1 Revised CRU Form
Chimpanzee Research Use Form
REVISED Attach 6_CRU Form Sample w screen shots (6)
CRU Form Sample with screen shots
OMB: 0925-0705
PROPOSED REVISIONS TO THE CHIMPANZEE RESEARCH USE FORM
Chimpanzee Research Use Form
This document presents the Chimpanzee Research Use Form in two ways.
Part 1 displays the screen shots from the CRU Reporting System with noted revisions
Part 2 provides the form in text only (for greater readability) in final version (no markup)
Part 1: Screen Shots from CRU Reporting System
First Screen After Login—Assignments - UNCHANGED
User sees assignments and clicks on CRU Report # (hotlink) to access the form. User submits one form per research application or proposal that involves use of chimpanzees or chimpanzee biomaterials.

Second Screen—Initiate CRU Report – REVISIONS ARE IN THE TEXT BELOW
Revisions indicated in highlighted areas
OMB No: 0925-0705, Expiration date: 10/31/2017
Privacy
Notice
This
collection of information is authorized by 42 U.S.C. 241, 282, 282b,
284a, and 48 CFR Subpart 15.3. Certain information provided through
this form may be used by NIH to identify and contact the submitter
about the proposed research.
Unless
otherwise specified in a written agreement between NIH and the
submitter, all information, including proprietary information,
submitted
through the form, attached to it, or referenced in it may be accessed
by the NIH, the CRUP, and/or the Council. NIH staff and contractors
who have signed confidentiality and non-disclosure statements will
use the information for administrative purposes, e.g., reviewing
materials for completeness, routing materials to the CRUP and the
Council, and managing subsequent steps related to discussions about
the proposed research.
Burden
Disclosure Statement
Public
reporting burden for this collection of information is estimated to
average
30 minutes
per response, including the time for reviewing instructions,
searching existing data sources, gathering and maintaining the data
needed, and completing and reviewing the collection of information.
An
agency may not conduct or sponsor, and a person is not required to
respond to, a collection of information unless it displays a
currently valid OMB control number.
Send comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden, to: NIH, Project Clearance Branch, 6705 Rockledge Drive,
MSC 7974, Bethesda, MD 20892-7974, ATTN:
PRA (0925-0705). Do not return the completed form to this
address.
Purpose
The purpose of this form is to obtain information needed by the NIH to assess whether the proposed research satisfies the NIH’s policy for research involving chimpanzees, as announced in the Federal Register and NIH Guide. These announcements inform the research community that NIH will support only “noninvasive” research involving chimpanzees. The NIH will consider the information submitted through this form prior to the agency making funding decisions or otherwise allowing the “noninvasive” research to begin. Completion of this form is a mandatory step toward receiving NIH support or approval for such research. Failure to complete this form by the due date may delay consideration by the NIH.
Who
Can Submit the Form
Only
applicants and offerors notified by the NIH are required to complete
this form. If the NIH has contacted you about completing this form,
it is because you have requested that the agency support or otherwise
approve a grant, contract, intramural project, or 3rd party research
activity involving chimpanzees or
chimpanzee biomaterials,
and the agency requires additional information before making funding
decisions or otherwise allowing the research to begin. The NIH
requires applicants and offerors to complete the form when the
proposed research:
Requests the use of chimpanzees (Pan troglodytes) or chimpanzee biomaterials in research, and
Is being considered for funding (grants), is in the competitive range (contracts), is scientifically meritorious and fundable (NIH intramural research), or was otherwise submitted for consideration (3rd party projects).
The form may be submitted by the Signing Official for an organization or his/her designee. The Signing Official is the individual, named by the applicant or offeror’s organization, authorized to act for the applicant or offeror and to assume the obligations imposed by applicable Federal laws, regulations, requirements, and conditions.
What
Information Gets Considered
Applicants
and offerors are required to describe their proposed research and how
it satisfies the agency announcement in NIH Guide NOT-OD-16-095.
Depending on the nature of the research, the NIH will determine if
the activity is “noninvasive” (e.g., observational
research or research involving stored biospecimens), or if the
research is “invasive.” Consideration is based solely on
the information provided in the Noninvasive Determination section of
this form. Although attachments may be submitted, they are optional.
The NIH may consult attached materials at its discretion. All
information pertinent to their consideration must be entered into the
Noninvasive Determination text boxes.
How
and When to Submit the Form and How to Revise Submitted
Information
Unless
otherwise specified by the NIH, applicants and offerors have 15
calendar days to submit the completed report after they have been
contacted by the NIH. Information must be submitted electronically
via the Chimpanzee Research Use Reporting System.
Questions about the online form can be emailed to the [email protected]. If the applicant or offeror notices that the report requires a change after it has been submitted, contact the NIH’s Division of Program Coordination, Planning, and Strategic Initiatives at [email protected]. Submissions that are incomplete by the due date may delay consideration by the NIH.
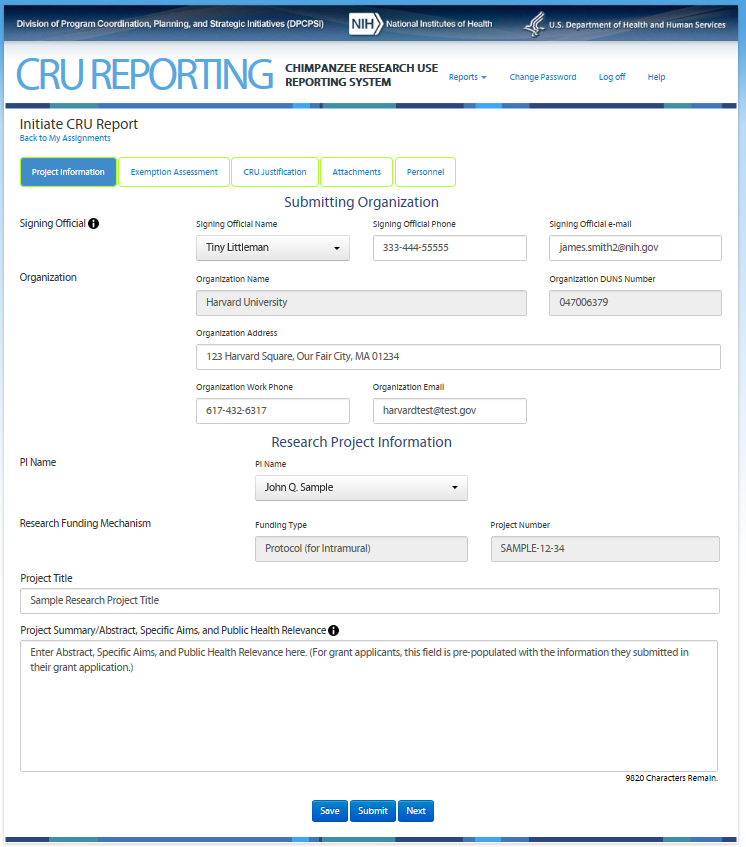
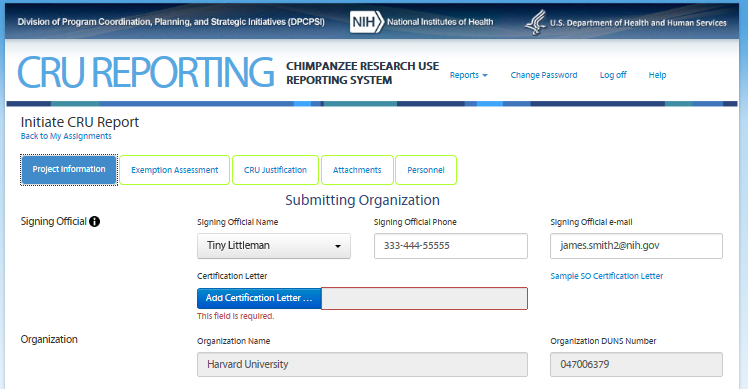
Third Screen—Project Information – REVISIONS ARE OUTLINED BELOW
For grant applicants (external to
NIH), the information on this screen is pre-populated with
information they submitted in the applicant’s grant
application. Note: On this screen the users see the icon
![]() that
allows them to access additional information about the item. The text
can be found in the revised CRU Report Guidance.
that
allows them to access additional information about the item. The text
can be found in the revised CRU Report Guidance.
Revisions to the Original Screen Shot Below
Tab “Exemption Determination” will change to “Noninvasive Determination”
Tabs “CRU Justification” and “Personnel” will be removed

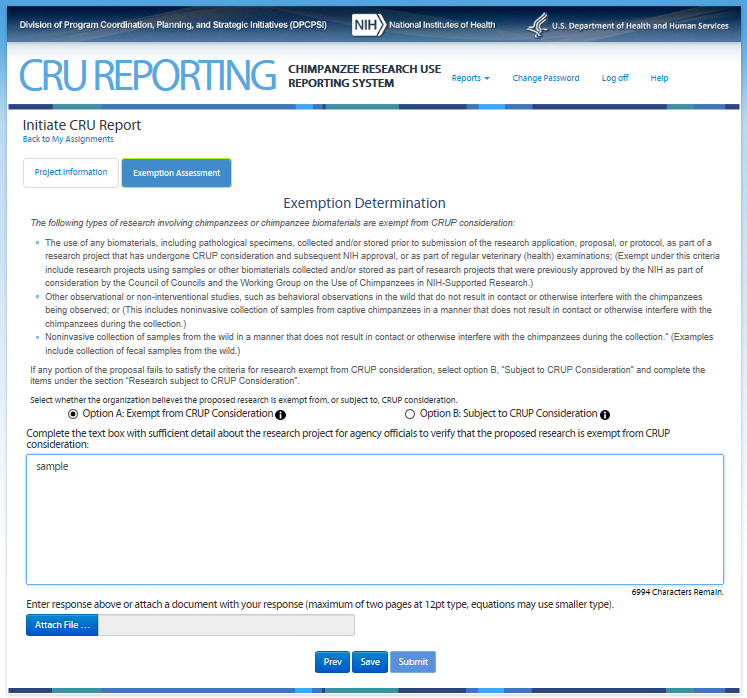
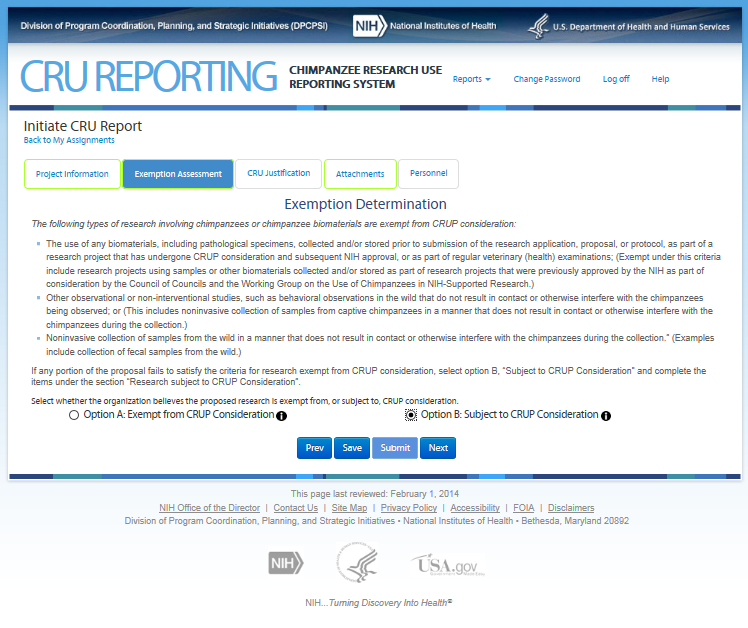
Fourth Screen—Exemption Assessment – REVISIONS ARE OUTLINED BELOW
This screen allows the user to
assess whether his/her proposed research is noninvasive. Note: On
this screen the users see the icon
![]() that
allows them to access additional information about the item. The text
can be found in the CRU Report Guidance.
that
allows them to access additional information about the item. The text
can be found in the CRU Report Guidance.
Revisions to the Original Screen Shot Below
Tab “Exemption Determination” renamed to “Noninvasive Determination”
“Option A: Exempt from CRUP Consideration” and “Option B: Subject to CRUP Consideration” removed.
Text changes follow
The following types of research involving chimpanzees or chimpanzee biomaterials are considered “noninvasive”:
Visual observation.
Behavioral studies designed to improve the establishment and maintenance of social groups. These activities may cause stress as a result of novel interactions between chimpanzees and caregivers, but they are not considered invasive as long as they are intended to maximize the well-being of the chimpanzees.
Medical examinations as deemed necessary to oversee the health of the chimpanzees, in the least invasive manner possible. Collection of samples routinely obtained during a physical examination for processing during this time is also considered noninvasive since a separate event is not required.
Administration and evaluation of environmental enrichment used to promote the psychological well-being of the chimpanzees.
Actions taken to provide essential medical treatment to an individual chimpanzee exhibiting symptoms of illness. This applies only to serious illness that cannot be treated while the chimpanzee remains within the colony.
Observational studies and collection of biomaterial in the wild without interfering with the chimpanzee.
Collections of biological materials (e.g., saliva, oral or other cavity specimens, urine, feces, or hair) obtained voluntarily from a chimpanzee that has been trained through positive reinforcement to cooperate in the collection. This excludes venipuncture or other more invasive methods.
If any portion of the proposal fails to satisfy the description of “noninvasive” research involving chimpanzees, contact your program officer or other federal official responsible for administering the award.
Complete the text box with sufficient detail about the research project for agency officials to verify that the proposed research is noninvasive.
****
After entering information into this section, the user may attach file and may submit the materials. User is then brought to Certifications and then the report is complete. In the original Chimpanzee Research Use Form, the user had the option to complete sections of the form relevant to research that formerly was subject to CRUP consideration, i.e., invasive research. These portions of the form would have been triggered by selecting “Option B: Subject to CRUP Consideration,” which are now obsolete.
Option B Screen: REVISION – OPTION B WILL BE REMOVED
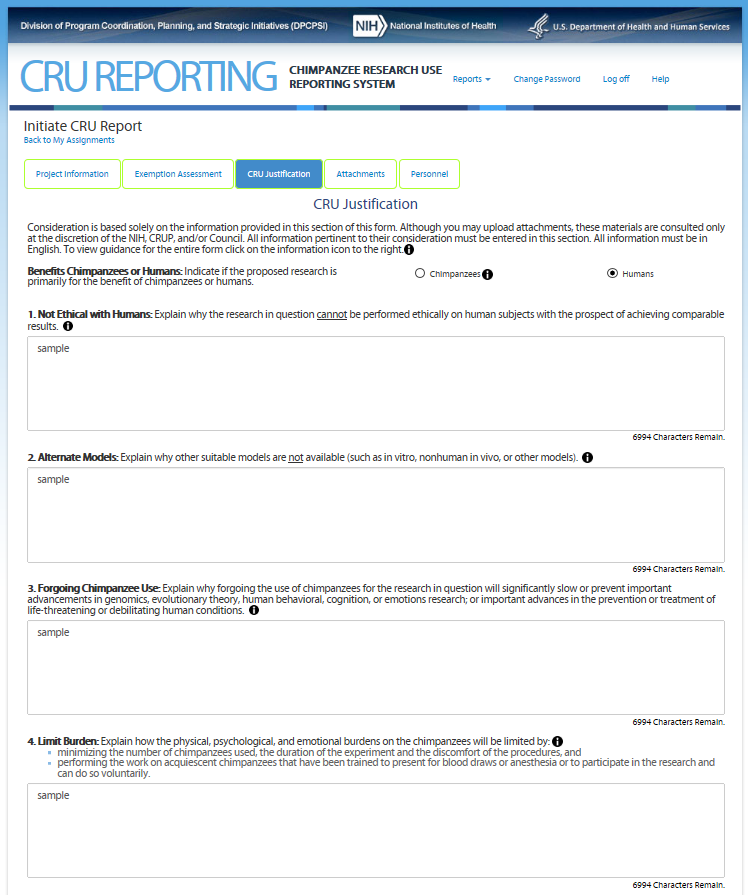
Fifth Screen—CRU Justification (top portion) – REVISION – WILL BE REMOVED
The Fifth Screen captures information to allow the Chimpanzee Research Use Panel to assess whether the proposed research is consistent with NIH interim policy on the use of chimpanzees.
Note: On this screen the users see
the icon
![]() that
allows them to access additional information about the item. The text
can be found in Attachment 8 CRU Report Guidance.
that
allows them to access additional information about the item. The text
can be found in Attachment 8 CRU Report Guidance.
Enumerated items 1 and 3 are available for entry only if “Humans” is selected. If “Chimpanzees” is selected an additional text box appears asking user to “Explain how the research is in the best interest of the chimpanzee and how it addresses the mission of the NIH.”
Fifth Screen—CRU Justification (continued) REVISION – WILL BE REMOVED
Note: On this screen the users see
the icon
![]() that
allows them to access additional information about the item. The text
can be found in Attachment 8 CRU Report Guidance.
that
allows them to access additional information about the item. The text
can be found in Attachment 8 CRU Report Guidance.
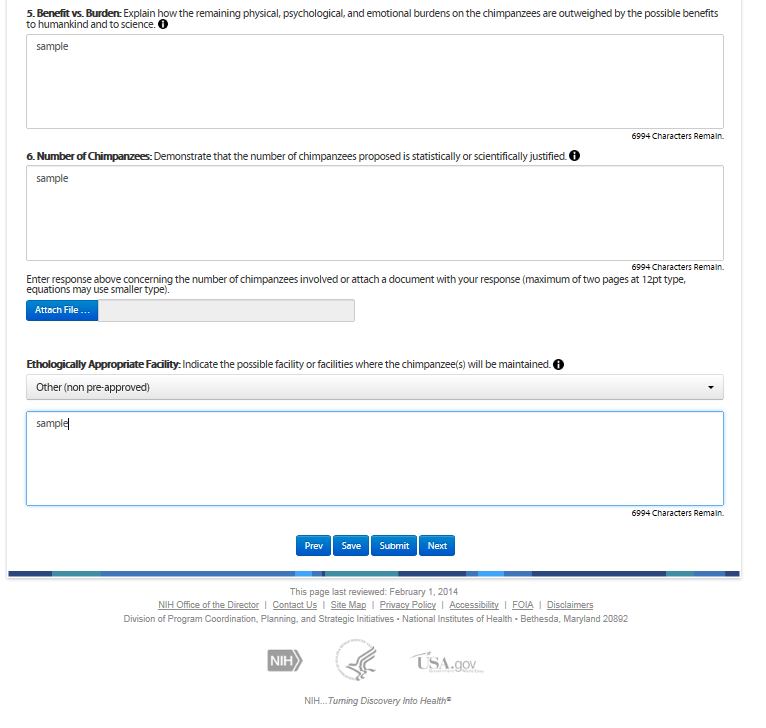
Sixth Screen—Attachments – REVISIONS OULINED BELOW
The screen allows the user to attach supplemental information, e.g., images. Attachments are optional.
Revisions
Minor text changes are below
Although NIH allows you to submit attachments, consideration of these materials by the NIH is discretionary. All pertinent information must be entered into the Noninvasive Determination tab. All information must be in English and attachments should be in pdf, image, or MS Word file types.
Applicants and offerors are strongly discouraged from resubmitting their grant application, contract proposal, or protocols as an attachment to the Chimpanzee Research Use Form.
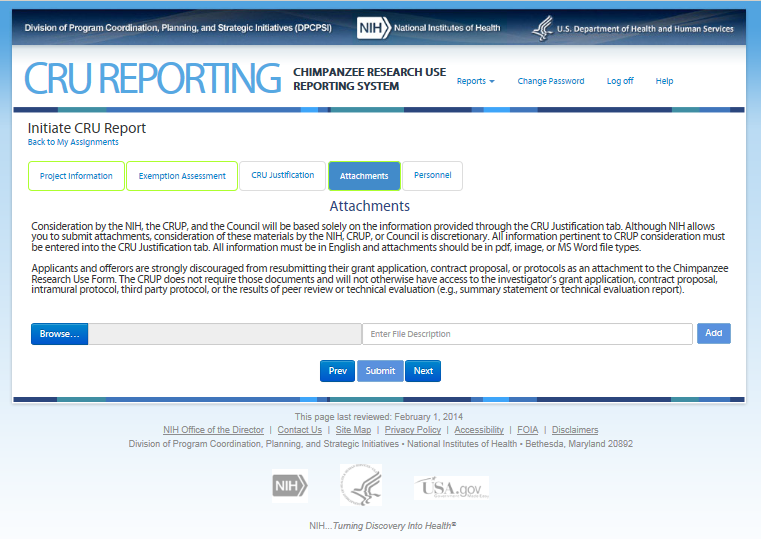
Seventh Screen—Personnel – REVISION – WILL BE REMOVED
This screen requires the user to provide information to NIH that will allow staff to avoid conflicts of interest when selecting members for the Chimpanzee Research Use Panel. Users may also upload a file (e.g., publications list) if preferred.
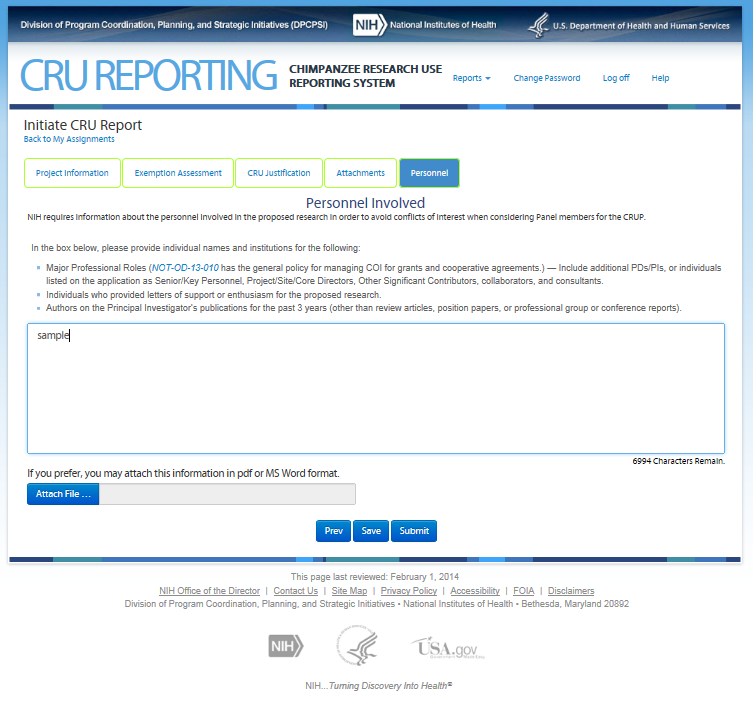
Eighth Screen—Certifications – REVISIONS OUTLINED BELOW
Upon hitting “Submit” the Signing Official is presented with the following certifications.
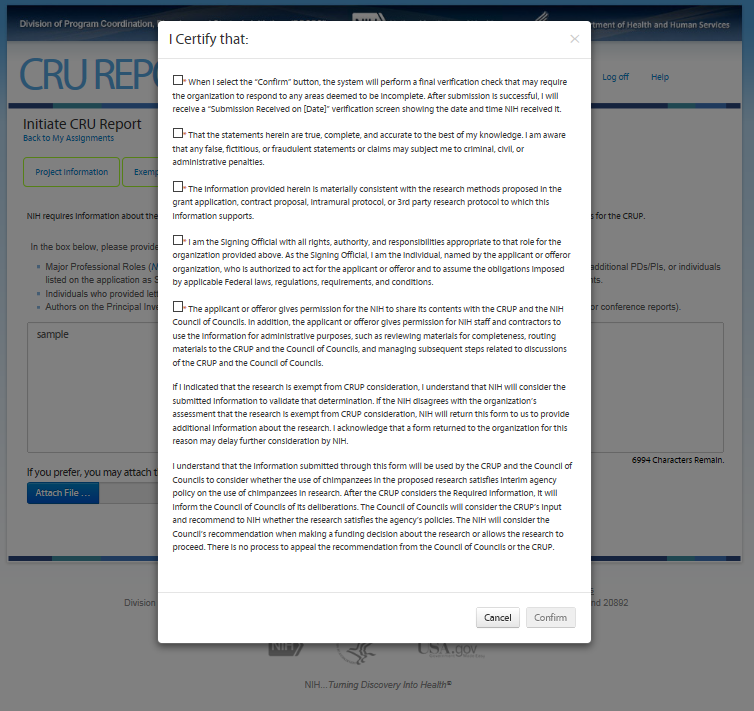
I certify that:
(1) I understand that I understand that I will receive a “Submission Received on [Date]” verification screen showing the date and time NIH received the submission. If I do not receive a verification screen after clicking "Confirm", I may contact [email protected] to confirm receipt by NIH.
(2) The statements herein are true, complete, and accurate to the best of my knowledge. I am aware that any false, fictitious, or fraudulent statements or claims may subject me to criminal, civil, or administrative penalties.
(3) The information provided herein is materially consistent with the research methods proposed in the grant application, contract proposal, intramural protocol, or 3rd party research protocol to which this information supports.
(4) I am the Signing Official with all rights, authority, and responsibilities appropriate to that role for the organization provided above. As the Signing Official, I am the individual, named by the applicant or offeror organization, who is authorized to act for the applicant or offeror and to assume the obligations imposed by applicable Federal laws, regulations, requirements, and conditions.
(5) The applicant or offeror gives permission for the NIH to share its contents within the agency and the NIH Council of Councils. In addition, the applicant or offeror gives permission for NIH staff and contractors to use the information for administrative purposes, such as reviewing materials for completeness, routing materials to the Council of Councils, and managing subsequent steps.
By indicating that the research is noninvasive, I understand that NIH will consider the submitted information to validate that determination. If the NIH disagrees with the organization’s assessment that the research is noninvasive, NIH consider the project to be invasive and suggest the investigator contact the program officer or other appropriate federal official. I acknowledge that NIH will not fund invasive research involving chimpanzees.
Intramural Project Review Acknowledgement: The Scientific Director has provided a statement verifying that the IC convened a review panel to assess the scientific merit of the protocol and determined that the protocol is scientifically highly meritorious and the IC Director has signed off that the project meets the IC’s scientific mission and priorities. [Conditional on the applicant identifying as an intramural researcher.
*****
If a user other than the Signing Official submits the form, he/she is presented with only the first certification check box plus the two paragraphs under number (5), and must attach the full certification text on agency letterhead signed by the Signing Official. In this case, the “Sample SO Certification Letter” link and a required attach file tool labeled “Add Certification Letter…” will appear on the Project Information screen to allow the user to attach the signed certification letter.
Part 2: Final Revised Text
(provided for readability)
Black text contains notes for the reader of this document. Blue text is what is onscreen.
First Screen After Login—Assignments
User sees assignments and clicks on CRU Report # (hotlink) to access the form. Users see the following information for the research project(s) they have in the system.
CRU Report #, Grant/Protocol/Contract #, Project title, PI Name, Status (Deadline)
Second Screen—Initiate CRU Report
OMB No: 0925-0705, Expiration date: 10/31/2017
Privacy
Notice
This
collection of information is authorized by 42 U.S.C. 241, 282, 282b,
284a, and 48 CFR Subpart 15.3. Certain information provided through
this form may be used by NIH to identify and contact the submitter
about the proposed research. Unless
otherwise specified in a written agreement between NIH and the
submitter, all information, including proprietary information,
submitted
through the form, attached to it, or referenced in it may be accessed
by the NIH, the CRUP, and/or the Council. NIH staff and contractors
who have signed confidentiality and non-disclosure statements will
use the information for administrative purposes, e.g., reviewing
materials for completeness, routing materials to the CRUP and the
Council, and managing subsequent steps related to discussions about
the proposed research.
Burden
Disclosure Statement
Public
reporting burden for this collection of information is estimated to
average 30 minutes per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information. An
agency may not conduct or sponsor, and a person is not required to
respond to, a collection of information unless it displays a
currently valid OMB control number.
Send comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden, to: NIH, Project Clearance Branch, 6705 Rockledge Drive,
MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-0705). Do
not return the completed form to this address.
Purpose
The purpose of this form is to obtain information needed by the NIH to assess whether the proposed research satisfies the NIH’s policy for research involving chimpanzees, as announced in the Federal Register and NIH Guide. These announcements inform the research community that NIH will support only “noninvasive” research involving chimpanzees.
The NIH will consider the information submitted through this form prior to the agency making funding decisions or otherwise allowing the “noninvasive” research to begin. Completion of this form is a mandatory step toward receiving NIH support or approval for such research. Failure to complete this form by the due date may delay consideration by the NIH.
Who
Can Submit the Form
Only
applicants and offerors notified by the NIH are required to complete
this form. If the NIH has contacted you about completing this form,
it is because you have requested that the agency support or otherwise
approve a grant, contract, intramural project, or 3rd party research
activity involving chimpanzees or chimpanzee biomaterials, and the
agency requires additional information before making funding
decisions or otherwise allowing the research to begin. The NIH
requires applicants and offerors to complete the form when the
proposed research:
Requests the use of chimpanzees (Pan troglodytes) or chimpanzee biomaterials in research, and
Is being considered for funding (grants), is in the competitive range (contracts), is scientifically meritorious and fundable (NIH intramural research), or was otherwise submitted for consideration (3rd party projects).
The form may be submitted by the Signing Official for an organization or his/her designee. The Signing Official is the individual, named by the applicant or offeror’s organization, authorized to act for the applicant or offeror and to assume the obligations imposed by applicable Federal laws, regulations, requirements, and conditions.
What
Information Gets Considered
Applicants
and offerors are required to describe their proposed research and how
it satisfies the agency announcement in NIH Guide NOT-OD-16-095.
Depending on the nature of the research, the NIH will determine if
the activity is “noninvasive” (e.g., observational
research or research involving stored biospecimens), or if the
research is “invasive.” Consideration is based solely on
the information provided in the Noninvasive Determination section of
this form. Although attachments may be submitted, they are optional.
The NIH may consult attached materials at its discretion. All
information pertinent to their consideration must be entered into the
Noninvasive Determination text boxes.
How
and When to Submit the Form and How to Revise Submitted
Information
Unless
otherwise specified by the NIH, applicants and offerors have 15
calendar days to submit the completed report after they have been
contacted by the NIH. Information must be submitted electronically
via the Chimpanzee Research Use Reporting System.
Questions about the online form can be emailed to the [email protected]. If the applicant or offeror notices that the report requires a change after it has been submitted, contact the NIH’s Division of Program Coordination, Planning, and Strategic Initiatives at [email protected]. Submissions that are incomplete by the due date may delay consideration by the NIH.
Third Screen—Project Information
For grant applicants (external to NIH), the information on this screen is pre-populated with information they submitted in their grant application. Prepopulate fields are designated with underline.
Note: On this screen the users see the icon
![]() that
allows them to access additional information about the item. The text
can be found in the CRU Report Guidance.
that
allows them to access additional information about the item. The text
can be found in the CRU Report Guidance.
Signing Official
Signing Official Name (dropdown) Signing Official Phone Signing Official e-mail
Organization
Organization Name Organization DUNS Number
Organization Address
Organization Work Phone Organization Email
PI Name
PI Name (dropdown)
Research Funding mechanism
Funding Type Project Number
Project Title
Project Summary/Abstract, Specific Aims, and
Public Health Relevance (Limit 10,000 characters)
![]()
Fourth Screen—Noninvasive Determination
Note: On this
screen the users see the icon
![]() that
allows them to access additional information about the item. The text
can be found in the CRU Report Guidance.
that
allows them to access additional information about the item. The text
can be found in the CRU Report Guidance.
Noninvasive Determination
The following types of research involving chimpanzees or chimpanzee biomaterials are considered “noninvasive”:
Visual observation.
Behavioral studies designed to improve the establishment and maintenance of social groups. These activities may cause stress as a result of novel interactions between chimpanzees and caregivers, but they are not considered invasive as long as they are intended to maximize the well-being of the chimpanzees.
Medical examinations as deemed necessary to oversee the health of the chimpanzees, in the least invasive manner possible. Collection of samples routinely obtained during a physical examination for processing during this time is also considered noninvasive since a separate event is not required.
Administration and evaluation of environmental enrichment used to promote the psychological well-being of the chimpanzees.
Actions taken to provide essential medical treatment to an individual chimpanzee exhibiting symptoms of illness. This applies only to serious illness that cannot be treated while the chimpanzee remains within the colony.
Observational studies and collection of biomaterial in the wild without interfering with the chimpanzee.
Collections of biological materials (e.g., saliva, oral or other cavity specimens, urine, feces, or hair) obtained voluntarily from a chimpanzee that has been trained through positive reinforcement to cooperate in the collection. This excludes venipuncture or other more invasive methods.
If any portion of the proposal fails to satisfy the description of “noninvasive” research involving chimpanzees, contact your program officer or other federal official responsible for administering the award.
Complete the text box with sufficient detail about the research project to agency officials to verity that the proposed research is noninvasive.
[text box] (Limit 7,000 characters)
Enter response above or attach a document with your response (maximum of two pages at 12pt type, equations may use smaller type).
[attach file option]
*****
User can then Submit and is then brought to Certifications and then the report is complete.
Fifth Screen—Certifications
Upon hitting “Submit” the Signing Official (SO) is presented with the following certifications. User must check each box to confirm.
I certify that:
 When I select the “Submit” button, the system will
perform a final verification check that may require the organization
to respond to any areas deemed to be incomplete. After submission is
successful, I will receive a “Submission Received on [Date]”
verification screen showing the date and time NIH received it.
When I select the “Submit” button, the system will
perform a final verification check that may require the organization
to respond to any areas deemed to be incomplete. After submission is
successful, I will receive a “Submission Received on [Date]”
verification screen showing the date and time NIH received it.
 That the statements herein are true, complete, and accurate to
the best of my knowledge. I am aware that any false, fictitious, or
fraudulent statements or claims may subject me to criminal, civil, or
administrative penalties.
That the statements herein are true, complete, and accurate to
the best of my knowledge. I am aware that any false, fictitious, or
fraudulent statements or claims may subject me to criminal, civil, or
administrative penalties.
 The information provided herein is materially consistent with the
research methods proposed in the grant application, contract
proposal, intramural protocol, or 3rd party research protocol to
which this information supports.
The information provided herein is materially consistent with the
research methods proposed in the grant application, contract
proposal, intramural protocol, or 3rd party research protocol to
which this information supports.
 I am the Signing Official with all rights, authority, and
responsibilities appropriate to that role for the organization
provided above. As the Signing Official, I am the individual, named
by the applicant or offeror organization, who is authorized to act
for the applicant or offeror and to assume the obligations imposed by
applicable Federal laws, regulations, requirements, and conditions.
I am the Signing Official with all rights, authority, and
responsibilities appropriate to that role for the organization
provided above. As the Signing Official, I am the individual, named
by the applicant or offeror organization, who is authorized to act
for the applicant or offeror and to assume the obligations imposed by
applicable Federal laws, regulations, requirements, and conditions.
 The applicant or offeror gives permission for the NIH to share
its contents within the agency and the NIH Council of Councils. In
addition, the applicant or offeror gives permission for NIH staff and
contractors to use the information for administrative purposes, such
as reviewing materials for completeness, routing materials to the
Council of Councils, and managing subsequent steps.
The applicant or offeror gives permission for the NIH to share
its contents within the agency and the NIH Council of Councils. In
addition, the applicant or offeror gives permission for NIH staff and
contractors to use the information for administrative purposes, such
as reviewing materials for completeness, routing materials to the
Council of Councils, and managing subsequent steps.
 By indicating that the research is noninvasive, I understand that
NIH will consider the submitted information to validate that
determination. If the NIH disagrees with the organization’s
assessment that the research is noninvasive, NIH consider the project
to be invasive and suggest the investigator contact the program
officer or other appropriate federal official. I acknowledge that NIH
will not fund invasive research involving chimpanzees.
By indicating that the research is noninvasive, I understand that
NIH will consider the submitted information to validate that
determination. If the NIH disagrees with the organization’s
assessment that the research is noninvasive, NIH consider the project
to be invasive and suggest the investigator contact the program
officer or other appropriate federal official. I acknowledge that NIH
will not fund invasive research involving chimpanzees.
 Intramural Project Review Acknowledgement: The Scientific Director
has provided a statement verifying that the IC convened a review
panel to assess the scientific merit of the protocol and determined
that the protocol is scientifically highly meritorious and the IC
Director has signed off that the project meets the IC’s
scientific mission and priorities. [Conditional on the applicant
identifying as an intramural researcher.
Intramural Project Review Acknowledgement: The Scientific Director
has provided a statement verifying that the IC convened a review
panel to assess the scientific merit of the protocol and determined
that the protocol is scientifically highly meritorious and the IC
Director has signed off that the project meets the IC’s
scientific mission and priorities. [Conditional on the applicant
identifying as an intramural researcher.
*****
If a user other than the Signing Official submits the form, he/she is presented with only the first certification check box plus the two paragraphs under number (5) and must attach the full certification text on agency letterhead signed by the Signing Official. In this case, the “Sample SO Certification Letter” link and a required attach file tool labeled “Add Certification Letter…” will appear on the Project Information screen to allow the user to attach the signed certification letter.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Smith, James (NIH/OD) [C] |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy