NYTS 2018-2020 SupSta Part A OMB (22 Mar 2018)_OMB April 24 2018
NYTS 2018-2020 SupSta Part A OMB (22 Mar 2018)_OMB April 24 2018.docx
National Youth Tobacco Surveys (NYTS) 2015-2017
OMB: 0920-0621
NATIONAL YOUTH TOBACCO SURVEY
2018 - 2020
OMB No. 0920-0621
Revision
SUPPORTING STATEMENT: PART
A
4/24/2018
Submitted by:
Ahmed Jamal, MBBS, MPH
Centers for Disease Control and Prevention
Office on Smoking and Health
Epidemiology Branch
4770 Buford Highway NE, MS-F 79
Atlanta, GA 30341
Phone: 770-488-5077
Fax: 770-488-5848
E-mail: [email protected]
Centers for Disease Control and Prevention
Department of Health and Human Services
TABLE OF CONTENTS
A. JUSTIFICATION
A.1. Circumstances Making the Collection of Information Necessary
A.2. Purpose and Use of Information Collection
A.3. Use of Improved Information Technology and Burden Reduction
A.4. Efforts to Identify Duplication and Use of Similar Information
A.5. Impact on Small Businesses or Other Small Entities
A.6. Consequences of Collecting the Information Less Frequently
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the
A.9. Explanation of Any Payment or Gift to Respondents
A.10. Assurance of Confidentiality Provided to Respondents
A.11. Justification for Sensitive Questions
A.12. Estimates of Annualized Burden Hours and Costs
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
A.14. Annualized Cost to the Government
A.15. Explanation for Program Changes or Adjustments
A.16. Plans for Tabulation and Publication and Project Time Schedule
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
A.19. Comments in Response to the Federal Register Notice and Efforts to Consult Outside of the Agency
REFERENCES
LIST OF ATTACHMENTS
Authorizing Legislation
B1. 60-Day Federal Register Notice
B2. Public Comment on the 60-Day Federal Register Notice and CDC Response
C. State Tobacco Control Reports that Cite National Youth Tobacco Survey Data
D. Publications from Prior Cycles of the National Youth Tobacco Survey
E1. State-level Recruitment Script for the National Youth Tobacco Survey
E2. State-level Recruitment Script for the National Youth Tobacco Survey Supplemental Document – State Letter of Invitation
F1. District-level Recruitment Script for the National Youth Tobacco Survey
F2. District-level Recruitment Script for the National Youth Tobacco Survey Supplemental Document – District Letter of Invitation
G1. School-level Recruitment Script for the National Youth Tobacco Survey
G2. School-level Recruitment Script for the National Youth Tobacco Survey Supplemental Documents – School Letter of Invitation and NYTS Fact Sheet for Schools
G3. School-level Recruitment Script for the National Youth Tobacco Survey Supplemental Documents – Letter to Agreeing Schools
H1. Data Collection Checklist for the National Youth Tobacco Survey
H2. Data Collection Checklist for the National Youth Tobacco Survey Supplemental Documents – Letter to Teachers in Participating Schools
I1. National Youth Tobacco Survey Questionnaire
I2. National Youth Tobacco Survey Questionnaire Supplemental Documents – Parental Permission Form Distribution Script
I3. National Youth Tobacco Survey Questionnaire Supplemental Documents – Parental Permission Form and Fact Sheet (English Version)
I4. National Youth Tobacco Survey Questionnaire Supplemental Documents – Parental Permission Form and Fact Sheet (Spanish Version)
I5. National Youth Tobacco Survey Questionnaire Supplemental Documents – Parental Permission Form Reminder Notice (English Version)
I6. National Youth Tobacco Survey Questionnaire Supplemental Documents – Parental Permission Form Reminder Notice (Spanish Version)
I7. National Youth Tobacco Survey Questionnaire Supplemental Documents – Questionnaire Administration Script
J. IRB Approval Letter
K. Sample Table Shells
L. Detailed Sampling and Weighting Plan
M. National Youth Tobacco Survey Non-Response Analysis Report, 2017
N. Cognitive Testing Results, 2017
O. External Peer Review of the National Youth Tobacco Survey Sampling Methodology, October 31, 2017
P1. Youth Cognitive Interview Guide – Understanding Use of Electronic Vapor Devices (EVDS) among Youth
P2. Cognitive Interview Assent Form, Individuals under 18 years of age - Understanding Use of Electronic Vapor Devices (EVDS) among Youth.
P3. Cognitive Interview Consent Form, Individuals 18 years of age - Understanding Use of Electronic Vapor Devices (EVDS) among Youth.
P4. Cognitive Testing: Parental Permission for minor child to be in a cognitive interview on understanding use of electronic vapor devices among youth
P5. Cognitive Testing Youth Materials – Electronic Nicotine Delivery Systems Key Facts
P6. Cognitive Testing Youth Materials – Tools for Quitting
P7. Cognitive Testing: Participant Screener – Understanding Use of Electronic Vapor Devices (EVDS) among Youth.
LIST OF TABLES
Table A.4 Characteristics of selected surveys of school-going youth or inclusive of school-aged youth, United States
Table A.12.a Estimated Annualized Burden Hours
Table A.12.b Annualized Estimated Cost to Respondents
Table A.14 Estimated Annualized Study Cost
Table A.15 Annualized Estimates for the 2018 NYTS, with changes since previous OMB approval
Table B.1 Distribution of Schools by Urban Status and School Type
Table B.2.a Quality Assurance Measures Before, During, and After Data Collection
Table B.2.b Major Means of Quality Control
Table B.3 Historical NYTS Participation Rates
LIST OF FIGURES
Figure A.4.a Relationship between NYTS and Other Surveys that are Inclusive of School-Aged Youth (e.g., PATH and NSDUH)
Figure A.4.b Relationship between NYTS and Other School-Based Surveys (e.g., YRBSS and MTF)

Goal of the study
The study is to design, conduct, and report on the school-based NYTS among 6th through 12th grade students in 2018, 2019, 2020, 2021, and 2022. The purpose of the survey is to assess student use of tobacco in a variety of forms; their knowledge of and attitudes toward tobacco; their exposure to secondhand tobacco smoke; and their exposure to influences that promote or discourage tobacco use, such as portrayals of tobacco in advertising and mass media, enforcement of age restrictions in the sales of tobacco to minors, provision of school- and community-based interventions, and access to supports in attempting to stop using tobacco.
Intended use of the resulting data
The NYTS data will be used to inform and evaluate the National Comprehensive Tobacco Control Program; inform progress towards achieving Healthy People 2020 objectives related to tobacco and youth; provide data to inform the Department of Health and Human Service’s Tobacco Control Strategic Action Plan, and provide national benchmark data for state-level Youth Tobacco Surveys and for comparison with the international community through the Global Youth Tobacco Survey.
Methods to be used to collect
Data for the NYTS shall be collected electronically via a digitally based self-administered questionnaire. However, if needed, at the discretion of the government the survey shall be collected via a paper-and-pencil self-administered questionnaires. For the NYTS, it is expected that a minimum of 24,000 students attending a minimum of 200 schools will participate.
The subpopulation to be studied
NYTS data will provide comparable data among subpopulations of youth such as non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanics, and others.
How data will be analyzed
The NYTS data will be weighted to be a nationally representative data. Data sets and documentation for the NYTS are available online. National trends and patterns of the distribution and determinants of tobacco use behaviors among youth enrolled in grades 6-12 could be compared by demographics and across years.
OVERVIEW
CDC requests OMB approval for three years to continue annual information collection for the National Youth Tobacco Survey (NYTS). The NYTS was previously conducted by CDC in 2004, 2006, 2009, and on an annual basis for years 2011-2017. The most recent OMB approval was for NYTS information collection in 2015, 2016, and 2017 (“2015 - 2017 National Youth Tobacco Survey (NYTS),” OMB no. 0920-0621, exp. 1/31/2018). The NYTS employs a repeat cross-sectional design to develop national estimates of tobacco use behaviors and exposure to pro- and anti-tobacco influences among students enrolled in grades 6-12. This Revision includes an update to the title of the information collection reflecting plans to conduct NYTS surveys annually in 2018, 2019, and 2020. There are no proposed changes in content from 2017 to 2018, but the burden allocation for instrument development and testing is for potential changes that might occur after 2018. The estimated burden per response has not changed.
A. JUSTIFICATION
A.1. CIRCUMSTANCES MAKING THE COLLECTION OF INFORMATION NECESSARY
This statement supports a request to obtain approval for the revision of a currently approved information collection request to conduct the school-based National Youth Tobacco Survey (NYTS) (OMB No. 0920-0621; exp. 1/31/2018). The NYTS is designed to assess the distribution and determinants of tobacco use behaviors among youth enrolled in grades 6-12. The information collection proposed in this request will use the current OMB-approved sampling strategy, recruitment methods, and data collection procedures to conduct the NYTS among nationally representative samples of students in public and private schools, enrolled in grades 6-12, during January through May of 2018, 2019, and 2020. The survey instruments for 2019 and 2020 may be revised to add items that are relevant to the present circumstances in tobacco prevention and control efforts among youth upon approval of this information collection request. The term of this request is to collect information annually for three years (2018-2020).
The NYTS is the only nationally representative survey of middle and high school students that focuses exclusively on tobacco use patterns and associated factors. In order to minimize duplication of data collection and the burden on survey participants, the CDC and the Food and Drug Administration (FDA) have collaborated to leverage the NYTS as a single data source to inform national objectives for tobacco use prevention and control among youth. The annual administration of NYTS has helped in the rapid identification of emerging trends, such as the increased use of electronic cigarettes (e-cigarettes) observed in 2014 and 2015 (CDC, 2013a; CDC, 2013b, CDC 2015), and allows for the development and inclusion of specific measures relevant to national objectives for tobacco prevention and control among youth.
CDC requests OMB approval to continue conducting the NYTS in 2018, 2019, and 2020. Survey methods remain the same as in past cycles of NYTS administration. Although the NYTS will be conducted with scannable questionnaires in 2018, CDC is committed to reducing burden and improving information gathering via advances in information technology. Thus, after 2018, CDC plans to conduct the NYTS electronically via a digitally based self-administered questionnaire which will improve respondents’ ability to complete the survey and should decrease burden as this platform will be familiar to most respondents. As a backup, data can be collected on optically scannable questionnaire booklets if necessary. A random sample of approximately 250 schools will be asked to participate in the 2018 NYTS. The probability of a school being selected is based on enrollment in grades 6 through 12. One or two classes (about 25 to 50 students) from each grade 6 through 12 will be selected randomly to take part in each school. Approximately 100 to 200 students are asked to participate in a school containing grades 9 through 12. In a school with grades 6 through 8, approximately 75 to 150 students are asked to participate. CDC expects a final minimum yield of 24,000 students. For a complete discussion of the survey’s sampling design and methods to evaluate non-response bias, please see Supporting Statement Part B and Attachment M.
The 2018 NYTS Questionnaire (Attachment I1) has 88 questions and is estimated to take 35-45 minutes to complete (approximately one class period). The number of questions in the 2018 NYTS questionnaire is comparable with previous cycles. Between 2011 and 2015, the NYTS had 81 questions, while there were 83 questions in 2016 and 88 questions in 2017. The increased number of questions in recent years reflects the detailed assessment of emerging tobacco products, such as electronic cigarettes (e-cigarettes) and hookah.
Background
CDC is responsible for leading and coordinating national strategic efforts aimed at preventing tobacco initiation, promoting tobacco cessation, protecting nonsmokers from secondhand smoke, and eliminating tobacco-related health disparities. A comprehensive tobacco control program must have surveillance and evaluation systems that can track and document a wide range of short-term, intermediate, and long-term intervention outcomes in the population, the data from which can inform public health program and policy efforts, as well as demonstrate programmatic and fiscal accountability (CDC, 2014a). The NYTS is a multifactorial survey that measures short-term outcomes (such as increased knowledge about the negative health consequences of tobacco use and exposure to secondhand smoke), intermediate-term outcomes (such as reduced access to tobacco products), and long-term outcomes (such as reduced tobacco use prevalence) (CDC, 2012b; CDC, 2014a; Starr, et al., 2005). As such, NYTS data are instrumental in providing the science base to inform evidenced-based approaches to public health interventions; stakeholder capacity to design, implement, and evaluate comprehensive tobacco control programs; and the facilitation of coordinated efforts among national and state partners.
The NYTS produces national estimates for the entire U.S. and provides important comparison data for other surveillance efforts. For example, many states conduct a Youth Tobacco Survey (YTS) to collect state-level tobacco use data, and a number of countries participate in the Global Youth Tobacco Survey (GYTS), which provides international tobacco use data. Since these surveys are comparable to the NYTS in methodology and content, states can measure their program’s progress relative to national trends. Similarly, CDC collaborates with the World Health Organization (WHO) in providing training and technical assistance to countries around the world in conducting the GYTS, which contains core questions found on both the YTS and the NYTS. Collectively, the NYTS, YTS, and GYTS are critical to CDC’s ongoing, comprehensive efforts to provide technical assistance to international, national, state, and local tobacco prevention and control efforts.
The NYTS is a comprehensive assessment of knowledge, attitudes, perceptions and behaviors related to multiple tobacco products (cigarettes, cigars, smokeless tobacco, pipe tobacco, e-cigarettes, hookahs, snus, dissolvable tobacco, and bidis) and also includes items about exposure to secondhand smoke, exposure to pro- and anti-tobacco influences such as portrayals of tobacco in advertising and mass media, provision of school- and community-based interventions, and enforcement of minors’ access laws. These data are essential to the design, implementation, and evaluation of comprehensive youth tobacco prevention and control programs by a variety of federal, state, and local stakeholders.
The justification for the continued implementation of the NYTS is based on three factors: (1) public health implications of tobacco use; (2) economic burden of tobacco use; and (3) mandates to monitor, reduce, and alter attitudes toward tobacco use and reduce exposure to pro-tobacco influences found in Section 301 of the Public Health Service Act (42 USC 241) (Attachment A).
Public Health Implications of Tobacco Use
Tobacco use remains the single leading preventable cause of disease and death in the United States. Age at initiation of smoking is an important indicator of future smoking behavior, as more than 87% of adult smokers report that they tried their first cigarette by the time they were 18 years of age (USDHHS, 2014). Additionally, people who start smoking when young are more likely to become strongly addicted to nicotine (USDHHS, 2014), and young people who try to quit using tobacco, experience the same nicotine withdrawal symptoms as adults who try to quit (USDHHS, 2012b). Also, nicotine exposure during adolescence may have lasting adverse consequences for brain development (USDHHS, 2014).
One of the newest nicotine-containing products on the U.S. market is electronic nicotine delivery systems (ENDS). ENDS include electronic cigarettes, vape pens, electronic hookahs and other similar devices. ENDS are battery-powered devices that provide doses of nicotine and other additives to the user in an aerosol and can contain flavorings that are particularly appealing to youth (e.g., fruit, mint, or chocolate). In 11 U.S. states, there were no restrictions on the sale of ENDS to minors in 2014 (CDC, 2014b). FDA’s deeming rule became effective on August 8, 2016, and extended FDA’s regulatory authority to all tobacco products, including ENDS, cigars, pipe tobacco, nicotine gels, and hookah (or water pipe) tobacco. As a result, ENDS could not be sold to minors now. However, states may have laws in effect that extend those sales restrictions past age 18 years. It also requires health warnings on roll-your-own tobacco, cigarette tobacco, and certain newly regulated tobacco products, and bans free samples. The new rule also restricts youth access to newly regulated tobacco products by: 1) not allowing products to be sold to those younger than 18 and requiring age verification via photo ID; and 2) not allowing tobacco products to be sold in vending machines (unless in an adult-only facility).
The 2015 NYTS revealed substantial increases in e-cigarette use among youths in grades 6 through 12 between 2011 and 2015, from 1.5% to 16.0% in high school students and 0.6% to 5.3% for middle school students (CDC, 2016). Since 2014, e-cigarettes have been the most commonly used tobacco product among youth (CDC 2015, CDC 2016). Moreover, concurrent use of multiple tobacco products is common with 13.0% of high school students using ≥2 tobacco products (CDC, 2016). Additionally, hookah (water pipe) is an increasing medium through which adolescents are consuming tobacco. The prevalence of hookah smoking among adolescents in 2013 was 21.4%, which is a significant increase, from 17.1% in 2010 and 18.3% in 2012 (NIDA, 2014). Past 30 day use of hookah among high school students also increased from 4.1% to 7.2% during 2011-2015 (CDC, 2016).
Taken as a whole, these data demonstrate the clear public health importance of monitoring tobacco use and related behaviors among adolescents, and further support the need for continued rigorous, scientific research and surveillance.
Costs of Tobacco Use
The economic impact of smoking and exposure to secondhand smoke is enormous in terms of increased medical costs, lost productivity, and other factors. Average annual cigarette smoking-attributable healthcare spending during 2006-2010 is estimated at $170 billion, >60% of which was financed through public health insurance programs (Xu et al. 2015). As this figure does not include costs from all tobacco use, it likely underestimates total healthcare spending due to tobacco use. In 2005-2009, furthermore, it is estimated that over $156 billion in lost productivity from premature death occurred due to cigarette smoking and exposure to secondhand smoke (USDHHS, 2014). This estimate likely underestimates the total value of lost productivity due to tobacco use, as it does not include losses due to morbidity (USDHHS, 2014).
Mandates to Monitor and/or Reduce Tobacco Use Among Youth
The justification for tobacco use surveillance among middle and high school students has strong Federal support. The NYTS provides data to support several strategic planning priorities for the U.S. Department of Health and Human Services (DHHS), including the Healthy People 2020 objectives (USDHHS, 2010b), CDC’s Budget Request Summary for FY 2015 (CDC, 2014c) on selected Government Performance and Results Act (GPRA) measures, DHHS’s Tobacco Control Strategic Action Plan (USDHHS, 2012a), and the Family Smoking Prevention and Tobacco Control Act. In addition to these strategic initiatives, CDC has identified tobacco use as one of its ten ‘winnable battles’ for public health, in other words, it is a public health priority with large-scale impact on health with known effective strategies to address it. (CDC, 2013c). Further information on these priorities follows below.
The Tobacco Use Chapter of Healthy People 2020 provides a framework and direction for public health activities to reduce tobacco use for the current decade. The NYTS is the established data source for Healthy People 2020 objective 18, which is to reduce the proportion of adolescents and young adults in grades 6 through 12 who are exposed to tobacco marketing; this objective has 4 sub-objectives pertaining to exposure to tobacco marketing through the following media and settings (USDHHS, 2010b):
Objective 18.1 — Internet;
Objective 18.2 — magazines and newspapers;
Objective 18.3 — movies and television;
Objective 18.4 — point of purchase.
The NYTS data are essential for creating historical context around Healthy People objectives and whether progress toward meeting these objectives has resumed or plateaued. In addition, the NYTS provides data that is complementary and supportive to other Healthy People Tobacco Use objectives as follows:
Objective 2—Reduce tobacco use by adolescents
The NYTS assesses use of a range of tobacco products, including those that have shown increasing popularity among youth in recent years, including hookah and e-cigarettes.
Objective 3—Reduce the initiation of tobacco use among children, adolescents, and young adults
The NYTS assesses not only initiation of tobacco use, but also a range of pro- and anti-tobacco influences; thereby enabling the identification of correlates of initiation (e.g., susceptibility, attitudes, and receptivity).
Objective 7—Increase cessation attempts by adolescent smokers
One goal of comprehensive tobacco prevention programs is to help people quit smoking. The NYTS assesses a range of factors associated with cessation intention, including number of cessation attempts, length of abstinence from tobacco use, symptoms of withdrawal and addiction, and use of cessation aids.
Objective 11— Reduce the proportion of nonsmokers exposed to secondhand smoke
NYTS assesses exposure to secondhand smoke in a variety of settings.
Objective 19— Reduce the illegal sales rate to minors through enforcement of laws prohibiting the sale of tobacco products to minors.
The NYTS assesses a range of factors related to access to tobacco products by minors, including point of purchase and requests for proof of age.
The Healthy People 2020 youth tobacco use objectives also are one of the DHHS Secretary’s 12 Leading Health Indicators (https://www.healthypeople.gov/2020/Leading-Health-Indicators, IOM, 2011). The Leading Health Indicators reflect the major public health concerns in the United States, and were chosen based upon their ability to motivate action, the availability of data to measure their progress, and their relevance as broad public health issues. Many states use YTS to collect these detailed data, with the added advantage of having comparable NYTS national data against which they can benchmark their findings.
In compliance with GPRA, CDC’s Online Performance Appendix focuses the agency’s priorities and directions for the future and assesses constituents’ requirements (CDC, 2014e). One of the focal areas in CDC’s Performance Appendix is to reduce the proportion of adolescents (grade 9 through 12) who are current cigarette smokers. The associated GPRA measure is the reduction of cigarette smoking among youth. CDC’s strategy for preventing tobacco use is a crosscutting approach that includes support for state programs, surveillance, prevention, research, evaluation, and health promotion. Only the NYTS gathers comprehensive national surveillance data among middle and high school students on tobacco use, including cigarette smoking, and on the influences promoting or discouraging tobacco use. Trend data underscore the importance of CDC’s focus on efforts related to the reduction of tobacco use among adolescents. From 2011 to 2012, prevalence of current tobacco and cigarette use declined among middle school (4.3% to 3.5%) and high school (15.8% to 14%) students; however a substantial proportion of youth tobacco use is comprised of products other than cigarettes, stressing the importance of monitoring and preventing new and emerging product use among youth (CDC, 2013b). For example, between 2011 and 2015, there has been a substantial increase in the use of e-cigarettes and hookahs among youth (CDC, 2016). The NYTS is an important tool used by CDC to provide support and technical assistance to state and national partners for comprehensive Tobacco Control Programs (TCP). NYTS data enable comprehensive evaluation of key state TCP short-term, intermediate, and long-term outcome indicators.
The annual administration of the NYTS will provide timely estimates of tobacco-use behaviors; exposure to tobacco marketing and advertising; compliance with tobacco-use policies, including minor’s access law to prevent underage tobacco purchases; exposure to new/expanding warning labels; levels of exposure to secondhand smoke; and social norms related to tobacco-use. It will address each of the four U.S. Department of Health and Human Service’s Tobacco Control Strategic Action Plan major action areas: 1) Leading by Example: Leveraging HHS Systems and Resources to Create a Society Free From Tobacco-Related Death and Disease; 2) Improving the Public’s Health: Strengthening the Implementation of Evidence-Based Interventions and Policies in States and Communities; 3) Engaging the Public: Changing Social Norms Around Tobacco Use, and; 4) Advancing Knowledge: Accelerating Research to Expand the Science Base and Monitor Progress (USDHHS, 2012a).
On June 22, 2009, the Family Smoking Prevention and Tobacco Control Act was signed into law. The Act amended Section 201 of the Food, Drug, and Cosmetic Act (FD&C) (21 U.S.C. 321) by inserting Chapter 9 (“Tobacco Products”), Section 901, which provided FDA with regulatory authority over the manufacture, distribution, and marketing of tobacco products, including the authority to promulgate tobacco product standards; regulate the labeling of tobacco products, including health warnings on tobacco product packages and in ads and removal of misleading descriptors such as “light”, “low”, and “mild”; require the testing and reporting of harmful or potentially harmful constituents (HPHC) by tobacco product brand and sub-brand; and restrict access to tobacco products, advertising, and promotions among youth. FDA’s deeming rule became effective on August 8, 2016, and extended the FDA’s regulatory authority to all tobacco products, including ENDS, cigars, pipe tobacco, nicotine gels, hookah (or water pipe) tobacco, and other tobacco products. CDC and FDA have collaborated in recent revisions to the NYTS instrument to prevent data duplication, enabling the leveraging of the CDC-initiated NYTS to collect information relevant to FDA’s regulatory authority, including awareness of tobacco product health warnings, perceptions about the harms of tobacco products, use of flavored tobacco products, symptoms of tobacco dependence, and ease of minor’s access to tobacco.
In addition to the strategic initiatives mandating the reduction of tobacco use by youth, CDC established a list of ten “Winnable Battles” in an effort to closely monitor emerging public health issues. These include:
These areas have been chosen based on the magnitude of the health problems, and the nation’s ability to make significant progress in improving outcomes. Each area is a leading cause of illness, injury, disability, or death, and/or represents enormous societal costs. In addition, there are evidence-based, scalable interventions already in place for each area that can be broadly implemented (CDC, 2014d).
Data collected through the NYTS can: (1) inform the development of health policy and guidelines that protect nonsmokers from secondhand smoke; (2) help researchers and policy makers to better understand youth exposure to pro-tobacco influences; (3) provide comprehensive tobacco use data to support well-funded tobacco control programs; (4) inform the implementation of other key evidence-based policies that will decrease the number of smokers and save lives. Completion of these steps will move CDC and the public health community one step closer toward eliminating tobacco use and achieving this winnable battle.
CDC requests OMB approval to conduct the NYTS in 2018, 2019, and 2020. The NYTS is conducted in a school-based setting. Respondents are students in grades 6-12. The survey instrument is updated annually if needed to reflect changes in the relevant product environment and emerging standards in terminology and classification of these products.
A.2 PURPOSE AND USE OF INFORMATION COLLECTION
The primary purpose of the NYTS is to collect information on the use of tobacco products; knowledge of and attitudes toward tobacco; exposure to secondhand smoke; and, exposure to pro- and anti-tobacco influences such as portrayals of tobacco in advertising and mass media, provision of school- and community-based interventions, enforcement of minors’ access laws. NYTS data will be used not only by CDC, but by several other Federal agencies, including FDA. Additionally, the information will have a broad use by state and local governments, nongovernmental organizations, and others in the private sector.
Survey Purposes
The specific aims of the survey are to:
Provide data for key cognitive (knowledge, intentions, and attitudes) and behavioral indicators related to the design, implementation, and evaluation of comprehensive tobacco prevention and control programs. Interim analyses conducted at various intervals before survey completion are done only for purposes of quality assurance/control.
Estimate the extent to which middle and high school students engage in tobacco use behaviors, as well as their exposure to influences promoting or discouraging tobacco use.
Assess the degree to which engaging in tobacco use behaviors and exposures to influences promoting or discouraging tobacco use varies as a function of sex, age, grade in school, and race/ethnicity.
Describe trends in tobacco use behaviors and pro- and anti-tobacco use influences. Assess the degree to which these trends vary as a function of sex, age, grade in school, and race/ethnicity.
Determine the interrelationships between tobacco use behaviors and exposure to pro- and anti-tobacco influences, and the degree to which these associations vary as a function of sex, age, grade in school, and race/ethnicity.
Provide data related to theory-driven constructs that can be useful for explaining tobacco use behavior, designing interventions, and evaluating intervention effectiveness.
Inform and monitor the impact of FDA’s policies, programs, and regulatory activities and assess progress towards achieving one of its core public health goals: preventing youth tobacco use.
Anticipated Uses of Results by CDC
NYTS data will be used by CDC’s Office on Smoking and Health, as well as, several other divisions within CDC, including the Division of Adolescent and School Health, the Division of Cancer Prevention and Control, and the Division of Oral Health.
Evaluation
Direct progress measurement related to one HP 2020 objective (which contains 4 sub-objectives) and one Leading Health Indicator.
Evaluate CDC’s Performance Plan in compliance with GPRA.
Assess trends in tobacco use among middle and high school students and exposure to pro- and anti-tobacco influences to determine the aggregate impact of tobacco prevention and control activities.
Provide data to evaluate the impact of comprehensive tobacco control programs on tobacco use by youth.
Research Synthesis
Provide states conducting the YTS with a national index that can be compared to state-specific short-term, intermediate, and long-term tobacco prevention and control outcome indicators.
Publish data in peer-reviewed publications and present at scientific meetings.
Identify research gaps in youth tobacco prevention and control.
Provide public health and education officials, youth, parents, and the general public with accurate information about tobacco use and exposure to pro- and anti-tobacco influences.
Provide U.S. data for WHO-sponsored international reports based on administration of the GYTS.
Provide data that are relevant and can be incorporated into a variety of government publications, including Surgeon General’s reports.
Policy
and Program Development
Provide stakeholders with information about tobacco use behaviors among middle school and high school students to help inform the identification and implementation of tobacco prevention and control interventions.
Determine how to best devise public information campaigns that take into account exposure to pro- and anti-tobacco influences among youth.
Technical Assistance
Help identify programs shown to be most effective in reducing tobacco use among youth.
Assist states in interpreting their YTS data against a national benchmark.
Provide evidence-based technical assistance to state and local departments of health and education.
Assess the need for new programs or modify existing programs that focus on preventing and reducing tobacco use among youth.
Assess the cumulative effects of multiple interventions and sources of information (school, family, community, and the media) on tobacco use behaviors among youth.
Anticipated Uses of Results by Other Federal Agencies and Departments
The survey results of the NYTS are of interest not only to CDC, but also to other Federal agencies and departments. For example:
DHHS uses NYTS data directly to track progress on one of the Healthy People 2020 objectives and one of the 12 Leading Health Indicators. USDHHS cited the NYTS in their August 2012 tobacco epidemic progress report: (USDHHS, 2012a) Ending the Tobacco Epidemic: Progress toward a Healthier Nation. Washington, DC: US Department of Health and Human Services. USDHHS also cited NYTS data in their 2014 Surgeon General’s Report: (USDHHS, 2014) The Health Consequences of Smoking- 50 years of Progress: A Report of the Surgeon General and their 2016 report, E-Cigarette Use Among Youth and Young Adults: A report of the Surgeon General (USDHHS, 2016).
FDA plans to use the NYTS data over time to inform its regulatory authority over the manufacture, distribution, and marketing of tobacco products. This includes the generation of national estimates of tobacco use and key tobacco-related measures among middle and high school students, such as tobacco product harm perceptions, exposure to marketing, and symptoms of tobacco dependence. In addition, data will be used to help monitor the impact of FDA regulatory activities, such as enforcement of youth tobacco product sales restrictions, restrictions on marketing and promotion, and changes to health warnings on cigarette and smokeless tobacco packages.
Health Resources and Services Administration (HRSA) identifies NYTS data as a source for credible and reliable youth data that provide a strong scientific aspect to Maternal and Child Health Bureau (MCHB) needs assessments in their Promising Practices in MCH Needs Assessment: A Guide Based on a National Study (USDHHS, HRSA, 2004) report In addition, NYTS data support HRSA, MCHB, and the American Academy of Pediatrics’ Bright Futures Health Supervision Guidelines formulate specific risk-reduction recommendations to prevent and assess tobacco use and exposure for infants, children, and adolescents.
National Institute on Drug Abuse (NIDA), in collaboration with FDA, is coordinating with CDC to harmonize tobacco-related measures in NYTS and the Population Assessment of Tobacco and Health study. These efforts are complementary to the missions of each agency while helping to prevent unnecessary data duplication.
National Cancer Institute (NCI) uses NYTS data to help inform its research, educational efforts, and demonstration projects focused on youth tobacco use prevention and the determinants of cessation. NYTS data are cited in NCI’s President’s Cancer Panel 2006-2007 Annual Report titled Promoting Healthy Lifestyles: Policy, Program, and Personal Recommendations for Reducing Cancer Risk (USDHHS, NIH, & NCI, 2007)
Office of the Surgeon General uses and reference the NYTS results to assess the need for focused use of resources for tobacco prevention and control efforts targeting youth that were articulated in Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. (USDHHS, 2012b), The Health Consequences of Smoking- 50 years of Progress: A Report of the Surgeon General (USDHHS, 2014), and E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General (USDHHS, 2016). NYTS data figured prominently in informing tobacco use trends in youth in all of these reports.
Use of Results by Those Outside Federal Agencies
Findings from the NYTS can also be used in a variety of ways by state and local governments, researchers, voluntary health organizations, physicians, teacher training institutions, educational administrators, health educators, teachers, and parents:
Policy analysts and researchers in the legislative and executive branches of government can use NYTS and YTS data to understand the relationships between tobacco use behaviors and exposure to pro- and anti-tobacco influences at national, state, and local levels, to evaluate existing policies, and to develop new policies based on evidence regarding effective tobacco use prevention and control programs.
Policy makers may evaluate findings from the study of these data to inform decisions related to policy approaches at national, state, and local levels.
The NYTS can provide an index against which state and local health and education agencies can compare their state YTS results. See Attachment C, State Tobacco Control Reports that Cite National Youth Tobacco Survey Data.
Findings can be used by state and local health and education agencies to assess disparities in tobacco use among racial/ethnic groups at the national level and make comparisons to the state and local levels.
The NYTS provides data that can help states evaluate age group targets for tobacco prevention media campaigns.
State Coordinated School Health programs can use NYTS data to gauge the success of their prevention efforts and work to identify areas of focus and ways to integrate prevention efforts.
State and local law enforcement officials can use findings from the NYTS to determine national compliance with the Synar Amendment, which bans the sale of tobacco products to youth aged <18 years.
Institutes of higher education can use findings from the NYTS in their teacher training programs to provide information on tobacco use behaviors and effectiveness of evidence-based tobacco prevention and control interventions.
State and local health departments can use the findings from the NYTS as a guide in developing local tobacco-related health promotion programs to measure progress toward meeting Healthy People 2020 objectives.
Family physicians, pediatricians, psychologists, and counselors can use findings from the NYTS to provide up-to-date information on tobacco use behaviors and factors that influence tobacco use for application in the adolescents they treat.
School administrators can use findings from the NYTS to provide information to assist them in justifying and planning educational programs to prevent tobacco use and capitalize on extant interventions that curtail use.
Health educators and other teachers can use findings from the NYTS to provide information that will bolster and provide a focus for their lesson plans and educational materials.
State and local education agencies have previously used NYTS results in creating awareness of risk behaviors, setting program goals, planning or modifying programs, developing staff development programs for teachers, and seeking/targeting funding.
Nongovernmental organizations and foundations have previously used NYTS data to characterize the problem of youth tobacco use and to evaluate interventions to decrease tobacco use. Examples include:
NYTS data were used in the American Cancer Society (ACS) 2015-2016 report Cancer Prevention & Early Detection: Facts and Figures 2015-2016 (ACS, 2015). In addition, ACS’ Cancer Action Network uses NYTS data to advocate for various public policies (e.g. raising the minimum age of sale to 21 for all tobacco products).
The Robert Wood Johnson Foundation (RWJF) funded a report that used NYTS data to highlight the need for tobacco prevention and control efforts among Asian American and Pacific Islander youth in their report Critical Policy Issues on Tobacco Prevention and Control for the Asian American and Pacific Islander Community (Asian Pacific Partners for Empowerment and Leadership, 2000). RWJF also cited the NYTS in a 2002 report concerning making tobacco relevant for Asian American and Pacific Island communities (Asian Pacific Partners for Empowerment and Leadership, 2002).
The California Cancer Research Fund for the University of California funded a report that cites NYTS data used to address tobacco use among Asian American, Native Hawaiian, and Pacific Island communities in California (The University of California, 2012).
American Legacy Foundation, now referred to as the Truth Initiative, (2000a-2000e, 2001a, 2001b, 2002, 2003a, 2003b, 2004, 2005) has used NYTS data in a series of “First Look” reports that address youth tobacco use and comprehensive tobacco control efforts. The ALF also used NYTS data in the 2005 study, Physician and dentist tobacco use counseling and adolescent smoking behavior: results from the 2000 National Youth Tobacco Survey (Shelley et al., 2005). Smoking among Asian American and Hawaiian/Pacific Islander youth: new data from the 2000 National Youth Tobacco Survey also cites the NYTS (Appleyard, Messeri, & Haviland, 2001), along with Tobacco Fact Sheet: Cigars, Cigarillos, and Little Cigars (American Legacy Foundation, 2012). Results from the 2014 NYTS contributed to a recent paper on frequency of current cigarette, cigar, smokeless, and e-cigarette use in the context of past 30-day and ever tobacco product use in U.S. middle and high school students (Villanti et al. 2016).
Professional organizations have previously used NYTS data to emphasize the importance of tobacco prevention efforts and monitor progress in tobacco control efforts and make policy recommendations. For example, the American Medical Association, used NYTS data to support their 2015 policy for a minimum legal purchase age of 21 for e-cigarettes (American Medical Association, 2015).
Parents and students can use findings from the NYTS posted through popular media including social networking sites, news outlets, and print media to better understand tobacco use behaviors and exposure to pro- and anti-tobacco influences among their children.
The Institute of Medicine cited NYTS data in their recent report on the Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products (IOM, 2015).
A.3 USE OF IMPROVED INFORMATION TECHNOLOGY AND BURDEN REDUCTION
Although the NYTS will be conducted with scannable questionnaires in 2018, CDC is committed to reducing burden and improving information gathering via advances in information technology, Thus, after 2018, CDC plans to conduct the NYTS electronically via a digitally based self-administered questionnaire which will improve respondents’ ability to complete the survey and should decrease burden as this platform will be familiar to most respondents. As a backup, data can be collected on optically scannable questionnaire booklets if necessary. The data cannot be accessed from currently existing automated databases. During questionnaire design, every effort has been made to limit respondent burden.
A.4 EFFORTS TO IDENTIFY DUPLICATION AND USE OF SIMILAR INFORMATION
CDC conducts ongoing searches of all major educational and health-related electronic databases, reviews related literature, consults with key outside partners and other experts, and maintains continuing communications with Federal agencies with related missions. To date, these efforts have not identified previous, current, or planned efforts to conduct comprehensive surveys of tobacco use behaviors, exposure to pro- and anti-tobacco influences, and key short-term and intermediate outcome indicators among a nationally representative sample of students in grades 6 through 12. Although many surveillance systems assess some aspects of tobacco use behaviors, the NYTS is inherently distinct from other existing population-level surveys that are conducted with different areas of emphasis and/or with different populations. Tobacco use is related to a wide spectrum of other health behaviors and health outcomes, and thus, is a critical measure to include in surveys of many topics among youth and adults. Most nationally-representative surveys focused on youth health behaviors do assess some aspects of tobacco product use. However, unlike the NYTS, all other national surveys, such as the Youth Risk Behavior Survey (YRBS; OMB No. 0920-0493, exp.11/30/2019), the National Survey on Drug Use and Health (NSDUH; OMB No. 0930-0110), Monitoring the Future (MTF), and the National Health and Nutrition Examination Survey (NHANES; OMB No: 0920-0950) are multi-risk factor surveys, meant to provide a broader snapshot of youths’ health and health behaviors. Thus, these surveys are limited in the number of questions they may ask about specific risk behaviors, such as tobacco use. Although tobacco-related questions on multi-risk factor surveys are important to provide information on youths’ health behaviors, they cannot meet the needs specific to the evaluation of tobacco prevention and control activities at the national level.
The Venn diagrams below illustrate how the study population of NYTS relates to other similar surveys.
Figure A.4.a – Relationship between NYTS and Other Surveys that are Inclusive of School-Aged Youth (e.g., PATH and NSDUH).
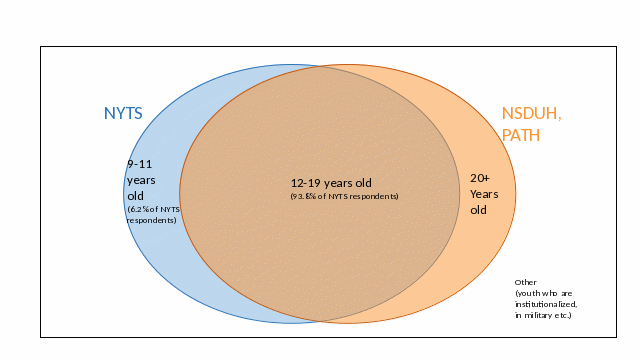
Figure A.4.b – Relationship between NYTS and Other School-Based Surveys (e.g., YRBSS and MTF)
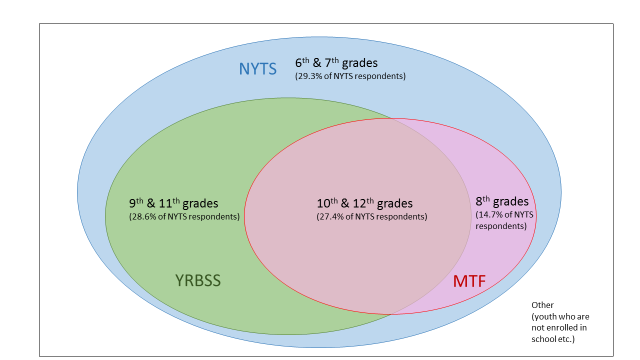
Table A.4 – Characteristics of selected surveys of school-going youth or inclusive of school-aged youth, United States
Characteristic |
NYTS |
YRBSS |
MTF |
PATH |
NSDUH |
Sponsor |
CDC |
CDC |
NIDA |
FDA, NIH |
SAHMSA |
Study design, periodicity |
Cross-sectional, annual |
Cross-sectional, biannual (every two years) |
Cross-sectional, annual |
Cohort, baseline sample drawn in 2013-2014 and was not replenished until Wave 4 (2016-2017) |
Cross-sectional, annual |
Study Setting |
School-based |
School-based |
School-based |
Household |
Household |
Study population |
Grades 6-12 |
Grades 9-12 |
Grades 8, 10, 12 |
Ages 12+ |
Ages 12+ |
Tobacco products assessed |
Cigarettes, cigars, roll-your-own tobacco, hookah, e-cigarettes, smokeless tobacco |
Same as in NYTS |
Same as in NYTS |
Same as in NYTS |
Less than in NYTS (does not include e-cigarettes or hookah) |
Other addictive behaviors independent of tobacco? (e.g., past 30-day heroin, or marijuana use) |
No |
Yes |
Yes |
Yes |
Yes |
Scope |
National |
National and state |
National |
National |
National, small-area estimates |
Uniqueness |
Only tobacco-specific survey of U.S. middle and high school students.
Tracks several HP2020 objectives |
Only survey to provide state-specific estimates of high school students |
Began in 1975; random sample of 12th grade students selected for biannual follow-up after high school (by mail) |
Has repeated observations (i.e., longitudinal) allowing for assessment of tobacco use trajectories and establishment of temporality between exposures and outcomes |
Broad spectrum of addictive behaviors assessed |
Limitations |
Lacks information on socio-economic status, academic performance, and other risky behaviors that are not tobacco-related; inability to generate state-specific estimates |
Only conducted every two years; States/ localities conducting YRBSS may delete or add questions at their discretion, thus, not all estimates may not be comparable or available across state samples. |
Data are unavailable for all grade levels (excludes 6-7th; 9th; and 11th grades). Lacks data on emerging tobacco products (such as hookah) |
Susceptible to loss to follow up, which could threaten external validity (generalizability) if attrition is systematic and not random |
No data on emerging tobacco products, e.g., hookah, e-cigarettes |
Thus, while there is duplication of several constructs across some of the surveillance systems, this does not constitute unnecessary duplication for several reasons (Table A.4). (1) These surveys have differences in study populations, periodicity of survey administration, study design, and intended uses. (2) Studies may contain differences in the measure of tobacco use. For example, NHANES (OMB No: 0920-0950) assesses use of certain tobacco products in the context of past 5-day use, while NYTS assesses within the context of past 30-day use. (3) There are unique data collected within each of the surveillance systems which allows for investigation of associations in a manner that cannot be done elsewhere.
The proposed NYTS data collection effort also is not duplicative of FDA’s campaign outcome evaluation studies (OMB numbers 0910-0788 and 0910-0753). Unlike NYTS, these outcome evaluation studies are designed to assess the effectiveness of public education campaigns that target specific youth subpopulations through such media as television, internet, and various social media. These outcome evaluation studies are not designed to obtain surveillance data on the general population of U.S. middle and high school youth, but are specific to each campaign and target audience; therefore, data from these studies cannot be used to make statistical inferences about U.S. middle and high school youth.
CDC assists states with the implementation of their own state youth tobacco surveys (YTS), however, substantial variation across jurisdictions in sampling techniques, questions, and survey administration procedures prohibit the calculation of national estimates from state-level results. Thus, the proposed data collection does not constitute unnecessary redundancy between NYTS and YTS.
Surveys for youth and adults often differ in questions assessed and survey modes by design. For NYTS, the survey is administered at schools because that provides the most secure setting for youth and it is also where most youth are during weekdays. This mode would not be suitable for adults. Similarly, some tobacco-use questions asked of youth, who are legally prohibited from purchasing tobacco and for whom tobacco use may be a recently acquired behavior, would not be appropriate for adults. This reinforces the need for youth-specific surveillance efforts, such as the NYTS, at the national level.
In the early 1990s the rapid rise in youth prevalence of tobacco use demonstrated the need for frequent assessments in order to identify such patterns in a timely manner in order to mitigate the damage. In addition, many changes are occurring in the tobacco control and tobacco product landscape, making it important to closely monitor their impacts on youth. In 2012, OMB approved the administration of the NYTS on an annual basis, and CDC and FDA began collaborating on ways to use the NYTS to help FDA inform its regulatory authority. NYTS instrument content is decided in collaboration between CDC and FDA in order to inform CDC’s non-regulatory public health approaches, and inform FDA’s regulatory activities. Thus, the survey is specifically being designed to avoid duplication while meeting the needs of both agencies. Beginning in 2012, questions were added to the survey specifically related to FDA’s regulatory authority, including awareness of tobacco product health warnings, perceptions about the harms of tobacco products, use of flavored tobacco products, symptoms of tobacco dependence, and ease of minors’ access to tobacco.
Since the 2015 NYTS, the CDC and FDA established a working group to obtain guidance and suggestions for new items on the questionnaire that would help facilitate the measurement of key data needed to address the missions of both agencies. Working group members include:
2018-2020 NYTS Consultants: Office on Smoking and Health, Centers for Disease Control and Prevention 4770 Buford Highway NE, Atlanta GA 30321 |
|
Linda J. Neff, Ph.D., M.S.P.H. Epidemiology Branch Chief Phone: 770-488-8647 E-mail: [email protected] |
David Homa, Ph.D., M.P.H. Senior Science Advisor for the Epidemiology Branch Phone: 770-488-3626 E-mail: [email protected] |
Brian A. King, Ph.D., M.P.H. Deputy Director For Research Translation Phone: 770-488-5107 E-mail: [email protected] |
Ahmed Jamal, MBBS, M.P.H. Team lead for Surveillance Phone: 770-488-5077 E-mail: [email protected] |
Sean Hu, MD, DrPH. Senior Epidemiologist Phone:
770-488-5845 |
Katrina Trivers, PhD, MSPH Team lead for Research Phone: 404-498-6861 Email: [email protected] |
2018-2020 NYTS Consultants: Center for Tobacco Products, Food and Drug Administration 10903 New Hampshire Avenue, Silver Spring, MD 20993 |
|
Bridget Ambrose, Ph.D., M.P.H. Epidemiology Branch Chief Phone: 301-796-4235 E-mail: [email protected] |
Benjamin Apelberg, Ph.D., M.H.S. Director, Division of Population Health Science Phone: 301-796-8869 E-mail: [email protected] |
Karen Cullen, Ph.D., M.P.H. Epidemiologist Phone: 240-402-4513 E-mail: [email protected] |
Kimberly Snyder, M.P.H. Social Scientist Phone: 240-402-2216 E-mail: [email protected] |
David Portnoy, Ph.D., MPH Social Science Team Lead Phone: 301-796-9298 E-mail: [email protected] |
Martha Engstrom, M.S. Evaluation Branch Chief Phone: 240-402-5387 E-mail: [email protected] |
In addition to CDC-FDA collaboration specific to the NYTS, enhanced review procedures were instituted in 2013 to promote overall efficiency and quality in federally-sponsored data collection relating to tobacco use and control. These efforts are coordinated through the HHS/Assistant Secretary for Planning and Evaluation (ASPE). An inter-agency workgroup was established under the HHS Data Council with representatives from HHS OPDIVS and programs collecting tobacco related data. The role of the group is to build infrastructure and connections to facilitate coordination and communication during the developmental stage of survey design to reduce duplication, improve response rates, reduce respondent burden, and promote standardization of estimates, where feasible. Representatives of the inter-agency workgroup have been consulted in the development of this ICR. Additional federal agencies consulted through this process include NCHS, NIH/NCI, NIH/NIDA, and SAMSHA.
The NYTS is the sole national comprehensive youth tobacco survey specifically designed to monitor and evaluate key short-term (knowledge and attitudes), intermediate (intentions), and long-term (behaviors) outcome indicators of comprehensive tobacco control programs and policies among a nationally representative sample of students in grades 6-12.
HHS/ASPE has approved submission of this ICR for the NYTS.
A.5 IMPACT ON SMALL BUSINESSES OR OTHER SMALL ENTITIES
The planned data collection does not involve small businesses or other small entities.
A.6 CONSEQUENCES OF COLLECTING THE INFORMATION LESS FREQUENTLY
The NYTS was initiated as a biennial survey in 1999. However, as witnessed during the past decade, youth tobacco use can increase or decrease rapidly, making biennial collection less optimal. On June 22, 2009, the Family Smoking Prevention and Tobacco Control Act was enacted, which gave FDA the authority to regulate the manufacturing, distribution, and marketing of tobacco products. FDA’s deeming rule became effective on August 8, 2016, and extended the FDA’s regulatory authority to all tobacco products, including ENDS, cigars, pipe tobacco, nicotine gels, hookah (or water pipe) tobacco, and other tobacco products. Under this authority, a number of regulatory and enforcement actions are underway, including the prohibition of certain types of tobacco advertising and promotion, prohibition of the sale of single cigarettes, elimination of flavors in cigarettes (other than menthol), and enforcement of youth access restrictions. In order to ensure that FDA's goal of protecting young people from tobacco use is achieved, annual data collection is necessary to monitor the impact of FDA's actions on public health, as well as to measure emerging public health issues (such as increased use of previously unregulated tobacco products, like the tripling of current use of e-cigarettes between 2013 and 2014 among all 6th to 12th grade students (CDC, 2013a)). The collection of annual data has been particularly important in the early years following FDA's regulatory authority as many regulations are being implemented in a short time frame. Rather than develop a completely new surveillance system to monitor measures critical to FDA regarding youth tobacco use, thereby increasing burden to the population, CDC and FDA partnered to leverage the existing NYTS system to collect annual data that will be useful to both federal agencies. The annual NYTS monitors tobacco product use among the nation’s youth and collects key information which will assist both CDC and FDA in ensuring that both agencies are protecting the public’s health. The collaboration between CDC and FDA in administering the NYTS annually will help both federal agencies, as well as other stakeholders whose mission it is to reduce tobacco use.
A.7 SPECIAL CIRCUMSTANCES RELATING TO THE GUIDELINE OF 5 CFR 1320.5
The data collection will be implemented in a manner consistent with 5 CFR 1320.5. No special circumstances are applicable to this proposed survey.
A.8 COMMENTS IN RESPONSE TO THE FEDERAL REGISTER NOTICE AND EFFORTS TO CONSULT OUTSIDE THE AGENCY
Federal Register Announcement
A 60-day Federal Register Notice (attachment B1) published in the Federal Register on October 13, 2017, Docket No. CDC-2017-0092, Document Citation 82FR 47740, Pages 47740-47741 (2 pages). CDC received and responded to 4 unique public comments (Attachment B2), related to this notice from both individuals and organizations that are outside of CDC. Within those four (4) unique sets of comments, CDC responded to 5 unique questions/comments.
Consultations
Consultations on the design, instrumentation, products, and statistical aspects of the NYTS have occurred at critical junctures during its original design and have continued since it originally received OMB clearance. The purposes of such consultations were to ensure the technical soundness and user relevance of survey results; to verify the importance, relevance, and accessibility of the information sought in the survey; to assess the clarity of instructions; and to minimize respondent burden.
In October 2016, OSH convened a panel of four external (independent) survey experts to discuss the strengths, weaknesses, and opportunities for improvement to the current NYTS methodology. The twofold objectives of the external peer review were: (1) to assess whether there is a more efficient and effective sampling methodology that would not negatively impact participation rates among U.S. middle and high schools. (2) To assess the enhancements to the survey that might yield valid and reliable national and sub-national estimates. Activities conducted as part of this external peer review included a pre-meeting web conference and in-person open and closed-door sessions. During the pre-meeting web conference, external reviewers were provided with an overview of the management and administration of the NYTS and were invited to submit written questions and comments in advance of the in-person meeting. During the in-person open session, the external experts had the opportunity to ask additional questions of OSH subject matter experts to help enhance their understanding of the survey’s intricacies and help them better formulate feedback and recommendations. The external experts also convened during a closed-door session to deliberate and discuss these recommendations amongst themselves (these experts were reminded that consensus was not being sought). The final recommendations provided by the panel covered different aspects of survey design and implementation, and included the sampling frame construction, sample design, ways to enhance response rates, the mode of survey administration, recruitment procedures, and weighting. Overall, the panel approved of the vast majority of the current NYTS protocol without changes. Other recommendations provided by the panel are either planned for the future (e.g., an electronic survey in lieu of paper and pencil administration), or cannot be implemented at this time because of resource limitations (e.g., subnational estimates).The final report containing the external peer review recommendations is provided as Attachment O.
Consultations with Various User Communities and Experts
Historically, the state YTS began as a questionnaire developed by and for a small group of state health departments for use in evaluating their tobacco prevention and control program expansions, funded largely by the Master Settlement Agreement. To facilitate state efforts to design, implement, and evaluate their tobacco use prevention and control programs, CDC provided technical assistance to states to enhance the relevance and decrease the respondent burden of the core YTS questionnaire. Thus, periodically, CDC met with representatives from a growing number of states to review their perceptions of the utility of data produced by the YTS, identify and remove redundancies, and identify the most relevant indicators. The core state YTS questionnaire in the summer of 1999 became the core for the first NYTS conducted in the fall of 1999. In February, 2005, CDC met with state and U.S. territory representatives to again solicit stakeholder input on the core YTS instrument.
Although Legacy (now the Truth Initiative) was responsible for the design, instrumentation, education products, and statistical aspects of the first three cycles of NYTS, Legacy actively consulted with CDC and other partners during each survey cycle. The purpose of these consultations was to ensure the technical soundness; to verify the importance, relevance, and accessibility of the information sought in the survey; to assess the clarity of instructions; and to minimize respondent burden.
The NYTS explicitly drew on a long tradition of consultations that occurred to support other CDC school-based data collections in that the NYTS inherited the lessons derived especially to: (1) develop and implement a sampling plan that efficiently oversamples racial and ethnic minority groups; (2) optimize institutional receptiveness toward the survey and (3) effectively field an anonymous classroom-based survey that can be understood readily by respondents.
A.9 EXPLANATION OF ANY PAYMENT OR GIFT TO RESPONDENTS
Schools will be given $500 in appreciation for their participation in NYTS, consistent with previous years of implementation of the NYTS (2012-2017). No payments will be offered or made to student respondents. OMB first suggested that CDC offer school incentives on school-based surveys as a means of improving school response rates and, thereby, improving the generalizability of results. Increasingly in recent years, school-based data collections, most of which do not fall under OMB review, have offered financial incentives to increase or maintain school participation rates. CDC believes that offering school incentives helps maintain, or slightly increase, school participation rates despite the growing number of competing, non-instructional demands placed on schools, including standardized testing.
A.10 ASSURANCE OF CONFIDENTIALITY PROVIDED TO RESPONDENTS
The CIO’s Information Systems Security Officer reviewed this submission and determined that the Privacy Act does not apply. This determination is based on the fact that the information that will be collected within NYTS is not considered a “record” as defined by the Privacy Act: it will not include individuals’ financial transactions, medical history, criminal or employment history, name, or the identifying number, symbol, or other identifier assigned to any individual, such as a finger or voice print or a photograph. NYTS data collection contractors will not have access to, nor collect personally identifiable information (PII). All data collected will be disseminated in the aggregate only. During recruitment, districts and schools will be informed that anonymity will be maintained throughout data collection; all data will be safeguarded closely; and that no institutional identifiers will be used in study reports. Anonymity will be mention to students and their parents on the parental permission forms.
After 2018, the data collected for this survey will be electronically via a digitally based self-administered questionnaire. Strict procedures will be in place to protect privacy and allow for anonymous participation. Additionally, for the survey using paper-and-pencil questionnaire, at the start of the survey administration sessions, professionally trained NYTS data collectors will instruct students to not put their names anywhere on the paper and pencil survey instrument (if used) and remind them that their responses will be treated in an anonymous manner (Questionnaire Administration Script, Attachment I7). At the conclusion of the survey administration session, students will be instructed to place their completed surveys in an envelope and seal it. The sealed individual student envelopes will then be deposited into a classroom-specific envelope. For the survey using a digitally based self-administered questionnaire, at the start of the survey administration sessions, professionally trained NYTS electronic data collectors will remind students that their responses will be captured anonymously (Questionnaire Administration Script, Attachment I7). At the conclusion of the survey administration session, students will be instructed to hand their tablet to the data collector. The students’ data will immediately be uploaded to the cloud database and erased from the tablet. As the NYTS electronic administration is completed in each selected class, the classroom-specific tablet will be stored in a school-specific box.
NYTS is required by law to notify parents of students selected for NYTS surveys that their child has been selected and that student participation is voluntary. Schools may use various processes to obtain parental permission, forms of notification (electronically, such as email, or a hard-copy letter) either provided by the state or developed by the school. However, the notification shall include the following elements:
this school will be participating in NYTS and your child’s classroom may be/is selected to participate;
a brief description of the nature and importance of NYTS;
all responses are confidential and results will not be reported to or about individual students or schools; and
your child may be excused from participation for any reason, is not required to finish the survey, and is not required to answer any test questions.
This data collection has received IRB approval from the CDC Human Research Protection Office (Attachment J). This approval is noted on the parental permission forms.
Overview of the Data Collection System
The NYTS will be conducted with a nationally representative sample of 6-12 grade students in public and private schools, during January through May of 2018, 2019, and 2020. Gaining access to and support for the survey involves a tiered approach. We begin by contacting State Education Agencies and State Departments of Health to notify them of the survey and request general guidance on working with the selected school districts and schools. We then contact selected districts to invite them to participate and obtain local approval to conduct the survey in selected schools. Once cleared at the school district level, selected schools will be invited to participate. After a school agrees to participate, a tailor-made plan for collection of data in the school will be developed (e.g., select classes, determine whether the survey will be administered to selected class sections simultaneously or in serial).
The NYTS takes about 35 minutes to complete. No individually identifiable information is collected on the survey (e.g., student name, class, school, etc.).
On the day of the survey, the data collector will bring all materials needed to conduct the survey. The data collector will work with the respective classroom teacher to determine which students have completed the necessary parental permission form process (using the Data Collection Checklist), and consequently are eligible to take the survey.
After the survey administered via a paper-and-pencil questionnaire is completed, students will be instructed to place their completed survey in an envelope and seal it. The sealed individual student envelopes will then be deposited into a classroom-specific envelope. As the NYTS administration is completed in each selected class, the classroom-specific envelope will be deposited in a school-specific envelope labeled with a school identification number (for weighting purposes only). Sealed school packets will be transmitted by the NYTS trained data collector to the data collection contractor’s survey processing center. For the survey administered via a digitally based self-administered questionnaire, at the conclusion of the survey administration session, students will be instructed to hand their tablet to the data collector. The students’ data will immediately be uploaded to the cloud database and erased from the tablet. As the NYTS electronic administration is completed in each selected class, the classroom-specific tablet will be stored in a school-specific box labeled with a school identification number (for weighting purposes only). Sealed school boxes will be transmitted by the NYTS electronic trained data collector to the data collection contractor’s survey processing center.
This information collection does not involve web-based data collection methods or refer respondents to websites.
Information to be Collected
The 2018 NYTS will be a self-administered, paper-and-pencil questionnaire consisting of 88 questions on a variety of tobacco related topics (Attachment I1). However, we anticipate that beginning in 2019, data will be collected electronically via a digitally based self-administered questionnaire. For the 2018 NYTS, it is expected that approximately 24,000 students attending at least 200 schools will participate. The questions include prevalence of tobacco product use, knowledge and attitudes, media and advertising, exposure to secondhand smoke, minors’ access and enforcement, school curriculum, and cessation.
Students who have obtained parental permission to participate, and are in classrooms selected to participate, will be asked to report about their tobacco use behaviors and behavioral determinants on the digitally based self-administered or paper-and-pencil questionnaire.
How Information will be Shared and for What Purpose
All selected schools, students, and their parents will be informed that anonymity will be maintained throughout data collection, that all data will be safeguarded closely, and that no institutional or individual identifiers will be used in study reports. Anonymity will be promised to students and their parents on parental permission forms. Students will be reminded that their responses are anonymous at the start of the survey administration session by a professionally trained NYTS data collector.
All contractor staff involved with the project are required to sign a non-disclosure, intellectual property, non-competition and non-solicitation agreement which is a statement of personal commitment to safeguard data obtained.
Impact of Proposed Collection on Respondent’s Privacy
Data collected from school administrators during recruitment is information that is already available in the public domain; school administrators will not provide personal information. The data collected on the NYTS are not identifiable.
As a means to monitor the parental permission form process and to ensure questionnaires are completed only by students for whom permission has been obtained, teachers are asked to enter student names on the Data Collection Checklist (similar to a class roll) (Appendix H1). Teachers can substitute any other information in place of student names (such as student ID numbers or letters) on the Data Collection Checklist as long as it will allow them to individually determine which students received parental permission to participate. This information will be conveyed to the data collector on the survey administration day.
The Data Collection Checklist is an optional tool to assist in managing the parental permission and student assent process. It will be destroyed at the end of the study. No individually identifiable information is collected on the NYTS survey (e.g., student name, class, school, etc.), therefore there is no way to connect students’ names to their response data.
Participation in the NYTS should pose little or no effect on the respondent’s privacy.
No individually identifiable information is collected on the NYTS survey (e.g., student name, class, school, etc.), therefore there is no way to connect students’ names to their response data.
Voluntary or Mandatory Nature of Participation
For the NYTS, participation is voluntary and respondents will be assured that there is no penalty if they decide not to respond, either to the information collection as a whole or to any particular question.
Opportunity to Consent to Sharing and Submission of Information
Although teachers are asked to record student names or another identifier on the Data Collection Checklist (Attachment H1), this information is only used to manage the parental permission and student assent process. Consent to record and provide this information to the CDC or data collection contractor will not be sought. The Data Collection Checklist will be destroyed at the end of the study.
At each school, local procedures for sending home parental permission forms will be followed. Schools will be asked to ensure permission forms are distributed at least 7 days before the survey administration. Teachers track the return of parental permission forms on the Data Collection Checklist to ensure that only students with parental permission participate. A waiver of written student assent was obtained for the participation of children because this research presents no more than minimal risk to subjects, parental permission is required for participation, the waiver will not adversely affect the rights and welfare of the students because they are free to decline to take part, and it is thought that some students may perceive they are not anonymous if they are required to provide stated assent and sign a consent/assent document. Students are told “Participating in this survey is voluntary and your grade in this class will not be affected, whether or not you answer the questions.” Completion of the survey implies student assent.
Information Security
CDC’s authorized data collection contractor has several security procedures in place to safeguard data. Data that are collected at school remain under the exclusive control of the contractor’s field staff until they are shipped to the contractor’s survey processing center. School personnel are not responsible for collecting and storing any data. The paper data will be stored in a locked file room (within a secured facility), accessible only to staff directly involved in the project, retained for three years after completion of the data collection, and then destroyed. For the future electronic mode of survey, at the conclusion of the survey administration session, students will be instructed to hand their tablet to the data collector. The students’ data will immediately be uploaded to the cloud database and erased from the tablet. Also, all electronic data will be stored on secured servers and will be accessible only to staff directly involved in the project.
Privacy Act Determination
Staff in the CDC Information Collection Review Office have reviewed this application and have determined that the Privacy Act does not apply. No identifying information will be retained in the data record that would enable an individual survey to be tracked back to a particular student.
A.11 JUSTIFICATION FOR SENSITIVE QUESTIONS
Seventy-four of the 88 questions on the NYTS are specific to tobacco-related issues (Attachment I1). Those pertaining to actual tobacco use, especially when asked of underage children, may be considered sensitive by some parents, students, or the school community. However, because getting accurate information on this topic is critical, the NYTS questionnaire must contain these sensitive questions. During the past 25 years, one of the primary responsibilities of CDC has been to monitor priority risk behaviors among youth. To monitor such behaviors, CDC must ask youth about them. Students are told in the instructions to the NYTS (Attachment I7) that “In order to help develop better education programs, educators and health officials must collect comprehensive data on the attitudes, knowledge, and behaviors of middle and high school students (grades 6‑12) with respect to tobacco, and on other influences that might make a youth susceptible to tobacco use in the future.” Students also are instructed to read the front cover of the questionnaire booklet which states, “This survey is about tobacco. We would like to know about you and the things you do that may affect your health. Your answers will be used for programs for young people like yourself.”
The remaining seven questions are demographic factors, two of which ask about race and ethnicity, and two of which are mandatory questions from Department of Health and Human Services Office on Minority Health. OMB considers questions about race and ethnicity to be sensitive. On October 30, 1997, the Office of Management and Budget (OMB) published "Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity" (Federal Register, 62 FR 58781 - 58790). The 1997 standards reflect a change in data collection policy, making it possible for Federal agencies to collect information that reflects the increasing diversity of the U.S. population stemming from growth in interracial marriages and immigration. Under this policy, federal agencies are required to offer respondents the option of selecting one or more race responses from a list of five designated racial categories. Additionally, the standards provide for the collection of data on whether or not a person is of "Hispanic or Latino" culture or origin. Such standards also foster comparability across data collections carried out by various agencies. The race and ethnicity questions in the NYTS follow all guidelines for the development of data collection questions, formats, and associated procedures to implement the 1997 standards.
The questions were developed in close cooperation with representatives from school systems across the nation and are presented in a straightforward and sensitive manner. Parental permission to participate in the NYTS will be obtained.
A.12 ESTIMATES OF ANNUALIZED BURDEN HOURS AND COSTS
Federal tobacco control and surveillance activities must adapt to a dynamic product environment. From time to time, CDC may modify instrument content to reflect changes in the federal government’s need for information to inform public health and regulatory activities. These modifications will be submitted to OMB through the Change Request mechanism.
Before requesting OMB approval of changes to the NYTS questionnaire, CDC may also conduct (i) cognitive testing of new questions or proposed changes in the wording of, or response options associated with individual questions, and/or (ii) pre-testing of the NYTS instrument as a whole, to ensure that burden per response remains compatible with administration in one class period (OMB No. 0920-0621). Detailed descriptions of these information collections will also be submitted to OMB under the Change Request mechanism.
Estimated Burden Hours
The estimated burden for this information collection is based on over 10 years of experience conducting the NYTS. The planned information collection involves administration of the NYTS questionnaire (Attachment I1) to independent samples of students in the spring of 2018. Respondents include state-level, district-level, and school-level administrators who provide information in the Recruitment Scripts for the NYTS (Attachments E1, F1, and G1), and teachers who complete the Data Collection Checklist for the NYTS (Attachment H1 & H2).
The NYTS will be conducted each year among a nationally representative sample of students attending public and private schools in grades 6 through 12. At state, school district, and school levels, the cooperation of educational administrators will be sought in recruitment of sampled schools. Burden estimates are based on expected sample sizes and budget under the current contract for the 2015-2017 NYTS cycle. These figures may be adjusted slightly when a new contract is put in place for the 2018-2020 cycles.
For the 2018 cycle of data collection, the total estimated number of respondents, by type, will include: state-level administrators (n=38), district-level administrators (n=153), and school-level administrators (n=240) who provide information in the Recruitment Script for the NYTS; teachers (n=973) who complete the Data Collection Checklist for the NYTS; and students (n=24,000) who receive instructions for and complete the NYTS questionnaire. There are no costs to respondents except their time.
The burden table also includes a new allocation of 101 annualized burden hours for instrument testing activities among students. Due to changes in the relevant product environment, patterns of use of tobacco and relevant products, or other factors, testing may be needed to assess new questions, changes in the wording of existing questions, or the response options associated with individual questions. The estimated burden hours per year was developed as follows. Cognitive testing of questionnaire content will typically be conducted in semi-structured interviews (table A.12.a: “cognitive testing”; 50 youth interviews per year @ 45 minutes per interview = 38 burden hours). These interviews will test new and existing NYTS questions for face and content validity. Briefly, in its approval of 0920-0621 on 1/27/15, OMB stated to The Centers for Disease Control and Prevention that “This clearance is based on the expectation that the survey instrument will be revised to maintain relevance with emerging tobacco use behaviors and control policies. Developmental testing of new survey content and incremental improvements to existing wording on the approved instrument (including dropping/adding of a small proportion of the questions), in a manner such that the overall content remains consistent with topics already covered, may be done through non-substantive changes.” Cognitive testing will investigate how well participants understand existing and proposed NYTS survey questions and response options related to tobacco use, such as for electronic vapor products and other emerging tobacco products. One-on-one cognitive interviews will last approximately 45 minutes. Interviewers will use a semi-structured interview guide (Attachment P1), and with the respondents’ assent (Attachment P2) or consent (Attachment P3), the interview will be audio recorded. Parental permission for participation in cognitive testing will be obtained for youth less than 18 years of age (Attachment P4). Youth who participate will be provided a $40 incentive to recognize their time burden and to cover travel costs. Educational materials will be provided to participants at the close of the cognitive testing interview (Attachment P5 and Attachment P6)
In addition, CDC may conduct pre-tests to ensure that each year’s NYTS questionnaire can be completed within one class period (table A.12.a: “survey pre-tests”; 30 tests per year @ 45 minutes/test = 23 hours). Finally, hours are allocated for screening of up to 300 youth prior to participation in cognitive testing and/or survey pre-testing activities (300 youth @ 8 minutes/response = 40 hours; Attachment P7). Respondent screening may be needed to ensure that testing is conducted with individuals whose characteristics are similar to the NYTS target population of youth in grades 6-12. The configuration of testing activities may vary from year to year. For example, some years we may require additional hours for cognitive testing versus survey pre-testing. However, for purposes of burden estimation, the total estimated number of respondents involved in testing is 300 and the adjusted average burden per response is 20 minutes/response. Each testing activity will be submitted to OMB as a Change Request.
The total burden estimated for the NYTS and associated support activities is 18,560 hours. These totals for this cycle are provided in Table A.12.a.
Estimated Cost to Respondents
There are no direct costs to the respondents themselves or to participating schools. However, the cost for administrators, teachers, and students can be calculated in terms of their time. In each category, the estimated respondent burden hours have been multiplied by an estimated average hourly salary for persons in that category. The U.S. Bureau of Labor Statistics is the source for hourly wages (http://www.bls.gov/oes/current/oes_nat.htm) (U.S. Bureau of Labor Statistics, 2016). The estimated burden cost in terms of the value of time students spend in responding are based on a minimum wage for students aged less than 20 years of $4.25/hour. The total estimated respondent burden cost for conducting the 2018 NYTS is $95,979 and is reported in Table A.12.b.
Table A.12.a – Estimated Annualized
Burden Hours
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden Per Response (In Hours) |
Total Burden (In Hours) |
State Administrators |
State-level Recruitment Script for the National Youth Tobacco Survey |
38 |
1 |
30/60 |
19 |
District Administrators |
District-level Recruitment Script for the National Youth Tobacco Survey |
153 |
1 |
30/60 |
77 |
School Administrators |
School-level Recruitment Script for the National Youth Tobacco Survey |
240 |
1 |
30/60 |
120 |
Teachers |
Data Collection Checklist for the National Youth Tobacco Survey |
973 |
1 |
15/60 |
243 |
Students |
National Youth Tobacco Survey |
24,000 |
1 |
45/60 |
18,000 |
Cognitive Testing |
50 |
1 |
45/60 |
38 |
|
Survey Pre-tests |
30 |
1 |
45/60 |
23 |
|
Testing Activities (screening) |
300 |
1 |
8/60 |
40 |
|
|
Total |
18,560 |
|||
Table A.12.b – Annualized Estimated Cost to Respondents
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden Per Response (In Hours) |
Hourly Wage Rate |
Total Respondent Costs |
State Administrators |
State-level Recruitment Script for the National Youth Tobacco Survey |
38 |
1 |
30/60 |
$51.46 |
$978 |
District Administrators |
District-level Recruitment Script for the National Youth Tobacco Survey |
153 |
1 |
30/60 |
$63.22 |
$4,836 |
School Administrators |
School-level Recruitment Script for the National Youth Tobacco Survey |
240 |
1 |
30/60 |
$52.50 |
$6,300 |
Teachers |
Data Collection Checklist for the National Youth Tobacco Survey |
973 |
1 |
15/60 |
$28.51 |
|
Students |
National Youth Tobacco Survey |
24,000 |
1 |
45/60 |
$4.25 |
$76,500 |
Cognitive Testing |
50 |
1 |
45/60 |
$4.25 |
$162 |
|
Survey Pre-tests |
30 |
1 |
45/60 |
$4.25 |
$98 |
|
Testing Activities (screening) |
300 |
1 |
8/60 |
$4.25 |
$170 |
|
|
Total |
$95,979 |
||||
A.13 ESTIMATES OF OTHER TOTAL ANNUAL COST BURDEN TO RESPONDENTS OR RECORD KEEPERS
There will be no respondent capital and maintenance costs.
A.14 ANNUALIZED COSTS TO THE GOVERNMENT
The study is funded under Contract No. XXXX-XXXX-XXX. The total contract award to XXX to conduct the 2018, 2019, and 2020 NYTS is $4,510,913. The estimated cost of the contract, annualized over the three years of this clearance request, is $2,799,930. These costs cover the activities in Table A.14 below. Some activities will be conducted during the pre-clearance period and others will occur post-clearance.
Additional costs will be incurred indirectly by the government in personnel costs of staff involved in oversight of the study and in conducting data analysis. It is estimated that two CDC employees will be involved for approximately 20% and 35% of their time (for federal personnel 100% time = 2,080 hours annually) at salaries of $58.09 and $46.43 per hour, respectively. The direct annual costs in CDC staff time will be approximately $24,165 + $33,801 = $57,966 annually. The total estimated annualized cost for the study, including the contract cost and federal government personnel cost, is $2,857,896.
Table
A.14 – Estimated Annualized Study Cost
Activity |
Cost |
Contract Costs |
|
Design and plan |
$213,097 |
Programming and developing |
$346,643 |
Recruitment and preparation |
$282,391 |
Printing and distribution |
$52,794 |
Recruiting and training |
$284,989 |
Collection of data |
$760,020 |
Processing, cleaning, weighing and developing data files |
$541,902 |
Dissemination and reporting of results |
$318,094 |
Subtotal |
|
Federal Employee Time Cost |
|
20% time for one FTE |
$24,165 |
35% time for one FTE |
$33,801 |
Subtotal |
$57,966 |
Total Estimated Annualized Cost to the Federal Government |
$2,857,896 |
*Components may not sum to this figure due to rounding.
A.15 EXPLANATION FOR PROGRAM CHANGES OR ADJUSTMENTS
Since the 2017 NYTS instrument well tracked patterns of use for both traditional tobacco products and emerging products, the 2018 NYTS instrument will remain the same as the one in 2017. So, there are no changes to the estimated burden per response or frequency of data collection for the survey instrument, the recruitment scripts, or the checklist used by teachers. For the 2018-2020 approval period, we are including an allocation of 101burden hours per year to allow for instrument testing activities.
All questions in the 1999-2017 NYTS surveys were tested for the paper-and-pencil questionnaire. Some question items were cognitively tested specifically for the NYTS while some question items were cognitively tested previously with other national surveys.
For the 2018 NYTS, three new questions specific to e-cigarettes were cognitively tested among 15 youths in September 2017. These questions included:
During the past 30 days, where did you get or buy the e-cigarettes that you have used? (Select one or more)
I have never tried an e-cigarette in the past 30 days
A gas station or convenience store
A grocery store
A drugstore
A mall or shopping center kiosk/stand
On the Internet
A vape shop or other store that only sells e-cigarettes
Some other place not listed here
From a family member
From a friend
From some other person that is not a family member or a friend
What are the reasons you have used e-cigarettes? (Select one or more)
I have never tried an e-cigarette
Friend or family member used them
To try to quit using other tobacco products, such as cigarettes
They cost less than other tobacco products, such as cigarettes
They are easier to get than other tobacco products, such as cigarettes
Famous people on TV or in movies use them
They are less harmful than other forms of tobacco, such as cigarettes
They are available in flavors, such as mint, candy, fruit, or chocolate
They can be used in areas where other tobacco products, such as cigarettes, are not allowed
I used them for some other reason
Have you ever used marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils in an e-cigarette?
I have never used an electronic product
Yes
No
Details on the methods and results of cognitive testing of these questions is provided as an attachment to these responses (Attachment N).
Beginning with the 2019 NYTS cycle, burden hours will go toward the development and cognitive testing of new questions. These questions will focus on emerging tobacco products or behaviors, and the awareness and evaluation of new or evolving tobacco-related policies and regulatory activities. CDC also will determine whether the burden of the questionnaire can be reduced by dropping questions that do not provide as valuable of information as the new questions.
We have increased the sample size in an effort to increase precision of estimates and allow for more robust subgroup analyses. This therefore increases the overall burden estimate slightly relative to previous cycles. The 2015-2017 NYTS approval was based on 21,605 annualized responses and 15,582 annualized burden hours. Current estimates for the 2018-2020 cycles of survey administration are based on estimates of 25,784 annualized responses and 18,560 annualized burden hours for 2018.
Table A.15 – Annualized Estimates for the 2018 NYTS, with changes since previous OMB approval
Type of Respondent |
Form Name |
No. of Respondents |
Change |
No. of Responses per Respondent |
Average Burden Per Response (In Hours) |
Total Burden (In Hours) |
Change |
State Administrators |
State-level Recruitment Script for the National Youth Tobacco Survey |
38 |
+3 |
1 |
30/60 |
19 |
+1 |
District Administrators |
District-level Recruitment Script for the National Youth Tobacco Survey |
153 |
+3 |
1 |
30/60 |
77 |
+2 |
School Administrators |
School-level Recruitment Script for the National Youth Tobacco Survey |
240 |
+20 |
1 |
30/60 |
120 |
+10 |
Teachers |
Data Collection Checklist for the National Youth Tobacco Survey |
973 |
0 |
1 |
15/60 |
243 |
0 |
Students |
National Youth Tobacco Survey |
24,000 |
+3,923 |
1 |
45/60 |
18,000 |
+2,942 |
Cognitive Testing |
50 |
+50 |
1 |
45/60 |
38 |
+38 |
|
Survey Pre-tests |
30 |
+30 |
1 |
45/60 |
23 |
+23 |
|
Testing Activities (screening) |
300 |
+150 |
1 |
8/60 |
40 |
-38 |
|
|
Total |
25,784 |
+4,179 |
|
18,560 |
+2,978 |
|
A.16 PLANS FOR TABULATION AND PUBLICATION AND PROJECT TIME SCHEDULE
A.16.a Tabulation Plans
Data will be tabulated in ways that will address the principal research purposes outlined in A.2. The planned analyses to be conducted are described briefly below:
Estimate the prevalence of tobacco use behaviors and behavioral determinants among middle and high school students overall and by sex, grade in school, and race/ethnicity--Descriptive statistics (percentages and confidence intervals) will be calculated to address this objective.
Assess whether tobacco use behaviors and behavioral determinants vary by sex, grade in school, and race/ethnicity--Cross tabulations, Chi-squared analyses, and regression analysis initially will be conducted to address this objective.
Determine the associations between tobacco use behaviors and behavioral determinants –Chi-squared and logistic regression analyses will be used.
Describe trends in tobacco use behaviors and behavioral determinants among middle and high school students overall and by sex, grade in school, and race/ethnicity--Multiple regression analyses that controls for sex, grade in school, and race/ethnicity and that simultaneously assesses linear and higher order time effects will be used.
Examine the effects of schools and local areas (school districts or PSUs) in estimating the prevalence of tobacco use-- multilevel models will be used.
Examples of the table shells that will be completed through analysis of the data are in Attachment K.
Publication Plans
CDC’s publication of data from prior cycles of NYTS was largely limited to the MMWR. Yearly MMWRs are typically published reporting an overview of all tobacco product use in middle and high School students. A MMWR article was published in 2016 describing characteristics of e-cigarette use among middle and high school students (CDC, 2016a). Trend analyses on the use of tobacco by middle and high schools students from 2011-2016 was cited in a recent MMWR published in June of 2017 (CDC, 2017). CDC will continue to publish NYTS results initially through the MMWR, which will be distributed to other Federal agencies, state and local health and education agencies, national health and education organizations, universities, and the general public. Additionally, NYTS results and a public use data set are available on the CDC web site at: http://www.cdc.gov/tobacco/data_statistics/surveys/NYTS/index.htm.
CDC and FDA also have released NYTS results through a variety of government publications, websites, peer-reviewed scientific journals, and annual conferences of national organizations focused on tobacco use, prevention and control, preventive medicine, public health, adolescent health, and epidemiology. A supplement was published in the American Journal of Preventive Medicine in 2014, with eight research articles co-authored by CDC and FDA describing new findings from the 2012 NYTS. An article was published in JAMA Pediatrics (Dutra & Glantz, 2014) to examine e-cigarette use and conventional cigarette smoking. In addition, data from the NYTS from 2000 through 2012 were used to assess patterns and trends of current tobacco use (cigarettes, cigars, and other tobacco products) among U.S. high school students (Arrazola et al., 2013). CDC hosted a podcast summarizing data on the popularity of emerging tobacco products, including e-cigarettes, among middle and high school students (Arrazola, R.A., 2013).
Time Schedule for the Project
The following represents our proposed schedule of activities for the NYTS, in terms of months after receipt of OMB clearance. The end date for data collection is constrained by the dates on which schools close for the summer. In addition, given that some twelfth grade students may be absent during the final weeks of the school year, it is highly desirable to complete data collection one months before schools close for the summer.
Key project dates will occur during the following time periods for the 2018 data collection:
Activity |
Time Period |
Recruit and schedule schools |
1 to 3 months after OMB clearance |
Print scannable questionnaires |
1 to 2 months after OMB clearance |
Train field data collectors |
2 months after OMB clearance |
Collect data |
2 to 5 months after OMB clearance |
Process data |
3 to 6 months after OMB clearance |
Weight/clean data |
7 to 8 months after OMB clearance |
Produce data file with documentation |
9 months after OMB clearance |
Analyze data |
10 to 11 months after OMB clearance |
Publish results |
15 to 17 months after OMB clearance |
Data collection is currently scheduled to occur during February through June, 2018. The time schedule for the 2019 and 2020 data collection will be analogous to that of the 2018 data collection. Results will be published in early 2019 initially in the MMWR, and subsequently in other publications.
A.17 REASON(S) DISPLAY OF OMB EXPIRATION DATE IS INAPPROPRIATE
The expiration date of OMB approval of the data collection will be displayed.
A.18 EXCEPTIONS TO CERTIFICATION FOR PAPERWORK REDUCTION ACT SUBMISSIONS
There are no exceptions to the certification.
A.19 COMMENTS IN RESPONSE TO THE FEDERAL REGISTER NOTICE (FRN) AND EFFORTS TO CONSULT OUTSIDE OF THE AGENCY
We responded to four public comments received through 60-day FRN. Below is a concise summary of the public comments that included questionnaire recommendations for the NYTS. CDC’s response to all received comments is provided in Attachment B2.
Public comment #1: Currently, the NYTS asks about the use of cigars, cigarillos, or little cigars together as one category. Truth Initiative recommends that the questions about cigar use be separated into the following categories: large cigars and cigarillos/little cigars.
CDC’s response: CDC and FDA will review and determine whether or how to incorporate the suggestion in the next revision of the survey, which will be in 2019.
Public comment #2: Currently, the NYTS states the following: “The next 11 questions are about electronic cigarettes or e-cigarettes. E-cigarettes are battery powered devices that usually contain a nicotine-based liquid that is vaporized and inhaled. You may also know them as e-cigs, vape-pens, hookah-pens, e-hookahs, e-cigars, e-pipes, personal vaporizers, or mods. Some brand examples include NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo, and Halo.” Truth Initiative recommends that JUUL be added as a brand example.
CDC’s response: CDC and FDA will review and determine whether or how to incorporate the suggestion in the next revision of the survey, which will be in 2019.
REFERENCES
American Cancer Society (2013a). Cancer Prevention & Early Detection: Facts and Figures 2013. Atlanta, GA: American Cancer Society, 2013.
American Cancer Society (2013b). Child and Teen Tobacco Use. Atlanta, GA: American Cancer Society.
American Cancer Society (2013c). Smokeless Tobacco: Who Uses Smokeless Tobacco? Retrieved from http://www.cancer.org/cancer/cancercauses/tobaccocancer/smokeless-tobacco
American Legacy Foundation (2000a). Cigarette Smoking Among Youth: Results from the 1999 National Youth Tobacco Survey. First Look Report 1. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2000b). Pathways to Established Smoking: Results from the 1999 National Youth Tobacco Survey. First Look Report 3. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2000c). The Relationship Between Cigarette Use and Other Tobacco Products: Results from the National Youth Tobacco Survey. First Look Report 4. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2000d). What Youth Think About Smoking: Results from the 1999 National Youth Tobacco Survey. First Look Report 2. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2000e). Youth Access to Cigarettes: Results from the 1999 National Youth Tobacco Survey. First Look Report 5. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2001a). Cigarette Smoking Among Youth: Results from the 2000 National Youth Tobacco Survey. First Look Report 7. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2001b). Youth Exposure to Environmental Tobacco Smoke. First Look Report 6. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2002). Using Multiple Strategies in Tobacco Use Prevention Education. First Look Report 8. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2003a). The Relationship between Cigarette Use and Other Tobacco Products: Results from the 2000 National Youth Tobacco Survey. First Look Report 10. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2003b). Youth Tobacco Cessation: Results from the 2000 National Youth Tobacco Survey. First Look Report 11. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2004). Cigarette Smoking among Youth: Results from the 2002 National Youth Tobacco Survey. First Look Report 13. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2005). Beyond Cigarettes: The Use Of Other Tobacco Products. First Look Report 15. Washington, DC: American Legacy Foundation.
American Legacy Foundation (2012). Tobacco Fact Sheet: Cigars, Cigarillos, and Little Cigars. Retrieved from http://www.legacyforhealth.org/content/download/642/7502/version/2/file/Fact_Sheet-Cigars_Cigarillos_LittleCigars.pdf
American Medical Association (2015). AMA Strengthens Policy on Electronic Cigarettes to Protect Youth. Retrieved from https://www.ama-assn.org/content/ama-strengthens-policy-electronic-cigarettes-further-protect-youth on June 8, 2017.
Appleyard, J., Messeri, P., & Haviland, D. (2001). Smoking among Asian American and Hawaiian/Pacific Islander youth: new data from the 2000 National Youth Tobacco Survey. Asian American Pacific Islander Journal of Health, 9(1), 5-14.
Apelberg,B., Backinger, C., Curry, S. (2014). Enhancing Youth Tobacco Surveillance to Inform Tobacco Product Regulation American Journal of Preventive Medicine, Volume 47, Issue 2 , S1 - S3.
Arrazola, R.A. (2013, November 21). Young Smokers. Centers for Disease Control and Prevention. Podcast retrieved from http://www2c.cdc.gov/podcasts/player.asp?f=8630364
Arrazola, R.A., Kuiper, N.M., & Dube, S.R. (2013). Patterns of Current Use of Tobacco Products Among U.S. High School Students for 2000-2012-Findings From the National Youth Tobacco Survey. Journal of Adolescent Health, 54(1), 54-60. http://dx.doi.org/10.1016/j.jadohealth.2013.08.003.
Asian Pacific Partners for Empowerment and Leadership (2000). Critical Policy Issues on Tobacco Prevention and Control for the Asian American and Pacific Islander Community. The Robert Wood Johnson Foundation.
Asian Pacific Partners for Empowerment and Leadership (2002). Making Tobacco Relevant for Asian American and Pacific Islander Communities. The Robert Wood Johnson Foundation, Centers for Disease Control and Prevention, Office on Smoking and Health, Campaign for Tobacco-Free Kids.
Aslam, N. & Bushra, R. (2010). Active Smoking in Adolescents of Karachi, Pakistan. Oman Medical Journal, 25(2), 142.Brook, J.S., Balka, E.B., Ning. Y, Brook, D.W (2007). Trajectories of cigarette smoking among African Americans and Puerto Ricans from adolescence to young adulthood: associations with dependence on alcohol and illegal drugs. Am J Addict. 2007 May-Jun;16(3):195-201. PubMed PMID: 17612823.
CDC (2001). Youth Tobacco Surveillance–United States, 2000. MMWR; 50(SS-4).
CDC (2005). Tobacco Use, Access, and Exposure to Tobacco in Media Among Middle and High Schools Students – United States, 2004. MMWR; 54(12):297-301.
CDC (2009). Cigarette Brand Preference Among Middle and High School Students Who Are Established Smokers- United States, 2004 and 2006. MMWR; 58(05): 112-115.
CDC (2010). Tobacco Use Among Middle and High School Students—United States, 2000-2009. MMWR; 59(33):1063-1068.
CDC (2012a). Current Tobacco Use Among Middle and High School Students – United States, 2011. MMWR; 61(31): 581-585.
CDC (2012b). National Youth Tobacco Survey. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. Available at http://www.cdc.gov/tobacco/data_statistics/surveys/nyts.
CDC (2013a). Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS. Tobacco Use Among Middle and High School Students — United States, 2011–2014. MMWR Morb Mortal Wkly Rep 2015 / 64(14);381-385.
CDC (2013c). Winnable Battles Progress Report- 2010-2015. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention.
CDC (2014a). Best Practices for Comprehensive Tobacco Control Programs – 2014. Atlanta, GA: U.S. Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
CDC (2014b). State Laws Prohibiting Sales to Minors and Indoor Use of Electronic Nicotine Delivery Systems — United States, November 2014. MMWR Morb Mortal Wkly Rep 2014;63(49);1145-1150. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6349a1.htm
CDC (2014c). Budget Request Summary- Fiscal Year 2015. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention.
CDC (2014d). Winnable Battles. Retrieved from http://www.cdc.gov/winnablebattles/
CDC (2014e). Annual Performance Report and Performance Plan- Fiscal Year 2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention.
CDC (2015). Arrazola RA, Singh T, Corey CG, et al. Tobacco Use Among Middle and High School Students — United States, 2011–2014. MMWR Morb Mortal Wkly Rep, 2015: 64(14);381-385.
CDC (2016a). Singh T, Kennedy S, Marynak K, Persoskie A, Melstrom P, King BA.
Characteristics of Electronic Cigarette Use Among Middle and High School Students - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016 Dec 30;65(5051):1425-1429. doi: 10.15585/mmwr.mm655051a2. PubMed PMID: 28033310.
CDC (2017). Jamal A, Gentzke A, Hu SS, et al. Tobacco Use Among Middle and High School Students — United States, 2011–2016. MMWR Morb Mortal Wkly Rep 2017;66:597–603. DOI: http://dx.doi.org/10.15585/mmwr.mm6623a1.
Dutra, L.M. & Glantz, S.A. (2014). Electronic Cigarettes and Conventional Cigarette Use Among US Adolescents: A Cross-sectional Study. JAMA Pediatrics, published online March 06, 2014. doi:10.1001/jamapediatrics.2013.5488.
FDA (2014). FDA Proposes to Extend Its Tobacco Authority to Additional Tobacco Products, including e-cigarettes. FDA NEWS RELEASE. N.p., 24 Apr. 2014. Web. 9 May 2014. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394667.htm
Institute of Medicine (2011). Leading Health Indicators for Healthy People 2020: Letter Report. Washington, DC: The National Academies Press.
IOM (Institute of Medicine). 2015. Public health implications of raising the minimum age of legal access to tobacco products. Washington, DC: The National Academies Press.
Lee S., Grana R.A., & Glantz, S.A. (2013). Electronic Cigarette Use Among Korean Adolescents: A Cross-Sectional Study of Market Penetration, Dual Use, and Relationship to Quit Attempts and Former Smoking. Published online November 25, 2013. Journal of Adolescent Health. doi:10.1016/j.jadohealth.2013.11.003.
Martin-Pujol, A., Fernandez, E., Schiaffino, A., Moncada, A., Ariza, C., Blanch, C., Martinez-Sanchez J.M., & the RESPIR-NET research group. (2013). Tobacco smoking, exposure to second-hand smoke, and asthma and wheezing in schoolchildren: a cross-sectional study. Acta Pediatric, 102(7), 305-309.
National Institute on Drug Abuse (2003). Youths’ Opportunities to Experiment Influence Later Use of Illegal Drugs. National Institute on Drug Abuse, 17(5).
National Institute on Drug Abuse (2014). Monitoring the Future national results on drug use: 1975-2013: Overview, Key Findings on Adolescent Drug Use. National Institute on Drug Abuse, National Institutes of Health. Ann Arbor, MI: Institute for Social Research, The University of Michigan.
Neergaard, J., Singh, P., Job, J., & Montgomery, S. (2007). Waterpipe smoking and nicotine exposure: A review of the current evidence. Nicotine and Tobacco Research, 9(10), 987–994.
Richardson, A., He, J.P., Curry, L., & Merikangas, K. (2012). Cigarette smoking and mood disorders in U.S. adolescents: Sex-specific associations with symptoms, diagnoses, impairment and health services use. Journal of Psychosomatic Research, 72(4), 269-275.
Shelley, D., Cantrell, J., Faulkner, D., Haviland, L., Healton, C., & Messeri, P. (2005). Physician and Dentist Tobacco use Counseling and Adolescent Smoking Behavior: Results from the 2000 National Youth Tobacco Survey. Pediatrics, 115(3), 719-725. American Legacy Foundation.
Smith, J.R., Novotny, T.E., Edland, S.D., Hofstetter, C.R., Lindsay, S.P., & Al-Delaimy, W.K. (2011). Determinants of Hookah Use among High School Students. Nicotine and Tobacco Research, (13)7, 565-572.
Starr, G., Rogers, T., Schooley, M., Porter, S., Wiesen, E., & Jamison, N. (2005). Key outcome indicators for evaluating comprehensive tobacco control programs. Atlanta, GA: Centers for Diesease Control and Prevention.
The University of California (2012). Tobacco Use among Asian American, Native Hawaiian and Pacific Islander Communities in California. The California Cancer Research Fund.
U.S. Bureau of Labor Statistics (2014). May 2013 National Occupational Employment and Wage Estimates, United States. Retrieved from http://www.bls.gov/oes/current/oes_nat.htm
Upadhyaya, H.P., Deas, D.D., Brady, K.T., & Kruesi, M. (2002). Cigarette smoking and psychiatric comorbidity in children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 41(11), 1294-1305.
USDHHS (2010a). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Service, Public Health Service, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
USDHHS (2010b). Healthy People 2020. Washington, D.C.: U.S. Department of Health and Human Services. Available at: http://healthypeople.gov/2020/default.aspx
USDHHS (2012a). Ending the Tobacco Epidemic: Progress toward a Healthier Nation. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health.
USDHHS (2012b). Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
USDHHS (2014). The Health Consequences of Smoking- 50 years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
USDHHS (2016). E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
USDHHS, HRSA (2004). Promising Practices in MCH Needs Assessment: A Guide Based on a National Study. U.S. Department of Health and Human Services, Health Resources and Services Administration.
USDHHS, NIH, & NCI (2007). NCI’s President’s Cancer Panel 2006-2007 Annual Report: Promoting Healthy Lifestyles: Policy, Program, and Personal Recommendations for Reducing Cancer Risk. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
USDHHS, NIH, & NIDA (2007). Director’s Report to the National Advisory Council on Drug Abuse. U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse.
Villanti AC, Pearson JL, Glasser AM, Johnson AL, Collins LK, Niaura RS, Abrams DB. Frequency of youth e-cigarette and tobacco use patterns in the U.S.: Measurement precision is critical to inform public health. Nicotine Tob Res. 2016 Dec 24.
Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF (2015). Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015
Mar;48(3):326-33.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | OMB |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy