PetNet Reporting Portal; FDA Form 3756
Pet Event Tracking Network (PETNet)--State, Federal Cooperation to Prevent Spread of Pet Food Related Diseases
0680 PetNet Revisions Screenshots 2014
PetNet Reporting Portal; FDA Form 3756
OMB: 0910-0680
Animal Feed Network – PETNet & LivestockNet Reporting Forms
Contents
Animal Feed Network – Public Facing Webpage 2
Animal Feed Network Log-in Webpage 3
Animal Feed Network – Portal Selection Webpage 3
PETNet Reporting Form - Instructions 8
PETNet OMB Language and References 9
LivestockNet Welcome Webpage 10
LivestockNet Reporting Form 10
LivestockNet Reporting Form - Instructions 14
LivestockNet OMB Language and References 15
Appendix A – PETNet Reporting Form Instructions 16
Appendix B – LivestockNet Menu Options for ‘Species Affected’ & ‘Class’ 17
Introduction
This document provides screenshots of the reporting forms housed within the two Animal Feed Network portals: PETNet and LivestockNet.
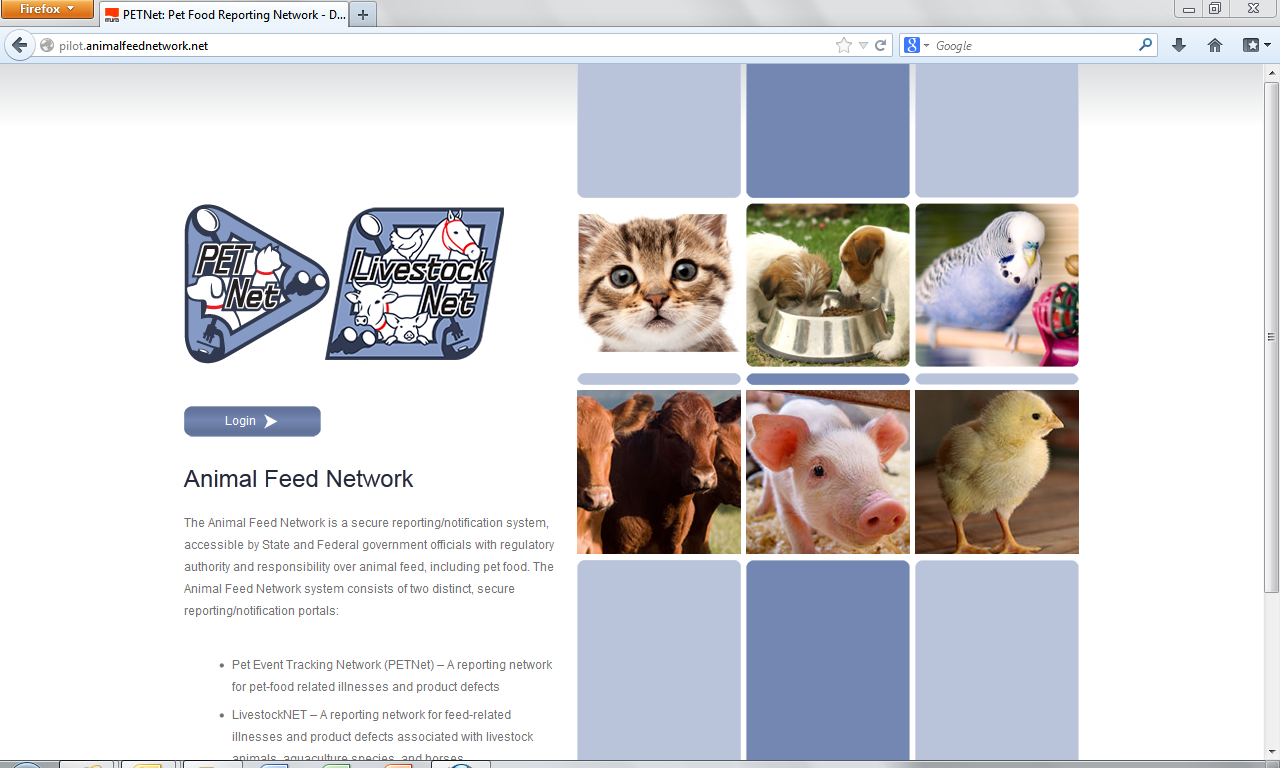
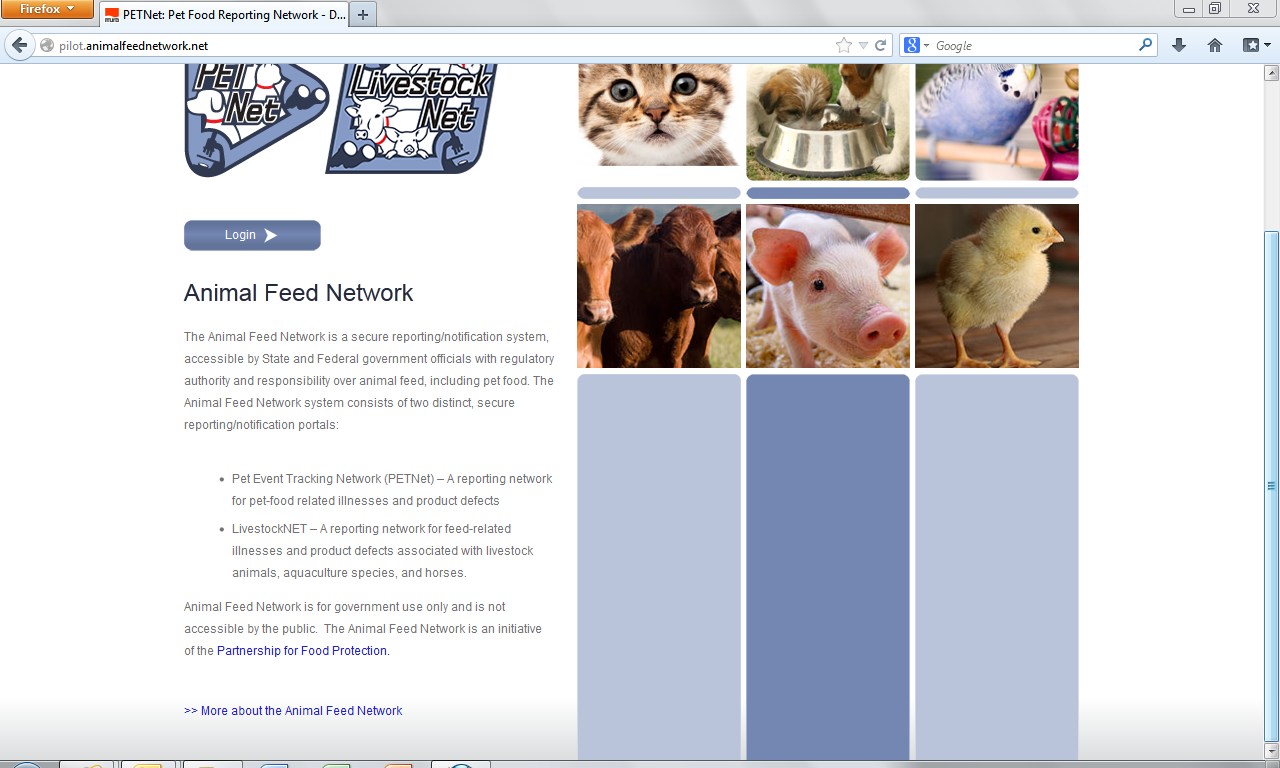
Animal Feed Network – Public Facing Webpage
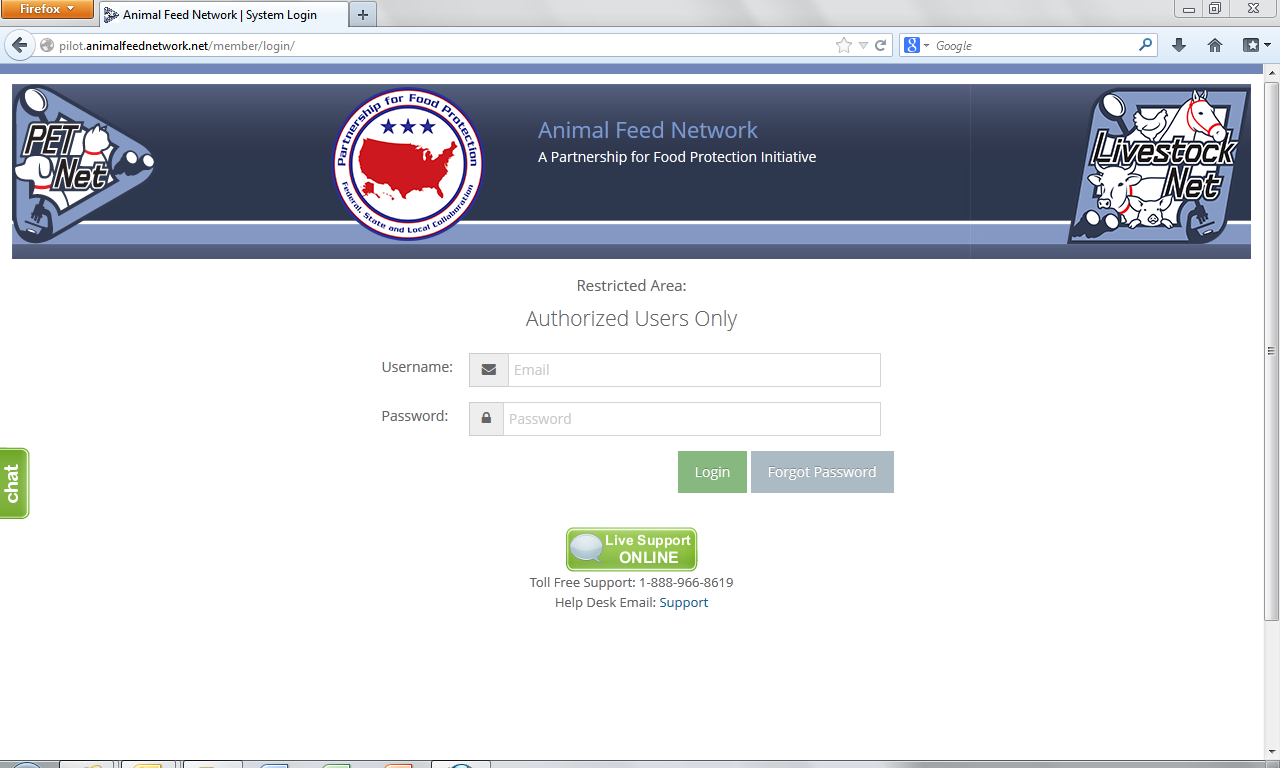
Animal Feed Network Log-in Webpage
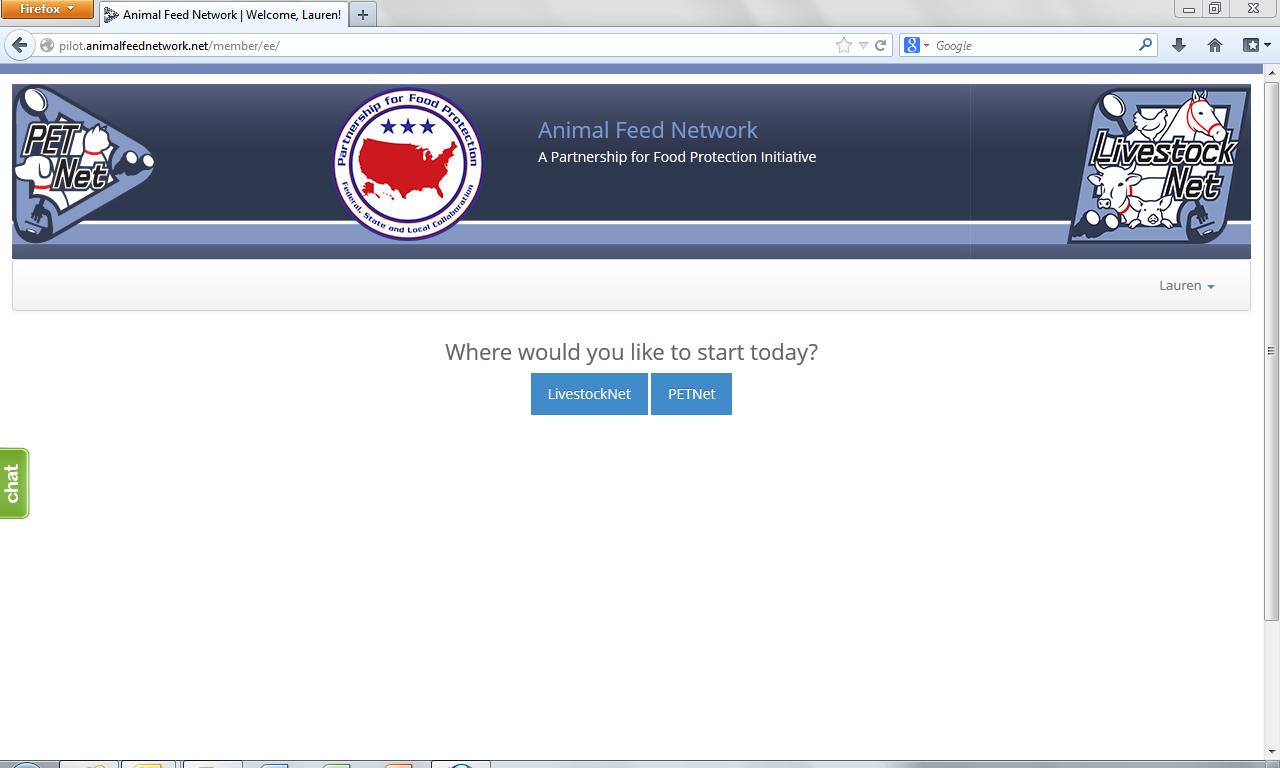
Animal Feed Network – Portal Selection Webpage
PETNet Welcome Webpage
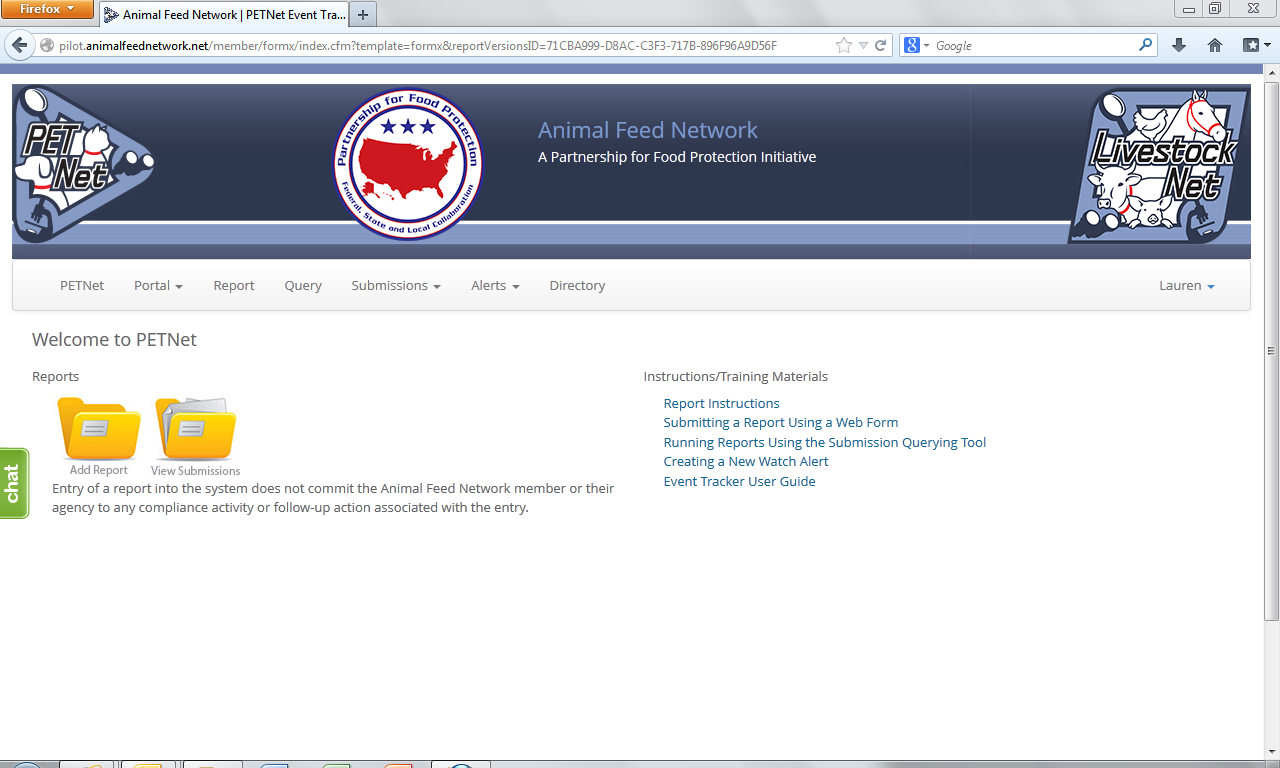
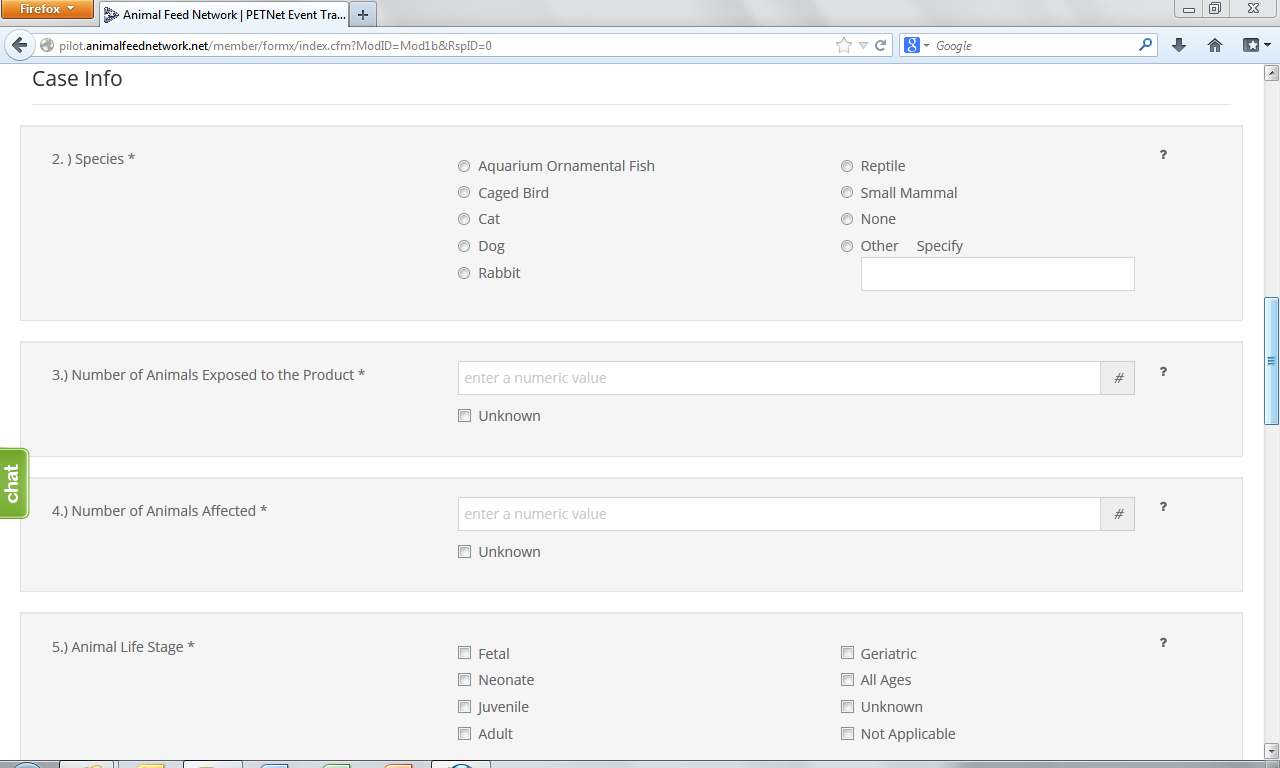
PETNet Reporting Form
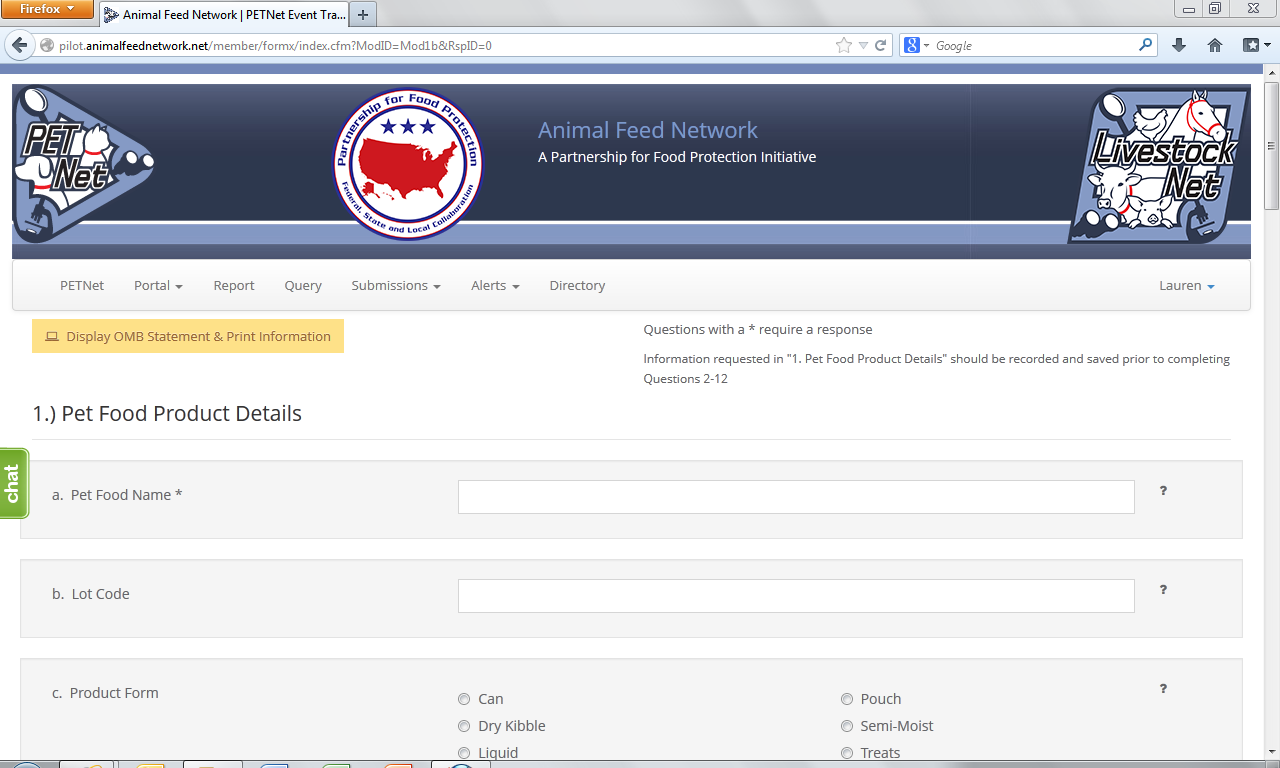
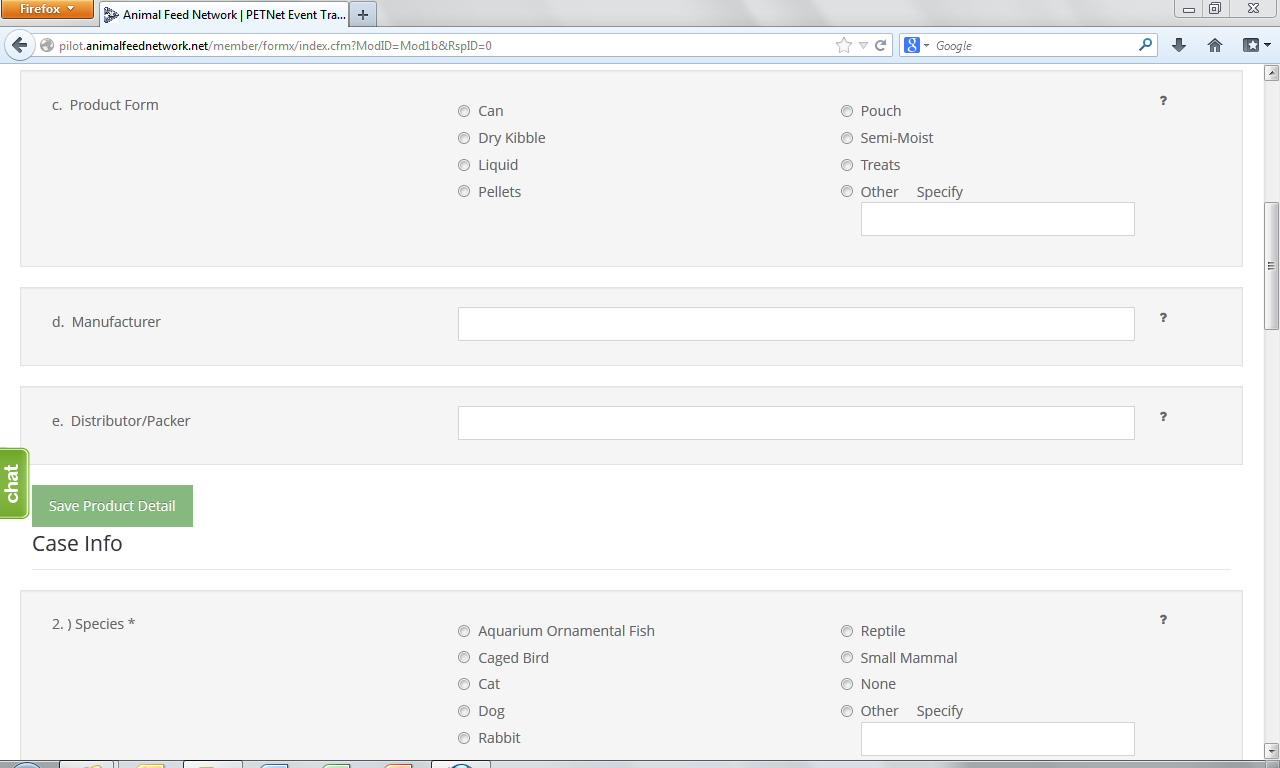
At this point, the user can opt to either select ‘Save Product Detail’ and enter additional pet food product details as needed/desired before proceeding to the section of the reporting form called ‘Case Info’ or proceed directly to ‘Case Info.’
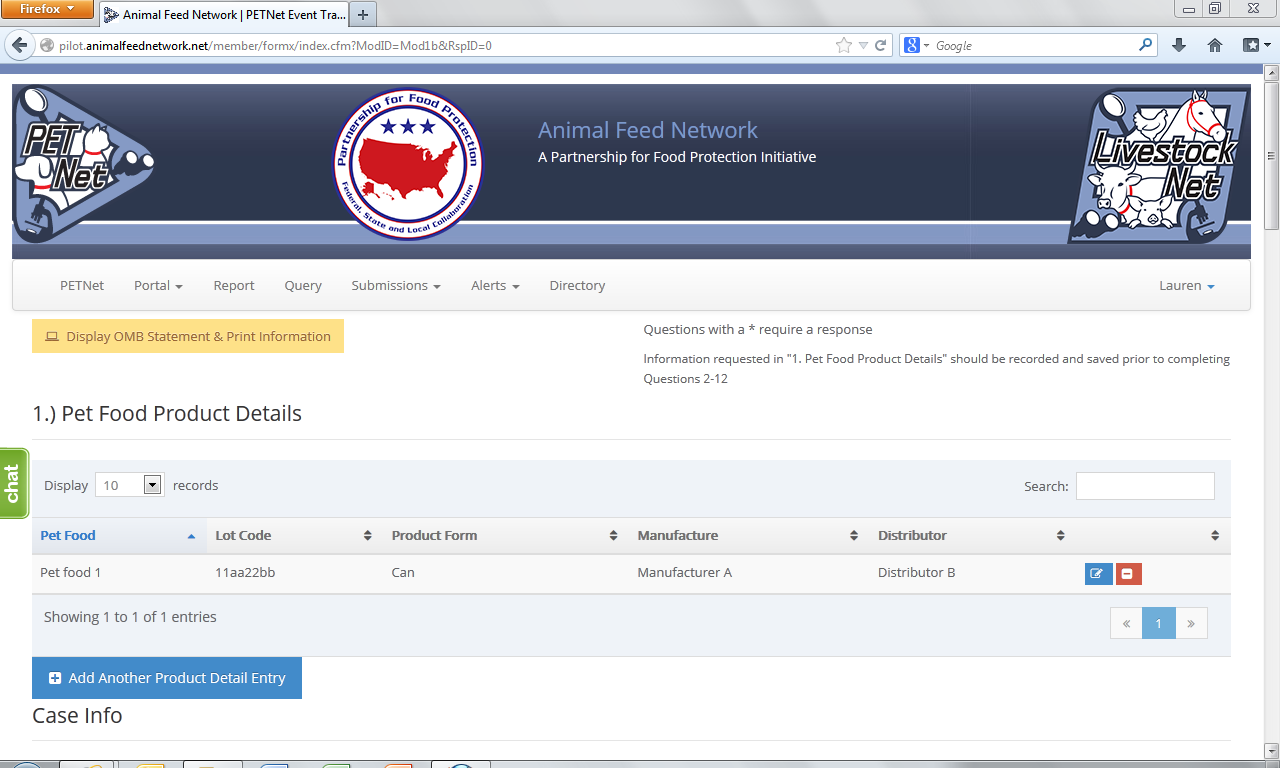
This form continues on the next page of this document.
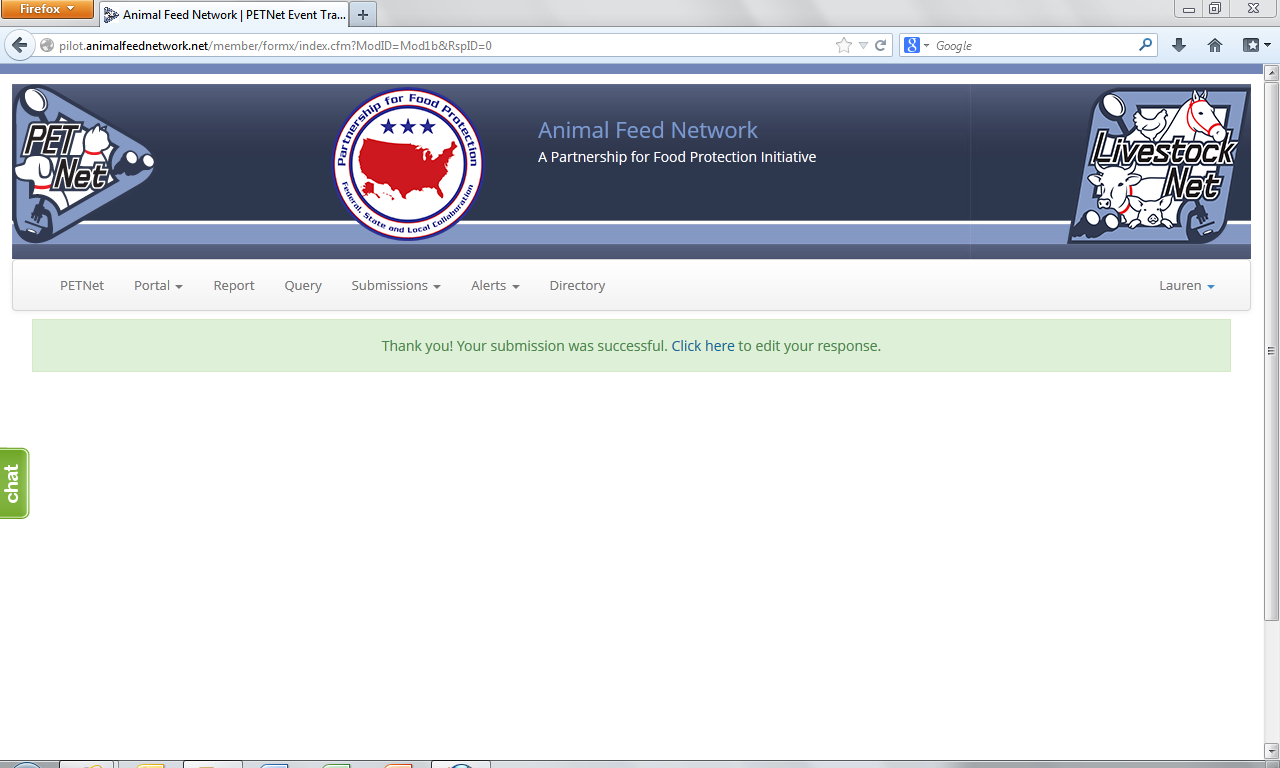
The following confirmation displays upon successful completion of the web form.
PETNet Reporting Form - Instructions
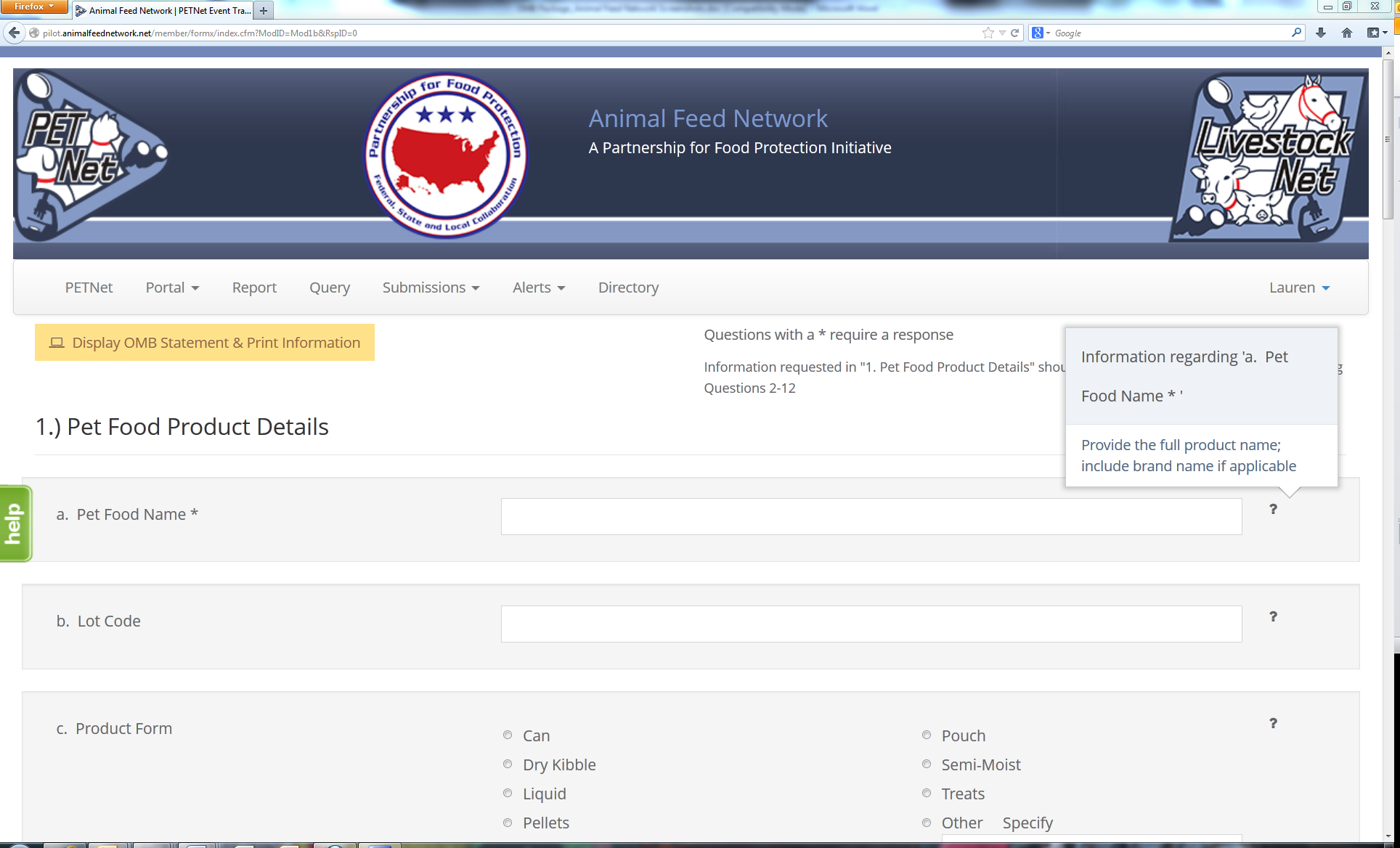
The question mark icons on the right hand side of the form’s data entry fields provide instructions for completing the form (visible when the user holds the cursor over the icon). Instructions for ‘Pet Food Name’ are displayed as an illustrative example for the purposes of this document. In lieu of individual screenshots for each question mark icon, we have included a summary of the instructions for data entry field in Appendix A.
PETNet OMB Language and References
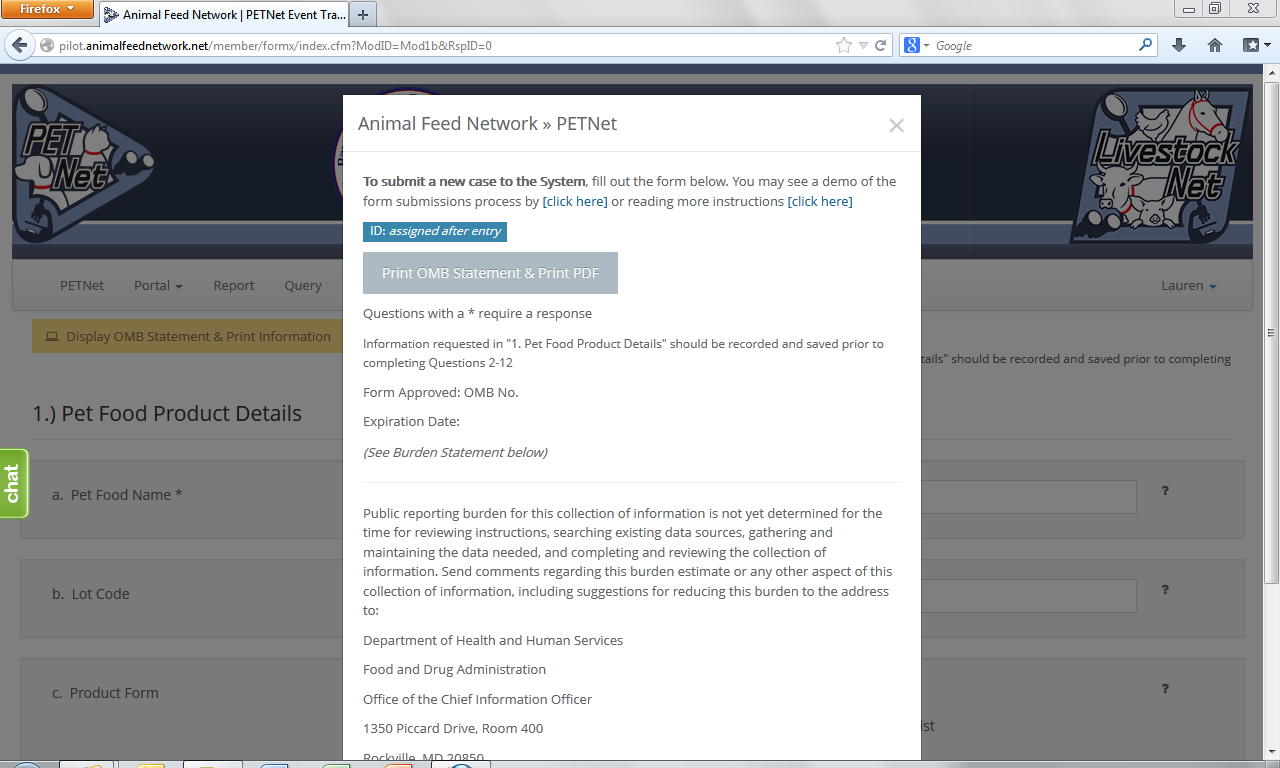
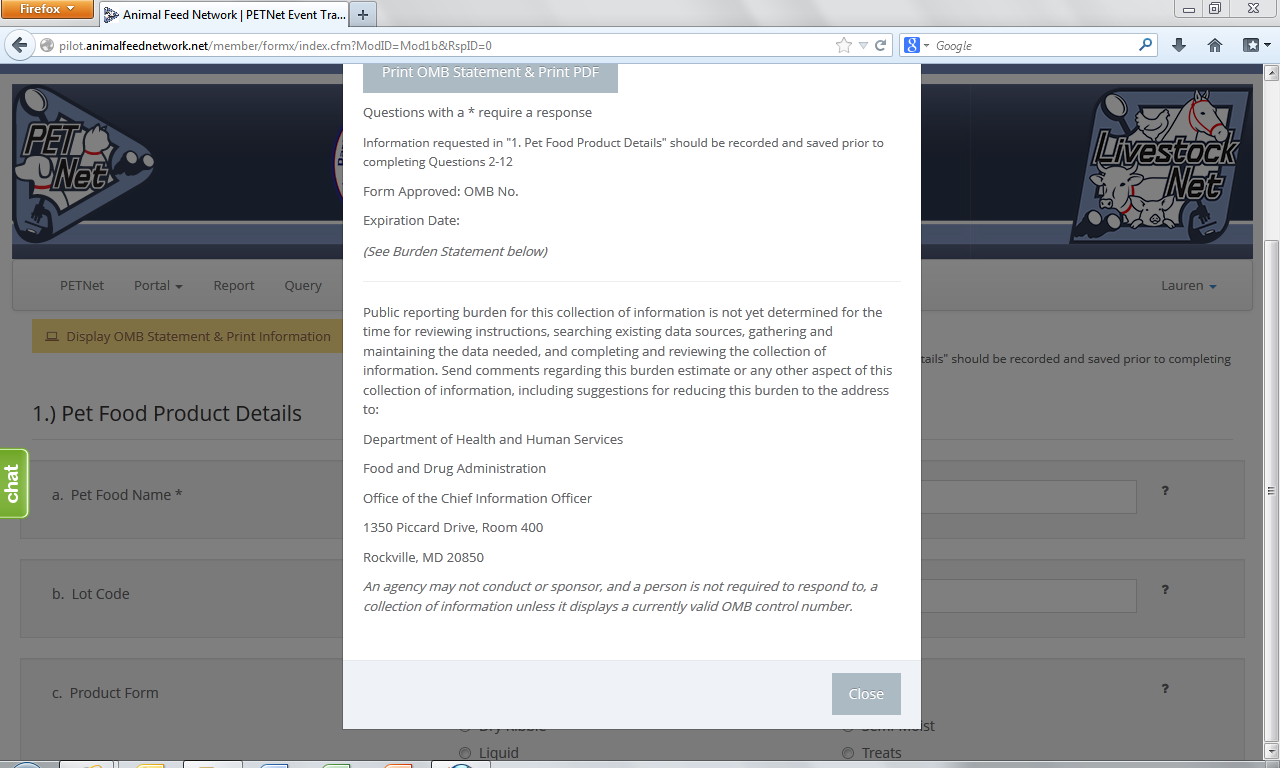
PETNet’s OMB references and burden statement can be viewed by clicking on the ‘Display OMB Statement & Print Information’ button on the web form. When this button is clicked, a pop-up window appears.

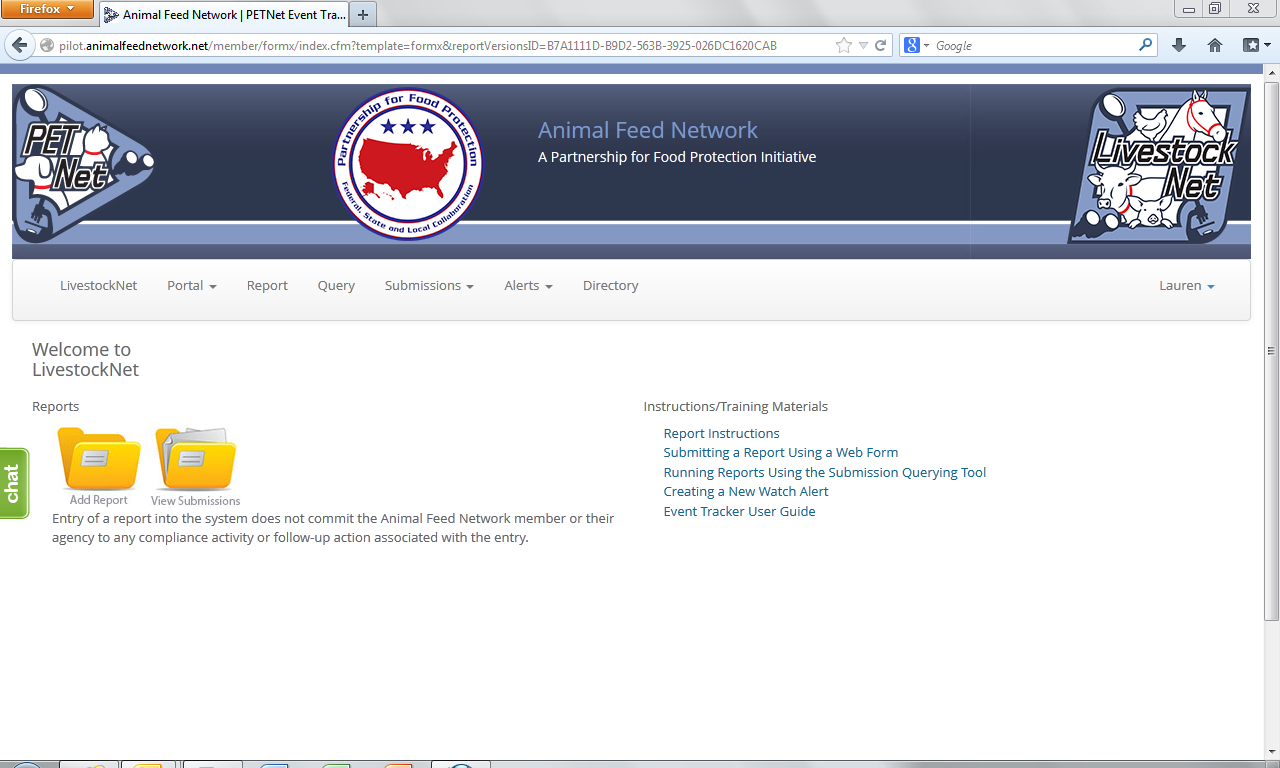
LivestockNet Welcome Webpage
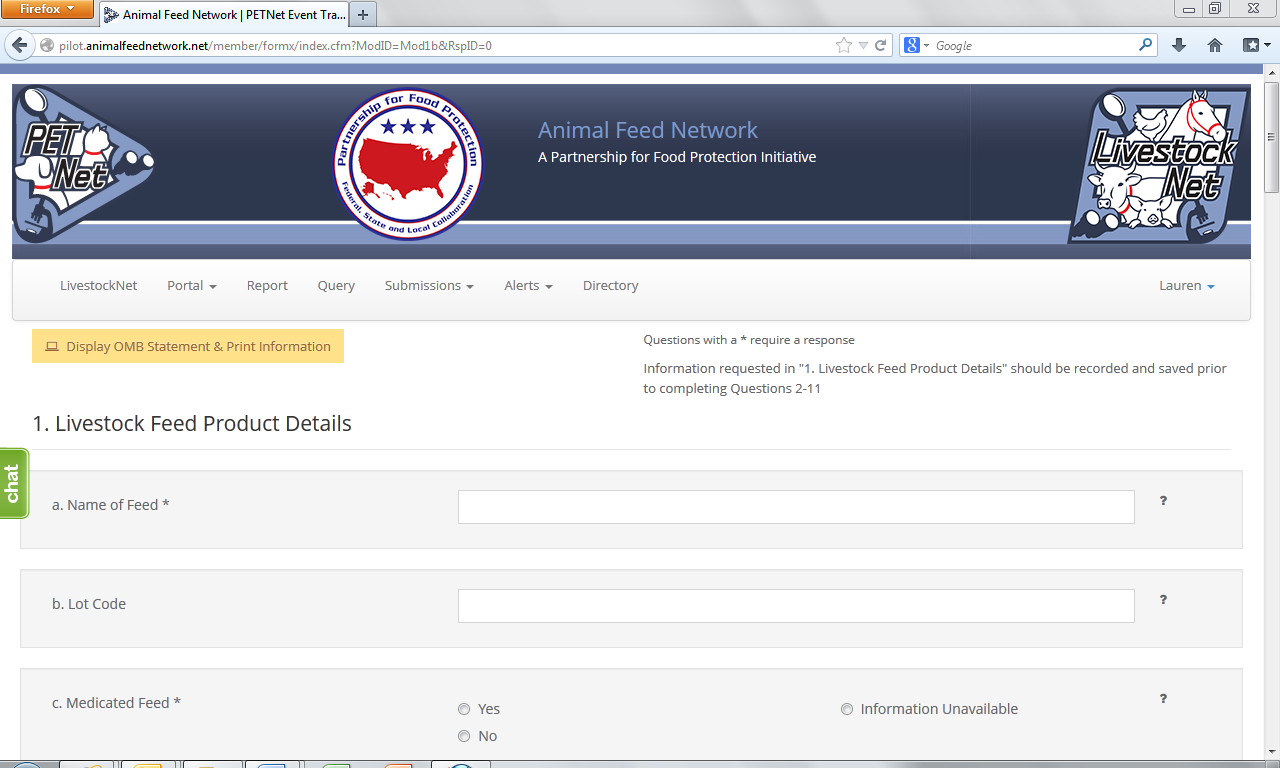
LivestockNet Reporting Form
This form continues on the next page of this document.
![]()
At this point, the user can opt to either select ‘Save Product Detail’ and enter additional livestock food product details as needed/desired before proceeding to the section of the reporting form called ‘Case Info’ or proceed directly to ‘Case Info.’
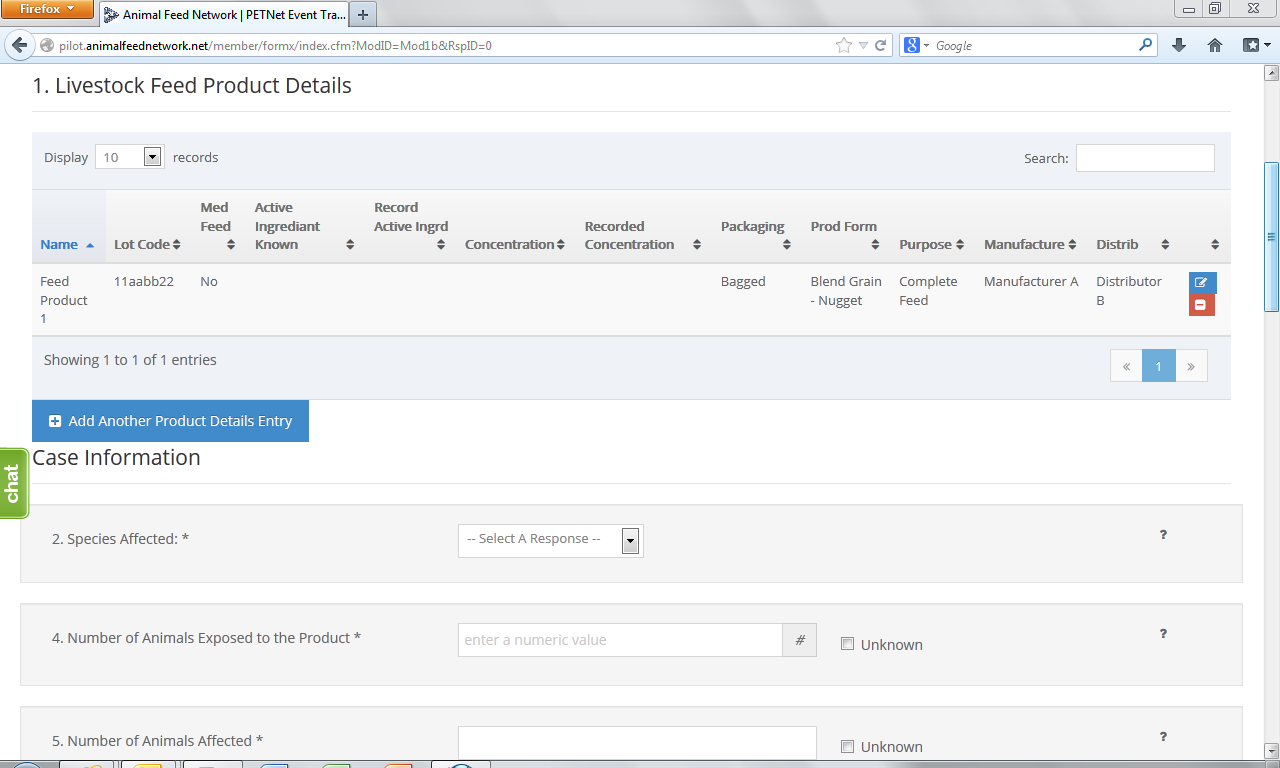
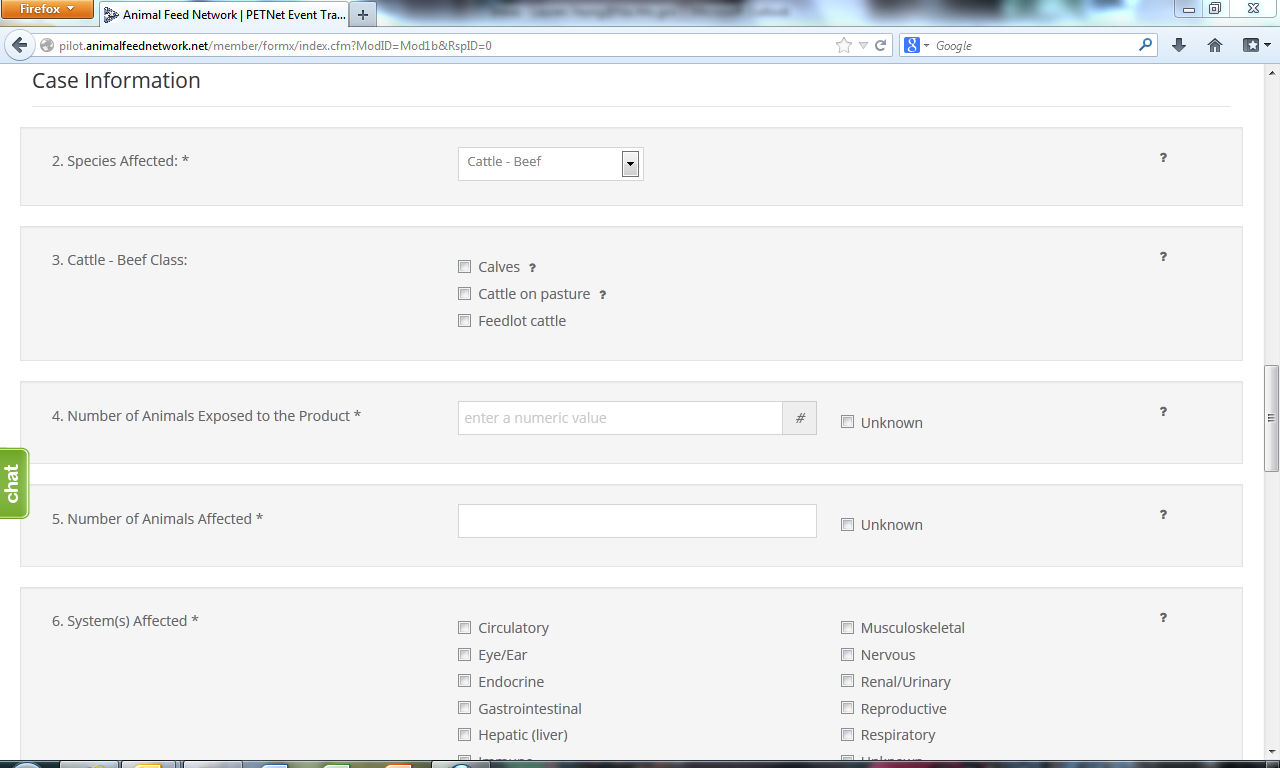
Upon reaching the ‘Species Affected’ field, the user must select from a drop down menu. Depending on the species selected, a list of classes specific to that species will appear. ‘Cattle-Beef’ has been selected as an illustrative example for the purposes of this document. In lieu of individual screenshots, we have included a table that lists the drop down menu options for ‘Species Affected’ and the corresponding ‘Class’ options for each species in Appendix B.
This form continues on the next page of this document.
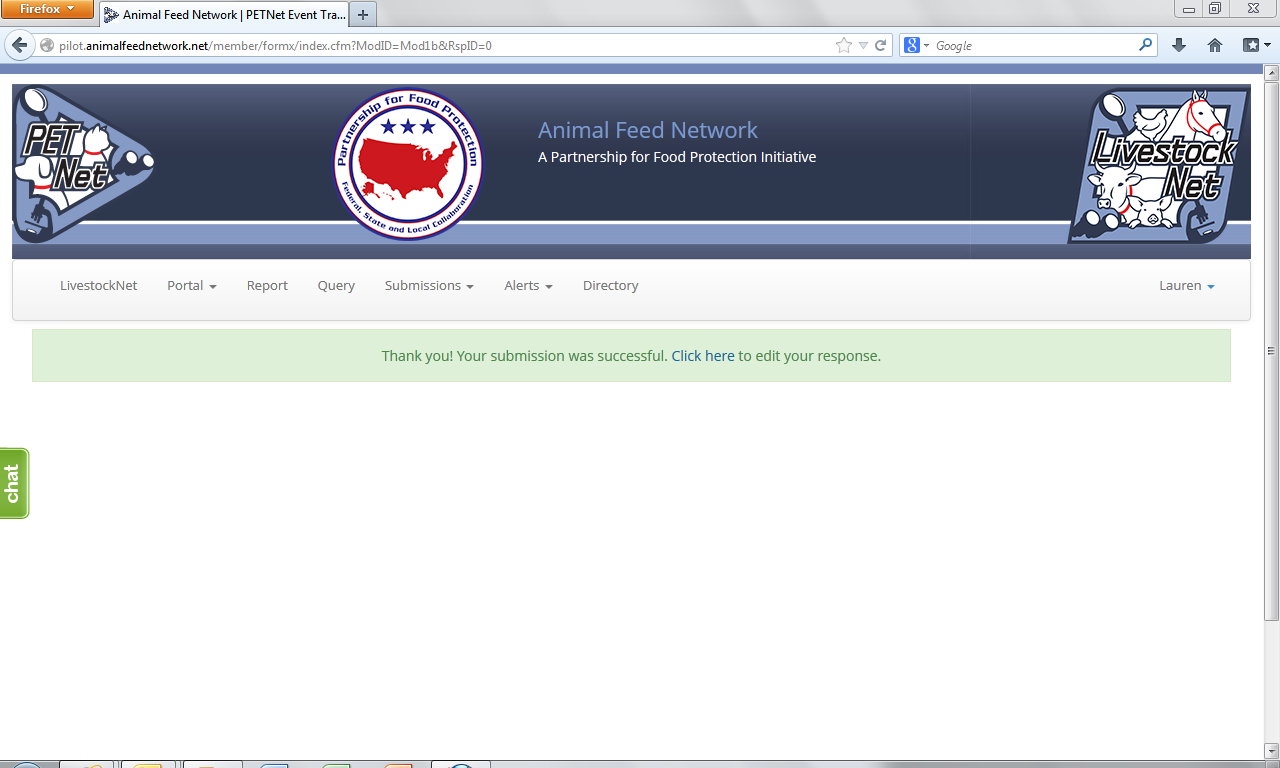
The following confirmation displays upon successful completion of the web form.
LivestockNet Reporting Form - Instructions
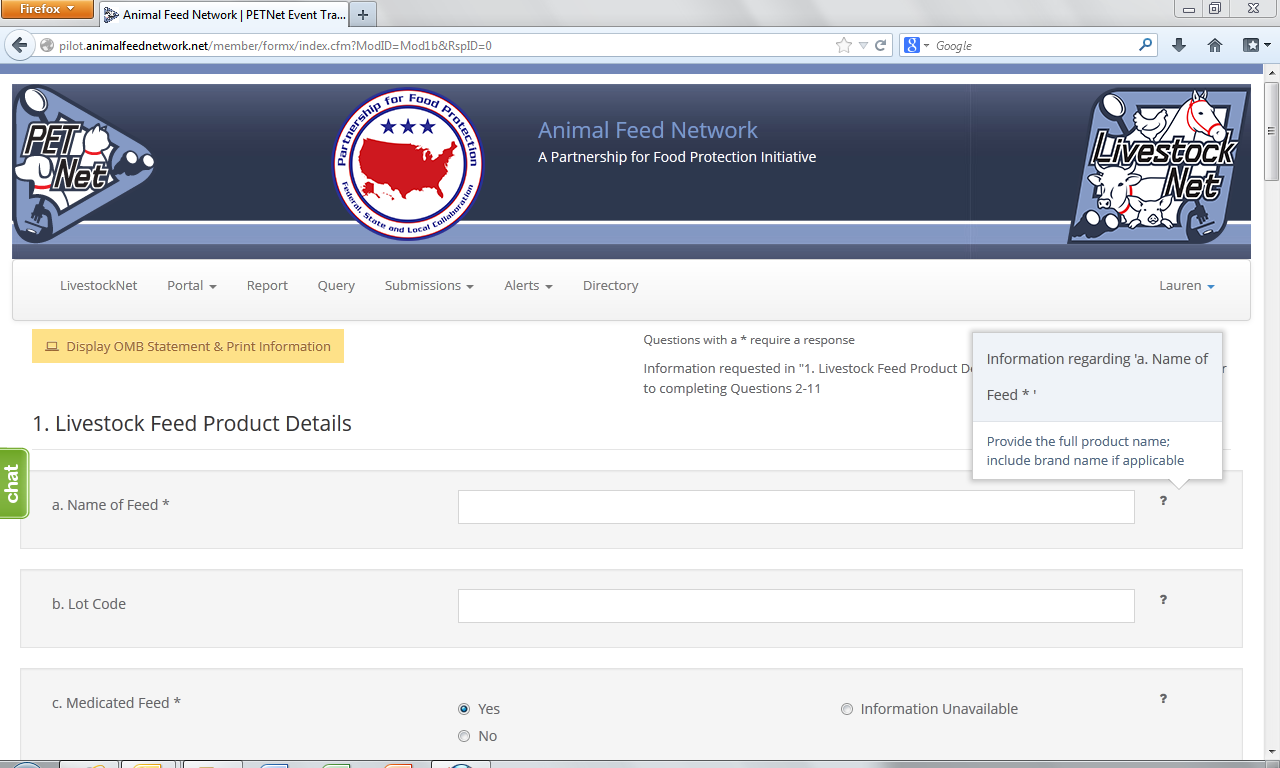
The question mark icons on the right hand side of the form data entry fields provide instructions for completing the form (visible when the user holds the cursor over the icon). Instructions for ‘Name of Feed’ are displayed as an illustrative example for the purposes of this document. In lieu of individual screenshots for each question mark icon, we have included a summary of the instructions for data entry field in Appendix C.
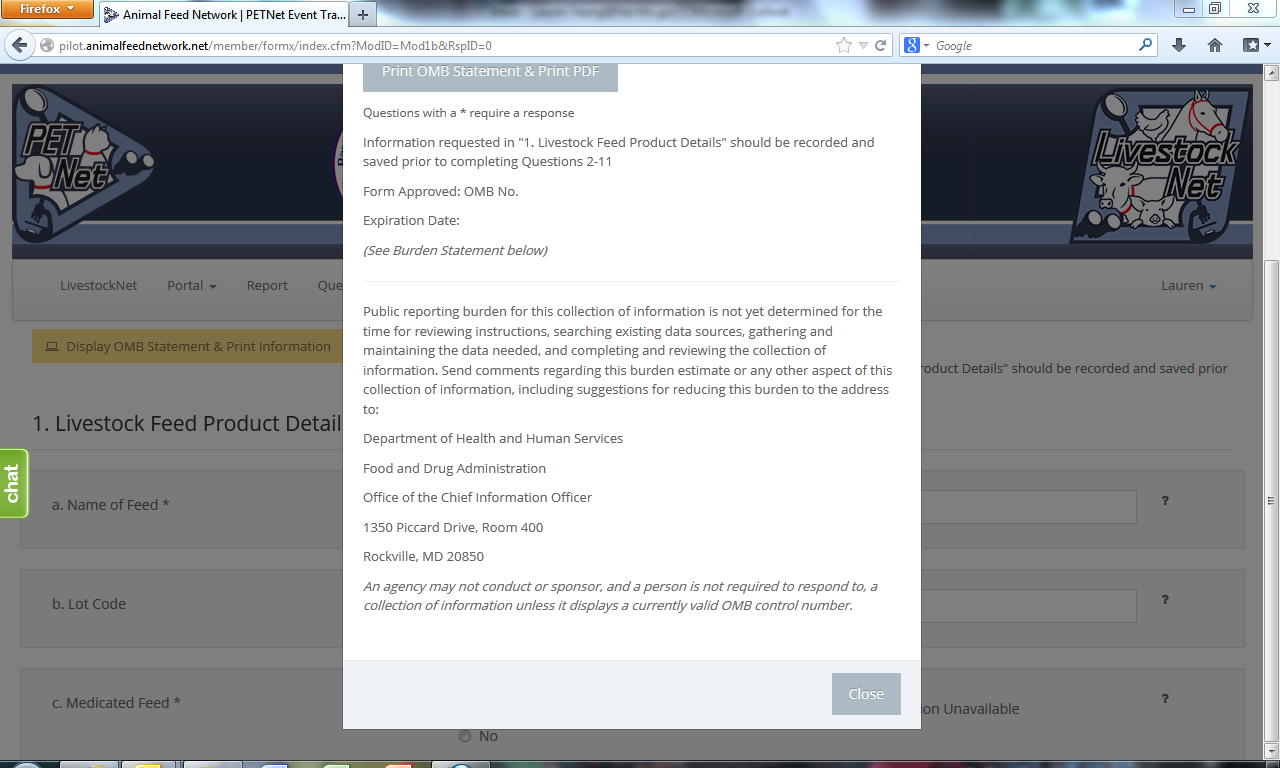
LivestockNet OMB Language and References
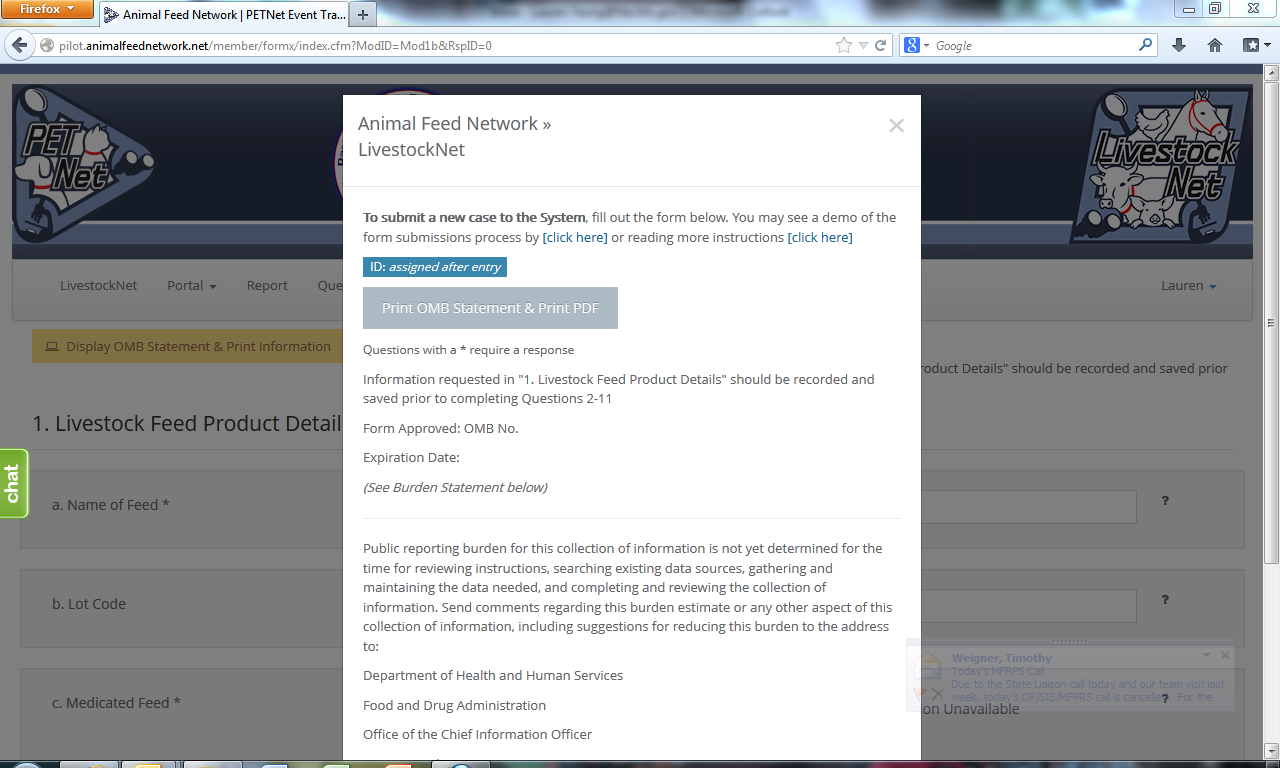
LivestockNet’s OMB references and burden statement can be viewed by clicking on the ‘Display OMB Statement & Print Information’ button on the web form. When this button is clicked, a pop-up window appears.

Appendix A – PETNet Reporting Form Instructions
1. Pet Food Product Details –
Pet Food Name*1 – Provide the full product name; include brand name if applicable
Lot Code – Enter the lot code, production code, batch number, or other identifying code to properly identify the affected product. If not known, enter “Unknown.”
Product Form – Select the type of packaging of the affected product.
Manufacturer – Enter the name of the manufacturer. If unknown, leave blank.
Distributor/Packer – Enter the name of the distributor/packer. If unknown, leave blank.
2. Species Affected* – Select one (1) species. If you select “Other,” specify in the space provided. Use one form per animal species affected. If reporting a product problem/defect only, select “None (product problem/defect report only).
3. Number Of Animals Exposed to the Product* – “Number of animals exposed to the product” refers to the number of animals at the specific location(s) included in this report that consumed or had access to the product. Enter the number or estimated number of animals exposed to product(s) listed in section 1. If no animal were exposed enter 0. If the number of animals is not known, select Unknown.
4. Number Of Animals Affected*– “Number of animals affected” refers to the number of animals at the specific location(s) included in this report that consumed or had access to the product and also exhibited clinical signs at or soon after consumption or exposure to the product. Enter the number or estimated number of animals affected. If no animals were affected enter “0.” If the number of animals is not known, select “Unknown.”
5. Animal Life Stage*– Select all that apply. If unknown, select “Unknown.” If reporting a product problem/defect only, select “Not applicable.”
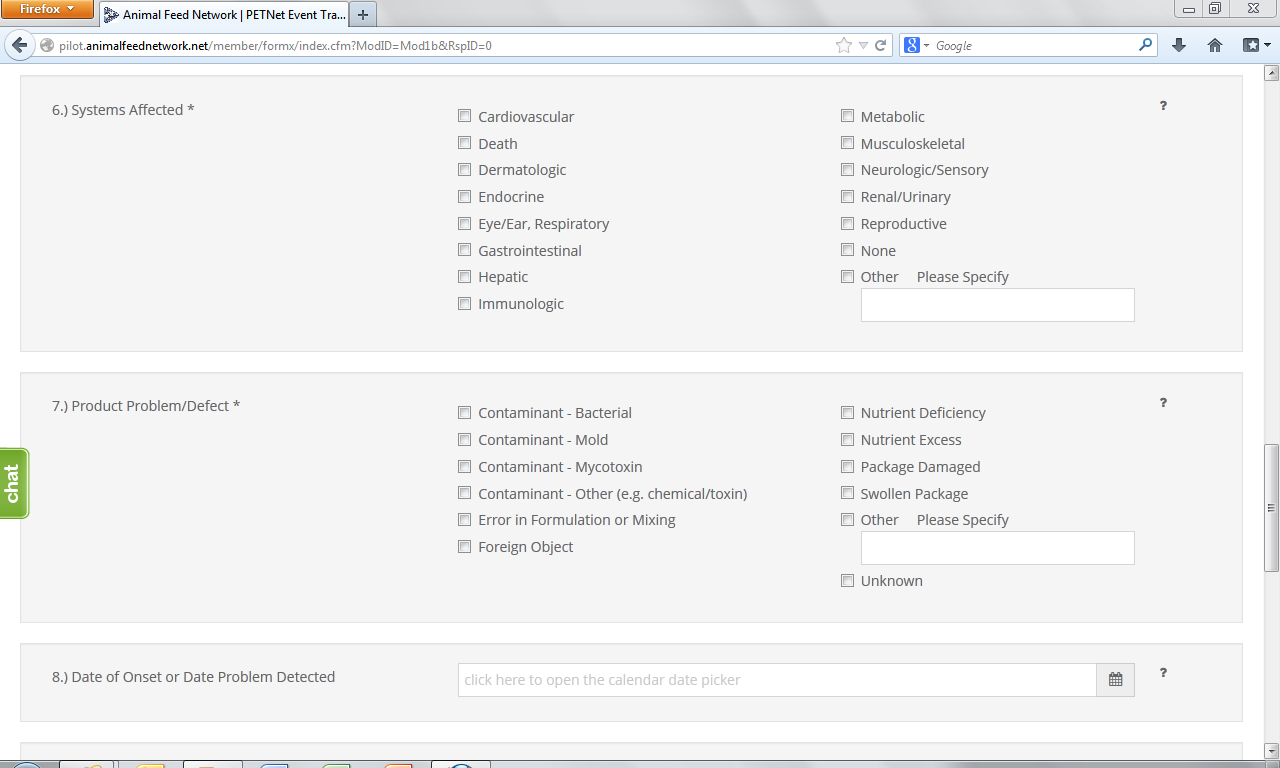
6. System(s) Affected* – Select all that apply. If no illness occurred, select “None.” If you select “Other,” specify in the space provided.
7. Product Problem/Defect*– Select all that apply. If unknown, select “Unknown.” If you select “Other,” specify in the space provided.
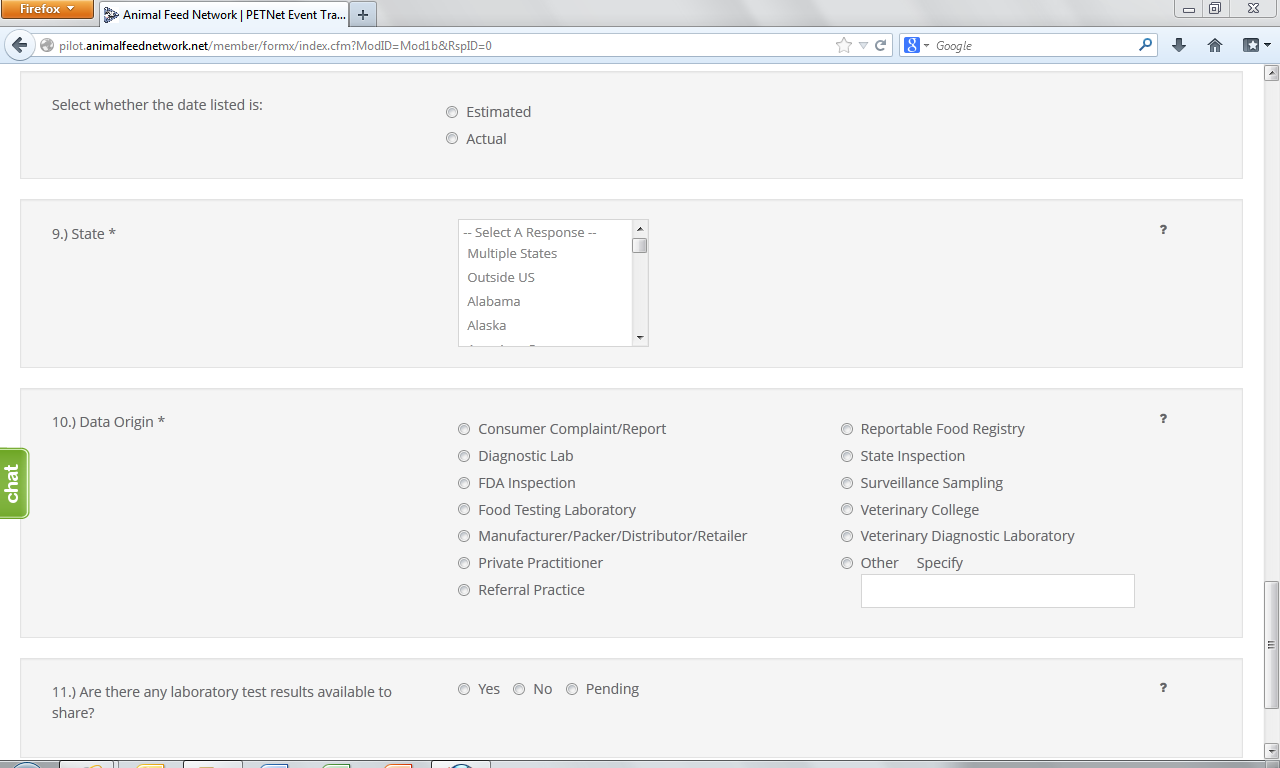
8. Date of Onset – Enter the date (in mm/dd/yyyy format) of onset of illness or the date the product problem/defect was first identified. Select whether the date listed is “Estimated” or “Actual.”
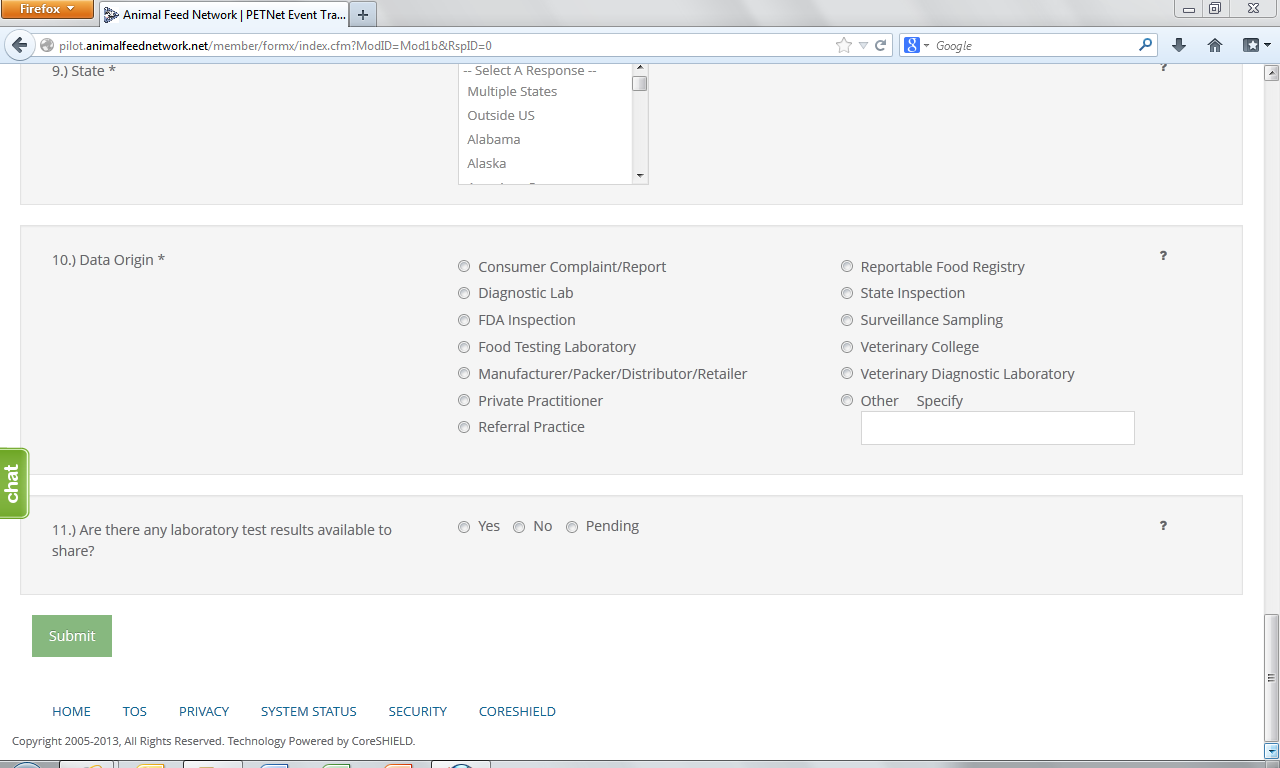
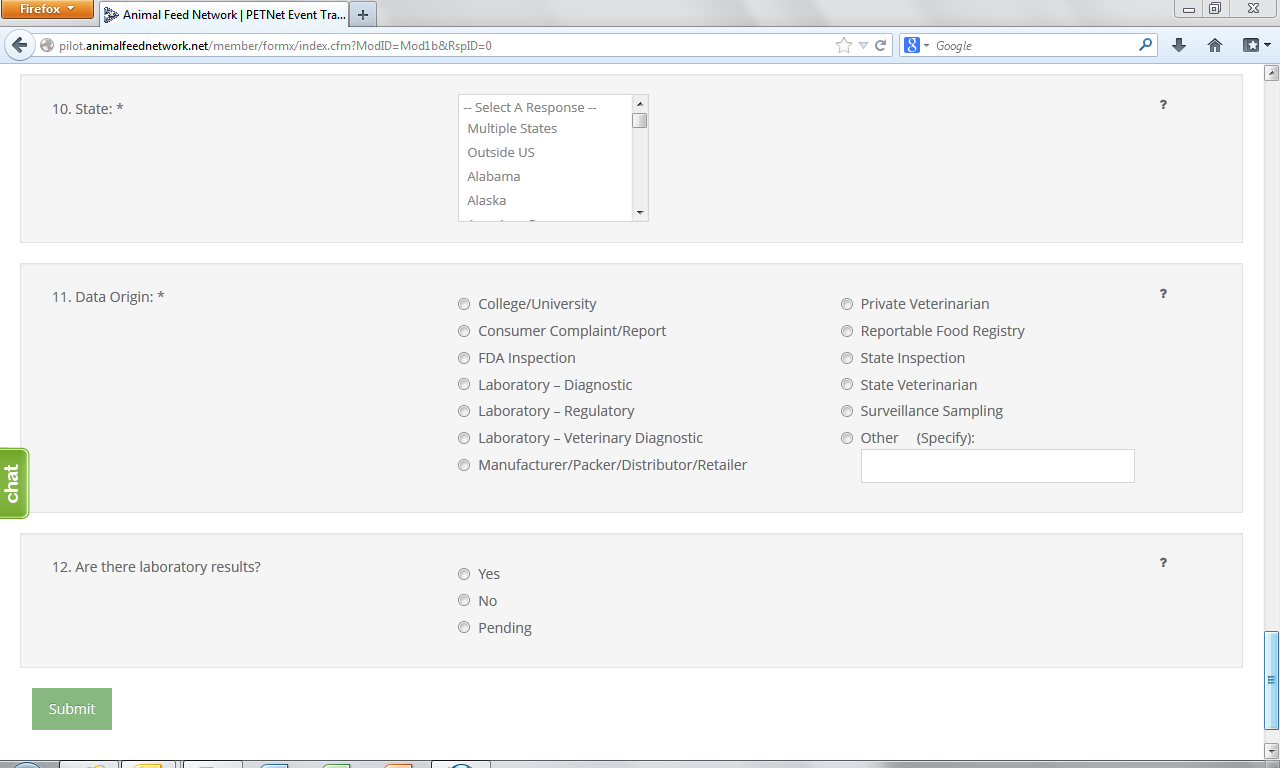
9. State* – Select from the drop down menu the State(s) where the incident was reported. If multiple States involved choose to either report each State using the Control (Ctrl) button or select “Multiple States.” Select the option of “Outside U.S.” if occurrence was outside U.S. borders.
10. Data Origin*– Select one (1) entry from among those listed.
11. Laboratory Test Results – Indicate whether there are laboratory results. If laboratory results are pending, select “Pending.”
Appendix B – LivestockNet Menu Options for ‘Species Affected’ & ‘Class’
SPECIES |
CLASS |
Swine |
Pre-Starter |
Starter |
|
Grower |
|
Finisher |
|
Gilts, Sows, Adult Boars |
|
Lactating Gilts & Sows |
|
Chicken - Layer |
Starting/Growing |
Finisher |
|
Laying |
|
Breeder |
|
Chicken - Broiler |
Starting/Growing |
Finisher |
|
Breeder |
|
Chicken - Broiler Breeder |
Starting/Growing |
Finishing |
|
Laying |
|
Turkey |
Starting/Growing |
Finisher |
|
Laying |
|
Breeder |
|
Cattle - Beef |
Calves |
Cattle on pasture |
|
Feedlot cattle |
|
Cattle - Dairy |
Veal milk replacer |
Herd milk replacer |
|
Starter |
|
Grower 1 (Growing Heifers, Bulls and Dairy Beef ≤ 12 mths of age) |
|
Grower 2 (Growing Heifers, Bulls and Dairy Beef > 12 mths of age) |
|
Lactating |
|
Non-Lactating |
|
Equine |
Growing |
Brood Mare |
|
Maintenance |
|
Performance (including Stallions) |
|
Goat |
Starter |
Grower |
|
Finisher |
|
Breeder |
|
Lactating |
SPECIES |
CLASS |
Sheep |
Starter |
Grower |
|
Finisher |
|
Breeder |
|
Lactating |
|
Ducks |
Starter |
Grower |
|
Finisher |
|
Breeder developer |
|
Breeder |
|
Geese |
Starter |
Grower |
|
Finisher |
|
Breeder developer |
|
Breeder |
|
Aquaculture Fin Fish |
Catfish |
Salmon |
|
Tilapia |
|
Trout |
|
Other (please specify) |
|
Aquaculture Shellfish |
Shrimp |
Crab |
|
Crawfish |
|
Other (please specify) |
|
Rabbit |
Grower |
Breeder |
|
Other (please specify) |
N/A |
None (product problem/defect report only) |
N/A |
Appendix C – LivestockNet Reporting Form Instructions
Livestock Feed Product Details
Name of Feed*2 – Provide the full product name; include brand name if applicable
Lot Code – Enter the lot code, production code, batch number, or other identifying code to properly identify the affected product. If not known, enter “Unknown”.
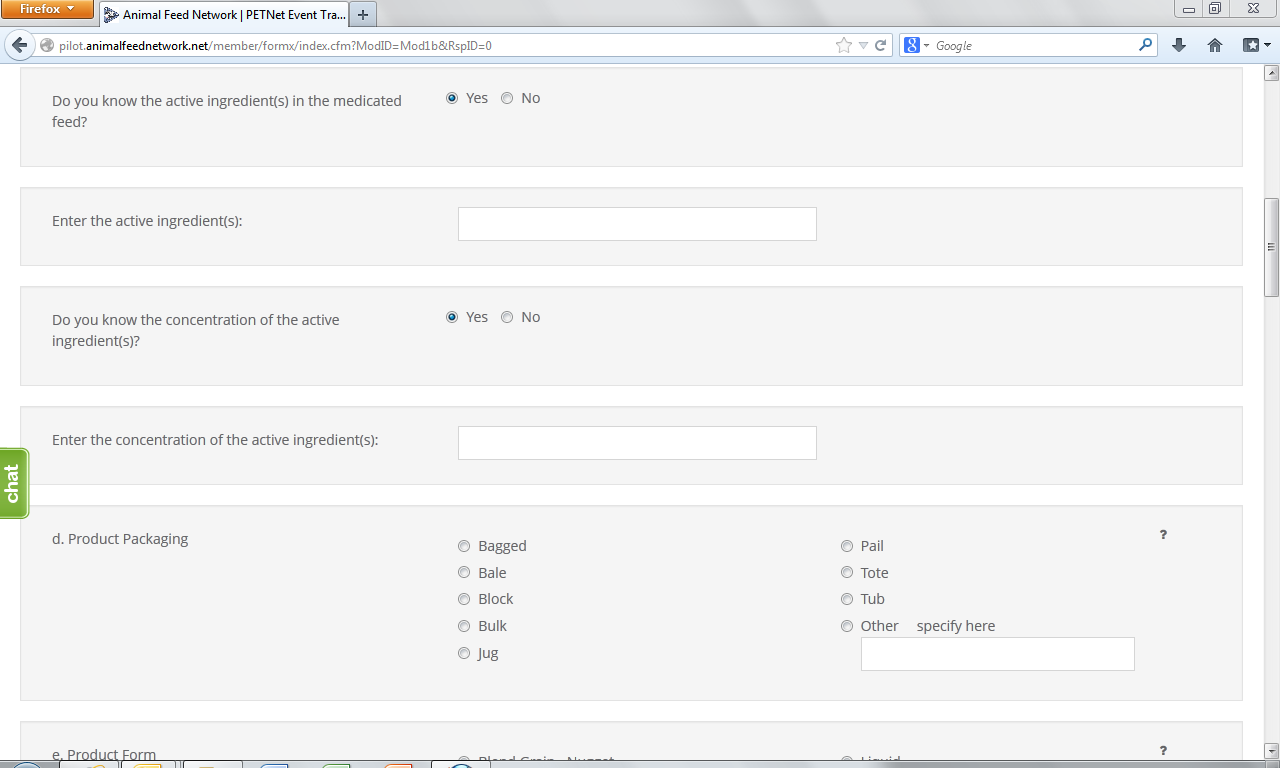
Medicated Feed* – Select whether the feed is a medicated feed, or if the information is unknown, select “Information Unavailable.” If a feed contains a medication, select “Yes” and enter the name(s) of the Active Ingredient(s). If an Active Ingredient is identified, enter the concentration (example: g of medication/ton of feed, percent of medication in feed, etc) of the Active Ingredient(s). If the Active Ingredient(s) or Concentration(s) are unknown, click on the corresponding box.
Product Packaging – Select the type of packaging of the affected product.
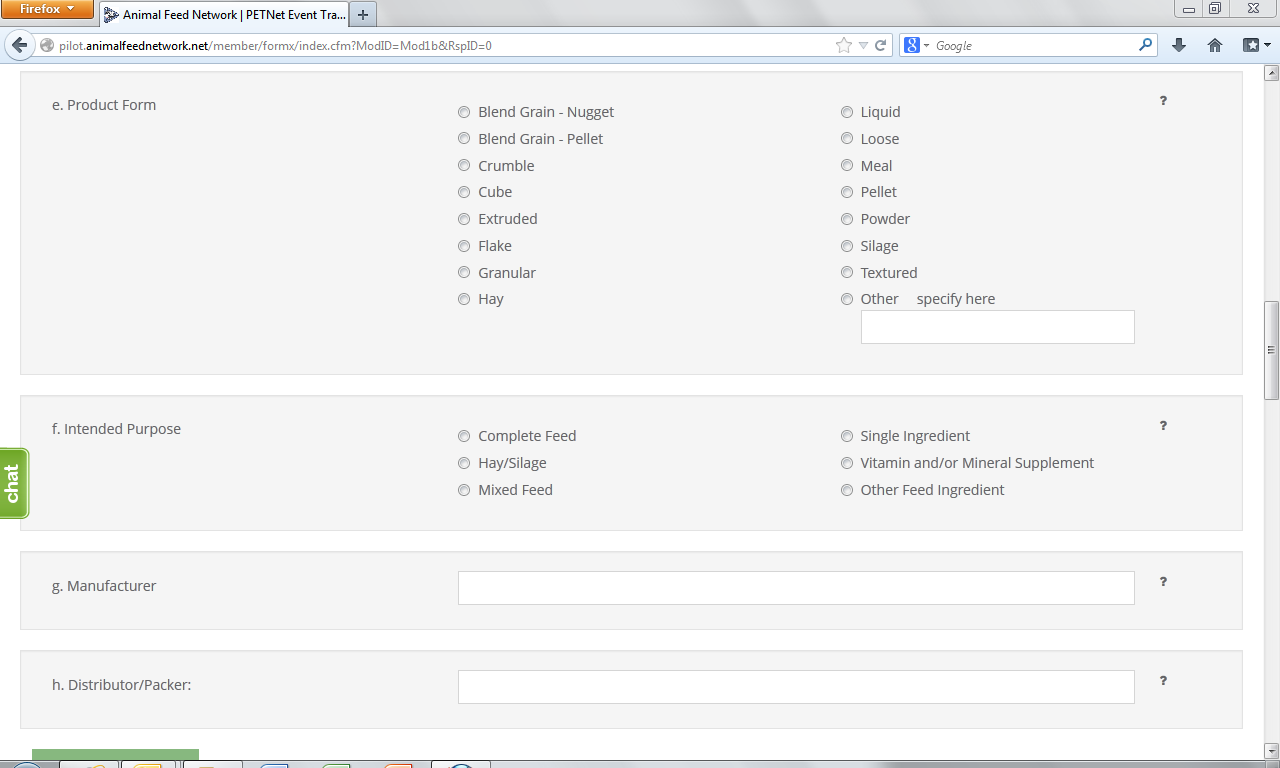
Product Form – Select the type of form the affected product was distributed as.
Intended Purpose – Select the appropriate designation for the affected product:
Complete Feed3: “A nutritionally adequate feed for animals other than man; by specific formula is compounded to be fed as the sole ration and is capable of maintaining life and/or promoting production without any additional substance being consumed except water”
Hay/Silage
Mixed Feed: (example: Mixed ration of soybean hulls and dried distillers grains)
Single Ingredient: (example: cottonseed hulls)
Vitamin or Mineral Supplement
Other Feed Ingredient: (example: flavoring, enzymes, flow agent, etc.)
Manufacturer – Enter the name of the manufacturer. If unknown, leave blank.
Distributor/Packer – Enter the name of the distributor/packer. If unknown, leave blank.
Species Affected* – Select one (1) species4. If you select “Other,” specify in the space provided. Use one form per animal species affected. If reporting a product problem/defect only, select “None (product problem/defect report only).”
Class – Enter the class of the animal affected. Select all that apply.
Number of Animals Exposed to the Product* – “Number of Animals Exposed to the Product” refers to the number of animals at the specific location(s) included in this report that consumed or had access to the product. Enter the number or estimated number of animals exposed to product(s) listed in section 1. If no animal were exposed enter “0.” If the number of animals is not known, select “Unknown.”
Number of Animals Affected* – “Number of Animals Affected” refers to the number of animals at the specific location(s) included in this report that consumed or had access to the product and also exhibited clinical signs at or soon after consumption or exposure to the product. Enter the number or estimated number of animals affected. If no animals were affected enter “0.” If the number of animals is not known, select “Unknown.”
System(s) Affected* – Select all that apply. If none, select “None.” If unknown, select “Unknown.”
Production Loss*– Select all that apply. If you select “Other”, specify in the space provided. If none, select “None.”
Product Problem/Defect* – Select all that apply. If you select “Other”, specify in the space provided. If unknown, select “Unknown.”
Date of Onset or Date Problem Detected – Enter the date (in mm/dd/yyyy format) of onset of illness or the date the product problem/defect was first identified. Select whether the date listed is “Estimated” or “Actual.”
State* – Select from the drop down menu the State(s) where the incident was reported. If multiple States involved choose to either report each State using the Control (Ctrl) button or select “Multiple States.” Select the option of “Outside U.S.” if occurrence was outside U.S. borders.
Data Origin* – Select one (1) entry from among those listed. If you select “Other”, specify in the space provided.
Laboratory Results – Indicate whether there are laboratory results. If laboratory results are pending, select “Pending.”
1 Items with an * require a response
2 Items with an * require a response
3 “Complete Feed” as defined in the Association of American Feed Control Officials (AAFCO) 2013 Official Publication, page 342.
4 Species utilized in this form were derived from the species and classes listed in the AAFCO 2013 Official Publication, pages 106-111.
Screenshots taken: 12/16/13 Page
| File Type | application/msword |
| Last Modified By | Bean, Domini |
| File Modified | 2014-02-28 |
| File Created | 2014-02-28 |
© 2026 OMB.report | Privacy Policy