5F Supplemental Instructions for Preparing an Institutional
PHS Applications and Pre-award Related Reporting (OD)
Attachment 5F PHS 398 Research Training Program Plan Form Instructions
398 Forms and Instructions (Electronic)
OMB: 0925-0001
PHS SF424 (R&R) Adobe Forms Version B Application Guide
8. Supplemental Instructions for Preparing an Institutional Ruth L. Kirschstein National Research Service Award (NRSA) Application
8.1 Introduction
All applicants must use the SF 424 (R&R) Application for Federal Assistance, following the instructional information in this section. The supplemental instructions found in this section (8) are for Institutional National Ruth L. Kirschstein National Research Service Award (NRSA) applications and include guidance and instructional information only when there is a difference in the required information to be submitted or there is a need for more specificity for the institutional research training program. Therefore, these supplemental instructions must be used along with the information found in Part I.1 – I.6 (see specific training grant review process at the end of Section 8) of this document.
These instructions apply to NIH-supported NRSA institutional research training programs (e.g., T32, T34, T35, T90). Some training programs are funded through Requests for Applications (RFAs) and may have special instructions.
Additionally, there are non-NRSA programs (e.g. T15, T37, D43, D71) which include research training under different regulatory authorities, and, while some of the information may be the same, it is important for individuals interested in those programs to carefully read the applicable Funding Opportunity Announcement (FOA) for specific program information and special application instructions. These training programs may have different eligibility requirements, submission dates, award provisions, and review criteria.
It is imperative that applicants become familiar with the NIH Research Training Activity code for which support is being requested, and applicants should carefully review the applicable FOA which contains more specific information associated with the award mechanism and the names of individuals that may be contacted for additional or clarifying information prior to submission of an application. Announcements for various training programs are issued periodically in the NIH Guide for Grants and Contracts, a weekly publication (http://grants.nih.gov/grants/guide/index.html).
This section includes instructions to be used when applying for competing (New, Renewal, Resubmission or Revision) institutional training grants, including both PHS Institutional Ruth L. Kirschstein National Research Service Awards (Kirschstein-NRSA) and non-NRSA awards. The contents include substitute both budget pages, and instructions for the Research Training Program Plan. Begin by reading the previous Sections 4 and 5, and then follow both sets of instructions.
Prior to preparing an application, review the Funding Opportunity Announcement (FOA) to which you are responding and consult with the appropriate PHS awarding component identified in the FOA. Current NIH-wide T32, T34 or T35 Kirschstein-NRSA Program Announcements (PA) are available at (http://grants.nih.gov/training/nrsa.htm). Note especially the eligibility requirements, submission dates, review criteria, award provisions, and payback provisions (when applicable). PAs are also issued periodically by the individual NIH Institutes or Centers in the NIH Guide for Grants and Contracts. This information is available from the appropriate PHS agency, from grantee offices of sponsored programs, or equivalent offices.
Please note that for Kirschstein-NRSA programs that include postdoctoral trainees, the Program Director must explain the terms of the payback service requirement to all prospective postdoctoral training candidates. A complete description of the service payback obligation is available in the relevant NRSA Program Announcement or the NIH Grants Policy Statement.
8.2 Institutional Research Training Programs
Prospective applicants are encouraged to review the T Kiosk for the most current program information. The T Kiosk includes information on NIH-wide Parent FOAs as well as IC-specific FOAs for a particular T programs. In addition, non-NRSA training programs are described here: http://grants.nih.gov/training/F_files_non_nrsa.htm.
8.3 Reserved
8.4 Specific Instructions for Institutional Training Grant Applications using the SF424 (R&R) Application
Standard instructions found in Parts I.1 – I.6 should be followed with the exceptions found in this section. Section numbers referenced below (e.g. 4.2 - 5.6) reflect those found in Part I.
8.4.1 Special Instructions for 4.2 Cover Component
Item 12. Proposed Project Start and Ending Dates
The usual starting date for an institutional Kirschstein-NRSA is July 1, but there are other possible starting dates. Consult the webpage of Standard Due Dates for Competing Applications (http://grants.nih.gov/grants/funding/submissionschedule.htm). Many PHS awarding components restrict submission and review dates to once a year. Applicants are strongly encouraged to contact the appropriate awarding component staff before submitting an application.
8.4.2 Special Instructions for 4.3 Research & Related Project/Performance Site Locations
List all of the locations where training, program management, and the research training experiences described in the Research Training Program Plan (8.7) will be performed. If a Project/Performance Site will be engaged in research involving human subjects, it is the responsibility of the applicant organization to assure that all Project/Performance Sites comply with the human subject protection regulations in 45 CFR part 46 and NIH policies for the protection of human subjects. For research involving live vertebrate animals, the applicant organization must supply information for all training sites where animals will be used by trainees. The applicant organization is responsible for assuring that all Project/Performance Sites have a current Animal Welfare Assurance and comply with the PHS Policy on Humane Care and Use of Laboratory Animals.
8.4.3 Special Instructions for 4.4 Research & Related Other Project Information Component
Item 1. Are Human Subjects Involved?
Check “Yes” if training plans include or potentially will include involvement of trainees in projects that include human subjects as defined by 45 CFR 46. Check “Yes” even if the proposed project is exempt from Regulations for the Protection of Human Subjects. If no activities involving human subjects are planned, check the No box, and skip the rest of block 1. This field is required.
The institution must ensure that trainees who will be involved in the design or conduct of research involving human subjects receive training in human subjects protections. It is the institution’s responsibility to ensure that trainees are properly supervised when working with human subjects.
In many instances, trainees supported by institutional training grants will be participating in research supported by research project grants for which the IRB approval or a determination of exemption exists. Existing IRB approval is sufficient for trainees, provided that the IRB determines the research would not be substantially modified by the participation of a trainee. The appropriate grants must be identified along with their IRB approval dates or exemption designation in Section 7 of the Research Training Program Plan.
Note that IRB approval information for the full training grant application is not required at the time of submission, but will be requested as Just-in-time (JIT) information prior to award. If an award is made and the research is not exempt from requirements stipulated in 45 CFR 46, and trainees will participate in research for which IRB review and approval does not otherwise exist, human subjects may not be involved and trainees may not participate in research involving human subjects unless the engaged institution has an approved FWA on file with OHRP, certification of the date of IRB approval has been submitted to and accepted by the PHS agency, and NIH requirements for human subjects protections have been addressed (see instructions in Part II, Supplemental Instructions for Preparing the Human Subjects Section of the Research Plan, and the NIH Grants Policy Statement (http://grants.nih.gov/grants/policy/nihgps_2011/index.htm)).
These policies apply to all Performance Sites.
Item 2. Are Vertebrate Animals Used?
Check “Yes” if training plans include or potentially will include trainees in projects involving the use of live vertebrate animals at any time during the proposed project period, either at the applicant organization or at any other training site or collaborating institution.
Otherwise, check the No box, and skip the rest of block 2. This field is required.
In many instances, trainees supported by institutional training grants will be participating in research supported by research project grants for which the IACUC review and approval exists. This existing IACUC approval is sufficient for trainees, provided that the research would not be substantially modified by the participation of a trainee. The appropriate grants must be identified along with their IACUC approval dates in Section 8 of the Research Training Program Plan.
Note that vertebrate animal approval information for the full training grant application is not required at the time of submission, but will be requested as Just-in-time (JIT) information prior to award. If an award is made and trainees will participate in research for which IACUC approval does not otherwise exist, vertebrate animals may not be involved and trainees may not participate in research utilizing vertebrate animals unless the institution has an approved Assurance on file with OLAW, certification of the date of IACUC approval has been submitted to and accepted by the PHS agency, and NIH requirements for the use of vertebrate animals have been addressed.
The institution must ensure that trainees are enrolled in the institution's animal welfare training and occupational health and safety programs for personnel who have contact with animals. It is the institution's responsibility to ensure that trainees are properly supervised when working with live vertebrate animals.
These policies apply to all Performance Sites.
Item 7. Project Summary/Abstract
Summarize the objectives, rationale and design of the research training program. Provide information regarding the research areas and scientific disciplines encompassed by the program. Include a brief description of the level(s) (i.e., undergraduate, predoctoral, postdoctoral, faculty) and duration of the proposed training, the projected number of participating trainees and their anticipated levels of experience. This section must be no longer than 30 lines of text and must follow the required font and margin specifications.
Item 8. Project Narrative
Using no more than two or three sentences, describe the relevance of this research training program to public health. In this section, use plain language that can be understood by a general, lay audience.
Item 9. Bibliography & References Cited
This item should be used only to cite references supporting the need, rationale, and approach for the training program described in the PHS 398 Research Training Program Plan. Note that the Literature Cited section of the Research Plan is captured in this section (unlike the placement in the PHS 398). Do not include lists of publications of project directors, mentors or trainees in this section, as this information will be included in the biosketches and Data Tables.
Item 10. Facilities & Other Resources
Describe the facilities and resources that will be used in the proposed training program. Indicate in what ways the applicant organization will support the program, financial or otherwise (e.g., supplementation of stipends, protected time for mentoring, support for student activities). This could also include, for example, space, shared laboratory facilities and equipment, funds for curriculum development, release time for the PD/PI and participating faculty, support for additional trainees in the program, or any other creative ways to improve the climate for the establishment and growth of the research training program.
Item 12. Other Attachments
Leave blank, unless specifically requested in the FOA.
8.4.4 Special Instructions for 4.5 Senior/Key Person Profile (Expanded) Component
Complete the Profile for the Program Director according to instructions in Section 4.5.
If multiple PD/PIs are proposed, explain in the Program Plan your rationale for how this will facilitate program administration. If your application involves Multiple PD/PIs, follow the directions in Section 4.5 to designate the Contact PI and to assign the PD/PI role to other senior/key persons. Additionally, the application must include a Multi-PD/PI Leadership Plan emphasizing how it will benefit the program and the trainees. Do not submit a leadership plan if you are not submitting a Multiple PD/PI application. See Part I Section 8.7, Items 3 and 10 for information associated with Multiple Program Directors.
Complete the profiles for other senior/key persons according to instructions in Section 4.5.
The Program Director(s) (in case of multiple PD/PIs), training faculty and any other individuals whose contributions are critical to the development, management and execution of the Research Training Program Plan in a substantive, measurable way (whether or not salaries are reimbursed) should be identified as senior/key persons. These would include co-Director(s), if applicable, and program staff. Since these efforts are not project related research endeavors, they should not be identified in Other Support information. Do not include proposed mentors and training faculty members (other than senior/key persons) in this section. Biographical Sketches for mentors and participating faculty will be included in the PHS 398 Research Training Program Plan Component, Section 8.7 Item 12, Participating Faculty Biosketches.
8.4.5 Special Instructions for 4.7 Research & Related Budget
This form is required for use in conjunction with the PHS 398 Training Budget for the R90 portion of T90/R90 applications, and is the only budget form that should be used for K12 applications. Otherwise this form should only be used when allowed or required in an FOA or IC-specific notice or announcement. Follow instructions in Section 4.7.
8.4.6 Special Instructions for 4.6 PHS 398 Cover Page Supplement
Item 2. Human Subjects
If you checked “Yes” to Human Subjects and “Yes” to Clinical Trial on the R&R Other Project Information form, you must check either “Yes” or “No” to indicate whether plans include or potentially include trainee participation in projects that are NIH-Defined Phase III Clinical Trials.
Item 4. Inventions and Patents
Not applicable – leave blank.
Item 5. Program Income
Check “No”.
Item 6. Human Embryonic Stem Cells (HESC)
Check “Yes” if training plans include or potentially will include involvement of trainees in projects that include human embryonic stem cells.
If “Yes”, list the 4-digit NIH Registration Number of the specific cell line(s) from the NIH Human Embryonic Cell Registry, or check the box indicating that the specific stem cell line cannot be referenced at this time.
Note that individual project HESC information is not required at the time of application, but will be requested as Just-in-time (JIT) information prior to award. At that time, the NIH will require information regarding project title, mentor and specific cell line(s) from the registry (http://stemcells.nih.gov/research/registry/defaultpage.asp) for each trainee utilizing human embryonic stem cells in a research project. Trainees may not participate in human embryonic stem cell related research until this information is provided.
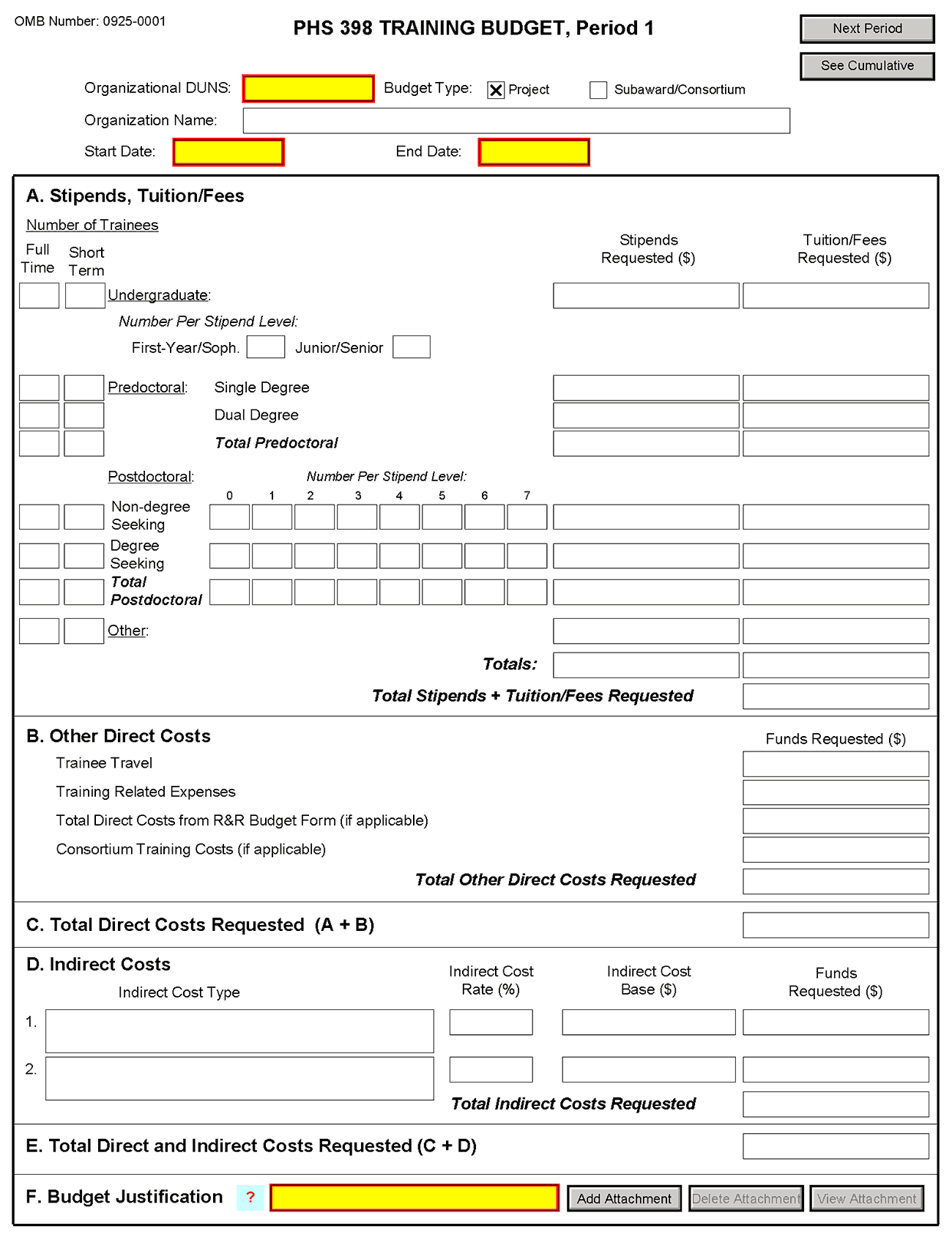
8.5 PHS 398 Training Budget
For NRSA training grant programs, use the PHS 398 Training Budget form pages and follow the instructions below. Refer to the relevant FOA or consult the PHS awarding component for current stipend levels and allowable costs.
For Non-NRSA training grant programs refer to the FOA for instructions regarding which Budget Form pages to use and how to complete them.
If you are requesting a budget of $500,000 direct costs or more for any year, contact the awarding component to determine whether you must obtain prior approval before submitting the application. Some Institutes/Centers do not require prior approval. (See Policy on the Acceptance for Review of Unsolicited Applications That Request $500,000 or More in Direct Costs.)
PHS 398 Training Budget, Periods 1 through 5
Part A. Stipends, Tuition/Fees
Enter the number of trainees, total stipend amount and total tuition/fees for each trainee category as appropriate. Use the current Institutional Kirschstein-NRSA stipend schedule, (http://grants.nih.gov/training/nrsa.htm). If a category contains different stipend levels, e.g., for varying levels of postdoctoral experience and/or varying appointment periods, itemize in the appropriate blocks. Enter the total stipends for all categories.
See http://grants.nih.gov/grants/guide/notice-files/NOT-OD-06-093.html for NIH policy regarding payment of tuition and fees. Tuition at the postdoctoral level is limited to that required for specified courses that are to be described in the Budget Justification (Part F.). Tuition and fees may be requested only to the extent that the same resident or nonresident tuition and fees are charged to regular non-Federally supported students and postdoctoral fellows. Where applicable, trainees should be divided into non-degree-seeking and degree-seeking categories. Note that health insurance is not included as part of this budget category. See the Training Related Expenses category below. Grantees should request full needs. The formula currently in effect will be applied by the NIH awarding component at the time an award is calculated.
Part B. Other Direct Costs
Enter the total costs for Trainee Travel, Training Related Expenses, Total Direct Costs from R&R Budget Form (if applicable) and Consortium Training Costs (if applicable).
Trainee Travel
Some NIH awarding components pay a flat rate per trainee for trainee travel for all long-term trainees. See the appropriate FOA and/or contact the awarding component to determine the amount provided for travel. In the budget justification, state the purpose of any travel, giving the number of trips involved, the destinations, and the number of trainees for whom funds are requested. PHS policy requires coach class air travel be used. Justify foreign travel in detail, describing its importance to the training experience. Enter the total amount requested in the Trainee Travel column.
Training Related Expenses (TRE)
Funds to defray other costs of training, such as health insurance (self-only or family), staff salaries, consultant costs, equipment, research supplies, staff travel, etc., are requested as a lump sum based on the amounts specified in the FOA and at http://grants.nih.gov/grants/guide/notice-files/NOT-OD-06-093.html for each predoctoral and postdoctoral trainee. Based on the number of trainees at the predetermined rate, enter the total dollar figure.
Health insurance (self-only or family, as applicable) is an allowable cost that may be requested as part of training related expenses, but only to the extent that the same health insurance fees are charged to regular non-Federally-supported students and postdoctoral fellows. The allowable TRE amount will be awarded as a lump sum. No further itemization or explanation is required.
The awarding Institute/Center will apply the Training Related Expenses level established for NRSA Institutional programs for the relevant fiscal year at the time of award.
Total Direct Costs from R&R Budget Form (if applicable)
Certain FOAs allow funds to cover costs for items other than those specified above. Use Research & Related Budget Pages, Sections A through I and K, to submit those costs. Total Direct Costs from the Research & Related Budget page should be inserted here. This line should not include any applicant indirect costs.
Consortium Training Costs (if applicable)
If training is occurring at more than one institution, and any transfer of funds between institutions occurs, the Training Subaward Budget Attachment Form should be used. (See Section 4.8). Total the direct costs from the Subaward Budget Attachment Forms and insert here. The applicant institution is responsible and accountable for any arrangements, expenditures, and submission of all required forms when more than one institution is involved in the research training program.
Part C. Total Direct Costs Requested
The sum of Sections A + B will be calculated automatically.
Part D. Indirect Costs
Facilities and Administrative (F&A) costs under Institutional Kirschstein-NRSAs, other than those issued to U.S., state, or local government agencies, will be awarded at 8%, excluding tuition/fees, equipment, and sub-grants and contracts in excess of $25,000. Equipment and consortium costs are also excluded from the F&A costs on those training grants where Training Related Expenses are not calculated and awarded on a lump-sum basis, such as the Minority Access to Research Careers Program (MARC) or Career Opportunities in Research (COR) Undergraduate Research Training Program. State and local government agencies will receive the full F&A cost rate.
Indirect Cost Type: Enter “F&A”
Indirect Cost Rate (%): Enter “8”
Indirect Cost Base ($): Enter the sum of Stipends and Total Other Direct Costs requested, regardless of whether those direct costs were listed on the PHS 398 Training Budget page or Research & Related Budget page. Indirect costs are not paid on Tuition/Fees, equipment, and sub-grants and contracts in excess of $25,000.
Funds Requested ($): Enter the product of Indirect Cost Rate multiplied by Indirect Cost Base.
Part E. Total Direct and Indirect Costs Requested (C+D)
The sum of Total Direct Costs Requested and Total Indirect Costs Requested will be calculated automatically.
Part F. Budget Justification
A detailed justification is to be attached only for the first budget period, but should reflect the entire budget period. Explain in detail the composition of any of the above items, as necessary. Itemize tuition and individual fees. If tuition varies, (e.g., in-state, out-of-state, student status) identify these separately. If tuition is requested for postdoctoral trainees, the specific courses must be described in the application. If trainee travel is not paid at a flat rate per trainee by the awarding component, state the purpose of any travel, giving the number of trips involved, the destinations, and the number of individuals for whom funds are requested, bearing in mind that PHS policy requires coach class air travel be used. For postdoctoral training slots, justify the stipend levels requested.
Any foreign travel must be justified in detail, describing its importance to the training experience and considering the type of opportunities available for training, how those opportunities differ from and complement those offered by the grantee institution, and the relationship of the proposed off-site training experience to the career stage of the grantee.
This budget justification should apply only to funds requested on the PHS 398 Training Budget form. When the Research & Related Budget Form is also used, two separate budget justifications are required, each covering the costs required in the particular budget component. Combining the information into a single upload is acceptable; however, each budget component requires a budget justification attachment so the same budget justification will need to be included in both budget components.
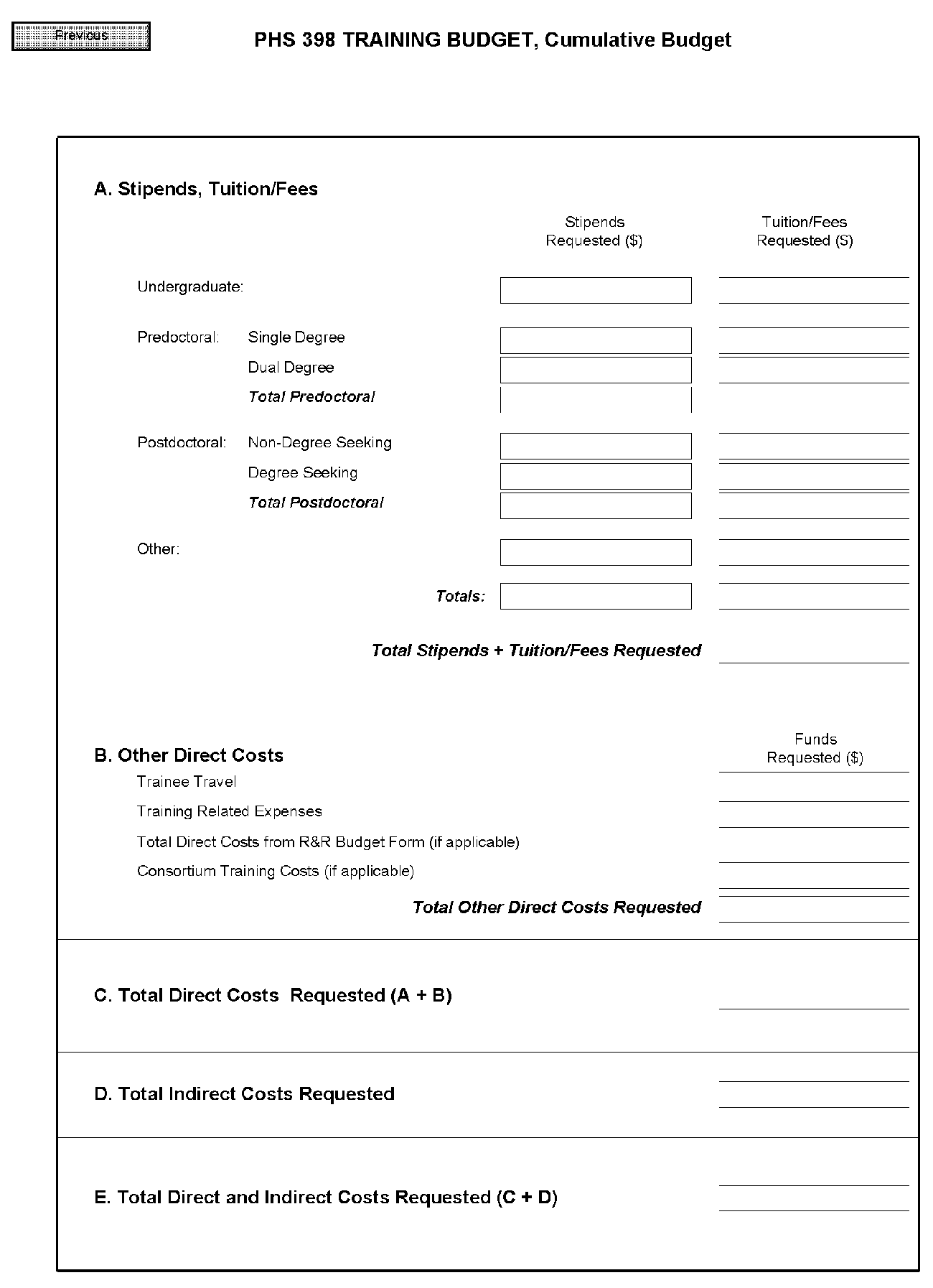
PHS 398 Training Budget, Cumulative Budget
All values on this form are calculated automatically. They present the summations of the amounts that you have entered previously, for each of the individual budget periods. Therefore, no data entry is allowed or required.
If any of the amounts displayed on this form appears to be incorrect, you may correct it by adjusting one or more of the values that contribute to that total. To make any such adjustments, you will need to revisit the appropriate budget period form(s) to enter corrected values.
8.6 PHS 398 Training Subaward Budget Attachment(s) Form
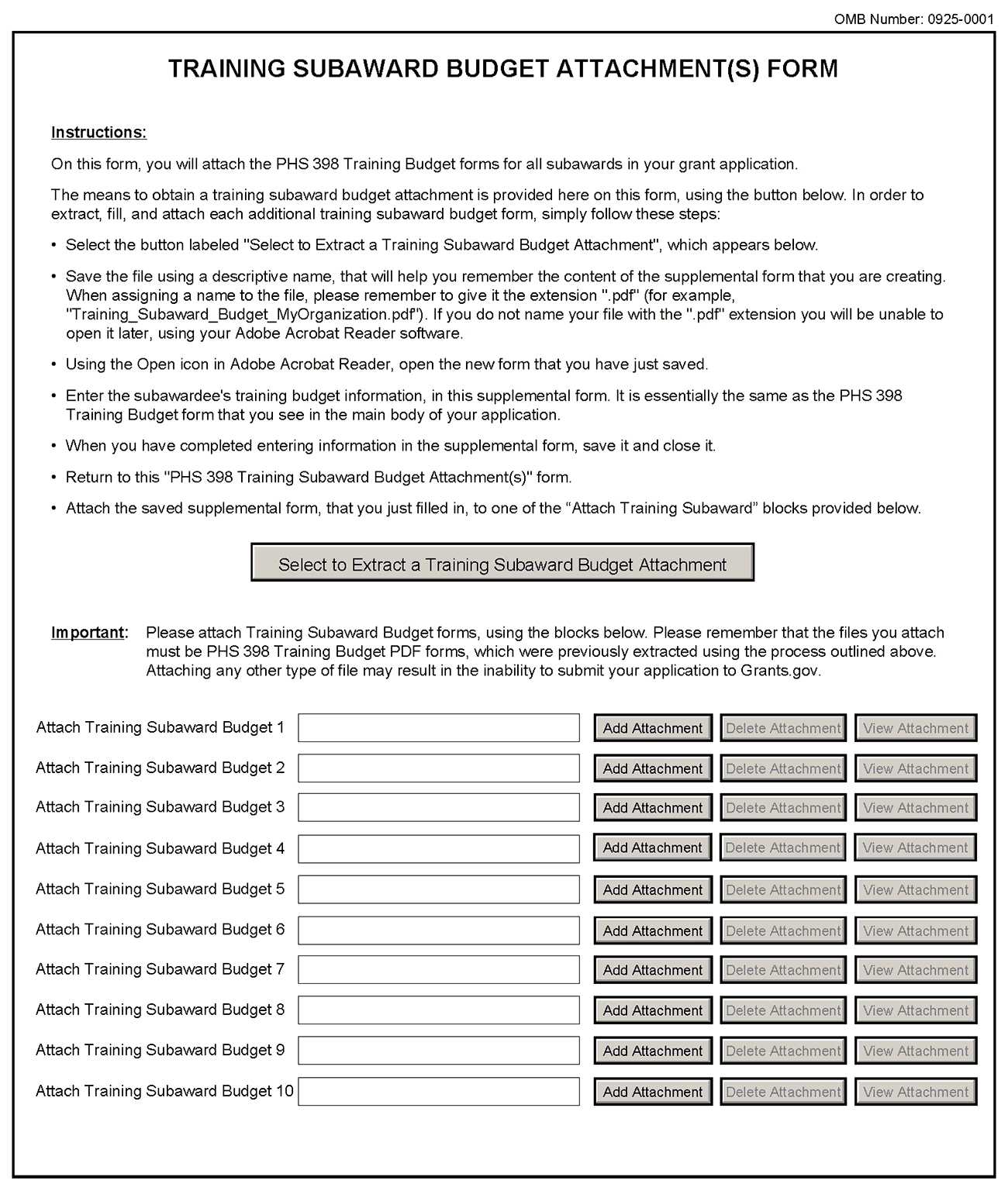
This form should be used when proposing subawards to other institutions. Complete the Subaward Budget for each contractor or collaborating institution. For NRSA programs, this is not common but is usually encountered when a portion of the training program takes place at a site other than the grantee institution via a collaborative or consortium arrangement. In such situations, the grantee institution is responsible and accountable for acceptable training arrangements, expenditure of funds and the submission of all required forms.
This component accommodates up to 10 separate subaward budgets. If you are submitting an application with >10 subaward budgets, budgets 11 and above should be converted to PDF and included as part of Section 8.5 Part F. Budget Justification, of the parent budget (PHS 398 Training Budget). Reminder, the sum of all subaward budgets; e.g., those attached separately and those provided as part of the budget justification, must be included in Part B. Consortium Training Costs on the PHS 398 Training Budget.
To start the process, the applicant organization should:
Select the Subaward Budget Attachment Form from the Optional Documents in the Grant Application Package.
Open the form, and click the “Select to Extract a Training Subaward Budget Attachment” button in the middle of the form. A “SAVE” dialog box appears.
Save the file locally using the first ten letters of the consortium organization’s name and use “.pdf” as the file extension. (The extracted file is an Adobe PDF file.) Once you have saved the file there is no need to extract another budget attachment. Doing so may cause you to lose any data already stored in the saved file.
E-mail the extracted, saved form to the consortium grantee. Note: consortium grantees must have installed Adobe Reader before they can complete the form. The consortium grantee should complete all the budget information as instructed in the R&R Budget component instructions in Section 4.7. Note: Organizational DUNS and Name of Organization fields must reflect that of the subaward/consortium grantee.
The consortium grantee must complete the budget component and e-mail it back to the applicant organization.
Return to the Subaward Budget Attachment Form and attach the consortium grantee’s budget to one of the blocks provided on the form.
Submitting Subaward Budgets that are not Active for all Periods of the Prime Grant
When submitting subaward budgets that are not active for all periods of the prime grant, fill out the subaward R&R Budget form and include only the number of periods for which the subaward is active. The budget period start/end dates reflected in each period should reflect the corresponding prime budget period start/end dates. This approach is the most workable solution to the limitations in existing forms that do not allow an “empty” budget period and do not allow submission of a subaward budget with zero effort to skip a budget period.
For example, suppose the prime has filled out a budget form with the following periods:
period 1 Jan 1, 2010 – Dec 31, 2010
period 2 Jan 1, 2011 – Dec 31, 2011
period 3 Jan 1, 2012 – Dec 31, 2012
period 4 Jan 1, 2013 – Dec 31, 2013
period 5 Jan 1, 2014 – Dec 31, 2014
Now, suppose there is a subaward that performs in support year 1 and does not become active again until support year 4. The subaward can fill out the first two periods of their budget form as follows:
period 1 Jan 1, 2010 – Dec 31, 2010 (dates correspond to prime period 1)
period 2 Jan 1, 2013 – Dec 31, 2013 (dates correspond to prime period 4)
It is not necessary that the budget period numbers between the prime and subaward match; the correlation is reflected in the dates. Do be careful, however, that the dates exactly match what is listed for the period in the prime budget.
Note this approach may cause a validation warning regarding the NIH $500,000 per year limit on direct costs, therefore you should document in both the cover letter and the subaward budget justification that the subaward is only active for specific periods of the prime. Appropriate NIH staff has access to the cover letter and reviewers have access to the budget justification. This documentation will make the date correlation immediately apparent and will help avoid any confusion.
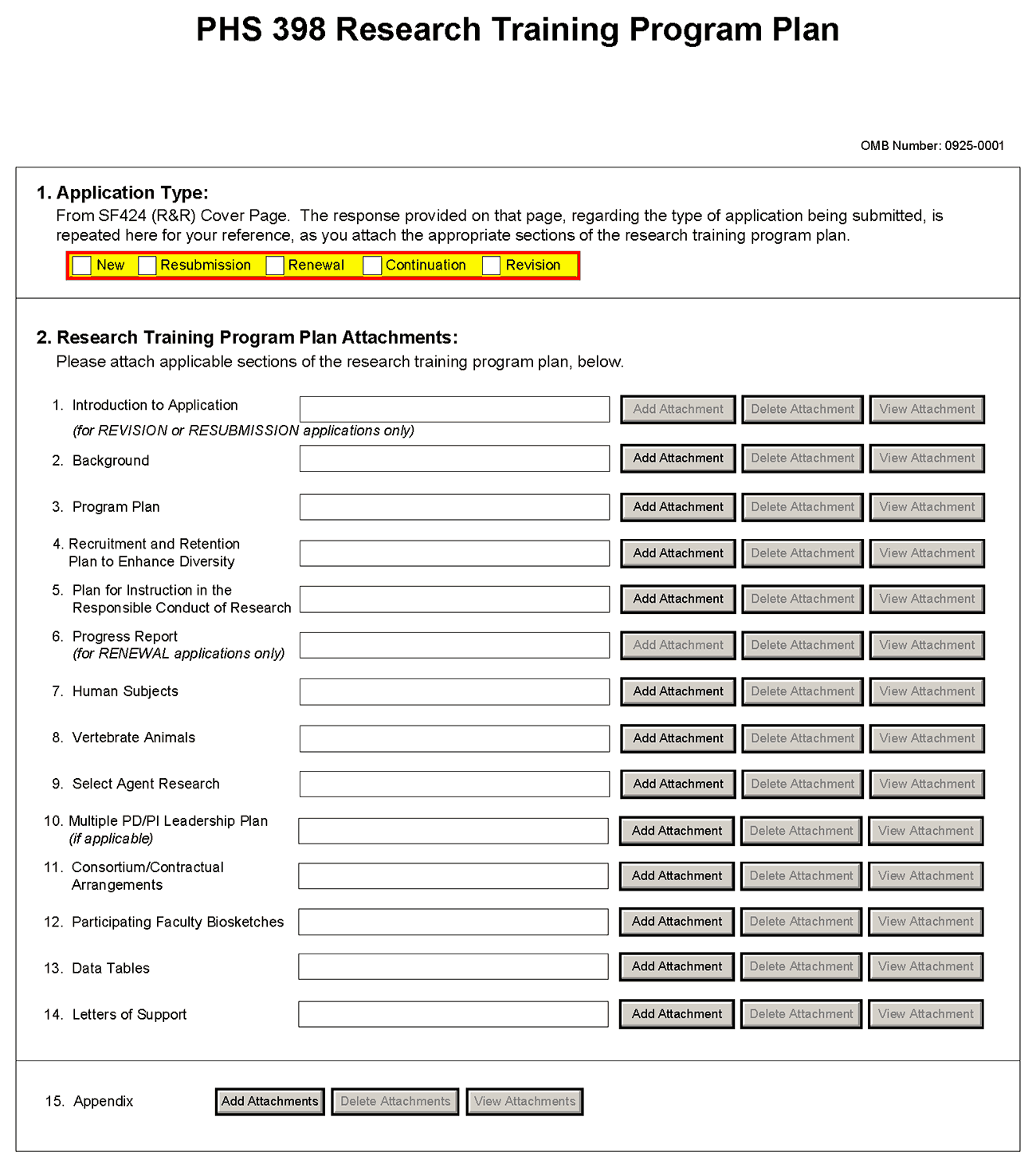
8.7 Research Training Program Plan Component
Before preparing the Research Training Program Plan, be sure to check the specific instructions in the Funding Opportunity Announcement (FOA) to which you are responding. Contact the appropriate PHS awarding component, which may have further advice or suggestions on completing your application, including the data tables mentioned below.
Note that there are page limits for certain sections. Information in Items 2.2 - 2.4, collectively, may not exceed 25 pages (note that this may span to 27 pages in the eRA Commons application image due to white space inserted at the end of sections when separating files). Information in items 2.5 - 2.14 is not part of the 25 page limitation. Please see NOT-OD-11-039 and NOT-OD-11-076. The information provided in required data tables (see below) will not be counted toward the page limitation. These tables should be numbered consecutively and titled as shown, even if some are not required by the PHS awarding component to which you are applying or in the FOA to which you are responding. Indicate by table number and title, those tables that are intentionally omitted. Additional tables that are not required may be included in the Research Training Program Plan, however, these tables will count as part of the 25 page limit. Additional tables not specified in these instructions should be identified by letter, rather than number to avoid confusion with the sequentially numbered required tables.
The instructions for Data Tables 1-12 are located on the OER Web site at http://grants.nih.gov/grants/ funding/424/index.htm#datatables. Please read the Introduction to the Data Tables before beginning to prepare your application. This section includes important definitions that should be used consistently both in the Data Tables and in all other parts of the application. The tables described in Item 2.13 should be included in the application at the point indicated and should not be inserted in the narrative for Items 2.2 – 2.5.
The Research Training Program Plan should include sufficient information needed for evaluation of the program, independent of any other document (e.g., previous application). Be specific and informative, and avoid redundancies.
Item 1 Application Type
This field is pre-populated from the SF424 (R&R) Cover Component. Corrections to this field must be made in that component.
Item 2 Research Training Program Plan Attachments
(See also Section 2.3.2 Creating PDFs for Text Attachments.)
Although many of the sections of this application are separate PDF attachments, page limitations referenced in the instructions and/or funding opportunity announcement must still be followed. Agency validations will include checks for page limits (and use of appropriate font). Some accommodation will be made for sections that, when combined, must fit within a specified limitation.
Text attachments should be generated using word processing software and then converted to PDF using PDF generating software. Avoid scanning text attachments to convert to PDF since that causes problems for the agency handling the application. In addition, be sure to save files with descriptive file names.
Do not include any information in a header or footer of the attachments. A header will be system-generated that references the name of the PD/PI. Page numbers for the footer will be system-generated in the complete application, with all pages sequentially numbered.
Since a number of reviewers will be reviewing applications as an electronic document and not a paper version, applicants are strongly encouraged to use only a standard, single-column format for the text. Avoid using a two-column format since it can cause difficulties when reviewing the document electronically.
Full-sized glossy photographs must only be included within the page limitations of the Research Training Plan. The maximum size of images to be included should be approximately 1200 x 1500 pixels using 256 colors. Figures must be readable as printed on an 8.5 x 11 inch page at normal (100%) scale.
Investigators must use image compression such as JPEG or PNG. Do not include figures or photographs as separate attachments either in the Appendix or elsewhere in the application.
Separate Attachments
Separate attachments have been designed for the Research Training Program Plan sections to maximize automatic validations conducted by the eRA system. When the application is received by the agency, all of the Research Training Program Plan sections will be concatenated in the appropriate order so that reviewers and agency staff will see a single cohesive Research Plan.
When attaching a PDF document to the actual forms, please note you are attaching an actual document, not just pointing to the location of an externally stored document. Therefore, if you revise the document after it has been attached, you must delete the previous attachment and then reattach the revised document to the application form. Use the “View Attachment” button to determine if the correct version has been attached.
Follow page limitations as specified in Funding Opportunity Announcements.
All applications and proposals for NIH funding must be self-contained within specified page limitations. Agency validations will include checks for page limits. Note that while these computer validations will help minimize incomplete and/or non-compliant applications, they do not replace the validations conducted by NIH staff. Applications found not to comply with the requirements may be delayed in the review process. Unless otherwise specified in an NIH solicitation, Internet Web site addresses (URLs) may not be used to provide information necessary to the review because reviewers are under no obligation to view the Internet sites. Moreover, reviewers are cautioned that they should not directly access an internet site as it could compromise their anonymity.
Notice of Proprietary Information
Applicants are discouraged from submitting information considered proprietary unless it is deemed essential for proper evaluation of the application. However, when the application contains information that constitutes trade secrets, or information that is commercial or financial, or information that is confidential or privileged, make sure you have checked the “Yes” box of question #3 in the “Other Project Information” component. Identify the pages in the application that contain this information by marking those paragraphs or lines with an asterisk (*) in the left-hand margin. Include a legend at the beginning of Section 2, similar to “The following sections marked with an asterisk contain proprietary/privileged information that (name of Applicant) requests not be released to persons outside the Government, except for purposes of review and evaluation.”
When information in the application constitutes trade secrets or information that is commercial or financial, or information that is confidential or privileged, it is furnished to the Government in confidence with the understanding that the information shall be used or disclosed only for evaluation of this application. If a grant is awarded as a result of or in connection with the submission of this application, the Government shall have the right to use or disclose the information to the extent authorized by law. This restriction does not limit the Government’s right to use the information if it is obtained without restriction from another source.
Begin each text section of the Research Training Program Plan with a section header (e.g., Introduction, Background, Program Plan, etc).
Field Name |
Instructions |
1. Introduction to Application (for Resubmission or Revision only) |
Use only if you are submitting an R&R Resubmission or Revision (Cover Page Item 8). The Introduction may not exceed 3 pages for resubmissions (previously known as a revision or amendment) or 1 page for revisions (previously known as competing supplements). See specific instructions in Section 2.7 “Resubmission” Applications and 2.8 “Revision” Applications concerning the content of the Introduction.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
2. Background |
The Background must fit within the combined 25-page limit for sections 2.2-2.4. Please see NOT-OD-11-039 and NOT-OD-11-076. Provide the rationale for the proposed research training program, relevant background history, and the need for the research training proposed. Indicate how the proposed program relates to current training activities at the applicant institution. Summarize the research training activities of the major participating unit(s) and department(s) represented in the proposed program. Complete and refer to the data reported in Tables 1-3: Table 1. Membership of Participating Departments/Programs Table 2. Participating Faculty Members Table 3. Institutional Training Grant Support Available to Participating Faculty Members, Departments, or Programs Use this data to document the environment in which the proposed training program will take place.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
3. Program Plan |
The Program Plan must fit within the combined 25-page limit for sections 2.2-2.4. Please see NOT-OD-11-039 and NOT-OD-11-076. a. Program Administration. Describe the Program Director's qualifications for providing leadership of the program, including relevant scientific background, current research areas, and experience in research training. Indicate the Program Director's percent effort in the proposed program. Describe the administrative structure of the program and the distribution of responsibilities within it, including the means by which the program director will obtain continuing advice with respect to the operation of the program. If multiple PD/PIs are proposed, explain in this section your rationale for how this will facilitate program administration. In addition, you must complete Item 2.10 Multiple PD/PI Leadership Plan. b. Program Faculty. Refer to the data presented in Table 2. Participating Faculty Members and elaborate as necessary to describe each faculty member's research that is relevant to the program and indicate how trainees will participate in the research. Describe the extent to which participating faculty members cooperated, interacted, and collaborated in the past, including joint publications and joint sponsorship of student research. Complete and refer to data in Tables 4-6: Table 4. Grant and Contract Support of the Participating Faculty Members Table 5. Pre and Postdoctoral Trainees of Participating Faculty Members Table 6. Publications of Research Completed by Trainees (or Potential Trainees) Use these tables to document the ability of the faculty to support the research activities of the proposed trainees, the training record of the faculty members, and the success of their trainees in generating publishable research results. For new applications, see the instructions for Table 6A or 6B, as applicable, and list publications for trainees who are representative of those who would be appointed if the grant is awarded. For Renewal applications, this data constitutes part of the Progress Report (see Item 2.6 Progress Report below). c. Proposed Training. Describe the proposed training program. State the training level and number of trainees. For postdoctoral trainees, indicate the proposed distribution by degree (e.g., M.D., Ph.D.). Describe course work and research opportunities, the extent to which trainees will participate directly in research, and the duration of training, i.e., usual period of time required to complete the training offered. Indicate how the individual disciplinary and/or departmental components of the program are integrated and coordinated and how they will relate to an individual trainee's experience. For training programs that emphasize research training for clinicians, describe the interactions with basic science departments and scientists. Include plans for ensuring that the training of these individuals will provide a substantive foundation for a competitive research career. Generally, a minimum of 2 years of research training is required for all postdoctoral trainees with health professional degrees. Describe fully any trainee’s access to and responsibility for patients, including time commitment. Provide representative examples of programs for individual trainees. Include curricula, degree requirements, didactic courses, laboratory experiences, qualifying examinations, and other training activities, such as seminars, journal clubs, etc. Describe how the preceptor and research problems are chosen, how each trainee's program will be guided, and how the trainee's performance will be monitored and evaluated. Include detailed mentoring plans as appropriate. If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line from the NIH hESC Registry cannot be identified, provide a strong justification for why an appropriate cell line cannot be chosen from the Registry at this time. d. Training Program Evaluation. Describe an evaluation plan to review and determine the quality and effectiveness of the training program. This should include plans to obtain feedback from current and former trainees to help identify weaknesses in the training program and to provide suggestions for program improvements. In addition, describe plans for assessing trainee’s career development and progression, including publications, degree completion, and post-training positions. Evaluation results are to be included in renewal (competing continuation) applications and as part of the Final Progress Report. e. Trainee Candidates. Describe recruitment plans, including the sources and availability of trainees; the qualifications of prospective trainees; and the criteria and procedures by which trainees will be selected. f. Institutional Environment and Commitment to Training. The administration of the applicant institution as well as all participating units and departments should include information in the application that documents institutional support and commitment to the goals of the research training program. The application should include a description of support (financial and otherwise) to be provided to the proposed program. This could include, for example, space, shared laboratory facilities and equipment, funds for curriculum development, release time for the PD/PI and/or participating faculty, support for additional trainees in the program, or any other creative ways to improve the climate for the establishment and growth of the research training program. Admissions and Completion RecordsFor programs that request only predoctoral trainee support, complete Table 7A. Admissions and Completion Records for the Participating Departments and Programs During the Past Five Years (Predoctoral Applicants). For programs that request only postdoctoral trainee support, complete Table 7B. Admissions and Completion Records for the Participating Departments and Programs During the Past Five Years (Postdoctoral Applicants). Programs requesting support for both predoctoral and postdoctoral trainees need to complete both Table 7A and Table 7B. Applicant institutions do not need to submit pre-enrollment data for individuals with disabilities and individuals from disadvantaged backgrounds in the first two data columns of these tables. However, all other information requested in the Table 7A and Table 7B must be provided as instructed. Use these tables to document the ability of the participating departments/programs to recruit and retain predoctoral and/or postdoctoral trainees through the completion of their training, the selectivity of the admissions process, the success of the departments/programs in recruitment and retention of trainees from diverse backgrounds, and to defend the requested number of training positions to be awarded. Qualifications of ApplicantsFor programs that request only predoctoral trainee support, complete Table 8A. Qualifications of Recent Predoctoral Applicants. For programs that request only postdoctoral trainee support, complete Table 8B. Qualifications of Recent Postdoctoral Applicants. Programs requesting support for both predoctoral and postdoctoral trainees need to complete both Table 8A and Table 8B. Use these tables to document the quality and depth of the applicant pools, including both Kirschstein-NRSA training grant eligible and non-Kirschstein-NRSA eligible applicants; the selectivity of the admissions process; the competitiveness of the program; and to justify the number of number of training positions requested. Current Trainee QualificationsFor programs that request only predoctoral trainee support, complete Table 9A. Qualifications of the Current Predoctoral Trainees Clearly Associated with the Training Program. For programs that request only postdoctoral trainee support, complete Table 9B. Qualifications of the Current Postdoctoral Trainees Clearly Associated with the Training Program. Programs that request both predoctoral and postdoctoral trainee support need to complete both Table 9A and Table 9B. Use these tables to document the number and quality of all trainees currently enrolled in the program, and their distribution by department and mentor; the selectivity of enrollment appointments to the training grant over the time period represented by the current program participants; and to defend the number of training positions to be awarded.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
This section must fit within the combined 25-page limit for sections 2.2-2.4. Please see NOT-OD-11-039 and NOT-OD-11-076. A Recruitment and Retention Plan to Enhance Diversity is required for all training grant activity codes except T34, T36, U2R, and all D-series activity codes. The NIH recognizes a unique and compelling need to promote diversity in the biomedical, behavioral, clinical and social sciences workforce. The NIH expects efforts to diversify the workforce to lead to the recruitment of the most talented researchers from all groups; to improve the quality of the educational and training environment; to balance and broaden the perspective in setting research priorities; to improve the ability to recruit subjects from diverse backgrounds into clinical research protocols; and to improve the Nation’s capacity to address and eliminate health disparities. Accordingly the NIH continues to encourage institutions to diversify their student and faculty populations and thus to increase the participation of individuals currently underrepresented in the biomedical, clinical, behavioral, and social sciences such as: individuals from underrepresented racial and ethnic groups, individuals with disabilities, and individuals from socially, culturally, economically, or educationally disadvantaged backgrounds that have inhibited their ability to pursue a career in health-related research. Institutions are encouraged to identify candidates who will increase diversity on a national or institutional basis. Definition of Diversity Recruitment GroupsThe NIH is particularly interested in encouraging the recruitment and retention of the following classes of candidates: A. Individuals from racial and ethnic groups that have been shown by the National Science Foundation to be underrepresented in health-related sciences on a national basis (see data at http://www.nsf.gov/statistics/showpub.cfm?TopID=2&SubID=27 and the report Women, Minorities, and Persons with Disabilities in Science and Engineering, 2007, p. 262). The following racial and ethnic groups have been shown to be underrepresented in biomedical research: African Americans, Hispanic Americas, Native Americans, Alaskan Natives, Hawaiian Natives, and natives of the U.S. Pacific Islands. In addition, it is recognized that under-representation can vary from setting to setting and individuals from racial or ethnic groups that can be convincingly demonstrated to be underrepresented by the grantee institution should be included in the recruitment and retention plan. B. Individuals with disabilities, who are defined as those with a physical or mental impairment that substantially limits one or more major life activities. C. Individuals from disadvantaged backgrounds who are defined as: 1. Individuals who come from a family with an annual income below established low-income thresholds. These thresholds are based on family size, published by the U.S. Bureau of the Census; adjusted annually for changes in the Consumer Price Index; and adjusted by the Secretary for use in all health professions programs. The Secretary periodically publishes these income levels at http://aspe.hhs.gov/poverty/index.shtml. For individuals from low income backgrounds, the institution must be able to demonstrate that such candidates (a) have qualified for Federal disadvantaged assistance; or (b) have received any of the following student loans: Health Professional Student Loans (HPSL), Loans for Disadvantaged Student Program; or have received scholarships from the U.S. Department of Health and Human Services under the Scholarship for Individuals with Exceptional Financial Need.
2. Individuals who come from a social, cultural, or educational
environment such as that found in certain rural or inner-city
environments that have demonstrably and recently directly
inhibited the individual from obtaining the knowledge, skills,
and abilities necessary to develop and participate in a research
career. New applications must include a description of plans to enhance recruitment of a diverse trainee pool and may wish to include data in support of past accomplishments. Renewal applications must include a detailed account of experiences in recruiting individuals from underrepresented groups during the previous funding period. Information should be included on both successful and unsuccessful recruitment strategies. History and Achievements. Describe efforts to recruit trainees from Diversity groups A, B, and C into the existing training program. For competing continuation/renewal applications, also describe past efforts to recruit and retain underrepresented minority students into training grant funded positions. Refer to the data presented in Table 1 and Table 7A and Table 7B that provided statistics on applications and admissions of these groups in comparison to the overall trainee pool. For Renewal applications, complete Table 10 Admissions and Completion Records for Underrepresented Minority (URM), Trainees with Disabilities, and Trainees from Disadvantaged Backgrounds Clearly Associated with the Training Program. New applicants may provide this data if they wish; however, it is not required of new applicants. Use this data to document the success of the program in recruiting and retaining trainees who are under-represented minorities, provide analysis of their support, and begin to establish a record of NIH training of other Diversity Recruitment groups. Proposed plans. Describe steps to be taken during the proposed award period regarding the identification, recruitment, and retention of graduate students and postdoctorates from underrepresented groups. Consider the success and/or failures of recruitment strategies used in the past. In particular, describe the specific efforts to be undertaken by the training program and how these might relate to the recruitment efforts of the medical school, graduate school, and/or the university at large. In most cases, institutional efforts alone will not satisfy the requirement to recruit individuals from underrepresented groups. Applications without a description of diversity recruitment efforts will be considered incomplete and may be delayed in the peer review process. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
|
5. Plan for Instruction in the Responsible Conduct of Research |
This section is limited to 3 pages. Please see NOT-OD-11-039. A plan for Instruction in the Responsible Conduct of Research (RCR) is required for all training grant activity codes except T36. Every trainee must receive instruction in the responsible conduct of research. See Part III Section 1.16 for information on the NIH Policy on Training in the Responsible Conduct of Research (RCR). New applications must include a plan for instruction in the responsible conduct of research. The plan should address how applicants plan to incorporate the five instructional components outlined in the NIH Policy on Training in the Responsible Conduct of Research: format, subject matter, faculty participation, duration, and frequency. In addition, the plan must describe how participation in RCR instruction will be monitored. In addition, Renewal applications must describe any changes in formal instruction over the past project period and plans for the future that address any weaknesses in the current RCR instruction. All training faculty who served as course directors, speakers, lecturers, and/or discussion leaders during the past project period must be named in the application. Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. This is a required attachment unless the FOA specifies otherwise. |
6. Progress Report (Renewal Applications Only) |
State the period covered and briefly describe the accomplishments of the training program. Describe any specific effects of this training program on curriculum and/or research directions. Describe how the funds provided under Training Related Expenses were used to benefit the program. For each trainee supported during the period covered, indicate their preceptor/mentor, and briefly summarize the research conducted by the trainee. For previous trainees appointed to the training grant and continuing in training, provide a brief statement of their status and progress toward completion of their training program. Refer to data presented in Table 6 Publications of Research Completed by Trainees (or Potential Trainees) and include in the table publications of trainees through the time that they complete their training for all trainees currently or previously supported by the training grant program regardless of whether support from this training grant is cited in the publication. Complete Table 11 Appointments to the Training Grant For Each Year of the Past Award (Renewal Applications Only). Use this table to document the utilization of awarded training positions. If any trainee positions were not filled, provide an explanation. For programs that request only predoctoral trainee support, complete Table 12A Predoctoral Trainees Supported by this Training Grant (Renewal Applications Only). For programs that request only postdoctoral trainee support, complete Table 12B Postdoctoral Trainees Supported by this Training Grant (Renewal Applications Only). Programs requesting support (or reporting on prior support) for both predoctoral and postdoctoral trainees need to complete both Table 12A and Table 12B. Use these tables to document how predoctoral training positions are used (i.e., distribution by mentor, year in program, years of support per trainee) and the success of the program in achieving the training objectives of the prior award period(s) for up to 10 years. Summarize this data in the text. If any postdoctoral trainee with a health professional degree appointed to the grant during the most recent award period received less than 2 years of research training, explain why. Where possible for past trainees, describe the extent of their current involvement in research, including research grant support and representative recent publications. Use the progress report narrative to provide information that is not readily presented in the required tables. Renewal applications must include a detailed account of experiences in recruiting individuals from underrepresented groups during the previous funding period. Information should be included on both successful and unsuccessful recruitment strategies. Renewal applications must describe the type of instructions provided in the current project period, the degree of student participation, the results of any assessments, and other relevant information.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
7. Human Subjects |
This section is required for applicants answering "yes" to the question "Are human subjects involved?" on the R&R Other Project Information form. If trainee participation in research involving human subjects is solely as part of other research projects that have received or will receive IRB review and approval, and no portion of the Training Grant Award will be used to support this research; provide a list of previously approved research projects (grant number, PD/PI, and project title) and their IRB approval dates or exemption designations. If plans are indefinite provide an explanation and prior to trainee participation in other research project grants, provide a list as indicated. If the training program involves definite plans for the participation of human subjects as defined in Part III.3, Human Subjects Research Definitions and Terms, outside of research projects that have received or will receive IRB review, follow the instructions in Part II, Supplemental Instructions for Preparing the Protection of Human Subjects Section of the Research Plan. The appropriate grants must be identified along with their IRB approval dates or exemption designation.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
8. Vertebrate Animals |
This section is required for applicants answering "yes" to the question "Are vertebrate animals involved?" on the R&R Other Project Information form. If the training program involves the use of live vertebrate animals solely as part of other research project grants, and no portion of the Training Grant Award will be used to support the purchase, use, or husbandry of live vertebrate animals in this research; provide a list of previously approved research projects (grant number, PD/PI, and project title) and IACUC approval dates. If plans are indefinite provide an explanation and prior to trainee participation in other research project grants, provide a list as indicated. If the training program involves definite plans to use live vertebrate animals outside of research projects that have received or will receive IACUC approval, follow the instructions in Part I, 5.5, (Research Plan Component), Item 10. Vertebrate Animals. The appropriate grants must be identified along with their IACUC approval dates.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
9. Select Agent Research |
If participating faculty proposed in the training program are conducting or plan to conduct research involving select agents in which trainees may participate, follow the instructions in Section 5.5, (Research Plan Component), Item 11. Select Agent Research.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
10. Multiple PD/PI Leadership Plan |
If you wish to submit a multiple PD/PI application, you must provide a Leadership Plan. Do not submit a leadership plan if you are not submitting a Multiple PD/PI application. For applications designating multiple PD/PIs, all such individuals must be assigned the PD/PI role on the Senior/Key Profile form, even those at organizations other than the applicant organization. Refer to the instructions in Section 5.5 (Research Plan Component), Item 12. Multiple PD/PI Leadership Plan. However, the emphasis in a training grant multiple PD leadership plan should be on how it will benefit the program and the trainees. A single Contact PD must be designated for the purpose of communicating with the NIH, although other individuals may contact the NIH on behalf of the Contact PD when necessary. Because training programs are intended to be coherent, NIH will not allocate the budget or training positions between multiple PDs. A single award will be made. Multiple PD plans should include reasonable numbers of PDs and each should be included for a specific purpose. Multiple-PD applications should not include all mentors of the training grant as PDs, except in unusual cases. For background information on the Multi-PD/PI initiative, see: http://grants.nih.gov/grants/multi_pi/index.htm.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
11. Consortium and Contractual Arrangements |
Describe any programmatic, fiscal, or administrative arrangements between the applicant organization and other participating organizations. See Section 5.5, Item 13, Consortium/Contractual Arrangements for additional guidance.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
12. Participating Faculty Biosketches |
Faculty Biosketches for participating faculty should follow the Additional NIH and other PHS Agencies Instructions for a Biographical Sketch, except that a personal statement is not required for participating faculty. These should be attached as a single document to avoid having to upload large numbers of separate documents. However, the Biosketches of the Program Director and other Senior/key Personnel should also be entered as described under SF424 (R&R) Section 4.5 Senior/Key Person Profile (Expanded) Component.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
13. Data Tables |
Instructions for Data Tables 1-12 mentioned above are located on the OER Web site at the following URL http://grants.nih.gov/grants/funding/424/index.htm#datatables. These instructions include an Introduction to the Data Tables that provides instructions applicable to all tables, specific instructions for each table, and Sample Data Tables. The Sample Data Tables illustrate the kind of data to include in each table for Kirschstein-NRSA training grant applications. Be sure to choose the Instruction and Blank Data Table set that corresponds to the type of application you are submitting, e.g., New, Renewal, or Revision Application, and the kind of training to be provided, e.g., predoctoral only, postdoctoral only, or pre and postdoctoral mixed. Modification of these instructions for use in non-Kirschstein-NRSA training grant applications will be included in relevant FOAs. Save this information in a single file in a location you remember. Start each numbered table on a new page. Bookmark the first page of each table by its table number (Table 1, Table 2, etc.) before you upload the file. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
14. Letters of Support |
Attach appropriate letters here from all individuals confirming their roles in the project. Letters documenting any agreements between the Program Director(s) and senior administration officials or other institutional officials are not required but may be included. For consultants, letters should include rate/charge for consulting services. The Program Director should check the FOA (particularly for non-NRSA programs) to determine if any program-specific letters of support are required.
Save this information in a single file in a location you remember. Click Add Attachment, browse to where you saved the file, select the file, and then click Open. |
15. Appendix |
Do not use the appendix to circumvent the page limitations of the Training Plan. All appendix material must be submitted as PDF attachments. A summary listing all of the items included in the appendix is required, and should be the first PDF file of the Appendix. Applications that do not follow the appendix requirements may be delayed in the review process. Research publications of trainees and mentors are not normally included as part of the Training Grant applications, but are allowed. Other types of publications reflecting on the activities of the program as a whole may also be included. When publications are allowed, appendix materials should be limited to those which are not publicly available, such as:
Publications that are publicly accessible must not be included in the appendix. For such publications, the URL or PMC submission identification numbers along with the full reference should be included as appropriate in the Progress Report section of the Research Training Program Plan, and/or in the Biographical Sketch. Do not include unpublished theses or abstracts/manuscripts submitted but not yet accepted for publication. Some materials other than publications that are unique to training grant applications (but not typically included in research grant applications) may be included as appendices. In general, the appendix may be used to provide samples of materials that are referred to in the body of the application, but are too cumbersome to include in the Research Training Program Plan without disrupting the narrative flow. Examples include: i. Syllabi for key courses, core courses and electives, including courses in Responsible Conduct of Research, Survival Skills for Research, etc.; ii. Retreat, seminar series, and other program activity agendas, rosters, and schedules; iii. Examples of forms used to document trainee progress and monitoring by the program; iv. Examples of materials used in recruitment and particularly recruitment and retention to enhance diversity of the student pool; v. Lists of meetings attended by students and their presentations; and vi. Student biosketches. As a reminder, tables other than the required Data Tables 1-12, must be incorporated into the 25 page limit of the Research Training Program Plan (items 2-4). These additional tables must not be included in the appendix materials. |
![]()
Once all data have been entered use the scroll bar to scroll up. You will be returned to the Grant Application Package screen. From this main screen, click on the form/document that you have just completed, and then click the => button. This will move the form/document to the Completed Documents box. To remove a form/document from the Completed Documents box, click the form/document name to select it, and then click the <= button. This will return the form/document to the Mandatory Documents or Optional Documents box.
8.8 Training Grant Peer Review Process
The goals of NIH-supported research training are to help ensure that a diverse pool of highly trained scientists is available in adequate numbers and in appropriate research areas to address the Nation’s biomedical, behavioral, and clinical research needs. The scientific review group will address and consider each of criteria below in assigning the application’s overall score, weighting them as appropriate for each application. Reviewers will first determine the quality of the proposed research training program, including information presented in the data tables and appendix, and then consider whether the requested number of trainee positions is appropriate for the program.
The general process information (Overview, Streamlining, and Dual-Level Peer Review) found in Part I.6 applies to training grant applications as well. However, the actual review criteria and other review considerations are different.
Review Criteria:
Training Program and Environment
Training Program Director/Principal Investigator (PD/PI)
Preceptors/Mentors
Trainees
Training Record
Additional Review Criteria:
Protection of Human Subjects from Research Risk
Inclusion of Women, Minorities and Children in Research
Care and Use of Vertebrate Animals in Research
Biohazards
Resubmission Applications
Renewal Applications
Additional Review Considerations:
Training in the Responsible Conduct of Research
Recruitment and Retention Plan to Enhance Diversity
Budget and Period of Support
Applicants should carefully review the applicable FOA for complete information associated with the peer review process. The FOA will describe essential information to be submitted for each of the above elements.
Part I: Instructions for
Preparing and Submitting an Application I-
| File Type | application/msword |
| Author | Leslie Dorman |
| Last Modified By | currend |
| File Modified | 2012-05-25 |
| File Created | 2012-05-23 |
© 2026 OMB.report | Privacy Policy