2 Attachment 5D PHS 398 Cover Page Supplement Instructions
PHS Applications and Pre-award Related Reporting (OD)
Attachment 5D PHS 398 Cover Page Supplement Instructions
PHS 398 Cover Page Supplement
OMB: 0925-0001
5. Completing PHS 398 Forms
5.1 Overview
In conjunction with the SF424 (R&R) forms, NIH and other PHS agencies grants applicants should also complete and submit additional forms titled “PHS 398.” Note the PHS 398 forms include additional data required by the agency for a complete application. While these are not identical to the PHS 398 application form pages, the PHS 398 reference is used to distinguish these additional data requirements from the data collected in the SF424 (R&R) forms. A complete application to NIH and other PHS agencies will include SF424 (R&R) and PHS 398 forms. The PHS 398 forms include:
PHS 398 Cover Page Supplement (this supplements the data requirements in the SF 424 R&R form)
PHS 398 Modular Budget Form (use only when a modular budget is submitted instead of a detailed budget)
PHS Inclusion Enrollment Report
Complete each form using the instructions provided below.
5.2 (Reserved)
5.3 Cover Page Supplement Form

Several types of applications require additional instructions to those found in this section:
Career Development Candidates should also refer to Section 7.4.7
Institutional Training Program Applicants should also refer to Section 8.4.6
1. Human Subjects Section
Field Name |
Instructions |
Clinical Trial? |
Check "yes" or "no" to indicate whether the project includes a clinical trial. See Supplemental Grant Application Instructions, Part III.3 for the specific definition. |
Agency-Defined Phase III Clinical Trial
|
Check “Yes” or “No” to indicate whether the project is an NIH-defined Phase III clinical trial. An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of evaluating an experimental intervention in comparison with a standard or controlled intervention or comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included. |
2. Vertebrate Animals Section
Field Name |
Instructions |
Are animals euthanized? |
Check "Yes" or "No" to indicate whether animals in the project are euthanized. |
If “Yes” to euthanasia Is method consistent with AVMA Guidelines? |
Check “Yes” or “No” to indicate whether the method of euthanasia is consistent with the American Veterinary Medical Association Guidelines for the Euthanasia of Animals. |
If “No” to AVMA Guidelines, describe method and provide a scientific justification. |
If you answered “No” to the question “Is method consistent with AVMA Guidelines?” describe the method of euthanasia and provide a scientific justification for its use. If you answered “Yes”, leave the section blank. |
3. *Disclosure Permission Statement Section
Field Name |
Instructions |
If this application does not result in an award, is the Government permitted to disclose the title of your proposed project, and the name, address, telephone number and e-mail address of the official signing for the applicant organization, to organizations that may be interested in contacting you for further information (e.g., possible collaborations, investment)?
|
Select "yes" or "no" to indicate whether disclosure permission is granted. This field is required. Your response will not affect any peer review or funding decisions. |
4. Program Income Section
Field Name |
Instructions |
Is program income anticipated during the periods for which the grant support is requested? |
If program income is anticipated during the periods for which the grant support is requested, check “Yes,” and then complete the section below. If no program income is anticipated, check “No” and leave the following section blank. |
Budget Period
|
If program income is anticipated, enter the budget periods in this column. If the application is funded, the Notice of Grant Award will provide specific instructions regarding the use of such income. |
Anticipated Amount ($) |
If program income is anticipated, enter the amount anticipated for each budget period listed. |
Source(s) |
If program income is anticipated, enter the source for each budget period listed. |
5. Human Embryonic Stem Cells Section
Field Name |
Instructions |
Does the proposed project involve human embryonic stem cells? |
If the proposed project involves human embryonic stem cells, check Yes and complete the section below. If the proposed project does not involve human embryonic stem cells, check No. |
Specific stem cell line cannot be referenced at this time. One from the registry will be used. |
If a specific line cannot be referenced at the time of application submission, check this box. Additionally, provide a strong justification for why an appropriate cell line is not available from the Registry at this time. The justification should be included as part of the Research Strategy or Program Plan as appropriate. |
Cell Line(s)
|
List in this section the 4-digit registration number of the specific cell line(s) from the NIH Human Embryonic Stem Cell Registry (e.g. 0123) |
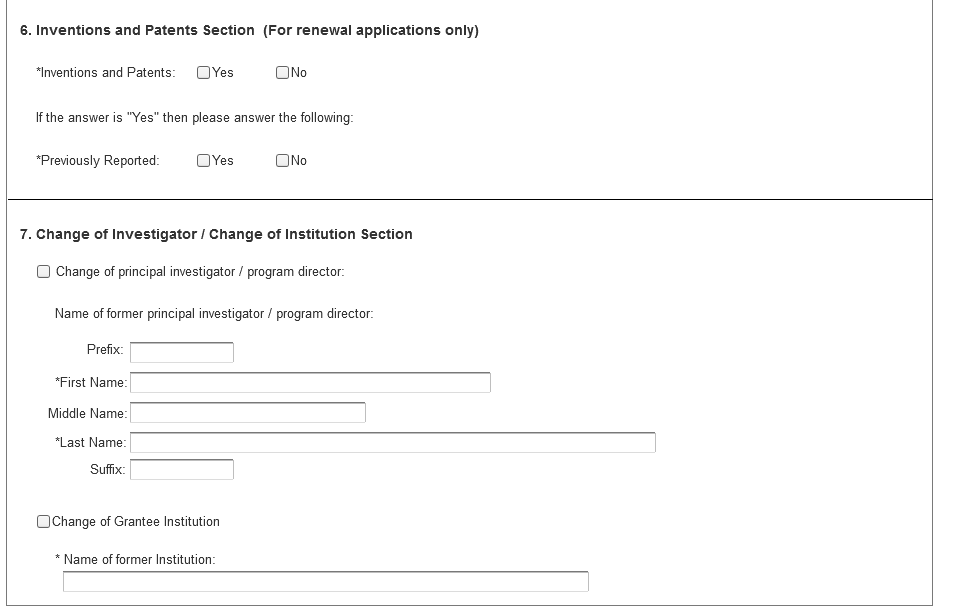
6. Inventions and Patents Section (For renewal applications only)
Field Name |
Instructions |
Inventions and Patents |
This block need only be completed if submitting an R&R “Renewal” application or a Resubmission of a Renewal application. If no inventions were conceived or reduced to practice during the course of work under this project, check “No.” The remaining parts of the item are then not applicable. If any inventions were conceived or reduced to practice during the previous period of support, check “Yes.” Note: NIH recipient organizations must promptly report inventions to the Extramural Inventions and Technology Resources Branch of the Office of Policy for Extramural Research Administration, OER, NIH, Bethesda, MD 20892-2750, (301) 435-1986. Invention reporting compliance according to regulations at 37 CFR 401.14 is described at http://www.iedison.gov.http://www.iedison.gov. The grantee is encouraged to submit reports electronically using Interagency Edison (http://www.iedison.gov). |
Previously Reported |
If the item above is checked "Yes", indicate whether this information has been reported previously to the PHS or to the applicant organization official responsible for patent matters. |
7. Change of Investigator/Change of Institution Section
Field Name |
Instructions |
Change of Program Director/Principal Investigator |
Check here, if this application reflects a change in principal investigator/program director from that indicated on a previous application This is not generally applicable to a "New" application.
|
Prefix |
If this application reflects a change in PD/PI, enter the name prefix (for example, Mr., Mrs., Rev.) of the former PD/PI. |
First Name |
If this application reflects a change in PD/PI, enter the first name of the former PD/PI. |
Middle Name |
If this application reflects a change in PD/PI, enter the middle name of the former PD/PI. |
Last Name |
If this application reflects a change in PD/PI, enter the last name of the former PD/PI. |
Suffix |
If this application reflects a change in PD/PI, provide the suffix (for example, Jr., Sr., PhD) of the former PD/PI. |
Change of Grantee Institution |
Check here, if this application reflects a change in grantee institution from that indicated on a previous application. This is not generally applicable to a "New" application. |
Name of Former Institution |
If this application reflects a change in grantee institution, insert the name of the former institution here. |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Harris, Stefanie (NIH/OD) [E] |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy