NHAMCS 2018-2020 Supporting Stat B 051118
NHAMCS 2018-2020 Supporting Stat B 051118.docx
National Hospital Ambulatory Medical Care Survey
OMB: 0920-0278
Supporting Statement B Request for Reinstatement with Change:
NATIONAL HOSPITAL AMBULATORY MEDICAL CARE SURVEY
OMB No. 0920-0278
Contact Information:
Brian Ward, PhD
Team Lead, Ambulatory Care Team
Ambulatory and Hospital Care Statistics Branch
Division of Health Care Statistics
National Center for Health Statistics/CDC
3311 Toledo Road, Room 3548
Hyattsville, MD 20782
301-458-4568
301-458-4693 (fax)
May 11, 2018



TABLE OF CONTENTS
Supporting Statement B – Collection of Information Employing Statistical Methods Pages
1. Respondent Universe and Sampling Methods 4
2. Procedures for the Collection of Information 5
3. Methods to Maximize Response Rates and Deal with Nonresponse 8
4. Tests of Procedures or Methods to be Undertaken 9
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data 9
Attachments:
Attachment A - Applicable Laws and Regulations
Attachment B.1 - 60-day Federal Registry Notice (FRN)
Attachment B.2 - 60-day FRN Public Comments
Attachment C - List of publications
Attachment D - List of Consultants
Attachment E - CDC Ethics Review Board Approval for Continuation of Protocol
Attachment F.1 - Introductory letter
Attachment F.2 - Reabstraction letter
Attachment G - Endorsement letters
Attachment H - Hospital Induction Interview (2018)
Attachment I - Ambulatory Unit Induction Interview (2018)
Attachment J. - Emergency Department Patient Record Form
Attachment K - Retrieving Medical Records
Attachment L - Reabstraction Telephone Call
Attachment M - Retrieving Medical Records (Reabstraction)
B. Collections of Information Employing Statistical Methods
The primary goal of the National Hospital Ambulatory Medical Care Survey (NHAMCS) has been to collect data on visits to emergency departments (EDs), outpatient departments (OPDs) and hospital-based ambulatory surgery locations (ASLs). But due to budgetary constraints, starting in 2018, NHAMCS will only collect information from hospital EDs. According to the 2015 NHAMCS, the estimated number of U.S. hospital ED visits was 136,943,000. The 2018-2020 NHAMCS sample of ED visits will use a three-stage probability design based on samples of geographic Primary Sampling Units (PSUs), hospitals within PSUs, and patient visits within those ambulatory units. We plan on fielding NHAMCS only until the National Hospital Care Survey (OMB No. 0920-0212, Exp. Date: 01/31/2019) is able to provide reliable national estimates.
1. Respondent Universe and Sampling Methods
The NHAMCS universe and sampling design are outlined at http://www.cdc.gov/nchs/ahcd/ahcd_estimation_procedures.htm#nhamcs_procedures. The universe for the NHAMCS consists of non-Federal, noninstitutional hospitals in the 50 states and District of Columbia, which have six or more beds staffed for inpatient use. These hospitals must specialize in general and medical, maternity, children’s general, or long term acute care, and have an average length of stay for all patients of less than 30 days. The hospital universe frame and sample were most recently updated for the 2017 NHAMCS using hospital data from the 2015 release of the Health Care Organizations (HCOs) database obtained from IMS Health, L.L.C. and data on annual visit volumes obtained from the American Hospital Database (AHD). The AHD data on visit volumes was used because IMS Health, L.L.C., no longer collects visit volumes for establishments in its databases.
The NHAMCS sample is a multi-stage design with a first stage sample consisting of two of the four PSU panels in the 1985-94 National Health Interview Survey (NHIS). The first-stage sample consists of 112 PSUs. From the sample PSUs, a stratified sample of approximately 600 hospitals was originally selected for the NHAMCS with hospital strata defined by whether hospitals had EDs and/or OPDs according to the sampling frame data. After the last update (in 2017), there are still about 600 hospitals in the total sample. Sample hospitals are randomly assigned to 16 panels with four-week reporting periods as described below. Because there are only 52 weeks in a year, only 13 of the 16 panels or about 450 hospitals are rotated into the sample annually. In each of these annual samples, we expect approximately 340 hospitals to have EDs and, thus, be in-scope for the 2018-2020 NHAMCS. This sample is sufficient to produce estimates with relative standard errors of 30 percent of less. See discussion of NCHS standards for reliability at http://www.cdc.gov/nchs/ahcd/ahcd_estimation_reliability.htm.
Hospitals
Non-Federal, short-stay (<30 days), and hospitals specializing in general and medical, maternity, children’s general, or long term acute care in the sample PSUs are eligible for inclusion in the sample. Institutional hospitals or hospitals with fewer than six beds for inpatient use are excluded from the sampling frame. Hospitals are stratified by whether they have an ED and/or an OPD and by certainty status (self-representing vs. non-self-representing) of the sample area for their location. Prior to sampling, hospitals are arrayed within PSUs by type of ownership (voluntary nonprofit, non-Federal government, proprietary) and size, where size is measured by volume of ED and OPD visits reported in the hospital sampling frame (which was most recently constructed using 2015 data from IMS Health and American Hospital Database). From the arrayed hospital list, five hospitals are selected in each PSU without replacement and with probability proportional to the visit volume. If there are five or fewer hospitals, then all hospitals in the PSU are selected.
A sample of approximately 600 hospitals is randomly divided into 16 groups of hospitals (that is, 37-38 hospitals in each group) in order to avoid hospitals participating during the same time period each year. Of these groups (or panels), only 13 are used within a given survey year. The hospital groups are assigned on a rotating basis to four-week reporting periods meaning that each hospital will be inducted approximately every 15 months. Substitution of the reporting period is not permitted. Based on the results of the 2015 NHAMCS, for which only the ED data are currently publicly available, the projected unweighted and weighted response rates for 2018 are 80% and 81% respectively.
Ambulatory Units
For the 2018-2020 cycles of NHAMCS, only the emergency departments/services will be inducted into the survey.
During the visit by a field representative to induct a hospital with an emergency department into the survey, a list of all emergency service areas (ESAs) is obtained. ESAs are defined as the smallest administrative unit of an ED where separate patient statistics are kept. It may be located on hospital grounds or operated off site by the hospital. All ESAs within a sample hospital are included.
Visits in all ED locations
Within the ED’s emergency service areas (ESAs), patient visits are systematically selected over the four-week reporting period assigned to the ED’s hospital. A visit is defined as a direct, personal exchange between an ambulatory patient and a physician, or a staff member acting under a physician's direction, for the purpose of seeking care and rendering health services. Visits solely for administrative purposes, such as payment of a bill, and visits in which no medical care is provided, such as visits to deliver a specimen, are out-of-scope.
Samples of approximately 100 visits are targeted from each hospital ED. If there are multiple ambulatory units within a targeted department, up to 30 visits are targeted from each unit in the survey. Sampling rates are determined from the number of patients seen during the reporting period and the desired number of sample records. This basic procedure is adapted, as necessary, to the record keeping systems of the particular hospitals. Previous NHAMCS studies have found that many ambulatory units keep their own logs which are used as the sampling frame for visits.
2. Procedures for the Collection of Information
Training
Training in data collection procedures is conducted at different times with four different types of staff. Census Bureau Headquarters staff are responsible for training the Regional Office staff. Regional Office staff have the primary responsibility for training the field representatives and supervising hospital data collection activities. Field representative training covers the following topics: inducting facilities, ensuring confidentiality by adhering to the Health Insurance Portability and Accountability Act (HIPAA), patient visit sampling, retrieving missing data, and medical record abstraction.
Census Bureau Headquarters staff are also responsible for writing the field manual which contains the following: the purposes of the survey; interviewing techniques; a description of the NHAMCS induction questionnaire and related forms; and the procedures for inducting hospitals, conducting hospital visits, patient visit sampling, and retrieving missing data.
Initial Contact
An introductory letter is sent from the Director of NCHS (Attachment F.1) to the chief executive officer of each sampled hospital. The letter describes the purpose of the survey, the authority for data collection, that participation is voluntary and that all collected information is confidential including the identity of the hospital [308(d) confidentiality requirements and Confidential Information Protection and Statistical Efficiency Act (PL-107-347)]. It also covers requirements related to HIPAA. At no time is patient consent required to obtain information. Letters of endorsement by the American College of Emergency Physicians (ACEP), Society for Academic Emergency Medicine (SAEM), Emergency Nurses Association (ENA), American College of Osteopathic Emergency Physicians (ACOEP), American Health Information Management Association (AHIMA) (Attachment G) are included in the mailing.
Hospital Induction
In an effort to improve visit volumes reported by sample hospitals, a new field operation was adopted in 2016 which requires that field representatives begin inducting the sampled hospital only after the reporting period has passed. In prior years, the telephone screener and induction stages were done about six-weeks before the reporting period began. The telephone screener call from the field representative is to ensure verification of hospital eligibility for the survey and to arrange for an appointment with the chief executive officer, the directors of the hospital department targeted, or whoever is designated as hospital liaison for this survey. During the meeting, the field representative explains the purpose of the survey, describes the data collection methods and length of the data collection period, and obtains both general descriptive information about the organization of the targeted hospital department(s), including specific information needed about service areas within the departments. Beginning in 2018, the Hospital Induction Interview (Attachment H) will be used to verify hospital sampling frame information, induct the sample hospitals, and obtain ED data.
Completion of Patient Record Forms
In order to decrease burden to facility staff and to facilitate the data collection procedures, field representatives will complete the Patient Record forms. For the 2018-2020 data collection, patient visit data are recorded for each sample visit using the ED Patient Record form (PRF) (Attachment J.1). Instructions on completing the PRFs and definitions of terms are provided in the computerized instrument through help screens.
The Patient Record forms for the NHAMCS routinely collect data on patient characteristics such as age, sex, race, and ethnicity, and visit characteristics such as date of visit, reason for visit in patient’s own words, physician diagnoses, medication provided or prescribed, and expected source of payment. Periodically specific items on diagnostic tests, procedures or non-medication therapies are added or deleted.
Monitoring Data Collection and Quality Control
Census Bureau Headquarters staff from the Associate Director for Demographic Programs-Survey Operations, National Ambulatory Medical Care Surveys, is responsible for overseeing the data collection. Census Bureau Headquarters staff, Field Division, is responsible for the supervision of staff in the Bureau’s Regional Offices who in turn supervise the field representatives. The field representative visits the eligible ESAs after the data collection period. An essential part of this effort is quality control which focuses on the completeness of the patient sampling frame, and assurance that a Patient Record form is completely filled out. Computerization of the Patient Record form has allowed for automated edits to be built into the instrument, so that keying errors are automatically detected as the data entry person is entering data.
In an effort to improve the completeness of PRFs collected, a new hospital case disposition system will be implemented starting in 2018. The new system only designates a ‘Complete’ code to a case if 50 percent or more of the expected PRFs are collected by a field representative. If less than 50 percent of the PRFs are collected, the case is designated a ‘Partial Complete’ code. And any cases without PRFs are assigned a ‘Refusal’ code.
Once a case is completed, the survey data are encrypted and sent to a secure Census Bureau database through a secure internet connection. The data are then sent to our keying and coding contractor who will do medical and drug coding on the verbatim text fields. Keying and data entry activities are performed under contract. All medical and drug coding, as well as all data entry operations, are subject to quality control procedures—specifically, a 10-percent quality control sample of survey records are independently keyed and coded. Computer edits for code ranges and inconsistencies are also performed.
Approximately five percent of completed hospitals will be approached for reabstraction. Hospitals that are selected for reabstraction receive a letter from the Branch Chief of the Division of Health Care Statistics, describing the study (Attachment F.2). Reabstraction is a quality control measure where for a completed hospital, data from 10 of the already abstracted patient records are systematically sampled and abstracted again by a different (usually more experienced) field representative. The patient’s medical record number is typically collected during the first abstraction, and that information enables the field representatives to locate already abstracted records for reabstraction. The medical record number is removed from the data file before it is transmitted to NCHS, and it is not retained at Census on any of their networks or systems. Data collected through reabstraction is analyzed at NCHS and the results of the analysis are used to improve the PRF questionnaires.
For some items, missing values are imputed by randomly assigning a value from Patient Record forms with similar characteristics. For the ED data, imputations for birth year and sex are based on ED volume, geographic region, immediacy with which patient should be seen, and the ICD-10-CM code (or sub-code) for primary diagnosis; for immediacy it is based on ED volume, region, and primary diagnosis. Starting with 2009 data, missing race information has been imputed using Census data on percent race for the patient’s zip code in a regression model.
Estimation Procedures
Estimation
procedures for NHAMCS are described at
http://www.cdc.gov/nchs/ahcd/ahcd_estimation_procedures.htm#nhamcs_procedures.
National estimates will be produced for visits to hospital EDs. The
estimation procedure has three basic components: (a) inflation by
reciprocals of the sampling selection probabilities, (b) adjustments
for nonresponse, and (c) calibration ratio adjustment. (The NHAMCS
hospital sample [n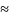 600]
is partitioned into 16 panels that are rotated into the sample over
periods of four weeks each so that only 13 panels are used in any one
year.) The calibration adjustments are based on visit counts
recorded by hospital in the most recent NHAMCS sampling frame.
600]
is partitioned into 16 panels that are rotated into the sample over
periods of four weeks each so that only 13 panels are used in any one
year.) The calibration adjustments are based on visit counts
recorded by hospital in the most recent NHAMCS sampling frame.
Beginning in 2004, the nonresponse adjustment factor was changed to account for the seasonality of the reporting period. Extra weights for nonresponding hospitals were shifted to responding hospitals in reporting periods within the same quarter of the year. The shift in nonresponse adjustment did not significantly affect any of the overall annual estimates.
Sampling Errors
Standard errors are calculated using a first-order Taylor series approximation methodi as applied in SUDAAN variance software.
3. Methods to Maximize Response Rates and Deal with Nonresponse
Based on the results of the 2015 NHAMCS, the projected unweighted and weighted response rates for 2018 are 80% and 81%, respectively, for the ED. Endorsements were solicited from several prominent national organizations, including ACEP, SAEM, ENA, ACOEP, and AHIMA. NCHS developed a participant web page at https://www.cdc.gov/nchs/ahcd/nhamcs_participant.htm, which gives a brief background on the NHAMCS, as well as provides information regarding selection and participation, confidentiality and privacy, the HIPAA Privacy Rule, new data components, data utilization, and contact information.
Data collection procedures are designed to minimize response burden, a major concern and influence on response rates. This survey does require commitment from a large number of persons within each hospital: the director, ambulatory unit directors, information systems technicians, and medical and clerical staff. Refusals to participate may occur at any one of the stages of induction or data collection. At the time of refusal, a refusal report is completed and the Census Bureau Regional Office is notified. Reasons for refusal vary considerably, necessitating refusal conversion procedures which are flexible and responsive to individual concerns. In general, the following survey features are stressed: the data are needed by the hospital and medical professions for a variety of purposes and do not exist elsewhere; all data about facilities, ambulatory units, and patients are kept confidential; and every effort is made to minimize the disruption of facility routine. Based on earlier experiences, these features are often persuasive in converting refusals.
4. Tests of Procedures or Methods to be Undertaken
Not applicable.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
The statistician responsible for the survey sample design is:
Iris Shimizu, Ph.D.
Mathematical Statistician
Statistical Research and Survey Design Staff
Division of Research and Methodology
National Center for Health Statistics
Telephone: 301-458‑4497
The data collection agent is the Census Bureau and the contact person is:
Lucinda Dalzell
Survey Director, National Ambulatory Medical Care Surveys
Office of the Associate Director for Demographic Programs
U.S. Census Bureau
Telephone: 301-763-1680
The data will be analyzed under the direction of:
Brian Ward, Ph.D.
Team Lead, Ambulatory Care Team
Ambulatory and Hospital Care Statistics Branch
Division of Health Care Statistics
National Center for Health Statistics
Telephone: 301-458-4568
i Williams RL. Taylor series linearization. In: Lavrakas PJ. Encyclopedia of Survey Research Methods. Thousand Oaks, CA: Sage; 2008.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy