Informed Consent Screenshots
File 6 -Card Sort Informed Consent Screenshots.docx
CDC and ATSDR Health Message Testing System
Informed Consent Screenshots
OMB: 0920-0572
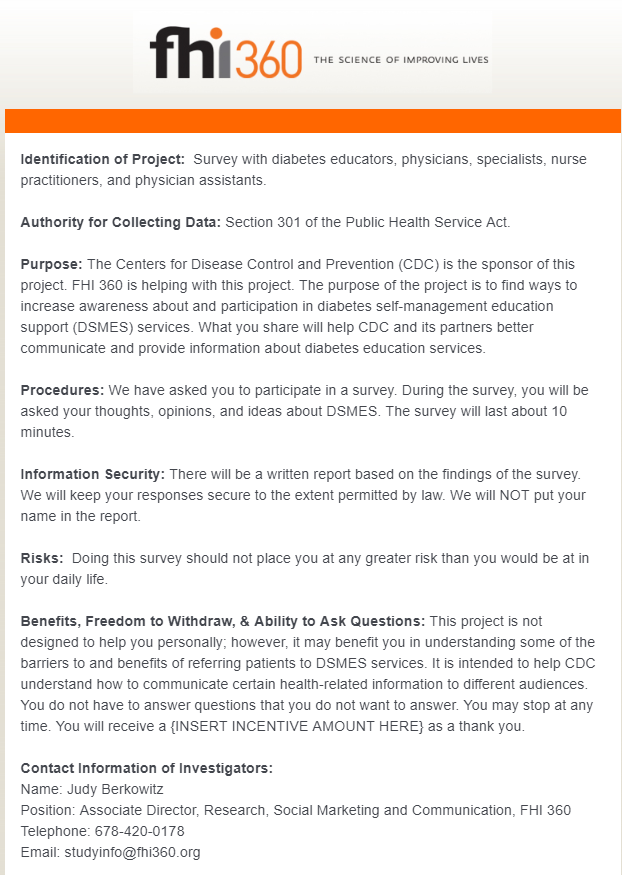
Screener for health care providers and diabetes educators (text and screenshots)
Identification of Project: Survey with diabetes educators, physicians, specialists, nurse practitioners, and physician assistants.
Authority for Collecting Data: Section 301 of the Public Health Service Act.
Purpose: The Centers for Disease Control and Prevention (CDC) is the sponsor of this project. FHI 360 is helping with this project. The purpose of the project is to find ways to increase awareness about and participation in diabetes self-management education and support (DSMES) services. What you share will help CDC and its partners better communicate and provide information about diabetes education services.
Procedures: We have asked you to participate in a survey. During the survey, you will be asked your thoughts, opinions, and ideas about DSMES. The survey will last about 10 minutes.
Information Security: There will be a written report based on the findings of the survey. We will keep your responses secure to the extent permitted by law. We will NOT put your name in the report.
Risks: Doing this survey should not place you at any greater risk than you would be at in your daily life.
Benefits, Freedom to Withdraw, & Ability to Ask Questions: This project is not designed to help you personally; however, it may benefit you in understanding some of the barriers to and benefits of referring patients to DSMES services. It is intended to help CDC understand how to communicate certain health-related information to different audiences. You do not have to answer questions that you do not want to answer. You may stop at any time. You will receive a {INSERT INCENTIVE AMOUNT HERE} as a thank you.
Contact Information of Investigators:
Name: Judy Berkowitz
Position: Associate Director, Research, Social Marketing and Communication, FHI 360
Telephone: 678-420-0178
Email: [email protected]
OIRE Contact for Concerns Regarding Your Rights as a Participant in this Research
Office of International Research Ethics (OIRE)
Telephone: (919) 405-1445
Email: [email protected]
OMB No. 0920-0572
Expiration Date 3/31/2021
Public reporting burden of this collection of information is estimated to average 10 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-0572).

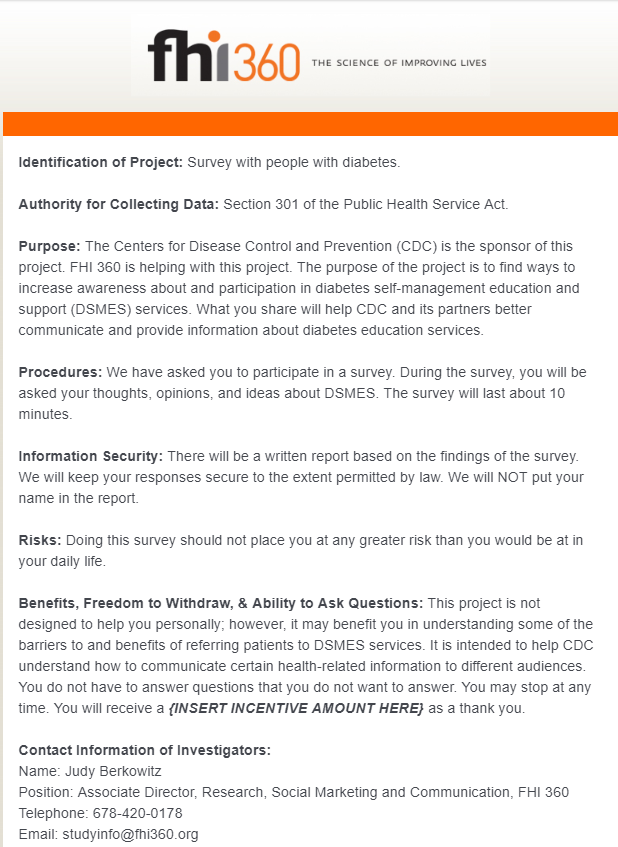
Screener for people with diabetes (text and screenshots)
Identification of Project: Survey with people with diabetes.
Authority for Collecting Data: Section 301 of the Public Health Service Act.
Purpose: The Centers for Disease Control and Prevention (CDC) is the sponsor of this project. FHI 360 is helping with this project. The purpose of the project is to find ways to increase awareness about and participation in diabetes self-management education and support (DSMES) services. What you share will help CDC and its partners better communicate and provide information about diabetes education services.
Procedures: We have asked you to participate in a survey. During the survey, you will be asked your thoughts, opinions, and ideas about DSMES. The survey will last about 10 minutes.
Information Security: There will be a written report based on the findings of the survey. We will keep your responses secure to the extent permitted by law. We will NOT put your name in the report.
Risks: Doing this survey should not place you at any greater risk than you would be at in your daily life.
Benefits, Freedom to Withdraw, & Ability to Ask Questions: This project is not designed to help you personally; however, it may benefit you in understanding some of the barriers to and benefits of referring patients to DSMES services. It is intended to help CDC understand how to communicate certain health-related information to different audiences. You do not have to answer questions that you do not want to answer. You may stop at any time. You will receive a {INSERT INCENTIVE AMOUNT HERE} as a thank you.
Contact Information of Investigators:
Name: Judy Berkowitz
Position: Associate Director, Research, Social Marketing and Communication, FHI 360
Telephone: 678-420-0178
Email: [email protected]
OIRE Contact for Concerns Regarding Your Rights as a Participant in this Research
Office of International Research Ethics (OIRE)
Telephone: (919) 405-1445
Email: [email protected]
OMB No. 0920-0572
Expiration Date 3/31/2021
Public reporting burden of this collection of information is estimated to average 10 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-0572).

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Katherine Dent |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy