Form 1 Ropr data collection instrument
American Recovery and Reinvestment Act "Developing a Registry of Registries"
Attachment B - RoPR Data Collection Instrument_v2_09-11-17 (3)
New RoPRR entered manually through self-registration
OMB: 0935-0203
Form
Approved
OMB No. 0935-XXXX
Exp. Date XX/XX/20XX
The RoPR data collection system is a web-based collection mechanism. The screenshots included in this document represent all sections that will be visible to users who enter information through the self-registration pathway. The online form has 20 sections.
Users who enter information through the ClinicalTrials.gov pathway (which already received OMB approval) will only see sections 1-8 and 20.
1)
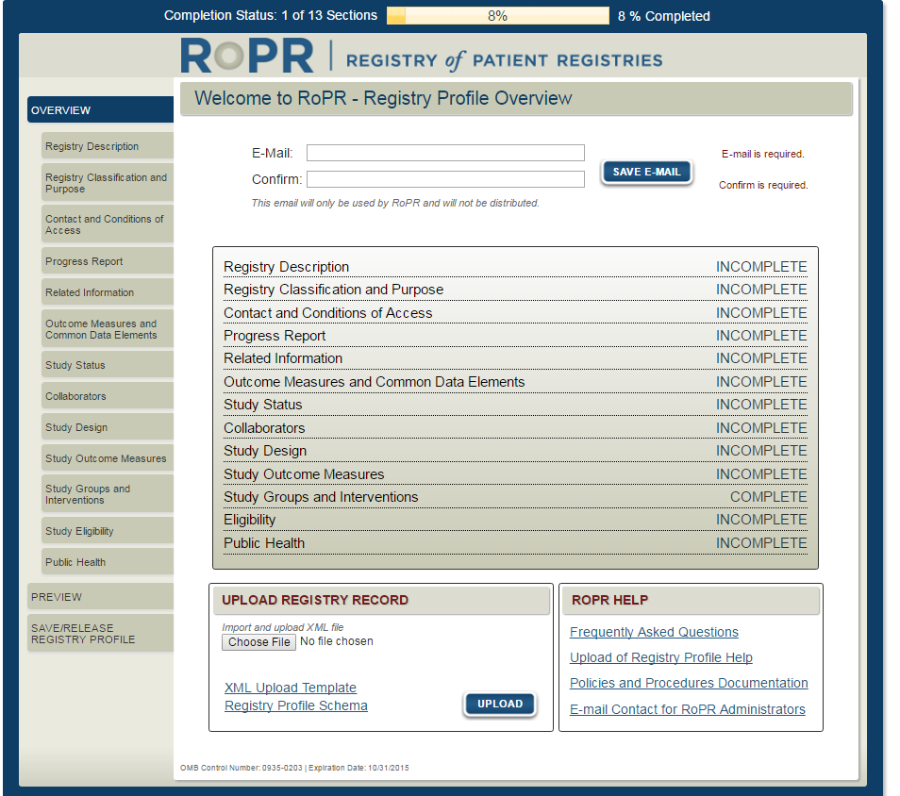
Public
reporting burden for this collection of information is estimated to
average 55
minutes per response, the estimated time required to complete
the survey. An agency may not conduct or sponsor, and a person
is not required to respond to, a collection of information unless it
displays a currently valid OMB control number. Send
comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden, to: AHRQ Reports Clearance Officer Attention: PRA,
Paperwork Reduction Project (0935-XXXX)
AHRQ,
5600 Fishers Lane, # 07W41A, Rockville, MD 20857.

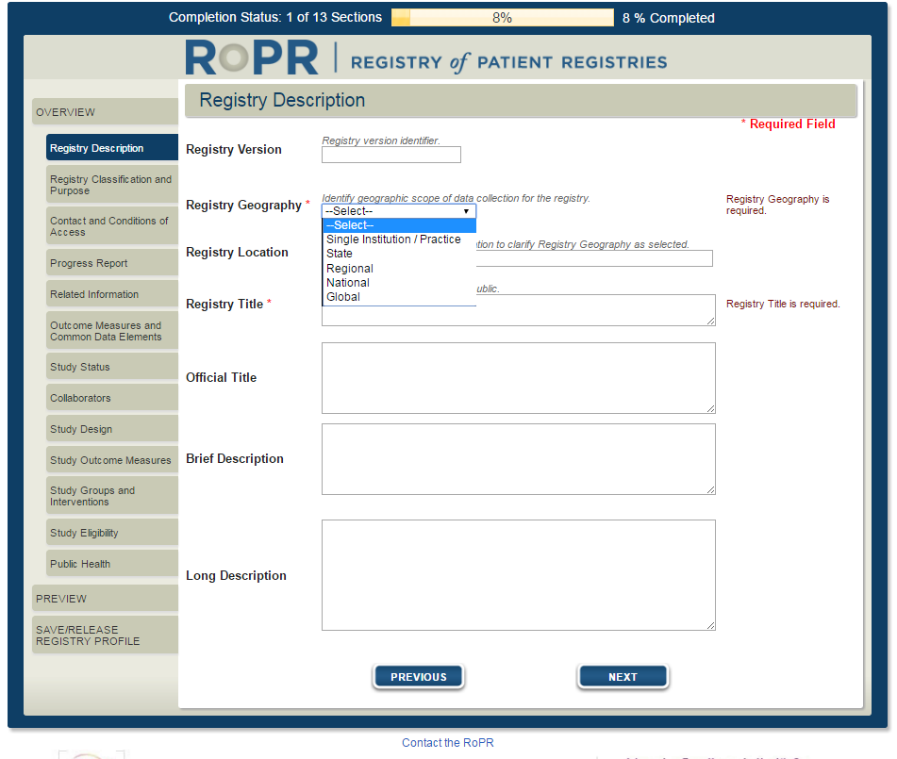
2)
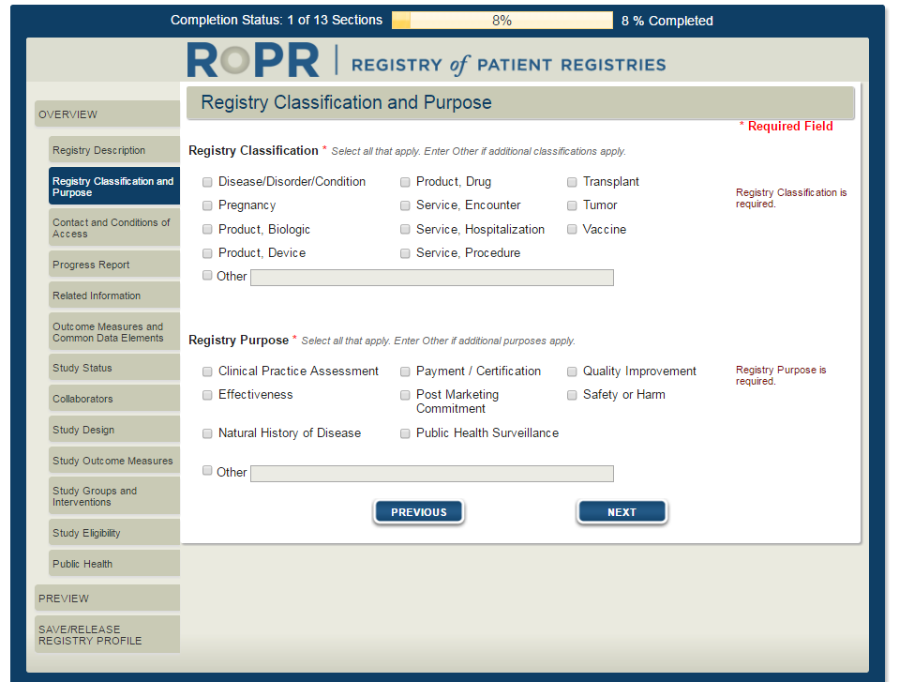
3a)
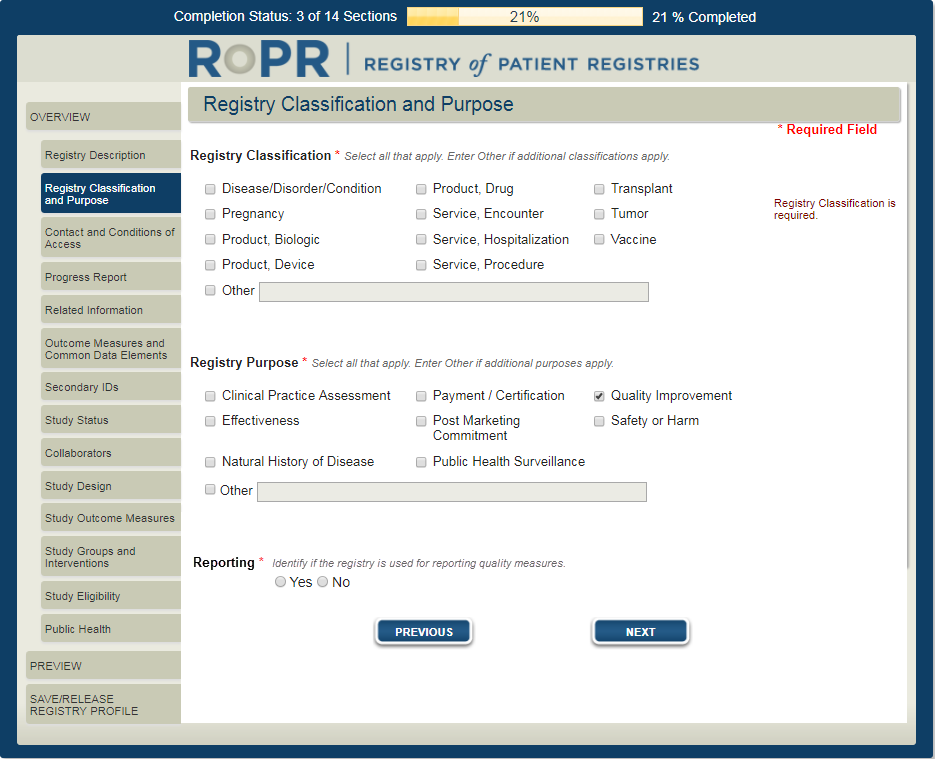
3b) Additional field shown if “quality improvement” purpose is selected
4)
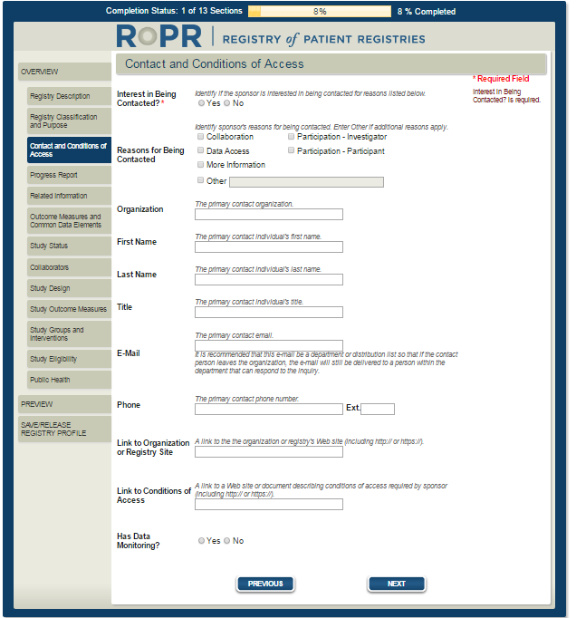
5)
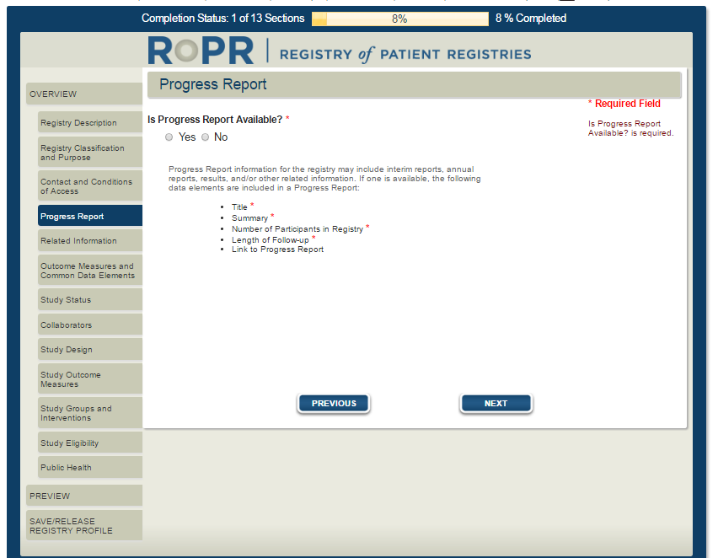
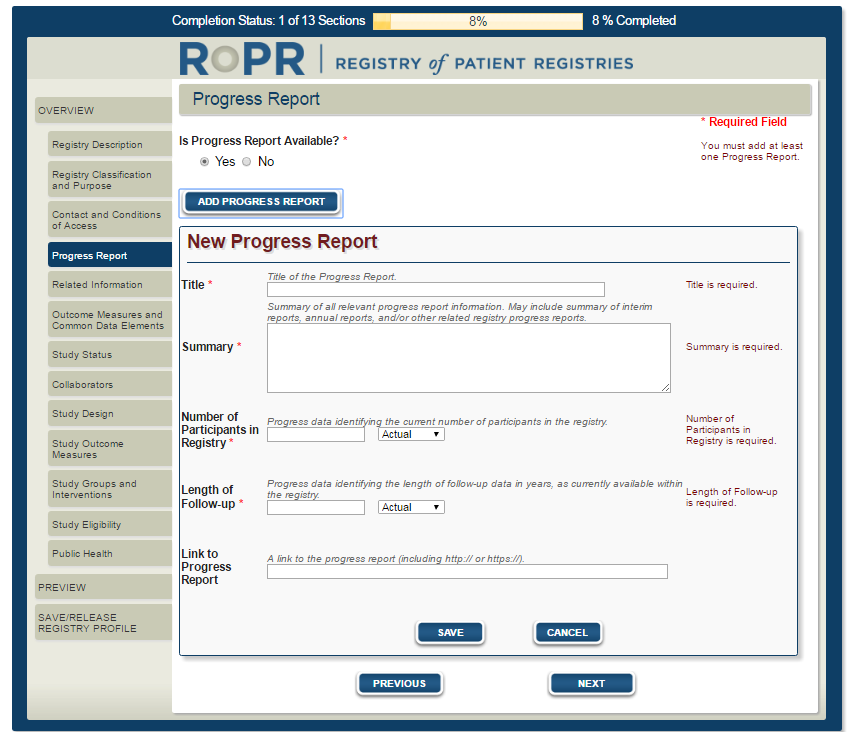
6)
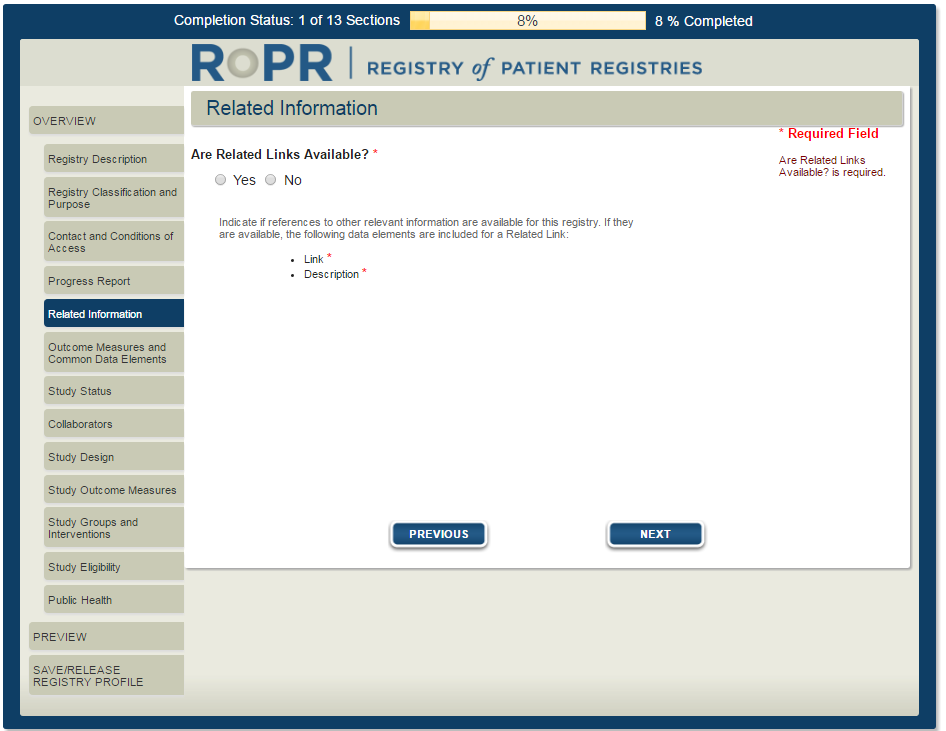
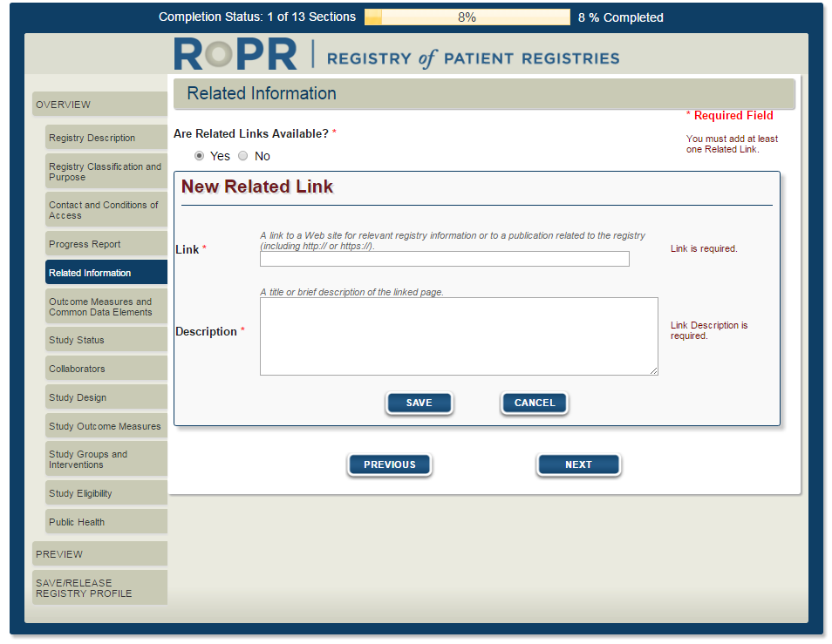
7)
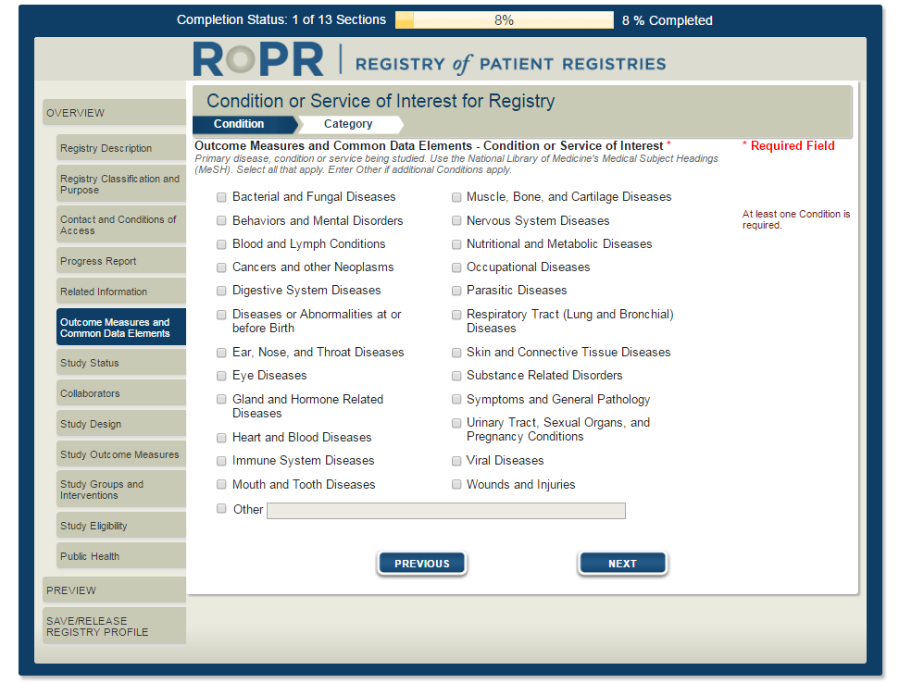
8)
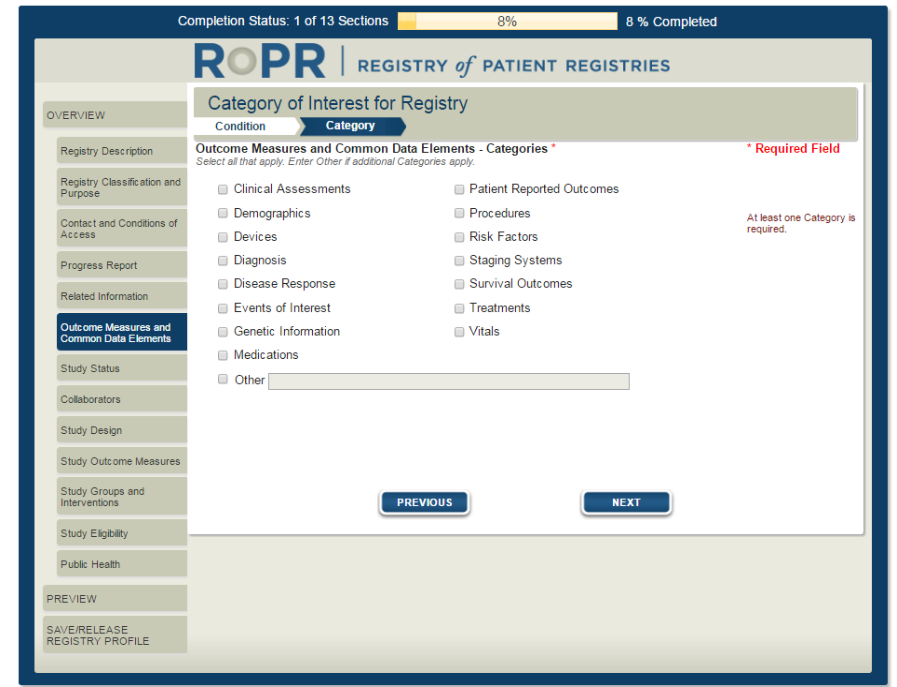
9)
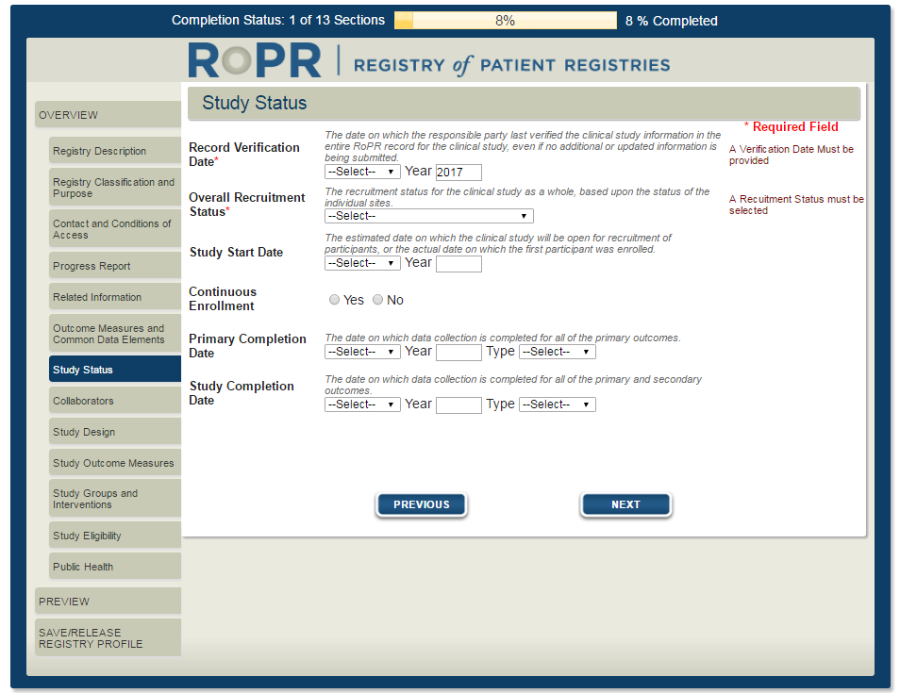
10)
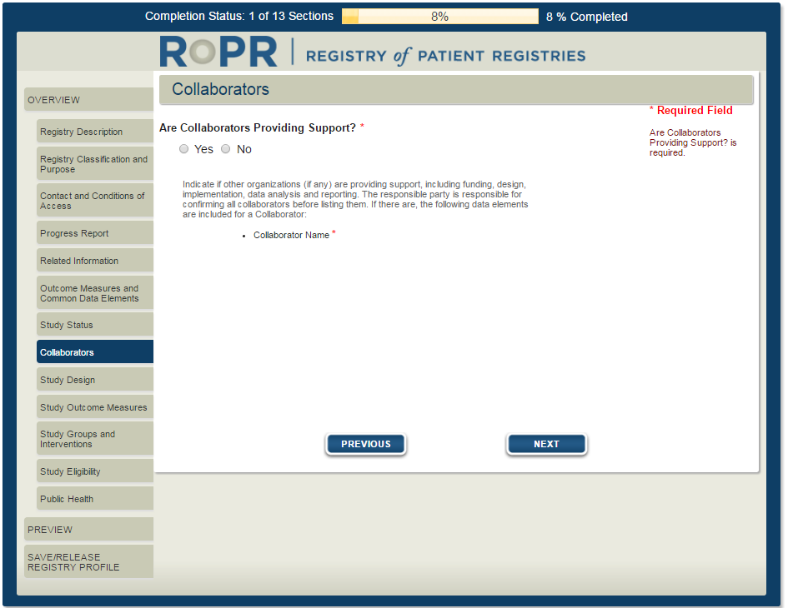
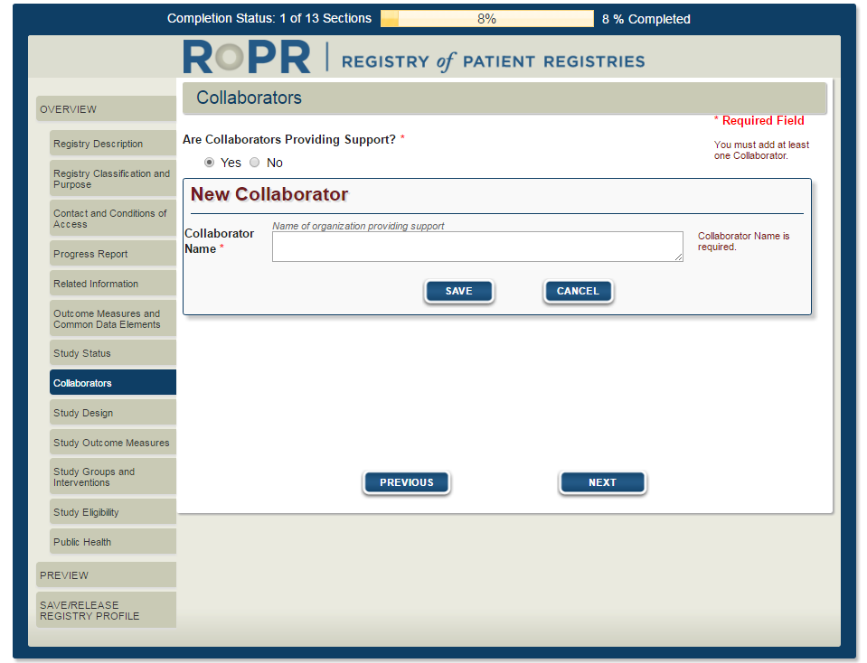
11)
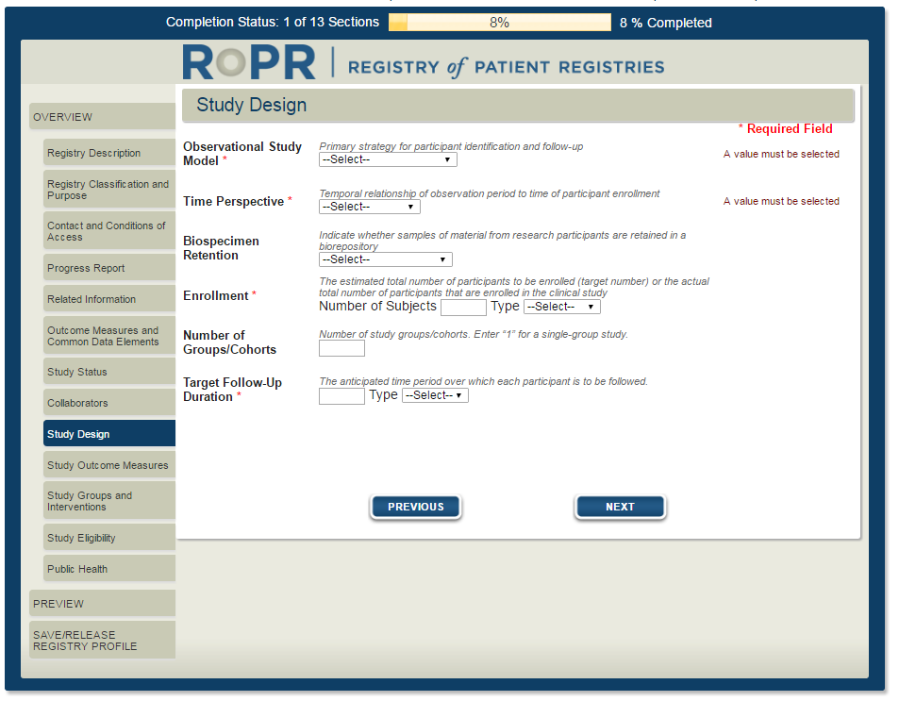
12)
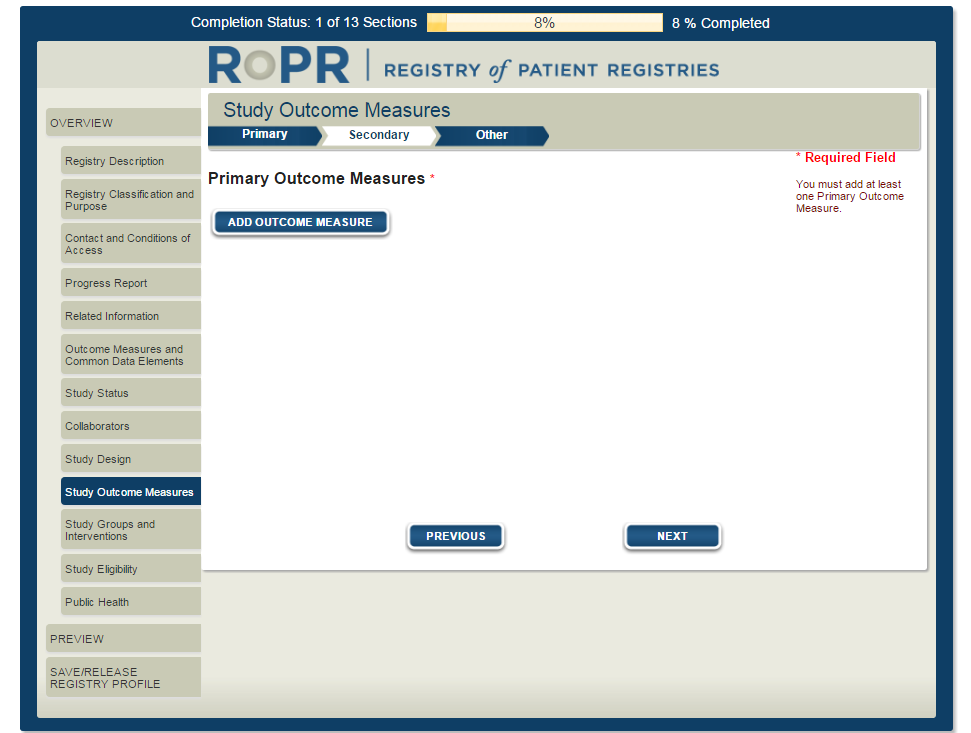
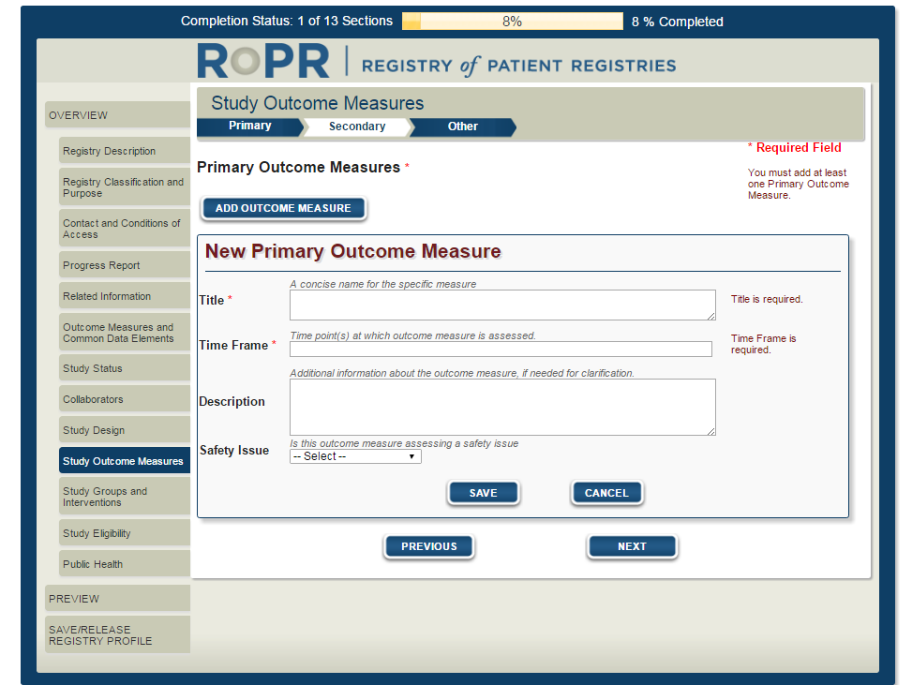
13)
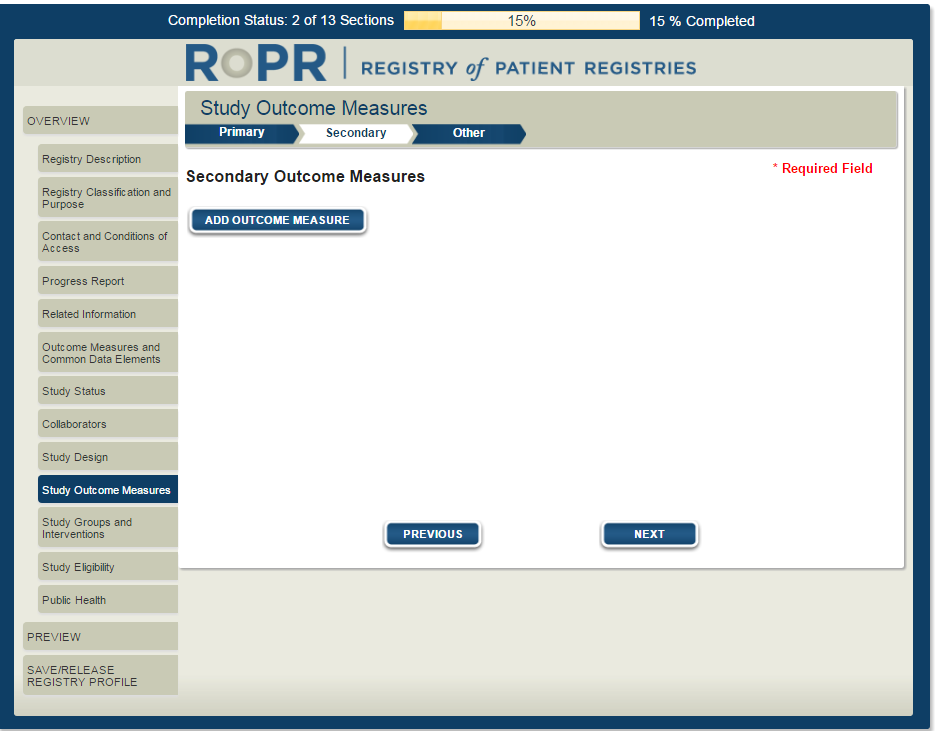
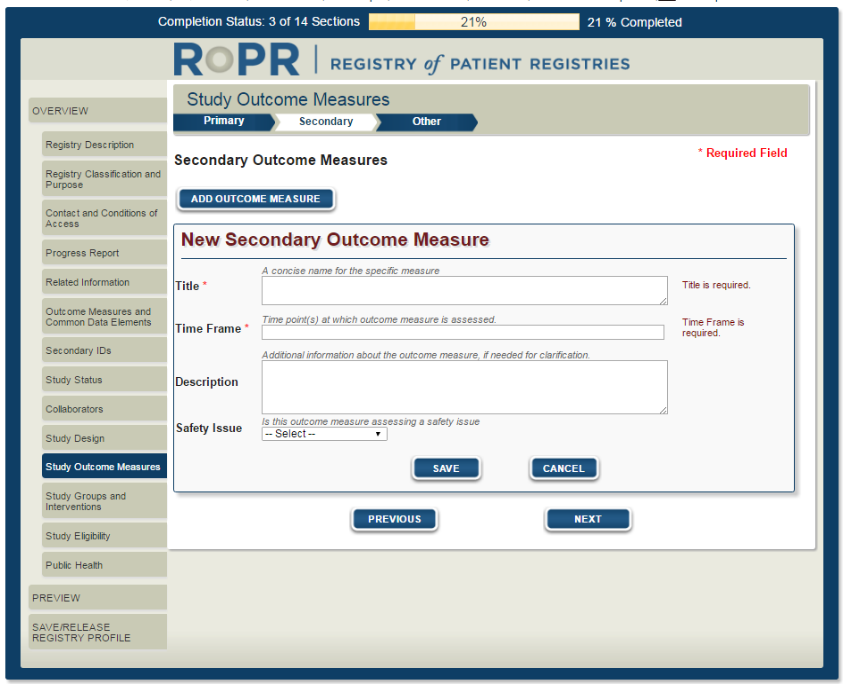
14)
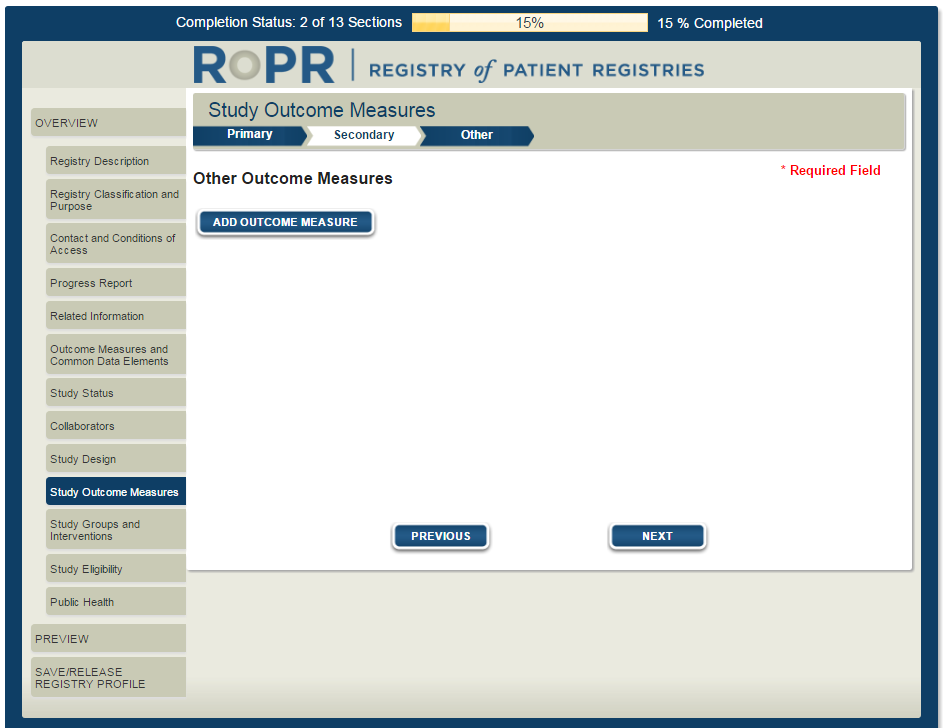
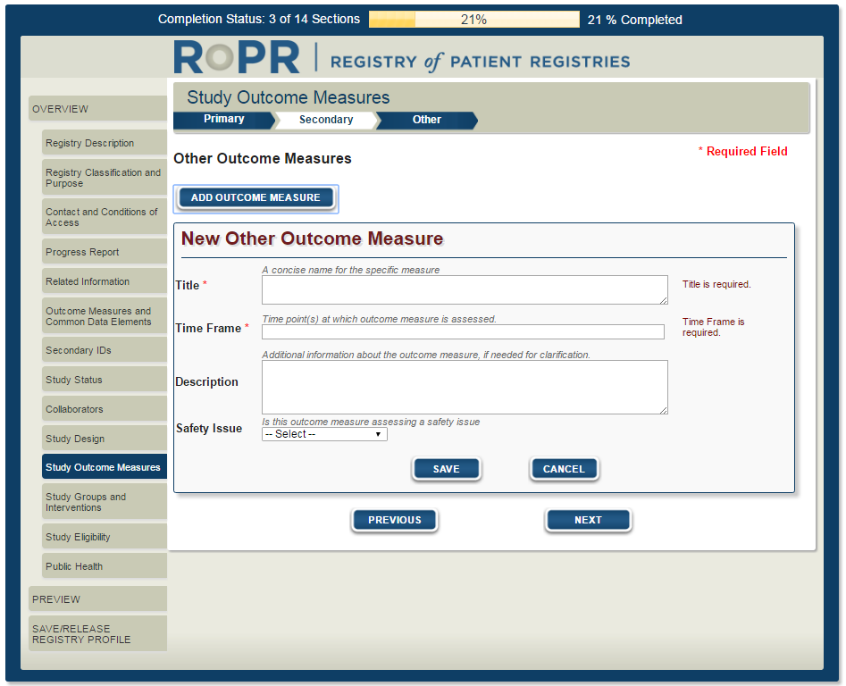
15)
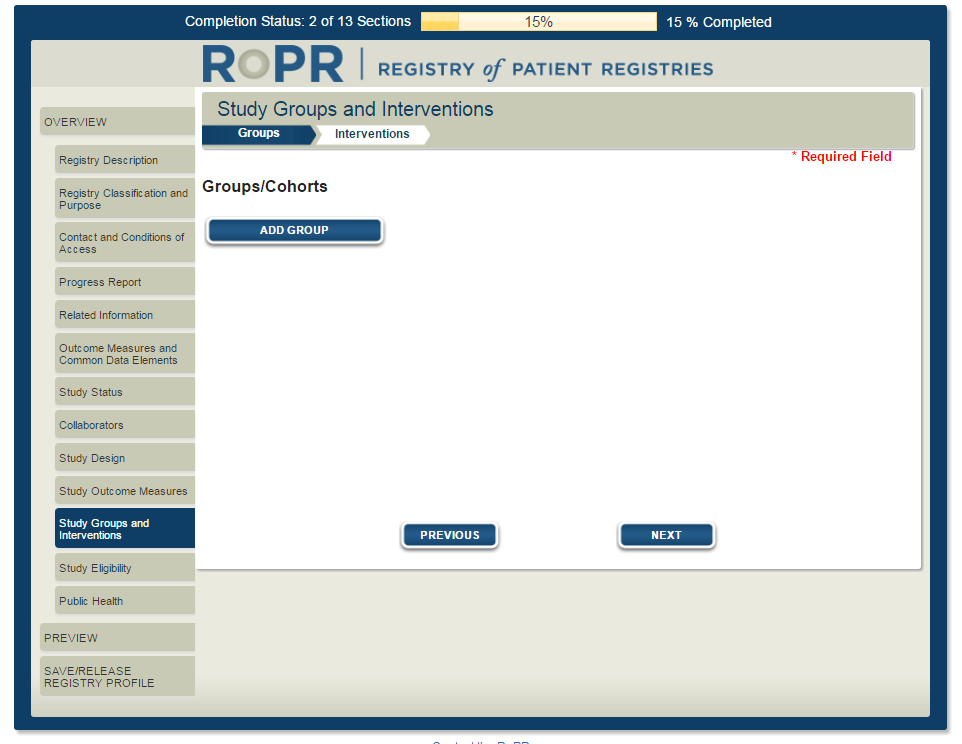
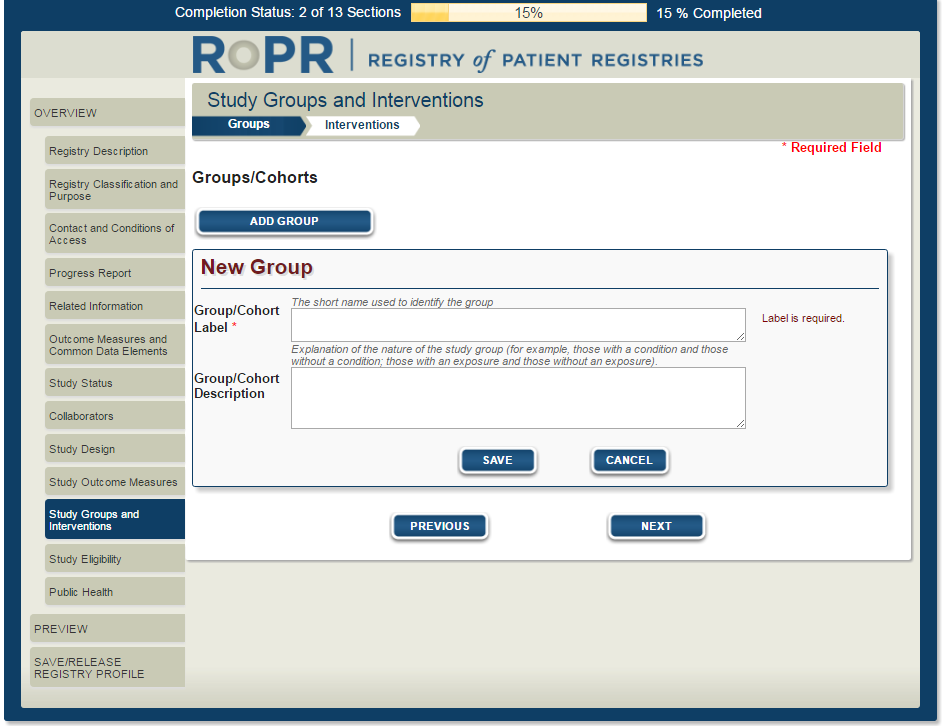
16)
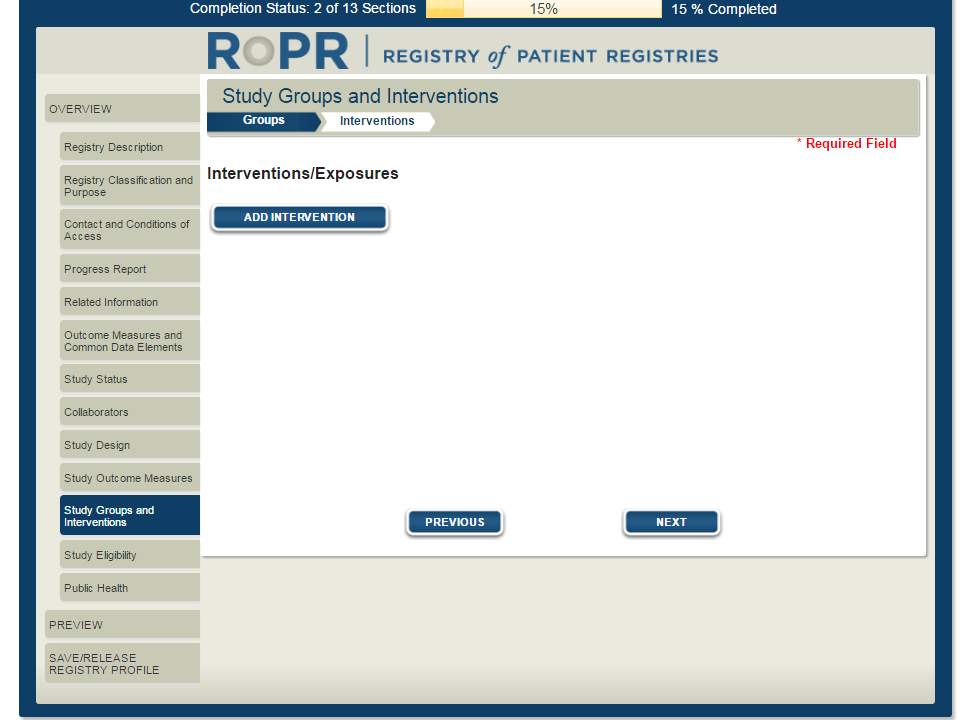
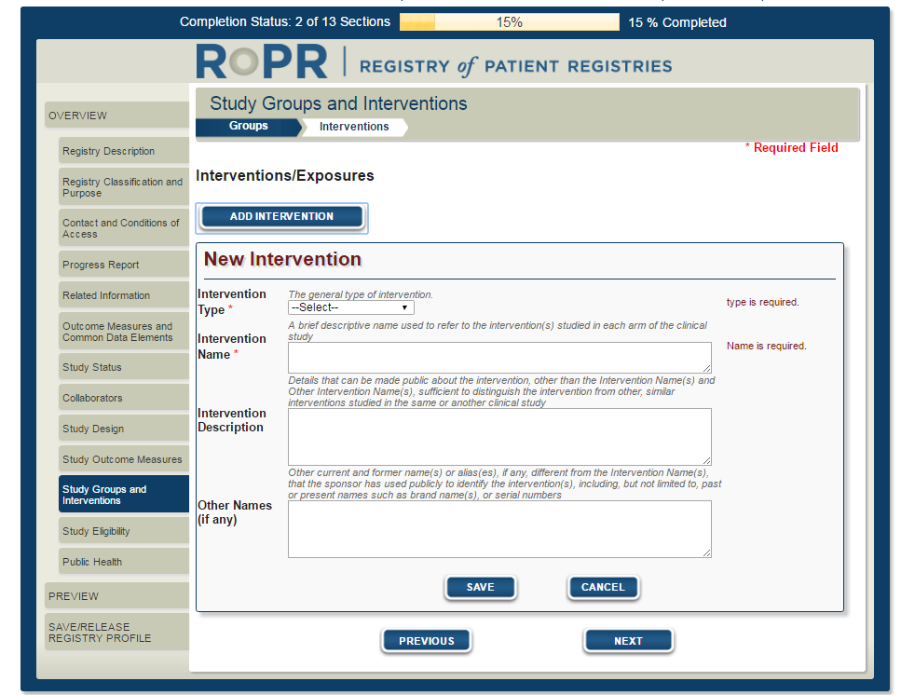
17)
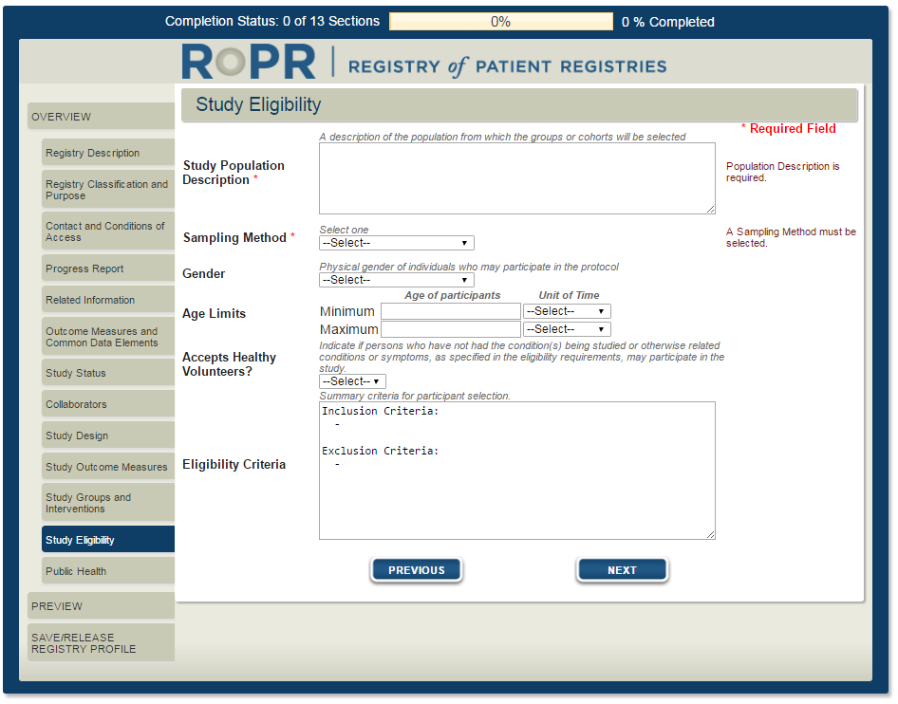
18)
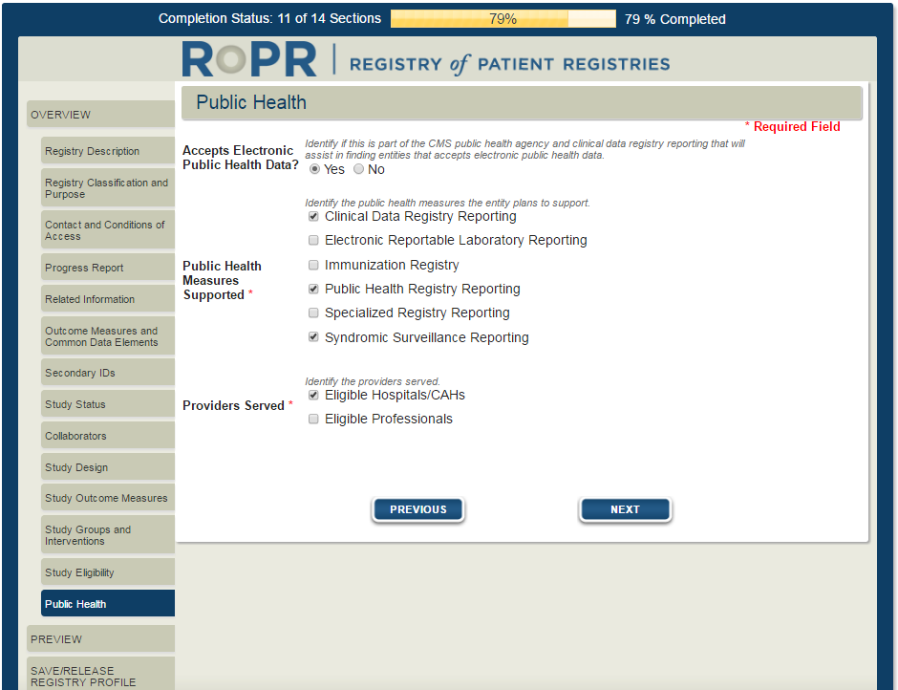
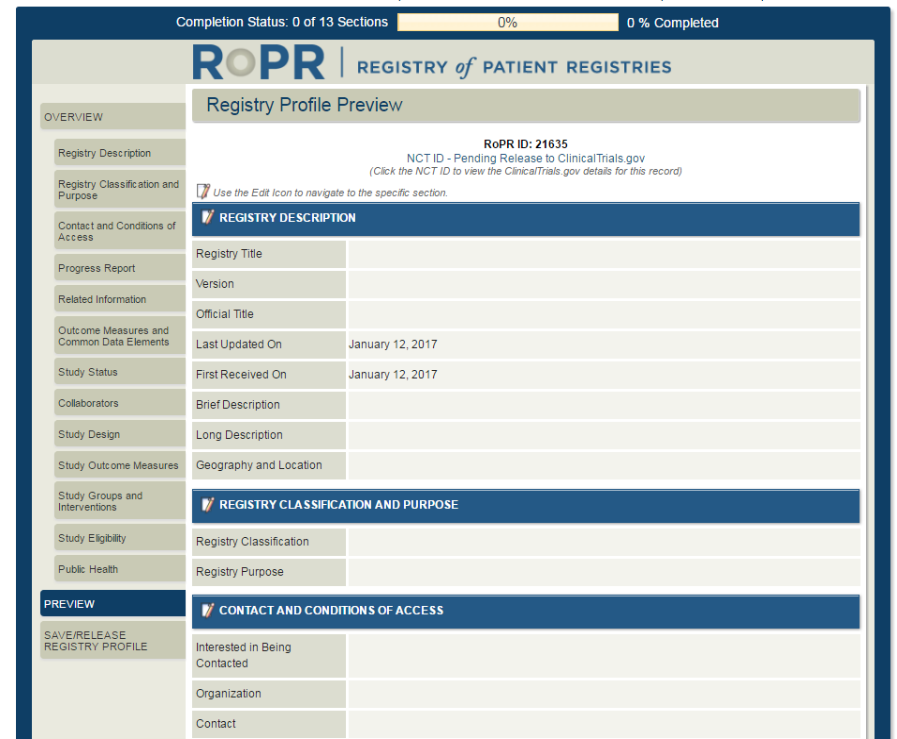
19)
20)
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Nelly Mentor |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy