HIV Quality Measures Performance Measure Module (HIVQM Module)
HIV Quality Measures Performance Measure Module (HIVQM Module)
HIVQM Manual PASSBACK FINAL 12-16
HIV Quality Measures Performance Measure Module (HIVQM Module)
OMB: 0906-0022

DRAFT 2016
Ryan White HIV/AIDS Program HIV Quality Measures (HIVQM)
Module
Instruction Manual

Public
Burden Statement: An agency may not conduct or sponsor, and a person
is not required to respond to, a collection of information unless it
displays a currently valid OMB control number. The OMB control
number for this project is XXXXXXXXXXX, and the expiration date is
XXXXXXXX. Public reporting burden for this collection of information
is estimated to average XX hours per respondent annually, including
the time for reviewing instructions, searching existing data
sources, gathering and maintaining the data needed, and completing
and reviewing the collection of information. Send comments regarding
this burden estimate or any other aspect of this collection of
information, including suggestions for reducing this burden, to HRSA
Reports Clearance Officer, 5600 Fishers Lane, Room 10-33, Rockville,
MD 20857.
HIV/AIDS
Bureau
Division
of Policy and Data
Health
Resources and Services Administration
U.S.
Department of Health and Human Services
5600
Fishers Lane, Room 7C-26
Rockville,
MD 20857
Table of Contents
Table of Contents i
Background 1
The HIV Quality Measure Module 2
What are the components of the HIVQM? 2
Which clients can be included in the HIVQM? 2
Who enters data in the HIVQM? 2
How do you access the HIVQM? 2
When can you enter data? 3
How is the HIVQM submitted to HAB? 3
Instructions for completing the hivqm Module 4
Step One: Access the most recent RSR Deliverable. 4
Step two: Access the HIVQM 5
Step three: Completing the HIVQM 6
Provider Information 6
Selecting Performance Measures 8
Entering Performance Data 9
Accessing HIVQM Summary Reports 11
Appendix A: Ryan White HIV/AIDS PROGRAM HIV Quality Measures 13
Background
The Ryan White HIV/AIDS Treatment Extension Act of 2009 (Public Law 111-87, October 30, 2009) provides the federal HIV programs in the Public Health Service (PHS) Act under Title XXVI flexibility to respond effectively to the changing epidemic. Its emphasis is on providing life-saving and life-extending services for people living with HIV (PLWH) across the country and on providing resources to targeted areas with the greatest need.
All Program Parts of the Ryan White HIV/AIDS Program (RWHAP) specify the Health Resources and Services Administration’s (HRSA’s) responsibilities in the allocation and administration of grant funds, as well as the evaluation of programs for the population served, and the improvement of the quality of care. Accurate records of the recipients receiving RWHAP funding, the services provided, and the clients served continue to be critical to the implementation of the statute and thus are necessary for HRSA to fulfill its responsibilities.
The Ryan White HIV/AIDS Program statute authorizes the use of grant funds to improve the quality, availability, and organization of HIV health care and support services. Specifically, recipients are required to establish a clinical quality management program (CQM) to:
assess the extent to which HIV services are consistent with the most recent Public Health Service guidelines, (otherwise known as the HHS guidelines) for the treatment of HIV disease and related opportunistic infections; and
develop strategies for ensuring that such services are consistent with the guidelines for improvement in the access to, and quality of HIV services.
Since 2007, HAB has released performance measures for recipients to use as guidance for their clinical quality management program. However, recipients are not required to use the HAB developed measures nor are they required to submit performance measure data. Recipients do report on some clinical data elements through the required Ryan White HIV/AIDS Program Services Report (RSR) on an annual basis; however, this information gives HAB only a snapshot of the quality of HIV services provided by recipients.
In 2013 the HIV/AIDS Bureau (HAB) introduced new HIV Performance Measures located at http://hab.hrsa.gov/deliverhivaidscare/habperformmeasures.html with the goals of:
Identifying core performance measures that are most critical to the care and treatment of people living with HIV;
Combining measures to address people of all ages living with HIV;
Aligning measures with U.S. Department of Health and Human Services priorities, guidelines, and initiatives;
Promoting relevant performance measures used in other federal programs;
Archiving performance measures; and
Monitoring progress toward achieving the goals identified in the National HIV/AIDS Strategy (NHAS).
The HIV Quality Measure Module
In addition, HAB developed the HIV Quality Measures (HIVQM) Module, a tool within the existing RSR portal to allow recipients to voluntarily enter aggregate data on the HAB Performance Measures. This tool provides recipients and their subrecipients an easy-to-use and structured platform to continually monitor their performance in serving clients, particularly in access to care and the provision of quality HIV services. It may be helpful to recipients and subrecipients as they set goals for performance measures and quality improvement projects. Finally, it also allows recipients to obtain reports that compare providers regionally and nationally against other providers. HRSA expects the HIVQM to better support clinical quality management, performance measurement, service delivery, and client monitoring at both the recipient and client levels, enhancing the quality and utility of the submitted data.
Recipients and service providers who participate in the Centers for Medicare and Medicaid Incentive Program, such as the Medicare and Medicaid Electronic Health Records Incentive Program and the Physician Quality Reporting System may also find the HIVQM helpful since data submitted are consistent to qualify and comply with the requirements from these programs.
What are the components of the HIVQM?
The HIVQM module is comprised of three parts:
The Provider Information consists of 4 pre-populated data points about the provider (taken from the latest RSR).
The Performance Measures is where recipients can choose and enter aggregate data on up to 44 clinical measures under nine main categories: 1) core, 2) all ages, 3) adolescents and adult, 4) HIV infected children, 5) HIV exposed children, 6) medical case management, 7) oral health, 8) ADAP and 9) system level. For more detailed information on these clinical measures, see Appendix A: Ryan White HIV Quality Measures, page 13.
The Summary Report is where recipients can generate reports of your own data as well as comparison reports with other recipients and/or subrecipients who have also entered data into the module, but will only be able to compare performance with those recipients and subrecipients that submit data into the database. The identity of the other recipients or subrecipients is not included in the comparison reports.
Which clients can be included in the HIVQM?
All clients who receive HIV services, regardless of funding source, can be included in the HIVQM.
Who enters data in the HIVQM?
The use of the HIVQM tool is voluntary, but it is strongly encouraged. The HIVQM is available for each recipient and sub-recipient who provides HIV care services and can enter their own data. However, recipients are able to complete the data entry in the HIVQM for any of their sub-recipients.
How do you access the HIVQM?
The HIVQM can be accessed through the existing RSR portal. See page 11 for detailed instructions.
When can you enter data?
The HIVQM is available to recipients and sub-recipients four times a year on the dates specified (cycles) in the table below. You may enter data at the beginning of the open date and until the closing date. You can enter data for one or more cycle.
Also, note that at every cycle you will be entering data for a specified 12-month period.
HIVQM Module Opens |
HIVQM Module Closes |
Measurement Year/ Period |
March 1, 2017 |
March 31, 2017 |
January 1, 2016 - December 31, 2016 |
June 1, 2017 |
June 30, 2017 |
April 1, 2016 – March 31, 2017 |
September 1, 2017 |
September 30, 2017 |
July 1, 2016 – June 30, 2017 |
December 1, 2017 |
December 31, 2017 |
October 1, 2016 – September 30, 2017 |
How is the HIVQM submitted to HAB?
Once you have entered and saved data in the Provider Information and the Performance Measures Sections, you have submitted your data. HAB will have access to the data at the conclusion of each HIVQM cycle.
|
Instructions for completing the hivqm Module
Each recipient and their sub-recipients will have access to the HIVQM. Those that receive funding from multiple parts, only need to access the module once to input data. For example: If an agency receives Part A and C funding, they will only need to input data once per reporting period. The Part A and C grant recipients of record will have access to that data.
Step One: Access the most recent RSR Deliverable.
(Recipient-providers only): Log in to the EHBs at https://grants.hrsa.gov/webexternal and navigate to the RSR Performance Report webpage.
I
 f
you need help navigating the EHBs to find your annual RSR,
contact the HRSA Contact Center at 1-877-464-4772.
f
you need help navigating the EHBs to find your annual RSR,
contact the HRSA Contact Center at 1-877-464-4772.Select the “grants folder” link for a grant which you receive RWHAP funds.
Select the “Performance Reports” link under the Submission heading and click “Start” or “Edit.”
(Service Providers only): Log in to the RSR web system at: https://performance.hrsa.gov/hab/regloginapp/admin/login.aspx?application=rsrApp
and navigate to RSR Performance Report webpage.
Step two: Access the HIVQM
On the bottom left side of the screen in the navigation panel select “HIVQM Inbox.” See Figure 1: Screenshot of the RSR Grantee Report Inbox.
Figure 1. Screenshot of the RSR Grantee Report Inbox

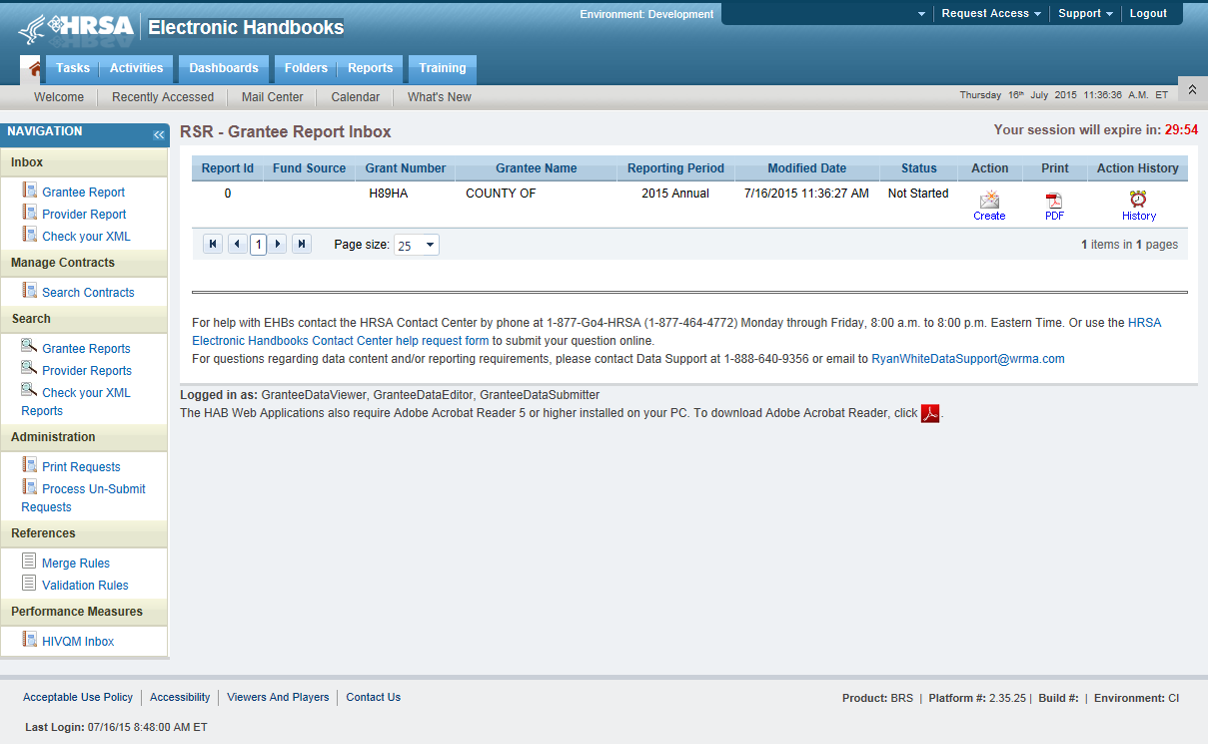
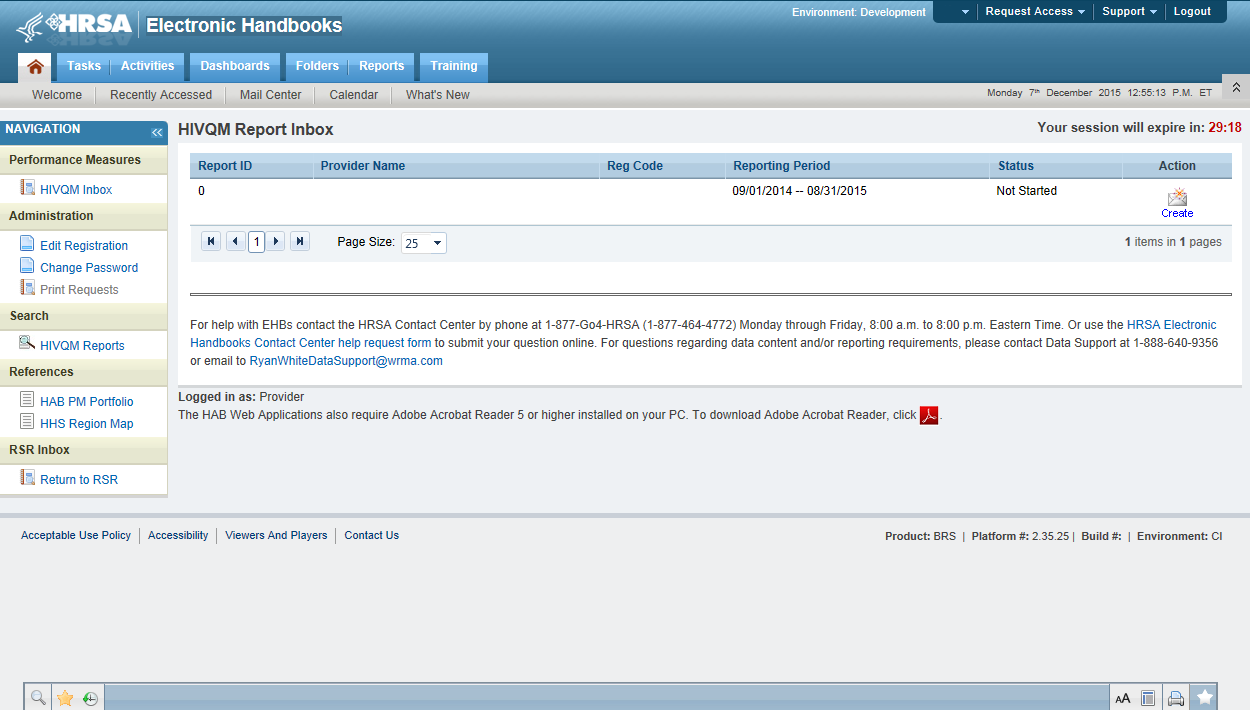
Once you are in the HIVQM Inbox, select the envelope icon on the right under the action column. See Figure 2: Screenshot of the HIVQM Inbox. This will take you to the Provider Information page of the HIVQM.
Figure 2. Screenshot of HIVQM Inbox

Step three: Completing the HIVQM
Provider Information
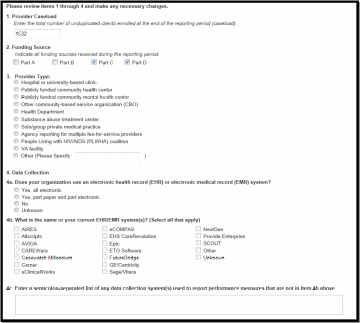
The Provider Information page will be pre-populated with data from your last RSR and consists of 4 items. Check the information already captured on the page and update any incorrect data. Below are the items and option responses. See Figure 3 for the Screenshot of the Provider Information page.
Provider Caseload: Enter the total number of unduplicated clients enrolled at the end of the cycle (caseload). You can enter a number up to 7 characters and must be greater than zero.
Funding Source: Indicate your agency’s funding sources received during the HIVQM cycle by select the corresponding checkboxes. You must select at least one funding source and can select more than one if applicable to your agency.
Part A
Part B
Part C
Part D
Provider Type: Indicate the agency type that best describes your agency by selecting the appropriate radio button. If you choose, Other, please specify a description. You must indicate at least one provider type.
Hospital or university based clinic
Publicly funded community health center
Publicly funded community mental health center
Other community based service organization (CBO)
Health Department
Substance abuse treatment center
Solo/group private medical practice
Agency submitting for multiple fee-for-service providers
People Living with HIV/AIDS (PLWHA) coalition
VA Facility
Other (Please specify.)
Data Collection: This item consists of 3 (a-c) data entries regarding your data collection system(s). You must enter data for a and b. You must enter data for c only if you selected, “Other”, in b.
Does your organization use a computerized data collection system? Select the appropriate radio button.
Yes, all electronic
Yes, part paper and part electronic
No
Unknown
What is the name of your current data collection system(s)? You can select all systems that your agency uses by selecting the checkboxes.
AIRES
Allscripts
AVIGA
CAREWare
Casewatch Millenium
Cerner
eClinicalWorks
eCOMPAS
EHS Care Revolution
Epic
ETO Software
FutureBridge
GE/Centricity
Sage/Vitera
NextGen
Provide Enterprise
SCOUT
Other
Unknown
If you selected, “Other”, in 4b, enter in the text field any data collection system(s) used to run performance measures that are not in item 4b above. Use a semicolon to separate multiple item lists.
Figure 3. HIVQM: Screenshot of the Provider Information Page
Once you have completed the Provider Information page, save your data by selecting the save button on the bottom right of the screen (not shown in Figure 3). If you did not enter data for all items, you will receive an error message to return to the item with missing data and correct it. You will not be able to save your data until all error messages have been addressed.
Selecting Performance Measures
Recipients and sub-recipients can choose 44 performance measures from 9 main categories. Recipients or service providers should prioritize and select measures that are most applicable to their agency, setting, patient population and epidemic. See Appendix A: Ryan White HIV Quality Measures for the list and descriptions. To select performance measures, click on the Select Measures link under HIVQM Navigation on the left side of the screen. See Figure 4: HIVQM: Performance Measure Selection Page.
Figure 4. HIVQM: Performance Measure Selection Page
Information
Icon
Expand
Icon




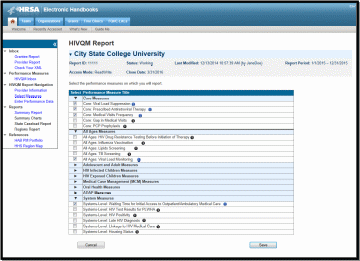
To see the performance measures under each main category, click on the expand icon on the left to expand your selections. Then choose the performance measures you will be entering data for by clicking on the corresponding checkbox. If you want more information about the performance measure, click on the information icon to the right and a pop-up window will display with additional information.
Once you have selected all the performance measures your agency would like to submit data on, save your data by selecting the save button in the lower right corner of the screen.
Entering Performance Data
Once you have saved your performance measures, you are ready to enter your data. On the left side of the screen in under HIVQM Navigation, select Enter Performance Data and the screen will refresh to the Data Entry page containing a table of all the performance measures that you selected from the Select Measures page. See Figure 5: HIVQM: Performance Measure Data Entry Page.
Figure 5. HIVQM: Performance Measure Data Entry Page
Information
Icon

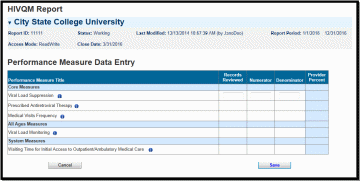
Complete the table by entering your data in the three columns to the right for records reviewed, numerator, and denominator.
Records reviewed should be the number of records that were assessed for the performance measure under review.
The denominator should include patients who should receive the care or service under review.
The numerator should include those patients who should and did receive the care or service under review.
For more program-related guidance on these numbers, you can click on the information icon to the right of the performance measure and a pop-up window will display with additional information.
In addition, below are some system tips to avoid receiving error messages when entering your data.
For records reviewed, you must enter a number less than or equal to your caseload number entered in the Provider Information page.
The records reviewed number must also be greater than or equal to the denominator.
The numerator must be less than or equal to the denominator.
If your numerator is less than 20% of the denominator, you will receive an alert to make sure that this number is correct. You can correct the numerator, or you can ignore the alert if the numerator is correct.
Once you have entered all your data, save your data by selecting the save button in the lower right corner of the screen. If you have entered invalid data (valid data is described above) in any of the columns, you will receive an error message. You will need to go back to your data entries and correct the errors before the system saves your data.
Once your data is accurate and saved, the page will refresh with the calculated “Provider Percent” populated on the last column. This is also your agency’s summary report.
Accessing HIVQM Summary Reports
After you have entered all your data on your selected performance measures, you can run comparison summary reports with other agencies. You will be able to compare your records reviewed, numerator, and denominator with other agencies regionally and nationally. To view reports, click on the Summary Report link under Reports on the left side of the screen. See Figure 6: HIVQM: Summary Report. You can view the following types of summary:
Only your selected performance measures
All performance measures
A single performance measure
You can choose which summary report you would like to see by clicking on the corresponding radio button on the Summary Report page. To choose a single performance measure, click on the radio button on the far right and click on the drop down menu for the desired performance measure. Once you have indicated your chosen report, a table will display comparison summary report. You can export your summary report via multiple formats (including PDF, Microsoft Excel and CSV format) by clicking on the icon in far left, next to the drop down menu.
Note that the reports will only represent data of agencies that submitted data into the HIVQM.
Figure 6. HIVQM: Summary Report
Export
Button



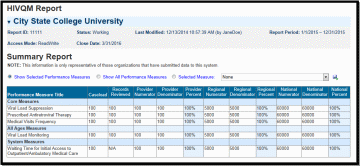
|
Appendix A: Ryan White HIV/AIDS PROGRAM HIV Quality Measures
The HAB HIV quality measures are divided into nine main categories:
Core Measures;
All Ages Measures;
Adolescent and Adult Measures;
HIV Infected Children Measures;
HIV Exposed Children Measures;
Medical Case Management (MCM) Measures;
Oral Health Measures;
ADAP Measures; and
System Measures.
Below are the descriptions of each performance measures under each category.
Core Measures
HIV Viral Load Suppression: Percentage of patients, regardless of age, with a diagnosis of HIV with a HIV viral load less than 200 copies/mL at last HIV viral load test during the measurement year.
Prescription of HIV Antiretroviral Therapy: Percentage of patients, regardless of age, with a diagnosis of HIV prescribed antiretroviral therapy1 for the treatment of HIV infection during the measurement year .
HIV Medical Visit Frequency: Percentage of patients, regardless of age, with a diagnosis of HIV who had at least one medical visit in each 6‐month period of the 24‐month measurement period with a minimum of 60 days between medical visits.
Gap in HIV Medical Visits: Percentage of patients, regardless of age, with a diagnosis of HIV who did not have a medical visit in the last 6 months of the measurement year.
Pneumocystis jiroveci Pneumonia (PCP) Prophylaxis: Percentage of patients aged 6 weeks or older with a diagnosis of HIV/AIDS, who were prescribed Pneumocystis jiroveci pneumonia (PCP) prophylaxis (Use the numerator and denomination that reflect patient population.)
All Ages Measures
HIV Drug Resistance Testing Before Initiation of Therapy: Percentage of patients, regardless of age, with a diagnosis of HIV who had an HIV drug resistance test performed before initiation of HIV antiretroviral therapy if therapy started during the measurement year.
Influenza Immunization: Percentage of patients aged 6 months and older seen for a visit between October 1 and March 31 who received an influenza immunization OR who reported previous receipt of an influenza immunization.
Lipid Screening: Percentage of patients, regardless of age, with a diagnosis of HIV who were prescribed HIV antiretroviral therapy and who had a fasting lipid panel during the measurement year.
Tuberculosis Screening: Percentage of patients aged 3 months and older with a diagnosis of HIV/AIDS, for whom there was documentation that a tuberculosis (TB) screening test was performed and results interpreted (for tuberculin skin tests) at least once since the diagnosis of HIV infection.
Adolescent and Adult Measures
Cervical Cancer Screening: Percentage of female patient with a diagnosis of HIV who have a Pap screening in the measurement year.
Chlamydia Screening: Percentage of patients with a diagnosis of HIV at risk for sexually transmitted infections (STI) who had a test for chlamydia within the measurement period.
Gonorrhea Screening: Percentage of patient with a diagnosis is of HIV at risk for sexually transmitted infection who had a test for gonorrhea within the measurement period.
Hepatitis B Screening: Percentage of patients, regardless of age, for whom Hepatitis B screening was performed at least once since the diagnosis of HIV/AIDS or for whom there is documented infection or immunity.
Hepatitis B Vaccination: Percentage of patients with a diagnosis of HIV who completed the vaccination series for Hepatitis B.
Hepatitis C Screening: Percentage of patients for whom Hepatitis C (HCV) screening was performed at least once since the diagnosis of HIV.
HIV Risk Counseling: Percentage of patients with a diagnosis of HIV who received HIV risk counseling1 in the measurement year.
Oral Exam: Percent of patients with a diagnosis of HIV who received an oral exam by a dentist at least once during the measurement year.
Pneumococcal Vaccination: Percentage of patients with a diagnosis of HIV who ever received pneumococcal vaccine.
Preventive Care and Screening: Screening for Clinical Depression and Follow‐Up Plan: Percentage of patients aged 12 years and older screened for clinical depression on the date of the encounter using an age appropriate standardized depression screening tool AND if positive, a follow‐up plan is documented on the date of the positive screen.
Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention: Percentage of patients aged 18 years and older who were screened for tobacco use one or more times within 24 months AND who received cessation counseling intervention if identified as a tobacco user.
Substance Abuse Screening: Percentage of patients aged 18 years and older who were screened for tobacco use one or more times within 24 months AND who received cessation counseling intervention if identified as a tobacco user.
Syphilis Screening: Percentage of adult patients with a diagnosis of HIV who had a test for syphilis performed within the measurement year.
HIV Infected Children Measures
MMR Vaccination: Percentage of pediatric patients with HIV infection who have had at least one dose of Measles, Mumps & Rubella (MMR) vaccine administered between 12-24 months of age.
HIV Exposed Children Measures
Diagnostic Testing to Exclude HIV Infection in Exposed Infants: Percentage of exposed infants born to HIV-infected women who received recommended virologic diagnostic testing for exclusion of HIV infection in the measurement year
Neonatal Zidovudine(ZDV) Prophylaxis: Percentage of infants born to HIV-infected women who were prescribed ZDV prophylaxis for HIV within 12 hours of birth during the measurement year
PCP Prophylaxis for HIV-Exposed Infants: Percentage of eligible infants with HIV-exposure who were prescribed PCP prophylaxis in the measurement year
Medical Case Management Measures
Medical Case Management: Care Plan: Percentage of medical case management patients, regardless of age, with a diagnosis of HIV who had a medical case management care plan1 developed and/or updated two or more times in the measurement year
Gap in HIV medical visits: Percentage of medical case management patients, regardless of age, with a diagnosis of HIV who did not have a medical visit in the last 6 months of the measurement year (that is documented in the medical case management record).
HIV Medical Visit Frequency: Percentage of medical case management patients, regardless of age, with a diagnosis of HIV who had at least one medical visit in each 6‐month period of the 24‐month measurement period with a minimum of 60 days between medical visits
Oral Health Measures
Dental and Medical History: Percentage of HIV-infected oral health patients who had a dental and medical health history (initial or updated) at least once in the measurement year.
Dental Treatment Plan: Percentage of HIV-infected oral health patients who had a dental treatment plan developed and/or updated at least once in the measurement year.
Oral Health Education: Percentage of HIV-infected oral health patients who received oral health education at least once in the measurement year.
Periodontal Screening or Examination: Percentage of HIV-infected oral health patients who had a periodontal screen or examination at least once in the measurement year.
Phase 1 Treatment Plan Completion: Percentage of HIV-infected oral health patients with a Phase 12 treatment plan that is completed within 12 months.
AIDS Drug Assistance Program (ADAP) Measures
Application Determination: Percent of ADAP applications approved or denied for new ADAP enrollment within 14 days (two weeks) of ADAP receiving a complete application in the measurement year
Application Recertification: Percentage of ADAP enrollees who are reviewed for continued ADAP eligibility two or more times in the measurement year.
Formulary: Percentage of new anti-retroviral classes that are included in the ADAP formulary within 90 days of the date of inclusion of new anti-retroviral classes in the PHS Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents during the measurement year.
Inappropriate Antiretroviral Regimen Components Resolved by ADAP: Percent of identified inappropriate antiretroviral (ARV) regimen components prescriptions that are resolved by the ADAP program during the measurement year.
System Measures
Waiting Time for Initial Access to Outpatient/Ambulatory Medical Care: Not available in 2016
HIV Test Results for PLWHA: Not available in 2016
HIV Positivity: Percentage of HIV positive tests in the measurement year.
Housing Status: Percentage of patients with an HIV diagnosis who were homeless or unstably housed in the 12-month measurement period
Late HIV Diagnosis: Percentage of patients with a diagnosis of Stage 3 HIV (AIDS) within 3 months of diagnosis of HIV.
Linkage to HIV Medical Care: Percentage of patients who attended a routine HIV medical care visit within 3 months of HIV diagnosis.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Michael J.E. Grier |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy