CMS-10639 Supporting Statement A NHSN CY 2019 (10-25-18)
CMS-10639 Supporting Statement A NHSN CY 2019 (10-25-18).docx
National Healthcare Safety Network (NHSN) Data Validation Study for the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) (CMS-10639)
OMB: 0938-1340
Supporting Statement – Part A
National Healthcare Safety Network (NHSN) Data Validation Study for the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP) (CMS-10639)
Background
Pursuant to section 1881(h) of the Social Security Act (the Act) as amended by section 153(h) of the Medicare Improvements for Patients and Providers Act (MIPPA), the Centers for Medicare and Medicaid Services (CMS) established the End-Stage Renal Disease Quality Incentive Program (ESRD QIP) starting in 2011. The ESRD QIP is the first value-based purchasing program established by CMS, and it is aimed at promoting patient health by providing a financial incentive for renal dialysis facilities to deliver high-quality care.
In implementing the End-Stage Renal Disease Quality Incentive Program, CMS believes that a successful quality incentive program will promote the delivery of high quality health care services in the renal dialysis facility setting. Under section 1881(h)(2) of the Act, the Secretary is required to specify quality measures for evaluating the quality of care ESRD patients receive at renal dialysis facilities. While the Act outlines a few mandatory measure topics, the Secretary is authorized to adopt measures on specified areas of medical topics determined appropriate by the Secretary (§1881(h)(2)). The ESRD QIP began in calendar year (CY) 2011 with an initial set of three quality measures and has dramatically increased its measure set over the intervening years through notice and comment rulemaking.
To score facility performance on quality measures, CMS must be able to collect data on these measures. CMS collects these data from multiple sources, including Medicare claims (OMB Control Number 0938-1197) and other tools such as the Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) Dialysis Event Protocol. To further expand the measures used to evaluate the quality of care provided to ESRD patients in renal dialysis facilities, CMS also collects data using the Consolidated Renal Operations in a Web-Enabled Network (CROWNWeb) System. The information collection requirements associated with the ESRD QIP measures that use data collected in CROWNWeb are covered under OMB Control Number 0938-1289. The information collection requirements associated with the NHSN Bloodstream Infection Modules are captured under OMB Control Number 0920-0666 (The National Healthcare Safety Network).
One of the critical elements of the ESRD QIP’s success is ensuring that the data submitted to calculate measure scores and Total Performance Scores (TPS) are accurate. In the CY 2015 ESRD PPS final rule, CMS finalized a feasibility study for Payment Year (PY) 2017 and PY 2018 to validate data reported to the CDC’s NHSN Dialysis Event Module for the NHSN Bloodstream Infection clinical measure. The purpose of this study was to compare the accuracy and frequency of dialysis event data reported to the NHSN system, to what was recorded in patient medical records for those years. Healthcare-Acquired Infections (HAI) are relatively rare, and CMS finalized that the feasibility study would target records with a higher probability of including a dialysis event, because this would enrich the validation sample while reducing the burden on facilities. For CY 2015, the feasibility study examined records from only 9 ESRD facilities.
The Feasibility Study in PY 2017 was conducted as follows: the validation contractor mailed CMS-approved formal request letters and packets to the nine selected facilities and asked for lists of all CY 2015 positive blood cultures, associated medical records, completed surveys, and patient cover sheets. The medical records were considered the “gold standard” for comparison purposes. Trained medical reviewers abstracted the data from the patient medical records. All nine selected facilities participated in the survey and submitted their positive blood culture templates. A total of 16 positive blood cultures were identified from those facilities. After abstracting the 16 patient charts, reviewers identified 21 reportable events. Due to the relatively small sample size, there were no instances where the chart reviewed was without an event and there were no events reported in NHSN for that patient during the time period. That would constitute a true negative. The facilities selected were also asked to complete a survey to provide information related to their participation with the feasibility study. They provided information regarding the processes used to track Dialysis Events as well as how their denominators were calculated. This information helped generalize findings and identify potential deficiencies and improvement opportunities. Based on the results of the feasibility study and the survey responses, the validation contractor made several recommendations for improvement opportunities. Specifically, they recommended:
additional training for Dialysis Event abstraction covering complex multiple dialysis events reporting as well as commonly found reporting errors.
a revision of the definition of a “match,” considering a match of the data if the report is within +/- 3 days of the event.
using a more robust study methodology, possibly expanding analysis from only one Dialysis Event to include the other two types of events and increasing the sample size to identify areas of required adjustment.
CMS took these recommendations and developed a more robust study to be implemented in the PY 2019 program. Due to the changes in the sample size and the methodology, described below, we developed a PRA package specific to the NHSN Data Validation work being done for the ESRD QIP beginning with the PY 2019 Program. We updated this PRA Package for the PY 2020 Program, and now we are updating the PRA Package for the PY 2021 Program, as discussed more fully below.
NHSN Data Validation for the ESRD QIP
CMS finalized a new methodology for the NHSN Data Validation work conducted in PY 2019 and later finalized a policy continuing use of that methodology with slight modifications to the sampling methodology. Additionally, for PY 2019, CMS finalized that the validation study would consist of 35 facilities—a policy that was continued for PY 2020.
For PY 2021, we are finalizing a policy in the CY 2019 ESRD PPS final rule to continue use of the PY 2020 study methodology, with some modifications. We are finalizing a proposal to increase the number of participating facilities from 35 to 150 facilities for the validation study conducted in CY 2019 for the PY 2021 study. Beginning with the PY 2021 study, we are also finalizing a policy to increase the number of collected records from 10 to 20 records. Further details on the methodology finalized for use in PY 2021 study are provided below. The purpose of this validation study is to compare the data entered by facilities into the CDC’s NHSN system against what is reported in medical records.
For the PY 2021 study, CMS is finalizing a policy to select 150 facilities to participate in an NHSN Dialysis Event validation study by submitting 20 patient records covering two quarters of data reported in CY 2019. A CMS contractor will send the selected facilities requests for medical records for all patients with “candidate events” during the evaluation period (i.e. patients who had any positive blood cultures; received any intravenous antimicrobials; had any pus, redness, or increased swelling at a vascular access site; and/or were admitted to a hospital during the evaluation period. Facilities will have 60 calendar days to respond to the request for medical records based on candidate events either electronically or on paper. If the contractor determines that additional medical records are needed to reach the 20-record threshold from a facility to validate whether the facility accurately reported the dialysis events, then the contactor will send a request for additional, randomly selected patient records from the facility. The facility will have 60 calendar days from the date of the letter to respond to the request. Through collaboration with the CDC for system and data access, the CMS contractor will utilize the methodology described above for reviewing and validating records from candidate events and randomly selected patients, to determine whether the facility reported dialysis events for those patients in accordance with the NHSN Dialysis Event Protocol. If a facility is selected to participate in the validation study but does not provide CMS with the requisite lists of positive blood cultures within 60 calendar days of receiving a request, then we will deduct 10 points from the facility’s TPS. Information from the validation study may be used in future years of the program to inform our consideration of future policies that would incorporate NHSN data accuracy into the scoring process.
Justification
Need and Legal Basis
Dialysis facilities, researchers, and patient advocacy groups as well as other stakeholders who have previously submitted public comments on the ESRD PPS Proposed Rule have expressed significant concerns about facilities not reporting dialysis events when they should be reported. These public comments, as well as a thorough review of data reported for the PY 2016 NHSN BSI clinical measure, and results from the NHSN data validation feasibility study, suggest that 23 percent of dialysis events are under-reported, and have clarified the delicate tradeoffs associated with incentivizing facilities to report and prevent dialysis events. Due to the small sample size of facilities that are validated through the ESRD QIP validation study, it is difficult to pinpoint exactly why the underreporting rate is high. We believe that the leading cause for underreporting is due to a lack of clear and consistent communication between hospitals and dialysis facilities. (In contrast, for example, our analysis of CROWNWeb data from the PY 2016 validation study showed an overall match rate of 92.2 percent among all facilities selected for participation). Complete and accurate reporting is critical to maintaining the integrity of the NHSN surveillance system, enables facilities to implement their own quality improvement initiatives, and enables the CDC to design and disseminate prevention strategies. To gain a more accurate understanding of the patient population and the data being submitted to NHSN, it is imperative that the data validation study be expanded to include a greater number of facilities. As noted above, we have finalized a proposal to expand the sample for NHSN validation, and we believe that this sample expansion is necessary to ensure that NHSN data are accurate and complete. Based on statistical analyses conducted by CDC, with an expected accuracy of 80% of dialysis events from facilities and setting the precision of the NHSN validation study to 95% confidence and 1% margin of error, we estimate that a total of 303 facilities and 6,072 chart reviews would be necessary to achieve the appropriate statistical power for the validation study. However, because that increase in sample size would represent a nearly tenfold increase in sampled facilities compared to current practice, we believe we should increase the sample size incrementally over two years. This PRA package focuses on the first year of our finalized expansion (the PY 2021 study).
Information Users
Section 1881(h) of the Act requires the Secretary, generally, to adopt a set of quality measures and assess the quality of care provided by renal dialysis facilities using those measures. The information that comes out of the validation study will be used by CMS and others to monitor and assess the accuracy of data reported to NSHN as part of the ESRD QIP. The information will also be used by CMS to identify areas where data accuracy could be improved and to direct its contractors to engage in activities designed to improve data accuracy in those areas (e.g., the development of targeted trainings to help facilities improve the accuracy of data reporting). Facilities, beneficiaries, and the public do not have access to validation results.
Use of Information Technology
The NHSN data validation study allows facilities to submit their quarterly data either electronically or on paper. These expenses would not exceed what is normally expended for a typical healthcare facility infection surveillance program. Paper forms are provided to facilities for data collection but they are not required to use them for entry of data into NHSN or for submission to CMS for the data validation study.
Duplication of Efforts/Similar Information
The purpose of the validation study is to review the medical records of the selected patients to ensure that the facility accurately reported any dialysis event data to the NHSN system. The patient medical record is the most accurate source of information regarding each patient’s condition and symptoms. The validation study does not require facilities to duplicate their effort and report identical data to CMS, rather it requires facilities to submit the complete patient record so that CMS can independently review and identify whether the facility properly reported dialysis events into NHSN according the CDC’s dialysis event protocol.
Small Businesses
Facilities treating 10 or fewer patients are excluded from NSHN Dialysis Event reporting. Individuals on peritoneal dialysis are also not included. Information collection requirements were designed to impose minimal burdens on small renal dialysis facilities subject to the ESRD QIP. Specifically, the NHSN system was created to allow small renal dialysis facilities to enter data via their web-based application rather than using paper-based data submission or employing a full electronic health record, which can be prohibitively expensive for these facilities. As a result, this effort facilitates small renal dialysis facilities’ collection and reporting of required data.
Less Frequent Collection
Each month, facilities report the number of maintenance hemodialysis outpatients who were dialyzed in the facility on the first two working days of the month, using the Denominators for Outpatient Dialysis form. This count is used to estimate the number of patients at the facility who are at risk of HAIs. Each month, facilities use a Dialysis Event form to report the details of each of three infection related dialysis events (IV antimicrobial starts, positive blood cultures, and evidence of local access site infection) that occurred among their patients. Due to the seasonal variability of bloodstream infections it is absolutely essential for facilities to report the full 12 months of data to reflect performance over the course of the entire performance period.
While the CDC requires that facilities report these data monthly under a previously approved PRA package (OMB Control Number 0920-0666), the proposed data validation study will require selected facilities to submit lists of dialysis events for the first two quarters of a given year. At this time, CMS is only able to validate the first two quarters of data due to operational constraints regarding when the data is available and when the scores and possible TPS reductions are calculated. Per the ESRD QIP guidelines, facilities are required to complete reporting for each quarter before the end of the subsequent quarter. This requirement hinders our ability to collect additional data and compute final scores prior to the start of the preview period.
Special Circumstances
The data validation study methodology is designed to collect quarterly data from dialysis facilities. Without this quarterly data, the CMS contractor will not be able to complete the data validation on a full year’s worth of data. We therefore believe that quarterly collection is most appropriate in order to appropriately complete the NHSN data validation study for the ESRD QIP.
Federal Register Notice/Outside Consultation
The CY 2019 ESRD PPS final rule served as the 30-day Federal Register notice. The rule published November 14, 2018 (83 FR 56922).
Payment or Gift to Respondent
Dialysis facilities are required to submit measure data to CMS as part of the Conditions for Coverage of End-Stage Renal Disease Facilities (see 42 CFR 494.180(h)). No additional payments or gifts will be given to respondents for compliance with the requirements of the NHSN Data Validation Study for the ESRD QIP. As noted above, if a facility is selected to participate in the validation study but does not provide CMS with the requisite medical records within 60 calendar days of receiving a request, then we would deduct 10 points from the facility’s TPS. This ten-point reduction may subject the facility to a payment penalty under the Program.
Confidentiality
CMS adheres to all confidentiality-related statutes, regulations, and agency policies. All information collected under ESRD QIP will conform to all applicable Federal laws and regulations and Federal, HHS, and CMS policies and standards as they relate to information security and data privacy. These laws and regulations may apply but are not limited to: The Privacy Act of 1974; the Federal Information Security Management Act of 2002; the Computer Fraud and Abuse Act of 1986; the Health Insurance Portability and Accountability Act of 1996; the EGovernment Act of 2002, the Clinger Cohen Act of 1996; the Medicare Modernization Act of 2003, and the corresponding implementing regulations. OMB Circular A–130, Management of Federal Resources, Appendix III, Security of Federal Automated Information Resources also applies. Federal, HHS, and CMS policies and standards include but are not limited to: All pertinent National Institute of Standards and Technology publications; the HHS Information Systems Program Handbook and the CMS Information Security Handbook.
SORN #: 09-70-0520 – ESRD Program Management and Medical Information System (PMMIS) published 6/17/2002 (67 FR 41244) and updated 5/8/2007 (72 FR 26126).
Sensitive Questions
There are no questions of a sensitive nature being collected as part of this data validation study.
Burden Estimates
Burden =

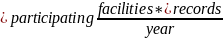 *
*
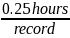 *
*
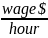
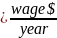
Table A. NHSN Data Validation Burden Estimate Elements
Burden Estimate Element |
PY 2021/CY 2019 |
Number of facilities participating in the NHSN Data Validation study, annually |
150 |
Estimated number of medical records per facility per year |
40 |
Time spent for record collection and submission per facility |
10 hours (approx. 0.25 hours per record) |
Hourly wage per hour engaged in data collection and submission plus overhead and benefits |
$41.18 |
Under the NHSN data validation studies finalized for PY 2021, we finalized a policy to sample records from 150 facilities. A CMS contractor will send these facilities quarterly requests.
To derive wage estimates, we used data from the U.S. Bureau of Labor Statistics’ (BLS) May 2017 National Occupational Employment and Wage Estimates.1 We anticipate that the labor required to collect and submit these data will be completed by either Medical Records and Health Information Technicians or similar administrative staff. The mean hourly wage of a Medical Records and Health Information Technician is $20.59. Fringe benefits and overhead are calculated at 100% using current HHS department-wide guidance on estimating the cost of fringe benefits and overhead. These are necessarily rough adjustments both because fringe benefits and overhead costs vary significantly from employer to employer and because methods of estimating these costs vary widely from study to study. Nonetheless, there is no practical alternative and we believe that these are reasonable estimation methods.
Using the assumptions described above, we estimate that an hourly labor cost of $41.18 as the basis of the wage estimates for all collection of information calculations in the ESRD QIP. We also estimate the total annual burden for the CY 2019/PY 2021 study is $61,770.
Table B. NHSN Data Validation Burden Per Facility
NHSN Data Validation Facilities CY 2017 |
Number of Facilities |
Number of Records Per Year |
Estimated Time Per Records |
Estimated Wage Plus Benefits Per Hour for Record Collection |
Annual Hour Burden Per Facility |
Annual Burden Per Facility |
PY 2021/CY 2019 |
||||||
NHSN Data Validation |
150 |
40 |
0.25 |
$41.18 |
10 |
$411.80 |
Table C. NHSN Total Data Validation Burden,
-
Basis
Annual Hour Burden
Annual Burden
PY 2021/CY 2019
Each Facility
10
$411.80
National
1500
$61,770.00
Capital Cost
There are no capital costs.
Cost to Federal Government
The cost to the Federal Government includes costs associated with the collection and validation of the data. The validation costs are an estimated $165,000 (FY) annually for the validation contract. The CDC maintains the NHSN system. The estimated cost to operate the validation contract includes 1/5 CMS staff at the GS-13 Level (approximate salary is $100,000). This results in a total estimated cost of $185,000) annually.
Changes to Burden
This is a revision to an existing application. The existing application covers the burden associated with the PY 2020 study, which had a total burden of $6,975.50 and 175 hours. The changes in burden associated with this amended application are due to the addition of the PY 2021 study, including the increase in the number of participating facilities and requested records as well as the update to the wage estimate. The annual hour burden associated with the PY 2021 study is 1,500. The annual burden associated with the PY 2021 study is $61,770.00
Publication/Tabulation Date
NHSN is an ongoing data collection system, and as such, does not have an annual timeline. The data are reported on a continuous basis by participating institutions and aggregated by CDC into a national database that is analyzed for two main purposes: to describe the epidemiologist of healthcare-associated adverse events, and to provide comparative data for populations with similar risks. Comparative data can be used by participating and non-participating healthcare institutions that collect their data using NHSN methodology.
CMS does not publish data validation results at this time.
Expiration Date
CMS will display the expiration date and the new OMB control number on the collection instruments.
Explain any exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
There are no exceptions to the certification statement “Certification for Paperwork Reduction Act Submissions” of OMB form 83-I.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Lallemand, Nicole |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy