Appendix D
2288ss03_Appendix D.docx
Pesticides Data Call In Program
Appendix D
OMB: 2070-0174
Appendix D
This document was originally published as Attachment G to a DCI ICR renewal document posted under the docket identification #EPA-HQ-OPP-2007-0923-0005. See docket in Regulations.gov. This document is republished in this appendix without change.
General Methodology Used to Estimate
Paperwork Burden Hours and Costs by the
Office of Pesticide Programs for Submission of
Required Data/Information for Responding to a Data Call-In Notice

Office of Pesticide Programs
October 2007

SECTION I 3
BACKGROUND 3
What is the purpose of this document? 3
Why does EPA issue DCIs? 3
How do PRA requirements relate to DCIs? 4
How does a DCI recipient respond to a DCI? 5
DCI burden activities tables 6
Table 1 6
Table 2 7
Variations of the response to a DCI 8
Data generation in response to a DCI 8
Non-data generation in responses a DCI 9
SECTION II 10
ESTIMATING PAPERWORK ACTVITIES OF DATA GENERATION 10
What are the key assumptions when estimating PRA burden hours and costs for data generation? 10
Paperwork burden is generally 35% of the cost of the study. 10
All registrants generate all DCI data 12
How are test cost estimates developed? 12
What if test cost estimates are unavailable? 12
What if the available test cost estimates vary? 13
What are the steps in calculating the paperwork burden (hours and costs)? 13 a. Calculate test costs 13
Distribute paperwork activities among labor categories. 13
Calculate paperwork burden hours from labor cost distribution. 14
Is the burden for those not generating data covered?. 15
Attachment A: Case Studies: Agency analysis of response costs of non-data generation 17
Study #1 Review of the Application for New and Amended Pesticide Registration, OMB No. 2070-0060, (EPA ICR No. 0277.14) for non-data generated burden hours and costs. 17
Study #2 Data Generation for Pesticide Reregistration ICR, OMB No. 2070-0107, (EPA ICR No. 1504.05) 19
Study #3 Economic Analysis for Proposed Changes in Data Requirements Rule for Biochemical and Microbial Pesticides – Cost Estimates for Data Waivers. 21
Attachment B: FIFRA estimated test cost chart and PRA cost estimates 22
Section I. Background
What is the purpose of this document?
The Paperwork Reduction Act (PRA), 44 U.S.C. 3501 et seq., requires federal agencies to estimate the “paperwork burden” for “information collection” activities. Under the PRA, “paperwork burden” means the total time, effort, or financial resources expended by persons to generate, maintain, retain, or disclose or provide information to or for a Federal Agency. Under the PRA, an “information collection” means any request for information made by a federal agency of ten or more respondents, and may include a request to report, retain records, or disclose information to third parties.
This document describes the methodology used by the Environmental Protection Agency’s (EPA) Office of Pesticide Programs (OPP) to estimate the paperwork burden hours and costs for stakeholders responding to Data Call-In (DCI) Notices issued by OPP under section 3(c)(2)(B) of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). The methodology is intended to provide a description of the process used by EPA to derive the estimated paperwork burden hours and costs associated with submitting a response to EPA under a DCI. The methodology presented in this document should also enable stakeholders to reproduce the burden estimates made by the Agency for DCI related collection activities. This increased transparency will enable the public to provide more substantive and meaningful feedback during public comment periods. This feedback will better enable the Agency to periodically amend the burden estimates. In addition, since the methodology presented here will be used by the Agency to estimate the paperwork burden for DCI requests, this document will serve as a reference for such calculations in the future.
Why does EPA issue DCIs?
With few exceptions, FIFRA requires EPA to evaluate all pesticides marketed and used in the United States to ensure that they will not pose unreasonable risks to human health and the environment. Pesticides that meet the requirements are granted a license or “registration” that permits their distribution, sale, and use according to specific use directions and requirements identified on the label. Under the Federal Food, Drug, and Cosmetic Act (FFDCA), EPA establishes tolerances (defined as maximum allowed pesticide residue levels) to specify the amount of the pesticide residue that can legally remain in or on food or animal feed, using a safety standard of a “reasonable certainty of no harm.”
EPA’s review and evaluation of a new, amended, or existing pesticide registration or tolerance require data of sufficient quality and quantity to characterize the pesticide’s hazards and the potential risk from its intended uses. The data requested by the Agency, including the data requested in the DCI, allow EPA to evaluate whether a pesticide meets the statutory standard for registration, and allow the Agency to establish the appropriate tolerance(s) for the pesticide under section 408 of the FFDCA.
Under section 3(c)(2)(B) of FIFRA EPA can require pesticide registrants to generate and submit data to the Agency, when such data are required to maintain an existing registration of a pesticide. EPA’s determination that additional data are needed could occur for various reasons, with the following four reasons being the most common:
The Reregistration Program: Section 4 of FIFRA requires EPA to reassess the health and safety data for all pesticide active ingredients registered before November 1, 1984, to determine whether these “older” pesticides meet the criteria for registration that would be expected of a pesticide being registered today for the first time. Section 4 directs EPA to use section 3(c)(2)(B) authority to obtain the required data.
The Registration Review Program: Section 3(g) of FIFRA contains provisions to ensure that each pesticide will be reviewed every 15 years to ensure that the pesticide continues to pose no risk of unreasonable adverse effects on human health or the environment. Section 3(g) instructs EPA to use the section 3(c)(2)(B) authority to obtain the required data.
The Special Review Program: Though rare, EPA may conduct a Special Review if EPA believes that a registered pesticide poses risks of unreasonable adverse effects on human health or the environment. Section 3(c)(2)(B) of FIFRA provides a means of obtaining any needed data.
Anticipated Residue/Percent Crop Treated Information: Under FFDCA, EPA can consider information on the anticipated levels of pesticide residues in food (the actual levels of pesticide residues that have been measured in food) and data on the actual percent of food treated with the pesticide chemical. The Agency must also provide for periodic reevaluation of this information. Under FFDCA section 408(b)(2)(E), EPA can issue a DCI for information relating to anticipated residues, and under section 408(b)(2)(F) EPA can issue a DCI for percent crop treated estimates.
How do the PRA requirements relate to DCIs?
The PRA states, “An agency may not conduct or sponsor, and a person is not required to respond to a “collection of information,” as defined at 5 CFR 1320.3(c), unless the “collection” displays a currently valid control number issued by the Office of Management and Budget (OMB).1 EPA’s issuance of a DCI under FIFRA section 3(c)(2)(B) is subject to the PRA requirements because the DCI is considered a “collection of information” under the PRA. To comply with the PRA requirements, EPA must submit an Information Collection Request (ICR) that provides specific information to OMB about the data that EPA intends to call in for a given pesticide, including: a list of required studies, the practical utility of the data, the estimated testing costs, and the estimated paperwork burden.
Under the PRA, “practical utility” means the actual, not merely the theoretical or potential, usefulness of information to or for an agency, taking into account its accuracy, validity, adequacy, and reliability, and the agency's ability to process the information it collects in a useful and timely fashion.2 “Burden” means the time, effort, or financial resources expended by persons to generate, maintain, or provide information to or for a Federal Agency.3” including resources to:
review instructions;
develop, acquire, install, and use technology and systems;
search data sources;
collect, review, validate, and verify information/data;
process and maintain information/data;
disclose and transmit/submit information/data;
change/adjust the existing ways of complying with any previously applicable instructions and requirements to comply with new requirements; and/or
train personnel.
How does a DCI recipient respond to a DCI?
Response to a DCI can generally be divided into three phases.
Phase 1: Initial response: After receiving a DCI, the recipient has 90 days to provide the initial response, which states how the recipient plans to comply with the DCI. A registrant may avoid generating the data if he qualifies for a generic data exemption (i.e., he uses a registered pesticide as the source of the active ingredient in his own product), cancels the product’s registration, submits or cites existing data, or requests a waiver.
Phase 2: Data generation: Unless the DCI recipient can cite existing studies or is granted a waiver by the Agency, the DCI recipient must then generate the required data.
Phase 3: Data submission: DCI recipient submits the studies/information to EPA.
DCI burden activities tables
Table 1 illustrates the paperwork
activities that would typically be performed by a DCI recipient
during each of the three phases. Note that the activities that are
likely to occur have been divided into three categories of duties:
managerial, technical and clerical. For this table, it was assumed
that the data generation was performed at the request of the DCI
recipient by a contract laboratory. Although DCI recipients can
certainly choose to generate the data themselves, the Agency believes
that the assumption used provides a sufficiently conservative
estimate, and does not expect the burden estimate for DCI recipients
to be more than the estimate. Table 1 includes only
those duties
 performed
directly by the DCI recipient.
performed
directly by the DCI recipient.
Table 1 – Response Phases
Managerial Duties |
Technical Duties |
Clerical Duties |
PHASE 1: INITIAL RESPONSE |
||
Read regulations
|
Read regulations
|
Complete other required paperwork |
Review EPA’s DCI notice |
Review EPA’s DCI notice |
|
Communicate with EPA |
|
Assist with review of internal company information |
Plan DCI response |
Search for existing data |
Assist with search for existing data |
Sign and send initial response forms to EPA |
|
Prepare initial response forms for submission |
Oversight of employee activities |
|
|
PHASE 2(a): DATA GENERATION USING CONTRACT LABORATORY |
||
Planning/oversight of employee and contract activities |
Plan the data collection activities with the laboratory |
Complete/file/archive other required paperwork |
Make decisions |
|
Electronic data entry |
Secure contract lab services and approve statement of work (SOW) |
Create test protocols for SOW |
|
Communicate with EPA |
Ensures contract laboratory maintains records and procedures during testing period in accordance with the Good Laboratory Practices (GLPs) |
|
Oversight of employee and contract activities |
Routine contact with testing laboratory which can include on- site visits |
|
|
Analyze interim report and/or monthly report |
|
|
Proof draft final report |
|
|
Generate acceptance report |
|
PHASE 3: DATA SUBMISSION TO EPA |
||
Sign-off on submission to EPA |
Draft summary of the data for cover letter |
Prepare submission to EPA |
Close-out contract |
|
|
Oversight of employee activities |
Complete other required paperwork |
Complete/file/archive other required paperwork |
Table 2 represents the PRA activities conducted at a contract laboratory. Data are generated by following standard operating procedures, which involve mostly technical duties. As part of their duties under Good Laboratory Practices (GLP), the scientists and technicians maintain logbooks and other records from which the final report is written. This table lists activities that occur in the conduct of studies where the test subjects are animals. Activities associated with animal care would not occur in studies where the test subject is inanimate, such as product chemistry studies, environmental fate studies or residue chemistry studies. Table 2
PHASE 2(b): PRA DATA GENERATION ACTIVITIES CONDUCTED AT THE CONTRACT LABORATORY* |
||
|
Technical Duties |
|
Individual animal care records |
||
Records on the rooms in which animals are housed and procedures are performed |
||
Necropsy records |
||
Equipment logbooks and computer-generated records such as chromatograms |
||
Records on preparation of analytical standards |
||
Freezer and storage area logbooks |
||
Chain-of-custody forms |
||
Quality control/quality assurance forms and review checks for accuracy |
||
Archive and transmittal of data forms |
||
*The Agency recognizes that in certain instances these activities might be conducted “in-house.”
VI. Variations of the response to a DCI
Because there are multiple ways of responding to a DCI, not all DCI recipients participate in all three phases. A registrant would participate only in phase 1 if they:
Voluntarily cancel the pesticide registration
Delete the uses of the product to which the requirements apply
Qualify for a generic data exemption
Request and receive a data waiver
Purchase/cite existing data
A registrant who purchases/cites existing data performs Phase 1 Initial response and Phase 3 Data Submission activities only. The initial response phase and the data submission phase are considered to have more-or-less fixed hours and costs since reading the regulations and preparing submissions to the Agency are independent of the type of information submitted.
Until the Agency receives the 90-day response letters to the DCI notice from the registrants indicating what studies, if any, they will conduct, it is not possible to accurately predict the total cost and burden of developing the data.
This methodology was prepared so that an average burden could be presented for a DCI recipient, regardless of the response they choose. Clearly, by assuming that all DCI recipients engage in all three activities the Agency has chosen to overestimate the burden for a DCI recipient who may not engage in any activities beyond Phase 1. The cost for DCI recipients who engage in a taskforce for data generation, voluntarily cancel the product or affected uses, submit or cite existing data, or are granted a waiver incur fewer burden hours and costs.
a. Data generation in response to a DCI
A registrant who chooses to generate data in response to the DCI may either:
generate and submit the required data on their own, or
generate and submit the required data as part of a taskforce.
Data generation is considered the most expensive of the three phases. However, the amount of the expense is highly dependent on the type of data required. Therefore, it is logical to assume that the more expensive the study, the greater the paperwork burden hours and costs. For a DCI recipient to generate and submit the required data on their own is the most expensive of the response scenarios. However, the cost for DCI respondents who pool their resources for data generation with other stakeholders is less than those who engage in data generation activities on their own. Therefore, the Agency encourages cost-sharing agreements among manufacturers of specific pesticide chemicals to minimize the duplication of laboratory tests conducted in response to a DCI. DCI notices explain the statutory provisions for cost sharing agreements for FIFRA.
b. Phase 1 and Phase 3 response activities for data generaters
Phase I and Phase 3 DCI response activities are a subset of the paperwork burden hours and costs estimates related to generating data to respond to a DCI notice. Unlike the wide variation of the costs for data generation, Phase 1 and Phase 3 response costs are more or less fixed costs. Generally, less than twenty-five (25) burden hours are spent on these activities at a cost of around $2,000 (indexed to 2006 dollars). Section II of this document presents a discussion of these burden hours and costs.
Section II. Estimating Paperwork Activities Of Data Generation
I. What are the key assumptions when estimating PRA burden hours and costs for data generation?
a. Paperwork activities are generally 35% of the cost of the study.
For more than a decade, EPA has been estimating all the paperwork burden hours and costs of responding to a DCI notice as approximately 35% of the cost of the study, see Figure - 1 Relationship of Test Costs to Response Phases. This formula allows the Agency to derive a reasonable estimate of for PRA activities (Phases 1, 2, and 3) by using the average estimated cost of specific tests. This approach was adopted because it allows the Agency to consider the potential for there to be more burdens related to a more complex study. The premise is that a more expensive test may cause the respondent to incur more burden hours and costs than a less expensive test would. This estimate is only applicable to DCI-related data generation. This percentage was developed from numerous sources of information including agency expertise, industry consultation, and repeated review by the public, industry, key stakeholders, and OMB on the Agency’s information collection activities.
EPA assumes that 35% of the cost of any given test reflects all burdens and costs necessary for the completion of the paperwork activities. The paperwork burden and cost fall into two general categories of activity burden, administrative and technical:
Administrative Paperwork Burden is defined as the labor time spent communicating and working with the Agency and planning a response to the DCI and the planning of data collection and submittal activities. Generally, the respondent will conduct collection activities listed in the Section 1, Table 1Response Phases (Phase 1 and Phase 3). The labor cost related to the Administrative category of paperwork burden is assumed to equal 2% of the total test cost (2% of total test cost = Administrative Paperwork Burden Cost).
Technical Paperwork Burden is the labor time needed to complete the paperwork associated with the initiation of testing, collecting and maintaining data, use of laboratory standards, data analysis, data compiling, data entry, oversight of contractor or employee activities, and decision-making. Generally, the respondent will conduct collection activities listed in Table 1-Response Phases (Phase 2(a)). This contract laboratory will conduct collection activities listed in Table 2 (Phase 2(b)). The labor cost for the technical category of paperwork burden is assumed to equal 33% of the total test cost. (33% of total test cost = Technical Paperwork Burden Cost)
Thus, [Administrative paperwork (2% of total test cost)] + [Technical paperwork (33% of total test cost)] = total test-related paperwork burden hours and costs
(35% of total test cost). This aggregate paperwork burden and cost estimate of 35% for data generation activities is used in a number of DCI-related Agency information collection activities, including:
Data Acquisition for Registration (OMB #2070-0122; EPA #1503); • Data Generation for Pesticide Reregistration (OMB #2070 - 0107; EPA #1504);
Data Call-Ins for Special Review and Registration Review Programs (OMB
#2070-0057; EPA #0922); and
Anticipated Residue/Percent Crop Treated (OMB #2070-0164; EPA #1911).
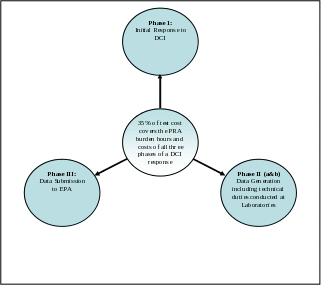
Figure - 1 Relationship of Test Costs to Response Phases
b. All registrants generate all DCI data
The paperwork activity estimates are based on the average cost of generating new data. The total cost of the paperwork burden hours and costs is equal to approximately 35% of the total costs to generate new data. This approach assumes:
Registrants generate all of the data as specified in the DCI notice.4
All data generation was performed by an independent laboratory.
Paperwork burden consists of an administrative (2%) and technical (33%) burden. These two categories relate to office and laboratory activities, respectively.
Paperwork burden is disaggregated by labor category as follows:5
Managerial (20%)
Technical (65%)
Clerical (15%)
Labor rates are derived from the parent ICR
Labor rates are “fully loaded6”
To estimate paperwork activities for each type of labor category (managerial, technical, and clerical), the disaggregated paperwork burden costs are divided by their corresponding labor rates ($/hr). EPA assumes that DCI respondents who generate all requested data on their own is the most expensive of the response options considered and represents the maximum potential estimate of overall burden.
How are test cost estimates developed?
The Agency maintains an archive of the basic FIFRA study cost estimates that were developed through surveys of independent testing laboratories, Agency economic analyses, and registrant comments during ICR renewal periods. To the extent possible, EPA uses multiple sources to provide test cost estimates, which are updated as needed. Attachment B contains a listing of the FIFRA study cost estimates currently on file with the Agency. The chart also provides the paperwork burden hour and cost estimates for specific studies.
What if test cost estimates are unavailable?
The Agency may request certain special studies or non-guideline studies that have not been previously required, and for which test cost estimates have not yet been calculated. If the estimated test cost is not readily available to the Agency at the time that a particular test is requested, EPA will estimate the cost on the basis of:
EPA staff expertise and experience;
similarity with another study protocol for which cost estimates are available;
type of study requested (e.g., animal studies, field monitoring, and other possible test parameters);
level of work that EPA expects will be involved in generating the data (high medium, or low effort required); and/or
time required to complete the test.
The Agency’s estimate would then serve as the default test cost for use in calculating the paperwork burden. EPA will update and revise these default test costs as more reliable estimates are obtained.
What if the available test cost estimates vary?
To the extent possible, EPA uses multiple sources to provide test cost estimates. If several cost estimate exist for a particular study, but the differing estimates fall within an acceptable range, EPA will use the average estimate as its estimate. For example, if test cost estimates for a study were quoted at $20,000, $30,000, and $40,000, these estimates would be considered to fall within an acceptable range, and the average of $30,000 would be used in EPA’s estimation of paperwork burden. If EPA finds that the quoted test costs for a given test varies widely among sources (i.e., the variation is not within a reasonable range), then EPA will make a case-by-case decision on how to estimate an average test cost using the criteria listed in section II-III (What if tests costs are unavailable).
What are the steps in calculating the paperwork burden (hours and costs)?
a. Calculate test costs
Using the EPA archive information of FIFRA study cost estimates, the Agency calculates the total paperwork burden hours and costs for a test as 35% of the total test cost (administrative paperwork burden as 2% and technical paperwork burden as 33% of the total test cost). This percent-based estimate of paperwork burden is reflective of expert opinion, information from industry, various proprietary information/data, and a general assessment of test costs.
b. Distribute paperwork activities among labor categories.
As an entity prepares a data generating response to the DCI notice, EPA assumes managerial, technical, and clerical staff will undertake certain activities. Paperwork burden costs are divided among managerial, technical, and clerical staff labor categories (see Table 3, below) to reasonably reflect, on average, the percent of work performed.
-
Table 3: Distribution of Paperwork Activities Across Labor Categories*
Labor category
% of Paperwork Activities Performed
Managerial
20%
Technical
65%
Clerical
15%
Using this percentage system, EPA can assign a paperwork activity cost to each labor category.
For example, study guideline 850.1735, Whole Sediment Acute Toxicity, has an estimated cost of $20,250. To assign a paperwork activity cost to each labor category, and to eventually arrive at a total estimate of paperwork burden in hours and costs, the steps below are taken.
Labor category activities:
Managerial labor: $1,417.50 = ($7,087.50 * 0.20) Technical labor: $4,606.88 = ($7,087.50 * 0.65) Clerical labor: $1,063.12 = ($7,087.50 * 0.15)
Total paperwork activities cost: = $7,087.50
EPA would estimate that $7,087.50 or 35% of the total test cost represents the cost of the total paperwork burden activities.
c. Calculate paperwork burden hours from labor cost distribution.
The second component for estimating DCI PRA activities is to estimate the average amount of time required to complete activities such as obtaining, compiling, preparing and submitting information to EPA. After distributing the paperwork costs among the managerial, technical and clerical labor categories, the paperwork burden hours are then derived by dividing the costs using fullyloaded wage rates ($/hour) compiled from the Department of Labor’s Bureau of Labor Statistics which are shown in Table 4.
-
Table 4: Fully-Loaded Hourly Wage Rates, by Labor Category*
Labor category
Rate ($/hour)
Managerial
$100.86
Technical
$64.80
Clerical
$33.05
To estimate paperwork burden in hours, using the hourly wage rates listed in Table 4, the steps below are taken.
Distribution of paperwork burden hours and costs for labor categories:
Managerial labor: 14.05 hours ($1,417.50) Technical labor: 71.04 hours ($4,606.88) Clerical labor: 32.17 hours ($1,063.12)
Total paperwork burden hours = 117.31 hours
VI. Is the burden for those not generating data covered?
As discussed in Section 1-VI, (Variations of the response to a DCI), there are multiple ways of responding to a DCI and not all DCI recipients will generate and submit data as part of the DCI response. Until the Agency receives the 90-day response letters to the DCI notice from the registrants indicating what studies, if any, they will conduct, it is not possible to predict the burden and costs of developing the data. Since the Agency cannot predict the number of DCI recipients who will actually generate data or the amount of data that might be submitted, EPA submits paperwork burden estimates to OMB for DCI response activities under all three Phases (1, 2, and 3). Therefore the Agency uses a default assumption that all DCI recipients will need to generate all of the data requested. The Agency recognizes that using this default assumption inflates the paperwork burden estimates associated with the DCI and renders an overstatement of the burden and cost. The Phase I and Phase 3 response activity burden hours and costs are accounted for in the existing DCI ICRs as a subset of the paperwork burden estimates for information collection activities that are related to generating data to respond to a DCI notice.7
In 2006, the Agency conducted a preliminary analysis of existing information from certain collections to estimate the PRA burden of paperwork activities that do not involve data generation, such as Phase 1 responses to DCIs . For the study, the Agency chose two ICRs in which PRA burden hours and costs for paperwork activities that do not involve data generation are clearly defined. The first, the
Application for New and Amended Pesticide Registration ICR, OMB No. 20700060, (EPA ICR No. 0277.14) represents the majority of paperwork activities that do not involve data generation for applications for pesticide registration and the second ICR, the Data Generation for Pesticide Reregistration ICR, OMB No. 2070-0107, (EPA ICR No. 1504.05) represents all DCI related PRA burden and costs. The subset of paperwork activities that do not involve data generation was tallied and the burden hours and costs from both ICRs were estimated. Finally, information from the Economic Analysis of the Proposed Change to Data Requirements Rule for Biochemical and Microbial Pesticides, September 2, 2005,8 was also used to extract costs for activities that do not involve data generation. Of these three studies, the estimates of costs of paperwork that do not involve data generation were 1) $1169.00; 2) $1417.00 and 3) $2000.00 per response respectively. The Agency believes these costs generally represent the range for paperwork burden and cost that registrants who are not generating data might be incur when creating Phase 1 responses. These analyses are discussed in detail in Attachment A.
Attachment A
Case Studies: Agency Analysis of Response Costs for
Phase 1 Responses
1. Study #1: Review of the Application for New and Amended Pesticide Registration, OMB No. 2070-0060, (EPA ICR No. 0277.14) representing burden hours and costs for paperwork activities that do not involve data generation for applications
for pesticide registration
In this study, EPA assumes:
Paperwork activities for applications for registration for “me-too” pesticides9 are similar to paperwork activities for developing a DCI response that does not entail generating new data or requesting a waiver.
Estimated burden and costs listed in Table A are fixed, consistent costs for every respondent, and are not derived from any test cost estimates.
Technical labor efforts are not calculated because the Agency assumes no technical burden would be involved in developing a DCI response that does not entail generating new data or requesting a waiver.10
The paperwork burden for phase 1 responses that are similar to activities in developing registration applications may be described as follows:
read and discuss test requirements (read the DCI letter to understand what data are to be submitted);
plan activities (includes time for reviewing internal company information);
complete paperwork (prepare necessary correspondence, documents to EPA);
Store/maintain information (maintain information submitted to the Agency in company files).
Paperwork burden for such activities may be disaggregated by labor
category as follows (percentages are approximations):
Managerial (65%)
Technical (0%)
Clerical (35%)
Labor rates are derived from the parent ICR.
Labor rates are fully loaded.
The supporting statement of the Application for New and Amended Pesticide Registration ICR characterizes the activities that would be needed for an application/notification. These activities are substantially similar to those of Phase 1: Initial Response that do not involve generating data or requesting a data waiver. The applicable activities are reproduced in Table A below:
Table A: Estimated Burden/Cost for Phase 1 Response* |
|
||||
Collection Activity |
Burden Hours |
Totals |
|||
Management |
Technical |
Clerical |
Hours |
Cost |
|
Read instructions |
7 |
0 |
0 |
7 |
938 |
Plan activities |
0.5 |
0 |
0 |
0.5 |
67 |
Complete Paperwork |
0 |
0 |
3 |
3 |
123 |
Store/maintain data |
0 |
0 |
1 |
1 |
41 |
Totals |
7.5 |
0 |
4 |
11.5 |
1169 |
(* Indexed to 2005 dollars)
After distributing the paperwork costs among the managerial and clerical staff labor categories, the paperwork burden hours are then derived by dividing the costs using the fully-loaded wage rates ($/hour) compiled in the parent ICR, Review of the Application for New and Amended Pesticide Registration, OMB No. 2070-0060, (EPA ICR No. 0277.14) approved by Office of Management and Budget, November 8, 2005.
2. Study #2: Data Generation for Pesticide Reregistration ICR, OMB No. 2070-0107, (EPA ICR No. 1504.05).
In this study EPA assumes:
The total paperwork burden hours and costs for Phase 1 responses are approximately 7 percent of the estimated average cost to generate new data.
Registrant responses to the DCI notice that do not involve data generation include voluntary cancellation, submitting or citing existing data, requesting a data waiver, or claiming eligibilitygeneric data exemption.
Paperwork activities that do not involve data generation may be described as follows:
read and discuss test requirements (read the DCI letter to understand what data are to be submitted);
plan activities (developing options for not generating new data includes reviewing internal company information for existing data);
complete paperwork (prepare necessary correspondence, documents and/or waiver requests to avoid having to submitting data to EPA);
record, maintain and file information (maintain information submitted to the Agency in company files).
Paperwork burden for Phase 1 DCI responses activities may be disaggregated by labor category as follows (percentages are approximations):
Managerial (50%)
Technical (5%)
Clerical (45%)
Labor rates are derived from the parent ICR 6) Labor rates are fully loaded.
Calculate Cost of Paperwork. Using the EPA maintained database of test cost estimates, the Agency calculates the total paperwork burden cost for a test as 7% of the total test cost because based on the percentage breakdown for paperwork burden applicable to non-data generation activities for this ICR. This percent-based estimate of paperwork burden is reflective of expert opinion, information from industry, various proprietary information/data, and a general assessment of test costs. These activities represent the Phase I DCI response activities discussed in section IV.
Distribute Paperwork Costs Among Labor Categories. As an entity prepares a response of non-data generation in response to the DCI notice, EPA assumes certain activities will be conducted by managerial, technical, and clerical staff. Paperwork activities are divided among these labor categories (see Table B below) to reasonably reflect, on average, the percent of work performed. These percentage breakdowns by labor category, derived from the parent ICR Data Generation for Pesticide Reregistration ICR, OMB No. 2070-0107, (EPA ICR No. 1504.05), are consistent for burden activities associated with confirmatory DCIs, product specific DCIs, and submission of voluntary studies cited within the parent ICR.
-
Table B: Distribution of Burden Across Labor Categories
Labor category
% of Paperwork Burden Activities Performed
Managerial
50%
Technical
5%
Clerical
45%
For example the study guideline 850.1735, Whole Sediment Acute Toxicity, has an estimated cost of $20,250. Estimates for paperwork cost for these non-data generation activities would be:
Managerial labor: $708.75 = ($1,417.50 * 0.50) Technical labor: $70.88 = ($1,417.50 * 0.05) Clerical labor: $637.87 = ($1,417.50 * 0.45)
Total labor: $1,417.50
EPA would estimate that $1,417.50 or 7% of the total test cost represents the cost of the total paperwork burden activities.
Calculate Paperwork Burden Hours From Labor Cost Distribution. After distributing the paperwork costs among the managerial, technical and clerical staff labor categories, the paperwork burden hours are then derived by dividing the costs using fully-loaded wage rates ($/hour) cited in the parent ICR and listed in Table C.
-
Table C: Fully-Loaded Hourly Wage Rates, by Labor Category*
Labor category
Rate ($/hour)
Managerial
$130
Technical
$88
Clerical
$40
(Labor costs cited in the parent ICR - Indexed to 2004 dollars)
To estimate paperwork burden in hours, using the hourly wage rates listed in Table C, the steps below are taken.
Managerial labor: 5.45 hours ($708.75 ÷ $130/hr) Technical labor: 0.81 hours ($70.88 ÷ $88/hr)
Clerical labor: 15.95 hours ($637.87 ÷ $40/hr)
Total hours: 22.21 hours
3. Study #3: Economic Analysis for Proposed Changes in Data Requirements Rule for Biochemical and Microbial Pesticides – Cost Estimates for Data Waivers.
Under 40 CFR 158.45, the Agency may waive data requirements on a case-bycase basis in response to specific requests by applicants. The Agency believes the paperwork burden and cost for developing a data waiver request is a relatively fixed cost. When the Agency analyzed burden and cost for data waivers in the Economic Analysis for Proposed Changes in Data Requirements Rule for Biochemical and Microbial Pesticides in 2005, the cost of applying for waivers was estimated to be approximately $2,000 per firm per registration action, regardless of the number of tests a waiver is applied for, if at least one waiver was granted. 11 EPA believes this unit of cost/burden may be applicable to the data waivers submitted to the Agency in response to a DCI. Under this assumption, all non-data generation paperwork activities would be placed at a fixed burden of 20 hours and the burden hours would be distributed as represented in Table D below.
Table D: Industry Estimated Burden/Cost Submitting a Data Waivers Response* |
|||||
Collection Activity |
Burden Hours |
Totals |
|||
Management |
Technical |
Clerical |
Hours |
Cost |
|
Research, Literature searches, Calculations & analysis |
|
10 |
|
10 |
1000 |
Compiling rational |
|
8 |
|
8 |
800 |
Review document file and submit |
1 |
|
1 |
2 |
200 |
Total |
|
|
|
20 |
2000 |
Attachment B
Chart: FIFRA Estimated Study Costs And Paperwork
Burden Hour And Cost Estimates
|
Test Information |
|
Paperwork Burden Totals (35% of study cost) |
|
|
|
Test Guideline/ Section |
Test Name |
|
|
|
|
|
|
|
|
|
|
|
|
Product Performance |
|
|
|
|
|
|
810.1000 |
Overview, Definitions, and General Considerations |
$100,000.0 |
$35,000.0 |
443.6 |
$100,000.0 |
$100,000.0 |
810.1550 |
Product Identity and Disclosure of Ingredients (Composition) (Chemical Identity) |
$223.0 |
$78.1 |
1.0 |
$223.0 |
$223.0 |
810.2100 |
Products for hard surfaces -EPA Disinfectant test |
$6,600.0 |
$2,310.0 |
29.3 |
$7,200.0 |
$6,000.0 |
810.2100 |
Products for hard surfaces - AOAC Fungicide test |
$1,600.0 |
$560.0 |
7.1 |
$2,000.0 |
$1,200.0 |
810.2100(b)&(i) |
Chemical Analysis |
$5,339.0 |
$1,868.7 |
23.7 |
$6,701.0 |
$3,976.0 |
810.2100(m)(2) |
Products for hard surfaces - AOAC Germicidal, detergent sanitizers |
$3,500.0 |
$1,225.0 |
15.5 |
$4,000.0 |
$3,000.0 |
810.21000(j) |
Products for hard surfaces -Sanitizer test non food |
$4,000.0 |
$1,400.0 |
17.7 |
$5,000.0 |
$3,000.0 |
810.2100b,c,d or i |
Products for hard surfaces -AOAC use dilution test, germicidal |
$6,000.0 |
$2,100.0 |
26.6 |
$7,000.0 |
$5,000.0 |
810.2100c,d,e |
Products for hard surfaces -AOAC Use dilution/germicidal spray/carrier |
$6,000.0 |
$2,100.0 |
26.6 |
$7,000.0 |
$5,000.0 |
810.2100(f) |
Products for hard surfaces - Fungicidal test |
$1,600.0 |
$560.0 |
7.1 |
$2,000.0 |
$1,200.0 |
810.2100(g) |
Products for hard surfaces - Virucidal activity method |
$4,000.0 |
$1,400.0 |
17.7 |
$6,000.0 |
$2,000.0 |
810.2100(g) |
Products for hard surfaces -AOAC Tuberculocidal test |
$3,250.0 |
$1,137.5 |
14.4 |
$5,000.0 |
$1,500.0 |
810.2100(l) |
Products for hard surfaces - Hard inanimate surface non food |
$4,000.0 |
$1,400.0 |
17.7 |
$5,000.0 |
$3,000.0 |
810.2200 |
Products for hard surfaces - AVG |
$6,187.0 |
$2,165.5 |
27.4 |
$6,187.0 |
$6,187.0 |
810.2200 - itemized |
Limited disinfectant Broad spectrum disinfectant Hospital disinfectant Fungicidal disinfectant Virucidal disinfectant Tuberculocidal disinfectant |
$4,201.0 |
$1,470.4 |
18.6 |
$5,010.0 |
$3,391.0 |
810.2200 - itemized |
$5,763.0 |
$2,017.1 |
25.6 |
$6,720.0 |
$4,806.0 |
|
810.2200 - itemized |
$5,993.0 |
$2,097.6 |
26.6 |
$7,000.0 |
$4,986.0 |
|
810.2200 - itemized |
$4,219.0 |
$1,476.7 |
18.7 |
$4,865.0 |
$3,572.0 |
|
810.2200 - itemized |
$13,068.0 |
$4,573.8 |
58.0 |
$19,574.0 |
$6,561.0 |
|
810.2200 - itemized |
$4,691.0 |
$1,641.9 |
20.8 |
$5,633.0 |
$6,748.0 |
|
810.2200 - itemized |
Additional bacteria Non-food contact Food contact - Halide products Food contact - Non-halide products Sanitizers for urinal and toilet bowl water and in-tank sanitizers Residual self-sanitizing - wet surfaces Sterilants |
$4,082.0 |
$1,428.7 |
18.1 |
$4,803.0 |
$3,361.0 |
810.2200 - itemized |
$5,198.0 |
$1,819.3 |
23.1 |
$6,026.0 |
$4,370.0 |
|
810.2200 - itemized |
$4,455.0 |
$1,559.3 |
19.8 |
$5,195.0 |
$3,714.0 |
|
810.2200 - itemized |
$6,086.0 |
$2,130.1 |
27.0 |
$7,301.0 |
$4,870.0 |
|
810.2200 - itemized |
$5,672.0 |
$1,985.2 |
25.2 |
$6,902.0 |
$4,441.0 |
|
810.2200 - itemized |
$5,210.0 |
$1,823.5 |
23.1 |
$3,969.0 |
$6,451.0 |
|
810.2200 - itemized |
$11,803.0 |
$4,131.1 |
52.4 |
$11,936.0 |
$11,669.0 |
|
810.2300b |
Products for fabrics/textiles -EPA Carpet Sanitizer |
$3,250.0 |
$1,137.5 |
14.4 |
$5,000.0 |
$1,500.0 |
810.2400 |
Products for air sanitizers |
$5,500.0 |
$1,925.0 |
24.4 |
$6,500.0 |
$4,500.0 |
810.2400(b)(j) |
Chemical Analysis |
$175.0 |
$61.3 |
0.8 |
$350.0 |
$0.0 |
810.2400(b)(l) |
Chemical Analysis |
$4,000.0 |
$1,400.0 |
17.7 |
$4,000.0 |
$4,000.0 |
810.2600 |
Products for microbial pests associated with human and animal waste |
$5,720.0 |
$2,002.0 |
25.4 |
$5,720.0 |
$5,720.0 |
810.2700(d) |
Products for treating water systems AOAC- water disinfectants pools |
$7,500.0 |
$2,625.0 |
33.3 |
$10,000.0 |
$5,000.0 |
810.3000 |
General considerations for Efficacy of invertebrate control agents |
$600.0 |
$210.0 |
2.7 |
$600.0 |
$600.0 |
810.3100 |
Soil treatments for imported fire ants |
$140,000.0 |
$49,000.0 |
621.1 |
$200,000.0 |
$80,000.0 |
810.3200 |
Livestock,poultry,fur and wool bearing animal treatments |
$30,000.0 |
$10,500.0 |
133.1 |
$50,000.0 |
$10,000.0 |
810.3300 |
Treatments to control pests of human and pets |
$50,000.0 |
$17,500.0 |
221.8 |
$60,000.0 |
$40,000.0 |
810.3400 |
Mosquito,blackfly and biting midge treatments |
$140,000.0 |
$49,000.0 |
621.1 |
$200,000.0 |
$80,000.0 |
810.3500 |
Premises Treatments |
$700,000.0 |
$245,000.0 |
3,105.3 |
$800,000.0 |
$600,000.0 |
810.3600 |
Structural Treatments |
$1,200.0 |
$420.0 |
5.3 |
$1,200.0 |
$1,200.0 |
810.3700 |
Insect repellants for human skin and outdoor premises |
$5,000.0 |
$1,750.0 |
22.2 |
$5,000.0 |
$5,000.0 |
810.3800 |
Methods for efficacy testing of termite baits |
$60,000.0 |
$21,000.0 |
266.2 |
$100,000.0 |
$20,000.0 |
|
|
|
|
|
|
|
Product Chemistry |
|
|
|
|
||
830.1550 |
Product identity and composition |
$233.0 |
$81.6 |
1.0 |
$223.0 |
|
830.1600 |
Description of materials used to produce the product |
$334.0 |
$116.9 |
1.5 |
$501.0 |
$167.0 |
830.1620 |
Description of production process |
$418.0 |
$146.3 |
1.9 |
$167.0 |
$668.0 |
830.1650 |
Description of formulation process |
$418.0 |
$146.3 |
1.9 |
$167.0 |
$668.0 |
830.1670 |
Discussion of formulation of impurities |
$418.0 |
$146.3 |
1.9 |
$167.0 |
$668.0 |
830.1700 |
Preliminary analysis |
$31,715.0 |
$11,100.3 |
140.7 |
$50,054.0 |
$8,875.0 |
830.1750 |
Certified limits |
$248.0 |
$86.8 |
1.1 |
$330.0 |
$165.0 |
830.1800 |
Enforcement analytical method |
$15,454.0 |
$5,408.9 |
68.6 |
$21,538.0 |
$9,371.0 |
830.1900 |
Submittal of samples |
$495.0 |
$173.3 |
2.2 |
$660.0 |
$330.0 |
830.6302 |
Color |
$700.0 |
$245.0 |
3.1 |
$1,000.0 |
$400.0 |
830.6303 |
Physical state |
$700.0 |
$245.0 |
3.1 |
$1,000.0 |
$400.0 |
830.6304 |
Odor |
$700.0 |
$245.0 |
3.1 |
$1,000.0 |
$400.0 |
830.6313 |
Stability to normal and elevated temperatures, metals, and metal ions |
$8,250.0 |
$2,887.5 |
36.6 |
$12,000.0 |
$4,500.0 |
830.6314 |
Oxidation/reduction: chemical incompatibility |
$2,994.0 |
$1,047.9 |
13.3 |
$3,044.0 |
$2,944.0 |
830.6315 |
Flammability |
$2,000.0 |
$700.0 |
8.9 |
$3,000.0 |
$1,000.0 |
830.6316 |
Explodability |
$4,163.0 |
$1,457.1 |
18.5 |
$4,163.0 |
$4,163.0 |
830.6317 |
Storage stability |
$11,500.0 |
$4,025.0 |
51.0 |
$15,000.0 |
$8,000.0 |
830.6319 |
|
|
Miscibility |
$1,100.0 |
$385.0 |
4.9 |
$1,500.0 |
$700.0 |
830.6320 |
|
|
Corrosion characteristics |
$2,750.0 |
$962.5 |
12.2 |
$3,500.0 |
$2,000.0 |
830.6321 |
|
|
Dielectric breakdown voltage |
$2,525.0 |
$883.8 |
11.2 |
$2,675.0 |
$2,375.0 |
830.7000 |
|
|
pH |
$750.0 |
$262.5 |
3.3 |
$1,000.0 |
$500.0 |
830.7050 |
|
|
UV/visible light absorption |
$2,022.0 |
$707.7 |
9.0 |
$2,072.0 |
$1,972.0 |
830.7100 |
|
|
Viscosity |
$1,400.0 |
$490.0 |
6.2 |
$2,000.0 |
$800.0 |
830.7200 |
|
|
Melting point/melting range |
$1,200.0 |
$420.0 |
5.3 |
$1,600.0 |
$800.0 |
830.7220 |
|
|
Boiling point/boiling range |
$1,500.0 |
$525.0 |
6.7 |
$2,000.0 |
$1,000.0 |
830.7300 |
|
|
Density/relative density/bulk density |
$1,400.0 |
$490.0 |
6.2 |
$2,000.0 |
$800.0 |
830.7370 |
|
|
Dissociation constants in water |
$4,845.0 |
$1,695.8 |
21.5 |
$4,845.0 |
$4,845.0 |
830.7520 |
|
|
Particle size, fiber length, and diameter distribution |
$1,333.0 |
$466.6 |
5.9 |
$1,333.0 |
$1,333.0 |
830.7550 |
|
|
Partition coefficient (n-octanol/water) - shake flask method |
$6,667.0 |
$2,333.5 |
29.6 |
$7,333.0 |
$6,000.0 |
830.7560 |
|
|
Partition coefficient (n-octanol/water) -generator column |
$6,667.0 |
$2,333.5 |
29.6 |
$7,333.0 |
$6,000.0 |
830.7570 |
|
|
Partition coefficient (n-octanol/water) -estimation chromatography |
$4,388.0 |
$1,535.8 |
19.5 |
$4,700.0 |
$4,075.0 |
830.7840 |
|
|
Water Solubility: column elution/shake flask |
$9,635.0 |
$3,372.3 |
42.7 |
$11,322.0 |
$7,947.0 |
830.7860 |
|
|
Water solubility |
$9,635.0 |
$3,372.3 |
42.7 |
$11,322.0 |
$7,947.0 |
830.7950 |
|
|
Vapor pressure |
$15,000.0 |
$5,250.0 |
66.5 |
$20,000.0 |
$10,000.0 |
|
|
|
|
|
|
|
|
|
Spray Drift |
|
|
|
|
|
|
|
|
840.1100 |
|
|
Spray droplet size spectrum |
$258,750.0 |
$90,562.5 |
1,147.9 |
$340,000.0 |
$177,500.0 |
840.1200 |
|
|
Spray drift field deposition |
$16,250.0 |
$5,687.5 |
72.1 |
$25,000.0 |
$7,500.0 |
|
|
|
|
|
|
|
|
|
Ecological Effects Tests |
|
|
|
|
|
|
|
|
850.1000 |
|
|
Use Profile |
$251.0 |
$87.9 |
1.1 |
$334.0 |
$167.0 |
850.1010 |
|
|
Aquatic invertebrate acute toxicity, freshwater daphnids |
$17,000.0 |
$5,950.0 |
75.4 |
$20,000.0 |
$14,000.0 |
850.1020 |
|
|
Gammarid acute toxicity test |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
850.1025 |
|
|
Oyster acute toxicity test |
$32,725.0 |
$11,453.8 |
145.2 |
$32,725.0 |
$7,550.0 |
850.1035 |
|
|
Mysid acute toxicity test |
$32,725.0 |
$11,453.8 |
145.2 |
$32,725.0 |
$7,550.0 |
850.1045 |
|
|
Penaeid acute toxicity test |
$32,725.0 |
$11,453.8 |
145.2 |
$32,725.0 |
$7,550.0 |
850.1055 |
|
|
Bivalve acute tox larval (embryo/larval) |
$32,725.0 |
$11,453.8 |
145.2 |
$32,725.0 |
$7,550.0 |
850.1075 |
|
|
Fish acute toxicity (freshwater) |
$17,000.0 |
$5,950.0 |
75.4 |
$28,061.0 |
$10,066.0 |
850.1075 |
|
|
Fish acute toxicity test (estaurine/marine) |
$20,138.0 |
$7,048.3 |
89.3 |
$20,138.0 |
$20,138.0 |
850.1300 |
|
|
Daphnid chronic toxicity test |
$118,063.0 |
$41,322.1 |
523.7 |
$162,800.0 |
$73,325.0 |
850.1350 |
|
|
Mysid chronic tox - aquatic invertebrate life-cycle (saltwater) |
$36,333.0 |
$12,716.6 |
161.2 |
$41,000.0 |
$31,667.0 |
850.1400 |
|
|
Fish early-life stage toxicity test (freshwater) |
$37,279.0 |
$13,047.7 |
165.4 |
$41,379.0 |
$33,179.0 |
850.1450 |
|
|
Fish early-life stage toxicity test (saltwater) |
$75,000.0 |
$26,250.0 |
332.7 |
$0.0 |
$0.0 |
850.1500 |
|
|
Fish life-cycle toxicity |
$512,500.0 |
$179,375.0 |
2,273.5 |
$650,000.0 |
$375,000.0 |
850.1710 |
|
|
Aquatic Bioavailability/Biomagnification: Oyster BCF |
$123,919.0 |
$43,371.7 |
549.7 |
$143,919.0 |
$103,919.0 |
850.1730 |
|
|
Aquatic Bioavailability/Biomagnification: Fish BCF |
$140,452.0 |
$49,158.2 |
623.1 |
$169,279.0 |
$111,624.0 |
850.1735 |
|
|
Whole sediment acute toxicity invertebrates (freshwater) |
$20,250.0 |
$7,087.5 |
89.8 |
$21,500.0 |
$26,000.0 |
850.1740 |
|
|
Whole sediment acute toxicity invertebrates (marine) |
$37,500.0 |
$13,125.0 |
166.4 |
$50,000.0 |
$25,000.0 |
850.1790 |
|
|
Chironomid sediment toxicity test |
$83,000.0 |
$29,050.0 |
368.2 |
$83,000.0 |
$83,000.0 |
850.1800 |
|
|
Tadpole/sediment subchronic toxicity test |
$195,856.0 |
$68,549.6 |
868.9 |
$195,856.0 |
$195,856.0 |
850.1850 |
|
|
Aquatic food chain transfer - Bioavailability |
$325,000.0 |
$113,750.0 |
1,441.8 |
$500,000.0 |
$150,000.0 |
850.1900 |
|
|
Generic freshwater microscosm test (laboratory) |
$295,000.0 |
$103,250.0 |
1,308.7 |
$360,000.0 |
$230,000.0 |
850.1925 |
Site-specific aquatic microcosm test (laboratory) |
$250,000.0 |
$87,500.0 |
1,109.0 |
$250,000.0 |
$250,000.0 |
850.1950 |
Simulated or actual field testing - field animal |
$512,500.0 |
$179,375.0 |
2,273.5 |
$650,000.0 |
$375,000.0 |
850.1950 |
Simulated or actual field testing - aquatic |
$600,000.0 |
$210,000.0 |
2,661.7 |
$700,000.0 |
$500,000.0 |
850.1950 |
Simulated or actual field testing - insect predators |
$87,500.0 |
$30,625.0 |
388.2 |
$100,000.0 |
$75,000.0 |
850.1950 |
Simulated or actual field testing - plants |
$62,500.0 |
$21,875.0 |
277.3 |
$75,000.0 |
$50,000.0 |
850.2100 |
Avian acute oral toxicity test |
$10,100.0 |
$3,535.0 |
44.8 |
$13,800.0 |
$6,400.0 |
850.2200 |
Avian dietary toxicity test |
$6,480.0 |
$2,268.0 |
28.7 |
$6,647.0 |
$6,313.0 |
850.2300 |
Avian reproduction test |
$168,250.0 |
$58,887.5 |
746.4 |
$215,500.0 |
$121,000.0 |
850.2400 |
Wild mammal acute toxicity |
$35,000.0 |
$12,250.0 |
155.3 |
$50,000.0 |
$20,000.0 |
850.2500 |
Simulated or actual field testing terrestrial wildlife |
$527,502.0 |
$184,625.7 |
2,340.1 |
$552,000.0 |
$502,000.0 |
850.2500 |
Simulated or actual field testing - birds |
$600,000.0 |
$210,000.0 |
2,661.7 |
$700,000.0 |
$500,000.0 |
850.3020 |
Honey bee acute contact toxicity |
$3,175.0 |
$1,111.3 |
14.1 |
$3,175.0 |
$3,175.0 |
850.3030 |
Honey bee toxicity of residues on foliage |
$13,368.0 |
$4,678.8 |
59.3 |
$16,670.0 |
$10,065.0 |
850.3040 |
Field testing for pollinators |
$47,500.0 |
$16,625.0 |
210.7 |
$65,000.0 |
$30,000.0 |
850.4000 |
Background - Nontarget plant testing |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
850.4025 |
Target area phytotoxicity |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
850.4100 |
Terrestrial plant toxicity (seedling emergence, Tier I) |
$14,625.0 |
$5,118.8 |
64.9 |
$15,625.0 |
$13,625.0 |
850.4150 |
Terrestrial plant toxicity (vegetative vigor, Tier I) |
$14,625.0 |
$5,118.8 |
64.9 |
$15,625.0 |
$13,625.0 |
850.4200 |
Seed germination/root elongation toxicity test |
$10,292.0 |
$3,602.2 |
45.7 |
$15,875.0 |
$4,709.0 |
850.4200 |
Seed germination/root elongation toxicity test |
$25,602.5 |
$8,960.9 |
113.6 |
$30,930.0 |
$20,275.0 |
850.4230 |
Early seed growth toxicity test |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
850.4225 |
Seedling emergence, Tier II |
$20,375.0 |
$7,131.3 |
90.4 |
$22,000.0 |
$18,750.0 |
850.4250 |
Vegetative vigor, Tier II |
$24,500.0 |
$8,575.0 |
108.7 |
$26,500.0 |
$22,500.0 |
850.4300 |
Terrestrial plants field study, Tier III |
$111,863.0 |
$39,152.1 |
496.2 |
$126,863.0 |
$26,500.0 |
850.4400 |
Aquatic plant toxicology test using Lemna spp., Tier I |
$35,155.0 |
$12,304.3 |
156.0 |
$39,525.0 |
$18,750.0 |
850.4400 |
Aquatic plant toxicology test using Lemna spp., Tier II |
$35,155.0 |
$18,632.2 |
236.2 |
$35,155.0 |
$35,155.0 |
850.4450 |
Aquatic plants field study, Tier III |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
850.5400 |
Algal Toxicity Tier I and Tier II |
$35,155.0 |
$12,304.3 |
156.0 |
$35,155.0 |
$35,155.0 |
na |
Acute toxicity to aquatic insects |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
na |
Aquatic insect life-cycle study |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
na |
Simulated or actual field testing for aquatic insects |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
na |
Nontarget insect testing - predators and parasites |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
na |
Nontarget insect testing - predators and parasites |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
|
|
|
|
|
|
|
Health Effects |
|
|
|
|
|
|
870.1100 |
Acute oral toxicity (rat) |
$3,473.0 |
$1,215.6 |
15.4 |
$4,015.0 |
$2,932.0 |
870.1200 |
Acute dermal toxicity |
$2,000.0 |
$700.0 |
8.9 |
$3,000.0 |
$1,000.0 |
870.1300 |
Acute inhalation toxicity (rat) |
$2,000.0 |
$700.0 |
8.9 |
$3,000.0 |
$1,000.0 |
870.1300 |
Acute inhalation tox (microbials) |
$12,000.0 |
$4,200.0 |
53.2 |
$20,000.0 |
$4,000.0 |
870.2400 |
Acute eye irritation (rabbit) |
$2,000.0 |
$700.0 |
8.9 |
$3,000.0 |
$1,000.0 |
870.2500 |
Acute dermal irritation |
$2,000.0 |
$700.0 |
8.9 |
$3,000.0 |
$1,000.0 |
870.2600 |
Skin (dermal) sensitization |
$8,000.0 |
$2,800.0 |
35.5 |
$10,000.0 |
$6,000.0 |
870.3100 |
90-day oral toxicity in rodents |
$138,106.0 |
$48,337.1 |
612.7 |
$142,517.0 |
$133,695.0 |
870.3150 |
90-day oral toxicity in non-rodents |
$221,047.0 |
$77,366.5 |
980.6 |
$221,047.0 |
$221,047.0 |
870.3200 |
21/28-day dermal toxicity |
$83,240.0 |
$29,134.0 |
369.3 |
$84,681.0 |
$81,798.0 |
870.3250 |
90-day dermal toxicity |
$137,094.0 |
$47,982.9 |
608.2 |
$138,114.0 |
$137,094.0 |
870.3465 |
90-day inhalation toxicity (rat) |
$300,000.0 |
$105,000.0 |
1,330.9 |
$350,000.0 |
$300,000.0 |
870.3700 |
Prenatal developmental toxicity study (rat and rabbit, preferred) |
$76,844.0 |
$26,895.4 |
340.9 |
$77,037.0 |
$76,844.0 |
870.3800 |
Reproduction and fertility effects (multigeneration) |
$378,479.0 |
$132,467.7 |
1,679.0 |
$381,233.0 |
$378,479.0 |
870.4100 |
Chronic tox (rodent and non-rodent) |
$950,000.0 |
$332,500.0 |
4,214.4 |
$1,100,000.0 |
$950,000.0 |
870.4200 |
Carcinogenicity (rat and mouse, preferred) |
$1,730,000.0 |
$605,500.0 |
7,674.6 |
$2,060,000.0 |
$1,730,000.0 |
870.4200 |
Carcinogenicity (microbials) |
$922,244.0 |
$322,785.4 |
4,091.2 |
$925,806.0 |
$922,244.0 |
870.5100 |
Bacterial reverse mutation assay |
$4,057.0 |
$1,420.0 |
18.0 |
$4,457.0 |
$4,057.0 |
870.5300 |
In vitro mammalian cell gene mutation test |
$18,654.0 |
$6,528.9 |
82.8 |
$19,767.0 |
$18,654.0 |
870.5375 |
In vitro mammalian chromosomal aberration test |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
870.5380 |
Mammalian spermatogonial chromosomal aberration test |
$19,382.0 |
$6,783.7 |
86.0 |
$19,382.0 |
$19,382.0 |
870.5385 |
Mammalian bone marrow chromosomal aberration test |
$30,004.0 |
$10,501.4 |
133.1 |
$30,004.0 |
$30,004.0 |
870.5395 |
Mammalian erthrocyte micronucleus test |
$20,477.0 |
$7,167.0 |
90.8 |
$20,594.0 |
$20,477.0 |
none |
Reference list of all studies/papers known to the applicant concerning mutagenicity |
$418.0 |
$146.3 |
1.9 |
$418.0 |
$418.0 |
870.5450 |
Rodent dominant lethal assay |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
870.5500 |
Bacterial DNA damage or repair tests |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
870.5550 |
Unscheduled DNA synthesis in mammalian cells in culture |
$30,000.0 |
$10,500.0 |
133.1 |
$0.0 |
$0.0 |
870.6100 |
Acute and 28 day delayed neurotoxicity organophosphorus substances (hen) |
$79,375.0 |
$27,781.3 |
352.1 |
$80,625.0 |
$72,125.0 |
870.6200 |
Acute neurotoxicity (rat) |
$89,596.0 |
$31,358.6 |
397.5 |
$91,680.0 |
$87,513.0 |
870.6200 |
90-day Neurotoxicity (rat) |
$184,039.0 |
$64,413.7 |
816.4 |
$186,410.0 |
$181,668.0 |
870.6300 |
Developmental neurotoxicity study |
$406,904.0 |
$142,416.4 |
1,805.1 |
$417,135.0 |
$396,904.0 |
870.6500 |
Schedule-controlled operant behavior |
$164,000.0 |
$57,400.0 |
727.5 |
$164,000.0 |
$164,000.0 |
870.6850 |
Peripheral nerve function |
$110,000.0 |
$38,500.0 |
488.0 |
$110,000.0 |
$110,000.0 |
870.6855 |
Neurophysiology: sensory evoked potentials |
$110,000.0 |
$38,500.0 |
488.0 |
$110,000.0 |
$110,000.0 |
870.7200 |
Companion animal safety |
$156,000.0 |
$54,600.0 |
692.0 |
$167,667.0 |
$144,333.0 |
870.7485 |
Metabolism and pharmacokinetics |
$182,729.0 |
$63,955.2 |
810.6 |
$217,729.0 |
$147,729.0 |
870.7600 |
Dermal penetration |
$147,529.0 |
$51,635.2 |
654.5 |
$175,346.0 |
$119,711.0 |
870.7800 |
Immunotoxicity |
$56,648.0 |
$19,826.8 |
251.3 |
$57,731.0 |
$55,565.0 |
|
|
|
|
|
|
|
Occupational and Residential Exposure |
|
|
|
|
|
|
875.1100 |
Dermal outdoor exposure |
$167,857.0 |
$58,750.0 |
744.6 |
$192,143.0 |
$143,571.0 |
875.1200 |
Dermal indoor exposure |
$126,429.0 |
$44,250.2 |
560.9 |
$150,714.0 |
$102,143.0 |
875.1300 |
Inhalation outdoor exposure |
$164,286.0 |
$57,500.1 |
728.8 |
$181,429.0 |
$147,143.0 |
875.1400 |
Inhalation indoor exposure |
$126,429.0 |
$44,250.2 |
560.9 |
$150,714.0 |
$102,143.0 |
875.1500 |
Biological monitoring |
$188,393.0 |
$65,937.6 |
835.7 |
$219,256.0 |
$157,500.0 |
875.1600 |
Application exposure data reporting and calculations |
$7,500.0 |
$2,625.0 |
33.3 |
$10,000.0 |
$5,000.0 |
875.1700 |
Product use information |
$3,000.0 |
$1,050.0 |
13.3 |
$4,000.0 |
$2,000.0 |
875.2100 |
Dislodgeable foliar residue dissipation and turf transferable residues |
$55,100.0 |
$19,285.0 |
244.4 |
$56,100.0 |
$54,100.0 |
875.2200 |
Soil residue dissipation |
$83,125.0 |
$29,093.8 |
368.8 |
$85,000.0 |
$81,250.0 |
875.2300 |
Indoor surface residue dissipation |
$35,000.0 |
$12,250.0 |
155.3 |
$35,000.0 |
|
875.2400 |
Dermal exposure |
$125,500.0 |
$43,925.0 |
556.7 |
$133,000.0 |
$118,000.0 |
875.2500 |
Inhalation exposure |
$73,000.0 |
$25,550.0 |
323.8 |
$81,000.0 |
$65,000.0 |
875.2600 |
Biological monitoring |
$166,875.0 |
$58,406.3 |
740.3 |
$191,667.0 |
$142,083.0 |
875.2700 |
Product use information |
$3,000.0 |
$1,050.0 |
13.3 |
$4,000.0 |
$2,000.0 |
875.2800 |
Description of human activity |
$3,000.0 |
$1,050.0 |
13.3 |
$4,000.0 |
$2,000.0 |
875.2900 |
Data reporting and calculations |
$3,000.0 |
$1,050.0 |
13.3 |
$4,000.0 |
$2,000.0 |
875.3000 |
Nondietary ingestion exposure |
$75,000.0 |
$26,250.0 |
332.7 |
$83,333.0 |
$66,667.0 |
|
|
|
|
|
|
|
Environmental Fate |
|
|
|
|
|
|
none |
Use Profile |
$251.0 |
$0.0 |
0.0 |
$334.0 |
$167.0 |
835.1230 |
Sediment and soil adsorption/desorption |
$23,750.0 |
$8,312.5 |
105.4 |
$24,583.0 |
$22,917.0 |
835.1240 |
Leaching and adsorption/desorption |
$46,780.0 |
$16,373.0 |
207.5 |
$51,880.0 |
$41,680.0 |
835.1410 |
Laboratory volatility |
$45,000.0 |
$15,750.0 |
199.6 |
$5,000.0 |
$40,000.0 |
835.2120 |
Hydrolysis |
$25,230.0 |
$8,830.5 |
111.9 |
$29,900.0 |
$20,560.0 |
835.2240 |
Photodegradation in water |
$46,875.0 |
$16,406.3 |
207.9 |
$47,875.0 |
$47,875.0 |
835.2370 |
Photodegradation in air |
$110,000.0 |
$38,500.0 |
488.0 |
$120,000.0 |
$100,000.0 |
835.2410 |
Photodegradation on soil |
$42,350.0 |
$14,822.5 |
187.9 |
$45,183.0 |
$39,517.0 |
835.4100 |
Aerobic soil metabolism |
$94,375.0 |
$33,031.3 |
418.7 |
$98,625.0 |
$90,125.0 |
835.4200 |
Anaerobic soil metabolism |
$71,300.0 |
$24,955.0 |
316.3 |
$71,300.0 |
$71,300.0 |
835.4300 |
Aerobic aquatic metabolism |
$44,475.0 |
$15,566.3 |
197.3 |
$47,350.0 |
$41,600.0 |
835.4400 |
Anaerobic aquatic metabolism |
$80,900.0 |
$28,315.0 |
358.9 |
$86,525.0 |
$72,275.0 |
835.6100 |
Terrestrial field dissipation |
$317,767.0 |
$111,218.5 |
1,409.7 |
$366,067.0 |
$269,467.0 |
835.6200 |
Aquatic field dissipation |
$267,250.0 |
$93,537.5 |
1,185.6 |
$354,000.0 |
$180,500.0 |
835.6300 |
Forestry dissipation |
$275,500.0 |
$96,425.0 |
1,222.2 |
$362,500.0 |
$188,500.0 |
835.6400 |
Combination and tank mixes |
$219,200.0 |
$76,720.0 |
972.4 |
$219,200.0 |
|
835.8100 |
Field volatility |
$230,900.0 |
$80,815.0 |
1,024.3 |
$230,900.0 |
|
835.7100 |
Groundwater Monitoring |
$1,225,000.0 |
$428,750.0 |
5,434.3 |
$2,000,000.0 |
$450,000.0 |
none |
Monitoring of representative U.S. waters |
$215,833.0 |
$75,541.6 |
957.5 |
$263,333.0 |
$168,333.0 |
none |
Leaching study |
$43,000.0 |
$15,050.0 |
190.8 |
$48,000.0 |
$38,000.0 |
|
|
|
|
|
|
|
Residue Chemistry |
|
|
|
|
|
|
860.1100 |
Chemical identity |
$1,250.0 |
$437.5 |
5.5 |
$2,000.0 |
$500.0 |
860.1200 |
Directions for use |
$4,000.0 |
$1,400.0 |
17.7 |
$5,000.0 |
$3,000.0 |
860.1300 |
Nature of the residue in plants |
$100,000.0 |
$35,000.0 |
443.6 |
$105,000.0 |
$95,000.0 |
860.1300 |
Nature of the residue in livestock |
$105,833.0 |
$37,041.6 |
469.5 |
$118,333.0 |
$93,333.0 |
860.1340 |
Residue analytical method - plants |
$22,125.0 |
$7,743.8 |
98.2 |
$23,250.0 |
$19,000.0 |
860.1340 |
Residue analytical method - livestock |
$65,500.0 |
$22,925.0 |
290.6 |
$76,800.0 |
$54,200.0 |
860.1360 |
Multiresidue method |
$24,000.0 |
$8,400.0 |
106.5 |
$25,667.0 |
$22,333.0 |
860.1380 |
Storage stability data |
$18,500.0 |
$6,475.0 |
82.1 |
$18,500.0 |
$18,500.0 |
860.1400 |
Water |
$53,750.0 |
$18,812.5 |
238.4 |
$55,000.0 |
$52,500.0 |
860.1400 |
Fish |
$104,000.0 |
$36,400.0 |
461.4 |
$130,000.0 |
$78,000.0 |
860.1400 |
Irrigated crops (one-crop) |
$18,000.0 |
$6,300.0 |
79.9 |
$20,500.0 |
$15,500.0 |
860.1460 |
Food handling |
$205,000.0 |
$71,750.0 |
909.4 |
$230,000.0 |
$180,000.0 |
860.1480 |
Meat/milk/poultry/eggs |
$149,000.0 |
$52,150.0 |
661.0 |
$152,333.0 |
$145,667.0 |
860.1500 |
Crop field trials |
$163,667.0 |
$57,283.5 |
726.1 |
$177,000.0 |
$150,333.0 |
860.1520 |
Processed food/feed |
$35,000.0 |
$12,250.0 |
155.3 |
$37,333.0 |
$32,667.0 |
860.1540 |
Reduction of Residues |
$15,000.0 |
$5,250.0 |
66.5 |
$20,000.0 |
$10,000.0 |
860.1550 |
Proposed tolerance |
$5,363.0 |
$1,877.1 |
23.8 |
$6,600.0 |
$4,124.0 |
860.1560 |
Reasonable grounds in support of the petition |
$10,000.0 |
$3,500.0 |
44.4 |
$15,000.0 |
$5,000.0 |
|
860.1650 |
Submittal of analytical reference standards |
$334.0 |
$116.9 |
1.5 |
$501.0 |
$167.0 |
|
860.1850 |
Confined accumulation in rotational crops |
$249,845.0 |
$87,445.8 |
1,108.4 |
$269,845.0 |
$229,845.0 |
|
860.1900 |
Field accumulation in rotational crops |
$137,500.0 |
$48,125.0 |
610.0 |
$125,000.0 |
$150,000.0 |
|
none |
Migration Studies |
$105,000.0 |
$36,750.0 |
465.8 |
$120,000.0 |
$90,000.0 |
|
|
|
|
|
|
|
|
|
Microbial Pesticides |
|
|
|
|
|
|
|
880.1100 |
Product identity |
$233.0 |
$81.6 |
1.0 |
$300.0 |
$165.0 |
|
880.1200 |
Description materials, production, formulation |
$908.0 |
$317.8 |
4.0 |
$1,650.0 |
$165.0 |
|
880.1400 |
Discussion of formation of impurities |
$330.0 |
$115.5 |
1.5 |
$495.0 |
$165.0 |
|
880.3800 |
Immune Response |
$85,000.0 |
$29,750.0 |
377.1 |
$100,000.0 |
$70,000.0 |
|
880.4350 |
Non-target insect testing |
$15,000.0 |
$5,250.0 |
66.5 |
$18,000.0 |
$12,000.0 |
|
880.4425 |
Dispenser - water leaching |
$25,000.0 |
$8,750.0 |
110.9 |
$30,000.0 |
$20,000.0 |
|
|
Hypersensitivity incidents |
$825.0 |
$288.8 |
3.7 |
$1,320.0 |
$330.0 |
|
885.1100 |
Product Identity |
$5,000.0 |
$1,750.0 |
22.2 |
$8,000.0 |
$2,000.0 |
|
885.1200a |
Manufacturing process |
$3,500.0 |
$1,225.0 |
15.5 |
$5,000.0 |
$2,000.0 |
|
885.1200b |
Deposition of samples |
$3,500.0 |
$1,225.0 |
15.5 |
$5,000.0 |
$2,000.0 |
|
885.1300 |
Discussion of formulation of unintentional ingredients |
$3,500.0 |
$1,225.0 |
15.5 |
$5,000.0 |
$2,000.0 |
|
885.1400 |
Analysis of samples |
$74,500.0 |
$26,075.0 |
330.5 |
$145,000.0 |
$4,000.0 |
|
885.1500 |
Certification of limits |
$350.0 |
$122.5 |
1.6 |
$500.0 |
$200.0 |
|
885.2000 |
Background for residue analysis of microbial pest control agents |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
|
885.2100 |
Chemical identity |
$660.0 |
$231.0 |
2.9 |
$825.0 |
$495.0 |
|
885.2200 |
Nature of the residue in plants |
$108,333.0 |
$37,916.6 |
480.6 |
$108,333.0 |
$108,333.0 |
|
885.2250 |
Nature of the residue in animals |
$117,144.0 |
$41,000.4 |
519.7 |
$123,750.0 |
$110,538.0 |
|
885.2300 |
Analytical method - plants |
$26,540.0 |
$9,289.0 |
117.7 |
$34,040.0 |
$19,040.0 |
|
885.2350 |
Analytical method - animals |
$43,908.0 |
$15,367.8 |
194.8 |
$56,325.0 |
$31,492.0 |
|
885.2400 |
Storage stability, plants |
$31,017.0 |
$10,856.0 |
137.6 |
$32,683.0 |
$29,350.0 |
|
885.2500 |
Magnitude of residue in plants |
$137,160.0 |
$48,006.0 |
608.5 |
$137,587.0 |
$136,733.0 |
|
885.2550 |
Magnitude of residue in meat/milk/poultry |
$157,663.0 |
$55,182.1 |
699.4 |
$162,425.0 |
$152,900.0 |
|
885.2600 |
Magnitude of residue in potable water, fish, and irrigated crops |
$221,442.0 |
$77,504.7 |
982.4 |
$245,225.0 |
$197,658.0 |
|
885.3000 |
Background Mammalian Infectivity/pathogenicity analysis |
$250,000.0 |
$87,500.0 |
1,109.0 |
$250,000.0 |
$250,000.0 |
|
885.3050 |
Acute oral toxicity/pathogenicity |
$33,500.0 |
$11,725.0 |
148.6 |
$41,000.0 |
$25,000.0 |
|
885.3150 |
Acute pulmonary toxicity/pathogenicity |
$37,500.0 |
$13,125.0 |
166.4 |
$50,000.0 |
$25,000.0 |
|
885.3200 |
Acute injection toxicity/pathogenicity (intravenous) |
$37,500.0 |
$13,125.0 |
166.4 |
$50,000.0 |
$25,000.0 |
|
885.3200 |
Acute injection toxicity/pathogenicity (intraperitoneal) |
$12,500.0 |
$4,375.0 |
55.5 |
$18,000.0 |
$7,000.0 |
|
885.3400 |
Hypersensitivity incidents |
$800.0 |
$280.0 |
3.5 |
$1,300.0 |
$300.0 |
|
885.3500 |
Cell Culture |
$30,000.0 |
$10,500.0 |
133.1 |
$35,000.0 |
$25,000.0 |
|
885.3550 |
Acute toxicity, TI |
$21,500.0 |
$7,525.0 |
95.4 |
$40,000.0 |
$3,000.0 |
|
885.3600 |
Subchronic toxicity/pathogenicity |
$150,000.0 |
$52,500.0 |
665.4 |
$200,000.0 |
$100,000.0 |
|
885.3650 |
Reproductive/fertility effects |
$162,500.0 |
$56,875.0 |
720.9 |
$20,000.0 |
$125,000.0 |
|
885.4050 |
Avian Oral, TI |
$15,000.0 |
$5,250.0 |
66.5 |
$18,000.0 |
$12,000.0 |
|
885.4100 |
Avian Inhalation toxicity/pathogenicity, TI |
$16,000.0 |
$5,600.0 |
71.0 |
$20,000.0 |
$12,000.0 |
|
885.4150 |
Wild mammal toxicity/pathogenicity,TI |
$65,000.0 |
$22,750.0 |
288.4 |
$80,000.0 |
$50,000.0 |
|
885.4200 |
Freshwater fish toxicity/pathogenicity,TI |
$37,500.0 |
$13,125.0 |
166.4 |
$45,000.0 |
$30,000.0 |
|
885.4240 |
Freshwater invertebrate toxicity/pathogenicity,TI |
$37,500.0 |
$13,125.0 |
166.4 |
$45,000.0 |
$30,000.0 |
|
|
885.4280 |
Estuarine/marine animal testing, TI |
$40,000.0 |
$14,000.0 |
177.4 |
$48,000.0 |
$32,000.0 |
|
885.4280 |
Estuarine/marine invertebrate testing,TI |
$40,000.0 |
$14,000.0 |
177.4 |
$48,000.0 |
$32,000.0 |
|
885.4300 |
Nontarget plant studies, TI |
$30,000.0 |
$10,500.0 |
133.1 |
$40,000.0 |
$20,000.0 |
|
885.4380 |
Honey bee testing |
$4,250.0 |
$1,487.5 |
18.9 |
$5,000.0 |
$3,500.0 |
|
885.4600 |
Avian chronic pathogenicity and reproduction, TIII |
$175,000.0 |
$61,250.0 |
776.3 |
$200,000.0 |
$150,000.0 |
|
885.4650 |
Aquatic invertebrate range testing,TIII |
$75,000.0 |
$26,250.0 |
332.7 |
$1,000.0 |
$50,000.0 |
|
885.4700 |
Fish life cycle studies,TIII |
$250,000.0 |
$87,500.0 |
1,109.0 |
$300,000.0 |
$200,000.0 |
|
885.4750 |
Aquatic ecosystem test |
$350,000.0 |
$122,500.0 |
1,552.7 |
$400,000.0 |
$300,000.0 |
|
885.5200 |
Terrestrial environmental expression tests |
$95,000.0 |
$33,250.0 |
421.4 |
$150,000.0 |
$40,000.0 |
|
885.5300 |
Freshwater environmental expression test |
$0.0 |
$0.0 |
0.0 |
$0.0 |
$0.0 |
|
885.5400 |
Marine or estaurine environmental expression tests |
$95,000.0 |
$33,250.0 |
421.4 |
$150,000.0 |
$40,000.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes:
|
|
Labor Rates in 2003 dollars, but data collected represents 2003 -2007 Clerical: $40/hr Technical: $88/hr Management: $130/hr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Color Code: |
|
|
|
|
|
|
|
|
|
Study has no cost estimate, source or year |
|
|
|
|
|
|
|
Test cost estimate has no source or year |
|
|
|
|
|
1 OMB is part of the Executive Office of the President.
2 5 CFR 1320.3(l)
3 5 CFR 1320.3(b)
4 Assumes registrants perform most of the PRA activities highlighted in, Section I-V, “DCI burden activity tables;” Table 1 – Response Phases
5 See Section I-V, “DCI burden activity tables;” Table 1 – Response Phases; which lists specific managerial, technical, and clerical duties.
6 “Fully loaded” labor rates are meant to be the estimated costs of wages, overhead, and benefits paid to an employee.
7 See Section 1-V “DCI burden activity tables;” Table 1 – Response Phases.
8 See Environmental Protection Agency proposed rule 40 CFR parts 158 and 172, Subparts L&M:
Data Requirements for Registration of Biochemical and Microbial Pesticides, (45 FR 12072 Wednesday March 8, 2006), section XVII Regulatory Assessment. For specific information refer to the docket EPA-HQ-OPP-2004-0415, document 6, U.S. EPA, 2005, “Economic Analysis of the Proposed Change to Data Requirements Rule for Biochemical and Microbial Pesticides,” FEAD/OPP/U.S. EPA, Washington, DC.
9 “Me too” pesticides are pesticides that are substantially similar to an existing pesticide. When applying for a “me-too” registration, a registrant would either cite existing data or claim eligibility for a formulator’s exemption. A registrant is eligible for a formulators exemption if he uses a registered pesticide product as the source of the active ingredient in his product. Little or no technical labor is involved for this type of response.
10 As an example, consider the DCI respondent who claims a generic data exemption (GDE) as their response to a DCI. A generic data exemption is the same as a formulator’s exemption. Little or no technical labor burden is involved for this type of response.
11 The Biopesticide Industry Alliance Regulatory Committee provided additional informal cost estimates to EPA. Burden breakdown and costs estimates would be: Research, literature searches, calculations, analysis etc. = 10 hours = $1000 writing; compiling the rationale = 8 hours = $800; and managerial/clerical = 2 hours = $200. For a total of $2,000.00 for each waiver. A general $100.00 per hour for consultant costs was provided to EPA.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Microsoft Word - EPA-HQ-OPP-2007-0923-DRAFT-0004 |
| Author | SYSTEM |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy