LOI2-BIO-19 - Integration of salivary analytes into the NCS: Evaluation of Feasibility, Efficiency, and Benefits
Biospecimen and Physical Measurements Formative Research Methodology Studies for the National Children?s Study (NICHD)
Attach S3 Goal 2_Saliva Collection Diagrams
LOI2-BIO-19 - Integration of salivary analytes into the NCS: Evaluation of Feasibility, Efficiency, and Benefits
OMB: 0925-0647
Attachment S3 Goal II Saliva Collection Instructions OMB #: 0925-0647
Expiration Date: 01/31/2015
SALIVA COLLECTION INSTRUCTIONS - MOTHER
Step 1: Record time |
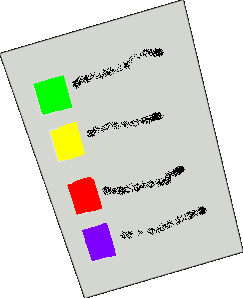
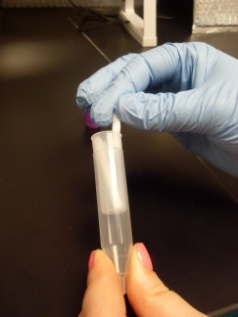
Step 2: Select tube |
||||
|
Record time on sample collection sheet
|
|
Match tube color with label on collection sheet |
||
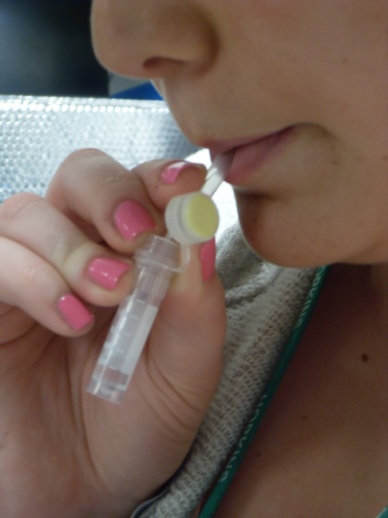
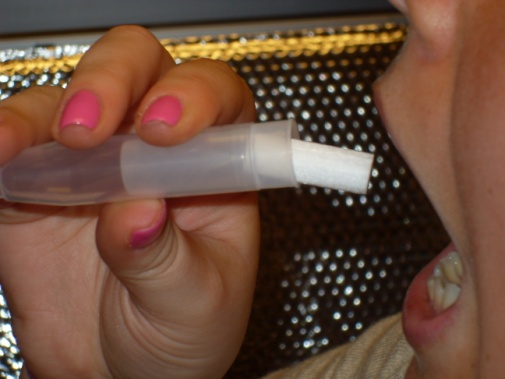
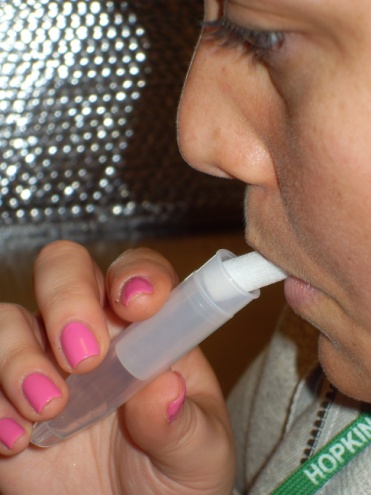
Step 3: Mother saliva collection (straw OR swab method) |
|||||
|
Straw method
1) Insert straw in mouth
2) Allow saliva to collect in tube (1mL)
3) Cap the tube, discard straw |
|
Swab method
1) Insert swab under tongue, close mouth
2) Hold for 2 minutes until saturated
3) Place swab in tube and cap the tube |
||
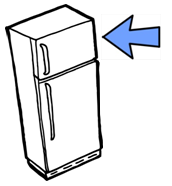
Step 4: Freeze sample |
Good Job! |
||||
|
Immediately place sample in freezer |
|
|||
SALIVA COLLECTION INSTRUCTIONS – INFANT/CHILD
Step 1: Record time |
Step 2: Select tube |
||||
|
Record time on sample collection sheet
|
|
Match tube color with label on collection sheet |
||
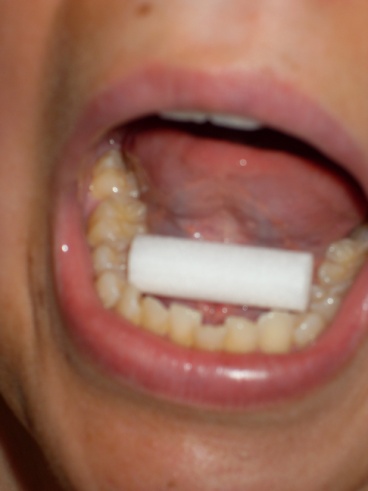
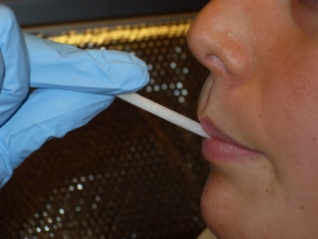
Step 4: Infant/Child saliva collection |
|||||
|
1) Firmly grasp one end of
swab with gloved hand 2) Place other end of swab under child’s tongue for 2 minutes
Do not let go of swab!
|
|
3) Switch sides of swab and repeat process
4) Fold swab
5) Place folded swab in tube and cap the tube
6) Discard glove |
||
Step 6: Freeze sample |
Good Job! |
||||
|
Immediately place sample in freezer |
|
|||
Public reporting burden for this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to: NIH, Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-0647*). Do not return the completed form to this address.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Jessica Bayer |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy