabilitation activities (e.g., new paint, carpeting, Energy Star appliances, IPM) should be only a few days.
For Comparison Homes: The baseline measurement will occur within one (1) week either before or after the baseline measurements were taken from the matched intervention home. Baseline part 2 will be collected in the home within one (1) week either before or after the baseline part 2 measurements were taken from the matched intervention home. Total time of study participation is approximately 1 year, although the exact time will vary depending upon the rehabilitation scenario. Residents will participate for the same amount of time as the matched group of intervention homes.
Table 4. Summary of clinical measurements
Factor |
Child with asthma (Age 7-12) |
Blood Baseline |
|
Urine Baseline Baseline (part 2 occurs after renovation is completed) 6-mo. follow-up 12-mo. follow-up |
|
Pulmonary Function Test Baseline Baseline (part 2 occurs after renovation is completed) 6-mo. follow-up 12-mo. follow-up |
|
Exhaled Nitric Oxide Baseline Baseline (part 2 occurs after renovation is completed) 6-mo. follow-up 12-mo. follow-up |
|
Respiratory Symptoms Questionnaire Baseline Baseline (part 2 occurs after renovation is completed) 6-mo. follow-up 12-mo. follow-up |
|
*Blood will be used for assessment of allergy status (IgE)
** Urine will be used for assessment of cotinine (marker of ETS exposure), pesticides, and VOC metabolites
Table 5. Summary of environmental measurements in homes*
Type of assessment |
Baseline |
Baseline part 2 (after renovation is completed) |
6-Month follow-up |
12-Month follow-up |
Allergens |
|
|
|
|
Fungi |
|
|
|
|
Pesticides |
|
|
|
|
VOCs |
|
|
|
|
Particulate Matter (PM2.5) |
|
|
|
|
Temperature |
|
|
|
|
Relative Humidity |
|
|
|
|
Air Exchange Rate |
|
|
|
|
* The mother/ primary caregiver’s home is the same as that of the child. Dust sampling will occur in kitchens and the children’s beds as well as those of the mother/ primary caregiver. The mother/ primary caregiver bed is sampled because it serves as a proxy of exposure to several of the indoor allergens. This proxy can help with characterization of the indoor environment especially in cases where limited dust is available from the child’s bed. Except for the pesticide measurements in the kitchen, all other measurements will be limited to the child’s bedroom.
Assessments for children: Upon enrollment, the technicians (with training provided by CDC) will collect all of the study baseline information from the primary caregiver during the initial visit. This includes: a home characteristics questionnaire, an environmental exposure assessment, and health questionnaire. For those children (age 7-12) who meet asthma inclusion criteria, we will also collect urine samples, a blood sample, nasal and throat swabs for assessment of acute respiratory illness (ARI), exhaled nitric oxide (eNO), and conduct pulmonary function testing by spirometry. Details regarding these assessments are provided below.
Questionnaires: Information will be collected on frequency and duration of asthma-related symptoms, healthcare utilization, school and work absences, and medication use. The home characteristics questionnaires administered to the enrollee’s mother/ primary caregiver will inquire about the type of building, heating and cooling of the home, furnishings, cleaning regimens, the presence of pets and pests, environmental smoke, and reports of dampness. Provenance of the questions is described in Part B.
Temperature and Relative Humidity Measurements: Temperature and relative humidity measurements for each home will be obtained during each home visit. A HOBO® continuous data logger (Onset Computer Corporation, Bourne, MA) will be placed on the floor in each home’s living room for one week, and continuous measurements (every 5 minutes) of temperature and relative humidity will be recorded.
Dust sampling: Sampling for allergens and fungi will be carried out by technicians using a standardized protocol. All field staff will be trained by CDC in the proper methods for sample collection and handling. Dust samples will be collected separately from kitchens and beds by using a canister vacuum cleaner. One dust sample will be collected from the kitchen, focusing on the baseboard area and perimeter of the oven and refrigerator, for a duration of 3 minutes. Another dust sample will be collected from the index child’s bed. Finally, a third dust sample will be collected from the bed of the mother/ primary caregiver. The mattress and pillows associated with the upper half of the bed will be vacuumed for 3 minutes. After sampling, each filter will be sealed in a sterile plastic tube and stored at -20oC until analysis for indoor allergens and fungi.
Indoor allergen analysis: Frozen dust samples will be transported to the laboratory at CDC. Samples will be analyzed dust mite (Der f 1 and Der p 1), cockroach, (Bla g 2), cat (Fel d 1), dog (Can f 1), rat (Rat n 1), and mouse allergens (Mus m 1) using commercially available multiplex immunoassays (Indoor Biotechnologies, Charlottesville, VA).
Fungi analysis: Dust samples from the beds will also be analyzed for a total biomass marker of fungi, ergosterol, by gas chromatography/mass spectrometry (GC/MS) (Park et al., 2008).
Volatile organic chemicals (VOCs): Continuous air monitoring will be conducted using passive diffusion dosimeters for VOCs (one for solvents and one for aldehydes). The passive dosimeters will be placed in each participating home for 5 days. Total VOCs will be quantified using GC/MS. Aldehydes will be desorbed from passive 2,4-dinitrophenylhydrazine (DNPH) treated media, and the derivatized aldehydes are to be analyzed by high-performance liquid chromatography (HPLC) (Adgate et al, 2004).
Pesticides: Dust samples will be collected by wiping a measured 12-inch square section of the floor along the baseboard in the kitchens. Samples will be gathered on gauze squares wetted with isopropanol and will be analyzed using GC/MS and HPLC/MS (Table 6). Common pyrethroid (cis and trans permethrin, cyfluthrin), organophosphate, and carbamate pesticides will be analyzed in addition to a synergist that is used uniquely in pyrethroid pesticides (piperonyl butoxide).
Table 6. A list of pesticides that EPA can measure in environmental samples.
Organochlorines |
Pyrethroids/Pyrethrins |
α- and γ- Chlordane |
Allerthrin |
Heptachlor |
Bifenthrin |
P,p=DDT |
Cyfluthrin I, II/III, IV |
P,p=DDE |
Cypermethrin I, II/III, IV |
Organophosphates |
Deltamethrin |
Chlorpyrifos |
Esfenvalerate |
Diazinon |
Fenpropathrin |
Malathion |
Imiprothrin |
Phenyl-Pyrazole |
Λ-cyhalothrin |
Fipronil |
Cis- and trans-Permethrin |
Other |
Pyrethrin I, II |
Piperonyl Butoxide |
Prallethrin |
|
Resmethrin |
|
Sumithrin |
|
Tetramethrin I, II |
Air Exchange Rates (AER): Air exchange rates can be quantified using non-toxic tracer gases such as SF6 and perfluorinated methylcyclohexane (PMCH). The method to be employed in this study will use the perfluorocarbon, PMCH. In brief, the method is accomplished by placing a sponge with a nontoxic tracer gas inside the home and allowing the gas to reach steady state (Dietz et al, 1982). With passive air sampling for a period of 12 hours up to one week, the PMCH is collected and then analyzed by gas chromatography and electron capture detector (GC/ECD). The range of quantification is 0.10 to 2.5 air changes per hour (ACH), and the upper limit of detection is about 3.0 ACH.
Particulate (PM2.5) Monitoring: Monitoring for particulate matter ≤ 2.5 µm (PM2.5) will be conducted in the child’s bedroom(at a height of 1.5 meter) using integrated sampling for a one week period during each home visit in order to enable for adjustment of seasonal variation (Breysse et al, 2005). Integrated samples will be collected using constant airflow portable sampling pumps designed for quiet indoor operation. Samples for PM2.5 will be collected on 37 mm, 1.0 µm pore-size PTFE membrane filters using single-stage Personal Modular Impactors (SKC, Inc.). The pump flow-rate will be calibrated at a flow rate equal to 3 L/min in the laboratory prior to the start of sampling and checked at the end of sampling with a BIOS DryCal DC-2 flow meter.
Outdoor air sampling: To obtain an estimate of outdoor PM and VOC exposure for each of the housing developments, we will conduct 1-week air sampling on rooftops under protected cover during winter, spring, summer and fall. These measurements will be repeated throughout the entire study period for a given city. These repeated measures should yield a better estimate of the average outdoor PM and VOC exposure and reduce the influence of local events that might give rise to extreme values.
Opportunity for real-time exposure assessment of VOCs and PM: CDC has an interagency agreement NIEHS to provide field-deployable units that measure particulate matter with an aerodynamic cutpoint of 2.5 µm (PM2.5), 1.0 µm (PM1.0), and VOCs to be used for field validation in a study of the potential environmental and health benefits associated green eco-friendly construction and maintenance practice in the Green Housing Study. These devices were developed as part of the NIH’s Gene- Environment Initiative (GEI), specifically the Sensors for Assessing Chemical Exposures (SACE program). NIH will provide up to five (5) field-deployable units from each of the selected SACE investigators that have developed sensors which are both 1) field-deployable and 2) capable of measuring analytes relevant to the Green Housing Study. These devices will collect measurement side-by-side with the traditional air sampling devices during each of the home visits. The advantage of these devices is that they can measure peaks of exposure that might not be captured with traditional integrated air sampling equipment. The peaks might be more closely related to the biomarkers that will be collected (e.g., VOC metabolites in urine and exhaled nitric oxide). Figures 4,5, and 6 below describe the three devices that will be used in the Green Housing Study.
Figure 4. The single-channel real-time PM2.5 monitor that will be used in the Green Housing Study.
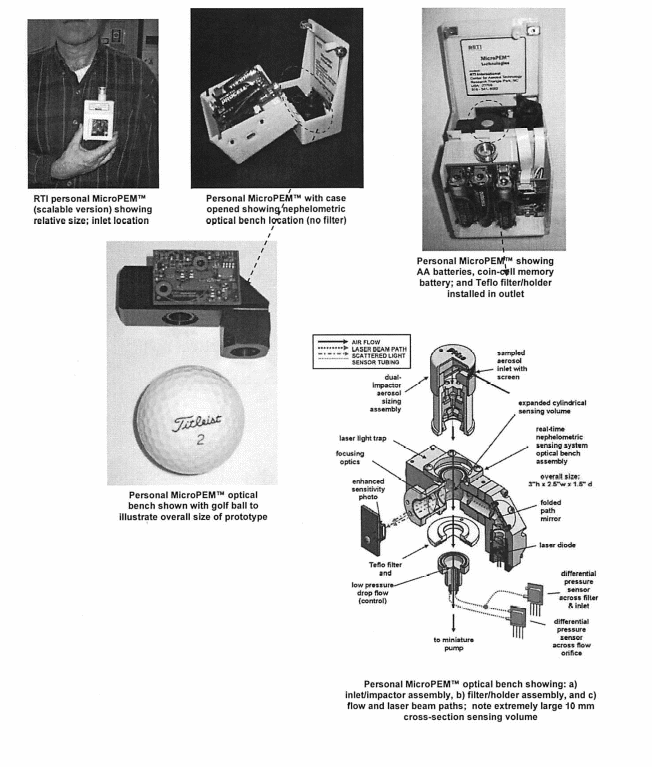
Figure 5. The dual-channel real-time PM1.0 and PM2.5 monitor that will be used in the Green Housing Study.
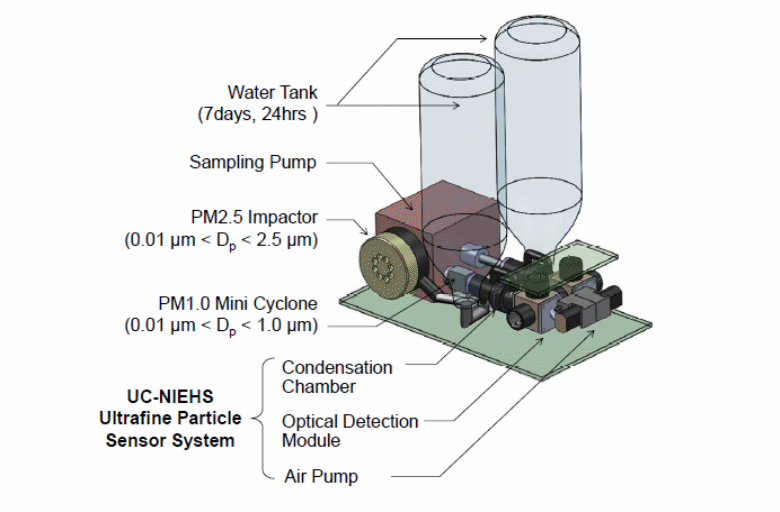
Figure 6. The real-time VOC monitor that will be used in the Green Housing Study.
Urine collection: Urine will be collected for two main purposes: 1) to assess recent ETS exposure via cotinine measurement); and 2) to assess biomarkers of pesticides and VOCs
(Tables 7 and 8). Urine analysis will be conducted by CDC’s National Center for Environmental Health, Division of Lab Sciences using standard methods (Baker et al, 2000, Matt et al, 1999, Ding et al, 2009).
Table 7. Urinary metabolites of VOCs measured by the CDC’s Division of Laboratory Sciences
|
Compound |
Parent Chemical |
DHBMA |
N-Acetyl-S- (3,4-Dihidroxybutyl)-L-Cysteine |
1,3 Butadiene |
MHBMA |
N-Acetyl-S- (1-Hydroxymethyl)-2-propenyl-L-Cysteine |
1,3 Butadiene |
CBMA |
N-Acetyl-S- (2-Carboxyethyl)-L-Cysteine |
Acrolein |
HPMA |
N-Acetyl-S- (3-Hydroxypropyl)-L-Cysteine |
Acrolein |
HEMA |
N-Acetyl-S- (2-Hydroxyethyl)-L-cysteine |
Acrylonitrile, Bromoethanol, chloroacetaldehyde, ethylene, chloroethylene, 1,2-dichloroethane, ethylene oxide, 1,2-dibromoethane, vinyl chloride |
PMA |
N-Acetyl-S-(phenyl)-L-cysteine |
Benzene |
BMA |
N-Acetyl-S- (benzyl)-L-Cysteine |
Toluene |
Table 8. Urinary metabolites of pesticides measured by the CDC’s Division of Laboratory Sciences
Compound |
Parent Chemical |
|
|
cis-2,2-(Dichloro)-2-dimethylvinyl cyclopropane carboxylic acid) (cis-DCCA)
|
Permethrin, cypermethrin, cyfluthrin
|
4-Fluoro-3-phenoxybenzoic acid (4F3PBA)
|
Cyfluthrin
|
Carbofuranphenol (2,3-dihydro-2,2-dimethy-7-hydroxybenzofuran) (CFP) |
Carbofuran, benfuracarb, carbosulfan
|
2-Isopropoxyphenol (IPP)
|
Propoxur
|
2-Isopropyl-4-methyl-6-hydroxypyrimidinol (IMPY)
|
Diazinon
|
para-Nitrophenol (PNP)
|
Parathion, methyl parathion, nitrobenzene
|
3,5,6-Trichloro-2-pyridinol (TCPy)
|
Chlorpyrifos |
Allergy testing: Allergen testing will be performed once at baseline following enrollment. We will use immunoCAP method to assess total and allergen-specific (dust mite, cockroach, cat, mouse, tree mix, grass mix, and weed mix) IgE antibodies in serum. Unfortunately, mold extracts used for measuring IgE are very poor (due to batch-to-batch variability), thus we will not be able to assess sensitization to mold.
Pulmonary function testing: Pulmonary function provides an objective outcome for determining improvements in respiratory health status following the intervention to decrease environmental asthma triggers in the home and improve asthma management. Spirometry (pulmonary function testing or PFTs) will be performed in children with a diagnosis of asthma who are 7-12 years of age. Study participants will be weighed and their heights will be measured using a calibrated scale prior to the start of each testing session. Standard spirometric measures, forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), the ratio of FEV1/FVC, forced expiratory flow between 25-75% of vital capacity (FEF25-75%), and peak expiratory flow (PEF), will be recorded for each patient (Hankinson et al, 1999). All children in this age range may not be able to successfully complete the forced expiratory maneuver required for this test, but attempts to test all children in this age range will be made. All PFT studies will be performed at each home visit to assess possible seasonal variation.
We will not conduct lung function tests on asthmatic children who are in distress; we will reschedule the visit. It is our experience that a phone call to the home approximately 1 hour before the scheduled visit serves not only as a reminder that our research assistants will be visiting the home, but also as an opportunity to inquire if the child will be at home and is ready for the tests (such as lung function, blood draw, etc). If during this phone call, the mother/ primary caregiver indicates that the child is in respiratory distress, then we will advise her to hang up and attend to her child and if necessary seek medical attention.
The technician who administers the lung function test in the home is not qualified to determine if the child’s lung function is impaired; accurate interpretation of test results requires review by a trained pediatric pulmonologist. We expect that it would take at least 2-3 months for the pulmonologist (site-specific) to review the lung function curves (typically done in batches)—by that time the lung function could have changed for that child. Lung function tests done in isolation (and at any given timepoint) without consideration of other clinical parameters are difficult to interpret. Therefore, we will mail the results of each of the lung function tests (as they become available) to the mother/ primary caregiver after review by the pulmonologist. The mother/ primary caregiver can then share this information (i.e., repeated lung function tests) with the child’s healthcare provider who can better interpret the lung function test results within the context of other relevant parameters (such as recent medication use) that would affect the child’s overall asthma management. These results will be provided to participating asthmatic children of both the green and comparison homes to avoid potential bias.
Documentation of the participating asthmatic child’s primary care provider will occur at the baseline home visit and participants who do not identify a primary care provider will be referred to one in their local area. A participant who contacts study staff with acute health concerns will be referred to his/her primary care provider or the Emergency Department.
Exhaled Nitric Oxide (eNO): eNO is a known marker of pulmonary inflammation and will provide a non-invasive means of assessing pulmonary inflammation in a large cohort that includes children (Buchvald et al., 2005; Cardinale et al., 2005; Pijnenburg, Hofhuis, Hop, & De Jongste, 2005). Measurement of exhaled nitric oxide will be obtained prior to lung function, and will be obtained according to the American Thoracic Society Guidelines (ATS, 2005). Nitric oxide concentrations will be measured using a chemiluminescent analyzer (NIOX TM System, Aerocrine, Sweden). This equipment is FDA-approved for clinical use in asthma management. Participants will be required to produce at least two reproducible exhalations.
Nasal and throat swabs: Children with asthma are commonly exposed to multiple indoor allergens and environmental tobacco smoke, multi-factorial exposures that may contribute to the increased asthma-related complications in this population. However, previous studies of environmental interventions for patients with asthma have not used objective measurements (i.e., PCR of nasal swabs) accounted for the role of acute respiratory illness (ARI) as triggers for asthma exacerbation (Morgan et al, 2004). Viral respiratory tract infections have been reported as important triggers for exacerbations of asthma in adults and children (Clark, 1979, Miller et al, 2008). Recent studies based on PCR assays support an important role of viral respiratory tract infections in acute asthma exacerbations (Khetsuriani et al, 2007). By accounting for the role of respiratory virus infections as triggers for asthma exacerbation, we may be able to find stronger associations when aiming to estimate the impact of environmental interventions on improvement of symptoms of asthma and decrease use of health care services. This is because respiratory virus infections may be associated (or interact) with study’s outcome and exposure measures, underestimating the effect of the intervention.
Mothers/ primary caregivers of the participating children with asthma will be trained to collect one nasal swab and one throat swab after 24-36 hours from onset of at least three of the following: fever, stuffy/runny nose, cough, sore throat, body aches, or tiredness, for more than 24 hours. It is estimated that children in this age group may have on average 4-5 episodes of ARI per year (Monto 2002). The specimens and an illness checklist will be collected on each occasion of a suspected ARI by using methods previously described by researchers (Esposito et al. 2010). The specimens can be stored in the participant’s refrigerator for up to one week before being picked up by the study coordinator. The study coordinator will be asked to collect the specimens within 1-2 days of being notified of the parent-collected specimens. At the time of swab pick-up, the trained research assistants will also collect a throat swab and another nasal swab from the child in order to validate the sample collected by the parent. The swabs will be combined and transported in either veal infusion broth (VIB) or Hank’s transport media on ice to the laboratory processing (within 24 hours). The specimens will then be stored at -70 C at local laboratory facilities before being sent to CDC. Specimens would be tested by RT-PCR for RSV, rhinovirus, influenza viruses, parainfluenza viruses, adenoviruses and human metapneumovirus at CDC’s Viral Respiratory Laboratory.
Assessment for mothers/ primary caregivers of children: The only measurement obtained will be questionnaire data regarding the impact of demographic characteristics and behaviors on the respiratory health of the participating child. Such behaviors include but are not limited to: smoking, cooking, and working in environments that could conceivably result in passive transport of chemicals and allergens.
Items of Information to be Collected
Data to be collected about the study participants will include: contact information, demographics, housing characteristics, environmental exposures, health outcomes, and healthcare utilization as listed in questionnaires (Appendices D1-13). We describe the Information in Identifiable Form (IFF) in Table 9.
Table 9. Information in Identifiable Form (IIF) collected during this study.
IIF category |
Collected by contractors but not sent to CDC |
Collected by contractors and sent to Green Housing Study staff at CDC |
name |
X |
|
date of birth |
|
X |
phone numbers |
X |
|
medical information and notes |
|
X |
biological specimens |
|
X |
e-mail address |
X |
|
employment status |
|
X |
home address |
|
X |
CDC requires the home address in order to geocode the home and adjust for influence of outdoor air pollution. (see section A10 for details).
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age
There is no website associated with this study. Therefore, there is no website content directed at children under 13 years of age.
A.2. Purpose and Use of Information Collection
The specific aims of this study are to: 1) conduct an exposure assessment of chemical and biological contaminants, pesticides, volatile organic compounds (VOCs), fungi, and indoor allergens in green vs. comparison housing; and 2) examine the relationship between living in green vs. comparison housing and asthma morbidity. Publications of the study results have the potential to be cited frequently by other researchers, and both CDC and HUD can use data from the Green Housing study to guide their Healthy Homes grantee’s activities via annual conferences and funding opportunities. In Table 10 below, we have justified the data collection in terms of positive needs and the negative consequences of not having the information, and we have emphasized the practical utility of the expected results to federal, state and local governments
Table 10. Justification and practical utility of the data collection.
Type of data collected |
Positive needs for having the information
|
Negative consequence of not having the information |
Practical utility to the government of the expected results |
Environmental exposures
|
This data will provide a direct measurement of environmental exposures in the homes of this sample of residents. |
Merely having health data will not allow us to know if any meaningful differences in health status were truly associated with differences in chemical/biological exposures that were related to green housing factors. One could assume that because health symptoms are improved, that the exposures would have been lower, but this would only be an assumption. |
This study will help CDC and HUD programs to advise their healthy homes, asthma, and child health grantees on which green criteria (if any) are positively associated with lower exposures. Subsequently, this will help grantees inform residents about which green housing practices and materials (if any) to implement in their homes not only for energy efficiency, but for lower exposures in their home, a place where people spend a significant proportion of their time. |
Health status |
This data will provide a direct measurement of health effects in this sample of residents. |
Merely having exposure data will not allow us to know if any meaningful improvements in health status will occur with green housing factors. One could assume that because exposures are lower, that the health would be better, but this would only be an assumption. |
This study will help CDC and HUD programs to advise their healthy homes and asthma, grantees on which green criteria (if any) are positively associated with health outcomes (e.g., asthma outcomes). Subsequently, this will help grantees inform residents in their communities on which green housing practices and materials (if any) to implement in their low-income urban multi-family homes not only for energy efficiency, but for improved health e.g., asthma outcomes). |
Healthcare utilization |
This data will provide a direct measurement of healthcare utilization by this sample of residents which enables us to more fully capture the burden of adverse health asthma outcomes.
|
If we did not collect data on healthcare utilization, then we would not be able to fully capture the burden of adverse health outcomes. |
This will help CDC identify possible alternatives to pharmaceuticals to decrease healthcare costs among low-income urban populations. It will inform Center for Medicare and Medicaid Services policies related to re-imbursement for preventative measures. |
Home Address |
We need to geocode the address so that we can use it to adjust for influence of outdoor air pollution. EPA currently has outdoor air pollution monitors in cities across the US. By knowing the exact location of our study participants’ homes, we can use EPA’s regional measurements in our statistical models of exposure and health outcomes. |
There is the possibility that even the greenest of homes could be located in a highly-polluted area which could overwhelm any potential health benefits of green housing factors.
If we do not adjust for outdoor air pollution, then we will not be able to tease out any effects of indoor green housing factors on respiratory symptoms of the study participants. |
Adjusting for outdoor air pollution will allow CDC and HUD to attribute improved respiratory health effects to green housing factors if they indeed exist. Subsequently, CDC and HUD can make informed recommendations about green building materials and practices that are connected to improved health outcomes. These recommendations could vary by city depending upon levels of outdoor air pollution. |
Date of birth |
We need to know the age of participants because age can influence health outcomes such as pulmonary function. |
If we were to ask contracted entities to strip the date of birth and give CDC only age, we believe that some data might come to us in a truncated/rounded form and this would make our statistical models inaccurate. To preclude differences by reporting site, CDC would have better control of modeling this very important variable. |
Accurate modeling of data is paramount to federal agencies defending and promoting their policies and recommendations. |
HUD has committed funds for the Green Housing Study to CDC via Interagency agreement (IAA) # I-PHI-01062. This IAA commitment for the next several years also leverages personnel and laboratory resources from CDC.
The proposed study will be conducted in low-income housing primarily in urban environments which is likely to have implications for the generalizability of our findings to suburban and rural residences. Also, it may not be appropriate to generalize our findings to children in families with higher socioeconomic status. However, this study will have the potential to improve the health outcomes of some of the most sensitive populations (low-income children with asthma).
Privacy Impact Assessment Information
The IIF collected during the course of the Green Housing Study is listed below in Table 11. While most of the IIF collected is for enrollment and follow-up activities, some data can be sensitive and will be described in detail below.
Table 11. Information in Identifiable Form (IIF) and intended uses
IFF category |
Collected by contractors but not sent to CDC |
Collected by contractors and sent to Green Housing Study staff at CDC |
Purpose |
name |
X |
|
Names are required for written informed consent. In addition, names aid both the study participant and the data collector during in-person and telephone questioning. |
date of birth |
|
X |
To determine eligibility and to also adjust for age in statistical analysis. |
phone numbers |
X |
|
To administer phone questionnaires. |
medical information and notes |
|
X |
To assess health outcomes for statistical analysis |
biological specimens |
|
X |
To assess health-related biomarkers for statistical analysis |
e-mail address |
X |
|
To serve as a secondary means of contacting study participants to administer questionnaires and schedule home visits for sampling |
employment status |
|
X |
To adjust for possible chemical exposures that could occur in the occupational environment. |
home address |
|
X |
To enable contractors to visit homes for sampling and also enable CDC to use geographic information systems (GIS) which can be used for adjusting for factors external to the home which could influence both exposures and health outcomes (e.g., outdoor air pollution). |
Data from paper questionnaires will be entered by the contracted data collectors into a database (e.g. Microsoft Access) which will also be password-protected. Dates of birth and home addresses are primary direct identifiers and the contractor’s removal of other direct identifiers (such as name, phone numbers, e-mail addresses) will minimize identification but not completely eliminate it. A unique Study ID will be assigned by the contractor as a key identifier for all study forms. The environmental and biological samples and measurements will only be identified by study ID. Contracted data collectors will maintain their paper files in locked cabinets and their electronic files will be stored on secured servers with password protection. Encrypted data files will be sent electronically to Green Housing Study investigators at CDC. Data will be stored on highly-secured CDC servers in Atlanta, GA. The servers are housed in a secure computer room complete with climate control, emergency power, and an uninterruptible power supply (UPS). Daily back-ups and integrated security are implemented through the CDC computer services infrastructure. All data access is password-protected, and all network communications use encryption. All servers and PCs that are part of the CDC infrastructure are protected by both host-based firewalls and software in order to prevent the undetected installation of "spyware". Only Green Housing Study investigators at CDC will be given access to read the encrypted data files.
CDC Green Housing Study investigators will receive electronic files with date of birth, medical information, biological specimens, employment status, and home address, identified by study ID number. While we acknowledge that home address is a unique identifier and the contractors will have the link to names and address, CDC Green Housing Study investigators are taking steps described in the previous paragraph to reduce the amount of individually-identifiable data maintained at CDC. If there were a breach of confidentiality for any of the above IIF, some effect on the respondent’s privacy could occur; however, the screening form will be the only form that contains name, home address, phone number, e-mail address, and study ID together; only the contracted data collectors will have this form. The contracted data collectors will only use name, phone number, e-mail address, and home address for locating the study participant and ensuring that follow-up questionnaires and clinical and environmental measurements are repeated accordingly. Contracted data collectors will be required to have human subjects training in accordance with their institution’s Institutional Review Board (IRB) and/or the CDC’s IRB. A component of human subjects training addresses data security measures.
A.3. Use of Improved Information Technology and Burden Reduction
Most of the data collection (i.e., 93%) from the study participants (i.e., the respondents) will be via paper forms; however, we are implementing text messaging to aid in monthly assessment of respiratory infections (i.e., 7% of data collection efforts). For the paper forms, the respondents will have minimal burden in providing their responses because they will not need to read questions nor write answers; the paid data collection contractors will record all of their verbal responses. The data collection contractors will then enter the survey data into an electronic database which will enable electronic transmission of data to CDC’s Green Housing Study researchers. We chose paper forms for most of the data collection because at this time, it is the least expensive method (as opposed to transcribing answers from voice recorders or paying for laptop/ notepad computers). The text messages given at months 1, 2, 4, 5, 7, 8, 10, and 11 will only take approximately 1 minute to respond to a few brief questions of respiratory infections, and they can be answered at the respondents’ convenience rather than relying upon direct interaction with the study team. We believe that this is an improvement over previous asthma studies that have relied upon a greater time period of recall between assessments.
A.4. Efforts to Identify Duplication and Use of Similar Information
CDC approached this in two ways: 1) we conducted a thorough literature search on green housing and health effects, and 2) we contacted subject matter experts from many different federal government agencies and private research organizations. In our literature search, we found that many studies had focused on relationships between housing characteristics and asthma, but none had specifically focused on how green housing factors were associated with these outcomes. The subject matter experts confirmed that a comprehensive evaluation of green housing factors and these health outcomes would be a novel and innovative approach to filling research gaps. The list of subject matter experts is listed in section A.8.
A.5. Impact on Small Businesses or Other Small Entities
The collection of this information does not directly impact small businesses or small entities.
A.6. Consequences of Collecting the Information Less Frequently
Some of the environmental and health outcome data are collected repeatedly (e.g., monthly, every 3 months or every 6 months) for several reasons: 1) to address seasonal variation in measurements; 2) to obtain better estimates of average exposure and/ or symptoms; and 3) to minimize recall bias. The technical obstacle to reducing the burden is as follows:
If we do not obtain valid estimates of exposure and health effects, then it will be difficult to accurately attribute any reduction in exposure and improvement in health to specific green practices and/or materials.
There are no legal obstacles to reducing the burden.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
The text of the Federal Register notice for this information collection, published in Federal Register Volume 75, Number 22, on February 3, 2010, is provided in Appendix B. One public comment was received in response to that notice and it is attached as Appendix C. No change occurred in response to this comment because the comment was only a request for the data collection plans which were then provided to the requestor.
B. During the design phase of the this study, CDC’s NCEH Healthy Homes and Lead Poisoning Prevention Branch reviewed published literature on green housing, and asthma and included consultation with researchers from HUD, EPA, other CDC branches (Division of Laboratory Sciences, Air Pollution and Respiratory Health Branch), and academic institutions. We have discussed availability of data and frequency of collection issues with subject matter experts (Table 12).
Table 12. List of experts consulted regarding study design and frequency of data collection
Name |
Title |
Affiliation |
Contact information |
Year of Consultation |
Peter Ashley, DrPH |
Director, Policy and Standards Division
|
U.S. Dept. of Housing and Urban Development |
Phone: 202-402-7595
|
2011 |
Karen Bradham, PhD |
Physical Scientist |
U.S. Environmental Protection Agency |
Phone: 919-541-9414 |
2009 |
Daniel Stout, PhD |
Biological Scientist |
U.S. Environmental Protection Agency |
Phone:919-541-5767 |
2009 |
Warren Friedman, PhD |
Senior Advisor to the Director |
U.S. Dept. of Housing and Urban Development |
Phone: 202-549-7868 |
2009 |
Dana Barr, PhD |
Branch Chief (Pesticide Laboratory) |
CDC/NCEH/DLS* |
Phone: 770-488-7886 |
2009 |
Benjamin Blount, PhD |
Branch Chief (VOC and Perchlorate Laboratory) |
CDC/NCEH/DLS* |
Phone: 770-488-7894 |
2009 |
John (Thomas) Bernert, PhD |
Branch Chief (Tobacco Exposure Biomarkers Section) |
CDC/NCEH/DLS* |
Phone: 770-488-7911 |
2009 |
Fuyuen Yip, PhD |
Team Lead |
CDC/NCEH/APHRB (Air Pollution and Respiratory Health Branch) |
Phone: 770-488-3719 |
2008 |
David Balshaw, PhD |
Project Scientist |
NIH, NIEHS |
Phone: 919-541-2448 |
2010 |
Sung-Roul Kim |
Research Associate |
Johns Hopkins University |
Phone: 011-82-2-380-7685 |
2009 |
Mark Mendell, PhD |
Staff Scientist |
Lawrence Berkeley National Laboratory |
Phone: 510-486-5762 |
2009 |
Brett Singer, PhD |
Staff Scientist |
Lawrence Berkeley National Laboratory |
Phone: 510-486-4779 |
2009 |
Kim Dietrich, PhD |
Professor |
Univ. of Cincinnati |
Phone: 513-558-0531 |
2009 |
Gary Adamkiewicz, PhD |
Research Scientist |
Harvard School of Public Health |
Phone: 617-384-8852 |
2008 |
Wanda Phipatanakul |
Assistant Professor |
Harvard Medical School |
Phone: 617-355-6117 |
2008 |
Robin Whyatt, DrPH |
Professor |
Columbia University |
Phone: 646-459-9609 |
2008 |
Andrew Gelman, PhD |
Professor of Statistics |
Columbia University |
Phone: 212-851-2142 |
2008 |
Elizabeth Matsui, MD |
Associate Professor |
Johns Hopkins University |
Phone: 410-955-5883 |
2010 |
Patrick Breysse, PhD |
Professor |
Johns Hopkins School of Public Health |
Phone: 410-955-3608 |
2010 |
Jeanne Moorman, MS |
Statistician |
CDC/NCEH/APHRB
|
Phone:770-488-3726 |
2011 |
Herman Mitchell, PhD |
Vice President & Senior Research Scientist |
Rho Federal Systems Division |
Phone: 919-408-8000 x 6223 |
2011 |
Lara Akinbami, MD |
Commander, U.S. Public Health Service |
CDC, National Center for Health Statistics |
Phone: 301-458-4306 |
2011 |
* CDC/NCEH/DLS = CDC, National Center for Environmental Health, Division of Laboratory Sciences
A.9 Explanation of Any Payment or Gift to Respondents
Study participants (mothers/ primary caregivers of children enrolled in study) will receive compensation (see Table 13) for their participation in the study and to successfully increase response rates. Many of the low-income families in the proposed cohort use “pay-as-you-go” cell phones. The Green Housing Study team researched several calling card providers and found that they range in costs. For example, one company offers pre-paid plans at 25 cents a minute and another for 60 minutes at $19.99. For this reason, compensation for the text messaging and phone calls will be provided to help defray the costs to the participants.
Table 13. Monetary compensation for study participants
Type of activity |
Time point |
Description of activities/ information/samples collected |
Time |
Amount of money |
Home visit
|
- Baseline
|
Explanation of the study (includes informed consent process), blood sample, urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
60 minutes |
$50
|
- Baseline part 2 |
urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
55 minutes |
$50
|
|
- 6 month follow-up |
urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
55 minutes |
$50 |
|
-12 month follow-up |
urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
55 minutes |
$50 |
|
Phone calls |
- 3 months - 9 months |
questionnaire |
5 minutes 5 minutes |
$2 $2 |
Text messages |
- 1, 2, 4, 5, 7, 8, 10, and 11 months |
Questionnaire. Each month, a series of 3 1-sentence texts will be sent to obtain this information, and the respondents will reply with 3 separate texts. |
1 minute for each month |
$2 each time (maximum = $16) |
* This time indicates the amount of time required for setting up the environmental sampling equipment. Some environmental sampling equipment will be left in home for 5 days, but will not require any supervision.
Each study site will likely have certain rules about how money can be disbursed to the participants. We would like to use a relatively new method which is a pre-paid credit card (e.g., VISA, MasterCard) which can enable the following:
One card can be given to each enrollee’s mother/primary caregiver at the beginning of the study.
The mother/primary caregiver will sign one receipt (at the beginning of the study) which acknowledges that the card will be uploaded with funds automatically (via a study site project coordinator) upon completion of each activity.
If the card is lost or stolen, the mothers/ primary caregivers can call the project coordinator who can cancel the card online. However, any funds that were missing from the lost or stolen card (prior to cancellation) will not be replaced. Only new funds will be added upon completion of each of the remaining study activities listed in the incentive table. The mother/primary caregiver will receive the replacement card at the next home visit.
Rather than using checks or cash, this option will enable immediate payment especially for phone call questionnaires, reduce number of receipts, minimize danger of study staff carrying large sums of money to home visits, improve accounting, eliminate the need for low-income participants to pay check cashing fees, and ensure that the study participant retains our study phone number (which will be written on back of card).
In Table 14, the results of the review of federal national household interview surveys are shown. In these studies, the incentive ranges from $140 to $230. Many of these studies involve medical examinations and blood/urine sampling.
Table 14. Burden, Incentive, and Response Rates in Federal Studies with Multiple Data Collection Formats
Study Name/Agency |
Year |
Study description |
Respondent burden |
Incentive |
Response rate |
Third National Health and Nutrition Examination Survey (NHANES III)/ CDC NCHS |
1988-1994 |
NHANES is designed to collect information about the health and diet of people in the United States to provide current statistical data on the amount, distribution, and effects of illness and disability in the United States. |
In-person interview, medical examination |
$230 (plus exam results) |
Interview=82% Exam=73% |
National Human Exposure Assessment Survey (NHEXAS) Region 5/ EPA |
1995-1997 |
A population-based pilot study of the exposure to metals, pesticides, volatile organic compounds, and other toxic chemicals of ~500 people in 3 US regions. |
Questionnaires, video-taped observations, duplicate diet samples, collection of blood and urine, measurements of air quality and soil and dust in and around the home |
$195 |
Questionnaire = 71.5% Visit 1 = 80% Visit 2 = 56.8% Visit 3 = 47.8% |
Minnesota Children's Pesticide Exposure Study (MNCPES)/ EPA |
1997 |
Study of multi-pathway and multi-pesticide exposures in children. The primary objective was to characterize children's exposure to selected pesticides through a combination of questionnaires, personal exposure measurements and monitoring of biological samples, environmental samples, and children's activity patterns. |
4-day duplicate diet samples, 6-days of personal air monitoring, keeping time and activity diaries, blood, urine and hair collections, videotaping. |
$195 (children given age-appropriate gifts and parents offered videotapes of their children) |
Telephone Screening = 67.5% |
School Health Initiative: Environment, Learning, Disease Study (SHIELD)/ EPA |
1999 |
School-based investigation of children's environmental health in economically disadvantaged urban neighborhoods of Minneapolis. |
Health questionnaires, 48-hour VOC sampling, blood draw, vacuum sampling in home, urine collections, school records review |
$140 (children given age-appropriate gifts) |
Recruitment= 56.7% (interviews/data collections ranged from 76-88%) |
Biologic Specimen-based Study of Dietary Measurement Error/ NCI |
1999 |
This study assessed dietary measurement error by comparing energy and protein intakes from two self-reported dietary data collection instruments (the NCI Diet History Questionnaire and the in-person 24-hour dietary recall interview) with two biomarkers (doubly labeled water and urinary nitrogen excretion) |
Three clinic visits. Dietary History Questionnaire, 24-hour dietary recall, height/weight measurements, physical activity questionnaires, urine collection, Doubly-labeled water dose, 24-hour urine collection |
$200 |
Telephone recruitment=79% Visit=100% (5 and 2 hours) |
A.10 Assurance of Confidentiality Provided to Respondents
Privacy Impact Assessment Information
This submission has been reviewed by ICRO, who determined that the Privacy Act does apply. The applicable System of Records Notice is 09-20-0136, Epidemiologic Studies and Surveillance of Disease Problems. While full names will not be sent to CDC, the contractors will have the capability of maintaining the link between name and study ID number; therefore, the privacy act does apply.
The Green Housing study staff (CDC and contractors) will make every effort to keep the data secure by a variety of methods. Data from paper questionnaires will be entered by the contracted data collectors into a database (e.g. Microsoft Access) which will be password-protected. Dates of birth and home addresses are primary direct identifiers and the contractor’s removal of other direct identifiers (such as name, phone numbers, e-mail addresses) will minimize identification but not completely eliminate it. A unique Study ID will be assigned by the contractor as a key identifier for all study forms. The environmental and biological samples and measurements will only be identified by study ID. The removal of these identifiers will help to minimize, but not completely eliminate, the ability to identify individual participants. Contracted data collectors will maintain their paper files in locked cabinets and their electronic files will be stored on secured servers with password protection. Encrypted data files will be sent electronically to Green Housing Study investigators at CDC. Data will be stored on highly-secured CDC servers in Atlanta, GA. The servers are housed in a secure computer room complete with climate control, emergency power, and an uninterruptible power supply (UPS). Daily back-ups and integrated security are implemented through the CDC computer services infrastructure. All data access is password-protected, and all network communications use encryption. All servers and PCs that are part of the CDC infrastructure are protected by both host-based firewalls and software in order to prevent the undetected installation of "spyware". At CDC, only Green Housing Study investigators will be given access to read the encrypted data files. CDC Green Housing Study investigators will receive electronic files with date of birth, medical information, biological specimens, employment status, and home address, identified by study ID number. While we acknowledge that home address is a unique identifier and the contractors will have the link to names and address, CDC Green Housing Study investigators are taking steps as described above in order to reduce the amount of individually-identifiable data maintained at CDC. If there were a breach of confidentiality for any of the above IIF at CDC, some effect on the respondent’s privacy could occur; however, all health and exposure information from questionnaires will only be identified by study ID. The screening form will be the only form that contains name, home address, phone number, e-mail address, and study ID together; only the contracted data collectors will have this form which will be filed in their locked cabinets and stored in their password-protected database.
After discussions with some housing tenant’s organization members and property managers, flyers (see Appendix H for a prototype of a recruitment flyer) were suggested as the optimal way to describe the study to the residents. Residents who express interest in the study can contact the site projector coordinator by telephone or e-mail. Subsequently, contracted staff (trained by CDC study investigators) will schedule a home visit with the residents. During this home visit, bilingual (English/Spanish or English/Chinese) study staff will describe the study again to the potential study participant. During this home visit, each resident’s eligibility will be assessed (i.e. the Screening Form will be filled out by the aforementioned staff based on responses from the mother/ primary caregiver). If a resident is eligible and is willing to participate, then the individual consent (or assent) form will be reviewed with the study participant in language (English, Spanish, or Chinese) appropriate to participant. If the resident agrees to participate, the consent form will be signed by both the participant and the interviewer obtaining consent. The consent form (Appendix F) describes the purpose of the study, what is expected of the participant during the study, intended uses of the data, study duration, alternatives to participation, data security and data sharing, compensation, and potential risks and benefits of the study. During the consent process, potential subjects are encouraged to ask questions. Participation in the study is voluntary, and withdrawal from the study has no influence on future healthcare. Assent will be obtained from children age 7-12. The assent form (Appendix G) is a simplified version of the consent form that is written at a level that a child (age 7-12) can understand and they are encouraged to ask any questions they might have about the study. The children ages 7-12 will be assenting to providing blood and urine samples for the study; they will not be asked to respond to survey questions—enrollees’ mothers/ primary caregivers will be providing that information. Copies of the consent and/or assent forms will be provided to the study participants. Contracted data collectors will be required to have human subjects training in accordance with their institution’s Institutional Review Board (IRB) and/or the CDC’s IRB. A component of human subjects training addresses data security measures.
During the consent process, CDC-trained interviewers will explain to the residents that participation in the study is voluntary and they may withdraw from the study at any time without negative consequences. The interviewers will also explain the intended uses of the data (i.e., to study how green housing affects respiratory outcomes), with whom information will be shared (i.e., Green Housing Study researchers), and the legal authority for the data collection (i.e., through the Public Health Service Act).
This study was originally approved by the CDC’s IRB (protocol #5587) on March 30, 2009 and then received a continuation on March 26, 2010 (Appendix E).
Data will be treated in a secure manner and will not be disclosed, unless otherwise compelled by law. The Information in Identifiable Form (IIF) collected during the course of the Green Housing Study is listed in section in Table 15. As described earlier Table 9 also describes the IIF, its intended uses, and who will have access to the IIF.
Table 15. Information in Identifiable Form (IIF) and intended uses
IIF category |
Collected by contractors but not sent to CDC |
Collected by contractors and sent to Green Housing Study staff at CDC |
Purpose |
name |
X |
|
Names are required for written informed consent. In addition, names aid both the study participant and the data collector during in-person and telephone questioning. |
date of birth |
|
X |
To determine eligibility and to also adjust for age in statistical analysis. |
phone numbers |
X |
|
To administer phone questionnaires. |
medical information and notes |
|
X |
To assess health outcomes for statistical analysis |
biological specimens |
|
X |
To assess health-related biomarkers for statistical analysis |
e-mail address |
X |
|
To serve as a secondary means of contacting study participants to administer questionnaires and schedule home visits for sampling |
employment status |
|
X |
To adjust for possible chemical exposures that could occur in the occupational environment. |
home address |
|
X |
To enable contractors to visit homes for sampling and also enable CDC to use geographic information systems (GIS) which can be used for adjusting for factors external to the home which could influence both exposures and health outcomes (e.g., outdoor air pollution). |
A.11 Justification for Sensitive Questions
Several questions in the questionnaires ask for information that could be considered sensitive by at least a segment of the general population (Table 16), but variables such as smoking and presence of cockroaches, mice, and rats are specifically geared toward factors that could be related to respiratory health. These items are necessary to assess the relationship between the presence of environmental exposures and the residents’ health (Chew et al. 1998). A copy of the questionnaires can be found in Appendix D (D1-D12). The interviewers are given detailed instructions within each of the questionnaires on how to collect the information, including skip patterns and when to probe for certain questions (e.g., types of inhaled corticosteroid medications typically used by the child with asthma). Interviewers will also be trained to be sensitive to any questions likely to cause discomfort, and the respondent will be informed of her right to refuse to answer any interview question.
Table 16. Questions of a possibly sensitive nature
Questions (possibly sensitive) |
Specific uses of information
|
|
|
Which one or more of the following would you say is your race? |
To adjust for race in statistical models. |
What is the highest level of school that you have completed or the highest degree that you have received? |
To adjust for socioeconomic status in statistical models. |
Which category represents the total combined income of all members of this family during the past 12 months? |
To adjust for socioeconomic status in statistical models. |
Do you smoke cigarettes? |
To adjust for smoking exposure in statistical models. Smoking could affect our environmental and clinical measurements. |
During the past 6 months, how often have you seen cockroaches in your household? |
To assess cockroach exposures pre- and post- interventions. |
During the past 6 months, how often have you seen mice in your household? |
To assess mouse exposures pre- and post- interventions. |
During the past 6 months, how often have you seen rats in your household? |
To assess rat exposures pre- and post- interventions. |
Explanation given to respondents: These questions are needed for this study and some of them have been shown to be associated with environmental exposures and health outcomes, so we need to take them into account.
A.12 Estimates of Annualized Burden Hours and Costs
As discussed in the Background section of this ICR, we hypothesize that children ages 7-12 with asthma who live in green housing, will have improved health outcomes as compared to those who live in comparison housing. Consequently, the respondents that will complete the questionnaires are mothers/ primary caregivers of enrolled children with asthma (ages 7-12 years).
Approximately 1000 adults will complete the screening forms. Kass et al (2009) obtained a screening percentage of 73% in their New York City Housing Authority intervention study. We estimate that after screening, 20% of households will not be eligible.
Two large-scale housing intervention studies in low-income neighborhoods that had a 1-year follow-up have reported response rates of 92-93% (Morgan et al. 2004; Persky et al. 2009). With an anticipated loss to follow-up in our study of 20%, we will recruit 832 households with asthmatic children to end up with 650 enrolled children with asthma (ages 7-12 years) . All health and environmental exposure information about children will be provided by their mothers/ primary caregivers (i.e., no children will fill out questionnaires). For the purposes of assessing potential burden, we are using the maximum of 832 mothers/ primary caregivers who could conceivably fill out the forms. The burden hours for each type of respondent are listed below in Table 17.
Each of the questionnaires was pilot-tested at CDC on nine predominantly college-educated CDC employee-volunteers during non-work hours. The pilot tests were administered by two Green Housing Study researchers. The results of our pilot testing are shown in Part B, Table 25. Based upon pilot testing, the questionnaires were revised to increase ease of understanding and speed of response. We conservatively estimated of the response times for our study participants (low-income mothers/ primary caregivers living in multifamily, urban housing) based on the average response times recorded during our pilot tests.
Table 17. Estimated Annualized Burden Hours
Forms |
Respondents |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response
|
Total |
Screening questionnaire |
Mothers/
primary caregivers |
1000 |
1 |
10/60 |
167 |
Baseline Questionnaire (Home Characteristics) |
Mothers/
primary caregivers |
832 |
1 |
15/60 |
208 |
Baseline Part 2 Questionnaire (Home Characteristics) |
Mothers/
primary caregivers |
832 |
1 |
5/60 |
69 |
Baseline Questionnaire (Demographics) |
Mothers/
primary caregivers |
832 |
1 |
5/60 |
69 |
Baseline Questionnaire (for Children with asthma 7-12 years) |
Mothers/
primary caregivers |
832 |
1 |
15/60 |
208 |
Monthly texts |
Mothers/
primary caregivers |
832 |
8 |
1/60 |
111 |
3 and 9-month Phone contact |
Mothers/
primary caregivers |
832 |
2 |
5/60 |
139 |
6 and 12-month Follow-up Questionnaire (for environment) |
Mothers/
primary caregivers |
832 |
2 |
10/60 |
277 |
6 and 12-month Follow-up Questionnaire (for Children with asthma 7-12 years) |
Mothers/
primary caregivers |
832 |
2 |
10/60 |
277 |
Time/Activity form (for Children with asthma 7-12 years) |
Mothers/
primary caregivers |
832 |
4 |
5/60 |
277 |
Time/Activity form (for mothers/ primary caregivers)
|
Mothers/
primary caregivers |
832 |
4 |
5/60 |
277 |
Illness Checklist |
Mothers/
primary caregivers |
832 |
4 |
5/60 |
277 |
Maximum number of respondents 1000 |
Total estimated burden hours 2,356 |
||||
We assumed earning potential for participants in our study (low-income mothers/ primary caregivers living in multifamily, urban housing) was minimum wage (as of May 11, 2011, the Federal minimum wage was $7.25 per hour (http://www.dol.gov/dol/topic/wages/minimumwage.htm) based on data provide by HUD regarding income of public housing residents (HUD 2009). From December 01, 2008 through March 31, 2010, the average income of residents living in public housing was $13,414 and 72% of the residents reported an income of $15,000 or less. For our study, we selected a conservative estimate of annualized burden cost (i.e., $7.25 per hour for one year of employment = $15,080). Therefore, the true annualized burden could be lower than the estimates in Table 18.
Table 18. Estimated Annualized Burden Costs
Forms |
Respondents |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response
|
Total |
Hourly Wage |
Total Respondent Costs |
Screening questionnaire |
Mothers/
primary caregivers |
1000 |
1 |
10/60 |
167 |
$7.25 |
$1210.75 |
Baseline Questionnaire (Home Characteristics) |
Mothers/
primary caregivers |
832 |
1 |
15/60 |
208 |
$7.25 |
$1508 |
Baseline Part 2 Questionnaire (Home Characteristics) |
Mothers/
primary caregivers |
832 |
1 |
5/60 |
69 |
$7.25 |
$500.25 |
Baseline Questionnaire (for Mother/ primary caregiver) |
Mothers/
primary caregivers |
832 |
1 |
5/60 |
69 |
$7.25 |
$500.25 |
Baseline Questionnaire (for Children with asthma 7-12 years) |
Mothers/
primary caregivers |
832 |
1 |
15/60 |
208 |
$7.25 |
$1508 |
Monthly texts |
Mothers/
primary caregivers |
832 |
8 |
1/60 |
111 |
$7.25 |
$804.75 |
3 and 9-month Phone contact |
Mothers/
primary caregivers |
832 |
2 |
5/60 |
139 |
$7.25 |
$1007.75 |
6 and 12-month Follow-up Questionnaire (for environment) |
Mothers/
primary caregivers |
832 |
2 |
10/60 |
277 |
$7.25 |
$2008.25 |
6 and 12-month Follow-up Questionnaire (for Children with asthma 7-12 years) |
Mothers/
primary caregivers |
832 |
2 |
10/60 |
277 |
$7.25 |
$2008.25 |
Time/Activity form (for Children with asthma 7-12 years) |
Mothers/
primary caregivers |
832 |
4 |
5/60 |
277 |
$7.25 |
$2008.25 |
Time/Activity form (for mothers/ primary caregivers)
|
Mothers/
primary caregivers |
832 |
4 |
5/60 |
277 |
$7.25 |
$2008.25 |
Illness Checklist |
Mothers/
primary caregivers |
832 |
4 |
5/60 |
277 |
$7.25 |
$2008.25 |
Total = |
$17,081.00 |
||||||
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record
Keepers
There is no anticipated cost burden to respondents resulting from the collection of information, except the costs associated with the respondents’ time. Respondents will not be required to incur (a) capital or start-up costs; or (b) operation and maintenance and purchase of services costs. Respondents will not be asked or required to keep any records.
A.14. Annualized Cost to the Government
The Green Housing Study will be conducted by CDC and contractors to be determined (TBD) via Inter-agency agreement (IAA) with HUD (#I-PHI-01062) (i.e., HUD will transfer the funds to CDC). The IAA with HUD is for 5-years, although we acknowledge that we can only apply for OMB approval for a 3-year period. Prior to the expiration of the initial 3-year OMB approval, we will file for a renewal.
The IAA for the 5-year study allots costs of $2,000,000 for subcontracting of the TBD staff, travel, interviewing, supplies, sample collection, laboratory analyses, data analysis, and reporting. The estimated cost for CDC personnel, study coordination, laboratory analysis, data analysis and oversight of the contractor’s work is $1,190,000 over a 5 yr period (Table 19 shows the annual costs). Another Federal Agency, HUD, will devote personnel, data interpretation, and travel, at a cost of $50,000, over the approximate 5-year period. The estimated total cost for the Green Housing Study is approximately $3,240,000, over the 5-year period.
Table 19. Overall Cost Estimate of Proposed Study
Category |
Annual Costs (dollars) |
CDC, including -three staff (GS-13) at 75% effort - travel for site visits |
Total = $238,000 $225,000 $13,000 |
HUD, including one staff (GS-14) and travel to Atlanta’s CDC office |
$10,000 |
TBD contractors, including all staff, travel, interviewing, supplies, sample collection, laboratory analyses, data analysis, and reporting. |
$400,000 |
Total costs |
$648,000 |
A. 15. Explanation for Program Changes or Adjustments
This is a new data collection.
A.16. Plans for Tabulation and Publication and Project Time Schedule
Reports associated with the study will include reports for respiratory outcomes. In addition to those reports, CDC will prepare at least three peer-reviewed journal articles of respiratory outcomes. CDC will also provide technical information and recommendations to various housing programs based on the findings of this study.
The research program will be conducted over a period of 5 years; however OMB clearance is being requested for 3 years. Prior to expiration of OMB clearance, Green Housing Study researchers will submit required documents to OMB in support of a renewal request. Table 20 shows the projected schedule of accomplishments and milestones for the study. Note, items in the table that will occur after the original OMB clearance period are noted with an asterisk; these items are scheduled to occur after the initial 3-year period and therefore will be predicated upon obtaining a renewal for OMB clearance.
Table 20. Project Time Schedule
Activity |
Months after OMB approval |
Select at least two study sites (with help of HUD) |
1 |
Subcontract the collection of data to the local study sites.
|
1 |
Train study staff from each site to collect environmental, survey, and clinical data |
1 |
Data collection |
2 |
Subcontract with laboratories to assay environmental samples and biomarkers collected during the study.
|
2 |
Summary of laboratory results from subcontracted institutions |
6, 12, 24, 36, 48*, 60* |
Summary of survey results from study sites |
6, 12, 24, 36, 48*, 60* |
Conduct statistical analysis
|
6, 12, 18, 24, 30, 36, 42*, 48*, 52*,60* |
Forms used for reporting study results back to participants and community |
6, 12, 60* |
Quarterly reporting: Provide draft quarterly reports within 21 days after the end of the quarter, which HUD shall review and comments within 10 days after receipt; and provide the quarterly report, within 7 days after receipt of HUD comments |
4,7,10,13,16,19,22,25,28,31,34, 37*,40*,43*,46*,49*, 52*, 55*, 58* |
Submit articles for peer review in journals |
12, 24, 36, 60* |
Final: Provide draft quarterly reports within 90 days after the end of the study, which HUD shall review and comments within 30 days after receipt; and provide the final report, within 21 days after receipt of HUD comments |
60* |
* Asterisked items are included here for completeness since much of the data analysis and dissemination of study findings will occur after the initial 3-year OMB approval timeframe.
The analysis plan includes the following: 1) descriptive statistics to show prevalence of environmental exposures and health outcomes (i.e., asthma morbidity) and 2) logistic and linear regressions to examine associations between environmental exposures such as indoor allergens, mold, pesticides, and VOCs and health outcomes. Detailed statistical analyses are described in section B.
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
The selection of study sites across the country will occur on a rolling basis over the course of the study. At each study site, contracted data collectors will collect data using CDC’s OMB-approved questionnaires. It is conceivable that data collection at one or more study sites will start or be continued from one OMB approval to the next. Consequently, to avoid the necessity of reprinting forms (with the new OMB expiration date), and thereby wasting paper, we request that the expiration date not be printed on the questionnaires.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
| File Type | application/msword |
| File Title | INFORMATION COLLECTION REQUEST FOR OMB REVIEW |
| Subject | AHHS draft ICR |
| Author | Other |
| Last Modified By | bbarker |
| File Modified | 2011-11-09 |
| File Created | 2011-11-09 |
© 2026 OMB.report | Privacy Policy