Form 659 2013 659 FDA Mobile
American Customer Satisfaction Index (ACSI) E-Government Website Customer Satisfaction Surveys
2013 659 FDA Mobile - Final to OMB.xlsx
2013 659 FDA Mobile - 2013 686 VA Medical Centers
OMB: 1090-0008
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 1090-0008 can be found here:
Document [xlsx]
Download: xlsx | pdf
Current Model Qsts
Current CQs
Element Rotations

Overview
Welcome and Thank You TextCurrent Model Qsts
Current CQs
Element Rotations
Sheet 1: Welcome and Thank You Text
| Model Instance Name: | 
|
||||||||||
| FDA Mobile | |||||||||||
| MID: | TBD | ||||||||||
| Date: | 8/6/2013 | ||||||||||
| Welcome and Thank You Text | |||||||||||
| Directions: | |||||||||||
| This welcome text is shown at the top of the questionnaire window and the thank you text at the bottom. This is a good place to mention the site/company/agency name so the visitor knows whom they are taking the survey for. Feel free to modify the standard Welcome and Thank you text shown in the boxes below. Please read comments before using any of the text. | |||||||||||
| Examples | |||||||||||
| Welcome Text Example | |||||||||||
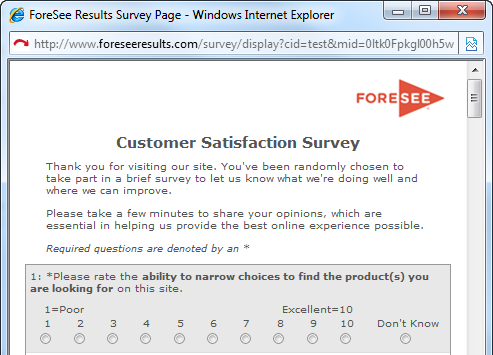
|
|||||||||||
| Welcome Text | |||||||||||
| Thank you for visiting our site on your mobile device. You've been randomly chosen to take part in a brief survey to let us know what we're doing well and where we can improve. Please take a few minutes to share your opinions, which are essential in helping us provide the best online experience possible. |
|||||||||||
| Thank You Text Example | |||||||||||
| DEFAULT Thank You Text | 
|
||||||||||
| Thank you for taking our survey and for helping us serve you better. We appreciate your input. | |||||||||||
Sheet 2: Current Model Qsts
| Model Instance Name: | |||||||||
| FDA Mobile | 
|
||||||||
| MID: | TBD | ||||||||
| Partitioned (Y/N)? | N | Element rotation scheme has been added | |||||||
| FPI Included(Y/N)? | N | ||||||||
| NOTE: All non-partitioned surveys will NOT be imputed and the elements will be rotated as a default unless otherwise specified and approved by Research. | |||||||||
| Date: | 8/6/2013 | ||||||||
| FDA Mobile | |||||||||
| Model questions utilize the ACSI methodology to determine scores and impacts | |||||||||
| ELEMENTS (drivers of satisfaction) | CUSTOMER SATISFACTION | FUTURE BEHAVIORS | FPI | ||||||
| MQ Label | MQ Label | MQ Label | Y? | ||||||
| Content (1=Poor, 10=Excellent, Don't Know) | Satisfaction | Return (1=Very Unlikely, 10=Very Likely) | N | ||||||
| Content - Accuracy | Please rate the accuracy of information on this mobile site. | Satisfaction - Overall | What is your overall satisfaction with this mobile site? (1=Very Dissatisfied, 10=Very Satisfied) |
Return to Mobile Site | How likely are you to return to this mobile site? | ||||
| Content - Quality | Please rate the quality of information on this mobile site. | Satisfaction - Expectations | How well does this mobile site meet your expectations? (1=Falls Short, 10=Exceeds) |
Recommend (1=Very Unlikely, 10=Very Likely) | N | ||||
| Functionality (1=Poor, 10=Excellent, Don't Know) | Satisfaction - Ideal | How does this site compare to your idea of an ideal mobile website? (1=Not Very Close, 10=Very Close) |
Recommend Mobile Site | How likely are you to recommend this mobile site to someone else? | |||||
| Functionality - Usefulness | Please rate the usefulness of the features (main site wide search tool, search by section, etc.) provided on this mobile site. | ||||||||
| Functionality - Variety | Please rate the variety of features (main site wide search tool, search by section, etc.) on this mobile site. | ||||||||
| Look and Feel (1=Poor, 10=Excellent, Don't Know) | |||||||||
| Look and Feel - Appeal | Please rate the visual appeal of this mobile site. | ||||||||
| Look and Feel - Readability | Please rate the readability of the pages on this mobile site. | ||||||||
| Navigation (1=Poor, 10=Excellent, Don't Know) | |||||||||
| Navigation - Organized | Please rate how well this mobile site is organized. | ||||||||
| Navigation - Options | Please rate the options available for navigating this mobile site. | ||||||||
| Site Performance (1=Poor, 10=Excellent, Don't Know) | |||||||||
| Site Performance - Loading | Please rate how quickly pages load on this mobile site. | 
|
|||||||
| Site Performance - Errors | Please rate the ability to load pages without getting error messages on this mobile site. | ||||||||

Sheet 3: Current CQs
| Model Instance Name: | ||||||||||||
| FDA Mobile | underlined & italicized: RE-ORDER | |||||||||||
| MID: TBD | pink: ADDITION | |||||||||||
| Date: | 8/6/2013 | blue + -->: REWORDING | ||||||||||
| FDA Mobile CUSTOM QUESTION LIST | ||||||||||||
| QID | Skip Logic Label | Question Text | Answer Choices (limited to 50 characters) |
Skip to | Type (select from list) | Single or Multi | Required Y/N |
Special Instructions | CQ Label | |||
| How frequently do you visit the FDA site on your mobile device? | This is my first time | Radio button, one-up vertical | Single | Y | Visit Frequency | |||||||
| Daily | ||||||||||||
| Weekly | ||||||||||||
| Monthly | ||||||||||||
| A couple times a year | ||||||||||||
| About once a year | ||||||||||||
| Which of the following categories best describes the information you were looking for? (Please select all that apply). | Food | Checkbox, one-up vertical | Multi | Y | OPS Group* | Topic | ||||||
| Drugs | ||||||||||||
| Medical Devices | ||||||||||||
| Radiation-Emitting Products | ||||||||||||
| Vaccines, Blood & Biologics | ||||||||||||
| Animal & Veterinary | ||||||||||||
| Cosmetics | ||||||||||||
| Tobacco Products | ||||||||||||
| Other, please specify: | A | Mutually Exclusive | ||||||||||
| A | Please describe the topic you were looking for: | Text area, no char limit | Single | N | OPS Group* | OE_Topic | ||||||
| Did you find the information you were looking for today? | Yes | Radio button, one-up vertical | Single | Y | Ability to Find | |||||||
| No | A | |||||||||||
| A | What were you unable to find on the FDA site today? | Text area, no char limit | Single | Y | OE_Ability to Find | |||||||
| For this visit to the FDA site, which of the following roles best describes you? | Regulated industry | Text area, no char limit | Single | Y | Role | |||||||
| Consumer | ||||||||||||
| Scientist, researcher | ||||||||||||
| Patient | ||||||||||||
| Caregiver, friend, family member of a person interested in health issues | ||||||||||||
| Physician | ||||||||||||
| Nurse, physician's assistant, nurse practitioner | ||||||||||||
| Pharmacist | ||||||||||||
| Other type of healthcare provider | ||||||||||||
| State or local public health professional | ||||||||||||
| Not-for-profit public health professional | ||||||||||||
| Consultant | ||||||||||||
| Attorney/Legal Counsel | ||||||||||||
| Educator, professor, teacher | ||||||||||||
| Student | ||||||||||||
| Journalist/Media | ||||||||||||
| Policymaker, legislator, staff | ||||||||||||
| FDA grantee | ||||||||||||
| FDA employee | ||||||||||||
| First responder | ||||||||||||
| Other, please specify: | A | |||||||||||
| A | Please describe your role: | Text area, no char limit | Single | N | OPS Group* | OE_Role | ||||||
| From what location are you accessing the FDA site on your mobile device: | I'm at home | Radio button, one-up vertical | Single | Y | Visit Location | |||||||
| I'm at work | ||||||||||||
| I'm at school | ||||||||||||
| Other | ||||||||||||
| Please provide one suggestion on how the FDA can improve this site: | Text area, no char limit | Single | Y | OE_One Improvement | ||||||||
Sheet 4: Element Rotations
| Base Element Order | Version 2 | Version 3 | Version 4 | Version 5 |
| Content | Site Performance | Navigation | Functionality | Look and Feel |
| Functionality | Navigation | Content | Site Performance | Navigation |
| Look and Feel | Look and Feel | Site Performance | Look and Feel | Content |
| Navigation | Functionality | Functionality | Content | Site Performance |
| Site Performance | Content | Look and Feel | Navigation | Functionality |
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy