Satisfaction Report
RefLab- A- Satisfaction Report.docx
Information Collections to Advance State, Tribal, Local and Territorial (STLT) Governmental Agency System Performance, Capacity, and Program Delivery
Satisfaction Report
OMB: 0920-0879
Attachment A
Customer Satisfaction Assessment of the CDC Infectious Diseases Reference Laboratories
Prepared by: Laboratory Quality Management Program, Office of the Director, National Center for Emerging and Zoonotic Infectious Diseases
July 2012
EXECUTIVE SUMMARY
When the CDC Infectious Diseases (ID) laboratories were inspected for Clinical Laboratory Improvement Amendment (CLIA) compliance in 2010, the Centers for Medicaid and Medicare Services (CMS) recommended a customer satisfaction questionnaire would benefit the laboratories and serve as a very good Quality Assurance tool.
A nine question survey was developed by the Laboratory Quality Management Program (LQMP) and the CLIA Subcommittee of the CDC ID Laboratories Quality Management System Steering Committee.
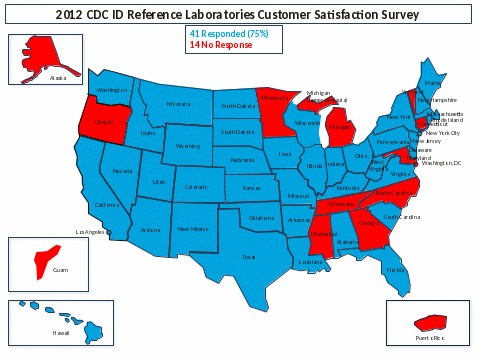
The questionnaire was sent to a total of 55 State and Territorial Public Health Laboratory (PHL) Directors on Monday, March 19, 2012. At the close of the survey on May 15, 2012, 41 responses had been received for a response rate of 75%.
The respondents were asked to rate aspects of CDC’s ID Reference Laboratory services. The choices were “Very Poor”, “Poor”, “Good”, “Very Good”, and “Not Applicable”.
The aspects rated and the summary responses were as follows:
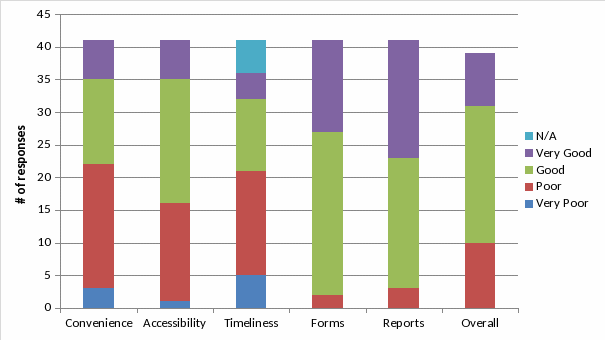
Convenience: 54% of respondents rated the ease of obtaining information about services offered as “Poor” or “Very Poor”.
Accessibility: 61% of respondents rated the ability to reach someone to interpret results as “Good” or “Very Good” while 39% rated accessibility as “Poor” or “Very Poor”
Timeliness: 52% of respondents stated that delivery of services within the time promised was “Poor” or “Very Poor”.
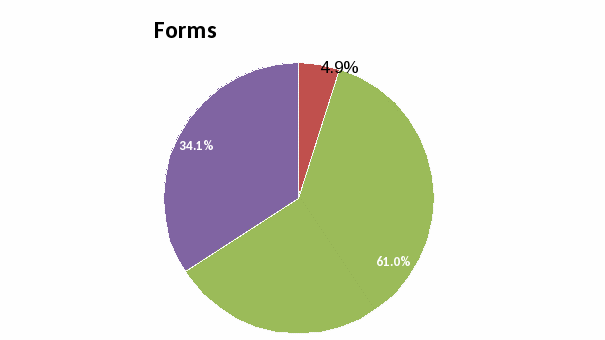
Forms: None of the respondents rated the ease of using the current specimen submission form as “Very Poor”. Most respondents rated this aspect as “Good” or “Very Good”.
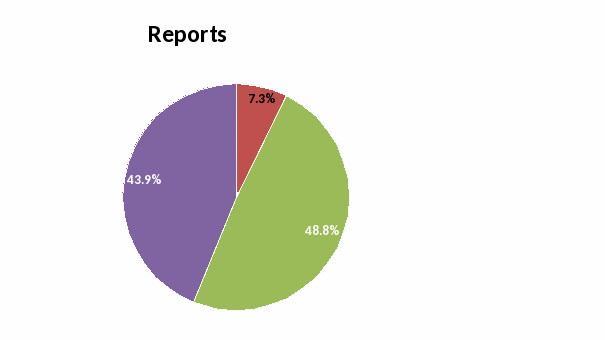
Reports: None of the respondents rated the ease of interpreting the current results forms as “Very Poor”. Most rated this aspect as “Good” or “Very Good”.
Overall: Although 75% of respondents rated CDC ID Reference Laboratory services as “Very Good” (21%) or “Good” (54%), 25% of respondents’ overall impression of the services offered by these laboratories was “Poor”.
The respondents were asked to respond to three further questions:
What are the three most important things CDC can do to improve the delivery of their ID reference laboratory services?
What are two things currently provided by CDC's ID reference laboratory services that are the most helpful to you?
What should be included in CDC's ID reference laboratory services that do not currently exist?
Suggestions for improvements:
Provide a directory of contact persons for each service (61%)
Provide a directory of services (specimen requirements and transport; turnaround time; frequency of testing, contact information) (56%)
Improve turnaround time (56%)
Improve access to reports (electronic reporting, secure email, online results) (27%)
Provide real-time information on frequency and projected turnaround times for individual tests (20%)
Improve communication when reporting is going to be delayed (15%)
Most useful services:
Provision of subject matter expertise and knowledge, and technical assistance (44%)
Provision of specialist testing not available elsewhere (32%)
Identification and confirmation of unusual isolates, and performance of molecular characterization (20%)
Suggestions for new services:
Provide a directory of services (specimen requirements and transport; turnaround time; frequency of testing, contact information) (22%)
Provide a directory of contact persons with phone numbers and email addresses organized by disease (12%)
Provide electronic laboratory reports (12%)
Response Plan:
The LQMP is developing a Laboratory Test Directory of Services that will be accessible to state PHLs and others on the CDC internet site. Each CDC ID laboratory will be asked to provide a list of tests offered, contact persons and their information, and specific details of test requirements and reporting.
An updated Specimen Submission Form has been created that will capture key information from the PHLs to enable test reports to be sent as an encrypted PDF via secure email. This will decrease the turnaround time for reporting results compared to the current practice of delivering results via the U.S. mail.
Selected PHLs will be asked to pilot these initiatives to ensure their functionality and that the end product meets their needs. Implementation will begin in FY 2013.
BACKGROUND
When the CDC Infectious Diseases (ID) laboratories were inspected for Clinical Laboratory Improvement Amendment (CLIA) compliance in 2010, the Centers for Medicaid and Medicare Services (CMS) recommended a customer satisfaction questionnaire would benefit the CDC laboratories and serve as a very good Quality Assurance tool.
METHOD
A nine question survey was developed by the Laboratory Quality Management Program (LQMP) and the CLIA Subcommittee of the CDC ID Laboratories Quality Management System Steering Committee. The questions were approved by OMB under the OSTLS Generic Information Collection Request (OMB No. 0920-0879) in February 2012. The questionnaire was sent to a total of 55 State and Territorial Public Health Laboratory (PHL) Directors on Monday, March 19, 2012 requesting input on the how the CDC ID Reference Laboratories are serving PHL needs.
The survey was distributed by email and utilized a web-based program, Survey Monkey, to collect the responses, which were due by Monday, April 20, 2012. The Association of Public Health Laboratories (APHL) assisted in providing email contact names and promoted the survey during their meeting at ICEID and through notices in their weekly eUpdate newsletter.
After the April closure date, a personalized email reminder was sent to the laboratories that had not responded, extending the due date another week and expressing our appreciation for their response. At the close of the survey on May 15, 2012, 41 responses had been received for a response rate of 75% (see map).
The first six questions allowed for easy checkbox answers. The respondents were asked to rate aspects of CDC’s ID Reference Laboratory services. The choices were “Very Poor”, “Poor”, “Good”, “Very Good”, and “Not Applicable”. The decision was made by the CLIA Subcommittee to omit a middle grade such as “Acceptable” to force an opinion from the respondents. The aspects rated were:
Convenience: Ease of obtaining information about services.
Accessibility: Ease of reaching someone to interpret results.
Timeliness: Was the service delivered within the time promised.
Forms: Ease of using current specimen submission form
Reports: Ease of reading and interpreting laboratory results
Overall: Overall impression of CDC’s ID reference laboratory services
The respondents were asked to respond to three further three open-ended, short answer questions:
What are the three most important things CDC can do to improve the delivery of their ID reference laboratory services?
What are two things currently provided by CDC's ID reference laboratory services that are the most helpful to you?
What should be included in CDC's ID reference laboratory services that do not currently exist?
RESULTS
Questions 1 - 6: Checkbox Answers
(Most frequent response in red)
|
Convenience |
Accessibility |
Timeliness |
Forms |
Reports |
Overall |
Very Poor |
3 |
1 |
5 |
0 |
0 |
0 |
Poor |
19 |
15 |
16 |
2 |
3 |
10 |
Good |
13 |
19 |
11 |
25 |
20 |
21 |
Very Good |
6 |
6 |
4 |
14 |
18 |
8 |
N/A |
0 |
0 |
5 |
0 |
0 |
0 |
No Answer |
0 |
0 |
0 |
0 |
0 |
2 |
Convenience:
53.6% of respondents rated the ease of obtaining information about
services offered as “Poor” or “Very Poor”.
This was reflected in the short answer questions (see below) where
the need for a Directory of Services was the most frequent request.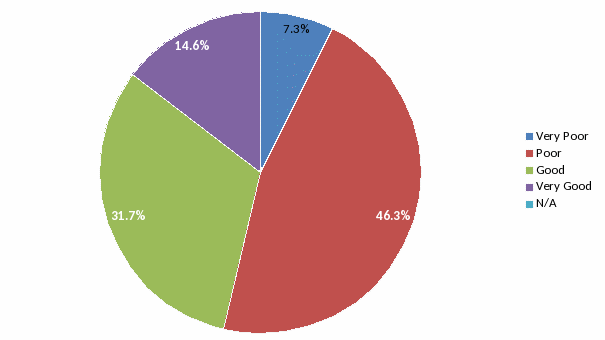
Timeliness: 52.1% of respondents stated that delivery of services within the time promised was “Poor” or “Very Poor”. This was reflected in the short answer questions (see below) with the request for improved turnaround time for testing.
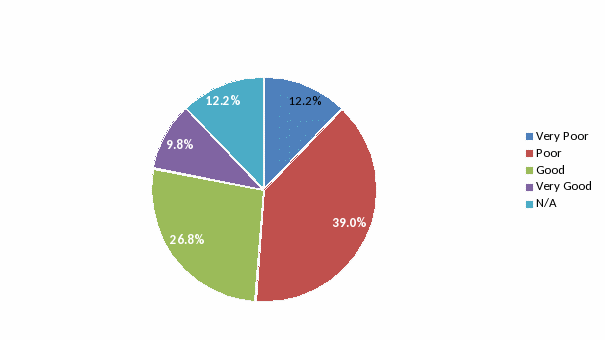
Accessibility: 39% of respondents rated the ability to reach someone to interpret results as “Poor” or “Very Poor”, while 61% rated accessibility as “Good” or “Very Good”. This was reflected with short answer questions (see below) where technical assistance and consultation were the most helpful services listed.

Forms and Reports: None of the respondents rated the ease of using the specimen submission form and interpreting the results form as “Very Poor”. The majority of the respondents rated these aspects as “Good” or “Very Good”. The short answer questions reflected their desire to have these forms and reports available electronically.
Overall: Although 75% of the respondents rated CDC ID Reference Laboratory services as “Very Good” (20.5%) and “Good” (53.8%), over 25% of respondents’ overall impression of CDC ID Reference Laboratories services was “Poor”.
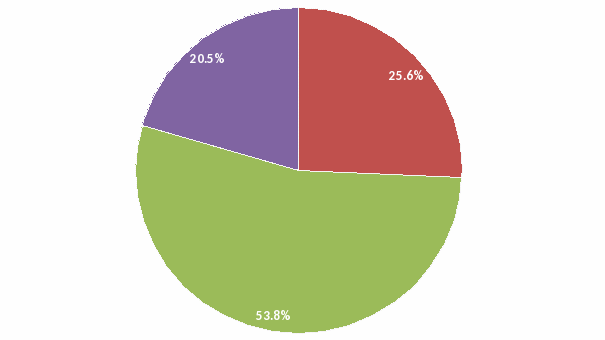
Questions 7 - 9: Short answer questions
The survey had three open-ended short answer questions to permit the public health laboratories to comment on areas needed for improvement and current services that are helpful. The comments were grouped according to similarity in nature and the top general comments are summarized below.
What are the three most important things CDC can do to improve the delivery of their ID reference laboratory services?
-
No. (%) respondents
Comment
25 (61%)
Provide a directory of contacts by services (phone and email)
23 (56%)
Provide a directory of services (specimen requirements & transport; turnaround time; frequency of testing, contact info)
23 (56%)
Improve turnaround time
11 (27%)
Improve access to reports (electronic reporting, secure email, online results)
8 (20%)
Post information on frequency and projected TAT for testing
6 (15%)
Improve communication of delays in reporting
Other comments included the need for published specimen submission forms with instructions on the web, preliminary reports and/or acknowledgement of specimen receipt, standardized report format, and the inclusion of methods used/biochemical tested on identification reports.
What are two things currently provided by CDC's ID reference laboratory services that are the most helpful to you?
-
No. (%) respondents
Comment
18 (44%)
Provides expertise & knowledge, technical assistance, and SME consultation
13 (32%)
Provides testing unavailable in-house
8 (20%)
Identifies and confirms unusual isolates and performs molecular characterization
Susceptibility testing of Mycobacterium tuberculosis and the telediagnosis service offered by DPDx were some of the most helpful services listed.
What should be included in CDC's ID reference laboratory services that do not currently exist?
-
No. (%) respondents
Comment
13 (32%)
Specific tests requested - none requested from multiple sites
9 (22%)
Directory of Services with testing available, specimen types, and TAT
5 (12%)
Directory of Contacts with phone and email addresses by disease
5 (12%)
Electronic Lab Reports/Paperless System/Web-based
11 (27%)
Nothing, no answer, not sure
Other general comments included standardized reporting, specimen tracking system, and training/webinars provided by SMEs.
Conclusions:
This inaugural Customer Satisfaction Survey indicates that the knowledge and technical services provided by the CDC ID Laboratories provide are invaluable to PHLs.
PHLs need an up-to-date Test Directory of Services and contact information to reach the appropriate person for advice and consultation. PHLs want faster turnaround times for testing, or at least better communication on expected turnaround time and notification of reporting delays. Our public health laboratory partners would like to be able to access their reports electronically. These are continuous quality improvements that should be accomplished in order to serve the public health community.
Response Plan:
The LQMP is developing a Laboratory Test Directory of Services that will be accessible to state PHLs and others on the CDC internet site. Each CDC ID laboratory will be asked to provide a list of tests offered, contact persons and their information, and specific details of test requirements and reporting.
An updated Specimen Submission Form has been created that will capture key information from the PHLs to enable test reports to be sent as an encrypted PDF via secure email. This will decrease the turnaround time for reporting results compared to the current practice of delivering results via the U.S. mail.
Selected PHLs will be asked to pilot these initiatives to ensure their functionality and that the end product meets their needs. Implementation will begin in FY 2013.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Thompson, Angela (CDC/OID/NCEZID) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-28 |
© 2026 OMB.report | Privacy Policy