CHATS Attachment E_revised_02272012
CHATS Attachment E_revised_02272012.docx
Children's Health after the Storms (CHATS)
CHATS Attachment E_revised_02272012
OMB: 0920-0925
ATTACHMENT E
ATTACHMENT E1
RTI IRB Approval


ATTACHMENT E2
CDC IRB Approval - Site Restriction

Memorandum
Date
d
July 29, 2011
From
Betty Wong, MPH, CHES
IRB C Administrator, Human Research Protection Office
Subject
Site Restricted: CDC IRB Approval of New Protocol #6115, "The Children’s Health after the Storms (CHATS)" (Expedited)
To
Fuyuen Yip, PhD, MPH
NCEH/EHHE
CDC’s IRB C has reviewed the request for approval of new protocol #6115, “The Children’s Health after the Storms (CHATS),” and has approved the protocol for the maximum allowable period of one year. CDC IRB approval will expire on 07/28/2012. The protocol was reviewed in accordance with the expedited review process outlined in 45 CFR 46.110(b)(1), categories 2-5 and 7. The IRB determined that the study poses no greater than minimal risk to subjects.
Collaborator Site Restriction: Study activities may not begin with the following collaborator/site until documentation indicating current IRB approval has been received by CDC’s Human Research Protection Office (HRPO) and the PI has been notified by HRPO that this restriction has been lifted:
Louisiana State University Health Sciences Center
If other institutions involved in this protocol are being awarded CDC funds through the CDC Procurement and Grants Office (PGO), you are required to send a copy of this IRB approval to the CDC PGO award specialist handling the award. You are also required to verify with the award specialist that the awardee has provided PGO with the required documentation and has approval to begin or continue research involving human subjects as described in this protocol.
As a reminder, the IRB must review and approve all human subjects’ research protocols at intervals appropriate to the degree of risk, but not less than once per year. There is no grace period beyond one year from the last IRB approval date. It is ultimately your responsibility to submit your research protocol for continuation review and approval by the IRB along with available IRB approvals from all collaborators. Please keep this approval in your protocol file as proof of IRB approval and as a reminder of the expiration date. To avoid lapses in approval of your research and the possible suspension of subject enrollment and/or termination of the protocol, please submit your continuation request along with all completed supporting documentation at least six weeks before the protocol's expiration date of 07/28/2012.
Any problems of a serious nature must be brought to the immediate attention of the CDC IRB, and any proposed changes to the protocol should be submitted as an amendment to the protocol for CDC IRB approval before they are implemented.
If you have any questions, please contact your National Center Human Subjects Contact or the CDC Human Research Protection Office (404) 639-4721 or e-mail: [email protected].
cc:
NCEH/ATSDR Human Subjects
Betsey Dunaway
Marlena Wald
ATTACHMENT E3
LSU IRB Approval
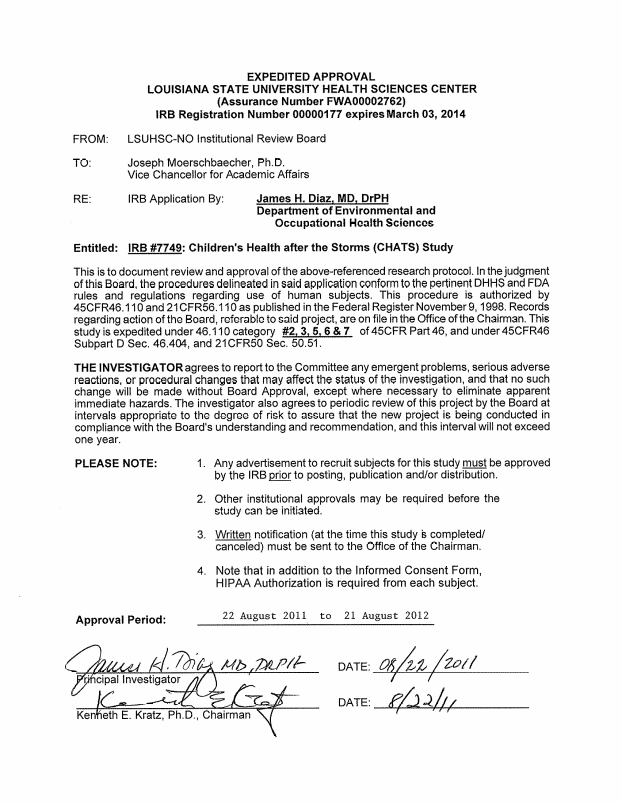 ATTACHMENT
E4
ATTACHMENT
E4
CDC IRB Approval Site Restriction Lifted

M emorandum
emorandum
J
Date
d
From
Betty Wong, MPH, CHES
IRB C Administrator, Human Research Protection Office
Subject
CDC IRB Approval of New Protocol #6115, "The Children’s Health after the Storms (CHATS)" (Expedited)
To
Fuyuen Yip, PhD, MPH
NCEH/EHHE
CDC’s IRB C has reviewed the request for approval of new protocol #6115, “The Children’s Health after the Storms (CHATS),” and has approved the protocol for the maximum allowable period of one year. CDC IRB approval will expire on 07/28/2012. The protocol was reviewed in accordance with the expedited review process outlined in 45 CFR 46.110(b)(1), categories 2-5 and 7. The IRB determined that the study poses no greater than minimal risk to subjects.
If other institutions involved in this protocol are being awarded CDC funds through the CDC Procurement and Grants Office (PGO), you are required to send a copy of this IRB approval to the CDC PGO award specialist handling the award. You are also required to verify with the award specialist that the awardee has provided PGO with the required documentation and has approval to begin or continue research involving human subjects as described in this protocol.
As a reminder, the IRB must review and approve all human subjects’ research protocols at intervals appropriate to the degree of risk, but not less than once per year. There is no grace period beyond one year from the last IRB approval date. It is ultimately your responsibility to submit your research protocol for continuation review and approval by the IRB along with available IRB approvals from all collaborators. Please keep this approval in your protocol file as proof of IRB approval and as a reminder of the expiration date. To avoid lapses in approval of your research and the possible suspension of subject enrollment and/or termination of the protocol, please submit your continuation request along with all completed supporting documentation at least six weeks before the protocol's expiration date of 07/28/2012.
Any problems of a serious nature must be brought to the immediate attention of the CDC IRB, and any proposed changes to the protocol should be submitted as an amendment to the protocol for CDC IRB approval before they are implemented.
If you have any questions, please contact your National Center Human Subjects’ Contact or the CDC Office of Scientific Integrity (404) 639-7570 or e-mail: [email protected].
cc:
NCEH/ATSDR Human Subjects
Betsey Dunaway
Marlena Wald
ATTACHMENT E5
RTI IRB Approval of Amendment Addressing CDC IRB Changes
 ATTACHMENT
E6
ATTACHMENT
E6
RTI IRB Continuing Notice Approval

IRB
Approval Notice E-
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Kim Adcock |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy