Form Application
Additional Infromation for the FY 2016-17 Uniform BG Application
TAB A FY_2016-2017_SABG__Plan_SSP_Amendment 09152016
Uniform BG Application Amendment
OMB: 0930-0370
FY 2016-2017 Uniform Application
Section III. Behavioral Health Assessment and Plan
Subsection C. Environmental Factors and Plan
Amendment
23. Syringe Services Program
The Substance Abuse Prevention and Treatment Block Grant (SABG) restriction1,2 on the use of federal funds for programs distributing sterile needles or syringes (referred to as syringe services programs (SSP)) was modified by the Consolidated Appropriations Act, 2016 (P.L. 114-113) signed by President Obama on December 18, 20153.
Section 520. Notwithstanding any other provisions of this Act, no funds appropriated in this Act shall be used to purchase sterile needles or syringes for the hypodermic injection of any illegal drug: Provided, that such limitation does not apply to the use of funds for elements of a program other than making such purchases if the relevant State or local health department, in consultation with the Centers for Disease Control and Prevention, determines that the State or local jurisdiction, as applicable, is experiencing, or is at risk for, a significant increase in hepatitis infections or an HIV outbreak due to injection drug use, and such program is operating in accordance with State and local law.
A state experiencing, or at risk for, a significant increase in hepatitis infections or an HIV outbreak due to injection drug use, (as determined by CDC), may propose to use SABG to fund elements of a SSP other than to purchase sterile needles or syringes. However, directing FY 2016 SABG funds to SSPs will require a modification of the 2016-2017 SABG Behavioral Assessment and Plan (Plan). States interested in directing SABG funds to SSPs must provide the information requested below and receive approval on the modification from the State Project Officer. Please note that the term used in the SABG statute and regulation, intravenous drug user (IVDU) is being replaced for the purposes of this discussion by the term now used by the federal government, persons who inject drugs (PWID).
States may consider making SABG funds available to either one or more entities to establish elements of a SSP or to establish a relationship with an existing SSP. States should keep in mind the related PWID SABG authorizing legislation and implementing regulation requirements when modifying the Plan, specifically, requirements to provide outreach to PWID, SUD treatment and recovery services for PWID, and to routinely collaborate with other healthcare providers, which may include HIV/STD clinics, public health providers, emergency departments, and mental health centers4. SAMHSA funds cannot be supplanted, in other words, used to fund an existing SSP so that state or other non-federal funds can then be used for another program.
In the first half of calendar year 2016 the federal government released three guidance documents regarding SSPs5: These documents can be found on the Aids.gov website: https://www.aids.gov/federal-resources/policies/syringe-services-programs/
Department of Health and Human Services Implementation Guidance to Support Certain Components of Syringe Services Programs, 2016 from The US Department of Health and Human Services, Office of HIV/AIDS and Infectious Disease Policyhttps://www.aids.gov/pdf/hhs-ssp-guidance.pdf
Centers for Disease Control and Prevention (CDC )Program Guidance for Implementing Certain Components of Syringe ServicesPrograms,2016 The Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention, Division of Hepatitis Prevention http://www.cdc.gov/hiv/pdf/risk/cdc-hiv-syringe-exchange-services.pdf5.
The Substance Abuse and Mental Health Services Administration (SAMHSA)-specific Guidance for States Requesting Use of Substance Abuse Prevention and Treatment Block Grant Funds to Implement SSPs http://www.samhsa.gov/sites/default/files/grants/ssp-guidance-state-block-grants.pdf
Please refer to the guidance documents above when requesting a modification to the state’s 2016-2017 Behavioral Health Assessment and Plan.
Please follow the steps listed below to modify the Plan:
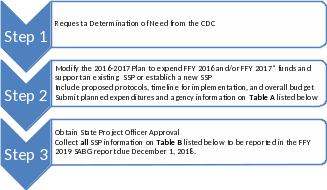
*FFY 2017 and future years subject to authorizing language in appropriation bills.
SSP Program Information
2016-2017 Behavioral Health Plan Amendment
TABLE A
Syringe Services Program (SSP) Agency Name |
Main Address of SSP |
Dollar amount of SABG funds used for SSP |
SUD treatment Provider Y or N |
# of locations (include any mobile locations) |
Narcan Provided Y or N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SSP Annual Data Collection
TABLE B
Syringe Services Program Name |
# of unique individuals served |
HIV Testing (Please enter total number of individuals served) |
Treatment for substance use conditions (Please enter total number of individuals served) |
Treatment for physical health (Please enter total number of individuals served) |
STD Testing (Please enter total number of individuals served) |
Hep C (Please enter total number of individuals served) |
|||||
ONSITE testing |
REFERRAL to testing |
ONSITE treatment |
REFERRAL to treatment |
ONSITE treatment |
REFERRAL to treatment |
ONSITE testing |
REFERRAL to testing |
ONSITE testing |
REFERRAL to testing |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
End Notes
1 Section 1923 (b) of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-23(b)) and 45 CFR § 96.126(e) requires entities that receive SABG funds to provide substance use disorder (SUD) treatment services to PWID to also conduct outreach activities to encourage such persons to undergo SUD treatment. Any state or jurisdiction that plans to re-obligate FY 2016 SABG funds previously made available such entities for the purposes of providing substance use disorder treatment services to PWID and outreach to such persons may submit an amendment to its plan to SAMHSA for the purpose of incorporating elements of a SSP in one or more such entities insofar as the plan amendment is applicable to the FY 2016 SABG funds only and is consistent with guidance issued by SAMHSA.
2Section 1931(a(1)(F) of Title XIX, Part B, Subpart II of the Public Health Service (PHS) Act (42 U.S.C.§ 300x-31(a)(1)(F)) and 45 CFR § 96.135(a)(6) explicitly prohibits the use of SABG funds to provide persons who inject drugs (PWID) with hypodermic needles or syringes so that such persons may inject illegal drugs unless the Surgeon General of the United States determines that a demonstration needle exchange program would be effective in reducing injection drug use and the risk of HIV transmission to others. On February 23, 2011, the Secretary of the U.S. Department of Health and Human Services published a notice in the Federal Register (76 FR 10038) indicating that the Surgeon General of the United States had made a determination that syringe services programs, when part of a comprehensive HIV prevention strategy, play a critical role in preventing HIV among PWID, facilitate entry into SUD treatment and primary care, and do not increase the illicit use of drugs.
3 Division H Departments of Labor, Health and Human Services and Education and Related Agencies, Title V General Provisions, Section 520 of the Consolidated Appropriations Act, 2016 (P.L. 114-113)
4 Section 1924(a) of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-24(a)) and 45 CFR § 96.127 requires entities that receives SABG funds to routinely make available, directly or through other public or nonprofit private entities, tuberculosis services as described in section 1924(b)(2) of the PHS Act to each person receiving SUD treatment and recovery services.
Section 1924(b) of Title XIX, Part B, Subpart II of the PHS Act (42 U.S.C. § 300x-24(b)) and 45 CFR 96.128 requires “designated states” as defined in Section 1924(b)(2) of the PHS Act to set-aside SABG funds to carry out 1 or more projects to make available early intervention services for HIV as defined in section 1924(b)(7)(B) at the sites at which persons are receiving SUD treatment and recovery services.
Section 1928(a) of Title XXI, Part B, Subpart II of the PHS Act (42 U.S.C. 300x-28(c)) and 45 CFR 96.132(c) requires states to ensure that substance abuse prevention and SUD treatment and recovery services providers coordinate such services with the provision of other services including, but not limited to, health services.
5 Department of Health and Human Services Implementation Guidance to Support Certain Components of Syringe Services Programs, 2016 describes a SSP as a comprehensive prevention program for PWID that includes the provision of sterile needles, syringes and other drug preparation equipment and disposal services, and some or all of the following services:
Comprehensive HIV risk reduction counseling related to sexual and injection and/or prescription drug misuse;
HIV, viral hepatitis, sexually transmitted diseases (STD), and tuberculosis (TB) screening;
Provision of naloxone (Narcan®) to reverse opiate overdoses;
Referral and linkage to HIV, viral hepatitis, STD, and TB prevention care and treatment services;
Referral and linkage to hepatitis A virus and hepatitis B virus vaccinations; and
Referral to SUD treatment and recovery services, primary medical care and mental health services.
Centers for Disease Control and Prevention (CDC) Program Guidance for Implementing Certain Components of Syringe Services Programs, 2016 includes a description of the elements of a SSP that can be supported with federal funds.
Personnel (e.g., program staff, as well as staff for planning, monitoring, evaluation, and quality assurance);
Supplies, exclusive of needles/syringes and devices solely used in the preparation of substances for illicit drug injection, e.g., cookers;
Testing kits for HCV and HIV;
Syringe disposal services (e.g., contract or other arrangement for disposal of bio-hazardous material);
Navigation services to ensure linkage to HIV and viral hepatitis prevention, treatment and care services, including antiretroviral therapy for HCV and HIV, pre-exposure prophylaxis, post-exposure prophylaxis, prevention of mother to child transmission and partner services; HAV and HBV vaccination, substance use disorder treatment, recovery support services and medical and mental health services;
Provision of naloxone to reverse opioid overdoses
Educational materials, including information about safer injection practices, overdose prevention and reversing a opioid overdose with naloxone, HIV and viral hepatitis prevention, treatment and care services, and mental health and substance use disorder treatment including medication-assisted treatment and recovery support services;
Condoms to reduce sexual risk of sexual transmission of HIV, viral hepatitis, and other STDs;
Communication and outreach activities; and
Planning and non-research evaluation activities.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Windows User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy