CBLS-ABLES SSA 20171018 clean
CBLS-ABLES SSA 20171018 clean.docx
Childhood Blood Lead Surveillance (CBLS) and Adult Blood Lead Epidemiology and Surveillance (ABLES)
OMB:
Childhood Blood Lead Surveillance (CBLS) and Adult Blood Lead Epidemiology and Surveillance (ABLES)
OMB Control No. 0920-NEW
New Information Collection Request
Supporting Statement Part A –
Justification
Program Official: Adrienne Ettinger, ScD
Title: Program Chief
Health Homes and Lead Poisoning Prevention Program (HHLPPP)
National Center for Environmental Health (NCEH)
Centers for Disease Control and Prevention (CDC)
Phone: 770-488-7492
Email: [email protected]
Fax: 770-488-3635
Date: October 18, 2017
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary 4
A.2. Purpose and Use of the Information Collection 5
A.3. Use of Improved Information Technology and Burden Reduction 16
A.4. Efforts to Identify Duplication and Use of Similar Information 17
A.5. Impact on Small Businesses or Other Small Entities 21
A.6. Consequences of Collecting the Information Less Frequently 21
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 21
A.9. Explanation of Any Payment or Gift to Respondents 24
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents 24
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions 34
A.12. Estimates of Annualized Burden Hours and Costs 35
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers 37
A.14. Annualized Cost to the Federal Government 38
A.15. Explanation for Program Changes or Adjustments 39
A.16. Plans for Tabulation and Publication and Project Time Schedule 41
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate 43
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions 43
Goal of the information
collection: The
goal of the National Center for Environmental Health (NCEH)
Childhood Blood Lead Surveillance (CBLS) Program is to promote
primary prevention of exposure to lead in children, and, as a
secondary prevention strategy, to prevent adverse health effects
when lead exposures occur in children, through improved program
management and oversight in respondent jurisdictions. The goal of
the National Institute for Occupational Safety and Health (NIOSH)
Adult Blood Lead Epidemiology and Surveillance (ABLES) Program is
to build state capacity for adult blood lead surveillance programs
to measure trends in adult blood lead levels and to prevent lead
over-exposures.
Together,
CBLS and ABLES
offer a coordinated, comprehensive, and systematic public health
approach to eliminate lead poisoning over the United States (U.S.)
population lifespan. Intended
use of the resulting data:
Through a new Fiscal Year 2017 (FY17) program announcement, NCEH
will promote primary prevention activities and will support
secondary prevention activities, including childhood blood lead
testing, surveillance, and targeted population-based interventions. The
NIOSH ABLES Program is a state-based surveillance program of
laboratory-reported adult blood lead levels. The program objective
is to build state capacity to initiate, expand, or improve adult
blood lead surveillance programs which can accurately measure
trends in adult blood lead levels and which can effectively
intervene to prevent lead over-exposures. By combining the data
collected from these two programs, CDC will be better able to
identify and intervene for both childhood and adult lead exposure. Methods
to be used to collect: NCEH
partners will submit standardized childhood lead screening data on
a quarterly basis via a secure encrypted file transfer protocol
(FTP) site. NCEH staff transfer the quarterly data into the
Childhood Blood Lead Surveillance (CBLS) system for processing,
dissemination, and results reporting. For the NIOSH ABLES Program,
partners will submit adult blood lead data on an annual basis
through secure email attachments, secure web accounts, or secure
encrypted FTP sites. Eventually, all ABLES partners will submit
data through secure encrypted FTP sites. Data will be stored on
secure CDC network drives. The ABLES Program may request
additional information from partners for data cleaning, management,
analysis, or reporting purposes. Subpopulation
of interest: The
respondents will include partners at State or local public health
agencies or their bona fide fiscal agents that will submit NCEH
childhood (n=48) and NIOSH adult blood lead surveillance data
(n=40). The CBLS is designed for blood lead surveillance among
children under 16 years of age. The majority of the NCEH respondent
activities focus on lead poisoning prevention among children, less
than 6 years of age (more specifically defined as less than 72
months). The NIOSH ABLES collection includes adults, 16 years of
age or over. About 95% of adults with elevated blood lead levels
are occupational when the exposure source is known. Occupational
exposures may affect child lead exposure in several ways including:
take-home (e.g., dust or residual) exposures from parents employed
in lead-related industries; pregnant and breastfeeding women who
are employed in lead-related industries may pass exposure to unborn
baby or breastfeeding infant; and older children employed on a
part-time or full-time basis in work that may involve lead (e.g.,
construction, commercial fishing). How
data will be analyzed: Data
will be analyzed by CDC staff to calculate descriptive statistics
and to explore factors that may be related to differences in
exposure levels.

A.1. Circumstances Making the Collection of Information Necessary
The National Center for Environmental Health (NCEH) is leading a new three-year information collection request (ICR), titled “Childhood Blood Lead Surveillance (CBLS) and Adult Blood Lead Epidemiology and Surveillance (ABLES),” that covers two Centers for Disease Control and Prevention (CDC) information collections, one for childhood blood lead surveillance by NCEH and another for adult blood lead surveillance by the National Institute for Occupational Safety and Health (NIOSH). Thus, blood lead surveillance over the human lifespan is covered under this single ICR.
The NCEH Healthy Homes and Lead Poisoning Prevention Program (HHLPPP) is sponsoring a new Fiscal Year 2017 (FY17) three-year cooperative agreement, titled “Lead Poisoning Prevention – Childhood Lead Poisoning Prevention – financed partially by Prevention and Public Health Funds” (FOA No. CDC-RFA-EH17-1701-PPHF17) (Attachment 3a). Based on available FY17 funds, NCEH is requesting PRA clearance for 48 respondents and an annual time burden of 760 hours.
There will be a one-year overlap between this new FY17 program and the fourth and final year of the existing FY14 cooperative agreement program titled “PPHF 2014: Lead Poisoning Prevention – Childhood Lead Poisoning Prevention – financed solely by 2014 Prevention and Public Health Funds” (Attachment 3b). During this overlapping program year, awardees will submit childhood blood lead surveillance data under one or the other ICR, but not under both.1
The NCEH CBLS collection is authorized under Sections 301(a), 317A, and 317B of the 1944 Public Health Service Act, as amended by the 1988 Lead Contamination Control Act. 2 In addition, this program is also authorized under Section 4002 of the Patient Protection and Affordable Care Act of 2010 (ACA), Public Law (PL) 111-148, (42 U.S.C. Section 300u-11), and under Section 2204 of the Water Infrastructure Improvements for the Nation (WIIN) Act of 2016 (PL 114-322) (Attachment 1a).
The NIOSH ABLES collection will be accounted for in this new ICR because it was previously included, but not fully described and its burden accounted for, under the Healthy Homes and Lead Poisoning Surveillance System (HHLPSS) ICR (OMB Control No. 0920-0931, expiration date 05/31/2018). The NIOSH ABLES Program is requesting approval for up to 40 respondents and an annual time burden of 280 hours. In total, both collections will require a total time burden of 1,040 hours. A discussion of the differences in the existing and the new blood lead surveillance ICRs is found in Sections A.10 and A.15.
The mission of NIOSH is to promote safety and health at work for all people through research and prevention. NIOSH was established under Section 22 (29 USC 671), and its general authority to conduct the ABLES collection is found in Section 20 (29 USC 669), of the 1970 Occupational Safety and Health Act (Public Law 91-596), as amended (Attachment 1b). Tracking of occupational hazards, exposures, injuries and illnesses has and continues to be an integral part of the NIOSH mission. NIOSH provides funding and expertise necessary for states to understand and prevent work-related risks. This support allows states to develop or increase state health department capacity to conduct occupational safety and health surveillance, as well as develop intervention and prevention programs.
Occupational exposures to adults may affect child lead exposure in several ways including: take-home (e.g., dust or residual) exposures from parents employed in lead-related industries; pregnant and breastfeeding women who are employed in lead-related industries may pass exposure to unborn baby or breastfeeding infant; and older children employed on a part-time or full-time basis in work that may involve lead (e.g., construction, commercial fishing). Hence, by combining the data collected from these two programs, CDC will be better able to identify and intervene for both childhood and adult lead exposure.
The 60-day Federal Register Notice was published on April 6, 2017 (Attachment 2) and is further discussed in Section A.8.
A.2. Purpose and Use of the Information Collection
The two CDC collections, under this single ICR, cover blood lead surveillance over the United States (U.S.) population lifespan, for children under 16 years of age, and for adults 16 years of age or over who may have entered the workforce. A summary of CBLS and ABLES Program milestones and accomplishments is found in Attachment 4. The NCEH CBLS and the NIOSH ABLES Programs together allow CDC to better identify and intervene for both childhood and adult lead exposure from lead paint, contaminated water, soil, dust or consumer products; for occupational exposures; and for “take home” exposures when workers bring lead contaminated clothing or equipment home to their children (NIOSH, 1995 & 2002; OSHA, 2014; Newman, 2015).
Table A.2.1. Healthy
People 2020 Goals Childhood
Blood Lead Surveillance System (CBLS) Environmental
Health Goal:
Promote
health for all through a healthy environment. Objective
EH-8
- Reduce blood lead levels in children. Objective
EH-8.1
- Reduce blood lead level in children aged 1–5 years. Objective
EH-8.2
- Reduce the mean blood lead levels in children. Objective
EH-20
- Reduce exposure to selected environmental chemicals in the
population, as measured by blood and urine concentrations of the
substances or their metabolites. Objective
EH-20.3
- Reduce exposure to lead in the population, as measured by blood
and urine concentrations of the substance or its metabolites. Objective
EH-22.1
- Increase the number of States, Territories, Tribes, and the
District of Columbia that monitor diseases or conditions that can
be caused by exposure to lead poisoning. Adult
Blood Lead Epidemiology and Surveillance (ABLES) Occupational
Safety and Health Goal:
Promote the health and safety of people at work through prevention
and early intervention. Objective
OSH-7
- Reduce the proportion of persons who have elevated blood lead
concentrations from work exposures.
Both NCEH and NIOSH collections are aligned
with the Healthy People 2020 Initiative (see
https://www.healthypeople.gov/)
(Table A.2.1).
CBLS Goals: Between 2007 and 2010, an estimated 535,000 children in the US had blood lead levels (BLLs) at or above the reference value for blood lead established for children by CDC in 2012 (≥5 µg/dL) (Wheeler, 2013; AAP, 2016). These children are at risk for the intellectual, behavioral, and academic deficits caused by lead. The primary source of lead exposure for children is their homes; some 38 million homes in the U.S. have lead-based paint hazards that can result in childhood lead poisoning. Low-income and minority children bear a disproportionate burden of this condition caused by unhealthy housing. In addition, some areas of the US report that as many as 35% of children identified with high BLLs are exposed to lead via sources other than lead-based paint in their homes, such as items decorated or made with lead, and drinking water.
The goal of the NCEH CBLS Program is to promote primary prevention of exposure to lead in children, and, as a secondary prevention strategy, to prevent adverse health effects when lead exposures occur in children, through improved program management and oversight in respondent jurisdictions for blood lead testing, surveillance, and targeted population-based interventions (Attachment 3a).
In addition, awardees will be expected to demonstrate that policies and systems are in place to identify lead-exposed children and link them to recommended services. More specifically, they will be expected to work closely with other agencies, partners, stakeholders and others serving children to ensure that a comprehensive system of referral, case management, follow up, and evaluation is in place for lead-exposed children.
ABLES Goals: The Occupational Safety and Health Administration (OSHA) estimates that approximately 804,000 workers in general industry and an additional 838,000 workers in construction are potentially exposed to lead. Workers are exposed to lead as a result of the production, use, maintenance, recycling, and disposal of lead material and products. Lead exposure occurs in most industry sectors including construction, manufacturing, wholesale trade, transportation, remediation and even recreation (OSHA, 2017).
The public health objective of the ABLES Program is identical to the Occupational Safety and Health Objective 7 in Healthy People 2020, which is to reduce the number of persons with BLLs >10 µg/dL from work exposures from 27.7 persons/100,000 in 2008 to 24.9 persons/100,000 employed adults by 2020. In 2015, the ABLES Program updated its case definition of elevated BLL to ≥5 µg/dL (NIOSH, 2015a), years after the Healthy People 2020 objective’s case definition (BLL ≥10µg/dL) was established. The ABLES Program seeks to accomplish the Healthy People 2020 objective by continuing to improve ABLES surveillance programs and working with state health and other agencies to effectively intervene and prevent further lead exposures. Intervention strategies implemented by ABLES-reporting states include conducting follow-up interviews with physicians, employers, and workers; investigating work sites; delivering technical assistance regarding exposure reduction or prevention; providing referrals for consultation and enforcement; and developing and disseminating educational materials and outreach programs (NIOSH, 2015b).
Primary Lead Poisoning Prevention Strategies
Childhood Primary Prevention: No safe threshold for BLL has been identified 3 (CDC, 2005; ATSDR, 2007). The American Academy of Pediatrics (AAP) Council on Environmental Health states that “[p]rimary prevention, reducing or eliminating the myriad sources of lead in the environment of children before exposure occurs, is the most reliable and cost-effective measure to protect children from lead toxicity” (AAP, 2016). The CDC Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP) underscores this position by stating “[T]he absence of an identified BLL without deleterious effects combined with the evidence that these effects, in the absence of other interventions, appear to be irreversible, underscores the critical importance of primary prevention” (ACCLPP, 2012).
The ACCLPP reiterated that “Primary prevention is a strategy that emphasizes the prevention of lead exposure, rather than a response to exposure after it has taken place. Primary prevention is necessary because the effects of lead appear to be irreversible. In the U.S., this strategy will largely require that children not live in older housing with lead-based paint hazards. Screening children for elevated BLLs and dealing with their housing only when their BLL is already elevated should no longer be acceptable practice” (ACCLPP, 2012).
Adult Worker Primary Prevention: Adult workers can be exposed to lead through inhalation, ingestion, and dermal absorption. Primary prevention of lead among workers can be achieved through engineering controls (e.g., local exhaust system), administrative controls (e.g., rotating workers’ schedule to minimize exposure time), safe work practices (e.g., showering and changing into clean clothes before leaving worksite), and use of personal protective equipment.
Secondary Lead Poisoning Prevention Strategies - Medical Management and Treatment Guidelines
Blood lead is the most widely available measure of exposure to lead for medical screening and treatment and for epidemiologic studies. It reflects the equilibrium between current and past lead exposure in bone and blood, and numerous studies have reported an association between BLLs and health outcomes (NTP, 2012). Since 1978, the CDC has published medical management and treatment guidelines when children with elevated BLLs are identified (CDC, 2002) and for clinical care of pregnant and lactating women (CDC, 2010). In 2016, the American Academy of Pediatrics (AAP) Council on Environmental Health, likewise, published its policy statement titled “Prevention of Childhood Lead Toxicity” with medical management treatment guidelines (AAP, 2016).
When primary prevention efforts fail to prevent and eliminate occupational cases of elevated BLLs, medical management practices are implemented to prevent harm to the extent possible. For workplace lead exposures, the Occupational Safety and Health Act Lead Standards give the examining physician broad flexibility to tailor special protective procedures to the needs of individual employees. Occupational medical management guidelines for lead were developed to parallel those previously recommended by CDC for children with elevated BLLs (CDC, 2002). Recommendations for medical management for elevated BLLs in workers are available from the Association of Occupational and Environmental Clinics (AOEC) with the Council of State and Territorial Epidemiologists (CSTE) (AOEC-CSTE, 2013), California Department of Public Health (CDPH) (CDPH, 2009), and the CSTE Occupational Health Surveillance Subcommittee (CSTE, 2013).
Reference Level for Blood Lead
Over the past four decades, there have been substantial efforts in environmental lead abatement, improved protection from occupational lead exposure, and a dramatic reduction in the prevalence of population BLLs over time. The CDC has reported the 1976-1980 US BLL mean in children, 6 months to 5 years, as 16.0 microgram per deciliter (μg/dL); and among adults, 18 to 74 years, 14.1 μg/dL (Annest, 1982). More recently, the CDC reported the 2009-2010 US BLL geometric means among children, 1 to 5 years, and among adults, 20 years and older, as 1.2 µg/dL for both age groups (CDC, 2015).
The lack of a safe threshold for BLL is supported by the 2012 National Toxicology Program (NTP) systematic review of studies of the relationship between health effects and low level BLLs. The NTP concluded that sufficient weight of evidence exists to presume that low level BLLs, both <10 μg/dL and <5 μg/dL for children and adults, are associated with adverse health outcomes (NTP, 2012).
Table A.2.1. Summary of NTP conclusions about health effects of low-level lead exposures
Life Stage |
Blood Lead Levels (BLLs) |
Health Effects |
Children |
<5 μg/dL |
Decreased academic achievement, IQ, and specific cognitive measures; increased incidence of attention-related behaviors and problem behaviors |
|
<10 μg/dL |
Delayed puberty, reduced postnatal growth, decreased IQ, and decreased hearing |
Adults |
<5 μg/dL |
Decreased glomerular filtration rate; maternal blood lead associated with reduced fetal growth |
|
<10 μg/dL |
Increased blood pressure, increased risk of hypertension, and increased incidence of essential tremor |
Lacking a safe threshold for BLL, and accounting for the steady decline in population BLLs over time, the ACLPP recommended a reference value based on the population distribution of blood lead among US children. The ACLPP recommended defining an elevated BLL as greater than or equal to the 97.5th percentile from two consecutive cycles of National Health and Nutrition Examination Survey (NHANES) (ACLPP, 2012). Adopting the ACLPP recommendation, CDC defined elevated BLL for children, aged 1 to 5 years, as ≥5 μg/dL, in 2012 (CDC, 2012). This reference value will undergo periodic reassessment by the CDC HHLPPP and its advisors.
In 2015, NIOSH updated the ABLES Program case definition for an elevated BLL among adults (16 years and older) to be a BLL ≥5 μg/dL of whole blood, in a venous blood specimen. The case definition was updated by NIOSH in response to CSTE’s recommendation (NIOSH, 2015a). This new case definition is used by CSTE (CSTE, 2015), and CDC’s National Notifiable Diseases Surveillance System (NNDSS, OMB Control No. 0920-0728, expiration date 01/31/2019) (NNDSS, 2017). NIOSH recommends that the most current guidelines for management of lead-exposed adults be implemented by the medical community at the current NIOSH reference value of 5 µg/dL, for adults, including pregnant women (NIOSH, 2015a; CDC, 2010).
From 2009 to November 2015, the NIOSH case definition for an elevated BLL was a BLL ≥10 µg/dL.
The HP2020 goal is to reduce the number of persons with BLLs ≥10 µg/dL from work exposures.
The Occupational Safety and Health Act Lead Standards require workers to be removed from lead exposure for BLLs ≥50 µg/dL (construction industry) or 60 µg/dL (general industry) and allow workers to return to work when the BLL is <40 µg/dL.
Further discussion of federal agencies following the CDC recommendations for blood lead reference values is found in Section A.4.
Program Accomplishments
HHLPSS ICR (OMB
Control No. 0920-0931, expiration date 05/31/2018)
2015
Terms of Clearance:
Clearance
is provided contingent on CDC advising its grantees/contractors of
the change in wording of the race/ethnicity and asthma questions
that were made during clearance.
Dissemination
of the aggregate data set and statistics generated from the
aggregate data set will always be accompanied by the following
caveats:
“These
data were collected for program management purposes. The data are
not generalizable at the national, state, or local level.
Furthermore, because inclusion criteria vary across grantees,
comparisons of aggregate statistics across programs can be
misleading (i.e., state policies and practices for blood lead
testing vary and local priorities drive decisions regarding which
homes receive assessments for other housing hazards). However,
descriptive statistics can be used to compare changes overtime in a
given area when the method by which housing units are chosen for
inclusion remains the same. With a thoughtful understanding of the
approach used to include housing units in a given location, HHLPPS
can be used to make associations between the number of individuals
in a given area and a specific housing hazard or health condition
and geographic descriptors such as poverty, age of housing,
tenancy, and health conditions.”
The HHLPPP anticipates the Terms of Clearance for this FY17 program will be the same as for the FY14 program, except for the requirement for advising awardees about the change in wording of race and ethnicity and asthma questions, which has been accomplished to date. The CBLS data will continue to be collected for program management purposes as described in the 2015 Terms of Clearance.
HHLPPP Accomplishments: In FY17, the HHLPPP will undergo its fourth and final year of the current FY14 cooperative agreement (FOA No. CDC-RFA-EH14-1408PPHF14). This final year will coincide with the first year of the new FY17 cooperative agreement (FOA No. CDC-RFA-EH17-1701PPHF17).
During the past three years, the HHLPPP met annually with FY14 program managers and worked with them to identify children at highest risk for lead exposure and targeted prevention activities to eliminate childhood lead poisoning in awardee jurisdictions.
During 2014-2017, the HHLPPP produced 17 publications (see list in References). Additional examples of the FY14 program accomplishments are below:
2014 (Year 1):
JT Lewis Site Investigation/Epi-Aid with ATSDR, EPA, and Philadelphia Department of Health (Emergency Epidemic Investigations; OMB Control No. 0920-0008; expiration date 07/31/2014).
Awardee Activities:
New York City identified lead hazards related to Bo Ying Compound, which is marketed for treatment of ailments in infants and children. The City reported its finding to the Food and Drug Administration (FDA). See Public Safety Alert at http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm416441.htm
2015 (Year 2):
HHLPPP completed data analysis for the JT Lewis Site Investigation/Epi-Aid in conjunction with the US Environmental Protection Agency (EPA) and the Philadelphia Health Department. The final report is in development.
Awardee Activities:
Rhode Island: used the cooperative agreement to create reports for all schools in the state showing screening rates in all schools.
New York City: created and shared a poster for the Chinese community on Bo Ying Compound. The compound, marketed for treatment of ailments in infants and children, may contain excessive levels of lead. The poster has been distributed throughout the Chinese communities in New York City and Washington State, supporting lead poisoning prevention efforts and showing collaborative effort from the cooperative agreement.
New Jersey: The program identified the need for a recovery project that increased residents’ knowledge of the connections between health and housing; ensured a competent health, social services, and housing professional workforce that is knowledgeable on the health impacts of housing conditions; and increased access to blood lead screenings for high-risk residents. The NJ Healthy Homes Training Center (HHTC), a public/private partnership between NJ DOH and Isles, Inc., a Trenton-based non-profit community development agency, developed a one-hour public education presentation “Creating a Healthy Home after a Hurricane or Flood” Local health departments conducted targeted screening using the LeadCare II blood lead analyzer. Statewide, in SFY2014, 2.9% of children less than 17 years of age had a blood lead level 5 µg/dL or greater. The recovery project identified a rate of 7.2% (95% confidence interval).
Oklahoma: The Oklahoma Childhood Lead Poisoning Prevention Program (OCLPPP) has had two meetings with the Oklahoma Health Care Authority (OHCA) to discuss a data sharing agreement to obtain a waiver of universal lead screening of all Medicaid children and focus on targeted screening for children who live in high-risk areas. The purpose is to compare the rates of children being screened on Medicaid versus those not on Medicaid. This can be compared to national screening levels of children on Medicaid and also to determine if there are areas in the state that have very low screening rates. These areas can be targeted to increase screening and ensure that decisions made on low risk and high-risk areas are data-driven. This will also allow OCLPPP to explore option for billing for services such as environmental investigations for children covered by Medicaid.
Wisconsin-Minnesota: Fraser Shipyard Lead Investigation Plan
Renovations in early 2015 at the Fraser Shipyard on the Wisconsin-Minnesota border resulted in elevated blood lead levels (EBLLs) in three employees working on the project. The investigation and response resulted in a large-scale collaboration between the Minnesota Department of Health (MDH), the Wisconsin Department of Health Services (WI DHS), OSHA, and Wisconsin Local Public Health Agencies (LPHAs). The collaboration took a three-pronged approach to the situation: a survey to assess the worksite and take home risk of lead exposure and lead exposure related symptoms; blood-lead sampling by health departments in both states to track blood lead levels of workers and their families; and environmental sampling by a certified professional for residences and vehicles from confirmed cases. Of the 357 workers who were potentially exposed to lead hazards at the shipyard, 233 had at least one blood lead screening. Worker protection and medical monitoring has increased due to this incident, and policies and procedures have also been reevaluated in light of the challenges faced throughout this event.
2016 (Year 3):
The HHLPPP supported CDC’s response to the lead contamination of drinking water in Flint, Michigan, including Emergency Operations Center (EOC) activation, data management, and community outreach.
Assisted Flint in response to the water contamination crisis with monitoring BLLs in more than 50% of the community’s children under 6 years of age, connecting more than 90% of children with elevated BLLs to case management.
Created an activity book to offer parents an interactive way to talk to their kids about lead in water.
Collaborated with leadership from the NCEH Communications team for an innovation project related to a CDC lead poisoning prevention program redesign (as part of the HHS Ignite Accelerator program).
Provided data that supported the U.S. Department of Housing and Urban Development’s plan to ban smoking in public housing.
Raised awareness about lead poisoning during October’s National Lead Poisoning Prevention Week via the tri-agency toolkit with customizable resources for state and local governments and organizations to develop their own campaigns.
Awardee Activities:
The District of Columbia program worked with the Centers for Medicaid and Medicare Services (CMS) and the College of American Pathologists (CAP) to have labs report blood sample type as a requirement for maintaining accreditation—this success contributes to more-accurate surveillance of pediatric BLLs nationwide, improving the knowledge of the true burden of lead poisoning.
New York City: In New York City, over 300,000 children under 6 years of age are tested annually. Since 2010, the number of children with blood lead levels at or above the 5 micrograms per deciliter (µg/dL) has decreased from 13,000 children in 2010 to 6,000 children in 2014. The lead surveillance program used small-area analyses to identify neighborhoods with higher blood lead levels. Within one neighborhood with a high rate of lead poisoning, the vast majority of children with the highest blood lead levels resided in an area home to a large Hasidic Jewish community. The lead program collaborated with local political and religious leaders and worked with trusted community based organizations to increase awareness about childhood lead poisoning prevention and all information was translated into Yiddish.
Rhode Island: In 2011, four core cities in Rhode Island reported having three times the number of children with elevated blood lead levels ≥10 µg/dL compared to other Rhode Island cities and towns. An estimated 80% of Rhode Island homes were built before 1978 and likely contain lead-based paint hazards, which can create lead dust hazards during renovations to these homes. Rhode Island implemented the US EPA’s 2010 Lead-Based Paint Renovation, Repair and Painting (RRP) Rule that requires workers to be certified and trained in the use of lead-safe work practices, and requires renovation, repair, and painting firms to be licensed by the department of health. Following the first full year of the program, there were 225 fewer children in Rhode Island with elevated blood levels and 180 cases of RRP violations were prosecuted for failure to obtain lead-safe certificates for rental units.
Louisiana: Louisiana state data demonstrated that some children attending Women and Infants Special Nutrition Projects (WIC clinics) do not receive routine health prevention services, and therefore are unlikely to receive blood lead testing at a medical clinic. The Louisiana lead program partnered with a New Orleans area WIC clinic to increase lead testing rates of children in Louisiana and to determine the percent of children tested during WIC clinic visits who had blood lead levels ≥5 µg/dL. By matching WIC client lists with surveillance data, the program demonstrated that WIC clinics are an efficient way to screen high-risk children who would not otherwise be tested. The program ensured blood lead testing for 1,395 children, 81% of whom had never had a previous test and has expanded to include WIC clinics in other high-risk areas of the state.
Washington: The Washington State Lead Poisoning Prevention Program convened an expert panel to develop risk-based childhood lead screening recommendations for use by clinicians in the state. The Program partnered with the Refugee Health Program to screen all refugee children between 6 months and 16 years of age as part of the refugee resettlement program. Over a three-year period from 2013 to 2016, they screened 3,275 children. Fourteen percent of the refugee children screened had elevated blood lead levels and 3% with blood lead levels greater than 10 µg/dL. These children were then able to be linked to appropriate follow-up services.
Mississippi: The Mississippi Lead Poisoning Prevention and Healthy Homes Program used CDC cooperative agreement funds to partner with six communities identified as high-risk areas for lead poisoning: the City of Meridian, the City of Jackson, the City of Hattiesburg, the City of West Point, the City of Moss Point, and the City of Yazoo City. Between July 1 and December 31, 2015, city partners facilitated lead poisoning prevention and health homes trainings, planned and conducted healthy homes community planning meetings featuring focused discussion on childhood lead poisoning prevention, and distributed health education materials to residents. Through focused campaigns, the communities distributed 2,000 lead poisoning prevention educational materials featuring details about lead testing and identifying lead sources. The communities also distributed 900 lead poisoning prevention toolkits to resident families. Since 2010, Mississippi has experienced an 18% increase in children tested for lead.
Ohio: In Marion County, Ohio staff at the local health department have worked with the OHHLPPP epidemiologist to create customized maps for their area depicting the high risk census tracts. These maps are being used to create posters to display to the public at organized events.
2017 (Year 3 Supplement):
Awardee Activities:
Illinois: Over the course of three years, the CLEAR-Win project assisted in lead-safe replacement of almost 8,000 windows at 379 properties containing 466 housing units. The 466 housing units included 251 children under the age of 6, the population most vulnerable to lead poisoning. In conjunction with Illinois’ study, HUD analyzed a sample of nearly 100 homes remediated in the CLEAR-Win program, comparing lead dust sampling performed at baseline and at one year. Lead dust wipe samples were collected by HUD before CLEAR-Win commenced, immediately after (clearance sampling), and nominally one year after CLEAR-Win’s work was completed. Between baseline and 1 year post intervention, geometric mean lead dust for interior floors, interior sills, and exterior troughs declined by 44%, 88% and 98% respectively. HUD’s results of CLEAR-Win’s pilot program in Illinois show that a state health department can successfully implement a window replacement program that dramatically reduces childhood lead exposure (Jacobs et al., 2016).
ABLES Accomplishments: In the past three years, the following program accomplishments were achieved:
In 2015, NIOSH updated the NIOSH ABLES Program’s case definition for an elevated BLL among adults (16 years and older) to be a BLL ≥5 μg/dL of whole blood, in response to CSTE’s recommendation.
Additionally, the ABLES Program secured continuing participation of state ABLES Programs through mutual partnership data sharing agreements, and continued to monitor the national and state burden of lead exposure using prevalence rates of elevated BLLs.
Nationwide data and findings from the ABLES Program are periodically published in the CDC’s Morbidity and Mortality Weekly Report and elsewhere. ABLES publications and data are available from the ABLES publications and data website.4
A.3. Use of Improved Information Technology and Burden Reduction
Reporting to the NCEH CBLS System: All CBLS reporting is done by electronic means. Up to 47 NCEH respondents will upload their “CBLS Variables – Text Files” (Attachment 5a) to the NCEH secure encrypted FTP site, on a quarterly basis with a one quarter lag. Based on past FY 14 experience with a single awardee, NCEH also anticipates that one respondent will report “CBLS Aggregate Records” in spreadsheet format for the FY 17 program (Attachment 5b).
Reporting to the ABLES System: All ABLES reporting is done by electronic means. State ABLES Programs submit an electronic data file (e.g., SAS, Access, CSV, Excel) to CDC on an annual basis. In addition to a “Brief Narrative Report,” ABLES respondents submit either “ABLES Case Records Form” or “ABLES Aggregate Records Form” annually (Attachments 5c & 5d, respectively). The format for electronic ABLES Case Records are attached (Attachment 5c1). Electronic reporting must be completed by the second quarter of the subsequent calendar year. The electronic file may be submitted through secure email, secure web login, or through encrypted secure FTP sites. Eventually, all ABLES partners will submit data through secure encrypted FTP sites.
A.4. Efforts to Identify Duplication and Use of Similar Information
In February 1991, the “Strategic Plan for the Elimination of Childhood Lead Poisoning” (HHS, 1991), recommended four immediately essential elements of the effort to eliminate childhood lead poisoning in the U.S., including establishment of national surveillance for children with elevated blood lead levels. CDC’s Childhood Lead Poisoning Prevention Program (CLPPP) began collecting surveillance data on blood lead levels in children less than 16 years of age in 1995 (Pertowski, 1994). Surveillance of individual children’s blood lead levels provides information on how well we are protecting all children from exposure to lead and also provides critical information needed to identify and care for those individual children who are already exposed. Blood lead screening and surveillance data provide the foundation for targeting prevention activities to high risk areas. Also in 1995, the Council of State and Territorial Epidemiologists (CSTE) designated elevated blood lead levels as the first noninfectious condition to be notifiable at the national level. Notifiable diseases and conditions are reportable to CDC on a voluntary basis for nationwide aggregation and monitoring (CDC, 2017).
The FY14 HHLPSS collects, and the FY17 CBLS will collect laboratory and clinician-reported BLL test results on individual children reported to participating state or local CLPPPs, with the exception of one respondent reporting test results in aggregate.
ABLES is a long-standing state-based surveillance program of laboratory-reported adult blood lead levels (BLLs). The program objective is to build state capacity to initiate, expand, or improve adult blood lead surveillance programs which can accurately measure trends in adult blood lead levels and which can effectively intervene to prevent lead over-exposures. Occupational exposures to adults may affect child lead exposure in several ways including: take-home (e.g., dust or residual) exposures from parents employed in lead-related industries; pregnant and breastfeeding women who are employed in lead-related industries may pass exposure to unborn baby or breastfeeding infant; and older children employed on a part-time or full-time basis in work that may involve lead (e.g., construction, commercial fishing). Hence, by combining the data collected from these two programs, CDC will be better able to identify and intervene for both childhood and adult lead exposure.
To coordinate their reporting and intervention activities for maximum efficiency, State ABLES Programs are strongly encouraged to develop effective working relationships with CLPPPs in their states. NIOSH encourages this collaboration to assist states better manage their software and IT resources. For the first time with this ICR, NIOSH and NCEH HHLPPP are working together to incorporate adult blood lead information with childhood lead data at the national level.
Other agencies, organizations, and/or CDC programs collect, or promote the collection of, blood lead data consistently with or following CDC’s CBLS and ABLES Program recommendations. However, with the exception of NHANES, all of the following data collections provide only aggregate summaries of the individual blood lead test results that are generated by State and local health departments through their support from CBLS and ABLES. NHANES is fundamentally different from these other collections as it produces nationally-representative estimates of the distribution of blood lead levels in the U.S. population based on blood lead testing in small, stratified, randomly-selected sample of adults and children.
CDC National Notifiable Diseases Surveillance System (NNDSS): CBLS and ABLES, with input from CSTE, both have consistent case definitions for elevated BLLs among children, less than 16 years of age, and adults, aged 16 years and over (NNDSS, 2017; NIOSH, 2015a; CSTE, 2015). The NIOSH ABLES Program is working to align adult blood lead surveillance with the NNDSS (OMB Control No. 0920-0728, expiration date 01/31/2019). Currently, NIOSH ABLES AND NCEH CBLS contribute annual reporting outside of direct electronic reporting to NNDSS, which uses the National Electronic Disease Surveillance System (NEDSS) Base System (NBS) architecture (CDC, 2016).5
CDC National Health and Nutrition Examination Survey (NHANES): CDC’s NHANES collects population-based data through a series of surveys administered since the early 1960’s on stratified, random samples of non-institutionalized adults and children in the U.S. These data are used to produce nationally-representative information on the health and nutritional status of the U.S. population. Since 1976, blood lead levels have been collected on a subset of NHANES participants and these data have been instrumental in developing policies to eliminate lead from gasoline and other consumer products. Recent survey data indicate the policy has been even more effective than originally envisioned, with a decline in elevated BLLs of more than 70% since the 1970s (CDC, 2014). Additionally, NHANES is a vital part of the CDC’s lead poisoning prevention policies to define a population-based blood lead reference values in children aged 1 to 5 years, as greater than or equal to the 97.5th percentile using two consecutive cycles of NHANES (ACCLPP, 2012; CDC, 2012).
The FY14 HHLPSS and the FY17 CBLS data, collected according to state and local policies, fundamentally differ from the NHANES estimates which are based on stratified random sampling designs. To reiterate the 2015 HHLPSS Terms of Clearance: “These (CBLS) data were collected for program management purposes. The data are not generalizable at the national, state, or local level.”
NCEH Tracking Program: The Tracking ICR is titled, “Environmental Public Health Tracking Network (Tracking Network)” (OMB Control No. 0920-1175, expiration date 04/30/2020).6 Aggregate childhood BLL data are currently submitted annually to the Tracking Program from its awardees under program announcements, CDC-RFA-EH14-1403 and CDC-RFA-EH14-1405, and for FY17, under CDC-RFA-EH17-1702. The Tracking BLL reporting is in the form of annual aggregate counts for children, in age categories from 0 to <72 months, and with BLL categories ranging from <5 to ≥70 µg/dL. The Tracking Program also asks whether the childhood BLLs are confirmed or not confirmed as defined by the CSTE (CSTE, 2015), and CDC’s NNDSS (OMB Control No. 0920-0728, expiration date 01/31/2019) (NNDSS, 2017).
The FY14 HHLPSS and the FY17 CBLS data fundamentally differ from the Tracking Program annual aggregate counts because HHLPSS and CBLS involve individual-level data (that is de-identified) used for program management. The Tracking Program emphasizes web-based dissemination of a large number of various environmental health indicators on environmental hazards, exposures, and health outcomes in aggregate form. There is some duplication of effort with CBLS since there are some states that participate in both CDC Programs; however, the duplication is minimal as the data required by the Tracking Program is readily available among CLPPP awardees. Thus, the Tracking annual aggregates come from Tracking awardees and volunteer programs, which are not HHLPPP awardees as well as Tracking awardees who are also CBLS awardees.
Centers for Medicare & Medicaid Services (CMS) 7,8,9: All children enrolled in Medicaid are required to receive blood lead screening tests at ages 12 months and 24 months. In addition, any child between ages 24 and 72 months with no record of a previous blood lead screening test must receive one. Completion of a risk assessment questionnaire does not meet the Medicaid requirement. The Medicaid requirement is met only when the two blood lead screening tests identified above (or a catch-up blood lead screening test) are conducted. These test results are captured by State-based CLPPPs, where these programs exist and as blood lead reporting laws require.
In addition, State Medicaid agencies are required to submit Early Periodic Screening, Diagnosis, and Treatment (EPSDT) data annually using the Form CMS-416, line 14, total number of screening blood lead tests (Annual EPSDT Participation Report; OMB Control No. 0938-0354; expiration date 06/30/2020), including the aggregate number of blood lead screening tests for children enrolled in Medicaid, from birth to age 6 years. The number of blood lead screenings provided is calculated using CPT code 83655 (“lead”) for a blood lead test. Follow-up blood tests performed on individuals who have been diagnosed with or are being treated for lead poisoning should not be counted. Alternatively, States may opt to include data collected from use of the HEDIS® measure developed by the National Committee for Quality Assurance to report blood lead screenings if their state has elected to use this performance measure (CMS, 2017).
The FY14 HHLPSS and the FY17 CBLS data fundamentally differ from the CMS data in that CBLS data is more complete since it does not restrict test results to those on children of a certain age or socioeconomic status. In addition, as stated previously, CBLS data represent individual-child data that is de-identified prior to submission as opposed to aggregate counts of tests or children tested.
CDC Reference Level Adoption
The following agencies and organizations have adopted the CDC recommended reference value for BLLs for children and for adults (currently ≥5 µg/dL) (CDC, 2012; NIOSH, 2015a):
American Academy of Pediatrics (AAP, 2016)
American College of Obstetricians and Gynecologists (ACOG, 2012)
Centers for Medicaid and Medicare Services (CMS, 2016)
U.S. Department of Housing and Urban Development (HUD, 2017)10
A.5. Impact on Small Businesses or Other Small Entities
Both NCEH and NIOSH are committed to collect only the minimum data necessary to carry out the goals for CBLS and ABLES.
The HHLPPP will only collect childhood BLLs directly from State and local health departments or their Bona Fide agents, not small businesses.
The ABLES Program will only collect adult BLLs directly from state departments of health, not small businesses.
A.6. Consequences of Collecting the Information Less Frequently
There are no technical or legal obstacles to reducing burden.
NCEH respondents will submit their data quarterly to CBLS as program requirements.
NIOSH respondents are requested to submit their ABLES data once per year through data sharing agreements.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances related to this information collection. This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day Federal Register Notice was published in the Federal Register on April 6, 2017, Vol. 82, No. 65, pp. 16839-41 (Attachment 2). CDC did not receive public comments related to this notice.
Within the past three years, the following consultations and meetings took place, among others not listed here.
2014-2017:
Biannual NCEH/ATSDR Board of Scientific Counselors
Jan 2017 – BSC Special Guest
Warren Friedman, PhD, CIH, SAIHA
Senior Advisor to the Director
HUD Office of Lead Hazard Control and Healthy Homes
Presented HUD’s 2017 final rule revising the agency’s criteria under the Lead Safe Housing Rule (LSHR) for the identification of children, under age six with elevated BLLs, in alignment with 2012 CDC reference value for elevated BLLs.
BSC Lead Poisoning Prevention Subcommittee, beginning March 2015
Annual Partners’ Meetings
Oct 2015 Meeting Regarding CSTE Position Statement on Elevated BLLs
The CSELS Surveillance Data Branch’s Surveillance Operations Team hosted a meeting with representatives from NIOSH and NCEH about CSTE position statement “15-EH-01: Public Health Reporting and National Notification for Elevated Blood Lead Levels.” NCEH approved the elevated childhood lead case definition; the group was awaiting final approval from NIOSH regarding the elevated adult blood lead surveillance case definition (CSTE, 2015).
NIOSH approval was obtained November 2015, with a confirmed venous BLL for adults (CDC, 2016).
Table A.8.1. NCEH/ATSDR Board of Scientific Counselors (as of 01/30/2017) |
|
CHAIR Melissa J. Perry
ScD, MHS |
DESIGNATED FEDERAL OFFICER William Cibulas,
PhD, MS |
MEMBERS |
|
Kenneth Aldous,
PhD |
Aaron Bernstein, MD
MPH |
Darryl Brown, PhD
MPA |
Suzanne Condon,
MSM |
Deborah A.
Cory-Slechta, Ph.D. |
Kim N. Dietrich,
Ph.D. |
Roberta Grant,
PhD |
Sharron E. LaFollette,
PhD |
Joyce M. Martin, MA
JD |
Ralph McCullers,
MPA |
John Meeker, ScD,
CIH |
Devon Payne-Sturges,
DrPH MPH |
Matthew J Strickland,
PhD MPH |
Phillip L. Williams,
PhD |
Nsedu Obot Witherspoon,
MPH |
|
FEDERAL EXPERTS |
|
Wayne E. Cascio, MD,
FACC, FAHA |
Bonnie S. Richter, MPH,
PhD |
Kristina Thayer,
PhD |
Douglas Trout,
MD |
Table A.8.2. NCEH/ATSDR BSC Lead Poisoning Prevention Subcommittee (as of 01/30/2017) https://www.atsdr.cdc.gov/science/lpp/lppmembership.html |
|
CHAIR Matthew Strickland,
BA, MA, MPH, PhD. |
DESIGNATED FEDERAL OFFICER William Cibulas,
PhD, MS, CAPT |
MEMBERS |
|
John G. Belt, MS |
Elizabeth A.
Colón |
Kim N. Dietrich,
Ph.D. |
Nathan M. Graber, MD,
MPH, FAAP |
Michael J. Kosnett, MD,
MPH |
Jennifer A. Lowry,
MD |
Patrick J. Parsons,
PhD, Chem., FRSC |
|
FEDERAL EXPERTS |
|
Mark A. Maddaloni, MS,
DrPH |
|
A.9. Explanation of Any Payment or Gift to Respondents
Respondents that submit data to CBLS or to ABLES are not provided payment for their data.
CBLS: Respondents will not receive payments or gifts for providing information. Data submission is required by NCEH under cooperative agreement (Attachment 3a).
ABLES: Respondents will not receive payments or gifts for providing information. Data submission to the NIOSH ABLES Program is completed through data sharing agreements on a voluntary basis.
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
Overview of the CBLS Data Collection System
The NCEH/ATSDR PRA Contact has reviewed this submission and has determined that the Privacy Act does apply. The applicable Privacy Act System of Records Notice is SORN 09-20-0136 “Epidemiologic Studies and Surveillance of Disease Problems” (retrievable by name and ID number).11 On October 4, 2017, the NCEH/ATSDR Information Systems Security Officer (ISSO) conducted an annual review of the CBLS and has determined that a new CDC Security Assessment and Authorizations (SA&A) is required. The FY17 CBLS data collection will not begin until the Authority to Operate (ATO) is granted (Attachment 6a).
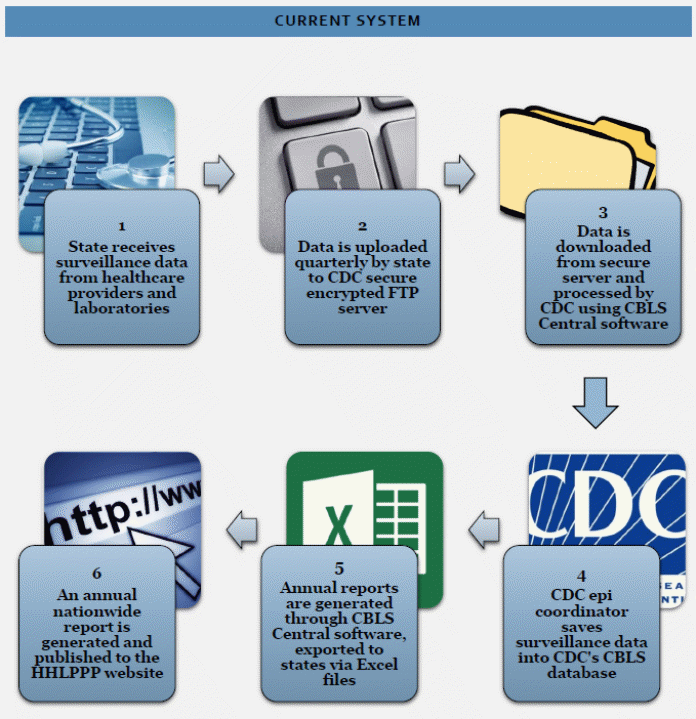
Figure 1. Overview of the Current Childhood Blood Lead Surveillance System
Figure 1 provides a graphical overview of the current CBLS data collection system.
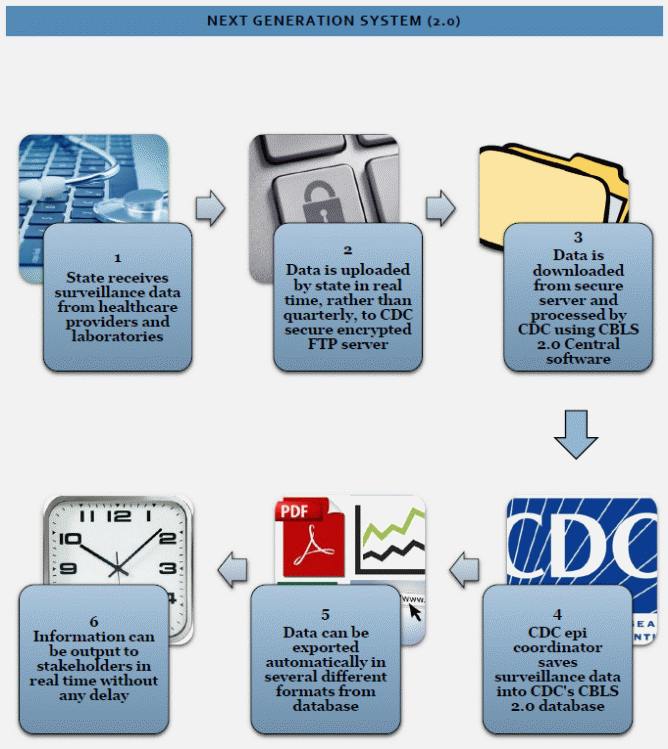
Figure 2. Overview of the Next Generation Childhood Blood Lead Surveillance System
The next generation CBLS will be a web-based system which is anticipated to go into production in Fall 2017 (Figure 2).
Reporting within Jurisdictions
The State or local health departments receive data from health care providers, laboratories, hospitals, or other facilities that analyze blood samples for lead and store those data on servers housed on their premises. Reporting is done through a variety of modes following the jurisdiction’s system design: 1) Health Level 7 (HL7) data format via secure, encrypted transfer; 2) Excel sheets via secure, encrypted FTP; or 3) secure delivery of paper records, such as via secure fax. The health departments are responsible for following all local or state personal privacy protection laws and state IT security protocols and processes, such as security options to enter data into password-protected Microsoft SQL databases.
This reporting from providers and laboratories to State or local CLPPPs is excluded from PRA burden under 5 CFR §1320.3(b)(2) and (b)(3),12 because public health law, statutes, or regulations require blood lead testing and reporting within state or local jurisdictions (NCSL, 2010). The reporting, recordkeeping, or disclosure activities needed to comply within jurisdictions are usual and customary, and would be required by law even in the absence of the federal requirement.
Required Quarterly Reporting to CBLS
As part of the FY14 and FY17 FOAs, the awardees are required to submit quarterly data to CDC by the final business day of the following quarter (e.g. data collected during the first quarter, is due on the final business day of the second quarter). All required data are extracted from their secure servers and transmitted to CDC via a secure FTP site. Data submitted in text files to CDC are processed and maintained in the CBLS database.
Attachment 5a (“CBLS Variables – Text Files”) provides a comprehensive list of all lead poisoning surveillance variables that will be submitted to CBLS as ASCII fixed field length (non-delimited) variable records. NCEH provides awardees with a protocol for quarterly data submission. The data are delivered in separate text files following format requirements that are processed into linkable tables using CBLS Central software. The Basic Format Table (Table 1) denotes the content for positions 1-12 that is included in each of the remaining tables: 2) Address Table; 3) Child Table (one record per patient); 4) Environmental Investigation Table (not systematically reported by all CLPPPs, due to differences in program and system design across jurisdictions); 5) Blood Lead Test Result Table (can have more than one record per patient with multiple tests); and 6) Child-to-Address Linking Table (optional). Each table has one or more key variables that can be used to join/merge the data between multiple tables. Three important linking variables are: 1) Program ID – identifies the awardee jurisdiction; 2) Child ID; and 3) Address ID, which may be used to create a unique ID per patient or per address. Attachment 5a provides the required data format for CBLS reporting.
Table A.10.1. Sample of Quarterly Text File Information Received by Table |
|||||
|
Positions 1-20 Table Links and All Else in Positions 21-109 |
||||
Basic Format
|
(Table 1) Child (File ID - CHI) |
(Table 2) Address (File ID – ADD) |
(Table 3) Lab Results (File ID – LAB) |
(Table 4) Investigation (File ID - INV) |
(Table 5) Child-to-Address Link (optional) (File ID – LNK) |
Positions 1-12* File ID* Reporting Quarter* Reporting Year* Program ID*
Positions 13-20† Child ID* or Address ID*
|
Positions 1-12* Child ID* Date of Birth* Sex* Ethnicity* Race* Chelated* Chelation Type* Funding Source* Lead source indicators* |
Positions 1-12* Address ID* City County FIPS Code* Zip Code State Census Tract History of Residential Renovation* |
Positions 1-12* Child ID* Address ID 21-28 Provider Type* Funding Source* Medicaid* (Y/N) Sample Date* Sample Analysis Date Sample Type* (capillary or venous) Test Result* (whole number) Reason for Test* Laboratory Type* (public health lab, commercial lab) Patient Age, in months Date Result Reported |
Positions 1-12* Address ID* Inspection Referral Date* Abatement Date Year Housing Built Ownership* Dwelling Type* Presence of Paint Hazards* XRF Paint Measures* Soil Lead Water Lead Industrial Hazard Nearby* |
Positions 1-12* Child ID* Address ID* Address Type* Date First Occupied* Date Last Occupied
|
* indicates required reporting. See Attachment 5a for complete list of reporting variables into CBLS. † Positions 13-20 are reserved for either the required Child ID or the Address ID linking variables depending on the type of record being submitted. |
|||||
In FY14, a single awardee reported annual summary “CBLS Aggregate Records” in spreadsheet format. Therefore, it is estimated that one awardee will report in aggregate again for FY 17. Reported quarterly summaries are: number of children tested, number and percentage of confirmed BLL ≥10 µg/dL, and number of BLLs by BLL categories, in µg/dL (5-9, 10-14, 15-19, 20-24, 25-44, 45-69, 70+, ≥5) (Attachment 5b).
Items of CBLS information to be collected: For the regular quarterly CBLS data submissions, CDC staff will receive electronic files with the following information in identifiable, or partially identifiable, form (IIF). All children and adults are assigned a unique identifier specific to their blood lead test records by the State or local program:
❑ Date of Birth
❑ Unique Address Identifier
❑ Medical Information and Notes (blood lead test results only)
❑ Laboratory Name
NCEH does not collect the following items of CBLS information:
❑ Patient Name
❑ Mailing Address
❑ Medical Record Numbers
❑ Financial Account Information
Optional Childhood Data Delivered to CDC
In special circumstances when State or local programs request technical assistance from CDC, or CDC makes a data request for its own sponsored projects, CDC will receive data that may include additional IIF that may be linked to the CBLS records delivered in the quarterly reports. In those situations, data will be transferred to CDC via secure FTP in the same manner as the quarterly data submissions. Data will be maintained on secure CDC servers.
Each request outside of the CBLS quarterly collection will undergo a separate research and PRA determination and is not covered by this ICR. However, the ability to receive IIF will be approved when the CDC Security Assessment and Authorization (SA&A) for CBLS is completed. The CBLS data collection will not begin until the Authority to Operate (ATO) is granted. Social security numbers are not provided to nor requested by CDC.
Items of optional childhood information to be collected:
❑ Name
❑ Mailing Address
❑ Phone Numbers
How CBLS information will be shared and for what purpose: Funded awardee programs will be informed with whom information will be shared (i.e., to HUD and/or EPA for enforcement of the Federal Lead Disclosure Rule Section 1018 of Title X13 and the Lead-Safe Housing Rule (24 CFR Part 35) 14), and the legal authority for the data collection (i.e., through the Public Health Service Act, the Affordable Care Act, and the WIIN Act – Attachment 1a).
Impact the proposed CBLS collection will have on respondent privacy: If there is a security breach for the data stored in CBLS at CDC, some effect on the respondent’s privacy could occur; however, to minimize this risk, there are a variety of safeguards in place as described in the applicable Privacy Act System of Records Notice (SORN 09-20-0136 “Epidemiologic Studies and Surveillance of Disease Problems”).15
Whether individuals are informed that providing the information for CBLS is voluntary or mandatory: Blood lead testing is not mandatory for all children (NCSL, 2010). Blood lead testing is mandatory for Medicaid enrolled children and varies by state for non-Medicaid enrolled children, typically by a determination of risk of exposure. Reporting blood lead test results to state agencies is guided by state regulations and is mandatory. However, providing information about the reporting mandate in clinical affairs is not uniform.
Opportunities to consent, if any, to sharing and submission of CBLS information: No consent form for the CBLS collection is required as the data are part of State or local surveillance efforts, under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) section on disclosures for public health activities.16 Consent to share with other federal agencies is not required when it involves enforcement of the Federal Lead Disclosure Rule Section 1018 of Title X and Lead-Safe Housing Rule (24 CFR Part 35).
How CBLS information will be secured: All collected data is secured in a password protected surveillance system. Only CDC staff will have access to the raw data in CBLS. Data from State or local programs are sent electronically to CDC via a secure FTP site. Physical controls will also be implemented. Data will be stored on highly-secured CDC servers in Atlanta, GA. Access to all CDC campuses is restricted by armed guards. The servers are housed in a secure computer room complete with climate control, emergency power, and an uninterruptible power supply (UPS). Daily back-ups and integrated security are implemented through the CDC computer services infrastructure. All data access is password-protected, and all network communications use encryption. All servers and PCs that are part of the CDC infrastructure are protected by both host-based firewalls and software in order to prevent the undetected installation of "spyware."
Overview of the ABLES Data Collection System
The NIOSH ISSO has reviewed this submission and has determined that the Privacy Act does apply. The applicable Privacy Act System of Records Notice is SORN 09-20-0147 “Occupational Health Epidemiological Studies and EEOICPA Program Records and WTC Health Program Records, HHS/CDC/NIOSH” (retrievable by name, assigned identification number, or social security number).17 The NIOSH ISSO has also determined that a PIA is required (Attachment 6b).
Adult blood lead data are submitted to NIOSH on an annual basis and maintained and analyzed by NIOSH staff. Attachment 5c provides a comprehensive list of individual “ABLES Case Records” that will be collected by NIOSH from about 80 percent of its respondents (n=32 of 40). Attachment 5d provides the aggregate fields anticipated to be reported to NIOSH from approximately 20 percent of its respondents (n=8 of 40). These two reporting options are offered by NIOSH due to differences in program capacity in the states.
State ABLES Collection and Reporting
For states to be eligible to participate in the ABLES Program, they must have mandatory state regulations requiring the reporting of adult BLLs by both public and private laboratories. State regulations require that the adult BLLs be reported to the state health department or another state health agency or department designated by the state to direct and coordinate the state’s adult lead poisoning surveillance program. This is the only entity in each state which can supply to CDC the data required for the ABLES Program.
State ABLES Programs 1) collect data on adult BLLs from laboratories and physicians through mandatory reporting; 2) assign unique identifiers to each adult to account for multiple BLL records per person, protect individual privacy, and permit longitudinal analyses; 3) follow-up on adults with BLLs ≥10 or ≥25 μg/dL18 via laboratories, health care providers, employers, or workers to ensure completeness of information (e.g., the industry in which the adult is employed and whether the exposure source is occupational, non-occupational, or both); 4) provide guidance and information to workers and employers to prevent lead exposures; and 5) submit individual or aggregated data annually to NIOSH.
State ABLES Programs submit an electronic data file each year (e.g., Excel spreadsheet) by the first or second quarter of the succeeding calendar year. States may submit data in two different data collection formats: 1) individual data records for each case, and 2) aggregated data in which only the final counts are provided (Attachments 5c & 5d). The data file includes:
1) the data in the prescribed format; and 2) a brief narrative report describing any notable lead surveillance activities during the year. The electronic file may be submitted through secure email, secure web login, or through encrypted secure FTP sites.
NIOSH ABLES Data Processing and Release
NIOSH consolidates data from reporting state ABLES Programs, conducts data quality control, analyzes the data, and disseminates the findings among stakeholders.
The report is derived from state tracking databases, which consist of test results submitted, by laboratories and related follow-up data on adults with elevated BLLs.
Items of ABLES information to be collected: All adults are assigned a unique identifier specific to their blood lead test records by state ABLES Programs. For ABLES, date of birth is included in the data files transmitted electronically to CDC. The date of birth is used to standardize the calculation of age because CDC has found that many state and local health departments vary in the format that they use for age (e.g., rounding errors up or down). CDC requires date of birth because the age will dictate which state/local program will complete follow-up of the individual (i.e. adult versus child). The age can also assist in identifying duplicate entries for a given ID. In addition, if a woman is of childbearing age, this could help state/local programs to target focused case management. Please see Attachment 5c for a full list of variables.
❑ State-assigned unique ID number for adult
❑ Date of Birth
❑ Sex
❑ Race
The ABLES data variables include other demographic information, such as occupation, and laboratory information, such as date of specimen collection, and BLL test result. Attachment 5d does not include identifiers, as data will be reported in aggregated counts.
How ABLES information will be shared and for what purpose: All ABLES information will be maintained by NIOSH. Upon request, ABLES information may be released to the State ABLES Program that originally provided the data or to other parties outside of NIOSH to the extent NIOSH is permitted to do so by law. All information not designated by State ABLES Programs as trade secret may be subject to automatic public disclosure under the Freedom of Information Act (FOIA). Additionally, it is understood that one of the purposes of the work performed by NIOSH is to obtain information that may be made available by NIOSH to industry and public through publication.
Impact the proposed ABLES collection will have on respondent privacy: If there were a security breach for the data stored in NIOSH/ABLES at CDC, some effect on the respondent’s privacy could occur; however, to minimize this risk, there are a variety of safeguards in place as described in the applicable Privacy Act System of Records Notices (SORN 09-20-0147 “Occupational Health Epidemiological Studies and EEOICPA Program Records”).
Whether individuals are informed that providing the information for ABLES is voluntary or mandatory: For states to be eligible to participate in the ABLES Program, they must have mandatory state regulations requiring the reporting of adult BLLs by both public and private laboratories. State regulations require that the adult BLLs be reported to the state health department or another state health agency or department designated by the state to direct and coordinate the state’s adult lead poisoning surveillance program. This is the only entity in each state which can supply to CDC the data required for the ABLES Program.
State ABLES Programs submit data to the ABLES Program on a voluntary basis. To encourage submission, NIOSH ABLES develops effective working relationships with State ABLES Programs by providing technical assistance and guidance in adult blood lead surveillance, prevention, and intervention.
Opportunities to consent, if any, to sharing and submission of ABLES information: Adult BLLs are collected based upon requirements by State or local entities. Under data sharing agreements, these entities share adult BLLs with ABLES for occupational surveillance. As a non-research activity, consent is not necessary. See Section A.11 for further discussion.
How ABLES information will be secured: Data received by NIOSH/ABLES are stored on password-protected servers inside CDC’s firewall and can only be accessed through encrypted CDC-issued laptops/computers or desktops. Additionally, only individuals who are part of the ABLES Program in NIOSH will have access to the ABLES secure drive.
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions
The NCEH/ATSDR Human Subjects Contact has reviewed the new FY17 three-year cooperative agreement (FOA No. CDC-RFA-EH17-1701-PPHF17), and has determined that this program is non-research and that review and approval by the CDC Institutional Review Board (IRB) is not required (Attachment 7a).
The NIOSH Human Subjects Contact has determined that the ABLES collection is non-research and that review and approval by the NIOSH IRB is not required (Attachment 7b).
The purpose of the NCEH and NIOSH activities is to identify and control a health problem, specifically lead exposures that may lead to adverse health outcomes for children and for adults.
Intended benefits of the projects are primarily or exclusively for the children or adults at risk for lead exposure.
The data collected are needed for State or local health departments to identify children or adults in need of referral for medical monitoring or management.
The knowledge that is generated does not extend beyond the scope of the activities; and project activities are not experimental.
CBLS Sensitive Questions: Questions that could be considered sensitive by at least a segment of the population such as information on pregnancy and race/ethnicity. These variables are integral to accomplishing the purpose of this surveillance system. Table A.11.1 describes the specific use of the possibly sensitive questions.
Table A.11.1. CBLS Sensitive Questions
Questions (possibly sensitive) |
Specific uses of information |
Pregnant at time of test? (at time of blood lead test) |
To assess prevalence of pregnant women with elevated blood lead, this provides important data for clinical follow up of women and their fetuses. |
Race/ethnicity? |
For targeting resources to subpopulations with high risk for elevated blood lead or housing risk factors |
ABLES Sensitive Questions: The only potentially sensitive information that ABLES collects are race and ethnicity. These variables are collected to identify subgroups that may have higher burden of occupational lead exposure.
A.12. Estimates of Annualized Burden Hours and Costs
The total time burden requested by the CDC is 1,040 hours.
CBLS Variables: NCEH respondents will be 48 cooperative agreement awardees from State or local health departments, or their bona fide agents, who have received funds to develop and implement a CLPPP. This new FY17 program will have an increase of 8 respondents over the 40 previously approved for the FY14 program (HHLPSS ICR, OMB Control No. 0920-0931, expiration date 05/31/2018).
The annual time burden requested by NCEH is 760 hours. It is estimated that 47 respondents will submit quarterly text files of case records (752 burden hours) and one respondent will submit aggregate records (8 burden hours).
ABLES Case Records: In-state principal investigators and laboratories routinely report adult BLL data to ABLES Programs according to their own state and local codes. Over the next three years, up to 40 participating state ABLES Programs will submit adult BLL data to NIOSH. Currently, 28 programs are reporting ABLES data; however, NIOSH aims to establish data sharing agreements with up to 12 additional programs over the next three years. On an annual basis, in addition to a brief narrative report of annual lead surveillance activities, ABLES respondents submit either case records or aggregate counts of annual adult blood lead test results. NIOSH estimates that 80 percent of their respondents (n=32) will spend 256 burden hours submitting case records and 20 percent (n=8) will spend 24 burden hours submitting aggregate records. The annual time burden requested by NIOSH is 280 hours.
Once the ABLES hours are approved in this new FY17 ICR, CDC will submit a revision request to remove the ABLES description from the HHLPSS ICR (OMB Control No. 0920-0931, expiration date 05/31/2018). Although a description of the ABLES collection was previously included, burden hours for ABLES were not requested or approved in PRA clearance for the 2015 HHLPSS extension ICR.
Table A.12.1. Estimated Annualized Burden Hours
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response |
Total |
State or Local Health Departments, or their Bona Fide Agents |
CBLS Variables – Text Files |
47 |
4 |
4 |
752 |
State or Local Health Departments, or their Bona Fide Agents |
CBLS Aggregate Records Form |
1 |
4 |
2 |
8 |
State or Local Health Departments, or their Bona Fide Agents |
ABLES Case Records Form and Brief Narrative Report |
32 |
1 |
8 |
256 |
State or Local Health Departments, or their Bona Fide Agents |
ABLES Aggregate Records Form and Brief Narrative Report |
8 |
1 |
3 |
24 |
Total |
1,040 |
||||
The annualized burden costs are $39,925.60.
The hourly wage for respondents is estimated to be $38.39 per hour. This is based on the May 2016 median hourly rate of pay for a computer programmer from the Bureau of Labor Statistics (see http://www.bls.gov/oes/current/oes151131.htm).
15-1131 Computer
Programmers -
Create,
modify, and test the code, forms, and script that allow computer
applications to run. Work from specifications drawn up by software
developers or other individuals. May assist software developers by
analyzing user needs and designing software solutions. May develop
and write computer programs to store, locate, and retrieve specific
documents, data, and information.
Table A.12.2. Estimated Annualized Burden Costs
Type of Respondents |
Form Name |
No. of Respondents |
Total |
Hourly Wage Rate |
Total Respondent Costs |
State or Local Health Departments, or their Bona Fide Agents |
CBLS Variables – Text Files |
47 |
752 |
$38.39 |
$28,869.28 |
State or Local Health Departments, or their Bona Fide Agents |
CBLS Aggregate Records Form |
1 |
8 |
$38.39 |
$307.12 |
State or Local Health Departments, or their Bona Fide Agents |
ABLES Case Records Form and Brief Narrative Report |
32 |
256 |
$38.39 |
$9,827.84 |
State or Local Health Departments, or their Bona Fide Agents |
ABLES Aggregate Records Form and Brief Narrative Report |
8 |
24 |
$38.39 |
$921.36 |
Total |
$39,925.60 |
||||
A.13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
CBLS: The estimate of the total annual cost burden to respondents or record keepers is based on the optional implementation of the NCEH HHLPSS software in State or local programs. The base software is provided at no cost to programs, and NCEH provides technical support for implementation at no cost:
Total capital and start-up cost component – approximately $40,000 for computer hardware and software. Many of the state or local programs (e.g., health departments) already have existing equipment that can be used.
Total operation and maintenance and purchase of services – maintenance of HHLPSS is approximately $5,000 per year. However, many of the state or local programs (e.g., health departments) already have existing computer servicing and software contracts in place and these can be used for HHLPPSS.
ABLES: There is no cost burden to respondents or record keepers for capital and start-up, operation, and maintenance per year.
A.14. Annualized Cost to the Federal Government
The combined annualized cost to the federal government for both CBLS and for ABLES is $7,780,833. The cost for each program is outlined below.
CBLS: The annualized estimated cost to the federal government is $7,730,833, and is based on the following:
The annual FY17 cooperative agreement program budget for surveillance activities is estimated to be $5,833,333.
This cooperative agreement cost is based on the three-year FY17 program budget of $35,000,000 (Attachment 3a).
One-half of the program budget is estimated to support program management and one half of the program budget is estimated to support surveillance activities, or $17,500,000 per program area for three years.
Therefore, the cost of surveillance activities is estimated to be $5,833,333 per year.
The annual federal personnel salary cost for surveillance activities is $510,000.
This salary estimate is based on a total annual cost of $1,275,000, based on the following positions: Program Chief, Deputy Program Chief, 6 Project Officers, 1 IT Specialist, 2 Epidemiologists, 1 Communications Specialist.19
Overall, 60 percent of NCEH personnel time is dedicated to program management ($765,000) and 40 percent of NCEH personnel time is dedicated to surveillance activities ($510,000).
Annual travel cost is $50,000 based on Project Officer site visits for 48 awardees, training, and meeting attendance.
Annual contract cost is $1,337,500, based on the following: IT Support, Data Management, Health Communication, and Training Contracts.
ABLES: The annualized estimated cost to the federal government is $50,000, and is based on the following:
NIOSH staff working in the ABLES Program includes four part-time subject matter experts that total 0.5 FTE. Their duties are management and oversight of the multi-state surveillance system (ABLES), including data management, data analysis, publication of findings, responding to public requests and providing technical assistance to state ABLES Programs. These employees spend approximately 1,044 hours per year working on the surveillance program. Using an estimated salary of $45 per hour, personnel costs will total $47,000 annually. One ABLES staff person also attends the annual national CSTE conference, and makes occasional trips to attend other meetings with ABLES stakeholders. States are providing data to the ABLES Program on a voluntary basis and no direct funding was provided by NIOSH to states in support of the ABLES Program.
-
Salaries
$47,000.00
Travel (Budget Estimate)
$3,000.00
Total Cost
$50,000.00
A.15. Explanation for Program Changes or Adjustments
Although this is a new ICR, NCEH is requesting approval for the following differences because of the overlap between concurrent Year 4 FY14 and the new FY17 program collections (Attachment 3a & 3b). The 4-year FY14 program will end at the end of Year 1 of the FY17 program:
Clarification of data delivery processes from awardee data management systems into the NCEH Childhood Blood Lead Surveillance (CBLS) system during the FY17 program. A more detailed discussion is found in Section A.10:
State and Local Systems: NCEH provides technical support but does not provide funding for system development. To promote standardization, NCEH provides basic HHLPSS software at no cost to state or local programs to manage their lead surveillance and other data such as for healthy homes programs. Thus, HHLPSS-adopted or equivalent systems are owned by partners. Their systems are customized for jurisdiction-specific program needs, and are subject to state or local legal codes and IT security requirements. Thus, data residing within these data systems and owned by the awardee programs are more accurately referred to as “HHLPSS Variables” because states may use their systems to collect healthy homes data in addition to lead surveillance data, The NCEH collection will continued to be called “HHLPSS Variables” for the duration of the FY14 program and the associated HHLPSS ICR (OMB Control No. 0920-0931, expiration date 05/31/2018).
CDC System: Partners are required to deliver a subset of their program data to the NCEH system. Specifically, the required quarterly reports include federally sponsored data on childhood blood lead screening. These data delivered to NCEH are more accurately referred to as “CBLS Variables – Text Files” or “CBLS Aggregate Records” for the FY17 program (Attachments 5a & 5b).
Beginning with the FY17 program, the form name in text format will be called “CBLS Variables” in the burden table and in operation to more accurately describe the collection that NCEH is sponsoring. The form is also revised to remove variables that reside only in partner systems and are not delivered to NCEH in CBLS.
In 2008-2010, the HHLPPP met with state and local healthy homes programs and asked them to identify those data elements related to healthy homes that they expected to collect under an expanded healthy homes program. As noted in the NCEH program milestones (Attachment 4), healthy homes information collections were never sponsored via cooperative agreement. Therefore, healthy homes variables will not be included in the revised “CBLS Variables – Text Files” (Attachment 5a) or in the “CBLS Aggregate Records” (Attachment 5b).
As described above, HHLPSS data may reside in respondent systems, but NCEH is currently collecting only “CBLS Variables – Text Files” or “CBLS Aggregate Records.” If healthy homes activities are approved in future program expansion, NCEH will submit an ICR for PRA clearance for the implementation of standardized healthy homes collections by the federal government.
Increased number of NCEH awardee respondents from 40 to 48, based on available funds.
Increased NCEH annual time burden from 640 to 760 hours (net increase of 120 hours), based on the increase in the number of respondents and adjustment of estimated time burden per quarterly response.
CDC is also taking this opportunity to request a title to more clearly show that the NIOSH ABLES Program is approved under this ICR. NIOSH is requesting the following revisions:
Provision of detailed descriptions previously omitted about the authority and scope of the ABLES information reporting procedures in the HHLPSS ICR (OMB Control No. 0920-0931, expiration date 05/31/2018), and including a description of the “ABLES Case Records Form” and the “ABLES Aggregate Records Form” (Attachments 5c & 5d).
Addition of 40 NIOSH respondents to the burden table, which were omitted from the previous HHLPSS ICRs in 2012 and 2015.
Addition of 280 hours for the NIOSH annual time burden, to correct for the previous omission.
Once approved in this new ICR, CDC will submit a change request to remove the discussion of ABLES from the existing HHLPSS ICR (OMB Control No. 0920-0931, expiration date 05/31/2018) to avoid duplication in PRA clearance.
In total, CDC is requesting approval for a total time burden of 1,040 hours (net increase of 400 hours) over the HHLPSS ICR (OMB Control No. 0920-0931, expiration date 05/31/2018).
A.16. Plans for Tabulation and Publication and Project Time Schedule
CBLS Project Timeline (Recurring timeline for 3 years)
CBLS Project Time Schedule |
|
Activity |
Time Schedule |
States deliver FY17 Q1 (Oct-Dec, 2017) data |
By end of FY17 Q2 (March 31, 2018) |
Q1 CBLS Data Cleaning and Quality Control Processing Reports sent to States |
FY17 Q3 (April-June, 2018) |
States deliver FY17 Q2 (Jan-Mar, 2018) data |
By end of FY17 Q3 (June 30, 2018) |
Q2 CBLS Data Cleaning and Quality Control Processing Reports sent to States |
FY17 Q4 (July-September, 2018) |
States deliver FY17 Q3 (Apr-Jun, 2018) data |
By end of FY17 Q4 (September 30, 2018) |
Q3 CBLS Data Cleaning and Quality Control Processing Reports sent to States |
FY18 Q1 (October-December, 2018) |
States deliver FY17 Q4 (Jul-Sep, 2018) data |
By end of FY18 Q1 (December 31, 2018) |
Q4 CBLS Data Cleaning and Quality Control Processing Reports sent to States |
FY18 Q2 (January-March, 2019) |
Annual Calendar Year 2017 Reports sent to States when States deliver FY18 Q1 (Oct-Dec, 2017) data. |
By end of FY18 Q2 (January-March, 2019) |
Post Annual Data on Web and/or Publish Annual Calendar Year Report |
By end of FY18 Q3 (April-June, 2019) |
CBLS Data Delivery and Processing Report Dissemination: The awardees are required to submit quarterly data to NCEH by the final business day of the following quarter (e.g. data collected during the first quarter, is due on the final business day of the second quarter). Data submitted in text files to NCEH are processed and maintained in the CBLS database. NCEH uses its processing software, CBLS Central, to perform data checks on awardee text files for required formatting. Text files are parsed into separate linkable data tables (e.g., Address, Child, Lab Results, and Investigation) (Attachment 5a). Processing reports are generated and sent to awardees, to indicate how many records were properly parsed and entered into the CBLS database and how many records were not loaded with an explanation of the rejection. Corrections from awardees are returned in the next quarterly report. Therefore, NCEH has a 1 to 2 quarter lag with on-time data delivery. CBLS Annual Reports are based on the calendar year and are sent to awardees at the end of the second quarter of the fiscal year.
CBLS Publications and Results Dissemination: CDC NCEH will share the de-identified data and/or results of the surveillance with interested parties through its website, publications, and peer-reviewed manuscripts. Public release of the Annual Calendar Year Data on the internet occurs in the middle of the next year and only with the authorization from awardees.20
Descriptive statistics (e.g., means and ranges for BLLs for children, aged less than 6 years) will be used to characterize the collected data. The descriptive statistics can be used to evaluate program progress in meeting stated goals, or identify subpopulations or small geographic areas where risk for specific housing conditions like older homes with lead-based paint is high. If identified, this information may be used to target resources to these areas. CBLS data will be reported in aggregate only and without IIF.
According to the 2015 Terms of Clearance, the limitations as well as the strengths of CBLS data must be described in each of the venues including that CBLS is not derived from a population-based representative sample.
“These data were collected for program management purposes. The data are not generalizable at the national, state, or local level. Furthermore, because inclusion criteria vary across grantees, comparisons of aggregate statistics across programs can be misleading (i.e., state policies and practices for blood lead testing vary and local priorities drive decisions regarding which homes receive assessments for other housing hazards). However, descriptive statistics can be used to compare changes overtime in a given area when the method by which housing units are chosen for inclusion remains the same. With a thoughtful understanding of the approach used to include housing units in a given location, HHLPPS can be used to make associations between the number of individuals in a given area and a specific housing hazard or health condition and geographic descriptors such as poverty, age of housing, tenancy, and health conditions.”
ABLES Project Timeline (Recurring timeline for 3 years)
ABLES Project Time Schedule |
|
Activity |
Time Schedule |
Request 2015 – 2016 data from States |
February – March 2017 |
Receive 2015 – 2016 data from States |
By June 30, 2017 |
Data Cleaning and Data Quality Control |
March – July 2017 |
Work on Report based on 2015 ABLES data |
July – August 2017 |
Post and/or Publish Report |
August – December 2017 |
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is appropriate.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
(AAP) American Academy of Pediatrics Council on Environmental Health. Prevention of Childhood Lead Toxicity. Pediatrics. 2016;138(1):e20161493
(ACCLPP) Advisory Committee on Childhood Lead Poisoning Prevention. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention (2012, January). Accessed 01/20/2017 at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf.
(ACOG) American College of Obstetricians and Gynecologists. Lead screening during pregnancy and lactation. Committee Opinion No. 533. Obstet Gynecol 2012;120:416–20. Accessed 01/27/2017 at http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Lead-Screening-During-Pregnancy-and-Lactation.
Alarcon WA. Elevated Blood Lead Levels among Employed Adults — United States, 1994–2012. MMWR Morb Mortal Wkly Rep. 2015 Oct 23;62(54):52-75. Accessed 01/20/2017 at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6254a4.htm.
Alarcon WA. Elevated Blood Lead Levels among Employed Adults — United States, 1994–2013. MMWR Morb Mortal Wkly Rep. 2016 Oct 14;63:59–65. Accessed 03/09/2017 at http://dx.doi.org/10.15585/mmwr.mm6355a5.
Annest JL, Mahaffey KR, Cox DH, Roberts J. Blood lead levels for persons 6 months-74 years of age: United States, 1976-80. Adv Data. 1982 May 12;(79):1-23.
(AOEC-CSTE) Association of Occupational and Environmental Clinics. 2007 Medical Management Guidelines for Lead-Exposed Adults with 2013 CSTE Medical Management Guidelines. (2013, October). Accessed 01/20/2016 at http://www.aoec.org/documents/positions/mmg_revision_with_cste_2013.pdf.
(ATSDR) Agency for Toxic Substances and Disease Registry. 2007. Toxicological Profile for Lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. Accessed 01/20/2017 at https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf.
(CDC) Centers for Disease Control and Prevention. Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Atlanta: CDC; 2002. Accessed 01/20/2017 at https://www.cdc.gov/nceh/lead/casemanagement/managingEBLLs.pdf.
(CDC) Centers for Disease Control and Prevention. Preventing Lead Poisoning in Young Children. Atlanta: 2005, August. Accessed 01/20/2017 at https://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf.
(CDC) Centers for Disease Control and Prevention. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women. Atlanta: 2010, November. Accessed 01/20/2017 at https://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf.
(CDC) Centers for Disease Control and Prevention. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention.” Atlanta: 2012, June. Accessed 01/20/2017 at https://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf.
(CDC) Centers for Disease Control and Prevention. (NHANES) National Health and Nutrition Examination Survey, 2013-2014: Overview – Let’s Improve Our Nation’s Health. Rockville: 2014. Accessed 01/30/2017 at https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013-14_overview_brochure.pdf
(CDC) Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. Atlanta: 2015, February. Accessed 01/20/2017 at https://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf.
(CDC) Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Diseases and Conditions – Lead, Elevated Blood Levels, Adult (≥16 Years) and Children (<16 Years). Atlanta: 2016. Accessed 01/20/2017 at https://wwwn.cdc.gov/nndss/conditions/lead-elevated-blood-levels/case-definition/2016/.
(CDC) Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Data Collection and Reporting. Atlanta: 2016Accessed 10/18/2017 at https://wwwn.cdc.gov/nndss/data-collection.html.
(CDPH) California Department of Public Health. Medical Guidelines for the Lead-Exposed Worker. Occupational Lead Poisoning Prevention Program (OLPPP) Richmond, CA: 2009. Accessed 01/20/2017 at http://www.cdph.ca.gov/programs/olppp/Documents/medgdln.pdf.
(CMS) Centers for Medicaid and Medicare Services. Coverage of Blood Lead Testing for Children Enrolled in Medicaid and the Children’s Health Insurance Program: Informational Bulletin. Baltimore: 2016, November. Accessed 01/20/2017 at https://www.medicaid.gov/federal-policy-guidance/downloads/cib113016.pdf.
(CMS) Centers for Medicaid and Medicare Services. Instructions for Completing Form CMS-416: Annual Early and Periodic Screening, Diagnostic, and Treatment (EPSDT) Participation Report. Baltimore: 2017, October. Accessed 10/18/2017 at https://www.medicaid.gov/medicaid/benefits/downloads/cms-416-instructions.pdf.
(CSELS) Center for Surveillance, Epidemiology, and Laboratory Services. CSELS Weekly Update: Week ending 10-02-2015 – DHIS Meeting Regarding CSTE Position Statement on Elevated Blood Lead Levels. 2015. Accessed 02/01/2017 at http://intranet.cdc.gov/ophss/csels/od/adc/docs/leadership_meetings/2015/CDC%20Senior%20Leadership%20Meeting%20Bullets%202015-10-02.pdf.
(CSTE) Council for State and Territorial Epidemiologists. Management Guidelines for Blood Lead Levels in Adults. Adopted CSTE Occupational Subcommittee, (2013, June). Accessed 01/20/2017 at http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/OccupationalHealth/ManagementGuidelinesforAdult.pdf.
(CSTE) Council for State and Territorial Epidemiologists. Public Health Reporting and National Notification for Elevated Blood Lead Levels (Position Statement 15-EH-01). (Revised 2015, December). Accessed 01/20/2017 at http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS1/15-EH-01_revised_12.4.15.pdf.
(HHS) U.S. Department of Health and Human Services. Strategic Plan for the Elimination of Childhood lead Poisoning. Developed for the Risk Management Subcommittee, Committee to Coordinate Environmental Health and Related Programs. Atlanta: 1991, February. Accessed 10/18/2011 at http://files.eric.ed.gov/fulltext/ED344700.pdf
(HUD) U.S. Department of Housing and Urban Development. Requirements for Notification, Evaluation and Reduction of Lead-Based Paint Hazards in Federally Owned Residential Property and Housing Receiving Federal Assistance; Response to Elevated Blood Lead Levels. Federal Register. 82(9);4152-72. [Final Rule - 24 CFR Part 35 - Lead Safe Housing Rule; Docket No. FR–5816–F–02]. Accessed 01/27/2017 at https://www.gpo.gov/fdsys/pkg/FR-2017-01-13/pdf/2017-00261.pdf.
Jacobs DE, Tobin M, Targos L, Clarkson D, Dixon S, Breysse J, et al. Window Replacement and Childhood Lead Exposure: Results from CLEAR-Win [unpublished]. 2016. Washington, DC: U.S. Department of Housing and Urban Development. 2015.
(NCSL) National Conference of State Legislatures. State Lead Poisoning Prevention Statutes. Denver: 2010 March. Compiled by Farquhar D.
Accessed 02/07/2017 at http://www.ncsl.org/documents/environ/stlaws10.pdf
Newman N, Jones C, Page E, Ceballos D, Oza A. Investigation of Childhood Lead Poisoning from Parental Take-Home Exposure from an Electronic Scrap Recycling Facility — Ohio, 2012. MMWR Morb Mortal Wkly Rep. 2015 Jul 17;64(27):743-5. Accessed 01/20/2017 at https://www.cdc.gov/mmwr/pdf/wk/mm6427.pdf.
(NIOSH) National Institute for Occupational Safety and Health. Report to Congress on Workers’ Home Contamination Study Conducted under the Workers’ Family Protection Act (29 U.S.C. 671a). DHHS NIOSH) Publication No. 95-123. Cincinnati: CDC; 1995, September. Accessed 01/20/2017 at https://www.cdc.gov/niosh/docs/95-123/pdfs/95-123.pdf.
(NIOSH) National Institute for Occupational Safety and Health. Protecting Workers’ Families: A Research Agenda. DHHS NIOSH) Publication No. 2002-113. Cincinnati: CDC; 2002, May. Accessed 01/20/2017 at https://www.cdc.gov/niosh/docs/2002-113/pdfs/2002-113.pdf.
(NIOSH) National Institute for Occupational Safety and Health. Adult Blood Lead Epidemiology and Surveillance (ABLES) acceptance memo Council for State and Territorial Epidemiologist (CSTE) case definition for an elevated blood lead level. Atlanta: 2015a, October. Internet site accessed 01/20/2017 at http://www.cdc.gov/niosh/topics/ABLES/description.html.
(NIOSH) National Institute for Occupational Safety and Health. Adult Blood Lead Epidemiology and Surveillance (ABLES) Program Description. Atlanta: 2015b, December. Internet site accessed 03/09/2017 at https://www.cdc.gov/niosh/topics/ables/description.html.
(NTP) National Toxicology Program. NTP Monograph on Health Effects of Low-Level Lead. Research Triangle Park: National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). 2012, June. Accessed 01/20/2017 at https://ntp.niehs.nih.gov/ntp/ohat/lead/final/monographhealtheffectslowlevellead_newissn_508.pdf.
(OSHA) Occupational Safety and Health Administration. Lead: If You Work Around Lead, Don't Take it Home! OSHA QuickCard™ (Publication 3680), (2014, June). Accessed 01/20/2017 at https://www.osha.gov/Publications/OSHA3680.pdf.
Pertowski C. Lead Poisoning. From Data to Action: CDC’s Public Health Surveillance for Women, Infants, and Children. Atlanta, GA: U.S. Department of Health and Human Services. Pp 311-9. 1994.
Wengrovitz AM, Brown MJ. Recommendations for Blood Lead Screening of Medicaid-eligible Children aged 1-5 Years: an Updated Approach to Targeting a Group at High Risk. MMWR Morb Mortal Wkly Rep. 2009 Aug 7;58(RR09):1-11. Accessed 01/30/2017 at https://www.cdc.gov/mmwr/pdf/rr/rr5809.pdf.
Wheeler W. Blood lead levels in children aged 1-5 years - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013 Apr 5;62(13):245-8. Accessed 03/03/2017 at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6213a3.htm.
2014-2017 HHLPPP Publications (n=17) |
2014 (n=4)
Basir M, Umar-Tsafe N, Getso K, Kaita IM, Nasidi A, Sani-Gwarzo N, et al. Assessment of blood lead levels among children aged ≤5 Years — Zamfara State, Nigeria, June–July 2012. Morbid Mortal Wkly Rep. 2014;63(15):325-7. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6315a2.htm?s_cid=mm6315a2_e [Brown MJ].
Ponder-Brookins P, Witt J, Steward J, Greenwell D, Chew GL, Samuel Y, et al. Incorporating community-based participatory research principles into environmental health research: challenges and lessons learned from a housing pilot study. J Environ Health. 2014;76(10):8-17. [Kennedy C, Brown, MJ] Available at https://www.ncbi.nlm.nih.gov/pubmed/24988659
Raymond J, Wheeler W, Brown MJ. Lead screening and prevalence of blood lead levels in children aged 1–2 years—Child Blood Lead Surveillance System, United States, 2002–2010 and National Health and Nutrition Examination Survey, United States, 1999–2010 and National Health and Nutrition Examination Survey, United States, 1999–2010. In: Use of selected clinical preventive services to improve the health of infants, children, and adolescents—United States, 1999–2011. MMWR 2014;63(Suppl 2). Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/su6302a6.htm.
Thurtle N, Greig J, Cooney L, Amitai Y, Ariti C, Brown MJ, et al. Description of 3,180 courses of chelation with dimercaptosuccinic acid in children ≤5 y with severe lead poisoning in Zamfara, Northern Nigeria: A retrospective analysis of programme data. PLoS Med 11(10): e1001739. Available at http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001739
|
2015 (n=6)
Educational Services for Children Affected by Lead Expert Panel. Educational interventions for children affected by lead. Atlanta: U.S. Department of Health and Human Services; 2015 [Brown MJ]. This publication is being used by numerous health agencies to collaborate and share with local Head Starts and school departments and education leaders.
Childhood Blood Lead Surveillance System (CBLS) 2014 data on the lead website.
Mason J, Wheeler W, Brown MJ. The economic burden of exposure to secondhand smoke for child and adult never smokers residing in U.S. public housing. Public Health Rep. 2015 130(3):230–44. This publication was mentioned on NPR as validation for HUD smoke‐free activities in public housing.
Raymond J, Brown MJ. Childhood blood lead levels—United States, 2007–2012. MMWR Morb Mortal Wkly Rep 2015;62(54):76‐80. Errata in: MMWR Morb Mortal Wkly Rep 2015;42(45):1277.
Kennedy C, Lordo R, Sucosky MS, Boehm R, Brown MJ. Evaluating the effectiveness of state specific lead‐based paint hazard risk reduction laws in preventing recurring incidences of lead poisoning in children. Int J Hyg Environ Health. 2016;219(1):110–7. Epub 2015 Oct 9.
Dignam T, García BR, De León M, Curtis G, Creanga AA, Azofeifa A, et al. Prevalence of Elevated Blood Lead Levels and Risk Factors Among Residents Younger Than 6 Years, Puerto Rico‐2010. J Public Health Manag Pract. 2016;22(1):E22–35. doi: 10.1097/PHH.0000000000000224. [Kennedy C, Brown MJ]
|
2016 (n=6)
Childhood Blood Lead Surveillance System (CBLS) 2015 data on the lead website.
Dignam T, García BR, De León M, Curtis G, Creanga AA, Azofeifa A, et al. Prevalence of elevated blood lead levels and risk factors among residents younger than 6 years, Puerto Rico-2010. J Public Health Manag Pract. 2016;22(1):E22–35. [Kennedy C, Brown MJ].
Kaufman JA, Brown MJ, Umar-Tsafe NT, Adbullah MB, Getso KI, Kaita IM, et al. Prevalence and risk factors of elevated blood lead in children in gold ore processing communities, Zamfara, Nigeria, 2012. J Health Pollut. 2016;6(11):2–8.
Kennedy C, Lordo R, Sucosky MS, Boehm R, Brown MJ. Evaluating the effectiveness of state specific lead-based paint hazard risk reduction laws in preventing recurring incidences of lead poisoning in children. Int J Hyg Environ Health. 2016;219(1):110–7. Epub 2015 Oct 9.
Kennedy C, Yard E, Dignam T, et al. Blood lead levels among children aged <6 Years—Flint, Michigan, 2013–2016. MMWR. 2016;65. [Buchanan S, Brown MJ, Raymond J, Rogers HS, Sarisky J]
Raymond J, Brown MJ. Blood lead levels in children aged <5 years — United States, 2007–2013. MMWR. 2016;63:66–72.
|
2017 (n=1)
Raymond J, Brown MJ. Childhood Blood Lead Levels in Children Aged <5 Years — United States, 2007–2014. MMWR. January 20, 2017 / 66; 1-10. |
List of Attachments
Attachment 1. Authorizing Legislation
1a. NCEH Authority
1b. NIOSH Authority
Attachment 2. 60-day Federal Register Notice
Attachment 3. Funding Opportunity Announcements
3a. FY17 NCEH CBLS FOA
3b. FY14 NCEH HHLPPP FOA
3c. 2015 HHLPSS Terms of Clearance
Attachment 4. Summary Table CBLS-ABLES Program Activities
Attachment 5. Data Collection Forms
5a. CBLS Variables – Text Files
5b. CBLS Aggregate Records Form
5c. ABLES Case Records Form and Brief Narrative Report
5c1. ABLES Case Records Format
5d. ABLES Aggregate Records Form and Brief Narrative Report
Attachment 6. Privacy Impact Assessment
6a. CBLS PIA
6b. ABLES PIA
Attachment 7. Research Determinations
7a. NCEH CBLS Research Determination
7b. NIOSH ABLES Research Determination
1 FOA No. CDC-RFA-EH14-1408PPHF14 is covered under “Healthy Home and Lead Poisoning Surveillance System (HHLPSS)” (OMB Control No. 0920-0931, expiration date 05/31/2018). NCEH will submit an extension ICR to cover the fourth and final year of the FY14 cooperative agreement. The HHLPSS ICR will be discontinued at the end of the program period. Partners who are awardees of both FY14 and FY17 programs, will report FY14 Year 4 data under the existing HHLPSS ICR, and FY17 Years 2 and 3, under this new ICR. Simultaneously, new FY17 program awardees will report all FY17 program data only under this new ICR.
2 The 1988 Lead Contamination Control Act amended the Public Health Service Act to authorize the Secretary of Health and Human Services to make grants to state and local governments for the initiation and expansion of community programs designed to: (1) screen infants and children for elevated blood lead levels; (2) assure referral for treatment of, and environmental intervention for, infants and children with such blood lead levels; and (3) provide education about childhood lead poisoning. It requires that grant priority be given to programs which will serve areas with a high incidence of elevated blood levels in infants and children. Accessed 01/20/2017 at https://www.congress.gov/bill/100th-congress/house-bill/4939.
3 The Agency for Toxic Substances and Disease Registry (ATSDR) has not developed Minimal Risk Levels (MRLs) for lead because a clear threshold for some of the more sensitive effects in humans has not been identified. An MRL is "an estimate of the daily human exposure to a dose of a chemical that is likely to be without an appreciable risk of adverse, noncancerous effects over a specified duration of exposure.” See “Section 2.3 – Lead Dose-Response Relationships” on page 31 and Appendix D on page D-1 from the ATSDR Toxicological Profile for Lead (2007) at https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf.
4 See https://wwwdev.cdc.gov/niosh/topics/ables/default.html. The most recent MMWR report can be found at: https://www.cdc.gov/mmwr/volumes/63/wr/mm6355a5.htm.
5 The National Electronic Disease Surveillance System (NEDSS) facilitates electronically transferring public health surveillance data from the healthcare system to public health departments. It is a conduit for exchanging information that supports NNDSS. Today, when states and territories voluntarily submit notifiable disease surveillance data electronically to CDC, they use data standards and electronic disease information systems and resources supported in part by NEDSS. This ensures that state data shared with CDC are submitted quickly, securely and in an understandable form. Accessed 01/31/2017 at https://wwwn.cdc.gov/nndss/nedss.html.
6 See Attachment 4b – Data Dictionary for Childhood Blood Lead Poisoning for the National Environmental Public Health Tracking Network at https://www.reginfo.gov/public/do/PRAViewICR?ref_nbr=201607-0920-005.
7 CMS: Accessed 01/27/2017 at https://www.medicaid.gov/federal-policy-guidance/downloads/cib113016.pdf
8 CMS: Accessed 01/27/2017 at https://www.medicaid.gov/federal-policy-guidance/downloads/cib-06-22-12.pdf
9 CMS: Accessed 01/27/2017 at https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/CMS416.pdf
10 HUD’s 2017 final rule revises the agency’s criteria under the Lead Safe Housing Rule (LSHR) for responding to the identification of children, under age six with elevated BLLs, who are residing in covered federally-assisted and federally-owned target housing. The final rule also addresses lead hazard evaluation and control for additional assisted housing units in the same properties as those in which children, under age six with elevated BLLs, have been discovered. The final rule adopts an approach that is consistent with the CDC’s reference range value for BLLs in children.
12 5 CFR § 1320.3 – Definitions: For purposes of implementing the PRA, the following terms are defined as follows in Paragraph (b):
(2) The time, effort, and financial resources necessary to comply with a collection of information that would be incurred by persons in the normal course of their activities (e.g., in compiling and maintaining business records) will be excluded from the “burden” if the agency demonstrates that the reporting, recordkeeping, or disclosure activities needed to comply are usual and customary.
(3) A collection of information conducted or sponsored by a Federal agency that is also conducted or sponsored by a unit of State, local, or tribal government is presumed to impose a Federal burden except to the extent that the agency shows that such State, local, or tribal requirement would be imposed even in the absence of a Federal requirement.
13 The Lead Disclosure Rule (Title X, Section 1018). This law requires the disclosure of known information on lead-based paint and lead-based paint hazards before the sale or lease of most housing built before 1978. Source: U.S. HUD
14 HUD’s Lead Safe Housing Rule. The U.S. Department of Housing and Urban Development’s Lead Safe Housing Rule applies to all target housing that is federally owned and applies to target housing receiving federal assistance. Source: U.S. HUD
16 See https://www.gpo.gov/fdsys/pkg/CFR-2011-title45-vol1/pdf/CFR-2011-title45-vol1-sec164-512.pdf and the discussion of 45 CFR 164.512(b) at https://privacyruleandresearch.nih.gov/pdf/ocr_publichealth.pdf.
18 NIOSH will track BLLs ≥5 μg/dL based on recent reference value adoption, and will continue to track BLLS ≥10 and ≥25 μg/dL because there is now a need to monitor the historic trends of occupational lead exposure based on BLLs ≥25 μg/dL. Prior to 2009, States were only obligated to report cases with BLL≥25 μg/dL. Cases with BLL ≥10 μg/dL were not reported until 2009. NIOSH will continue to work with reporting states to receive updated reporting standards as authorized by state regulations in the future.
19 Based on OPM Atlanta Locality Pay for Grade and Step 5 Salary Table at https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2017/ATL.pdf.
20 National estimates are provided at https://www.cdc.gov/nceh/lead/data/Chart_Website_StateConfirmedByYear_1997_2015.pdf.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Davis, Stephanie I. (CDC/ONDIEH/NCEH) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy