SS Part A_CRCCP_2-22-17
SS Part A_CRCCP_2-22-17.docx
Colorectal Cancer Control Program (CRCCP) Monitoring Activities
OMB: 0920-1074
Colorectal Cancer Control Program (CRCCP) Monitoring Activities
REVISION
Supporting Statement – Section A
Program Official/Project Officer
Dara Schlueter, MPH
Health Scientist
Division of Cancer Prevention and Control
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
4770 Buford Highway, Mailstop F-76
Atlanta, GA 30341
Phone: 770-488-4241
Fax: 770-488-3230
Table of Contents
A. Justification
A1. Circumstances Making the Collection of Information Necessary
A2. Purpose and Use of the Information Collection
A3. Use of Improved Information Technology and Burden Reduction
A4. Efforts to Identify Duplication and Use of Similar Information
A5. Impact on Small Businesses or Other Small Entities
A6. Consequences of Collecting the Information Less Frequently
A7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
A8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A9. Explanation of Any Payment or Gift to Respondents
A10. Assurance of Confidentiality Provided by Respondents
A11. Justification for Sensitive Questions
A12. Estimates of Annualized Burden Hours and Costs
A13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
A14. Annualized Cost to the Government
A15. Explanation for Program Changes or Adjustments
A16. Plans for Tabulation and Publication and Project Time Schedule
A17. Reason(s) Display of OMB Expiration Date is Inappropriate
A18. Exceptions to Certification for Paperwork Reduction Act Submissions
The goal of this
annual survey is to systematically collect information about the
implementation of program activities from each of the 29 CRCCP
awardees for Component 1 and each of the 6 awardees for Component 2.
We will be using descriptive statistics to produce grantee reports
for use by CDC for program management and technical assistance
planning, as well as for the grantees’ own program
improvement.
Note: Attachments are included as separate files as instructed.
Attachment 1: Authorizing Legislation
Attachment 2: CRCCP Logic Models
Attachment 3a: CRCCP Annual Grantee Survey (screenshots)
Attachment 3b: Survey Introductory Email
Attachment 3c: Survey Reminder Email
Attachment 4a: CRCCP Clinic-Level Data Collection Instrument (screenshots)
Attachment 4b: CRCCP Clinic-Level Data Dictionary
Attachment 4c: CRCCP Clinic-Level Data Collection Introductory Email
Attachment 5: CRCCP Data Collection Revision Matrix
Attachment 6: 60-Day Federal Register Announcement
The
goal of this information collection is to systematically collect
information about the implementation and outcomes of the CRCCP, a
program including 30 grantees.
CDC
will use resulting information to monitor the implementation of
CRCCP activities and evaluate outcomes achieved across all
grantees.
CDC
will conduct an annual grantee survey and collect clinic-level
information from grantees’ health system partners.
The
subpopulation for the grantee survey is the 30 CRCCP Program
Managers/Program Directors. Clinic-level information, including a
CRC screening rate, represents clinics serving clients ages 50-75
in partner health systems. The information will be reported to CDC
by the CRCCP Program Managers/Program Directors.
CDC
will use descriptive statistics to produce reports for CDC program
management and CRCCP grantees.
ABSTRACT
CDC is requesting a revision to OMB No. 0920-1074, an information collection for the Colorectal Cancer Control Program (CRCCP). The CRCCP Funding Opportunity Announcement (FOA) DP15-1502, a 5-year cooperative agreement issued in 2015, funds 30 grantees (previously 31) to provide CRC screening and related support services. The information collection plan includes an annual grantee survey and clinic-level information collection. Based on CDC’s data collection experience in program year 1 and feedback from grantees and subject matter experts, DCPC has incorporated changes to the CRCCP data collection effort which streamlines both the annual grantee survey and clinic-level data collection. CDC anticipates that these changes will reduce the overall burden to grantees. The revised information collection will continue to allow CDC to provide routine monitoring feedback to grantees based on their information submissions, tailor technical assistance as needed, support program planning, and assess program outcomes. OMB approval is requested for three years.
A. Justification
A1. Circumstances Making the Collection of Information Necessary
CDC is requesting a revision to OMB No. 0920-1074. In Program Year 1 (July 1, 2015 – June 30, 2016), the information collection consisted of an annual grantee survey and clinic-level information collection. The annual grantee survey has been redesigned for Program Years 2-5 to eliminate survey items that are more appropriately captured at the clinic – as opposed to grantee – level. The overall length of the survey was reduced from 132 questions to 73 questions. Clinic-level data collection has been redesigned to add several data variables to capture additional and more specific information about clinic-level program implementation and monitoring/evaluation. The overall length of clinic-level information collection increased from 116 variables to 125 variables. The estimated annualized burden is expected to decrease. OMB approval is requested for three years.
Colorectal cancer (CRC) is the second leading cause of death from cancer in the United States among cancers that affect both men and women. CRC screening has been shown to reduce incidence of and death from the disease. Screening for CRC can detect disease early when treatment is more effective and prevent cancer by finding and removing precancerous polyps. Of individuals diagnosed with early stage CRC, more than 90% live five or more years. Despite strong evidence supporting screening, only 65% of adults currently report being up-to-date with CRC screening as recommended by the U.S. Preventive Services Task Force, with more than 22 million age-eligible adults estimated to be untested. To reduce CRC morbidity, mortality, and associated costs, use of CRC screening tests must be increased among age-eligible adults with the lowest CRC screening rates.
To address this, the Division of Cancer Prevention and Control issued the Colorectal Cancer Control Program (CRCCP): Organized Approaches to Increase Colorectal Cancer Screening (DP15-1502) (hereafter referred to as CRCCP), a 5-year cooperative agreement aimed at increasing CRC screening rates among an applicant defined target population of persons aged 50-75 years of age within a partner health system serving a defined geographical area or disparate population. The CRCCP has two distinct program components:
Component 1:
Funding for component 1 is limited to partnerships with health systems to implement up to four priority evidence-based interventions (EBIs) described in the Guide to Community Preventive Services as well as other supporting strategies. Grantees must implement at least two EBIs in each partnering health system.
Component 2: Funding for component 2 is used by grantees to provide direct screening and follow-up clinical services for a limited number of individuals aged 50-64 in the program’s priority population who are asymptomatic, at average risk for CRC, have inadequate or no health insurance for CRC screening, and are low income.
The CRCCP funds a total of 30 grantees (previously 31), including 22 state governments or bona-fide agents, 7 universities, and 1 tribal organization. All 30 grantees receive Component 1 funding to implement four priority evidence-based interventions (EBIs; provider assessment and feedback, provider reminders, patient reminders, and reducing structural barriers) along with supporting strategies (patient navigation, small media, and activities facilitating community-clinical linkages) in order to increase clinic-level CRC screening rates within partner health systems. Six of the 30 grantees also received Component 2 funding to support clinical service delivery. Component 2 grantees fund health care providers in their jurisdiction to deliver CRC screening, diagnostic evaluation, and treatment referrals for those diagnosed with cancer.
Based on data collection conducted in Program Year 1 and feedback received from grantees and subject matter experts, CRCCP information collection has been revised. Specifically, CDC determined that several items within the annual grantee survey are more appropriately assessed at the clinic level, and clinic-level data variables were expanded to capture more detailed information regarding program implementation and evaluation for the remaining program years. CDC is authorized to collect information by the Public Health Service Act (see Attachment 1 – Authorizing Legislation).
A2. Purpose and Use of the Information Collection
CDC is required by DP-15-1502 to monitor and evaluate both process and outcome measures for the CRCCP. Grantees are required to report information to CDC to support these efforts. In redesigning the CRCCP, CDC developed detailed program logic models, one for each component, to reflect processes and expected outcomes over time (see Attachment 2– CRCCP Logic Models). The logic models guided development of the monitoring and evaluation plan for the CRCCP which focuses on programmatic efforts at the health system clinic level where CDC can more effectively monitor changes in screening rates over time and assess program effectiveness.
Two forms of information collection have been implemented to assess program processes and outcomes – an annual grantee survey and clinic-level data collection. In Program Year 1, the annual survey was used to monitor grantee use of the four priority EBIs required by CDC and supporting activities (SAs) implemented in partner health systems, with questions that assessed: (1) program management, (2) implementation activities, including use of priority EBIs and SAs, (3) health information technology (IT), (4) partnerships, (5) data use, (6) training and technical assistance (TA), and (7) clinical service delivery (for programs receiving Component 2 funding only). For Program Years 2-5, CDC proposes use of an updated CRCCP Annual Grantee Survey (see Attachment 3a – CRCCP Annual Grantee Survey) that eliminates survey items related to implementation activities as these data are more accurately reported at the clinic level. Survey questions are of various types, including dichotomous, multiple response, and free text. CDC will conduct the CRCCP annual grantee survey among all 30 grantees following the end of each program year.
Clinic-level data collection assesses CRCCP’s primary outcome of interest - CRC screening rates within partner health system clinics. CRCCP grantees collect and report CRCCP clinic-level information for all health system partners’ primary care clinic sites. Health systems typically include multiple primary care clinic sites. As health system partnerships are established, grantees collect baseline information about the clinic setting, patient population characteristics, CRC screening rates, and EBI/supporting activities implementation. Grantees then collect and report a CRC screening rate and updated information about EBI/SA implementation annually for the duration of the FOA. For Program Years 2-5, CDC proposes an updated CRCCP clinic-level data collection that includes additional items to collect additional information and details on program implementation, as well as monitoring/evaluation at the clinic level (see Attachment 4a – CRCCP Clinic-Level Data Collection Instrument (screenshots) and Attachment 4b – CRCCP Clinic-Level Data Dictionary). Clinic-level information will help CDC to describe program reach, the clinic settings, characteristics of the population being served, program activities implemented at the clinic-level, and changes in CRC screening rates over time.
Exhibit 1 illustrates the key changes proposed for CRCCP information collection. Updates to both the annual grantee survey and the clinic-level data collection ensure that only the most pertinent data are collected at both the grantee- and clinic-levels. Based on our experiences in program year 1, CDC found that many of the program implementation details gathered via grantee-level survey were difficult for grantees to report and did not derive high quality data. Conversely, we found that those same data items would be more appropriately reported on the clinic level, where clinic-level staff are better able to determine and report more specific details on program implementation (and potentially monitoring/evaluation) activities. Revisions to both the annual grantee survey and the clinic-level data collection reflect this rationale. See Attachment 5: CRCCP Data Collection Revision Matrix for a list of specific changes by section for each instrument, and rationale for each change.
Exhibit 1: Key Changes to CRCCP Information Collection Plan
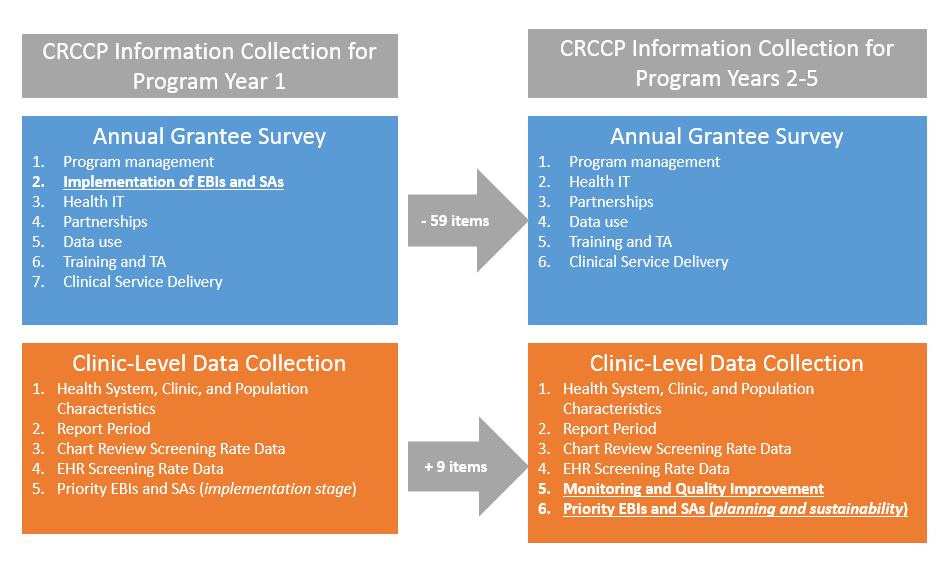
Together,
the proposed information collection activities are expected to
contribute to a more effective CRCCP and strengthen CDC’s
ability to demonstrate program results. These monitoring activities
will also help to identify successful activities that need to be
maintained, replicated, or expanded, as well as provide insight into
areas needing improvement.
The scope of information collection is limited to monitoring the public health activities and experiences of CRCCP grantees acting in their official capacity. Personal identifying information will not be collected, and the information collection will not yield information that can be generalized. As such, this information collection will not require IRB review. CDC will use this information to better understand the range of experiences among grantees and as one of many inputs into decision-making and/or program management. In addition, the findings will be reported back to the grantees to help them identify successful implementation models and focus networking for shared experiences, lessons learned, and best practices.
A3. Use of Improved Information Technology and Burden Reduction
Grantee survey information will be collected annually via a web-based questionnaire allowing respondents to complete and submit their responses electronically. Clinic-level information will be collected annually through a web-based instrument. (See Attachment 4a - CRCCP Clinic-Level Collection Instrument (screenshots)). Both methods use pre-existing web infrastructure and tools already in place for the CRCCP grantees. These methods were chosen to minimize the overall burden on respondents.
A4. Efforts to Identify Duplication and Use of Similar Information
The information to be collected from the CRCCP grantees are unique to the current program and, therefore, not duplicative of other efforts. The revised CRCCP survey eliminates survey items about implementation activities (i.e., use of EBIs and SAs) at the grantee level. Based on grantee feedback, CDC anticipates that grantees will be able to report higher quality implementation data at the clinic level. The revised CRCCP Clinic-Level Data Collection includes additional items to capture new data on implementation of the EBIs and SAs at the clinic level. There is an existing, complimentary information collection to monitor Component 2 service delivery (OMB No: 0920-0745 exp. 12/31/2018).
A5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this information collection.
A6. Consequences of Collecting the Information Less Frequently
The purpose of this request is to ensure collection of information that is not otherwise available in a current, time sensitive, or standardized format to specific or emergent priorities of HHS and CDC. CRCCP information collection was previously approved; however, this request proposes use of a revised CRCCP Annual Grantee Survey (Attachment 3a) and revised CRCCP Clinic-Level Data Collection (see Attachment 4b – CRCCP Clinic-Level Data Dictionary). Revisions to these instruments are based on CDC’s data collection experience in program year 1, as well as feedback from grantees in the field and subject matter experts, and are designed to streamline data collection. Without this information collection, there would be:
No systematic information collection regarding the implementation of program activities and outcomes, as required in the current FOA.
No systematic assessment of training and technical assistance needs.
No systematic assessment of monitoring and evaluation efforts at the clinic level.
Less effective and less timely assessment of implementation partners and their program activities.
Fewer resources from which to make data-driven decisions that are often required of CDC as well as required of its grantees.
OMB approval is requested for three years. There are no legal obstacles to reduce the burden.
A7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances with this information collection package. This request fully complies with the regulation 5 CFR 1320.5 and will be voluntary.
A8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
Table A.8.1. Individuals Who Have Provided Consultation on the Project
Consultant |
Title |
Affiliation |
Phone |
Year of Consult |
|
Cam Escoffery
|
Associate Professor |
Emory University |
404-727-4701 |
2015 |
|
Peggy Hannon |
Director, Associate Professor |
University of Washington School of Public Health |
206-616-7859 |
2015 |
|
Thuy Vu |
Research Coordinator |
University of Washington School of Public Health |
206-616-4724 |
2015 |
|
Annette Maxwell
|
Professor |
University of California – Los Angeles
|
310-794-9282 |
2015 |
|
Kathleen McNamara |
Associate Vice President, Clinical Affairs |
National Association of Community Health Centers (NACHC) |
301-347-0400 |
2015 |
|
Ben Reisler |
Clinical Data Specialist |
National Association of Community Health Centers (NACHC) |
301-347-0411 |
Notice of this project was published in the Federal Register on December 30, 2016 in Vol. 81, No 251, pages 96457-96459. (See Attachment 6 – 60-Day Federal Register Announcement). No public comments were received.
A9. Explanation of Any Payment or Gift to Respondents
CDC will not provide payments or gifts to respondents.
A10. Assurance of Confidentiality Provided by Respondents
This submission has been reviewed by CDC’s Information System Security Office, who determined that the Privacy Act does not apply. Activities do not involve the collection of individually identifiable information, and all information is programmatic in nature.
Overview of the Information Collection System
CDC proposes to collect information in two forms from Colorectal Cancer Control Program (CRCCP) grantees, who are 30 state governments or bona-fide agents, universities and tribal organizations. The information collection will support monitoring and evaluation of program implementation and outcomes of the CRCCP. The two forms include a CRCCP annual grantee survey and a set of CRCCP clinic-level information acquired through health system partnerships. Program policy requires grantees to report both forms of information to CDC.
The CRCCP annual grantee survey information collection consists of a web-based questionnaire designed to collect grantee-level information from all 30 CRCCP grantees. CDC proposes use of an updated CRCCP Annual Grantee Survey (see Attachment 3a– CRCCP Annual Grantee Survey) that eliminates survey items related to implementation activities as this information is more appropriately collected at the clinic level. The Program Director or Manager for each cooperative agreement will serve as the survey respondent. Contact information for the grantee is obtained from program administrative systems and used to distribute survey introductory and reminder emails (See Attachment 3b - Survey Introductory Email and Attachment 3c – Survey Reminder Email). The CDC contactor will manage primary information collection and send respondents a unique link to an online instrument, and not to a website, that will enable grantees access to view and enter their survey information. After receiving responses to the survey, the contractor will prepare a validated analysis file and set of reports for CDC to assist in interpreting results. CDC will prepare and distribute grantee-specific and CRCCP summary feedback reports. The web-based information collection instrument software will be developed using an open-source product called LimeSurvey (limesurvey.org). This effort will build on a pre-existing survey information collection and set of development tools used for another national cancer screening program also administered by CDC’s Division of Cancer Prevention and Control: The Annual Survey of the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) Grantees’ Program Implementation (OMB No. 0920-1046 exp. 1/31/2018).
The clinic-level information collection consists of information on each clinic site where CRCCP program interventions are implemented. Grantees establish formal agreements with health systems to implement evidence-based interventions to increase CRC screening within clinics where primary care preventive services are delivered or supported. CDC proposes use of revised CRCCP Clinic-Level Data Collection (see Attachment 4b – CRCCP Clinic-Level Data Dictionary) that better complements the annual grantee survey by gathering data previously captured through the survey, including information on program implementation at the clinic level, as well as new variables related to monitoring and evaluation efforts. Information will be reported through a web-based information entry instrument accessible to grantees on the pre-existing secure CRCCP program website (www.crccp.org) to simplify the reporting process with centralized information collection, validation, access control and technical support (Attachment 4a - CRCCP Clinic-Level Collection Instrument (screenshots)).
Each data collection will be implemented within three months after the end of each grantee program year, which runs from July-June. For both information collections, the contractor will host the instrument and archive information on secure network servers. The contractor will aggregate and validate the information for quality and completeness, and prepare an analysis file and reports for delivery to CDC.
Items of information to be collected
The information to be collected is programmatic in nature and does not involve research with human subjects. IRB approval is not required. No personally identifiable information is collected.
The revised grantee survey consists of 6 sections (compared to 7 sections in the previous version). The number of items completed by a respondent will vary due to skip logic. Questions are of various types including dichotomous, multiple response, and open-ended. To reduce burden, survey items collected in Program Year 1 to assess implementation of EBIs and SAs have been removed as collection of this information at the clinic level is more meaningful. There are also a limited number of questions requiring open-ended or narrative responses. The six sections of the survey include:
Respondent background information
Program management
Health IT and partnerships
Data use
Training and technical assistance
CRC screening delivery (for component 2 grantees only)
The revised clinic-level data collection instrument consists of 9 sections (as opposed to the previous version consisting of 8 sections). A number of items have been added to several sections to gather additional and more detailed information related to implementation of the EBIs and SAs, as well as monitoring/evaluation efforts at the clinic level (see Attachment 4b). Clinic-level information includes:
Record identification
Partner health system characteristics
Clinic characteristics
Clinic and patient population characteristics
Reporting period (for screening rates)
Chart review screening rate data
Electronic Health Record (EHR) screening rate
Data monitoring and quality improvement
Priority evidence-based EBIs and SAs
How information is shared and for what purpose
For both information collections, each grantee respondent will receive a customized feedback report relating to their program. Grantees will not have access to other grantees’ submissions or individualized reports. Program summary information and CRCCP aggregate results (e.g., performance ranges) will be shared across programs for grantees to compare performance and identify networking opportunities with others engaging in similar activities.
Information will be used by CDC to monitor and evaluate the CRCCP, provide feedback to grantees and stakeholders on program processes and outcomes, and inform program planning decisions for future programs. DCPC investigators will prepare formal reports periodically. CDC does not plan to create a public use dataset given the programmatic nature of the information and its strict application for monitoring the 30 CRCCP grantees. Program Announcement CDC-RFA-DP15-1502, the CRCCP funding announcement, requires that CDC monitor and evaluate CRCCP processes (i.e., implementation) and outcomes.
Statement of impact on the respondent’s privacy
The annual grantee survey includes programmatic information and does not contain direct personal identifiers. As such, the information collection will have little or no effect on the respondent’s privacy.
The clinic-level information identifies the partner health system and clinic by name and includes the clinic address – both of which are publicly available. The name, in addition to an assigned ID, are used to ensure accurate identification of the clinic when reporting longitudinal (annual) information, and to compare clinic implementation activities with grantee work plans. Except for in feedback reports to grantees, CDC will not identify the name of the Health System or Clinic partner. Information is treated in a secure manner and will not be disclosed, unless otherwise compelled by law.
Opportunities to consent to sharing and submission of information
Survey respondents are notified that their information will be maintained in a secure manner and that they will receive individualized feedback reports for their use. There are no advisements that relate to data sharing since CDC has no plans to share information or develop a public-use data set. There is no impact on the respondent’s privacy.
How information is secured
Both information collections are secured by technical, physical, and administrative safeguards as outlined below.
Technical
All data reside on a dedicated server on the contractor’s local area network behind the contractor’s firewall and is password protected on its own security domain. Access to the server is limited to the contractor’s authorized project staff. No non-project staff is allowed access to the data. All of the contractor’s project staff is required to sign a confidentiality agreement before passwords and keys are assigned.
Access to the CRCCP program website is restricted via a password-protected secure website. Access to grantee-specific reports and clinic-level data entry systems (Attachment 4a - CRCCP Clinic-Level Collection Instrument (screenshots)) are further restricted within the website. Each grantee has its own directory location, so no grantee has access to another grantee’s information. The CRCCP program website utilizes the Hypertext Transfer Protocol Secure (HTTPS) to ensure secure connections. In addition, the website will enable Strict Transport Security (HSTS), which is in compliance with OMB memorandum M-15-13, Policy to Require Secure Connections across Federal Websites and Web Services.
Once information has been compiled by the contractor and delivered to CDC via courier, all data are maintained for restricted access on CDC’s secure LAN server with access permission grantee by the CDC CRCCP data manager.
Physical
The contractor’s server is housed in a secure facility with restricted access.
Receipt and processing logs are maintained to document data receipt, file processing and report production. All reports and electronic storage media containing grantee information are stored under lock and key when not in use and will be destroyed when no longer needed.
Once data have been compiled by the contractor and delivered to CDC, all datasets are maintained for restricted access on a secure LAN server, which is housed in a secure facility. All CDC staff are issued identification badges and access to the building is controlled by key cards.
Administrative
CDC and contract staff have developed and implemented an information system security plan to ensure that the information is kept secure. Periodic review and update of the contractor’s security processes is conducted to adjust for needed changes and will be amended as needed to maintain the continued security of the information.
The contractual agreement between CDC and the contractor includes non-disclosure terms. The contractor’s project security team oversees operations to prevent unauthorized disclosure of the CRCCP data.
Once the information have been delivered to CDC, data are housed on CDC’s secure LAN server and restricted access is controlled by the CRCCP data manager.
A11. Justification for Sensitive Questions
No information will be collected that are of personal or sensitive nature. IRB approval is not required.
A12. Estimates of Annualized Burden Hours and Costs
The total number of grantees decreased from 31 grantees in PY1 to 30 grantees in PY2. The estimated burden hours for the PY1 CRCCP annual grantee survey (132 survey questions) was based on a pilot test of the information collection instruments by 5 public health professionals. In the pilot test, the average time to complete the instrument, including time for reviewing instructions and completing the instrument, was approximately 45 minutes. The updated burden hours for the revised CRCCP annual grantee survey (73 survey questions) is based on a 47% reduction in survey items. Therefore, the updated estimated burden is 24 minutes per response.
The estimated burden hours for the PY1 CRCCP clinic-level information collection tool (116 data variables) was based on a pilot test of the information collection instrument by 4 public health professionals. In the pilot test, the average time to complete the instrument was approximately 30 minutes. The updated burden hours for the CRCCP clinic-level information collection tool (125 data variables) is based on an 8% increase in data variables. The updated estimated burden 32 minutes per response. CDC estimates an average of 12 responses per grantee annually to correspond with the number of health system partners. The overall burden estimate for both data collections decreases from 210 to 204 burden hours.
Estimates for the average hourly wage for respondents are based on the Department of Labor (DOL) National Compensation Survey estimate for management occupations – medical and health services managers in state government (http://www.bls.gov/ncs/ocs/sp/nctb1349.pdf). Based on DOL data, an average hourly wage of $57.11 is estimated for all respondents.
Table A.12.A. Estimated Annualized Burden Hours
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden per Response (in hr) |
Total Burden (in hr) |
CRCCP Grantees |
CRCCP Annual Grantee Survey |
30 |
1 |
24/60 |
12 |
CRCCP Clinic-level Information Collection Template |
30
|
12 |
32/60 |
192 |
|
|
Total |
204 |
|||
Table A.12.B. Estimated Annualized Burden Costs
Type of Respondent |
Form Name |
Number of Respondents |
Total Burden Hours |
Hourly Wage Rate |
Total Cost |
CRCCP Grantees |
CRCCP Annual Grantee Survey |
30 |
12 |
$57.11 |
$685 |
CRCCP Clinic-level Information Collection Template |
30 |
192 |
$57.11 |
$10,965 |
|
Total |
|
|
|
|
$11,650 |
A13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There will be no direct costs to the respondents other than their time to participate in each information collection.
A14. Annualized Cost to the Government
Total operations and maintenance costs includes work performed by both the contractor and CDC personnel. Salary cost of CDC staff include an FTE (GS-13) to lead the project and coordinate all related activities of each information collection as well as another FTE (GS-12) to help with data management, analysis and report preparation. One hundred and twenty hours of staff time was estimated for each FTE annually for this information collection. Cost of the contractor represents an estimated 35% ($154,913) of total annual contract funds ($491,788) allocated for CRCCP data management activities. The estimated cost to the federal government is $165,209. Table A.14 describes how the cost estimate was calculated.
Table A.14. Estimated Annualized Cost to the Federal Government
Staff (FTE) |
Average Hours per Collection |
Average Hourly Rate |
Average Cost |
Health Scientist (GS-13) Lead health scientist to prepare OMB package; overall coordination; and consult on information collection, analysis, report preparation |
120 |
$45.97 |
$5516 |
Health Scientist (GS-12) Data management support, analysis, report preparation |
120 |
$39.83 |
$4780 |
Contractor Costs |
|
|
|
Annualized Cost of Contract with Information Management Services Responsible for building web-based application, information collection, coding and entry, quality control, analysis, report preparation |
|
|
$154,913 |
Estimated Total Cost of Information Collection |
$165,209 |
||
A15. Explanation for Program Changes or Adjustments
This is a request to reinstate with change OMB No. 0920-1074. For the CRCCP annual grantee survey, CDC proposes use of a revised survey instrument (see Attachment 3a – CRCCP Annual Grantee Survey) that eliminates data collection related to program implementation of EBIs and SAs as these are appropriately assessed at the clinic level. These changes reduce burden related to the survey among grantees from 24 to 12 hours. The revised clinic-level data collection instrument (see Attachment 4a – CRCCP Clinic-Level Data Collection Instrument (screenshots)) includes additional items related to implementation of the EBIs and SAs, as well as monitoring and evaluation, at the clinic level. These changes increase burden related to clinic-level data collection from 186 to 198 hours. The overall burden decreases from 210 to 204 burden hours.
Table A.15. Changes in Information Collection
|
Previous Approval |
Proposed Changes for Current Revision |
||||
Information Collection Instrument |
No. Respondents |
No. Burden Hrs. |
No. Respondents |
No. Burden Hrs. |
Change in Respondents |
Change in Burden Hrs. |
CRCCP Annual Grantee Survey |
31 |
24 |
30 |
12 |
-1 |
-12 |
CRCCP Clinic-level Information Collection Template |
31 |
186 |
30 |
192 |
-1 |
+6 |
Total: |
-6 |
|||||
A16. Plans for Tabulation and Publication and Project Time Schedule
The CRCCP Annual Grantee Survey and CRCCP Clinic-Level Information Collection Template will be completed annually within 3 months after the end of each program year (July – September). Data validation, analysis, and report preparation and dissemination will follow. A summary timeline is provided below:
Estimated Project Time Schedule
Activity |
Time Schedule |
Introductory emails for CRCCP Annual Grantee Survey sent to respondents with link to survey, information collection begins. CRCCP Clinic-Level Data Template available for reporting, information collection begins. |
Begin 1-3 months after end of program year, information collection continued for up to 6 weeks |
Survey reminder emails sent to non-responders (survey only) |
10 days after introductory letters sent |
Data Validation |
Completed 1 month after end of information collection |
Data Analyses |
Completed 4 months after end of information collection |
Report Preparation |
Completed 6 months after end of information collection |
Report Dissemination |
Completed 7 months after end of information collection |
A17. Reason(s) Display of OMB Expiration Date is Inappropriate
We are requesting no exemption.
A18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification. These activities comply with the requirements in 5 CFR 1320.9.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | gel2 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy