CMS-10525 - Supporting Statement A [rev 12-20-2016 by OSORA PRA]
CMS-10525 - Supporting Statement A [rev 12-20-2016 by OSORA PRA].docx
Program of all-Inclusive Care for the Elderly PACE Quality Data Entry in the CMS Health Plan Monitoring System (HPMS) (CMS-10525)
OMB: 0938-1264
Supporting Statement Part A
Program of all-Inclusive Care for the Elderly PACE Quality Data Entry in
CMS Health Plan Monitoring System
CMS-10525, OMB 0938-1264
Note: This information collection request is currently approved by OMB under the title, “Health Plan Monitoring System Level I and Level II Data Entry for the Program of all-Inclusive Care for the Elderly.” This iteration revises the title as set out above. The OMB control number and the CMS ID number are unchanged. Additional changes are discussed below under section 15.
Background
Program of All-Inclusive Care for the Elderly (PACE) organizations coordinate the care of each participant enrolled in the program based on his or her individual needs with the goal of enabling older individuals to remain in their community. To be eligible to enroll in PACE, an individual must: be 55 or older, live in the service area of a PACE organization (PO), need a nursing home-level of care (as certified by the state in which he or she lives), and be able to live safely in the community with assistance from PACE (42 CFR 460.150(b)).
PACE is a program that provides comprehensive care. An interdisciplinary team of health professionals provides individuals with coordinated care. The overall quality of care is analyzed by information collected and reported to the Centers for Medicare & Medicaid Services (CMS) related to specific quality data that may cause potential or actual harm. CMS requires that these quality data be reported to CMS. CMS analyzes the quality data to identify opportunities to improve the quality of care, safety and PACE sustainability and growth.
CMS’s overall goal for collecting quality data is to measure healthcare quality processes outcomes, participant perceptions and organization structure and or /systems that are associated with the ability to provide high-quality health care and/or that relate to one or more quality goals for health care improvement (CMS, 2014).
Currently, quality data reporting is identified as Level I or Level II reporting. Level I reporting requirements refer to data that POs regularly report to CMS via the CMS Health Plan Management System (HPMS) and the Division of Medicare Advantage operations (DMAO) portal. (Please see Appendix A for the list of quality data categories.)
Level II reporting requirements apply specifically to unusual incidents that result in serious adverse participant outcomes, or negative national or regional notoriety related to PACE. For example, Level II reportable incidents may include certain deaths, infectious disease outbreaks, pressure related ulcer/injury acquired or traumatic injuries while enrolled in the PACE program. POs have been collecting, submitting and reporting Level I and Level II data to CMS and State administering agencies (SAA) since 1999.
Additionally, CMS including quality measure data collection as a data source. CMS is establishing adopted National Quality Forum (NQF) modified for PACE quality measurement use. Quality measures are quality data that is used for assessing observations, treatment, processes, experience, and/or outcomes of participant care.
In this PRA package, we are making title changes from Level I and Level II to PACE Quality Data. The PACE Quality Data title will be used to reference all PACE data collections. Additionally, we plan to include three (Falls, Falls with Injury and Pressure Ulcer Prevalence) PACE Quality measures adopted from the NQF as PACE quality data collection. We are also requesting in this PRA package to collect all PACE quality data into HPMS. All quality data will be used to improve quality for participants in PACE. There will be a separate PRA package that will explain the definitions of each (3) quality measure. POs will be educated on data criteria, entry and report data quarterly.
A. Justification
Need and Legal Basis
CMS requests a three-year clearance from the Office of Management and Budget (OMB) under the Paperwork Reduction Act of 1995 for POs to collect quality data into the CMS HPMS for purposes related to improving quality of care. Quality data is necessary for ensuring participant protection and creating quality improvement processes and programs.
CMS is focused on quality improvement and providing quality healthcare for Medicare beneficiaries. It is critical that POs have established benchmarks to compare and contrast care and quality outcomes against POs or other organizations with a similar, frail population. Quality data collection will enable POs and CMS to identify opportunities for measurement and evaluation of quality of care to improve health outcomes for PACE participants.
The legal basis is as follows:
Statutory Section
1894(b)(2)(A) of the Social Security Act requires a written plan to be developed by the PO of quality assurance and improvement, procedures for implementing such a plan.
Regulations at 42 CFR Part 460
§460.140(a) requires a PO to meet external quality assessment and reporting requirements, as specified by CMS or State administering agency, in accordance with §460.202.
§460.200(a) requires a PO to collect data, maintain records, and submit reports as required by CMS and the State administering agency.
§460.200(b) requires a PO to allow CMS and the State administering agency access to data and records including, but not limited to, Participants Health Outcomes Data.
§460.202 requires a PO to meet external quality assessment and reporting requirements, as specified by CMS or State administering agency. A PACE organization must establish and maintain a health information system that collects, analyzes, integrates, and reports data necessary to measure the organization’s performance, including outcomes of care furnished to participants. A PACE organization must furnish data and information pertaining to its provision of participants’ care in the manner and at the time intervals specified by CMS and the SAA. The items collected are specified in the PACE program agreement.
Information Users
The primary purpose of this action is to collect consistent data among all POs with the goal of using the data to analyze and identify overall quality improvement strategies for enhancing quality of care provided to PACE participants.
3. Use of Information Technology
The data is collected at the PACE site and then submitted into HPMS via excel file uploads and drop down selections submissions. POs are provided login identification codes and this code is considered their signature and is required by HPMS.
4. Duplication of Effort
This information collection does not duplicate any other effort and the information cannot be obtained from any other source.
5. Small Businesses
This collection should not impact small businesses or other small entities.
6. Less Frequent Collection
Quality assurance and participant safeguards are at risk without the collection of quality data. PACE programs will be required to submit quality data on a quarterly basis for the purposes of identifying risk for harm and areas for POs quality improvement. If this data is not submitted, CMS and POs cannot adequately assess their performance and participants are at increased risk for harm.
7. Special Circumstances
There are no special circumstances that would require an information collection to be conducted in a manner that requires respondents to:
Report information to the agency more often than quarterly;
Prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Submit more than an original and two copies of any document;
Retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
Collect data in connection with a statistical survey that is not designed to produce valid and reliable results that can be generalized to the universe of study,
Use a statistical data classification that has not been reviewed and approved by OMB;
Include a pledge of confidentiality that is not supported by authority established in statute or regulation that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
Submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
8. Federal Register/Outside Consultation
The 60-day notice published in the Federal Register on June 13, 2016 (81 FR 38187). Comments were received. The comments and our response have been added to this PRA package.
Based on previous and recent public comment which claimed that our burden estimates were too low, we have reassessed our figures and have revised them accordingly. For instance, this iteration adjusts the number of entries by adding 50 Level I entries and 85 Level II entries. The number of data categories remains unchanged.
9. Payments/Gifts to Respondents
Quality data collection do not include incentive payments or gifts.
10. Confidentiality
POs are aware and informed of the confidentiality of their data collection, recording, and data entry under 42 U.S.C. 1306, 20 CFR parts 401 and 422, 5 U.S.C. 552 (Freedom of Information Act), 5 U.S.C. 552a (Privacy Act of 1974), and OMB Circular No. A-130.
11. Sensitive Questions
There are no sensitive questions associated with this collection. Specifically, the collection does not solicit questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private.
12. Burden Estimates (Hours & Wages)
Wage Estimates
We estimate $36.00/hr which is based on data from the survey among PACE staff that currently holds the Clinical Quality positions (total 10 PACE Quality personnel from different regions surveyed as of June 2015).
Burden Estimates
Exhibit 1 shows the estimated annual time for PACE Quality Data (formally known as Level I and Level II) and PACE Quality Measures HPMS data entry.
Data Entry |
Number of possible data entries/PO |
Estimated Number of data categories per data entry |
Hours per data entry |
Annual Burden Hours (per PO) |
Annual Burden Hours (aggregate: for 120 POs) |
PACE Quality Data Entry - Level I |
80 |
8 |
2.0 |
1,280 |
153,600 |
PACE Quality Data Entry - Level II |
125 |
10 |
1.0 |
1,250 |
150,000 |
Quality Measure(s) Data Entry |
90 |
10 |
0.25 |
225 |
27,000 |
Total |
295 |
28 |
varies |
2,115 |
330,600 |
Exhibit 2 sets out the estimated annualized cost for quality managers to enter PACE Quality Data (Formally known as Level I, Level II) and Quality Measures data into HPMS.
Data Entry
|
Annual Burden Hours (per PO) |
Hourly Wage |
Annual Cost Per PO ($) |
Total Cost (aggregate: for 120 POs) ($) |
PACE Quality Data Entry - Level I |
1,280 |
$36.00/hr |
46,080 |
5,529,600 |
PACE Quality Data Entry - Level II |
1,250 |
45,000 |
5,400,000 |
|
Quality Measure(s) Data Entry |
225 |
8,100 |
972,000 |
|
Total |
2,115 |
$36.00/hr |
99,180 |
11,901,600 |
Information Collection Instruments/Instruction/Guidance Documents
PACE Level I Reporting Guidance, Issued Feb 2016
PACE Level II Reporting Guidance HPMS Memo, Issued June, 2015
PACE Level II Reporting Guidance, Issued July 2015
Additional Information (Part 1: Instructions)
Additional Information (Part 2: Instructions)
Additional Information (Part 3: Example of Data Entry Format)
13. Capital Costs
There is no capital cost for this data collection and entry.
14. Cost to Federal Government
For the cost estimates provided below, wages correlate to one government employee who is/will frequently monitor and analyze HPMS PACE Quality Data formally Level I, Level II and Quality Measure Data.
Exhibit 3 shows the annualized cost burden to the Federal Government to analyze HPMS Quality Data formally known as Level I, Level II, and Quality Measure Data. We estimate that weekly reviews will require 8-10 hours by a nurse consultant.
Data Entry
|
Total Burden Hours |
Hourly Wage Rate* |
Total Burden Cost ($) |
PACE Quality Data - Level I Data Review |
110 |
$44.15/hr |
4,856.50 |
PACE Quality Data - Level II Data Review |
210 |
9,271.50 |
|
Quality Measure(s) Data Review |
115 |
5,077.25 |
|
Total |
435 |
$44.15/hr |
19,205.25 |
*The hourly rate is based on Office of Personnel Management’s General Schedule for the Washington-Baltimore-Arlington locality (effective January 2016) for a GS-13 step 1 nurse consultant (see https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2016/DCB_h.pdf).
15. Program and Burden Changes
This information collection request is currently approved by OMB under the title, “Health Plan Monitoring System Level I and Level II Data Entry for the Program of all-Inclusive Care for the Elderly.” This iteration revises the title to read, “Program of all-Inclusive Care for the Elderly PACE Quality Data Entry in CMS Health Plan Monitoring System.”
Similarly, Level I and Level II are changed to PACE Quality Data. The PACE Quality Data title will be used to reference all PACE data collections.
There are several data quality changes (see Appendix A for a list of this iteration’s quality data) which have no impact on our currently approved burden estimates. The changes were made to mimic industry terminology and standards. More specifically, there was a name change from Prospective enrollees to Denials, there was no change in the intent, however we HPMS to capture the same information. For Census, this information was collected in the HPMS but was not listed under as a certain individual title and therefore the technology within the HPMS system allowed for a title to be created in the system for the number POs in a PO (which is called census). While Immunizations has always been collected, it was inadvertently omitted from the currently approved information collection request. Unusual incidents and Reporting requirements/all reporting is not a new requirement, it’s the old terminology relating to Level I and Level II as Unusual incidents.
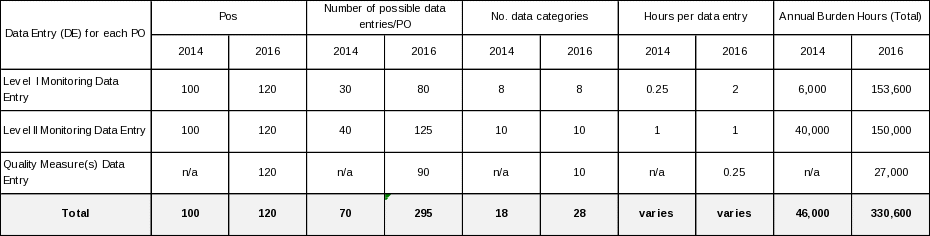
As demonstrated in the following table, we are:
(1) Adjusting our estimated number of respondents from 100 POs to 120 POs.
(2) We are adding three (Falls, Falls with Injury and Pressure Ulcer Prevalence) PACE Quality measures adopted from the NQF.
(3) While the number of data categories is unchanged, this iteration adds 50 Level I entries and 85 Level II entries. The adjustment is in response to public comment.
(4) Based on previous and current public comments we are adjusting Level I response time from 0.25 hr to 2.0 hr.
(5) Correcting our calculation for the number of responses which should amount to: # POs x annual frequency of reporting.
(6) We propose to collect all PACE quality data into HPMS. Currently, Level I data is entered into HPMS as a narrative for each requirement. The change includes entering all PACE quality data collected in HPMS from a pick list/drop down selection format.
There will be a separate PRA package that will explain the definitions of each of the three (3) quality measures. We are setting out the implementation burden based on the results of the beta testing for feasibility (approved by OMB under the Fast Track control number 0938-1185). The testing showed that to implement these quality requirements into HPMS, POs are able to collect data with less effort that’s being requested to enter data into HPMS. Also, we are streamlining the data entry process, currently POs entry data in two areas HPMS and in the DMAO portal. We want move away from this process and have one data system (HPMS). In addition, each measure goes under review of a Technical Expert Panel, Public Comment, and the MCAG Medical Officer.
16. Publication/Tabulation Dates
CMS does plan to publish data of benchmarks and overall quality data maybe released for public viewing. No publication dates at this time. However, aggregate data results will be computed for POs in HPMS to establish and evaluate quality initiatives, improvements, benchmarking and comparison among POs and other like services and programs.
17. Expiration Date
The expiration date will be displayed.
18. Certification Statement
There are no exceptions to the certification statement.
B. Collections of Information Employing Statistical Methods
CMS will not be employing statistical methods and statistical surveys will not be used in these data collections. However, quality measures data will have statistical data classifications. This data collection does not require the use of statistical data classification reviewed and approved by OMB.
Appendix A
PACE Quality Data (Formally Level I & Level II Reporting Requirements)
Grievances
Appeals
Enrollments/Census/Disenrollment
Readmissions
Emergency Visits (Unscheduled) Care
Denials
Immunizations
Burns
Abuse
Adverse Drug Reactions
Adverse Outcomes
Deaths
Elopement
Equipment-Related Occurrences
Falls/ With Injury
Fires/Other Disasters
Food-borne Outbreaks
Infectious Disease Outbreaks
Media-Related Event
Medication-Related Occurrences
Motor Vehicle Accidents
Pressure Ulcer/Injury
Restraint Use
Suicide and Suicide Attempts
Quality Measures
Falls
Falls With Injury
Pressure Ulcer/Injury Prevalence
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | TAMIKA GLADNEY |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy