Tree Testing FDA.Gov
Fast Track Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery
For OMB_Tree Testing FDADotGov 110717 RLM
Tree Testing FDA.Gov
OMB: 0990-0379
Form Approved
OMB# 0990-0379
Exp. Date 09/30/2020
For OMB: Tree Testing FDA.gov
Welcome
Welcome to this TreeJack study, and thank you for agreeing to participate!
The activity shouldn't take longer than 10 to 15 minutes to complete.
Your response will help us to organize the content on our website. Find out how on the next page...
Instructions
Here's how it works:
You will be asked to find a certain item and presented with a list of links.
Click through the list until you arrive at one that you think helps you complete the task.
If you take a wrong turn, you can go back by clicking one of the links above.
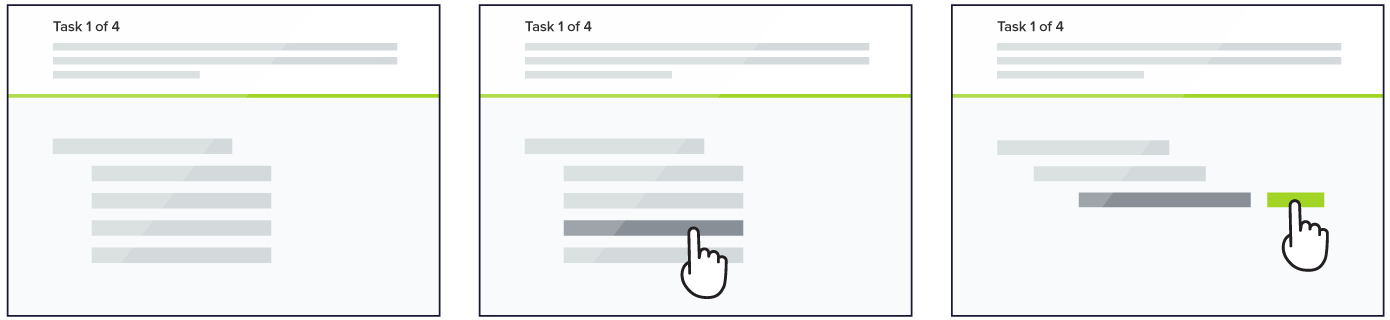
One thing you will notice as you go through the tasks is that the order of the links will change. This is just something we are doing during testing and will not be a part of the new site structure.
Randomizing the lists will help us more easily evaluate the paths people take to find information and spot possible issues with how items are placed or labelled.
As you go through the tasks, please remember there are no right or wrong answers. Our goal is to help site visitors find the information they need quickly & easily, and your efforts today are critical to our reaching that goal.
Thank you in advance. Now let's get started!
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0990-0379. The time required to complete this information collection is estimated to average 15 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: U.S. Department of Health & Human Services, OS/OCIO/PRA, 200 Independence Ave., S.W., Suite 336-E, Washington D.C. 20201, Attention: PRA Reports Clearance Officer.
Tasks
Task #1
Locate where you can report an allergic reaction to a food product.
Report a Problem > How Do I Report a Problem?
Task #2
Locate information about how to dispose of unused medicines
What FDA Covers > Drugs & Cosmetics
Task #3
Locate info about job opportunities at FDA
About FDA > Careers @ FDA
Task #4
Locate information about government regulation of vapes and e-cigs.
What FDA Covers > Tobacco
Laws, Regulations and Guidance > Laws & Regulations
Task #5
Locate FDA webinars for healthcare professionals.
Training & Education > Webinars
Task #6
Locate information about safe food handling in restaurants
What FDA Covers > Food & Dietary Supplements
Laws, Regulations and Guidance > Guidance for Industry
Task #7
Locate FDA guidance on required labelling for cosmetics packaging.
What FDA Covers > Drugs & Cosmetics
Laws, Regulations and Guidance > Guidance for Industry
Task #8
Locate studies or publications from FDA about evaluating vaccine safety.
What FDA Covers> Biologics & Vaccines
Science & Research
Task #9
Locate information about the food labels for consumers (Nutrition Facts).
What FDA Covers > Food & Dietary Supplements
Task #10
Locate a recently issued FDA warning letter to a manufacturer.
Enforcement and Inspections > Enforcement
Task #11
Locate recent pet food recalls.
What FDA Covers > Animal & Veterinary
News and Events > Recalls
Task #12
Locate FDA's approved drug products listing (the Orange Book).
What FDA Covers > Drugs & Cosmetics
Post Study Questions
Thank you for completing the sorting exercise. The task will help FDA to learn more about how to best organize information for website users.
We appreciate your time and ask that you answer a few questions.
1. Which of the following descriptions would you use to describe yourself when visiting the FDA.gov website? Please check all that apply:
Consumer
Patient
Caregiver
Family or friend of a patient
Researcher or educator
Journalist or member of the press
Federal government employee
Industry or business professional
Other ___________
2. Have you visited the FDA.gov website this year?
Yes
No
Cannot recall
3. Is FDA.gov a website you do or might visit for professional reasons for personal reasons, for both professional and personal reasons?
Personal
Professional
Both
Neither
4. Thinking of the current FDA.gov website, how easy or difficult is it to understand the labels used to organize the information on FDA.gov?
Very easy
Easy
Neither easy nor difficult
Difficult
Very difficult
5. Please tell us more about your rating.
6. When you think about looking for information on the current FDA.gov website, how confident are you that you can find the information you might need?
Likert item
Very confident
Confident
Neutral
Not very confident
Not confident at all
7. Please tell us more about your confidence rating.
8. Thinking of the way information was categorized on FDA.gov website during this exercise, how confident are you that you could find the information you might need in your new structure?
Very confident
Confident
Neutral
Not very confident
Not confident at all
9. Final comments:
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Mayeaux, Rebecca [USA] |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy