MIPS PRA Supporting Statement Part B 5.26.2016 clean
MIPS PRA Supporting Statement Part B 5.26.2016 clean.docx
(CMS-10621) Merit-based Incentive Payment System
OMB: 0938-1314
Supporting Statement – Part B
Merit-Based Incentive Payment System (MIPS)
CMS-XXXX, OCN XXXX-XXXX
Collections of Information Employing Statistical Methods
Introduction
The Centers for Medicare & Medicaid Services (CMS) seeks approval to collect, process, and analyze data for the purposes of implementing the Merit-based Incentive Payment System (MIPS), one of two paths for providers available through the proposed Quality Payment Program authorized by the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). The Quality Payment Program would replace a patchwork system of Medicare reporting programs with a flexible system that allows MIPS eligible clinicians to choose from two paths that link quality to payments: the Merit-Based Incentive Payment System (MIPS) and Advanced Alternative Payment Models. The MIPS is a new program that combines parts of the Physician Quality Reporting System (PQRS), the Value Modifier (VM or Value-based Payment Modifier), and the Medicare Electronic Health Record (EHR) incentive program into one single program in which MIPS eligible clinicians and groups will be measured on four performance categories. The four performance categories are quality, resource use, clinical practice improvement activities (CPIA), and advancing care information (related to meaningful use of certified EHR technology). Under the APM path, clinicians participating in certain kinds of APMs (Advanced APMs) may become qualifying APM participants (QPs) and excluded from MIPS. QPs will receive lump-sum incentive payments equal to 5 percent of their prior year’s payments.
The data collected under this PRA will be used for research, evaluation, and measure assessment and refinement activities. Specifically, CMS plans to use the data to produce annual statistical reports that will describe the reporting experience of MIPS eligible clinicians as a whole and subgroups of MIPS eligible clinicians.1 The data will also be utilized to fulfill a MACRA requirement in which GAO must perform a MIPS evaluation to submit to Congress by October 1, 20212. Further, CMS will build on existing PQRS processes to monitor and assess measures on ongoing basis to ensure their soundness and appropriateness for continued use in the MIPS. As required by the MACRA, the ongoing measure assessment and monitoring process will be used to refine, add, and drop measures as appropriate.
This Supporting Statement Part B discusses nine Information Collections (ICs) related the evaluation and implementation of the MIPS:
Four ICs related to MIPS quality performance category submission by MIPS eligible clinicians:
Quality measures--Claims submission method
Quality measures-- Qualified registry and Qualified Clinical Data Registry (QCDR) submission method
Quality measures-- EHR submission method
Quality measures—CMS Web Interface submission method
Qualified Registry or QCDR self-nomination
MIPS data validation
Advancing care information performance category
CPIA performance category
Partial QP election
This Supporting Statement Part B is organized as follows: each section below discusses the relevant statistical information for all nine ICs. The four quality performance category submission mechanisms are generally discussed as a group under the quality performance reporting category header, whereas the remaining five ICs are discussed individually under separate headers.
1. Describe (including a numerical estimate) the potential respondent universe and any sampling or other respondent selection method to be used. Data on the number of entities (e.g., establishments, State and local government units, households, or persons) in the universe covered by the collection and in the corresponding sample are to be provided in tabular form for the universe as a whole and for each of the strata in the proposed sample. Indicate expected response rates for the collection as a whole. If the collection had been conducted previously, include the actual response rate achieved during the last collection.
Quality Performance Category Data Submission
Potential respondent universe and response rates
Because historical participation rates for quality data submission under PQRS have never reached 100 percent, we anticipate that MIPS will not achieve full participation. Under the 2014 PQRS, 834,358 of approximately 1.3 million eligible professionals (EPs) (including those who belonged to group practices that participated under the group practice reporting option (GPRO), EPs within an accountable care organization (ACO) participating under the Shared Savings Program or Pioneer ACO Model, and EPs participating through the comprehensive primary care (CPC) initiative) participated. The 2014 PQRS participation rate was 62.8 percent, quadruple the 15 percent participation rate in the program’s first year (2007).3
Given that the majority of MIPS quality performance category measures have been previously used under PQRS, we assume that clinicians who previously submitted quality measures under PQRS will continue to do so under MIPS, either as voluntary reporters or as MIPS eligible clinicians required to report. We assume that some clinicians will voluntarily submit quality performance category data because MACRA permits any eligible professional (EP) under PQRS who is not a MIPS eligible clinician the option to volunteer to report on applicable measures and activities under MIPS. 4 Voluntary reporters will be scored and receive performance feedback under MIPS, but will not be subject to payment adjustments.
Because the MIPS program has not yet been implemented, we estimate the potential universe of MIPS eligible clinicians required to report and the potential universe of clinicians who can voluntarily report using 2014 data from the PQRS, VM Program and other CMS data. The potential universe of MIPS eligible clinicians subject to reporting requirements includes between approximately 716,613 and 775,613 (among the 1,009,623 MIPS eligible clinicians).
The potential universe of EPs who may voluntarily report to MIPS includes between approximately 444,263 and 503,605 EPs5, including approximately 187,990 Medicare professionals not in eligible specialties, 225,615 EPs in eligible specialties that fall under the proposed low-volume threshold,6 and between approximately 30,658 and 90,000 QPs.7 Clinicians may voluntarily submit MIPS quality data for multiple reasons, including (a) to get feedback on their performance relative to national benchmarks or (b) because some clinicians will not be informed if they are MIPS eligible clinicians until after the end of the performance period. Specifically, small groups and solo practitioners will not be informed if they met the low-volume threshold for the MIPS performance period 2017 until early 2018. Similarly, Advanced APM participants will not be informed if they met the performance period 2017 QP threshold to be exempt from MIPS reporting or the partial QP threshold for opting out of MIPS reporting until early 2018.
As discussed above, we assume that the 834,358 of Medicare EPs that submitted PQRS quality data in 2014 will continue to submit quality data under MIPS. We estimate that 81 percent of EPs submitting quality data will be MIPS eligible clinicians. The remaining 19 percent of EPs will submit quality data voluntarily, including Medicare professionals not in eligible specialties (11 percent), and clinicians in eligible specialties meeting the low-volume threshold (8 percent). We assume that the participating MIPS eligible clinicians and voluntary reporters will continue to use the quality data submission mechanism that they used under PQRS. Specifically, we assume that the number of clinicians using different quality data submission mechanisms will include:
299,169 clinicians submitting as individuals through the claims mechanism.
214,590 clinicians submitting as individuals through qualified registry or QCDR mechanisms.
77,241 clinicians submitting through EHR mechanisms.
112,467 clinicians not in APMs and submitting as 300 groups through the CMS Web Interface.
139,921 clinicians participating in 332 Shared Savings Program ACOs and submitting through the CMS Web Interface.
20 participants in Next Gen ACO Model submitting through the CMS Web Interface.8
Sampling for quality data submission
Sampling will be used to select patients for the CMS Web Interface. For the other quality measure submission mechanisms, sampling at the patient level will likely be precluded by the proposed completeness criteria for other quality measure submission mechanisms. Individual MIPS eligible clinicians or groups submitting data on quality measures using QCDRs, qualified registries, or via EHR will be required to report on at least 90 percent of the MIPS eligible clinician or group’s patients that meet the measure’s denominator criteria, regardless of payer for the performance period. In other words, for these submission mechanisms, we would expect to receive quality data for both Medicare and non-Medicare patients. Individual MIPS eligible clinicians submitting data on quality measures data using Medicare Part B claims, would report on at least 80 percent of the Medicare Part B patients seen during the performance period to which the measure applies.
For groups, Shared Savings Program ACOs, and Next Generation ACOs (entities) submitting quality measures through the CMS Web Interface, we plan to use a similar sampling method to that employed in the PQRS GPRO Web Interface and developed for the Physician Group Practice (PGP) Demonstration. Entities must report on all patient care measures and modules included in the CMS Web Interface submission mechanism. For each patient care measure and module, the CMS Web Interface provides a sample of ranked and assigned beneficiaries. Entities must populate data fields for the first 248 consecutively ranked and assigned beneficiaries in the order in which they appear in the entities sample for each module or patient care measure. If the pool of eligible assigned beneficiaries is less than 248, then entities must report on 100 percent of assigned beneficiaries. 9
QCDR or Qualified Registry Self-nomination
We anticipate that the 98 qualified registries and 49 QCDRs qualified to report quality measures data for the 2015 PQRS will self-nominate to submit data on behalf of MIPS eligible clinicians and groups.10 Under the Notice of Proposed Rulemaking, we are proposing to expand qualified registries’ and QCDRs’ capabilities by allowing them to submit data on measures, activities, or objectives for any of the following MIPS performance categories:
Quality;
CPIA; or
Advancing care information, if the MIPS eligible clinician or group is using certified EHR technology.
MIPS Data Validation Survey
The MIPS data validation survey will be designed and administered to identify and address problems with data handling, data accuracy, and incorrect payments for the MIPS program. The survey will build on the PQRS data validation survey. Under MIPS, the survey’s topics will be expanded beyond validation of quality measures to include CPIA and potentially the advancing care information performance category data. Hence we submit data on the statistical design for the PQRS data validation survey here because the MIPS data validation survey will share many of these design elements.
Because the data submitted by, or on behalf of, MIPS eligible clinicians to the MIPS program is used to calculate payment adjustments, it is critical that this data is accurate. Additionally, the data will be used to generate performance feedback for MIPS eligible clinicians and, in some cases, will be posted publicly on the CMS website, further supporting the need for accurate and complete data.
The ultimate use of the quality performance category data is to improve the quality of care for Medicare beneficiaries. This aligns with the CMS mission and helps to make health care more cost-effective and efficient. To determine if data quality issues exist and if the payment adjustments are correct, additional information is required. Surveys are one tool that will be used to collect this data, and they will be sent to the following submission entities: groups using the CMS Web Interface, qualified registries, QCDRs, health IT vendors, and MIPS eligible clinicians submitting via the EHR and claims submission options.
The survey is completely automated and was designed with simplicity as a core requirement – it does not require a login and can be accessed via a link provided in a survey invitation email. There is no Protected Health Information (PHI) or Personally Identifiable Information (PII) submitted in the survey. In order to minimize the burden on the participant community, the number of questions in a survey will not exceed 30. The majority of the questions in the survey are “point and click,” allowing the participant to complete the survey quickly. There is a feedback section included in the survey, which allows for free-form text entry and document upload; however, document uploads are not required.
Sampling, as it relates to the MIPS data validation survey, will limit the number of entities that receive the survey and, consequently, the data examined to identify errors and incorrect payments made from the MIPS data. The samples are generated following the analytical process described in statistical methodology section below. For the first MIPS performance period, we plan to sample as many as 500 entities (MIPS eligible clinicians or groups).
Data Submission for Advancing Care Information and CPIA Performance Categories
Advancing care information performance category data will not be submitted separately by EPs in most cases as was required under the Medicare EHR Incentive Program. MIPS eligible clinicians and groups will submit advancing care information performance category data using the same data submission mechanism they have selected for other MIPS performance categories including attestation, QCDR, qualified registry, EHR, or CMS Web Interface (groups of 25 or more), or they may choose another MIPS data submission mechanism. Based on historical data and 2015 EHR meaningful use attestation, we estimate that approximately 436,500 MIPS eligible clinicians not participating in APMs will submit advancing care information performance category data to MIPS. As noted in Supporting Statement Part A, we also estimated that Advancing Care Information Data will be submitted by 24,925 billing TINs representing 140,341 MIPS eligible clinicians participating in 434 Shared Savings Program ACOs and 55,000 MIPS eligible clinicians participating in other APMs.
Requirements for data submission regarding CPIAs are new, and we do not have historical data which is directly relevant. MIPS eligible clinicians and groups may submit CPIA performance category data using the same data submission mechanism they have selected for other MIPS performance categories including attestation, QCDR, qualified registry, EHR, or CMS Web Interface (groups of 25 or more), or they may choose another MIPS data submission mechanism. We anticipate that the rates of participation in CPIA performance category data submission will be comparable to those of quality performance category data submission. For MIPS eligible clinicians not part of an APM, we assume that the number of MIPS eligible clinicians submitting quality performance category data as part of a group will be approximately the same as the number of EPs reporting PQRS data through the GPRO Web Interface in 2014. We estimate that there could be as many as 595,100 MIPS eligible clinicians submitting CPIA performance category data as individuals. We estimate that approximately 112,500 MIPS eligible clinicians comprising 300 groups may submit data at the group level. As noted in Supporting Statement Part A, we also estimated that CPIA data will be submitted by 24,925 billing TINS representing 140,341 MIPS eligible clinicians participating in 434 Shared Savings Program ACOs and 55,000 MIPS eligible clinicians participating in other APMs.
Data Submission for Partial QP Election for Advanced APM participants
We do not anticipate using sampling for the data submission for Partial QP Elections for APMs. One representative from each APM Entity will make an election on behalf of all APM Entity participants.
2. Describe the procedures for the collection of information including:
- Statistical methodology for stratification and sample selection,
- Estimation procedure,
- Degree of accuracy needed for the purpose described in the justification,
- Unusual problems requiring specialized sampling procedures, and
- Any use of periodic (less frequent than annual) data collection cycles to reduce burden.
Quality Performance Category
For the quality performance category data submission, Table 1 provides information regarding the proposed performance period, sampling, and completeness criteria for the four of the five data submission mechanisms for MIPS eligible clinicians and groups to submit quality measures data for the 2019 MIPS payment adjustment. The requirements for the fifth quality data submission mechanism, CAHPS for MIPS, will be discussed in a separate PRA Package.
TABLE 1: Summary of Proposed Quality Data Submission Criteria for MIPS via Part B Claims, QCDR, Qualified Registry, EHR and CMS Web Interface
Performance Period |
Measure Type |
Submission Mechanism |
Submission Criteria, including Sampling |
Data Completeness |
Jan 1 – Dec 31 |
Individual MIPS eligible clinicians |
Part B Claims |
Report at least six measures including one cross-cutting measure and at least one outcome measure, or if an outcome measure is not available report one other high priority measure; if less than six measures apply then report on each measure that is applicable. MIPS eligible clinicians and groups will have to select their measures from either the list of all MIPS Measures in Table A or a set of specialty specific measures in Table E. |
80 percent of MIPS eligible clinician’s patients |
Jan 1 – Dec 31 |
Individual MIPS eligible clinicians or Groups |
QCDR Qualified Registry EHR |
Report at least six measures including one cross-cutting measure and at least one outcome measure, or if an outcome measure is not available report one other high priority measure; if less than six measures apply then report on each measure that is applicable MIPS eligible clinicians and groups will have to select their measures from either the list of all MIPS Measures in Table A or a set of specialty specific measures in Table E.
|
90 percent of MIPS eligible clinician’s or groups patients |
Jan 1 – Dec 31 |
Groups of 25 or more eligible clinicians |
CMS Web Interface |
Report on all measures included in the CMS Web Interface; AND populate data fields for the first 248 consecutively ranked and assigned Medicare beneficiaries in the order in which they appear in the group’s sample for each module/measure. If the pool of eligible assigned beneficiaries is less than 248, then the group would report on 100 percent of assigned beneficiaries. |
Sampling requirements for their Medicare Part B patients |
QCDR or Qualified Registry Self-nomination
We do not anticipate using sampling for the web-based submission of the QCDR and qualified registry self-nomination form. One representative from each QCDR or qualified registry will submit the form.
MIPS Data Validation Survey
The MIPS data validation survey will build on the PQRS data validation survey. Details on the methodology on the PQRS data validation survey are provided as follows. The methodology begins with the Measures Analytics Phase. During this phase, the data validation contractor conducts detailed literature reviews, environmental scans, and analyses, such as comparison of results against national benchmarks and year-to-year comparisons, to assess the validity of submitted quality results at various levels of granularity. Where feasible, the analyses performed during the Measure Indicator Analysis phase are completed for each of the applicable submission options. The outcomes of these analyses define the business rules for measure submission and validation that are incorporated into rule sets during the Data Analytics phase. Where feasible, the contractor re-uses the rules developed in previous option years by applying required updates or changes.
During the Data Analytics Phase, the data validation contractor applies the rules to the data, via the Cross Industry Standard Process for Data Mining (CRISP-DM) process to derive the samples for the Electronic Survey (Survey). The CRISP-DM process, which is the most widely adopted data mining life cycle model, uses discrete phases to increase data understanding, and in turn, increase the precision of the analytics that are applied during each subsequent phase. By following the CRISP-DM life cycle model, the contractor ensures that a robust process is used to look at the data from many angles to detect and submit data inaccuracies. During this process, the groups and MIPS eligible clinicians are “scored” based on how many rules they did or did not violate.
The phases in the CRISP-DM process are shown in Table 1 below.
Table 1: CRISP-DM Process
# |
CRISP-DM Phases |
Description |
1 |
Business Understanding |
This initial phase focuses on understanding the project objectives and requirements from a business perspective, and then converting this knowledge into a data mining problem definition and a preliminary plan designed to achieve the objectives. |
2 |
Data Understanding |
The data understanding phase starts with an initial data collection and proceeds with activities to get familiar with the data, to identify data quality problems, to discover first insights into the data, or to detect interesting subsets to form hypotheses for hidden information. |
3 |
Data Preparation |
The data preparation phase covers all activities to construct the final dataset (data that will be fed into the modeling tools) from the initial raw data. Data preparation tasks are likely to be performed multiple times, and not in any prescribed order. Tasks include selecting tables, records, and attributes, as well as transforming and cleaning data for modeling tools. |
4 |
Modeling |
In this phase, various modeling techniques are selected and applied, and their parameters are calibrated to optimal values. Typically, there are several techniques for the same data mining problem type. Some techniques have specific requirements based on the form of the data. Therefore, stepping back to the data preparation phase is often needed. |
5 |
Evaluation |
At this stage in the project, models are built that appear to have high quality from a data analysis perspective. Before proceeding to final deployment of the model, it is important to evaluate the model more thoroughly and review the steps executed to construct the model, to be certain it properly achieves the business objectives. A key objective is to determine if there is some important business issue that has not been sufficiently considered. At the end of this phase, a decision on the use of the data mining results should be reached. |
6 |
Deployment |
Creating the model is generally not the end of the project. Even if the purpose of the model is to increase knowledge of the data, the knowledge gained must be organized and presented in a way that the customer can use. Depending on the requirements, the deployment phase can be as simple as generating a submit data or as complex as implementing a repeatable data mining process. In many cases it will be the customer, not the data analyst, who will carry out the deployment steps. However, even if the analyst will not carry out the deployment effort, it is important for the customer to understand up front the actions which will need to be carried out to make use of the created models. |
During the modeling phase, the business rules are translated into SAS code and the code is applied to the data mart. At the highest level, the data modeling process is iterative in nature, as shown in Figure 1below.
Figure 1: CRISP-DM Process
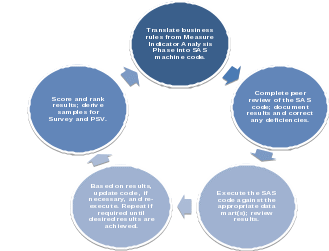
As stated above, when the rules are executed against the data mart, the entities are scored based on how many rules they did or did not violate. The results are then ranked and the samples derived for survey. The intent of this process is two-fold: to identify sources of reporting error and develop recommendations for corrective action(s) and to identify candidates for payment recoupment.
The final step in the data modeling process is to use the experience gained to further refine the rules with the expectation that it will help CMS better identify reporting errors and incorrect payments in future years.
Data Submission for Advancing Care Information and CPIA Performance Categories
We do not anticipate using sampling for the advancing care information and CPIA performance categories. For MIPS eligible clinicians reporting as groups, one representative from each group will submit data on behalf of the entire group.
Data Submission for Partial QP Election for Advanced APM participants
We do not anticipate using sampling for the data submission for Partial QP Elections for APMs. One representative from each APM Entity will submit data on behalf of all APM participants.
3. Describe methods to maximize response rates and to deal with issues of non-response. The accuracy and reliability of information collected must be shown to be adequate for intended uses. For collections based on sampling, a special justification must be provided for any collection that will not yield 'reliable' data that can be generalized to the universe studied.
Quality Performance Category Data Submission
We believe that in addition to being eligible for payment adjustments through MIPS, providing MIPS eligible clinicians and groups with multiple submission options will help to maximize response rates.
We expect additional experience with submission under MIPS to clarify optimal sample sizes and submission criteria for use in future performance periods. We will continually evaluate our policies on sampling and notify the public through future notice and comment rulemaking if we make substantive changes. As we evaluate our policies, we plan to continue a dialogue with stakeholders to discuss opportunities for program efficiency and flexibility.
QCDR or Qualified Registry Self-nomination
We assume that QCDRs and qualified registries that self-nominated for PQRS in the past will self-nominate to submit data on behalf of MIPS eligible clinicians and groups. We believe our proposal to allow qualified registries and QCDRs to submit data for three MIPS performance categories will result in their continued engagement under the MIPS.
MIPS Data Validation Survey
We anticipate that the response rate for the MIPS data validation survey will be similar to the 86 percent response rate experienced in the most recent year of the PQRS data validation survey.
Knowing that timely and appropriate communication encourages participation by the survey participants, survey respondents will receive the initial invitation email and frequent reminder notices. In addition, prior to implementing a new survey, the survey contractor will conduct live demonstrations of the survey functionality with the participant community to increase their comfort level with the survey and encourage successful participation.
Regarding non-response, the contractor will follow-up with participants regularly to remind them that survey participation is required and to ensure that they are on track to complete the survey within the prescribed timeframes.
Appendices E and F contain sample communications, referenced above, that we will plan to share with the MIPS eligible clinician community as part of the MIPS data validation survey. These materials are based on the communication materials for the current PQRS data validation survey.
Data Submission for Advancing Care Information and CPIA Performance Categories
We believe that in addition to being eligible for payment adjustments through MIPS, providing MIPS eligible clinicians and groups with multiple submission options will help to maximize response rates in the advancing care information and CPIA performance categories. Further, we anticipate the advancing care information performance category will have a higher response rate for MIPS eligible clinicians than its predecessor, the Medicare EHR Incentive Program, because it allows for groups as well as individual MIPS eligible clinician reporting.
Data Submission for Partial QP Election for Advanced APM participants
We believe that the opportunity to opt into MIPS reporting and payment adjustments will maximize Advanced APM Entities’ response rates for partial QP elections on behalf of their model participants.
4. Describe any tests of procedures or methods to be undertaken. Testing is encouraged as an effective means of refining collections of information to minimize burden and improve utility. Tests must be approved if they call for answers to identical questions from 10 or more respondents. A proposed test or set of tests may be submitted for approval separately or in combination with the main collection of information.
Quality Performance Category
As stated above, we expect that the initial experience with MIPS will clarify optimal sample sizes and submission criteria for use in future performance periods. We will continually evaluate our policies based on our analysis of the MIPS and other data. For group submission through the CMS Web Interface, we note that the methodology was derived from commercially available methods used to compute quality measures in the commercial and Medicare managed care environment and was previously used under the PQRS GPRO Web Interface.
QCDR or Qualified Registry Self-nomination
As noted above, we plan to modify the QCDR and qualified registry self-nomination process so that they can submit data on behalf of MIPS eligible clinicians and groups for three performance categories: quality, CPIA, and advancing care information performance category data (if the MIPS eligible clinician or group is using certified EHR technology).
Prior to the implementation of the modified QCDR and qualified registry self-nomination process via web-based user interface, testing with fewer than 10 respondents will be completed to ensure that the self-nomination process is functioning as designed.
MIPS Data Validation Survey
Prior to the implementation of new surveys, testing with fewer than 10 respondents will be completed to ensure that the survey is functioning as designed.
Advancing Care Information and CPIA Performance Categories
As stated above, we expect that our initial experience with MIPS will clarify optimal data submission criteria for use in future performance periods. We will continually evaluate our policies based on our analysis of the MIPS and other data.
Partial QP Election for Advanced APM Entities
Prior to the implementation of the Partial QP election data via a web-based user interface, testing with fewer than 10 respondents will be completed to ensure that the data submission tool is functioning as designed.
5. Provide the name and telephone number of individuals consulted on statistical aspects of the design and the name of the agency unit, contractor(s), grantee(s), or other person(s) who will actually collect and/or analyze the information for the agency.
Quality, Advancing Care Information, and CPIA Performance Category Data
We anticipate that a contractor (TBD) will analyze information collected from individual MIPS eligible clinicians submitting data to the quality, advancing care information, and CPIA performance categories.
CMS Web Interface Quality Performance Category Submission
As noted above, we expect that the statistical methods for the CMS Web Interface data submission option will be very similar to those developed for the GPRO Web Interface data submission option. The methods were adopted from the PGP demonstration, the National Committee for Quality Assurance (NCQA) and RTI International were consulted on the development of the sampling methodology. A contractor (TBD) will administer the sampling methodology for the CMS Web Interface.
QCDR or Qualified Registry Self-nomination
Because a statistical design will not be used, no statistical experts were consulted on the QCDR or Qualified Registry self-nomination process.
MIPS Data Validation Survey
We expect that the statistical design of the MIPS data validation survey will be very similar to those for the PQRS data validation survey. IBM Corporation and NCQA were consulted on the statistical aspects of the design of the PQRS data validation survey.
Data Submission for Partial QP Election for Advanced APM Entities
Because a statistical design will not be used, no statistical experts were consulted on the partial QP Election for Advanced APMs.
1 The MIPS annual statistical reports will be modeled after two existing annual reports, the PQRS Experience Report and the Value Modifier Report.
2 MACRA mandates that the GAO evaluate and make recommendations regarding the Composite Performance Scores and the impact of technical assistance.
3 The 2014 PQRS data are from the most recent PQRS Experience Report, available at: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/pqrs/analysisandpayment.html
4 Section 1848(q)(1)(C) of the Act defines a MIPS eligible clinician for payment years 1 and 2 as a physician, physician’s assistant, nurse practitioner, or clinical nurse anesthetist, or a group that includes such clinicians. Specialties not listed as eligible in the Act for payment period 1 or 2 include: Audiologists, Certified Nurse Midwives, Clinical Psychologists/Counselors, Clinical Social Workers, Physical/Occupational Therapists, and Registered Dieticians/Nutritionists.
5 We provide an upper and lower bound estimate of our range of MIPS eligible clinicians because of uncertainty as to the number of clinicians who will join new and existing Advanced Alternative Payment Models in 2016. For more details on the estimated number of MIPS eligible clinicians, see the Regulatory Impact Analysis in the Notice of Proposed Rule Making.
6 The proposed low-volume threshold is less than $10,000 in Medicare Allowable charges and fewer than 100 Medicare patients,
7 The Qualifying APM participant estimate also accounts for certain Partial Qualifying APM participants. We provide an upper and lower bound estimate of QPs because of uncertainty as to the number of clinicians who will join new and existing Advanced Alternative Payment Models in 2016. For more details on the estimated number of QPs, see the Regulatory Impact Analysis in the Notice of Proposed Rule Making.
8 We are assuming that the number of clinicians who will participate in MIPS quality reporting via the NextGen ACO Model in performance period 2017 will be equal to the number who participated in PQRS through Pioneer ACO Model in 2014. The Next Generation ACO Model is not included in 2014 PQRS data because it was launched in 2015. The Pioneer ACO model ends in 2016.
9As noted above, the CMS Web Interface will use similar sampling specifications as under the PQRS GPRO Web Interface. For additional information on sampling under the PQRS GPRO Web Interface Reporting Option, see https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/GPRO_Web_Interface.html
10 The full list of qualified registries for 2015 is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/Downloads/2015QualifiedRegistries.pdf and the full list of QCDRs is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/Downloads/2015QCDRPosting.pdf.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Supporting Statement – Part B |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy