Form Q_NlmClinTr_Web_Br Q_NlmClinTr_Web_Browse_20160920 (2)
E-Government Website Customer Satisfaction Surveys
Q_NlmClinTr_Web_Browse_20160920 (2).xlsx
Mapping and Questionnaire Documents IA 30824 A0 (NLM)
OMB: 1090-0008
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 1090-0008 can be found here:
Document [xlsx]
Download: xlsx | pdf
Current Model Qsts
Current Custom Qsts
Custom Qsts (1-9-13)
Custom Qsts (11-11-11)

Overview
Welcome and Thank You TextCurrent Model Qsts
Current Custom Qsts
Custom Qsts (1-9-13)
Custom Qsts (11-11-11)
Sheet 1: Welcome and Thank You Text
| Model Instance Name: NLM Clinical Trials | 
|
||||||||||
| #REF! | |||||||||||
| MID: | #REF! | ||||||||||
| Date: 2-14-2011 | 2/14/2011 | ||||||||||
| Welcome and Thank You Text | |||||||||||
| Directions: | |||||||||||
| This welcome text is shown at the top of the questionnaire window and the thank you text at the bottom. This is a good place to mention the site/company/agency name so the visitor knows whom they are taking the survey for. Feel free to modify the standard Welcome text shown in the box below. | |||||||||||
| Examples | |||||||||||
| Welcome Text Example | |||||||||||
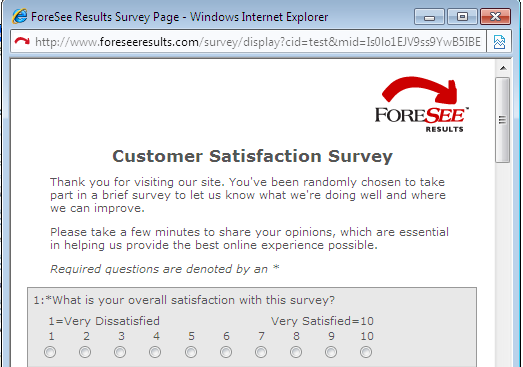
|
|||||||||||
| Welcome Text | |||||||||||
| Thank you for visiting our site. You've been randomly chosen to take part in a brief survey to let us know what we're doing well and where we can improve. Please take a few minutes to share your opinions, which are essential in helping us provide the best online experience possible. |
|||||||||||
| Thank You Text Example | |||||||||||
| Thank You Text | 
|
||||||||||
| Thank you for taking our survey - and for helping us serve you better. Please note you will not receive a response from us based on your survey comments. If you would like us to contact you about your feedback, please visit the Contact Us section of our web site. |
|||||||||||
Sheet 2: Current Model Qsts
| Model Instance Name: | |||||
| NLM Clinical Trials v2 | |||||
| MID: | hRlIhMU4IRp1NowpYUdwEw== | ||||
| Date: | 2/14/2011 | ||||
| \ | |||||
| Model questions utilize the ACSI methodology to determine scores and impacts | |||||
| ELEMENTS (drivers of satisfaction) | CUSTOMER SATISFACTION | FUTURE BEHAVIORS | |||
| Content (1=Poor, 10=Excellent, Don't Know) | Satisfaction | Return (1=Very Unlikely, 10=Very Likely) | |||
| 1 | Please rate the accuracy of information on ClinicalTrials.gov. | 23 | What is your overall satisfaction with ClinicalTrials.gov? (1=Very Dissatisfied, 10=Very Satisfied) |
26 | How likely are you to return to ClinicalTrials.gov? |
| 2 | Please rate the quality of information on ClinicalTrials.gov. | 24 | How well does this site meet your expectations? (1=Falls Short, 10=Exceeds) |
Recommend (1=Very Unlikely, 10=Very Likely) | |
| 3 | Please rate the freshness of content on ClinicalTrials.gov. | 25 | How does this site compare to your idea of an ideal website? (1=Not Very Close, 10=Very Close) |
27 | How likely are you to recommend this site to someone else? |
| Look and Feel (1=Poor, 10=Excellent, Don't Know) | Primary Resource (1=Very Unlikely, 10=Very Likely) | ||||
| 4 | Please rate the visual appeal of this site. | How likely are you to use ClinicalTrials.gov as your primary resource for clinical trials information? | |||
| 5 | Please rate the balance of graphics and text on this site. | ||||
| 6 | Please rate the readability of the pages on this site. | ||||
| Navigation (1=Poor, 10=Excellent, Don't Know) | |||||
| 7 | Please rate how well this site is organized. | ||||
| 8 | Please rate the options available for navigating this site. | 
|
|||
| 9 | Please rate how well the ClinicalTrials.gov layout helps you find what you are looking for. | ||||
| 10 | Please rate the number of clicks to get where you want on ClinicalTrials.gov. | ||||
| Site Performance (1=Poor, 10=Excellent, Don't Know) | |||||
| 11 | Please rate how quickly pages load on this site. | ||||
| 12 | Please rate the consistency of speed from page to page on ClinicalTrials.gov. | ||||
| 13 | Please rate the ability to load pages without getting error messages on ClinicalTrials.gov. | ||||
| Search (1=Poor, 10=Excellent, Don't Know) | |||||
| 14 | Please rate the relevance of search results on ClinicalTrials.gov. | ||||
| 15 | Please rate the organization of search results on this site. | ||||
| 16 | Please rate how well the search results help you decide what to select. | ||||
| 17 | Please rate how well the search feature helps you to narrow the results to find what you want. | ||||

Sheet 3: Current Custom Qsts
| Model Instance Name: | |||||||||||
| NLM Clinical Trials v2 | underlined & italicized: RE-ORDER | ||||||||||
| MID: hRlIhMU4IRp1NowpYUdwEw== | pink: ADDITION | ||||||||||
| Date: | 3/1/2008 | blue + -->: REWORDING | |||||||||
| partitioned - yes | |||||||||||
| NLM Clinical Trials v2 CUSTOM QUESTION LIST | |||||||||||
| QID | Skip Logic Label | Question Text | Answer Choices (limited to 50 characters) |
Skip to | Type (select from list) | Single or Multi | Required Y/N |
Special Instructions | CQ Label | ||
| EDO09660 | How did you first learn about ClinicalTrials.gov? | Search engine (e.g., Google, Yahoo) | Radio button, one-up vertical | Single | Y | Randomize | Awareness Source | ||||
| Someone recommended it | |||||||||||
| Social media (e.g., Twitter, Facebook) | OPS Group | ||||||||||
| Television or radio | |||||||||||
| Medical journal article | |||||||||||
| Newspaper, magazine, or press release | |||||||||||
| Other government agency | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09661 | A | Other source | Text field, <100 char | N | Other Awareness Source | ||||||
| EDO09662 | How frequently do you visit ClinicalTrials.gov? | First time | Radio button, one-up vertical | Single | Y | Skip Logic Group | Frequency of Visit | ||||
| Daily | A, B | ||||||||||
| Several times a week | A, B | ||||||||||
| About once a week | A, B | ||||||||||
| Several times a month | A, B | ||||||||||
| About once a month | A, B | ||||||||||
| Every few months | A, B | ||||||||||
| EDO09663 | Which of the following best describes your role visiting ClinicalTrials.gov today? | Patient | A, G | Radio button, one-up vertical | S | Y | Skip Logic Group | Role | |||
| Family or Friend of Patient | B, G | ||||||||||
| Healthy Person | B, G | ||||||||||
| Clinical Trial Staff | C, H | ||||||||||
| Health Care Provider (e.g., nurse, physician, etc.) | D, H | ||||||||||
| Scientist/Researcher | E, H | ||||||||||
| Librarian or Information Professional | F, H | ||||||||||
| Student/Educator | F, H | ||||||||||
| Clinical Research Support (e.g. regulatory affairs) | F, H | ||||||||||
| Medical Communications | F, H | ||||||||||
| Institutional Review Board (IRB) or Ethics Committee Member | F, H | ||||||||||
| Other, please specify: | I,F,H | ||||||||||
| EDO09664 | I | What best describes your role? | Text area, no char limit | N | Skip Logic Group | Other role | |||||
| EDO09665 | A | Which of the following best describes the reason you are visiting the site today? | Trying to learn more about a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Patient | |||
| Interest in volunteering for a clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Other, please specify: | J,G | ||||||||||
| EDO09666 | J | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Patient Visit | |||||
| EDO09667 | B | Which of the following best describes the reason you are visiting the site today? | Concerned about family member or friend with a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Healthy person | |||
| Interest in volunteering for a clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Other, please specify: | K | ||||||||||
| EDO09668 | K | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Healthy Person Visit | |||||
| EDO09669 | C | Which of the following best describes the reason you are visiting the site today? | Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Clinical Trial Staff | |||
| Interest in a particular clinical trial | |||||||||||
| Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Other, please specify: | L | ||||||||||
| EDO09670 | L | What best describes your reason for visit? | Text area, no char limit | Skip Logic Group | Other Clinical Trials Staff | ||||||
| EDO09671 | D | Which of the following best describes the reason you are visiting the site today? | Trying to find a clinical trial for a patient with a specific disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Health Care Provider | |||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Other, please specify: | M | ||||||||||
| EDO09672 | M | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Health Care Provider | |||||
| EDO09673 | E | Which of the following best describes the reason you are visiting the site today? | Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | Radio button, one-up vertical | S | Y | Visit Reason-Scientist | ||||
| Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | |||||||||||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Identify unpublished trials | |||||||||||
| Other, please specify: | N | ||||||||||
| EDO09674 | N | What best describes your reason for visit? | Text area, no char limit | Skip Logic Group | Other Scientist/Researcher | ||||||
| EDO09675 | F | Which of the following best describes the reason you are visiting the site today? | Trying to learn more about a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Other/Generic | |||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | |||||||||||
| Trying to learn about clinical research | |||||||||||
| Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | |||||||||||
| Interest in a particular clinical trial | |||||||||||
| Other, please specify: | O | ||||||||||
| EDO09676 | O | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other | |||||
| EDO09677 | G | What do you plan to do with the information you are looking for on ClinicalTrials.gov? (select all that apply) | Contact trials that I found | Checkbox, one-up vertical | M | Y | Skip Logic Group | Infornmation Use-Patient | |||
| Discuss with family or friends | Randomize | ||||||||||
| Discuss with a doctor or other health care provider | |||||||||||
| Seek more information on a disease, condition, diagnosis, or treatment | |||||||||||
| Give information to family member or friend | |||||||||||
| Look for trials in other registries | |||||||||||
| Didn't find what I wanted | |||||||||||
| Not sure yet | |||||||||||
| Explore what you have to offer (just browsing) | |||||||||||
| Other, please specify: | P | ||||||||||
| EDO09678 | P | What do you plan to do with the information you are looking for? | Text area, no char limit | N | Skip Logic Group | Other-Information Use Patient | |||||
| EDO09679 | H | What do you plan to do with the information you are looking for on ClinicalTrials.gov? (select all that apply) | Refer a patient to clinical trial | Checkbox, one-up vertical | Skip Logic Group | Information Use-Non-Patient | |||||
| Use information for a project or presentation | |||||||||||
| Improve my understanding of clinical research | Randomize | ||||||||||
| Support new or current research projects | |||||||||||
| Explore or support business opportunities in the field of biomedical research | |||||||||||
| Seek further information from other websites | |||||||||||
| Seek further information from ClinicalTrials.gov | |||||||||||
| Include in systematic review | |||||||||||
| Compare information with published trial results | |||||||||||
| Didn't find what I wanted | |||||||||||
| Not sure yet | |||||||||||
| Other, please specify: | Q | ||||||||||
| EDO09680 | Q | What do you plan to do with the information you are looking for? | Text area, no char limit | N | Skip Logic Group | Other-Information Use Non-Patient | |||||
| EDO09681 | Were you able to find what you were looking for? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Find Information | |||
| No | B | ||||||||||
| I was not looking for anything in particular/Just browsing | |||||||||||
| EDO09682 | A | Did the information you found meet your needs? | Yes | Radio button, one-up vertical | Skip Logic Group | Info Met Needs | |||||
| No, please specify | C | ||||||||||
| EDO09683 | B | What specifically were you looking for that you did not find? | Text area, no char limit | N | Skip Logic Group | Information Seeking | |||||
| EDO09684 | C | Describe why the information did not meet your needs. | Text area, no char limit | N | Skip Logic Group | Info Did Not Meet Needs | |||||
| EDO09685 | If you used the search feature of this site today to find your information, what method did you use? | Search Box (type in key words) | Checkbox, one-up vertical | M | Y | OPS Group | Search Experience | ||||
| Basic Search (including the search box at the top right-hand corner of each page) | Randomize | ||||||||||
| Advanced Search (searching within specific fields, such as disease, location, treatment, sponsor) | |||||||||||
| Studies by Topic (browse by condition, drug intervention, sponsor) | |||||||||||
| Studies by Map | |||||||||||
| I did not use the search feature today | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09686 | A | Other Search Experience | Text field, <100 char | N | Other Search Experience | ||||||
| EDO09687 | Please describe your experience with the navigation process on this site: | I did not encounter any difficulties | Checkbox, one-up vertical | M | Y | OPS Group | |||||
| Could not navigate back to previous information | |||||||||||
| Would often feel lost, not know where I was | Randomize | ||||||||||
| Too many links or navigational choices | |||||||||||
| Links did not take me where I expected | |||||||||||
| Had technical difficulties (e.g. broken links, error messages) | |||||||||||
| Links/labels are difficult to understand | |||||||||||
| I used the search feature | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09688 | A | Other Navigation Experience | Text field, <100 char | N | Other Navigation Experience | ||||||
| EDO09689 | Did you look at any summary results data tables on ClinicalTrials.gov today? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Data Table Results | |||
| No | B | ||||||||||
| I did not know that ClinicalTrials.gov includes studies with summary results information | |||||||||||
| EDO09690 | A | Did you find the summary results data tables useful? | Yes | Radio button, one-up vertical | S | Y | Skip Logic Group | Data Table Results Useful | |||
| No, please specify: | B | ||||||||||
| EDO09691 | B | Why did you not find the results useful? | Text area, no char limit | N | Skip Logic Group | Data Table Results Not Useful | |||||
| EDO09692 | C | Have you looked for the results of a clinical trial in the past week? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Clinical Trials Past Week | ||
| No | |||||||||||
| EDO09693 | A | Where did you look for information about the results of a clinical trial? | MEDLINE/PubMed | Checkbox, one-up vertical | S | Y | Skip Logic Group | Clinical Trial Sources | |||
| ClinicalTrials.gov summary results data tables | |||||||||||
| Medical journal article | |||||||||||
| Newspaper, magazine, or press release | |||||||||||
| Other, please specify | B | ||||||||||
| EDO09694 | B | Where did you look for clinical trial results? | Text area, no char limit | N | Skip Logic Group | Other Clinical Trial Sources | |||||
| EDO09695 | Please suggest one improvement you would like to see on ClinicalTrials.gov: | Text area, no char limit | N | Improvements | |||||||
Sheet 4: Custom Qsts (1-9-13)
| Model Instance Name: | |||||||||||
| NLM Clinical Trials v2 | underlined & italicized: RE-ORDER | ||||||||||
| MID: hRlIhMU4IRp1NowpYUdwEw== | pink: ADDITION | ||||||||||
| Date: | 3/1/2008 | blue + -->: REWORDING | |||||||||
| partitioned - yes | |||||||||||
| NLM Clinical Trials v2 CUSTOM QUESTION LIST | |||||||||||
| QID | Skip Logic Label | Question Text | Answer Choices (limited to 50 characters) |
Skip to | Type (select from list) | Single or Multi | Required Y/N |
Special Instructions | CQ Label | ||
| EDO09660 | How did you first learn about ClinicalTrials.gov? | Search engine (e.g., Google, Yahoo) | Radio button, one-up vertical | Single | Y | Randomize | Awareness Source | ||||
| Someone recommended it | |||||||||||
| Social media (e.g., Twitter, Facebook) | OPS Group | ||||||||||
| Television or radio | |||||||||||
| Medical journal article | |||||||||||
| Newspaper, magazine, or press release | |||||||||||
| Other government agency | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09661 | A | Other source | Text field, <100 char | N | Other Awareness Source | ||||||
| EDO09662 | How frequently do you visit ClinicalTrials.gov? | First time | Radio button, one-up vertical | Single | Y | Skip Logic Group | Frequency of Visit | ||||
| Daily | A, B | ||||||||||
| Several times a week | A, B | ||||||||||
| About once a week | A, B | ||||||||||
| Several times a month | A, B | ||||||||||
| About once a month | A, B | ||||||||||
| Every few months | A, B | ||||||||||
| A | Please select the statement which best describes your experience with the new ClinicalTrials.gov site redesign: | It is easier to find what I am looking for | Radio button, one-up vertical | Single | Y | Skip Logic Group | Redesign Experience | ||||
| It is harder to find what I am looking for | C | ||||||||||
| My experience is no different when looking for information on the new site | |||||||||||
| Don’t Know | |||||||||||
| C | Please specify why it is harder to find what you are looking for. | Text area, no char limit | N | Skip Logic Group | Hard to Find Expereince | ||||||
| B | Please tell us what you think of the redesigned ClinicalTrials.gov site. | Text area, no char limit | N | Skip Logic Group | Redesign Comments | ||||||
| EDO09663 | Which of the following best describes your role visiting ClinicalTrials.gov today? | Patient | A, G | Radio button, one-up vertical | S | Y | Skip Logic Group | Role | |||
| Family or Friend of Patient | B, G | ||||||||||
| Healthy Person | B, G | ||||||||||
| Clinical Trial Staff | C, H | ||||||||||
| Health Care Provider (e.g., nurse, physician, etc.) | D, H | ||||||||||
| Scientist/Researcher | E, H | ||||||||||
| Librarian or Information Professional | F, H | ||||||||||
| Student/Educator | F, H | ||||||||||
| Clinical Research Support (e.g. regulatory affairs) | F, H | ||||||||||
| Medical Communications | F, H | ||||||||||
| Institutional Review Board (IRB) or Ethics Committee Member | F, H | ||||||||||
| Other, please specify: | I,F,H | ||||||||||
| EDO09664 | I | What best describes your role? | Text area, no char limit | N | Skip Logic Group | Other role | |||||
| EDO09665 | A | Which of the following best describes the reason you are visiting the site today? | Trying to learn more about a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Patient | |||
| Interest in volunteering for a clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Other, please specify: | J,G | ||||||||||
| EDO09666 | J | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Patient Visit | |||||
| EDO09667 | B | Which of the following best describes the reason you are visiting the site today? | Concerned about family member or friend with a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Healthy person | |||
| Interest in volunteering for a clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Other, please specify: | K | ||||||||||
| EDO09668 | K | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Healthy Person Visit | |||||
| EDO09669 | C | Which of the following best describes the reason you are visiting the site today? | Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Clinical Trial Staff | |||
| Interest in a particular clinical trial | |||||||||||
| Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Other, please specify: | L | ||||||||||
| EDO09670 | L | What best describes your reason for visit? | Text area, no char limit | Skip Logic Group | Other Clinical Trials Staff | ||||||
| EDO09671 | D | Which of the following best describes the reason you are visiting the site today? | Trying to find a clinical trial for a patient with a specific disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Health Care Provider | |||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Other, please specify: | M | ||||||||||
| EDO09672 | M | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Health Care Provider | |||||
| EDO09673 | E | Which of the following best describes the reason you are visiting the site today? | Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | Radio button, one-up vertical | S | Y | Visit Reason-Scientist | ||||
| Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | |||||||||||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Identify unpublished trials | |||||||||||
| Other, please specify: | N | ||||||||||
| EDO09674 | N | What best describes your reason for visit? | Text area, no char limit | Skip Logic Group | Other Scientist/Researcher | ||||||
| EDO09675 | F | Which of the following best describes the reason you are visiting the site today? | Trying to learn more about a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Other/Generic | |||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | |||||||||||
| Trying to learn about clinical research | |||||||||||
| Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | |||||||||||
| Interest in a particular clinical trial | |||||||||||
| Other, please specify: | O | ||||||||||
| EDO09676 | O | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other | |||||
| EDO09677 | G | What do you plan to do with the information you are looking for on ClinicalTrials.gov? (select all that apply) | Contact trials that I found | Checkbox, one-up vertical | M | Y | Skip Logic Group | Infornmation Use-Patient | |||
| Discuss with family or friends | Randomize | ||||||||||
| Discuss with a doctor or other health care provider | |||||||||||
| Seek more information on a disease, condition, diagnosis, or treatment | |||||||||||
| Give information to family member or friend | |||||||||||
| Look for trials in other registries | |||||||||||
| Didn't find what I wanted | |||||||||||
| Not sure yet | |||||||||||
| Explore what you have to offer (just browsing) | |||||||||||
| Other, please specify: | P | ||||||||||
| EDO09678 | P | What do you plan to do with the information you are looking for? | Text area, no char limit | N | Skip Logic Group | Other-Information Use Patient | |||||
| EDO09679 | H | What do you plan to do with the information you are looking for on ClinicalTrials.gov? (select all that apply) | Refer a patient to clinical trial | Checkbox, one-up vertical | Skip Logic Group | Information Use-Non-Patient | |||||
| Use information for a project or presentation | |||||||||||
| Improve my understanding of clinical research | Randomize | ||||||||||
| Support new or current research projects | |||||||||||
| Explore or support business opportunities in the field of biomedical research | |||||||||||
| Seek further information from other websites | |||||||||||
| Seek further information from ClinicalTrials.gov | |||||||||||
| Include in systematic review | |||||||||||
| Compare information with published trial results | |||||||||||
| Didn't find what I wanted | |||||||||||
| Not sure yet | |||||||||||
| Other, please specify: | Q | ||||||||||
| EDO09680 | Q | What do you plan to do with the information you are looking for? | Text area, no char limit | N | Skip Logic Group | Other-Information Use Non-Patient | |||||
| EDO09681 | Were you able to find what you were looking for? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Find Information | |||
| No | B | ||||||||||
| I was not looking for anything in particular/Just browsing | |||||||||||
| EDO09682 | A | Did the information you found meet your needs? | Yes | Radio button, one-up vertical | Skip Logic Group | Info Met Needs | |||||
| No, please specify | C | ||||||||||
| EDO09683 | B | What specifically were you looking for that you did not find? | Text area, no char limit | N | Skip Logic Group | Information Seeking | |||||
| EDO09684 | C | Describe why the information did not meet your needs. | Text area, no char limit | N | Skip Logic Group | Info Did Not Meet Needs | |||||
| EDO09685 | If you used the search feature of this site today to find your information, what method did you use? | Search Box (type in key words) | Checkbox, one-up vertical | M | Y | OPS Group | Search Experience | ||||
| Basic Search (including the search box at the top right-hand corner of each page) | Randomize | ||||||||||
| Advanced Search (searching within specific fields, such as disease, location, treatment, sponsor) | |||||||||||
| Studies by Topic (browse by condition, drug intervention, sponsor) | |||||||||||
| Studies by Map | |||||||||||
| I did not use the search feature today | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09686 | A | Other Search Experience | Text field, <100 char | N | Other Search Experience | ||||||
| EDO09687 | Please describe your experience with the navigation process on this site: | I did not encounter any difficulties | Checkbox, one-up vertical | M | Y | OPS Group | |||||
| Could not navigate back to previous information | |||||||||||
| Would often feel lost, not know where I was | Randomize | ||||||||||
| Too many links or navigational choices | |||||||||||
| Links did not take me where I expected | |||||||||||
| Had technical difficulties (e.g. broken links, error messages) | |||||||||||
| Links/labels are difficult to understand | |||||||||||
| I used the search feature | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09688 | A | Other Navigation Experience | Text field, <100 char | N | Other Navigation Experience | ||||||
| EDO09689 | Did you look at any summary results data tables on ClinicalTrials.gov today? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Data Table Results | |||
| No | B | ||||||||||
| I did not know that ClinicalTrials.gov includes studies with summary results information | |||||||||||
| EDO09690 | A | Did you find the summary results data tables useful? | Yes | Radio button, one-up vertical | S | Y | Skip Logic Group | Data Table Results Useful | |||
| No, please specify: | B | ||||||||||
| EDO09691 | B | Why did you not find the results useful? | Text area, no char limit | N | Skip Logic Group | Data Table Results Not Useful | |||||
| EDO09692 | C | Have you looked for the results of a clinical trial in the past week? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Clinical Trials Past Week | ||
| No | |||||||||||
| EDO09693 | A | Where did you look for information about the results of a clinical trial? | MEDLINE/PubMed | Checkbox, one-up vertical | S | Y | Skip Logic Group | Clinical Trial Sources | |||
| ClinicalTrials.gov summary results data tables | |||||||||||
| Medical journal article | |||||||||||
| Newspaper, magazine, or press release | |||||||||||
| Other, please specify | B | ||||||||||
| EDO09694 | B | Where did you look for clinical trial results? | Text area, no char limit | N | Skip Logic Group | Other Clinical Trial Sources | |||||
| EDO09695 | Please suggest one improvement you would like to see on ClinicalTrials.gov: | Text area, no char limit | N | Improvements | |||||||
Sheet 5: Custom Qsts (11-11-11)
| Model Instance Name: | |||||||||||
| NLM Clinical Trials v2 | underlined & italicized: RE-ORDER | ||||||||||
| MID: hRlIhMU4IRp1NowpYUdwEw== | pink: ADDITION | ||||||||||
| Date: | 3/1/2008 | blue + -->: REWORDING | |||||||||
| partitioned - yes | |||||||||||
| NLM Clinical Trials v2 CUSTOM QUESTION LIST | |||||||||||
| QID | Skip Logic Label | Question Text | Answer Choices (limited to 50 characters) |
Skip to | Type (select from list) | Single or Multi | Required Y/N |
Special Instructions | CQ Label | ||
| EDO09660 | How did you first learn about ClinicalTrials.gov? | Search engine (e.g., Google, Yahoo) | Radio button, one-up vertical | Single | Y | Randomize | Awareness Source | ||||
| Someone recommended it | |||||||||||
| Social media (e.g., Twitter, Facebook) | OPS Group | ||||||||||
| Television or radio | |||||||||||
| Medical journal article | |||||||||||
| Newspaper, magazine, or press release | |||||||||||
| Other government agency | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09661 | A | Other source | Text field, <100 char | N | Other Awareness Source | ||||||
| EDO09662 | How frequently do you visit ClinicalTrials.gov? | First time | Radio button, one-up vertical | Single | Y | Frequency of Visit | |||||
| Daily | |||||||||||
| Several times a week | |||||||||||
| About once a week | |||||||||||
| Several times a month | |||||||||||
| About once a month | |||||||||||
| Every few months | |||||||||||
| EDO09663 | Which of the following best describes your role visiting ClinicalTrials.gov today? | Patient | A, G | Radio button, one-up vertical | S | Y | Skip Logic Group | Role | |||
| Family or Friend of Patient | B, G | ||||||||||
| Healthy Person | B, G | ||||||||||
| Clinical Trial Staff | C, H | ||||||||||
| Health Care Provider (e.g., nurse, physician, etc.) | D, H | ||||||||||
| Scientist/Researcher | E, H | ||||||||||
| Librarian or Information Professional | F, H | ||||||||||
| Student/Educator | F, H | ||||||||||
| Clinical Research Support (e.g. regulatory affairs) | F, H | ||||||||||
| Medical Communications | F, H | ||||||||||
| Institutional Review Board (IRB) or Ethics Committee Member | F, H | ||||||||||
| Other, please specify: | I,F,H | ||||||||||
| EDO09664 | I | What best describes your role? | Text area, no char limit | N | Skip Logic Group | Other role | |||||
| EDO09665 | A | Which of the following best describes the reason you are visiting the site today? | Trying to learn more about a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Patient | |||
| Interest in volunteering for a clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Other, please specify: | J,G | ||||||||||
| EDO09666 | J | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Patient Visit | |||||
| EDO09667 | B | Which of the following best describes the reason you are visiting the site today? | Concerned about family member or friend with a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Healthy person | |||
| Interest in volunteering for a clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Other, please specify: | K | ||||||||||
| EDO09668 | K | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Healthy Person Visit | |||||
| EDO09669 | C | Which of the following best describes the reason you are visiting the site today? | Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Clinical Trial Staff | |||
| Interest in a particular clinical trial | |||||||||||
| Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Other, please specify: | L | ||||||||||
| EDO09670 | L | What best describes your reason for visit? | Text area, no char limit | Skip Logic Group | Other Clinical Trials Staff | ||||||
| EDO09671 | D | Which of the following best describes the reason you are visiting the site today? | Trying to find a clinical trial for a patient with a specific disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Health Care Provider | |||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Other, please specify: | M | ||||||||||
| EDO09672 | M | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other Health Care Provider | |||||
| EDO09673 | E | Which of the following best describes the reason you are visiting the site today? | Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | Radio button, one-up vertical | S | Y | Visit Reason-Scientist | ||||
| Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | |||||||||||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions for a disease or condition | |||||||||||
| Identify unpublished trials | |||||||||||
| Other, please specify: | N | ||||||||||
| EDO09674 | N | What best describes your reason for visit? | Text area, no char limit | Skip Logic Group | Other Scientist/Researcher | ||||||
| EDO09675 | F | Which of the following best describes the reason you are visiting the site today? | Trying to learn more about a disease or condition | Radio button, one-up vertical | S | Y | Skip Logic Group | Visit Reason-Other/Generic | |||
| Interest in a particular clinical trial | |||||||||||
| Trying to learn more about experimental interventions (e.g., drugs, medical devices) for a disease or condition | |||||||||||
| Interest in certain types of clinical trials (e.g. randomized controlled trials, observational studies) | |||||||||||
| Trying to learn about clinical research | |||||||||||
| Trying to learn about ClinicalTrials.gov policies (e.g., how to register, report results) | |||||||||||
| Interest in a particular clinical trial | |||||||||||
| Other, please specify: | O | ||||||||||
| EDO09676 | O | What best describes your reason for visit? | Text area, no char limit | N | Skip Logic Group | Other | |||||
| EDO09677 | G | What do you plan to do with the information you are looking for on ClinicalTrials.gov? (select all that apply) | Contact trials that I found | Checkbox, one-up vertical | M | Y | Skip Logic Group | Infornmation Use-Patient | |||
| Discuss with family or friends | Randomize | ||||||||||
| Discuss with a doctor or other health care provider | |||||||||||
| Seek more information on a disease, condition, diagnosis, or treatment | |||||||||||
| Give information to family member or friend | |||||||||||
| Look for trials in other registries | |||||||||||
| Didn't find what I wanted | |||||||||||
| Not sure yet | |||||||||||
| Explore what you have to offer (just browsing) | |||||||||||
| Other, please specify: | P | ||||||||||
| EDO09678 | P | What do you plan to do with the information you are looking for? | Text area, no char limit | N | Skip Logic Group | Other-Information Use Patient | |||||
| EDO09679 | H | What do you plan to do with the information you are looking for on ClinicalTrials.gov? (select all that apply) | Refer a patient to clinical trial | Checkbox, one-up vertical | Skip Logic Group | Information Use-Non-Patient | |||||
| Use information for a project or presentation | |||||||||||
| Improve my understanding of clinical research | Randomize | ||||||||||
| Support new or current research projects | |||||||||||
| Explore or support business opportunities in the field of biomedical research | |||||||||||
| Seek further information from other websites | |||||||||||
| Seek further information from ClinicalTrials.gov | |||||||||||
| Include in systematic review | |||||||||||
| Compare information with published trial results | |||||||||||
| Didn't find what I wanted | |||||||||||
| Not sure yet | |||||||||||
| Other, please specify: | Q | ||||||||||
| EDO09680 | Q | What do you plan to do with the information you are looking for? | Text area, no char limit | N | Skip Logic Group | Other-Information Use Non-Patient | |||||
| EDO09681 | Were you able to find what you were looking for? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Find Information | |||
| No | B | ||||||||||
| I was not looking for anything in particular/Just browsing | |||||||||||
| EDO09682 | A | Did the information you found meet your needs? | Yes | Radio button, one-up vertical | Skip Logic Group | Info Met Needs | |||||
| No, please specify | C | ||||||||||
| EDO09683 | B | What specifically were you looking for that you did not find? | Text area, no char limit | N | Skip Logic Group | Information Seeking | |||||
| EDO09684 | C | Describe why the information did not meet your needs. | Text area, no char limit | N | Skip Logic Group | Info Did Not Meet Needs | |||||
| EDO09685 | If you used the search feature of this site today to find your information, what method did you use? | Search Box (type in key words) | Checkbox, one-up vertical | M | Y | OPS Group | Search Experience | ||||
| Basic Search (including the search box at the top right-hand corner of each page) | Randomize | ||||||||||
| Advanced Search (searching within specific fields, such as disease, location, treatment, sponsor) | |||||||||||
| Studies by Topic (browse by condition, drug intervention, sponsor) | |||||||||||
| Studies by Map | |||||||||||
| I did not use the search feature today | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09686 | A | Other Search Experience | Text field, <100 char | N | Other Search Experience | ||||||
| EDO09687 | Please describe your experience with the navigation process on this site: | I did not encounter any difficulties | Checkbox, one-up vertical | M | Y | OPS Group | |||||
| Could not navigate back to previous information | |||||||||||
| Would often feel lost, not know where I was | Randomize | ||||||||||
| Too many links or navigational choices | |||||||||||
| Links did not take me where I expected | |||||||||||
| Had technical difficulties (e.g. broken links, error messages) | |||||||||||
| Links/labels are difficult to understand | |||||||||||
| I used the search feature | |||||||||||
| Other, please specify: | A | ||||||||||
| EDO09688 | A | Other Navigation Experience | Text field, <100 char | N | Other Navigation Experience | ||||||
| EDO09689 | Did you look at any summary results data tables on ClinicalTrials.gov today? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Data Table Results | |||
| No | B | ||||||||||
| I did not know that ClinicalTrials.gov includes studies with summary results information | |||||||||||
| EDO09690 | A | Did you find the summary results data tables useful? | Yes | Radio button, one-up vertical | S | Y | Skip Logic Group | Data Table Results Useful | |||
| No, please specify: | B | ||||||||||
| EDO09691 | B | Why did you not find the results useful? | Text area, no char limit | N | Skip Logic Group | Data Table Results Not Useful | |||||
| EDO09692 | C | Have you looked for the results of a clinical trial in the past week? | Yes | A | Radio button, one-up vertical | S | Y | Skip Logic Group | Clinical Trials Past Week | ||
| No | |||||||||||
| EDO09693 | A | Where did you look for information about the results of a clinical trial? | MEDLINE/PubMed | Checkbox, one-up vertical | S | Y | Skip Logic Group | Clinical Trial Sources | |||
| ClinicalTrials.gov summary results data tables | |||||||||||
| Medical journal article | |||||||||||
| Newspaper, magazine, or press release | |||||||||||
| Other, please specify | B | ||||||||||
| EDO09694 | B | Where did you look for clinical trial results? | Text area, no char limit | N | Skip Logic Group | Other Clinical Trial Sources | |||||
| EDO09695 | Please suggest one improvement you would like to see on ClinicalTrials.gov: | Text area, no char limit | N | Improvements | |||||||
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy