Form 6A Completing PHS Fellowship Specific Components
PHS Applications and Pre-award Related Reporting (OD)
Attachment 6A PHS 398 Fellowship Supplemental Form Instructions
PHS Fellowship Supplemental Form (electronic)
OMB: 0925-0001
PHS SF424 (R&R) Individual Fellowship Application Guide
5. Completing PHS Fellowship Specific Components
5.1 Overview
In conjunction with the SF424 (R&R) components, NIH and AHRQ Fellowship applicants should also complete and submit additional components in the “PHS Fellowship Supplemental Form.” Note the PHS Fellowship components include additional data required by the agency for a complete application. While these are not identical to the PHS Fellowship application form pages, the PHS Fellowship reference is used to distinguish these additional data requirements from the data collected in the SF424 (R&R) components. A complete application to NIH and AHRQ will include SF424 (R&R) and PHS Fellowship components.
5.2 (Reserved)
5.3 PHS Fellowship Supplemental Form
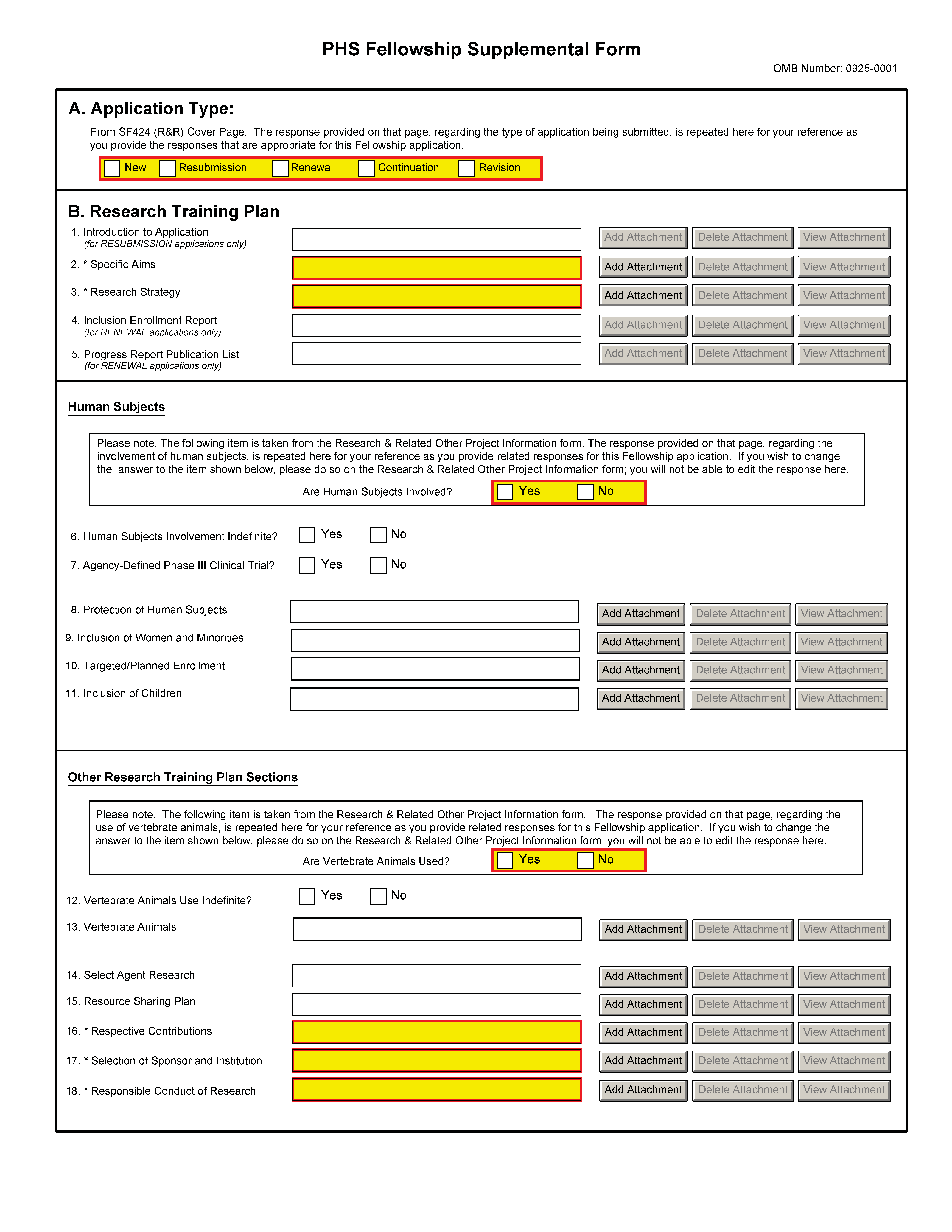
It is strongly recommended that fellowship applicants and sponsors speak with a PHS Program Official for Institute or Center (IC) specific guidance before preparing this application. A list of contacts specifically for extramural training at the NIH ICs can also be found at: http://grants.nih.gov/training/ tac_training_contacts.doc. For AHRQ, see http://www.ahrq.gov/fund/training/trgstaff.htm. Individuals always are encouraged to check these Web sites for the most current contact information.
Note: Required fields on the PHS Fellowship Supplemental Form are noted with an asterisk(*).
Field Name |
Instructions |
A. Application Type |
This field is pre-populated from the SF424 (R&R) Cover Component. Corrections to this field must be made in that component. |
B. Research Training Plan |
The Research Training Plan should include sufficient information needed for evaluation of the project, independent of any other document (e.g., previous application). Be specific and informative, and avoid redundancies. This section should be well-formulated and presented in sufficient detail that it can be evaluated for both its research training potential and scientific merit. It is important that it be developed in collaboration with your sponsor, but it should be written by you, the fellowship applicant. Research Training Plan Attachments (Also, see Section 2.3.2 - Creating PDFs for Text Attachments and Section 2.6 - Format Specifications for Text (PDF) Attachments of the SF424(R&R) Application Guide) Although many of the sections of this application are separate PDF attachments, page limitations referenced in the instructions and/or funding opportunity announcement must still be followed. Agency validations will include checks for page limits (and use of appropriate font). Some accommodation will be made for sections that, when combined, must fit within a specified limitation. NIH and other PHS agencies require all text attachments to the SF424(R&R) application forms to be submitted as PDF files. Text attachments should be generated using word processing software and then converted to PDF using PDF generating software. Avoid scanning text attachments to convert to PDF since that causes problems for the agency handling the application. Scanning paper documents, without the proper Optical Character Recognition (OCR) process, will hamper automated processing of your application for NIH analysis and reporting. Do not include any information in a header or footer of the attachments. A header will be system-generated that references the name of the PD/PI. Page numbers for the footer will be system-generated in the complete application, with all pages sequentially numbered. Since a number of reviewers will be reviewing applications as an electronic document and not a paper version, fellowship applicants are strongly encouraged to use only a standard, single-column format for the text. Avoid using a two-column format since it can cause difficulties when reviewing the document electronically. Full-sized glossy photographs of material such as electron micrographs or gels must only be included within the page limitations of the Research Strategy section. The maximum size of images to be included should be approximately 1200 x 1500 pixels using 256 colors. Figures must be readable as printed on an 8.5 x 11 inch page at normal (100%) scale. Investigators must use image compression such as JPEG or PMG. Do not include figures or photographs as separate attachments either in the Appendix or elsewhere in the application. Separate Attachments Separate attachments have been designed for the Research Training Plan sections to maximize automatic validations conducted by the eRA system. When the application is received by the agency, all of the Research Training Plan sections will be placed in the appropriate order so that reviewers and agency staff will see a single cohesive Research Training Plan. When attaching a PDF document to the actual forms, please note you are attaching an actual document, not just pointing to the location of an externally stored document. Therefore, if you revise the document after it has been attached, you must delete the previous attachment and then reattach the revised document to the application form. Use the “View Attachment” button to determine if the correct version has been attached. Page Limitations All applications for funding must be self-contained within specified page limitations. Agency validations will include checks for page limits. Note that while these computer validations will help minimize incomplete and/or non-compliant applications, they do not replace the validations conducted by Agency staff. Applications found not to comply with the requirements may be delayed in the review process. Applicants are prohibited from using the Appendix to circumvent page limitations in any section of the application for which a page limit applies. For additional information regarding Appendix material and page limits, please refer to the NIH Guide Notice NOT-OD-11-080, http://grants.nih.gov/grants/guide/notice-files/NOT-OD-11-080.html. The following page limits apply to fellowship applicants only, unless specified otherwise in the FOA. All page limits include all tables, graphs, figures, diagrams and charts.
Be succinct and remember that there is no requirement to use all six pages allotted to the Research Strategy. Note that the Research Training Plan PDF may include graphic images of gels, micrographs, photographs, etc,; however these images may not be included in the Appendix (except when part of a qualifying publication). Unless otherwise specified in a PHS solicitation, Internet Web site addresses (URLs) may not be used to provide information necessary to the review, except for reference citations, because reviewers are under no obligation to view the Internet sites. Moreover, reviewers are cautioned that they should not directly access an internet site as it could compromise their anonymity. Note: Begin each text section of the Research Training Plan with a section header (e.g., Introduction, Specific Aims, Research Strategy, etc.). Research Training Plan of Resubmission Applications A resubmission application must include substantial changes. If the summary statement cites weaknesses specifically to the Research Training Plan, identify these changes in the resubmitted Research Training Plan clearly by bracketing, indenting, or changing typography, unless the changes are so extensive as to include most of the text. This exception should be explained in the Introduction. Do not underline or shade changes. Application processing may be delayed or the application may be returned if it does not comply with all of these requirements. Include sufficient information to permit an effective review without reviewers having to refer to any previous application. |
1. Introduction to Application (Resubmission Applications Only) |
The Introduction (resubmissions only) is limited to one page unless specified in the FOA. Attach for all resubmission applications an Introduction of no more than one page that summarizes the substantial additions, deletions, and changes. The Introduction must also include responses to criticisms and issues raised in the summary statement for the previous application. See specific instructions in Part I Section 2.7, Resubmission Applications, on the content of the Introduction. First time (new) applications should not include an Introduction unless specified in the FOA. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
2. Specific Aims |
Specific Aims are limited to one page. State concisely the goals of the proposed research and summarize the expected outcome(s), including the impact that the results of the proposed research will exert on the research field(s) involved. List succinctly the specific objectives of the research proposed, e.g., to test a stated hypothesis, create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice, address a critical barrier to progress in the field, or develop new technology. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open.
|
3. Research Strategy |
This item is limited to six pages. Organize the Research Strategy in the specified order using the instructions provided below. Start each section with the appropriate section heading — Significance, Innovation, Approach. Cite published experimental details in the Research Strategy section and provide the full reference in the Bibliography and References Cited section (Part I Section 4.4.9). Follow the page limits for the Research Strategy in the table of page limits (Table 2.6-1), unless specified otherwise in the FOA. Note that the page limit for this attachment will be validated as a single file. (a) Significance
(b) Innovation - Fellowship applications should not include an Innovation section unless specified in the FOA. (c) Approach
If an applicant has multiple Specific Aims, then the applicant may address Significance, Innovation and Approach for each Specific Aim individually, or may address Significance, Innovation and Approach for all of the Specific Aims collectively. As applicable, also include the following information as part of the Research Strategy, keeping within the three sections listed above: Significance, Innovation, and Approach. Preliminary Studies for New Applications. For new applications, include information on preliminary studies, if any. Discuss the applicant's preliminary studies, data and/or experience pertinent to this application. When applicable, provide a succinct account of published and unpublished results, indicating progress toward their achievement. Progress Report for Renewal Applications. Renewal applications for individual fellowships are rare. You should consult with your program official before preparing such an application. In the rare instance that you are submitting a renewal application, provide a Progress Report. Provide the beginning and ending dates for the period covered since the last competitive review. Summarize the specific aims of the previous project period and the importance of the findings, and emphasize the progress made toward their achievement. Explain any significant changes to the specific aims and any new directions including changes resulting from significant budget reductions. A list of publications, manuscripts accepted for publication, patents, and other printed materials should be included in Section 5; do not include that information here. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
4. Inclusion Enrollment Report (for RENEWAL applications only) |
In the rare instance that you are submitting a renewal application, and it involves clinical research, then you must report on the enrollment of research subjects and their distribution by ethnicity/race and sex/gender. See Part II, Section 4.3 for more detailed instructions on which Target and Enrollment Report or Table to use. (Not part of the page limitations of the Research Training Plan.) Note for applications using the Adobe B (not B-1) package only: If submitting a resubmission of a renewal, this upload will not be available. Instead, the Inclusion Enrollment Report required in the PHS Fellowship Supplemental Form component (Item 4) should be saved as an individual pdf file titled “Inclusion_Enrollment_Report.pdf” and attached in Item 12 (Other Attachments) of the SF424 (R&R) Other Project Information component. |
5. Progress Report Publication List (for RENEWAL applications only) |
In the rare instance that you are submitting a renewal application, list the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, and other printed materials that have resulted from the project since it was last reviewed competitively. For NIH applications only, when citing articles that fall under the NIH Public Access Policy, http://publicaccess.nih.gov/, were authored or co-authored by the fellowship applicant and arose from NIH support, provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for each article. If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate “PMC Journal – In Process.” A list of these journals is posted at: http://publicaccess.nih.gov/submit_process_journals.htm. Citations that are not covered by the NIH Public Access Policy, but are publicly available in a free, online format may include URLs or PMCID numbers along with the full reference (note that copies of these publications are not accepted as appendix material, see F. Appendix below). Note for applications using the Adobe B (not B-1) package only: If submitting a resubmission of a renewal, this upload will not be available. Instead, the Progress Report Publication List (Item 5) should be saved as an individual pdf file titled “Progress_Report_Publication_List.pdf” and attached in Item 12 (Other Attachments) of the SF424 (R&R) Other Project Information component. |
Human Subjects |
Prefilled from the Research and Related Other Project Information form. If activities involving human subjects are not planned at any time during the proposed project at any performance site, skip the remainder of the block and continue to Other Research Training Plan Sections. If you have indicated “Yes” for Human Subjects involvement, consult with your Sponsor and Administrative Officials at the Sponsoring Institution before completing this section, and refer to Part II Supplemental Instructions for Preparing the Human Subjects Section of the Research Training Plan. Human subjects requirements may apply even if you are obtaining specimens/data from collaborators or if you are subcontracting the human research to another organization. For all research involving human subjects, a part of the peer review process will include careful consideration of protections from research risks, as well as the appropriate inclusion of women, minorities, and children. The Scientific Review Group (SRG) will assess the adequacy of safeguards of the rights and welfare of research participants, and the appropriate inclusion of women, minorities, and children, based on the information in the application. The evaluation of the inclusion plans will be factored into the overall score that the SRGs award for scientific and technical merit of the application. Much of the information on the protection of human subjects that you are required to provide in the Fellowship application is identical to information that you will be required to provide for IRB review at your own institution. Do not use the protection of human subjects section to circumvent the page limits of the Research Strategy. |
6. Human Subjects Involvement Indefinite? |
Check “Yes” if at the time of application plans to involve human subjects are unknown. If an award is made, the fellow may not participate in human subjects research until an updated research training plan is submitted and approved by the awarding component. Such a plan must be developed in consultation with the sponsor. Certification of the date of IRB approval must also be submitted before the fellow can participate in human subjects research. |
7. Agency-Defined Phase III Clinical Trial? |
Check the “Yes” or “No” box to indicate whether the project is an NIH-defined Phase III clinical trial. An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of either evaluating an experimental intervention in comparison with a standard or control intervention or of comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included. |
8. Protection of Human Subjects |
This section is required for applicants answering “yes” to the question “Are human subjects involved?” on the R&R Other Project Information form. If the answer is “No” to the question but the proposed research involves human specimens and/or data from subjects applicants must provide a justification in this section for the claim that no human subjects are involved. Do not use the protection of human subjects section to circumvent the page limits of the Research Strategy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
9. Inclusion of Women and Minorities |
Refer to Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan. This section is required for applicants answering “yes” to the question “Are human subjects involved?” on the R&R Other Project Information form and the research does not fall under Exemption 4. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
10. Targeted/ Planned Enrollment (Clinical Research Only) |
If this application involves the Inclusion of Women and Minorities, complete the Targeted/Planned Enrollment Table for each protocol; see Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan, Section 4.3. For applicants answering “Yes” to the question “Are human subjects involved?” on the R&R Other Project Information Form and the research does not fall under Exemption 4, complete the Targeted/Planned Enrollment Table for each protocol. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
11. Inclusion of Children |
Refer to Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan, Sections 4.4 and 5.7. For applicants answering “Yes” to the question “Are human subjects involved” on the R&R Other Project Information Form and the research does not fall under Section 4, this section is required . Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
Other Research Training Plan Sections |
Consult with your Sponsor and Administrative Officials at the Sponsoring Institution before completing Items 13 through 17 and 19. |
Are Vertebrate Animals Used? |
Prefilled from the Research and Other Project Information form. If activities involving vertebrate animals are not planned at any time during the proposed project at any performance site, indicate no and skip items #13 and #14. |
12. Vertebrate Animals Use Indefinite? |
If the sponsoring institution has an approved Animal Welfare Assurance on file with the NIH Office of Laboratory Animal Welfare (OLAW) but, at the time of application, plans for the involvement of vertebrate animals are so indefinite that IACUC review and approval are not feasible, check "Yes." If an award is made, vertebrate animals may not be involved until a verification of the date of IACUC approval has been submitted to the NIH IC or AHRQ. |
13. Vertebrate Animals |
If Vertebrate Animals are involved in the project, address each of the five points below. This section should be a concise, complete description of the animals and proposed procedures. While additional details may be included in the Research Strategy, the responses to the five required points below must be cohesive and include sufficient detail to allow evaluation by peer reviewers and NIH staff. If all or part of the proposed research involving vertebrate animals will take place at alternate sites (such as project/performance or collaborating site(s)), identify those sites and describe the activities at those locations. Although no specific page limitation applies to this section of the application, be succinct. Failure to address the following five points will result in the application being designated as incomplete and will be grounds for the PHS to defer the application from the peer review round. Alternatively, the application’s impact/priority score may be negatively affected. If the involvement of animals is indefinite, provide an explanation and indicate when it is anticipated that animals will be used. If an award is made, prior to the involvement of animals the grantee must submit to the NIH awarding office detailed information as required in points 1-5 above and verification of IACUC approval. If the grantee does not have an Animal Welfare Assurance then an appropriate Assurance will be required (See Part III, Section 2.2 Vertebrate Animals for more information). The five points are as follows: 1. Provide a detailed description of the proposed use of the animals in the work outlined in the “Research Strategy” section. Identify the species, strains, ages, sex, and numbers of animals to be used in the proposed work. 2. Justify the use of animals, the choice of species, and the numbers to be used. If animals are in short supply, costly, or to be used in large numbers, provide an additional rationale for their selection and numbers. 3. Provide information on the veterinary care of the animals involved. 4. Describe the procedures for ensuring that discomfort, distress, pain, and injury will be limited to that which is unavoidable in the conduct of scientifically sound research. Describe the use of analgesic, anesthetic, and tranquilizing drugs and/or comfortable restraining devices, where appropriate, to minimize discomfort, distress, pain, and injury. 5. Describe any method of euthanasia to be used and the reasons for its selection. State whether this method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association (AVMA) Guidelines on Euthanasia. If not, present a justification for not following the recommendations. Do not use the vertebrate animal section to circumvent the page limits of the Research Strategy. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
14. Select Agent Research |
Select agents are hazardous biological agents and toxins that have been identified by DHHS or USDA as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. CDC maintains a list of these agents. See http://www.cdc.gov/od/sap/docs/salist.pdf. If the activities proposed in the application involve only the use of a strain(s) of select agents which has been excluded from the list of select agents and toxins as per 42 CFR 73.3, the select agent requirements do not apply. Use this section to identify the strain(s) of the select agent that will be used and note that it has been excluded from this list. The CDC maintains a list of exclusions at http://www.cdc.gov/od/sap/sap/exclusion.htm. If the strain(s) is not currently excluded from the list of select agents and toxins but you have applied or intend to apply to DHHS for an exclusion from the list, use this section to indicate the status of your request or your intent to apply for an exclusion and provide a brief justification for the exclusion. If any of the activities proposed in your application involve the use of select agents at any time during the proposed project period, either at the applicant organization or at any other performance site, address the following three points for each site at which select agent research will take place. Although no specific page limitation applies to this section, be succinct. 1. Identify the select agent(s) to be used in the proposed research. 2. Provide the registration status of all entities* where select agent(s) will be used.
*An “entity” is defined in 42 CFR 73.1 as “any government agency (Federal, State, or local), academic institution, corporation, company, partnership, society, association, firm, sole proprietorship, or other legal entity.” 3. Provide a description of all facilities where the select agent(s) will be used.
If you are responding to a specific funding opportunity announcement, address any requirements specified by the FOA. Reviewers will assess the information provided in this Section, and any questions associated with select agent research will need to be addressed prior to award. Save this file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
15. Resource Sharing Plan(s) |
NIH considers the sharing of unique research resources developed through NIH-sponsored research an important means to enhance the value and further the advancement of the research. When resources have been developed with NIH funds and the associated research findings published or provided to NIH, it is important that they be made readily available for research purposes to qualified individuals within the scientific community. See Part III, 1.5 Sharing Research Resources. 1. Data Sharing Plan: Investigators seeking $500,000 or more in direct costs (exclusive of consortium F&A) in any year are expected to include a brief 1-paragraph description of how final research data will be shared, or explain why data-sharing is not possible. Specific Funding Opportunity Announcements may require that all applications include this information regardless of the dollar level. Applicants are encouraged to read the specific opportunity carefully and discuss their data-sharing plan with their program contact at the time they negotiate an agreement with the Institute/Center (IC) staff to accept assignment of their application. See Data-Sharing Policy or http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html. 2. Sharing Model Organisms: If the development of model organisms is anticipated, attach a description of a specific plan for sharing and distributing unique model organism research resources or state appropriate reasons why such sharing is restricted or not possible. For many individual fellowships it is anticipated that plans of this nature would have already been reported to the NIH by your sponsor in his/her research application. When this has occurred, indicate so in this section and include the appropriate grant number. For additional information on this policy, see Sharing Model Organisms in Part III, 1.3.2. 3. Genome-Wide Association Studies (GWAS): Applicants seeking funding for a genome-wide association study are expected to provide a plan for submission of GWAS data to the NIH-designated GWAS data repository, or provide an appropriate explanation why submission to the repository is not possible. GWAS is defined as any study of genetic variation across the entire genome that is designed to identify genetic associations with observable traits (e.g., blood pressure or weight) or the presence or absence of a disease or condition. For further information see Policy for Sharing of Data Obtained in NIH Supported or Conducted Genome-Wide Association Studies (NOT-OD-07-088) and http://gwas.nih.gov/. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
16. Respective Contributions |
This item is limited to one page. Describe the collaborative process between you and your sponsor/co-sponsor in the development, review, and editing of this research training plan. Discuss the respective roles in accomplishing the proposed research. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
17. Selection of Sponsor and Institution |
This item is limited to one page. Describe the rationale/justification for the selection of the sponsor and institution. 1. Explain why the sponsor, co-sponsor (if any), and institution were selected to accomplish the research training goals. If the proposed research training is to take place at a site other than the sponsoring organization, provide an explanation here. 2. Doctorate or Current Institution. (For postdoctoral and senior fellows only) Since training is expected to broaden a fellow's perspective, postdoctoral fellowship applicants requesting training at either their doctorate institution or at the institution where they have been training for more than a year must explain why further training at that institution would be valuable. Individuals applying for Senior Fellowships who are requesting training at the institution at which they are employed should provide a similar explanation. 3. Foreign Institution. If you are proposing a research training experience at a foreign institution, show that the foreign institution and sponsor offer special opportunities for training that are not currently available in the United States. Key factors in the selection of a foreign institution should be described. If applicable, the need for and level of proficiency in reading, speaking, and comprehending the foreign language should be addressed. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
18. Responsible Conduct of Research |
This item is limited to one page. Note: No award will be made if an application lacks this component. Every fellow must receive instruction in the responsible conduct of research ((http://grants.nih.gov/grants/guide/notice-files/NOT-OD-10-019.html) appropriate to the career stage of the applicant. The section must document prior participation or instruction in responsible conduct of research during the applicant's current career stage (including the date instruction was last completed) and propose a plan to either receive instruction in responsible conduct of research or participate as a course lecturer, etc., depending on the applicant's career stage. Applications must include a description, limited to no more than one page, of the sponsoring institution’s plans to provide, and the fellowship applicant’s plans for obtaining, instruction in the responsible conduct of research, including the format, subject matter, faculty participation, duration and frequency of instruction. The plan should be tailored to the needs of the fellow, and may go beyond formal institutional courses and provide opportunities for the individual to develop their own scholarly understanding of the ethical issues associated with their research activities and their impact on society. The role of the sponsor in the instruction in responsible conduct of research must be described. See Part III, 1.16 for additional information. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
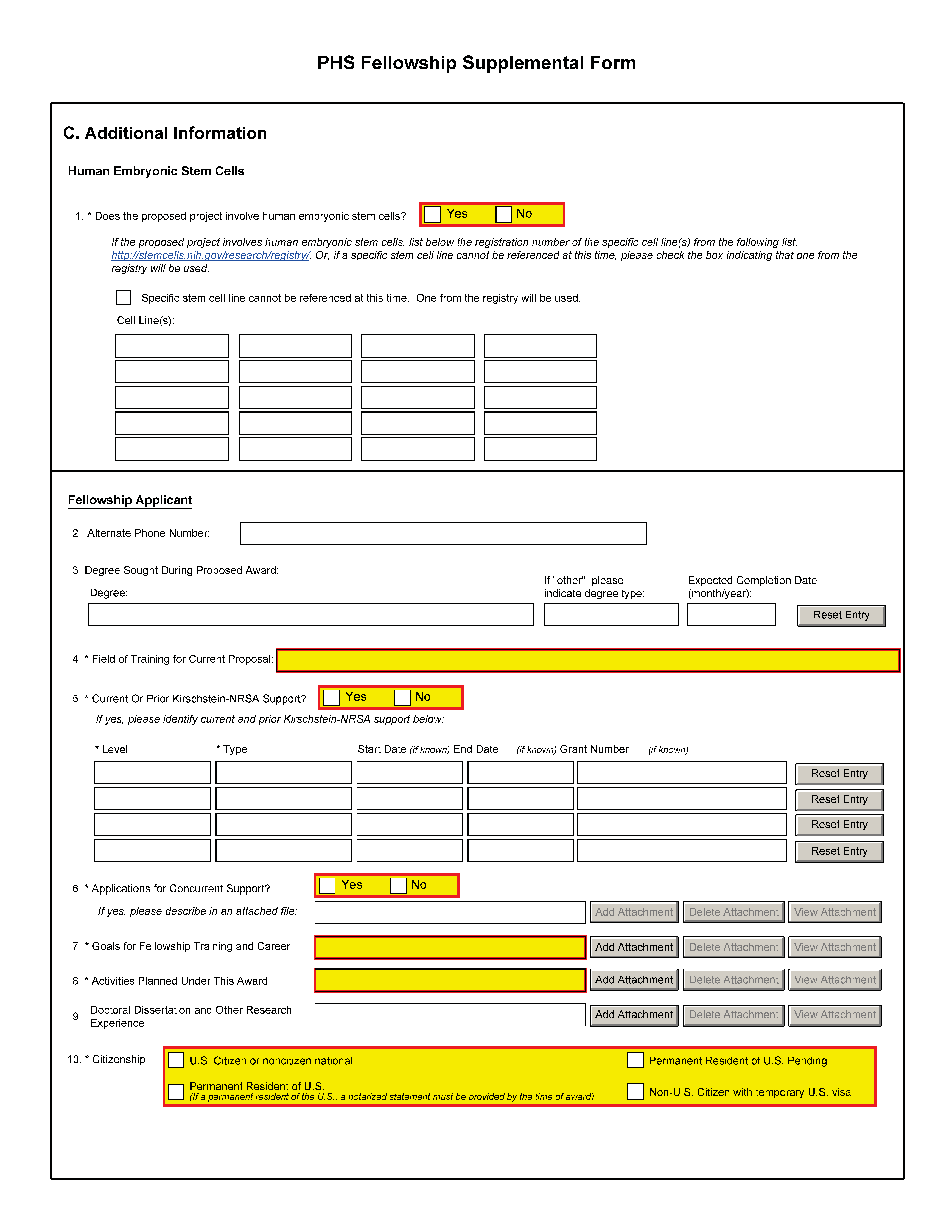
C. Additional Information |
|
1. Human Embryonic Stem Cells |
Indicate “Yes” if the proposed research involves human embryonic stem cells. See http://stemcells.nih.gov/index.asp for a definition of human embryonic stem cells. If the proposed project involves human embryonic stem cells, list in this section the 4-digit NIH Registration Number of the specific cell line(s) from the NIH Human Embryonic Stem Cell Registry found at: http://grants.nih.gov/stem_cells/registry/current.htm. If a specific stem cell line cannot be referenced at the time of application submission, check the box provided to indicate that one from the registry will be used. |
Fellowship Applicant |
|
2. Alternate Phone Number |
Enter an alternate phone number (e.g., cell phone) where the fellowship applicant can be reached on matters relating to this application for fellowship support. This should be a different number than provided in the PD/PI contact information in the Cover Component. |
3. Degree Sought During Proposed Award |
Complete if applicable. Completion of the degree requirements should be coordinated with the sponsor. The fellowship applicant must select the degree from the drop down menu and also enter the month and year of the expected completion date. If the degree is not on the drop down menu, please mark “Other” and indicate the type of degree in the space provided. |
4. Field of Training for Current Proposal |
Indicate the proposed area of research training according to the Fields of Training (FOT) codes listed in the drop down menu. Provide the FOT code that best describes the proposed area of research training from the FOT codes listed in the instructions. Select the subcategory descriptor, unless the broader category (in bold uppercase) fits best. If the FOT listing does not provide a good descriptor, select “Other.” (This information is used for reporting purposes only and is not used for study section assignments.) |
5. Current or Prior Kirschstein-NRSA Support?
|
This item is limited to one page. If “Yes”, identify the current and/or prior Kirschstein-NRSA support from the drop down menu, up to four entries. Define level of support as either predoctoral or postdoctoral level (not the level of experience). The type of support is either individual fellowship or institutional research training grant indicated on the drop down menu. Enter the start and end dates (if known) of the support (month, day, and year) and the grant number (if known) of the current and/or prior support (e.g., T32 GM123456 or F31 HL345678). An individual cannot receive more than 5 years of cumulative predoctoral Kirschstein-NRSA support and 3 years cumulative postdoctoral Kirschstein-NRSA support (the total of Institutional Grants and Individual Fellowships) without a waiver from the NIH IC. The NIH ICs have different policies on waiving the statutory limits on support. Therefore, the fellowship applicant must request a waiver from the probable funding IC before requesting a period of support that would exceed these limits. The fellow’s sponsor and a sponsoring institution official must endorse the request, and it must include justification and specify the amount of additional support for which approval is sought. Individuals seeking additional support beyond the third year of postdoctoral support are strongly advised to consult with their awarding IC Program Officer before submitting a waiver request. It is important to read carefully the applicable FOA that may have an overall approval to exceed these limits (e.g., the F30 combined M.D./Ph.D. program allows for up to 6 years of predoctoral support). Promptly report to the NIH IC to which this application is assigned any additional NRSA support received while this application is pending. |
6. Applications for Concurrent Support? |
This item is limited to one page. Check the appropriate answer, indicating “Yes” if the fellowship applicant has applied or will be applying for other support that would run concurrently with the period covered by this application. Include the type, dates, source(s) and amount in the attachment document. The fellowship applicant must promptly report to the NIH IC to which this application is assigned, or AHRQ, any support resulting from other such applications. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
7. Goals for Fellowship Training and Career |
This item is limited to one page. The fellowship applicant must describe his/her overall career goals, and explain how the proposed research training will enable the attainment of these goals. Identify the skills, theories, conceptual approaches, etc. to be learned or enhanced during the award. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
8. Activities Planned Under This Award |
This item is limited to one page. The fellowship applicant must describe by year the activities (research, coursework, etc.) he/she will be involved in under the proposed award and estimate the percentage of time to be devoted to each activity, based on a normal working day for a full-time fellow as defined by the sponsoring institution. The percentage should total 100 for each year. Also, briefly explain activities other than research and relate them to the proposed research training. For postdoctoral fellowships (F32), do not exceed three years. For senior fellowships (F33) do not exceed two years. Predoctoral fellowships (F31), including fellowship applicants for the M.D./Ph.D. (F30) program may reflect up to six years if allowed by the applicable FOA. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
9. Doctoral Dissertation and Research Experience |
This item is limited to two pages. Summarize your research experience (limited to 2 pages) in chronological order. Advanced graduate students, who have (or will have) completed their comprehensive examinations by the time of award must also include a narrative of their doctoral dissertation (may be preliminary). If you have no research experience, list other scientific experience. Do not list academic courses. In summarizing their research experience, Postdoctoral and Senior Fellowship applicants should include the areas studied and conclusions drawn. Postdoctoral fellowship applicants should also specify which areas of research were part of their thesis or dissertation and which, if any, were part of a previous postdoctoral project. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
10. Citizenship |
Fellowship applicants must check the appropriate box. To be eligible for a Kirschstein-NRSA Individual Fellowship (F30, F31, F32, F33), the fellowship applicant must be a U.S. citizen, a non-citizen national, or have been lawfully admitted to the U.S. for permanent residence before the award is issued. U.S. non-citizen nationals are persons born in lands that are not States but are under U.S. sovereignty, jurisdiction, or administration, e.g., American Samoa. Individuals on temporary student visas are not eligible for NRSA support. If the fellowship applicant has been lawfully admitted for permanent residence, i.e., is in possession of a Permanent Resident Card (USCIS Form I-551) or other legal verification of such status, the fellowship applicant should check the “Permanent Resident of U.S.” box. Before the award is issued, a permanent resident will be required to submit a notarized statement that a licensed notary has seen the fellowship applicant’s valid Permanent Resident Card (USCIS Form I-551) or other valid verification from the U.S. Immigration and Naturalization Service of legal admission to the U.S. If the fellowship applicant is a non-citizen of the U.S. who has applied for, but not yet been granted legal admission to the U.S. as a permanent resident, the applicant should check the “Permanent Resident of U.S. Pending” box, understanding that no award will be issued until such time as the required permanent residency has been established and the required documentation submitted to NIH or AHRQ. If the fellowship applicant is applying for a non-NRSA fellowship program supported by the NIH, for which citizenship or permanent residency is not required (e.g., Fogarty International Center programs), the fellowship applicant must have in his/her possession a valid visa allowing him/her to remain in the U.S. (or in a foreign research training setting, if applicable) long enough to be productive on the proposed fellowship project. It is the responsibility of the sponsoring institution to determine and retain documentation indicating that the individual fellowship applicant’s visa will allow him/her to reside in the proposed research training setting for the period of time necessary to complete the proposed fellowship. The fellowship applicant should check the “Non-U.S. Citizen with temporary U.S. visa” box. Verification may be requested by the NIH IC prior to issuance of an award. In general, it is highly recommended that all non-U.S. citizens adhere to specific requirements as stated in the FOA or contact the appropriate individual listed on the FOA. |
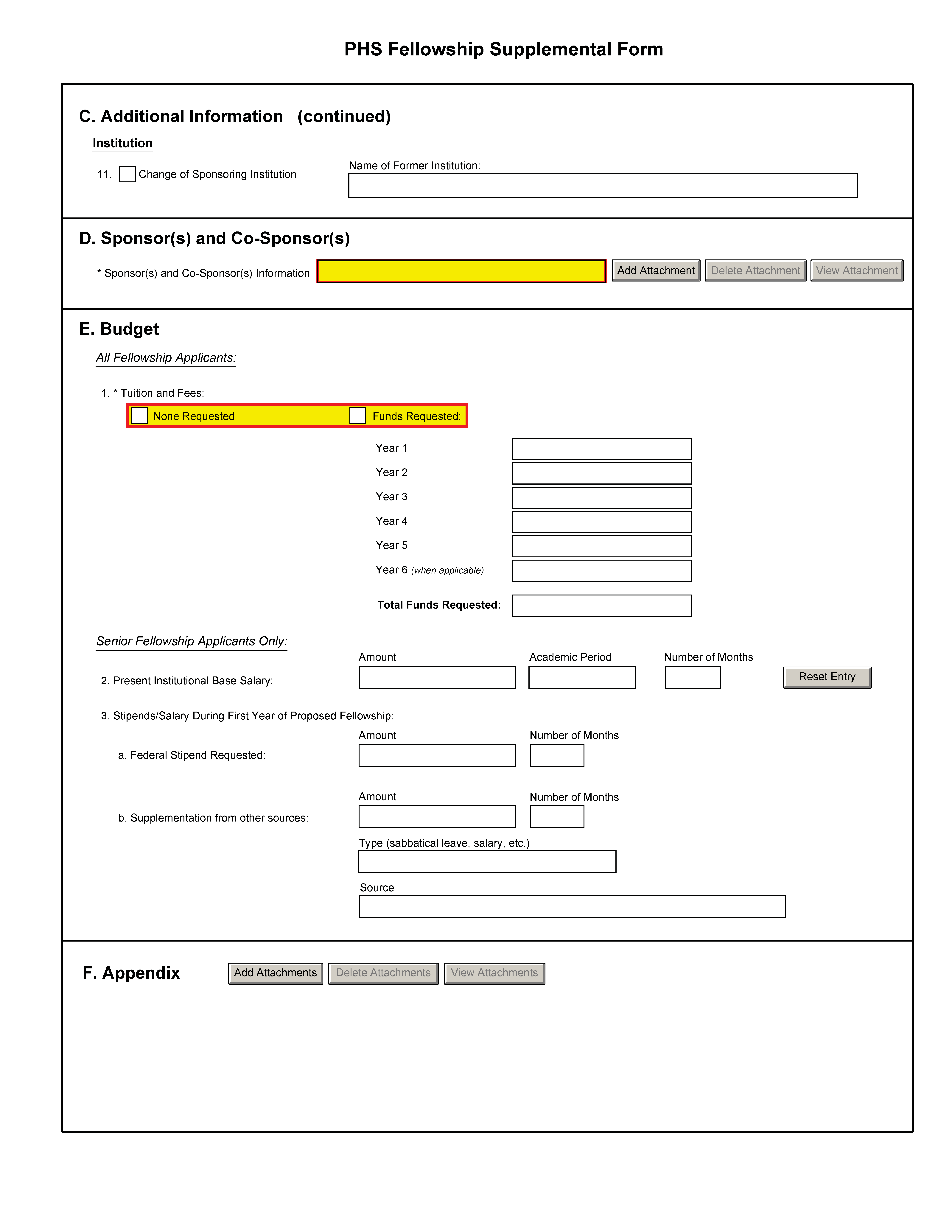
Institution |
|
11. Change of Sponsoring Institution |
The fellowship applicant must indicate if this application is being submitted with a change of sponsoring institution. If the fellowship applicant checks the box, the name of the former sponsoring institution must be provided. |
D. Sponsor and Co-Sponsor Information |
For applications using the Adobe B-1(not B) package only: Sponsor and any Co-Sponsor(s) (if any) Information is limited to 6 pages. Create a heading at the top of the first page titled “Section II--Sponsor and Co-Sponsor Information.” Complete these items as comprehensively as possible so that a meaningful evaluation of the training environment can be made by the reviewers. a. Research Support Available In a table, list all current and pending research and research training support specifically available to the applicant for this particular training experience. Include funding source, complete identifying number, title of the research or training program, and name of the principal investigator, dates, and amount of the award. Include this information for any co-sponsor as well. b. Sponsor's/Co-Sponsor’s Previous Fellows/Trainees Give the total number of predoctoral and postdoctoral individuals previously sponsored. Select up to five that are representative and, for those five, provide their present employing organizations and position titles or occupations. Include this information for any co-sponsor as well. c. Training Plan, Environment, Research Facilities Describe the research training plan that you have developed specifically for the Fellowship applicant. Include items such as classes, seminars, and opportunities for interaction with other groups and scientists. Describe the research environment and available research facilities and equipment. Indicate the relationship of the proposed research training to the applicant's career goals. Describe the skills and techniques that the applicant will learn. Relate these to the applicant's career goals. d. Number of Fellows/Trainees to be Supervised During the Fellowship Indicate whether pre- or postdoctoral. Include this information for any co-sponsor as well. e. Applicant's Qualifications and Potential for a Research Career Describe how the Fellowship applicant is suited for this research training opportunity based on his/her academic record and research experience level, including how the research training plan, and your own expertise as the sponsor will assist in producing an independent researcher. |
E. Budget |
|
All Fellowship Applicants: 1. Tuition and Fees: |
All fellowship applicants should list the estimated costs of tuition and fees. Postdoctoral and senior fellowship applicants should list the costs associated with courses planned that support the research training experience and are identified and described in the attachment for the Research Strategy section of the Research Training Plan in Section B. If no tuition and fees are being requested, check the box provided. With the exception of senior fellowship applicants, no additional budget information is required. The final stipend and institutional allowance will be determined at the time of award. In accordance with NIH Guide NOT-OD-06-093, funds to offset the costs of health insurance (self or family, as appropriate) are included in the standard Institutional Allowance, and not to be requested as part of Tuition and Fees. |
Senior Fellowship Applicants Only: 2. Present Institutional Base Salary: |
Senior fellowship applicants must provide their present base salary and indicate the period of time on which the salary is determined (e.g., academic year of 9 months, full-time 12 months, etc. The number may not be more than 12, but may include a decimal indicating partial months (e.g., 9.5). |
Senior Fellowship Applicants Only: 3. Stipend/ Salary During First Year of Proposed Fellowship: |
a. Federal Stipend Requested: Fellowship applicants must insert the stipend being requested for the initial period of support and the number of months. b. Supplementation from other sources: Fellowship applicants should enter the anticipated amount and the length of time associated with the amount. Enter also the type of supplementation expected (e.g., salary, sabbatical leave, etc.) and the source of such funding. |
F. Appendix |
Only one copy of appendix material should be included. A maximum of 10 PDF attachments is allowed in the Appendix. Grants.gov defaults to a maximum of 10 separate attachments. If more than 10 appendix attachments are needed, combine the remaining information into attachment #10. Note that this is the total number of appendix items, not the total number of publications. Publications that are publicly accessible must not be included in the appendix. When allowed there is a limit of three publications that are not publicly available (see below for further details and check the FOA for any specific instructions), though not all fellowship activity codes allow publications to be included in the appendix. Do not use the Appendix to circumvent the page limitations of the Research Strategy section of the Research Training Plan or any other section of the application for which a page limit applies. For additional information regarding Appendix material and page limits, please refer to the NIH Guide Notice NOT-OD-11-080, http://grants.nih.gov/grants/guide/notice-files/NOT-OD-11-080.html. Appendix material may not appear in the assembled application in the order attached, so it is important to use filenames for attachments that are descriptive of the content. A summary sheet listing all of the items included in the appendix is also encouraged but not required. When including a summary sheet, it should be included in the first appendix attachment. Applications that do not follow the appendix requirements may be delayed in the review process. New, resubmission, renewal, and revision applications may include the following materials in the Appendix:
Do not include unpublished theses, or abstracts/manuscripts submitted (but not yet accepted) for publication.
Items that must not be included in the appendix:
Publications that are publicly accessible. For such publications, the URL or PMC submission identification numbers along with the full reference should be included as appropriate in the Bibliography and References cited section, the Progress Report Publication List section, and/or the Biographical Sketch section. |
Once all data have been entered use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. To remove a document from the Submission box, click the document name to select it and then click the Move Form to Delete button. This will return the document to the Mandatory Documents Submission List or Optional Documents Submission List.
5.4 Letters of Reference (must be submitted electronically through the eRA Commons)
IMPORTANT NOTE: This section contains instructions for both the Fellowship Applicant (Part A) and the Referees (Part B). Applicants are urged to read both sections carefully so they are able to provide accurate instructions to the Referees. Failure to submit all of the required references may result in the application being returned to you without review.
Part A. Instructions for Fellowship Applicants:
Be sure to list the names of those individuals submitting Letters of Reference on your behalf in the cover letter.
Letters of Reference are an important component of the application for the Individual Fellowship Awards. Applicants must arrange to have at least three (and no more than five) references submitted on their behalf to the eRA Commons Web site at https://commons.era.nih.gov/commons/reference/submitRefereeInformation.jsp.
Letters of Reference are due by the application receipt deadline date. Although previously NIH provided a 5 business days grace period for the receipt of Letters of Reference after the application receipt due date, the new policy eliminates the grace period. More information can be found in NIH Guide Notice NOT-OD-11-047.
Your referees should be carefully selected. Only those individuals who can make the most meaningful comments about your qualifications for a research career should be used. The sponsor of this application cannot be counted as a reference. The sponsor's recommendation is included as part of the application (See Sponsor/Co-Sponsor Information). Whenever possible, select at least one referee who is not in your current department. For postdoctoral applications, if not submitting a reference from your dissertation advisor or chief of service, provide an explanation in Item 12 – Other Attachments on the SF424 (R&R) Other Project Information Form. Also for postdoctoral applications, references from graduate or medical school are preferred over those from undergraduate school. Note: For resubmission applications, it is critical that NEW (updated) Letters of Reference be submitted providing an up-to-date evaluation of the Fellowship applicant’s potential to develop as an independent and productive researcher, and the continued need for additional supervised research experience.
Electronic submission of a Letter of Reference is a separate process from submitting an application electronically. Letters of Reference are submitted directly through the eRA Commons and do not use Grants.gov. Therefore, this process requires that the referee provide information including (a) the PD/PI (Fellowship applicant) Commons user name, (b) the PD/PI first and last name as they appear on the PD/PI’s Commons account, and (c) the number assigned to the Funding Opportunity Announcement (FOA) under which the Fellowship applicant is applying. The FOA number provided to referees must be the FOA number under which the application is submitted; otherwise, the Letters of Reference will not be matched up with the application in the eRA Commons.
Request reference letters only from individuals who will be able to submit them in time for the application receipt date. Consider any factor (e.g., illness or extended vacation) that might cause an inordinate delay. Give these reference instructions to the referees well in advance of the application submission date.
For electronically submitted applications that involve separately submitted confidential Letters of Reference, applicants can monitor the submission of reference letters – though not access the actual documents through their eRA Commons Personal Profile. Failure to provide references may delay processing of your application or may result in the application being returned to you without review.
Once the letter has been submitted, confirmation e-mails will be sent to both the referee and the applicant following electronic submission. The confirmation sent to the applicant will include the referee’s name and the date the letter was submitted. The confirmation sent to the referee will include the referee and applicant’s names, a confirmation number, and the date the letter was submitted.
The applicant and the AOR/SO may check the status of submitted letters by logging into their Commons account and accessing the “check status” screen for this application. The applicant is responsible for reviewing the status of submitted Letters of Reference and contacting referees to ensure that letters are submitted by the receipt deadline. While the applicant is able to check on the status of the submitted Letters of Reference, the letters are confidential and he/she will not have access to the letters themselves. Note, Letters of Reference can be submitted at any time prior to the receipt deadline. It is not necessary to wait until after the application is submitted before Letters of Reference are submitted; the two submissions are distinct.
Applicants should provide the following instructions to their referees. Applicants can direct their referees to the Individual Fellowship Application Guide SF424 (R&R) at http://grants.nih.gov/grants/funding/424/index.htm, then go to Section 5.4, Part B. Instructions for Referees.
Part B. Instructions for Referees:
Letters of Reference must be submitted to the eRA Commons at https://commons.era.nih.gov/commons/reference/submitRefereeInformation.jsp, and may be submitted any time after the Funding Opportunity Announcement opens and not later than the application receipt due date.
Failure to submit the required reference in the appropriate format may result in the application being returned to the applicant without review.
Please put the name of the fellowship applicant at the top of the letter. Also, be sure to include your name and title in the letter.
When you are finished with the letter, return to the eRA Commons page (https://commons.era.nih.gov/commons/reference/submitRefereeInformation.jsp) and complete the following required information:
Referee First Name (Required)
Referee Last Name (Required)
Referee MI Name (middle initial) (Not Required)
Referee E-mail (Required)
Referee Institution/Affiliation (Required)
Referee Department (Required)
PD/PI (Fellowship applicant) Commons User ID (Required)
PD/PI’s Last Name, as it appears on the PD/PI’s Commons account (Required) (will be validated to ensure they match)
Funding Opportunity Announcement Number (Required and must match the number of the FOA under which the application is being submitted)
Reference Letter Confirmation Number (Required only if resubmitting a reference letter; not required otherwise)
Fellowship Letter of Reference – two pages maximum. Complete the letter using word processing software and then convert to PDF using PDF generating software. Avoid scanning text attachments to convert to PDF since that causes problems for the agency handling the application. Additional tips for creating PDF files can be found at http://grants.nih.gov/grants/ElectronicReceipt/pdf_guidelines.htm.
Note that the Letter of Reference can be submitted at any time prior to the receipt deadline. It is not necessary to wait until after the application is submitted before the Letter of Reference is submitted; the two submissions are distinct. After you have submitted your Letter of Reference, both you and the applicant will receive a confirmation of receipt by e-mail. Your e-mail confirmation will include a Reference Letter Confirmation Number. The Confirmation Number will be required when resubmitting reference letters. Please print the confirmation e-mail for your records.
5.5 The Peer Review Process
Overview
NIH policy is intended to ensure that applications for funding submitted to the NIH are evaluated on the basis of a process that is fair, equitable, timely, and conducted in a manner free of bias. The NIH dual peer review system is mandated by statute in accordance with section 492 of the Public Health Service Act and federal regulations governing "Scientific Peer Review of Research Grant Applications and Research and Development Contract Proposals" (42 CFR part 52h).
The first level of review is carried out by a Scientific Review Group (SRG) composed primarily of non-federal scientists who have expertise in relevant scientific disciplines and current research areas. The second level of review of fellowship applications is performed by senior staff of the potential awarding IC. Only the NIH IC may make actual funding decisions.
A detailed description of what happens to your individual fellowship application after it is received for peer review can be found at the following location: http://cms.csr.nih.gov/AboutCSR/OverviewofPeerReviewProcess.htm. Additional information about charters and membership of SRGs, Councils, and Boards can be obtained from the appropriate agency.
Discussed and Not Discussed Applications
The initial scientific peer review of individual fellowship applications will also include a process in which only those applications deemed by the reviewers to have the highest scientific and technical merit, generally the better half of the applications under review, will be discussed at the SRG meeting, assigned an impact/priority score, and receive a second level review. Applications in the lower half are reviewed by SRG members but they are not discussed or assigned numerical impact/priority scores at the SRG meeting. This process allows the reviewers to focus their discussion on the most meritorious applications.
Before the review meeting, each reviewer and discussant assigned to an application will give a separate score for each of the five core review criteria and a preliminary impact/priority score for that application (see below). The preliminary impact/priority scores will be used to determine which Fellowship applications will be discussed.
Scoring
SRG members are instructed to evaluate individual fellowship applications by addressing the scored review criteria (see below) and additional review criteria as applicable for the application. However, Funding Opportunity Announcements (FOAs) may list different and/or additional review criteria and considerations.
For each application that is discussed, a final overall impact/priority score will be given by each eligible committee member (without conflicts of interest) following the panel discussion. Each member’s impact/priority score will reflect his/her evaluation of the overall impact of the project in its entirety, rather than an arithmetic formula applied to the reviewer’s scores given to each criterion. The final impact/priority score for each discussed application will be determined by calculating the arithmetic average of all the eligible members’ impact/priority scores, and multiplying the average by 10.
As part of the initial merit review, and regardless of whether an application is discussed or not discussed , all applicants will receive a written critique, called a Summary Statement, unless stated otherwise in the FOA. The Summary Statement represents a combination of the reviewers' written comments and scores for individual criteria. The Summary Statement for discussed applications includes the SRO's summary of the members' discussion during the SRG meeting; the final impact/priority score; the recommendations of the SRG, including budget recommendations; and administrative notes of special considerations. For applications that are not discussed by the full committee, the scores of the assigned reviewers and discussants for the scored review criteria will be reported individually on the Summary Statement. Numerical impact/priority scores are not given for applications that are not discussed.
Individual Fellowship Application Review Criteria
Overall Impact/Merit. Reviewers will provide an overall impact/priority score to reflect their assessment of the likelihood that the fellowship will enhance the applicant’s potential for, and commitment to, a productive independent scientific research career in a health-related field, in consideration of the scored and additional review criteria (as applicable for the project proposed).
Scored Review Criteria. Reviewers will consider each of the five review criteria below in the determination of scientific and technical merit, and give a separate score for each.
The following review criteria are applicable to F31 and F32 applications. For review criteria pertaining to other individual fellowship applications (e.g., F05, F30, F33), please refer to the specific FOA.
Fellowship Applicant: Are the applicant’s academic record and research experience of high quality? Does the applicant have the potential to develop as an independent and productive researcher in biomedical, behavioral or clinical science?
Sponsor(s), Collaborator(s), and Consultant(s): Are the sponsor(s) research qualifications (including successful competition for research support) and track record of mentoring appropriate for the proposed fellowship? Are there (1) evidence of a match between the research interests of the applicant and the sponsor (including an understanding of the applicant’s research training needs) and (2) a demonstrated ability and commitment of the sponsor to assist in meeting these needs? Are the qualifications of any collaborator(s) and/or consultant(s), including their complementary expertise and previous experience in fostering the training of fellows, appropriate for the proposed research project?
Research Training Plan: Is the proposed research plan of high scientific quality, and does it relate to the applicant’s training plan? Is the training plan consistent with the candidate’s stage of research development? Will the research training plan provide the applicant with individualized and supervised experiences that will develop research skills needed for his/her independent and productive research career?
Training Potential: Does the proposed research training plan have the potential to provide the fellow with the requisite individualized and supervised experiences that will develop his/her research skills? Does the proposed research training have the potential to serve as a sound foundation that will lead the fellow to an independent and productive career?
Institutional Environment and Commitment to Training: Are the research facilities, resources (e.g. equipment, laboratory space, computer time, subject populations), and training opportunities adequate and appropriate? Is the institutional environment for the scientific development of the applicant of high quality, and is there appropriate institutional commitment to fostering the fellows’ training as an independent and productive researcher?
As applicable for the project proposed, reviewers will consider the following additional terms in the determination of scientific and technical merit, but will not give separate scores for these items.
Protections for Human Subjects. For research that involves human subjects but does not involve one of the six categories of research that are exempt under 45 CFR part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the six categories of research that are exempt under 45 CFR part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials.
Inclusion of Women, Minorities, and Children. When the proposed project involves clinical research, the committee will evaluate the proposed plans for inclusion of minorities and members of both genders, as well as the inclusion of children.
Vertebrate Animals. The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following five points: 1) proposed use of the animals, and species, strains, ages, sex, and numbers to be used; 2) justifications for the use of animals and for the appropriateness of the species and numbers proposed; 3) adequacy of veterinary care; 4) procedures for limiting discomfort, distress, pain and injury to that which is unavoidable in the conduct of scientifically sound research including the use of analgesic, anesthetic, and tranquilizing drugs and/or comfortable restraining devices; and 5) methods of euthanasia and reason for selection if not consistent with the AVMA Guidelines on Euthanasia. For additional information, see http://grants.nih.gov/grants/olaw/VASchecklist.pdf.
Biohazards. Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmission Applications. When reviewing a resubmission application, the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
Renewal Applications. When reviewing a renewal application, the committee will consider the progress made in the last funding period.
Additional Review Considerations. As applicable for the project proposed, reviewers will address each of the following items, but will not give scores for these items and should not consider them in providing an overall impact/priority score.
Training in the Responsible Conduct of Research. Reviewers will evaluate plans for instruction in responsible conduct of research as well as the past record of instruction in responsible conduct of research, where applicable. Reviewers will specifically address the five Instructional Components (Format, Subject Matter, Faculty Participation, Duration and Frequency of Instruction), as detailed in NOT-OD-10-019. The review of this consideration will be guided by the principles set forth in NOT-OD-10-019. Plans and past record will be rated as ACCEPTABLE or UNACCEPTABLE.
Applications from Foreign Organizations. Reviewers will assess whether the research training presents special opportunities and clearly described scientific advantages for the applicant, through the use of talent (e.g., mentor), resources, populations (if applicable), or training environment that are not readily available in the United States or augment existing U.S. talent and/or resources.
Select Agent Research. Reviewers will assess the information provided in this section of the application, including 1) the select agent(s) to be used in the proposed research, 2) the registration status of all entities where select agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of select agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the select agent(s).
Resource Sharing Plans. Reviewers will comment on whether the following Resource Sharing Plans, or the rationale for not sharing the following types of resources, are reasonable: 1) Data Sharing Plan (http://grants.nih.gov/grants/policy/data_sharing/data_sharing_guidance.htm); 2) Sharing Model Organisms (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-04-042.html); and 3) Genome Wide Association Studies (GWAS) (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-07-088.html).
Budget and Period of Support. Reviewers will consider whether the requested period of support is fully justified and reasonable in relation to the proposed fellowship training.
Dual-Level Peer Review
The second level review of Fellowship applications is performed by senior staff of the potential awarding component (Institute, Center, or other unit). Fellowship applications are not required to undergo Advisory Council/Board review.
Part I: Instructions for Preparing and Submitting an Application
I-
| File Type | application/msword |
| Author | Leslie Dorman |
| Last Modified By | Leslie Dorman |
| File Modified | 2012-05-25 |
| File Created | 2012-05-25 |
© 2026 OMB.report | Privacy Policy