1-EI Generic ICR SS Part A 20130326
1-EI Generic ICR SS Part A 20130326.docx
Generic Clearance of ATSDR Exposure Investigations (EI) formerly NCEH/ATSDR Exposure Investigations (EI)
OMB: 0923-0040
Exposure Investigations: Supporting Statement A
Supporting
Statement (Part A: Justification) of the Request for
OMB Review
and Approval of
Generic Clearance of
ATSDR Exposure Investigations (EI)
0923-0040
Reinstatement with Change
March 2013
Science Support Branch (SSB)
Division of Community and Health Investigations (DCHI)
Agency for Toxic Substances and Disease Registry (ATSDR)
Program Official:
Peter J. Kowalski, MPH, CIH
Lead, Exposure Investigations Team
Science Support Branch
Division of Community Health Investigations
Agency for Toxic Substances and Disease Registry
4770
Buford Hwy NE, MS F59
Atlanta, GA 30341
Phone: 770-488-0776
Fax: 770-488-1542
Email: [email protected]
Point of Contact:
Karen M. Scruton, MS
Environmental Health Scientist, Exposure Investigations Team
Science Support Branch
Division of Community Health Investigation
Agency for Toxic Substances and Disease Registry
4770
Buford Hwy NE, MS F59
Atlanta, GA 30341
Phone: 770-488-1325
Fax: 770-488-1542
Email: [email protected]
Table of Contents
A.1 Circumstances Making the Collection of Information Necessary 4
A.1.1. Privacy Impact Assessment 8
Overview of the Data Collection System 8
Items of Information to be Collected 10
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age 11
A.2 Purpose and Use of Information Collection 12
A.2.1. Privacy Impact Assessment 13
A.3 Use of Improved Information Technology and Burden Reduction 13
A.4 Efforts to Identify Duplication and Use of Similar Information 13
A.5 Impact on Small Businesses or Other Small Entities 14
A.6 Consequences of Collecting the Information Less Frequently 15
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 15
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 15
A.9 Explanation of Any Payment or Gift to Respondents 16
A.10 Assurance of Confidentiality Provided to Respondents 16
A.10.1. Privacy Impact Assessment Information 17
A.11 Justification for Sensitive Questions 18
A.12 Estimates of Annualized Burden Hours and Costs 18
A. Estimates of Annualized Burden Hours 18
B. Annualized Cost to Respondents 19
A.13 Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 19
A.14. Annualized Cost to the Federal Government 19
A.15. Explanation for Program Changes or Adjustments 19
A.16. Plans for Tabulation and Publication and Project Time Schedule 20
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate 20
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions 20
List of Attachments
1: Authorizing Legislation – CERCLA
2: 60-day Federal Register Notice
3: Chemical Exposure Question Bank
4: Map of EPA and ATSDR Regions
A. Justification
A.1 Circumstances
Making the Collection of Information Necessary
The classification of this information collection request (ICR) is as a reinstatement with change of the National Center for Environmental Health/Agency for Toxic Substances and Disease Registry (NCEH/ATSDR) Exposure Investigations (EI), OMB Control Number (OCN) 0923-0040. The 60-day Federal Register notice for the reinstatement of OCN 0923-0040 is provided as Attachment 2.
ATSDR requests a change to a three-year “generic” clearance to allow the Agency to carry out its public health activities in a more timely and efficient manner. The Agency also requests a title change to read – Generic Clearance of ATSDR Exposure Investigations (EI) under the authority of the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA), commonly known as the "Superfund" Act, as amended by the Superfund Amendments and Reauthorization Act (SARA) of 1986 (Attachment 1).1 Under CERCLA, ATSDR works closely with the U.S. Environmental Protection Agency (EPA) to evaluate the presence and nature of hazardous substances at specific sites and the levels at which these substances may pose a threat to human health. ATSDR works to prevent or reduce further exposure and the illnesses that result from such hazardous substances.
Due to the 2012 ATSDR reorganization (Federal Register, Volume 77 No. 221, 15 Nov 2012, Pages 68125-7), the agency is requesting that OMB allow three simultaneous review tracks (Eastern, Central, Western) to expedite review and approval of these time-sensitive submittals from across the nation. The justification for this request is further discussed in Section A.1.1.
ATSDR Public Health Assessment Process and the Role of the Exposure Investigation
The ATSDR Division of Community Health and Investigation (DCHI) conducts public health assessments (PHAs) at sites when requested by the U.S. EPA, states, organizations, or individual petitioners. The 2005 ATSDR PHA Guidance Manual is available at, http://www.atsdr.cdc.gov/HAC/PHAManual/toc.html. The purpose of the agency’s PHA process is to find out whether people have been, are being, or may be exposed to hazardous substances and, if so whether that exposure is harmful, or potentially harmful, and should therefore be stopped or reduced. The process also serves as a mechanism through which the agency responds to specific community health concerns related to hazardous waste sites.
In summary, the PHA process includes the following steps:
Obtaining Site Information;
Involving and Communicating with the Community;
Exposure Evaluation;
Health Outcome Evaluation; and
Determining Conclusions and Recommendations

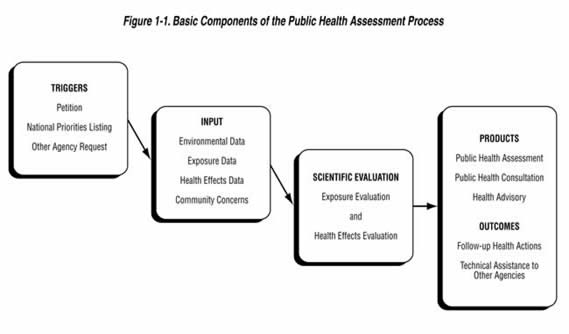
Exposure assessment is the hallmark of the PHA process. ATSDR scientists review environmental data to see how much contamination is at a site, where it is, and how people might come into contact with it. Generally, ATSDR does not collect its own environmental sampling data but reviews information provided by federal and state government agencies and/or their contractors, potentially responsible parties, and the public. When adequate environmental or exposure information does not exist to assess human exposures and possible related health effects, ATSDR will indicate what further environmental sampling may be needed and may collect environmental and biological samples, when appropriate.
Therefore, as part of the PHA process, ATSDR uses EIs to fill data gaps that are essential for evaluating whether communities are exposed to contaminants and whether a health hazard is present. The EI team conducts point of human-contact sampling focused on geographic areas where exposures are expected to be high. EIs may include environmental or biological sampling, or both (ATSDR, 2005).
Environmental sampling may include ambient air, personal air, indoor air, dust, soil, sediment, biota including food sources, ground water, tap water and surface water sampling. Depending on individual site characteristics, the sampling period may vary from days to several months.
Biological sampling may include, but not limited to, blood and urine sampling for exposure biomarkers.
Relevant health symptoms and medical information related to site-specific exposures may be assessed.
Most EIs sampling events are completed over a period of days to months and are a one-time occurrence.
An EI aims to identify the most highly exposed individuals and measure their exposure. The results of the investigation are site-specific and apply only to the participants from the site. An EI is not considered a health study. The participants’ results are not intended to be generalized to other populations and other communities. No participants from external comparison groups are included in the data collection. As a public service, EIs provide individual exposure information back to the participants.
EIs are also used as the basis to implement appropriate public health actions that reduce exposure to communities. Examples of the use of EIs in informing responsive public health action and in reducing exposure of the public include:
Example 1: Excel Dairy, MN – Concentrated Animal Feed Operation (CAFO)
Nearby residents complained of odor & symptoms (e.g., eye and throat irritation, headache, nausea)
Conducted emergency sampling of Hydrogen Sulfide (H2S) outdoor air – some H2S levels were higher than emergency response guidelines (>400 exceedances of MN standard)
Determined exposure to emissions from the waste lagoons are a Public Health Hazard for individuals living nearby (30 to 40 people)
State environmental agency continued air monitoring of emissions from the facility
The USEPA and the Minnesota Attorney General’s office used ATSDR’s EI data to support enforcement actions against the dairy
Those actions results in the dairy’s closure while the dairy operators sought ways to reduce the emissions (e.g., apply permanent covers for manure lagoons and eliminate land manure applications)
As a result, MN businesses must comply with ambient air standards for 9 contaminants at their property line
ATSDR provided congressional briefing on CAFOs
http://www.health.state.mn.us/divs/eh/hazardous/sites/marshall/exceldairy/exceldairyhcfull.pdf
Example 2: Penn Tex Resources, IL – Oil Field
Nearby residents complained of H2S emissions
Sampled H2S in outdoor and indoor air in community
2,000 – 3,000 people directly affected
Peak H2S levels in community ranged from 53 to 1,500 ppb; many were above acute Minimum Risk Level (MRL) of 70 parts per billion (ppb)
Determined two large areas were at increased risk of respiratory and neurological health impacts
U.S. Attorney brought civil action pursuant to the Clean Air Act
A Consent Decree was signed in April 2007
Engineering remedies were implemented
The State of Illinois changed laws for oil well fields
EPA awarded ATSDR staff the Gold Medal
http://www.atsdr.cdc.gov/HAC/pha/PennTex_HC_3-19-08/PennTex_HC_3-19-08.pdf
http://regulations.vlex.com/vid/consent-judgments-penntex-illinois-26944001
The Exposure Investigation Criteria and Recommendation Process
Within ATSDR, the DCHI Science Support Branch (SSB) EI Team is a multidisciplinary group of 6-8 scientists with expertise in environmental health science and engineering, industrial hygiene, epidemiology, toxicology, and medicine. In order for an EI to be conducted four criteria must be met for the EI to be approved. The criteria are:
Can an exposed population be identified?
Does a data gap exist that affects the ability to determine if a health hazard exists?
Can an EI be designed that will address this data gap?
Will the EI results impact the public health decision for the site?
If the answers to these questions indicate that an EI would allow ATSDR to make a better-informed public health call, the DCHI EI Team may conduct agency-led EIs. The SSB also coordinates and lends technical assistance to states, tribal, and territorial health departments that conduct their own EIs. Currently, ATSDR is administering a cooperative agreement program called the “ATSDR’s Partnership to Promote Localized Efforts to Reduce Environmental Exposure (APPLETREE) Program” (FY11-FY14 Award No. TS11-1101) which sponsors state-led nonresearch EIs. ATSDR anticipates that future DCHI cooperative agreement programs will also include this long-established PHA process and underlying EI activity under the scope of this generic clearance. If future revisions to the scope of the generic clearance are necessary, ATSDR will request OMB review and approval for changes.
After the team develops and conducts an EI, they evaluate the results and communicate their public health findings and recommendations to the community. If exposures are found at levels that might cause health concerns, the following may be recommended:
Reduction or elimination of exposure,
Expanded sampling to identify the extent of exposure/contamination,
Prevention or identification of adverse health effects, and
Further applied research.
In order to continue this necessary public health function, ATSDR is requesting approval of this generic OMB submission. Public health concerns leading to requests for an EI can be time-critical to document that an exposure exists and to address a number of public health hazard categories, such as urgent public health hazards.2 The EI team will ensure that OMB can perform a timely and expedited review for individual EIs under this generic clearance by submitting a standardized review packet that will provide all necessary information.
A.1.1. Privacy Impact Assessment
All of ATSDR’s biological assessments and some of the environmental assessments involve participants. ATSDR provides the participants with information on the EI process and what it can and cannot determine. After providing the participants this information, ATSDR asks for their consent to participate in the EI. Participation is completely voluntary; participants can stop participating in the EI at any time.
Overview of the Data Collection System
The primary objectives of the information collected for EIs under this generic information request are to assess exposures to environmental contaminants. Data obtained during EIs include biological (blood, urine and other biological samples) and environmental (water, air, soil and other environmental media) sampling. Information obtained from the participants assists the team in determining if exposure has occurred or is occurring. For each EI, a data collection system will include all of the measurements and procedures that are proposed to address data gaps in biological and environmental sampling.
The data collection system for EIs will be characterized by the following:
Who can use the EI Generic Clearance?
The DCHI SSB EI team and the ATSDR staff and partners in the DCHI cooperative agreement program will use the EI Generic Clearance for OMB submittals for each EI.
The DCHI cooperative agreement operates across ten ATSDR regions across the nation. In 2012, the site work in the ten regions were functionally reorganized (Federal Register, Volume 77 No. 221, 15 Nov 2012, Pages 68125-7; see https://www.federalregister.gov/articles/2012/11/15/2012-27533/statement-of-organization-functions-and-delegations-of-authority). ATSDR DCHI was divided into three functional units that administer its ten regions and its cooperative agreement program: Eastern Branch, Central Branch and Western Branch. The DCHI Science Support Branch (SSB) supports all three DCHI branches. It is uncertain at this time how many EIs across the states, regions, and branches will require an expedited approval at the same time. Given that EIs require timely approvals to investigate urgent public health hazards, we are requesting that OMB allow three simultaneous review tracks (Eastern, Central, and Western) to expedite a timely review of these time-sensitive submittals. Attachment 4 provides a map of the EPA regions and ATSDR branches.
Who can be included as part of the EI Generic Clearance?
EI participants will vary based on the nature of the EI but will likely include community members that are concerned about being exposed to environmental contamination. As previously described, the EI will be conducted if the exposed population can be identified at the site. Investigations tend to focus on the most highly exposed at the site, such as those living in proximity to the site. On occasion, small businesses may be included as EI participants. Based on past experience, we estimate that 2 percent of the EI participants per year may involve small businesses.
What types of questions may be asked as part of the EI Generic Clearance?
Attachment 3 provides a bank of questions that may be used to evaluate chemical exposure of individuals and communities for EIs. Attachment 3 provides the type of questions that may be used in the EI process to evaluate and interpret the results of the EI. It is not required that the questions in Attachment 3 be used in the EI, but these questions will be considered. Further details regarding the types of questions that may be included as part of the EI are discussed in the following sections.
What are the benefits to using the EI Generic Clearance?
The benefits to using the EI Generic Clearance include providing a standardized review package to be used for OMB submittal for each EI. The template will provide all needed information in a clear, concise document to expedite OMB review.
Information is generally gathered in a face-to-face interview with potentially exposed participants, but could occasionally be administered by phone or mail. Only those questions pertaining to a specific contaminant exposure route are asked in an investigation. In addition, only questions needed to determine the extent of exposure in a particular situation are asked. With these data, we can assess the presence or absence of a specific exposure and estimate how long and how frequently people have had contact with the chemical(s) of interest. The responses also provide data about exposure to other sources of the chemical(s).
General contact information (name, address, phone number, email) and comparison information on physical attributes (height, weight, age, race, gender) can account for approximately 20 questions per investigation. Some of this information is investigation-specific; not all of this data is collected for every investigation.
Although some of the information is entered on paper, where practical, we load the information collection form onto a laptop and record the answers electronically. ATSDR computers comply with the HHS Standard 2008-0007.001S for encryption. We generally interview people in their homes.
All environmental and biological sampling will be overseen by the federal or state EI lead. Environmental samples will be collected by appropriate EI personnel (assistance from state and local partners and contractors may be obtained) and shipped directly to the appropriate laboratories for analysis. Biological samples and documentation will be obtained by trained personnel, such as RNs, and shipped directly to qualified laboratories for analysis. Appropriate Quality Assurance Plans will be prepared and implemented by ATSDR, states, and contractors, as appropriate.
Items of Information to be Collected
Collecting identifying information is necessary to facilitate personal contact with participants, to obtain their consent to participate and to provide them with results. The information is also used by ATSDR to better interpret the results of the sampling. ATSDR uses the information only to contact respondents. Data is treated in a private manner, unless otherwise compelled by law.
ATSDR collects contact information (e.g., name, address, phone number, email address) to provide the participant with their individual results. General information, which includes height, weight, age/date of birth, race, gender, etc., may also be collected primarily on biological investigations to assist with results interpretation.
ATSDR asks participants questions about recreational or occupational activities that could increase their potential exposure to the contaminants under investigation. In addition, ATSDR collects information on other possible sources of chemical(s) exposure such as medicines taken, foods eaten, hobbies and jobs done, etc. That information represents their individual exposure history. Attachment 3 provides a bank of questions that may be used to compile the situation-specific EI.
Examples of the information that may be collected during environmental and biological sampling events are provided in Tables A-1.1 and A-1.2.
Table A-1.1
EI Activities Requiring Information from Property Owner or Resident for Environmental Sampling
Information Collection Methods |
Examples of Needed Information |
Demographic questionnaire (see Question Bank provided in Attachment 3 for suggested questions – use of these questions is optional) |
|
Household questionnaire (see Question Bank provided in Attachment 3 for suggested questions – use of these questions is optional)
|
|
Visual inventory of home by Field Interviewer |
|
Table A-1.2
EI Activities Requiring Information from Property Owner or Resident for Biological Sampling
Information Collection Methods |
Examples of Needed Information |
Demographic questionnaire (see Question Bank provided in Attachment 3 for suggested questions – use of these questions is optional) |
|
Household questionnaire (see Question Bank provided in Attachment 3 for suggested questions – use of these questions is optional)
|
|
Visual inventory of home by Field Interviewer |
|
Biological sampling of participant by appropriate health professional |
specific environmental contaminants
|
Symptom or medical information questionnaire (see Question Bank provided in Attachment 3 for suggested questions – use of these questions is optional) |
- Symptom or medical information related to exposure of interest |
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age
There are no web sites related to Exposure Investigations for children under 13 years of age.
A.2 Purpose and Use of Information Collection
Section A.1.1, Overview of the Collection System, provides information on the data collection procedures that will be used in the EI process. Data from ATSDR’s EI reports are used by public health professionals, environmental risk managers, and other decision makers to determine if current conditions warrant additional sampling or intervention to minimize or eliminate human exposure. They can also be useful in determining the source and extent of the exposures. After an environmental cleanup or other intervention to reduce exposure is completed, EIs can be useful in assessing the effectiveness of those actions. For past and future exposures, EIs can use exposure-dose reconstruction analysis (environmental sampling information and computer models) to estimate the contaminant levels that people may have been exposed to in the past or may be exposed to in the future.
ATSDR will produce needed information regarding important state and local public health issues to support public health action. Further, ATSDR expects to use these findings to improve our understanding of the public health impacts posed by environmental contaminants so that public health interventions may be implemented as quickly as possible. The results of the EI are generally not generalizable and are applicable only to the sampled participants.
EIs can involve environmental sampling (e.g., air, water, soil, or food), biological sampling (e.g., urine, blood, hair samples) or both. Standard air modeling to augment environmental data also been conducted for EIs. The number of participants in an individual EI generally ranges from 10 to 100.
Where needed, questions will be developed for the purpose of gathering information for the EI to assess environmental exposures (Tables A.1-1 and A.1-2). In deciding where and from whom to gather information, ATSDR considers the following:
Can we test the most appropriate and highly exposed population?
Can we identify vulnerable populations, including children or those more susceptible to specific contaminants?
Should we test children and other sensitive populations? For example, it may not be appropriate to collect the required sample (70-ml blood) for dioxins from a small child, pregnant woman, or an anemic or underweight person.
If we do pre- and post-testing to check intervention or environmental remediation effectiveness, how will participants be selected if the original participants are not available?
ATSDR asks approximately 12-20 questions per investigation that are pertinent to environmental exposure. This number can vary depending on the number of chemicals being investigated, the route of exposure (breathing, eating, touching), and number of other sources of the chemical(s) (e.g., products used, jobs done). Attachment 3 provides a list of potential questions that may be used in the EI, although their use is not required.
Topic areas for the questions shown in Attachment 3 include the following:
General Information: name, address, phone number, email, height, weight, age, race, ethnicity, gender
Media specific: air (indoor/outdoor), water (water source, plumbing), soil, and food
Other sources: occupational, hobbies, household chemical uses and house construction characteristics, lifestyle (e.g., smoking), medicines, symptoms, and/or health conditions, and foods
A.2.1. Privacy Impact Assessment
ATSDR only collects information that will help us interpret the laboratory data and recognize likely exposure scenarios. Once we conduct an EI, we match the unique answers given by participants with their laboratory results or environmental samples to determine whether intervention is needed on an individual level. The information collection is therefore inherently person- or location-specific. Section A.1 provides two examples of instances where the results of the EI has resulted in changes that have reduced exposure of communities to environmental contaminants.
Data are treated to protect privacy; access to computer files is password-protected and access is limited to authorized EI personnel, including contractors. All staff working on the project agrees to safeguard the data and not to make unauthorized disclosures. Published reports may present responses in aggregate form and no individuals are identified by name.
A.3 Use of Improved Information Technology and Burden Reduction
Generally, ATSDR interviews people in their homes either in-person or over the phone. Where practical, we will record the results of the interview electronically as we are interviewing the participants. The use of electronic data collection as compared to paper collection has steadily increased with time. Any data on laptops will be encrypted in accordance with information systems security requirements for safeguarding personally identifiable information. That information is stored in a secure database along with the laboratory and/or modeling results.
Several procedures may be used to sign up participants for the EI, such as newsletters or recruitment posters. Usually, the participants are targeted for inclusion in the EI and initial contact is made with potential participants through mail or phone.
A.4 Efforts to Identify Duplication and Use of Similar Information
ATSDR determined through literature and internet searches, discussions with other public health and environmental professionals, and attendance at meetings that other agencies are asking or have asked similar questions. However, their questions and resulting data are being used for population-based research and modeling, policy setting, or behavioral change through education. Since our information collection is inherently person- or location-specific, we cannot use the results of national probability surveys to inform our site-specific work. Again, the intent of the EI is not to generalize information to represent population based data, but to match the unique answers given by participants with their laboratory results or environmental samples to determine whether individual intervention is needed. We have, however, found some of the questions from other federal agencies’ surveys useful to identify appropriate chemical exposure questions. We have also used reference values from the Fourth National Report on Human Exposure to Environmental Chemicals as national comparison values for EI participant results (see http://www.cdc.gov/exposurereport/).
Below is a list of the agencies that we determined are asking similar questions; although, these surveys differ from ATSDR EIs as nationally representative.
NHAP=EPA National Human Activity Pattern Survey (NHAPS) - This EPA survey was a two-year probability-based telephone survey (n = 9,386) of exposure-related human activities in the United States. The primary purpose of NHAPS was to provide comprehensive and current exposure information over broad geographical and temporal scales, particularly for use in probabilistic population exposure models. NHAPS was conducted on a virtually daily basis from late September 1992 through September 1994. [ http://eetd.lbl.gov/IED/viaq/pubs/LBNL-47713.pdf]
NHEXAS=EPA National Human Exposure Assessment Survey – In the early 1990's, EPA initiated this population-based pilot study of the exposure of over 500 people in three areas of the U.S. to metals, pesticides, volatile organic compounds, and other toxic chemicals. Measurements were made of the air people breathed, the foods and beverages they consumed, and the soil and dust in/near their home. Ultimately, the EPA anticipates that the information gained from NHEXAS will help individuals, communities, states, the EPA, and other organizations understand the greatest health risks from various chemicals and decide whether steps to reduce those risks are needed. [http://www.epa.gov/heasd/edrb/nhexas.html]
NHANES = National Health and Nutrition Examination Survey – Teams of doctors, dentists, nutritionists, and health technicians go out to communities across the United States for the National Health and Nutrition Examination Survey (NHANES), which is updated annually. Since the early 1990s, CDC has surveyed approximately 5,000 people /year. Data from direct examination, testing, and measurement of national samples of the civilian noninstitutionalized population provide the basis for (1) estimates of the medically defined prevalence of specific diseases in the United States and the distribution of the population with respect to physical, physiological, and psychological characteristics and (2) analysis of relationships among the various measurements without reference to an explicit finite universe of persons (OMB approval #0920-0237) [ http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/questexam09_10.htm].
A.5 Impact on Small Businesses or Other Small Entities
Every effort is made to minimize the burden on all participants in EIs. Very few of our EIs (approximately 2 percent) have involved small businesses. On occasion, ATSDR has asked for participation from employees and attendees of daycare facilities and schools. Participation in an EI is voluntary and ATSDR strives to keep our questions to the minimum needed to interpret our results. When such an entity will be involved in an EI, ATSDR will identify these in each request for approval under this generic clearance.
A.6 Consequences of Collecting the Information Less Frequently
The vast majority of EIs are a one-time sampling or modeling event related to a specific exposure situation. At times, the results of the first sampling event require that we collect additional samples (either environmental or biological). Participants in EIs are generally asked one set of questions per sampling event. If we need to conduct additional sampling (e.g., to assess the effectiveness of an intervention), we would request that the participants answer another set of appropriate questions.
If ATSDR determines that additional investigation is needed, ATSDR would obtain a separate OMB clearance in order to specifically conduct that investigation.
There are no legal obstacles to reduce the burden.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances associated with this data collection. The data collection will fully comply with the guidelines of 5 CFR 1320.5.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day Federal Register notice was published in the Federal Register, Vol. 77, No. 61 on Thursday, March 29, 2012 (Attachment 2).
Below is a list of individuals and groups outside of the agency who were consulted to obtain their views on the availability of data, the clarity of instructions and information, and the completeness of the material.
Sharon Lee, Division of Environmental & Occupational Disease Control,
CA Dept of Health Services
& Facilitator of the Interstate Chemical Terrorism Workgroup
1515 Clay St., Suite 1901
Oakland CA 94612
Phone (510) 622-4478
Lee, Sharon (DHS-EHIB) [[email protected]]
– ATSDR solicited comments through her from the Interstate Chemical Terrorism Workgroup and requested information on other surveys. More than 30 state representatives reviewed the chemical exposure questions. ATSDR received oral and written comments from 10 representatives and added questions from other surveys. (2006)
Laura Fenster, PhD., Epidemiologist
Occupational Health Branch
CA Dept of Health Services
1515 Clay St., Suite 1901
Oakland CA 94612
Phone (510) 622-4448
Fenster, Laura (DHS-DEODC-OHB) [[email protected]]
- reviewed the chemical exposure questions and provided comments. She also provided information on other surveys and ATSDR incorporated some of the questions into the chemical exposure questions. (2006)
Bruce Bernard, M.D., M.P.H.
Medical Section Chief
Hazard Evaluations and Technical Assistance Branch
Div of Surveillance, Hazard Evaluations & Field Studies
National Institute for Occupational Safety and Health (NIOSH)
Centers for Disease Control and Prevention (CDC)
4676 Columbia Parkway R-10
Cincinnati, Ohio 45226
Phone (513) 841-4589
– provided review of portions of the package pertaining to occupational exposure. (2006)
A.9 Explanation of Any Payment or Gift to Respondents
In general, participants will not be reimbursed for their participation in an EI. Under some circumstances, there may be exceptions. In these cases, the Agency may provide stipends of up to $75.
If respondents participate in these kinds of studies remotely, via phone, or Internet, any proposed stipend needs to be justified to OMB and must be considerably less than that provided to respondents in in-person studies, who have to travel to the agency or other facility to participate. If such information collections include hard-to-reach groups and the agency plans to offer non-standard stipends, the Agency will provide OMB with additional justifications in the request for clearance of these specific activities.
A.10 Assurance of Confidentiality Provided to Respondents
Institutional Review Board
Federal Regulations for Protection of Human Subjects (45 CFR 46) state that “research means a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” In contrast, EIs are generally intended to be systematic investigations but are not designed to develop or contribute to generalizable knowledge. However, ATSDR does require that participants in EIs be fully informed of the potential risks and benefits of their participation and that the privacy of the participants’ information be protected.
Thus, EIs are generally a nonresearch activity and human subjects review by an Institutional Review Board (IRB) will not be required. On occasion as indicated in the Public Health Assessment Guidance Manual (ATSDR, 2005), state or CDC IRB review will be obtained if required by the purpose or methods of the EI (e.g., if a potential research question is asked, if vulnerable populations will be involved, or if circumstances would be considered greater than minimal risk in a research setting). All ATSDR EIs are reviewed by the NCEH/ATSDR Human Subjects Coordinator who is designated to make human subjects research-or-non-research determinations on a case-by-case basis. All IRB forms that are submitted for EIs will include all appropriate information from the Privacy Act including authority and purpose for collecting the data, with whom identifiable information will be shared, the voluntary nature of the information collection and the effect upon the respondent for not participating.3
A.10.1. Privacy Impact Assessment Information
A. It has been determined that the Privacy Act is applicable. The applicable System of Records Notice (SORN) is ATSDR’s broad SORN covering the majority of investigations involving personally identifiable information conducted by the Agency, 09-19-0001, “Records of Persons Exposed or Potentially Exposed to Hazardous or Toxic Substances.”
B. Identifying information such as name, address, phone number and email are collected. ATSDR uses the information only to contact respondents. Identifying information is necessary to facilitate the personal contact with respondents to conduct the survey, to obtain consent to participate, and to provide them their results.
All identifying information maintained by the agency will be managed by ATSDR and is subject to the ATSDR Comprehensive Record Control Schedule (CRCS), B-371, which contains authorized disposition instructions for ATSDR's administrative and program records.
For EIs completed by states, Sunshine Laws may apply. Sunshine Laws require openness in government, which may result in personal identification being accessible by the general public. For those states with Sunshine Laws, the consent form will include a statement indicating that these laws may apply.
Data are treated in a private manner, unless otherwise compelled by law. The paper document containing personal identifiers are kept in locked file cabinets at ATSDR. Access to computer files is password-protected and access is limited to authorized EI personnel. All staff working on the project agree to safeguard the data and not to make unauthorized disclosures. Any data on laptops will be encrypted in accordance with information systems security requirements for safeguarding personally identifiable information. Data are safeguarded in accordance with applicable statutes. Responses in published reports are presented in aggregate form and no individuals are identified by name.
C. Respondent Consent - Although EIs are not systematic investigations, all participants will be informed of the potential risks and benefits and will be asked to consent as participants in an EI. An EI-specific consent form will be prepared with all necessary information.
D. Voluntary Nature - Respondents are told that their participation in the EI is voluntary and they may refuse to answer any of the questions.
A.11 Justification for Sensitive Questions
ATSDR sometimes gathers information about individual characteristics (e.g., gender, age, weight, ethnicity, and race) to assist with interpretation for biological samples. For example, if ethnicity and race information is collected, the individual’s laboratory results are compared to similar ethnicity and race results in the National Report on Human Exposure to Environmental Chemicals (see citation above). Beyond that, generally, questions of a sensitive nature are not asked.
Occasionally, we may need to ask questions on symptoms, medical outcomes, or drug and medication use to assist us in interpreting an individual’s laboratory results. ATSDR may also ask questions pertaining to recent or current pregnancy status for one of two reasons: 1) pregnancy makes a woman and her unborn child more vulnerable to the effects of some chemicals (e.g., lead) or 2) some blood tests require a large quantity of blood. For example it is generally difficult to collect a 70-ml blood sample for dioxins from a small child, pregnant woman, or an anemic or underweight person.
Social security numbers are not needed nor will be requested.
A.12 Estimates of Annualized Burden Hours and Costs
A. Estimates of Annualized Burden Hours
Typically, ATSDR conducts approximately 12 EIs nationwide each year requiring a survey. Generally, the number of participants per investigation ranges from 10 to 100. Therefore, we estimate that the maximum total number of respondents annually is 1200 (12x100). Generally, we ask the questions once.
The time burden per respondent is estimated at 30 minutes. A typical survey may include up to 20 general questions taking less than 30 seconds each to respond and 20 more in-depth exposure specific questions requiring less than one minute each. This estimate is consistent with our results from EIs conducted in the past few years. The total estimated annual burden hours are 600.
Estimated Annualized Burden Hours
Type of Respondents |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (in hours) |
Total Burden (In Hours) |
EI participants |
1200 |
1 |
30/60 |
B. Annualized Cost to Respondents
Using a rate of $21.35/hr, the annualized cost to respondents for the hour burdens for the collection of information is $12,810. The hourly wage rate is based on the U.S. Department of Labor, Bureau of Labor Statistics’ most current statistics [May 2010 National Occupational Employment and Wage Estimates United States, last updated April 6, 2011].
Estimated Annualized Burden Costs
Type of Respondent |
Total Burden Hours |
No. Responses per Respondent |
Hourly Wage Rate |
Total Respondent Costs |
EI participants |
600 |
1 |
$21.35 |
$12,810 |
A.13 Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There are no other total annual cost burden to respondents or record keepers.
A.14. Annualized Cost to the Federal Government
Costs for ATSDR personnel and cooperative agreement state personnel were estimated based on experience with previous EI activities.
For the past 3 years, the annual budget for EIs has been $960,000. This includes: FTEs (including benefits), contractors, travel, per diem, and laboratory, supply, and equipment costs. We expect the budget to remain unchanged for the next three years.
A.15. Explanation for Program Changes or Adjustments
The burden hours have increased from 375 hours in the current inventory to 600 hours due to the addition of EIs conducted by cooperative agreement states requiring a survey each year.
A.16. Plans for Tabulation and Publication and Project Time Schedule
A.16-1 Project Time Schedule
A project Time Schedule will be provided for each EI submitted under the generic clearance. The Time Schedule for the EI will be variable, based on site-specific conditions. The time frame for collecting the environmental and/or biological data for an EI can range from one day to several months, depending on the sampled medium and complexity of the EI. The following is a general schedule that is anticipated for most EIs.
Activity Time Schedule
Start of data collection………………………………………1 month after OMB approval
Field work…………………………………………………...1-8 months after OMB approval
Analysis…………………………………………………...... 8-12 months after OMB approval
Respond to participants ………………………………......... 12-18 months after OMB approval
Written Report………………………………………........... 18-36 months after OMB approval
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
We are not requesting an exemption to the display of the expiration date.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to certification for Paperwork Reduction Act.
1 This correction is requested because NCEH activities are not authorized under CERCLA.
2 Public health hazard categories - Public health hazard categories are statements used in PHAs about whether people could be harmed by conditions present at the site in the past, present, or future. One or more hazard categories might be appropriate for each site. There are five public health hazard categories (ATSDR, 2005). 1) No public health hazard - A category used in ATSDR's public health assessment documents for sites where people have never and will never come into contact with harmful amounts of site-related substances. 2) No apparent public health hazard - A category used in ATSDR's public health assessments for sites where human exposure to contaminated media might be occurring, might have occurred in the past, or might occur in the future, but where the exposure is not expected to cause any harmful health effects. 3) Indeterminate public health hazard - The category used in ATSDR's public health assessment documents when a professional judgment about the level of health hazard cannot be made because information critical to such a decision is lacking. 4) Public health hazard - A category used in ATSDR's public health assessments for sites that pose a public health hazard because of long-term exposures (greater than 1 year) to sufficiently high levels of hazardous substances or radionuclides that could result in harmful health effects. 5) Urgent public health hazard - A category used in ATSDR's public health assessments for sites where short-term exposures (less than 1 year) to hazardous substances or conditions could result in harmful health effects that require rapid intervention.
3 The APPLETREE cooperative agreement program (Award No. TS11-1101) specifies that funded state, tribal, and territorial partners are limited to conducting nonresearch EIs. For these, ATSDR may provide technical assistance. The ATSDR Science Support Branch may lead either research or nonresearch investigations.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Exposure Investigations: Supporting Statement A |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy