Sampling and Analysis Plan (SAP)
Att17_ApxD_SAP_FINAL.docx
ATSDR Exposure Investigations (EIs)
Sampling and Analysis Plan (SAP)
OMB: 0923-0048
Appendix D: Sampling and Analysis Plan
Sampling and Analysis Plan
Environmental Sampling (Multi-media) for ATSDR’s Supplemental Exposure Investigation: Per- and Polyfluoroalkyl Substances (PFAS)
Table of Contents
1. Introduction 1
2.0 Project Data Quality Objectives 2
2.1 Project Objectives and Problem Definition 2
3.0 Field Methods and Procedures 3
3.1 Indoor Dust (Filtered Composite Samples) 3
3.1.2 Sampling Point Selection 3
3.1.3 Analyte and Method Selection 3
3.1.5 Sample Labeling and Shipping 5
3.2.2 Sampling Point Selection 6
3.2.3 Analyte and Method Selection 7
3.2.5 Sample Labeling and Shipping 9
3.3.2 Sampling Point Selection 11
3.3.3 Analyte and Method Selection 11
3.3.5 Sample Labeling and Shipping 14
3.4.2 Sampling Point Selection 14
3.4.3 Analyte and Method Selection 14
3.4.5 Sample Labeling and Shipping 15
3.5.2 Sampling Point Selection 17
3.5.3 Analyte and Method Selection 18
3.5.5 Sample Labeling and Shipping 20
3.6.2 Sampling Point Selection 21
3.6.3 Analyte and Method Selection 21
3.6.5 Sample Labeling and Shipping 23
3.7.2 Sampling Point Selection 24
3.7.3 Analyte and Method Selection 24
3.7.5 Sample Labeling and Shipping 25
3.8.2 Sample Selection Point 26
3.8.3 Analyte and Method Selection 27
3.8.5 Sample Labeling and Shipping 28
3.9 PFAS Cross Contamination Prevention Best Practices 29
3.11 Sample Handling and Custody 30
3.12 Inspection of Supplies and Consumables 30
4.0 Analytes and Method Selection 30
4.1 Quality Control Samples 33
4.2 Field and Equipment Blanks 33
4.3 Duplicate and Split Samples 33
5.0 Sampling Logistics 34
5.1 Field Sampling Schedule 34
6.0 References 35
1.1 Background and Purpose
Relatively scant information is available in the literature indicating whether non-drinking water exposures may contribute to per- and polyfluoroalkyl substances (PFAS) body burdens. Under an interagency agreement, the Agency for Toxic Substances (ATSDR) and U.S. Environmental Protection Agency (EPA) are conducting a supplemental exposure investigation (EI) to identify potential non-drinking water contributors of PFAS body burdens. The agencies will conduct the supplemental EI in two of the eight communities with drinking water contamination included in CDC/ATSDR’s PFAS exposure assessments (EAs).
This document represents the sampling and analysis plan (SAP) and details the procedural and analytical methods to be followed when conducting sampling as part of this EI. CDC/ATSDR will be responsible for obtaining head of household consent prior to proceeding with administration of questionnaires and the environmental sampling within homes.
1.2 EI Overview
Air (indoor and outdoor), dust (filtered and bulk), surface wipes, silicone wristbands, soil, and produce samples will be collected and analyzed for PFAS and PFAS precursors in accordance with prescribed methods described in this SAP. The EI will also include the administration of a questionnaire that will focus on better defining exposure to PFAS in the drinking water and to obtain additional information on non-drinking water exposure to PFAS sources, including use of consumer products and potential dietary exposure.
CDC/ATSDR will collect samples from a subset of participating households at two of CDC/ATSDR’s PFAS EA locations (Hampden County, MA, and New Castle County, DE). Households will be identified and scheduled by recruiting strategies outlined in CDC/ATSDR’s EI Protocol. The targeted number of households by sample type are as follows:
Sample Type |
Hampden County, MA |
New Castle County, DE |
||
Ambient Air (within community) |
<4 |
<4 |
||
Indoor Air |
20 |
20 |
||
Indoor Dust (filtered) |
80 |
40 |
||
Indoor Dust (bulk) |
20 |
20 |
||
Surface Wipes |
20 |
20 |
||
Soil |
20 |
20 |
||
Wristbands |
20 |
20 |
||
Produce |
21 |
21 |
||
2.0 Project Data Quality Objectives
2.1 Project Objectives and Problem Definition
The primary objective of this plan is to ensure that the samples will be collected in a consistent manner and will be of the quality necessary to support CDC/ATSDR evaluation of PFAS exposure in the selected communities. CDC/ATSDR’s overall goal in this EI is to determine the presence or absence of PFAS in selected media as well as the nature of detected PFAS. The intention is not to generate data needed to address regulatory issues.
2.2 Data Quality Objectives
The project Data Quality Objectives (DQOs) help determine how good data must be to achieve a project’s specific technical goals and objectives. This EI will use DQOs to develop the criteria that the data collection design should satisfy, including where to conduct sampling, the number of samples to collect, and the overall representativeness, completeness, and comparability of data. Eurofins have supplied ATSDR and EPA with a copy of their proprietary procedures for complete transparency. All laboratory analysis will be conducted with established procedures for quality assurance and control, including the use of an appropriate number of QC samples.
Data representativeness
To help ensure that environmental samples collected are representative of the household that is being sampled, the sampling team will (1) collect dust and wipe samples from the primary living spaces as identified by the homeowner (e.g., living room, family room, television room, kitchen, bedroom) in which participants spend the most time; (2) locate indoor air sampling devices in one of the primary living spaces; and (3) implement soil sampling using incremental sampling approaches in areas of the yard accessed by children and other family members (the decision or exposure unit). For produce, sample selection and representativeness will be dictated by availability and variety of local produce.
Data completeness
The sampling team will ensure that complete samples sufficient to run the requested analyses are collected, that the necessary quality control samples are collected and that laboratory calibration records are provided with the sampling report to determine the reliability of the sample data. Every effort will be made to reschedule sampling times should homeowners not be available for the scheduled data collection time to help ensure all of the selected households are sampled.
Data comparability
Data sets will be checked for comparability. Comparability is a qualitative measure of the confidence with which data sets can be compared. All analytical data received from the contract laboratories will be reviewed to ensure analyses have been completed in accordance with documented analytical procedures and by reporting the results in the standard units of measure as required in the methods.
3.0 Field Methods and Procedures
This section presents planned procedures and methods for conducting sampling activities.
The household sampling locations will be a subset of PFAS EA participating households. Summary tables with community-specific locations, house identification numbers, contact numbers, and the household-specific sampling plan will be provided to the environmental sampling team ahead of scheduled appointments. The exact sampling locations within each household will be identified at the time of field sampling and recorded in the project-specific environmental sample collection form (Appendix D.1). Any deviations to the sampling plan will be documented on the form.
3.1 Indoor Dust (Filtered Composite Samples)
3.1.1 Field Equipment
0.8 μm pore size polycarbonate filters placed in open-face 37-mm filter cassettes
Gast Rotary Vane Pump Model 1532 or similar
Gilian Bubble Flow Meter model 800285
Silicon tubing 3/16" ID x 3/8" OD x 0.094" wall
Electronic Tesso TPS-100 scale
250 mL HDPE wide-mouth bottles, supplied by the lab
Wooden 2 square foot (ft2) sampling template
PFAS free masking tape
Field forms
Chain of Custody (COC) forms, supplied by the lab
Powderless nitrile gloves
PFAS-free resealable bags
Coolers, supplied by the lab
Ice
3.1.2 Sampling Point Selection
Two or three composite dust samples will be collected from the floor of three locations inside each selected home – the primary living space as identified by the homeowner (e.g., living room, family room, television room), the kitchen, and the bedroom in which EI participants spend the most time. Participants will be instructed by the scheduler to not vacuum carpeting or sweep floors for at least 5 days prior to the scheduled visit to enable the sampling team to collect enough dust for analysis.
A filtered dust sample will be taken at all EI participants’ homes: 80 homes in MA and 40 homes in DE.
3.1.3 Analyte and Method Selection
All filtered composite dust samples will be analyzed for targeted PFAS (by LC-MS/MS). FTOH analysis (by GC-MS/MS) will be conducted on samples collected in 10 percent of the selected households, and TOF analysis (by combustion ion chromatography) will be conducted on samples collected in 10 percent of the selected households. See Section 4.0 for more information.
3.1.4 Sample Collection
Filtered dust samples will be collected in every household included in the EI. Household dust samples will be collected using a high-volume air sampler (Gast RotaryVane Pump Model 1532 or similar) with a flow rate of 15 L/min. The sampler will be calibrated against a bubble meter (Gilian Bubble FlowMeter model 800285, Gilian Instrument Corp.). Dust will be collected on 0.8 μm pore size polycarbonate filters (model 738 PC, Zefon Analytical Accessories, St. Petersburg, Fla.) placed in open-face 37-mm filter cassettes. The filters will be pre-tested to ensure they are PFAS free. See photographs below.


Samples will be taken from both hard and soft surfaces, with mats, carpets, and area rugs being the preferred sampling surfaces. Samples will be taken from easily accessible floor surfaces, but sampling staff may ask permission of the homeowner to temporarily move small items to gain access to more floor space.
Field staff will use a wooden 2 ft2 sampling template and have PFAS-free masking tape on hand to mark off the sampling area. The total surface area, as well as the surface types on which the sample was taken will be recorded on environmental sample collection form. Field staff will attempt to collect samples with a minimum of 1-gram of total dust each in the open-faced cassettes from each home, vacuuming the same one 2 ft2 of carpet or other surface at least four times (vertically, horizontally, circles with the cassette) with slightly overlapping passes.
The initial weight of each filter cassettes will be recorded in the field using an electronic Tesso TPS-100 scale. The initial recorded weight will be compared to the post-sampling weight of the filter cassette to determine when enough sample has been collected at each household. The change in sample weight will be recorded. Once sufficient sample volume has been collected, the filter cassette will be capped and placed in a certified pre-cleaned HDPE wide-mouth bottle. Each bottle will be labeled with the appropriate sample identification number prior to shipping.
While CDC/ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to planned sampling methodology. For instance, additional locations may need to be sampled to collect a minimum of 1 gram of dust from each household. Field staff will continue to sample additional 2 ft2 areas until the 1-gram minimum composite sample volume has been collected. In addition, if a filter cassette is observed full or overloaded of the filter, a new cassette will be used, and the contract laboratory will composite with the initial filter cassette in the laboratory.
Sampling teams will conduct all sampling activities in a manner to minimize potential contamination and cross-contamination of samples. Sampling staff will wear new nitrile gloves at each sampling point to avoid exposure to pollutants and other chemical, physical, and biological hazards, and to prevent cross-contamination of samples. Sampling staff will take care not to touch the insides of filter cassettes, bottles or lids, and caps during sampling. Sampling staff will use a new section of silicon tubing at each home to limit cross-contamination of samples.
Any deviations to the sampling plan will be documented on the environmental sample collection form and communicated to CDC/ATSDR. Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will also be recorded on the environmental sample collection form.
3.1.5 Sample Labeling and Shipping
A coded label provided by the laboratory that provides the sample ID and project ID will be affixed to each sample container.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location (e.g., locked hotel room, office, or vehicle) and stored at <4°C under low light conditions (i.e., in coolers on wet ice) until they are shipped. Samples will be shipped using the instructions below.
After sampling, insert sample containers into certified pre-cleaned HDPE bottles.
Ensure sample labels are firmly affixed to the HDPE bottles, then tape over the bottles with clear packing tape.
Place the bottles inside a one-gallon PFAS-free resealable bag.
Place a large garbage bag in the interior of the cooler. This bag will be your “outer liner.” No water or ice should be placed outside this bag.
Pour in some wet ice in a single layer to cover the bottom of the outer liner.
Add a second large garbage bag to the cooler so that it fits inside the outer liner. The second bag will be your “inner liner.”
Place bagged samples inside the inner liner.
Tie a knot at the top of the inner bag around the sample containers.
Pour ice onto and around the inner liner to fill up any empty spaces on the outside of the inner liner until the cooler is full. The ice should fill up about 30-50% of the content of cooler. Make sure that there are enough loose ends to tie the outer liner in a knot.
Tie a knot at the top of the outer liner in a manner that ensures there will be no leakage.
Place completed COC in a Ziploc bag and place in the cooler on top of the outer liner.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.2 Indoor Air
An integrated indoor air sample will be collected from the main living space of each selected home over the course of up to 7 days. Each sample will be collected using a low-volume pumping device (active sampling). ATSDR will collect samples for PFAS (30 semi-volatiles) in 20 homes and FTOH (volatiles) in a subset of (n=5) of those homes. Quality assurance samples (duplicate samples, field blanks) are listed in Table D.1.
The sampling and analysis approach, modified for PFAS, is based in part on methods described in EPA (2017) (https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=335764&Lab=NERL) and Roth et al. (2020) (https://pubs.acs.org/doi/suppl/10.1021/acs.estlett.0c00052/suppl_file/ez0c00052_si_001.pdf; https://pubs.acs.org/doi/suppl/10.1021/acs.estlett.0c00052/suppl_file/ez0c00052_si_001.pdf).
Sampling team members will be fully trained in the air collection requirements and equipment operating procedures described herein prior to deployment.
3.2.1 Field Equipment
Clean and spiked sample media, supplied by the laboratory (polyurethane foam [PUF]/XAD cartridge for semi-volatile PFAS and thermal desorption tube for volatile fractions)
Sampling pumps (e.g., SKC “AirChek Touch” or comparable pump), with battery backup
Flow meters
Silicon tubing
Metal sampling box (e.g., 12” (W)× 8” (D) × 4” (H) equipped with holes for power cord, sampling tube, exhaust, and locking clasp, and box key)
Extech 44450 pocket humidity/temperature pen
Power strip
Standard laboratory ring stand with vertical support of 100 cm
Fixed position two-pronged clamp to hold the sorbent tube
PFAS-free resealable bags (anticipated to be part of the sample kit provided by the lab)
Flow-rate adapter
Zip ties
Powderless nitrile gloves
Field forms
Chain of custody (COC) forms, supplied by the lab
Coolers, supplied by the lab
PFAS-free ice packs
3.2.2 Sampling Point Selection
An active air sampler will be placed in selected homes based on the following considerations:
The first priority is selecting the room of primary occupancy by the participant (the room where the participant spends most of his/her time throughout the day other than a bedroom). The room of primary occupancy is likely to include the den or living room. When the primary room is unavailable, then select a secondary room such as the kitchen or a bedroom to locate the sampling device. Record the room identity on the field form.
Following the room selection, the sampling team will identify the location where the indoor sampler will be placed. Pumps are typically placed in out-of-the-way locations away from the center of the room in order to present as little interference to the participants as possible. Factors contributing to location selection include: the dimension of the room and its furnishings, the presence of children or pets who might tamper with the device, occupant activity in the room, ventilation, potential disturbance to the occupants by the noise generated by the pump, location of outlets and trip hazards associated with the stretching of extension cords.
Sample media will be positioned at breathing zone height (~5 feet).
For duplicate samples, sampling pumps will be collocated within their boxes by placing each in adjacent positions at the base of the ring stand. Both sampling cartridges can be located on the same horizontal support.
The field staff will record the sampler location within the room in ink on the field form.
While CDC/ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to planned sampler installation/placement. The sampling team will record any deviations from the plan or observations that may impact data collection.
3.2.3 Analyte and Method Selection
Indoor air samples will be analyzed for a targeted list of 30 semi-volatile PFAS and fluorotelomer alcohols (FTOH). Samples will be collected for the semi-volatile PFAS analytes using PUF/XAD/PUF cartridges while a separate sample, more appropriate for the volatile FTOH, will be collected using thermal desorption cartridges with appropriate sorbents. Preliminary testing is being performed for both methods to optimize flow rates, sampling durations, and sample volumes to achieve the best detection limits possible for residential sample collection without analyte breakthrough. See Section 4.0 for more information on laboratory methods and method detection limits.
3.2.4 Sample Collection
Sample collection steps are adapted from EPA [2017]. Standard operating procedures will be refined and expanded as needed once method validation testing is complete, sampling equipment and sampling media are confirmed, and target flow rates and sample duration are determined.
Installation
Set up the metal sampling box (designed to tamper proof the pump and deaden the noise associated with the pump). The box will contain the sampling pump with power cord. Make sure a 3-foot length of silicon tubing connected to the sample pump inlet extends through the hole on the side of the sampling box.
If more than a single wall outlet is required, plug the power strip into the nearest outlet. Run an extension cord from the nearest electrical outlet to provide power to the sampling equipment. Plug the extension cord into the power strip. Make sure that the sampling pump is plugged into the power strip. Ensure that all electrical connections are secure. Using 3” wide PFAS-free tape, secure the cord(s) in such a manner as to ensure it does not pose a trip hazard to the occupants or those involved in the sampling event.
Make sure the AirCheck Touch is firmly plugged into the charging cradle and the red (charging) or green (charged) LED is illuminated on the front of the charging cradle.
Don a clean pair of disposable powderless, nitrile gloves.
Retrieve the sampling media from its respective plastic bag.
Remove the caps/tips of the sampling media.
Connect the media to the silicon tubing that is connected to the inlet of the sampling pump. Secure both tubing attachments with a zip tie.
Hook a horizontal clamp assembly to the upright post of the ring stand.
Place the sampling box next to the base of the ring stand.
Attach the sampler cartridge/tube to the clamp with the open inlet facing down.
Calibration
Instructions for the selected pump will be followed. In general, the field staff will adhere to the following procedures (for the AirCheck Touch), all of which will be tested and further documented as needed prior to deployment.
Activate the pump by pressing the recessed power button on the side of pump.
Unlatch and raise the protective screen cover on the pump.
Touch the flowrate screen on the top of the AirCheck Touch to bring up the “Set Flow” screen. Use the up and down arrows to adjust the flowrate to the desired flowrate for the sampling event (to be determined through pre-deployment testing and validation studies and anticipated to be between 4 and 5 L/minute).
Connect the NIST traceable flow meter to the media inlet using the flow rate adapter. The flowrate adapter, connected in-line to the flow meter, simply slides onto the glass housing of the open-faced cartridge prior to calibration procedures.
Allow the pump to run for at least five minutes to warm up.
Press the ON button to turn on the flow meter and an initializing screen will display. Press and hold the Read button until a reading starts, then release. This will begin an automatic continuous read session. The display will indicate the current flow reading (shown as Flow on the left upper corner of the LCD screen), average flow reading of 10 readings and number of readings up to 10 times.
If the AirCheck Touch display flowrate and the NIST traceable flowmeter reading differs by more than 2%, press the tools icon on the top right of the AirCheck Touch screen to enter into the “Manual Cal” screen.
Adjust the flowrate by pressing the up and down arrows on the “Manual Cal” screen to adjust the flowrate to match the flowrate of the NIST traceable flowmeter. Touch the check mark key to enter the new flowrate value and leave the “Manual Cal” screen. Touch the checkmark on the next screen to accept the new value.
Once the flowrate is set in the proper range, let the pump run for 2 minutes. Press and hold the Read button on the flow meter to initiate multiple readings, then release. Record the average flow measurement (the average flow value of the 10 readings as provided as a readout on the device) and sample ID on the sample collection record.
Remove the flow meter from the cartridge inlet.
Record all calibration data in a field notebook.
Sampling
After the sampling media (cartridge/tube) is installed and the appropriate flowrate has been set/calibrated, begin sampling by pressing the Green “Start Sample” button.
Record the start time and all pertinent information on the sample collection form.
Close the lid of the AirCheck Touch. Close, latch, and lock the outer box.
Sample Recovery
After the designated sampling period, unlock and open the outer sampling box. Raise the screen cover of the AirCheck Touch.
Reconnect the NIST traceable flow meter to the inlet of the cartridge. No adjustment to the pump is required at this step and only flow readings are collected. The average flow measurement (average flow value in 10 readings) will be recorded on the sample collection sheet.
Record the accumulated time and sample volume from the AirCheck Touch display on the sample collection form. Press the red “Stop” button to end the sampling. The Accumulated time and volume will reset when the pump is stopped. the field staff (using nitrile gloved hands) will reconnect the flow meter to the inlet of the cartridge. No adjustment to the pump is required at this step and only flow estimates are collected. The average flow measurement (average flow value in 10 readings) will be recorded on the sample collection sheet.
Record the sample end date and time on the sample collection sheet.
Replace the Split Housing/Inlet from the plastic bag onto the sampler cartridge and place the caps on both ends (inlet and outlet) of the cartridge. Place a self-adhesive, previously prepared label on the sample housing. The identifying label should include the unique sample code and the collection date.
Place the sampling media in a PFAS-free resealable plastic bag.
Any deviations to the sampling plan will be documented on the environmental sample collection form. Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will also be recorded on the environmental sample collection form.
3.2.5 Sample Labeling and Shipping
A coded label provided by the laboratory will be affixed to each sample that provides the sample ID and project ID.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule and any specific requirements dictated by the laboratory or method. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location and stored at <4°C in a field freezer (shipped to or acquired at the field site) until they are shipped. Samples will be shipped using the instructions below.
Place bagged frozen ice packs (PFAS-free) in a single layer to cover the bottom of the cooler.
Place double bagged samples on top of the ice packs.
Place more bagged frozen icepacks in the cooler to fill the extra space.
Place completed COC in a Ziploc bag and place in the cooler on top of the ice packs.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.3 Ambient Air
Integrated samples will be collected in ambient (outdoor) air at a single location for up to 7 days. Samples will be collected using one high-volume sampler and two (collocated) low-volume samplers. ATSDR will use the same equipment and procedures used for collecting the indoor air samples as described in Section 3.2. This section describes minor adaptations to the low-volume sampling approach in the ambient setting and the procedures for ambient air sampling using the high-volume sampler.
High-volume sampling approaches will be guided by EPA Method TO-13A, Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air [EPA 1999].
Sampling team members will be fully trained in the air collection requirements and equipment operating procedures described herein prior to deployment.
3.3.1 Field Equipment
Clean and spiked sample media, supplied by the laboratory (PUF/XAD cartridge for semi-volatile PFAS and thermal desorption tube for volatile fractions)
SKC AirChek Touch or comparable pump (2 low-volume samplers)
Flow meters
Silicon tubing
Weatherproof sampling box (e.g., 12” (W)× 8” (D) × 4” (H) equipped with holes for power cord, sampling tube, exhaust, and locking clasp, and box key)
Extech 44450 pocket humidity/temperature pen
Handheld GPS unit
Power strip
Standard laboratory ring stand with vertical support of 100 cm
Fixed position two-pronged clamp to hold the sorbent tube
PFAS-free resealable bags (anticipated to be part of the sample kit provided by the lab)
Flow-rate adapter
Zip ties
Tisch Environmental Hi-Volume TE-1000 type sampler
Installation tools
Adjustable resistance plate for Hi-Volume sampler
Hi-Volume sampler motor (replacement)
Hi-Volume sampler motor brushes (replacement)
Water manometer
Field forms
Chain of custody (COC) forms, supplied by the lab
Powderless nitrile gloves, cotton gloves
Coolers, supplied by the lab
PFAS-free icepacks
3.3.2 Sampling Point Selection
Sampling apparatus for ambient data collection will be situated in a centralized location within the EA sampling frame within the community. Location selection will be based considerations such as power source, security, and minimizing noise disturbances, which ATSDR will evaluate during pre-sampling reconnaissance.
Field staff will record the ambient sampling equipment location (lat/long) on the field form and take pictures, if possible, in a 360 degree arc to record sampling location.
Temporary fencing and signage may be installed to ensure the equipment is secured and there is no potential for tampering with samples.
While ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to anticipated sampler installation/placement. The sampling team will record any deviations from the plan or observations that may impact data collection.
3.3.3 Analyte and Method Selection
Outdoor air samples will be analyzed for a targeted list of 30 semi-volatile PFAS and fluorotelomer alcohols (FTOH). Samples will be collected for the semi-volatile PFAS analytes using PUF/XAD/PUF cartridges while a separate sample, more appropriate for the volatile FTOH, will be collected using thermal desorption cartridges with appropriate sorbents. Preliminary testing is being performed for both methods to optimize flow rates, sampling durations, and sample volumes to achieve the best detection limits possible for residential sample collection without analyte breakthrough. See Section 4.0 for more information on laboratory methods and method detection limits.
3.3.4 Sample Collection
High-volume samples: A Tisch Environmental Hi-Volume TE-1000 type sampler will be used to collect PFAS samples daily. We will collect 24-hour integrated samples and follow EPA Method TO-13A guidelines [EPA 1999] for sampler setup.
Low-volume samples: The calibration, installation, and breakdown procedures are the same as those described in Section 3.2
Installation/Set Up
Install the Hi-Volume sampling system so that the inlet is between 2-15m above level ground.
A set of stakes will be used to hold the Hi-Volume sampling system in place during the duration of sampling and prevent tipping.
No more than 100 feet of extension cord should be used for the sampling system and it should be plugged into a dedicated 110v AC outlet.
The Hi-Volume sampler should be installed at least 2 times the distance from any obstruction and have 360 degrees of unrestricted airflow.
The Hi-Volume sampler should not be installed under the dripline of any vegetation if possible.
Calibration
Once the Hi-Volume samplers are set up at the sampling location, a Calibrated Orifice Transfer standard will be used to calibrate the Hi-Volume sampler as described in EPA Method TO-13A, Section 11.2.2.
Record the calibration orifice ID, sampler ID, location, date, time, ambient temperature, and ambient barometric pressure.
Record the orifice manometer inches for each reading associated with Hi-Volume sampler magnehelic readings associated with 70, 60, 50, 40, 30, 20, and 10 inches.
Calculate the magnehelic flowrate and desired magnahelic setpoint per the calculations found in EPA Method TO-13A, Section 11.2.2.
All calibration records will be recorded in a field notebook.
Sampling
Open the lid of the Hi-Volume sampler. Remove the sampling head by lifting up on the two rings and the base of the sampling head releasing the latches that hold it in place.
With the sampling head unscrew the lower assembly from the sample head. While wearing cotton gloves remove the PUF/XAD-2 trap/cartridge from its shipping container and open the sealed packaging.
Remove the end caps from the trap and retain in the shipping container for later use.
Inspect the sampling media for any damage during shipment. Any chip or crack in the surface of the trap will cause it to not seal appropriately inside the sample head assembly.
Insert the trap into the lower assembly of the sample head so that the glass frit is at the bottom. Take care not to spill the PUF or XAD-2 contents while installing. Screw the lower assembly back to the upper assembly making sure the rubber fittings on each end seat onto the lips of the PUF/XAD-2 trap.
Remount the sample head back into the Hi-Volume sampler and press down on the ringed latches until the lock into place.
Open the upper assembly filter holder. Place the lower Teflon offset ring on the bottom of the filter holder above the screen. Open the glass fiber filter shipping container. With a pair of clean forceps, carefully transfer the glass fiber from the shipping container to the center of the screen on the upper assembly filter holder. Place the upper Teflon offset ring on top of the filter aligning the edges with the filter. Place the top portion of the filter holder assembly back on top and clamp the filter holder assembly back together.
Record all appropriate information on to the sample data sheet (ambient temperature, barometric pressure, elapsed time meter reading, sampler number, filter number, and PUF cartridge number).
Turn on the sampler and allow to warm-up for approximately 5 minutes.
Record the sampler magnahelic reading on the sample data sheet and turn the sampler off.
Set the program clamp on the elapsed timer wheel taking care to set the correct "OFF" clamp at the appropriate times. These will be set for a 24-hour sample duration.
The sampler will switch on at the appropriate time. Close and lock the Hi-Volume sampler lid and door.
Sample Recovery
Sample recovery procedures will generally follow EPA Method TO-13A, Section 11.3.4.
After the end of the sampling period, turn the Hi-Volume sampler on and wait five minutes for the sampler to warm up.
Record the Hi-Volume sampler magnahelic reading on the sample data sheet then turn the sampler off.
Record the following information on the sample data sheet: ambient temperature, barometric pressure, elapsed time meter reading.
Inspect the site area to ensure that nothing has changed that could bias the sample collection. Inspect the shelter and ancillary equipment to ensure it is functional in in good working condition.
Carefully unclamp and remove the filter using a pair of clean forceps. Then unscrew the upper sampling head assembly. Remove the PUF/XAD-2 trap wearing a pair of cotton gloves. Fold the glass fiber filter twice and place it inside the PUF/XAD-2 trap. Then recap and repackage the trap into the shipping container.
Inspect the site area to ensure that nothing has changed that could bias the sample collection. Inspect the shelter and ancillary equipment to ensure it is functional in in good working condition.
3.3.5 Sample Labeling and Shipping
See Section 3.2.5 for low volume samples. Follow similar procedures for all ambient samples, placing samples with ice packs in designated coolers, and include sample data sheets.
3.4 Bulk Dust
3.4.1 Field Equipment
PFAS-free resealable bags
Pre-cleaned large forceps
250 mL HDPE wide-mouth bottles, supplied by the lab
Extra 2-gallon heavy duty resealable plastic bags
Scissors
10 x 1g aliquots of SRM 2585, supplied by the lab.
Field forms
COC forms, supplied by the lab
Dust mask (disposable)
Powderless nitrile gloves
Coolers, supplied by the lab
Ice
3.4.2 Sampling Point Selection
A bulk dust sample will be collected from the participant’s vacuum cleaner. Procedures are adapted from the National Children’s Study Environmental Vacuum Bag Dust Technician Collect SOP (National Children’s Study, n.d.) and EPA’s Field Collection Standard Operating Procedures for an EPA Pilot Study Evaluating Personal, Housing, and Community Factors Influencing Children’s Potential Exposures to Indoor Contaminants at Various Lifestages (EPA Pilot Study Add-On to the Green Housing Study) (U.S. EPA 2017).
During scheduling participants will be instructed not to empty/dispose of dust in their vacuum, but not to vacuum at least five days prior to the appointment (see Section 3.3, filtered dust collection).
3.4.3 Analyte and Method Selection
All bulk dust samples will be analyzed for targeted PFAS (by LC-MS/MS), FTOH analysis (by GC-MS/MS), TOF analysis (by combustion ion chromatography), and supplemented by total oxidizable precursor (TOP) assay. SRM 2585 will be used as a reference for bulk dust. See Section 4.0 for more information.
3.4.4 Sample Collection
Confirm with the participant that they have and use a vacuum cleaner(s) in the residence. If a vacuum does not exist or has not been used, no sample can be collected. Document the reason on the environmental data collection form.
Ask the participant to show you the vacuum cleaner that is used most frequently inside the home. If this vacuum cleaner is a hand-held vacuum, ask the participant if there is a full-sized canister or upright vacuum that is also used. Only collect the sample from a hand-held vacuum if it is the only vacuum in the home.
Don a clean pair of powder-free nitrile gloves.
For vacuums with a bag:
Carefully remove the bag from the vacuum cleaner. Removing the vacuum bag from some vacuums may not be intuitive. If needed, ask the participant for help, or to remove the bag for you.
Place the contents of the vacuum bag into the 250 mL HDPE wide-mouth jar. If the contents are dense and do not pour out easily, reach in and grab the dust with the large forceps if necessary. Make sure you have removed as much of the fine dust as possible. Shake the container, if necessary. Collect all of the dust, if possible. Otherwise, make sure the sample collected is representative of the dust in the bag or container (i.e., includes both coarse and fine particles).
Affix the sample ID label onto the jar.
For vacuums without a bag, with a reusable bag, or a central vacuum system:
Carefully remove the vacuum dust collection container or reusable bag. Removing the vacuum dust collection container or reusable bag from some vacuums may not be intuitive. If needed, ask the participant for help, or to remove the container or bag for you.
Holding the vacuum dust collection container over the bag, pour the dust into the 250 mL HDPE wide-mouth jar. If the contents are dense and do not pour out easily, reach in and grab the dust with the forceps if necessary. Make sure you have removed as much of the fine dust as possible. Shake the container, if necessary. Collect all of the dust, if possible. Otherwise, make sure the sample collected is representative of the dust in the bag or container (i.e., includes both coarse and fine particles).
Affix the sample ID label onto the jar.
Record any notes on the collection form.
Place the vacuum bag sample into the cooler with ice.
Clean the work area thoroughly.
While CDC/ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to planned sampling methodology. For instance, the participant’s vacuum may be empty. If the participant’s vacuum is empty, a “spot” vacuum sample may be collected after the filtered sample is collected.
Any deviations to the sampling plan will be documented on the environmental sample collection form and communicated to CDC/ATSDR. Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will also be recorded on the environmental sample collection form.
3.4.5 Sample Labeling and Shipping
A coded label provided by the laboratory that provides the sample ID and project ID will be affixed to each sample container.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location (e.g., locked hotel room, office, or vehicle) and stored at <4°C under low light conditions (i.e., in coolers on wet ice) until they are shipped. Samples will be shipped using the instructions below.
Ensure sample labels are firmly affixed to the HDPE bottles, then tape over the bottles with clear packing tape.
Place the bottles inside a one-gallon PFAS-free resealable bag.
Place a large garbage bag in the interior of the cooler. This bag will be your “outer liner.” No water or ice should be placed outside this bag.
Pour in some wet ice in a single layer to cover the bottom of the outer liner.
Add a second large garbage bag to the cooler so that it fits inside the outer liner. The second bag will be your “inner liner.”
Place bagged samples inside the inner liner.
Tie a knot at the top of the inner bag around the sample containers.
Pour ice onto and around the inner liner to fill up any empty spaces on the outside of the inner liner until the cooler is full. The ice should fill up about 30-50% of the content of cooler. Make sure that there are enough loose ends to tie the outer liner in a knot.
Tie a knot at the top of the outer liner in a manner that ensures there will be no leakage.
Place completed COC in a Ziploc bag and place in the cooler on top of the outer liner.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.5 Soil
CDC/ATSDR will collect surface soil samples at 20 properties within each community using an incremental sampling methodology (ISM) approach. This sampling method involves collecting and combining many equal mass increments of soil (i.e., increment samples) across a specific area or volume of soil (e.g., an exposure unit) into a single representative sampled for laboratory analysis (i.e., bulk ISM sample). The combined sample is sieved and ground to obtain a consistent particle size and then subsampled and processed by the laboratory following specific protocols. Due to the sampling density afforded by collecting many increments, ISM samples can provide more precise and representative estimates of an exposure unit’s average contaminant concentrations than other sampling approaches.
The approach described below is based on CDC/ATSDR’s Exposure Point Concentration Guidance for Non-discrete Sampling (2021, in development) and guidance developed by the following entities:
Interstate Technology Regulatory Council (ITRC), 2020: https://ism-2.itrcweb.org/
Hawaii Department of Health, 2016: http://www.hawaiidoh.org/tgm.aspx?p=0402a.aspx
The sample team is encouraged to review CDC/ATSDR’s guidance and the two other documents listed above prior to sample collection for additional background on this sampling approach.
3.5.1 Field Equipment
2-gallon PFAS-free resealable bags to hold the ISM soil samples
Cold Regions Research and Engineering Laboratory (CRREL) multi-increment sampler with a three centimeter probe to collect the ISM soil samples (2 wrenches will be needed to adjust the CRREL tool) (ERDC/CRREL SR-09-1, User's Manual for the CRREL Multi-Increment Sampling Tool (erwiki.net)
Stainless steel wool pads or a parts-cleaning brush with stainless bristles to remove adhered soil from the CRREL sampler between sampling locations
PFAS-free cleaning solution to decontaminate sampling equipment between locations. All water used to decontaminate/clean off sampling equipment should be confirmed to not contain any PFAS prior to use. Laboratory supplied PFAS-free deionized water is preferred. Commercially available deionized water in a high density polyethylene (HDPE) container may be used if verified to be PFAS-free.
Clean PFAS-free paper towels to wipe down the CRREL sampler
Measuring tape to determine increment sampling locations
Global Positioning System (GPS) unit
Pin flags or posts for identifying sampling locations
Field forms to document sampling activities and field conditions
Scale to measure increment samples and bulk ISM samples
COC forms, supplied by the lab
Powderless nitrile gloves
Coolers, supplied by the lab
Sample labels
Large garbage bag for packing the samples for shipment
Ice for shipping
3.5.2 Sampling Point Selection
ISM increments will be collected throughout the identified exposure unit (i.e., the area that people could come into contact with contaminants in soil on a regular basis) at each property1. For some properties, this may include the front, side, and back areas of the properties—if individuals are expected to access all areas of the properties equally. For others, this may encompass a more limited space (e.g., the backyard only if there is no access to the front yard).
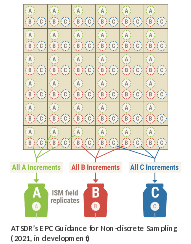 Many
possible sampling designs can be applied for ISM sample collection,
each with the goal of yielding unbiased estimates of average
concentrations. Systematic random grid sampling, a common and
reliable method, will be used for this program. Under this design,
the position of the first increment sample is randomly selected and
the remaining ISM increment collection points are determined by a
sampling grid based off that first point. If field duplicates or
triplicates are collected, the same grid pattern is used, but based
on a new randomly selected starting point.
Many
possible sampling designs can be applied for ISM sample collection,
each with the goal of yielding unbiased estimates of average
concentrations. Systematic random grid sampling, a common and
reliable method, will be used for this program. Under this design,
the position of the first increment sample is randomly selected and
the remaining ISM increment collection points are determined by a
sampling grid based off that first point. If field duplicates or
triplicates are collected, the same grid pattern is used, but based
on a new randomly selected starting point.
An example of this sampling pattern with two field replicates is shown in the figure to the right. For this example, increment samples are collected and combined into three field replicate ISM samples. In this case, a sampler collects equal volume increments at all A locations and then combines those increments into a single ISM sample. This process is repeated for all B and then C sampling locations if field replicates were to be collected. Note that field replicates will only be collected at a subset of properties (see Section 3.5.4).
3.5.3 Analyte and Method Selection
ISM soil samples will be analyzed for targeted PFAS via LC-MS/MS. FTOH analysis (by GC-MS/MS) will be conducted on samples collected in 50 percent of the selected households, and TOF analysis (by combustion ion chromatography) will be conducted on the remaining 50% of the selected households. See Section 4.0 for more detail.
ISM soil samples will be subsampled and processed in the laboratory following standard ISM protocols (HDOH 2016; ISM 2020). This will involve sieving the samples to a particle size of less than two millimeters, and then drying and grinding the samples prior to subsampling (e.g., using a two dimensional Japanese slab cake) and then extracting material for laboratory analysis.
3.5.4 Sample Collection
CDC/ATSDR will collect an ISM soil sample at each property with the goal of obtaining an ISM sample that contains analytes in the same proportion as soil throughout the given exposure unit.
For this program, each ISM sample will be comprised of 80 increments collected in an unbiased manner and at a depth of two centimeters throughout the identified exposure unit for a given property. Each individual soil increment that is collected will weigh between 30 and 40 grams. The target bulk sample mass for each of the resulting combined samples is 3 kilograms (30-40 grams X 80 increments = approximately 3 kilograms). This bulk mass will ensure that there is sufficient sample mass available for analysis after the laboratory sieves the combined samples to less than two millimeters particle size.
At a subset of properties, ISM field replicates will be collected. Field replicates consist of separate ISM samples collected and processed from the same exposure unit. For this program, triplicate ISM field samples will be collected at approximately 20% of participating properties in each community (i.e., three to five properties per community).
A two-person team will collect the ISM samples. One person will collect the increments while the other holds the resealable sample collection bags and keeps track of the number of increments. Sample procedures are as follows.
Check the local weather forecast prior to mobilization. Sample collection should be completed during stable weather conditions to ensure moisture consistency between replicates and increments, as well as uniform soil density.
Outline the boundaries of the exposure unit with field tape and measure the dimensions of the exposure unit with a tape measure or equivalent. If possible, record the location of the corners of the exposure unit (or enough points to delineate the exposure unit’s shape if irregular) with a GPS unit
Estimate the overall area of the exposure unit using the GPS unit or manual measurements.
Estimate the approximate increment spacing using Equation 1 below for 80 increments and the area determined in the previous step.
Equation 1
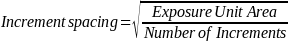
Subdivide the exposure unit into uniform sized cells based on the desired number of increments (i.e., 80 increments) and increment spacing calculated in the previous step. This will create a “sample grid”. Systematic sampling requires that increment sampling locations be evenly spaced between all axes of the “sample grid”, to the extent feasible in the field.
Place pins/flags in the ground to mark the start of each cell/row for easy identification of the 80 increment sample locations within the “sample grid”. Alternative, these markings can be logged in the GPS unit.
Use a systematic random sampling approach to identify the specific increment sample location for the first cell of the “sample grid.” Note that actual increment collection locations reflect a random offset of the grid, with increments collected from an identical (i.e., systematic) location within each cell (HDOH 2016). That means that the initial increment is collected from a random location within the first cell. The location selected within that first cell will be the same location that is sampled in each of the subsequent cells.
Use a coring device (i.e., a CRREL sampler with a three centimeter probe – see image below) to collect increments in each cell of the “sample grid.” Increment samples should be collected at a depth of 2 centimeters and have a mass between 30 and 40 grams. Additional information on how to operate the CRREL sampler is available at: ERDC/CRREL SR-09-1, User's Manual for the CRREL Multi-Increment Sampling Tool (erwiki.net)
 Begin
by collecting a sample from a cell located at one corner of the
“sample grid” and then placing that sample into a
2-gallon resealable bag.
Begin
by collecting a sample from a cell located at one corner of the
“sample grid” and then placing that sample into a
2-gallon resealable bag.
Repeat this process at each of the other 79 cells, following the pre-determined sample path throughout the “sample grid”, and place each subsequent increment sample into the same resealable bag. The combined sample containing all 80 increments represents the bulk ISM sample, which should have a final mass of approximately 3 kilograms. This is the sample that will be sent to the laboratory. Note that large sticks, stones, and other particles can be removed from the ISM bulk sample prior to shipment to the laboratory.
Place the ISM bulk sample inside of another 2-gallon resealable bag to protect the sample during transport to the laboratory.
At a subset of properties (i.e., 20% of properties within a given community), repeat this process throughout the same “sample grid” in order to collect two additional field replicates. For this, shift the increment sample location within each cell in a systematic manner for both replicates.
Sampling equipment can be used repeatedly within an exposure unit/property without decontamination but should be decontaminated between exposure units/properties and also between replicates (at the subset of properties where field replicates are to be collected). Before starting ISM sample collection at a new exposure unit/property or for field replicates, the CRREL sampler (and any other tools used to collect increment samples) should be cleaned of soil particles, decontaminated with a triple rinse of PFAS-free deionized water, and then dried with PFAS-free paper towels.
While CDC/ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to planned sampling methodology. If participants ask the sampling team to stop at any time, the sampling team will stop sampling. Any deviations to the sampling plan will be documented on environmental sample collection forms. Conditions during sample collection, including the weather, any unusual odors, and a brief description of the visual appearance of the sample will also be recorded. For decontamination of the sampler tip between households, the tip will be rinsed with water and a methanol wipe and allowed to dry before reuse.
3.5.5 Sample Labeling and Shipping
A coded label provided by the laboratory that provides the sample ID and project ID will be affixed to each ISM sample container.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location (e.g., locked hotel room, office, or vehicle) and stored at <4°C under low light conditions (i.e., in coolers on wet ice) until they are shipped. Samples will be packed and shipped following the instructions below.
Place a large garbage bag in the interior of the cooler. This bag will be your “outer liner.” No water or ice should be placed outside this bag.
Pour in some wet ice in a single layer to cover the bottom of the outer liner.
Add a second large garbage bag to the cooler so that it fits inside the outer liner. The second bag will be your “inner liner.”
Place bagged ISM soil samples inside the inner liner.
Tie a knot at the top of the inner bag around the sample containers.
Pour ice onto and around the inner liner to fill up any empty spaces on the outside of the inner liner until the cooler is full. The ice should fill up about 30-50% of the content of cooler. Make sure that there are enough loose ends to tie the outer liner in a knot.
Tie a knot at the top of the outer liner in a manner that ensures there will be no leakage.
Place completed COC in a Ziploc bag and place in the cooler on top of the outer liner.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.6 Surface Wipes
3.6.1 Field Equipment
Glass fiber wipes in HDPE jars, both supplied by the lab
1 liter of methanol (MeOH) or other solvent, supplied by the lab
Wooden sampling template (minimum 10cm x 10cm/4in x 4in)
KimWipes
PFAS-free masking tape to tape down template
Trash bags
Ruler or measuring tape
Field forms
COC forms, supplied by the lab
Powderless nitrile gloves
Coolers, supplied by the lab
Ice
3.6.2 Sampling Point Selection
Wipes will be taken at two locations within the home, along with appropriate QC samples. Samples may be taken in areas that may have residue from the use of PFAS-containing products or areas where dust may accumulate, such as kitchen counters or shelves in a high-traffic area that may accumulate dust. The scheduler will have instructed the participant to not dust, vacuum or sweep floors for at least 5 days prior to the scheduled visit.
3.6.3 Analyte and Method Selection
Wipe samples will be analyzed for targeted PFAS via LC-MS/MS. FTOH analysis (by GC-MS/MS) will be conducted on samples collected in 50 percent of the selected households, and TOF analysis (by combustion ion chromatography) will be conducted on samples collected in the remaining 50 percent of the selected households. See Section 4.0 for more detail.
3.6.4 Sample Collection
Field staff will follow the Department of Housing and Urban Development (HUD) protocol for Wipe Sampling of Settled Dust for Lead Determination (https://www.hud.gov/sites/documents/LBPH-40.PDF), adapting the elements specific to collecting samples for PFAS analysis. Procedures extracted from the HUD protocol follow.
ATSDR will collect two samples within each home: one from the kitchen counter to evaluate potential cleaning products and food packaging and one from a closet area within the home to evaluate PFAS on clothing items. Sampling staff may ask permission of the homeowner to temporarily move small items to gain access to more space.
Identify the sample area (the area to be wiped). Do not touch the sample area. Clean the wooden template with a KimWipe. Carefully place the clean template on the sample area and, to keep it from moving while wiping, tape the outside edges to the surface. Minimize disturbance of dust in the sample area.
Don a pair of powderless nitrile gloves
Retrieve a wipe from a HDPE jar, and moisten it with methanol.
Place the moistened wipe at one corner of the area to be sampled with the wipe fully opened and flat on the surface.
Make the first wipe pass, top-to-bottom: With the fingers together, grasp the wipe between the thumb and the palm. Press down firmly, but not excessively with the fingers and, if the wipe is large enough, the palm. Proceed to wipe top-to-bottom with as many “S”-like motions as are necessary to completely cover the entire sample area. Exerting excessive pressure on the wipe will cause it to curl. Exerting too little pressure will result in poor collection of dust. Do not use only the fingertips to hold down the wipe, because there will not be complete contact with the surface and some dust may be missed. Attempt to pick up all dust from the sample area.
Do not cross the template, but be sure to wipe the entire sample area. It is permissible to touch the template with the wipe, but not the surface beyond.
Make the second wipe pass, side-to-side: Fold the wipe in half with the contaminated side facing inward. (You may straighten the wipe by laying it on the sample area, contaminated side up, and folding it over.) Be sure not to spill dust when folding. Once folded, place the wipe in the top corner of the sample area and press down firmly with the fingers (and the palm if the folded wipe is large enough). Repeat wiping the area with “S”-like or “Z”-like motions, but on the second pass, move in a side-to-side direction. Attempt to pick up all dust. Do not touch the contaminated side of the wipe with the hand or fingers. Do not shake the wipe in an attempt to straighten it out, since dust may be lost during shaking.
Make the third wipe pass, top-to-bottom, as was done for the first pass: Fold the wipe into a quarter of the pad with the contaminated side facing inward. (You may straighten the wipe by laying it on the sample area, contaminated side up, and folding it over.) Be sure not to spill dust when folding. Once folded, place the wipe in the top corner of the sample area and press down firmly with the fingers (and the palm if the folded wipe is large enough). Repeat wiping the area with “S”-like or “Z”-like motions, but on the second pass, move in a top-to-bottom direction. Attempt to pick up all dust. Do not touch the contaminated side of the wipe with the hand or fingers. Do not shake the wipe in an attempt to straighten it out, since dust may be lost during shaking.
After collecting as much dust as possible with the wipe, fold the wipe with the contaminated side facing inward again, and insert aseptically (without touching anything else) into the HDPE jar.
Measure or record the area of the wiped area. If the template was used, record the area of the template. If a custom area was used, measure the dimensions of the surface area wiped to the nearest eighth of an inch using a tape measure or a ruler. Record specific measurements for each area wiped on the field sampling form.
After sampling, remove all masking tape and put it in a trash bag. Before removing the last pair of disposable gloves, put all other contaminated gloves and other sampling debris used for the sampling period into a trash bag. Then remove the last pair of gloves and put them in the trash bag. Remove the trash bag when leaving the dwelling. Do not throw away gloves, wipes, etc. inside the dwelling unit where they could be accessible to young children. The plastic bag and gloves may be a suffocation hazard.
Sampling teams will conduct all sampling activities in a manner to minimize potential contamination and cross-contamination of samples. Sampling staff will wear new nitrile gloves at each sampling point to avoid exposure to pollutants and other chemical, physical, and biological hazards, and to prevent cross-contamination of samples. Sampling staff will take care not to touch the insides of bottles or lids.
While CDC/ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to planned sampling methodology. For instance, a larger sampling area may need to be used if there is no visible dust in the sampling area. If this is the case, use PFAS-free masking tape to mark a larger sampling area and be sure to record the sample area to the nearest eighth of an inch using a tape measure or a ruler. Record specific measurements for each area wiped on the field sampling form.
Any deviations to the sampling plan will be documented on the environmental sample collection form and communicated to CDC/ATSDR. Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will also be recorded on the environmental sample collection form.
3.6.5 Sample Labeling and Shipping
A coded label provided by the laboratory that provides the sample ID and project ID will be affixed to each sample container.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location (e.g., locked hotel room, office, or vehicle) and stored at <4°C under low light conditions (i.e., in coolers on wet ice) until they are shipped. Samples will be shipped using the instructions below.
After sampling, insert sample containers into certified pre-cleaned HDPE bottles.
Ensure sample labels are firmly affixed to the HDPE bottles, then tape over the bottles with clear packing tape.
Place the bottles inside a one-gallon PFAS-free resealable bag.
Place a large garbage bag in the interior of the cooler. This bag will be your “outer liner.” No water or ice should be placed outside this bag.
Pour in some wet ice in a single layer to cover the bottom of the outer liner.
Add a second large garbage bag to the cooler so that it fits inside the outer liner. The second bag will be your “inner liner.”
Place bagged samples inside the inner liner.
Tie a knot at the top of the inner bag around the sample containers.
Pour ice onto and around the inner liner to fill up any empty spaces on the outside of the inner liner until the cooler is full. The ice should fill up about 30-50% of the content of cooler. Make sure that there are enough loose ends to tie the outer liner in a knot.
Tie a knot at the top of the outer liner in a manner that ensures there will be no leakage.
Place completed COC in a Ziploc bag and place in the cooler on top of the outer liner.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.7 Silicone Wristbands
3.7.1 Field Equipment
KimWipes
Stainless-steel forceps
Silicone wristbands, pre-cleaned by lab
125 mL HDPE bottles, supplied by the lab
Field forms
COC forms, supplied by the lab
Powderless nitrile gloves
Coolers, supplied by the lab
Ice
3.7.2 Sampling Point Selection
One adult per household will be requested to wear two to three wristbands. This should be the person who signs the consent or their designee (e.g., a spouse).
3.7.3 Analyte and Method Selection
Silicone wristbands will be analyzed for targeted PFAS via LC-MS/MS. FTOH analysis (by GC-MS/MS) will be conducted on samples collected in 50 percent of the selected households, and TOF analysis will be conducted on samples collected in the remaining 50 percent of the selected households. See Section 4.0 for more detail.
3.7.4 Sample Collection
A sample will be collected using silicone wristbands as a passive sampler. One participant per selected household will receive two to three wristbands designated for passively collecting PFAS.
Participants will be instructed to wear wristbands continuously for 7 days, but to remove the wristband during showering, bathing, or swimming.
For drop off at the participant’s home:
Don a pair of nitrile gloves.
Unscrew the wristband jars.
Hand the two wristbands to the participant.
Allow the participant to place the PFAS wristbands on their wrist.
Hand the participant an instruction sheet for wearing the wristbands and talk them through the instructions.
For pick up at the participant’s home:
Don a pair of nitrile gloves.
Obtain the 125mL wristband jars from storage.
Unscrew the wristband jar.
Ask the participant to remove their wristbands.
Take the wristbands from the participant.
Place the wristbands in individual jars and label the jars, specifying the required analysis.
Place the jars on wet ice.
While CDC/ATSDR does not anticipate sampling difficulties, site-specific conditions may result in modifications to planned sampling methodology. For example, some participants’ wrists may be too big or small for the wristbands. In such cases, CDC/ATSDR will consider asking another adult household member to wear the wristbands.
Sampling teams will conduct all sampling activities in a manner to minimize potential contamination and cross-contamination of samples. Sampling staff will take care not to touch the insides of bottles or lids during sampling.
Any deviations to the sampling plan will be documented on the environmental sample collection form. Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will also be recorded on the environmental sample collection form.
3.7.5 Sample Labeling and Shipping
A coded label provided by the laboratory will be affixed to each sample container that provides the sample ID and project ID.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location (e.g., locked hotel room, office, or vehicle) and stored at <4°C under low light conditions (i.e., in coolers on wet ice) until they are shipped. Samples will be shipped using the instructions below.
After sampling, insert sample containers into certified pre-cleaned HDPE bottles.
Ensure sample labels are firmly affixed to the HDPE bottles, then tape over the bottles with clear packing tape.
Place the bottles inside a one-gallon PFAS-free resealable bag.
Place a large garbage bag in the interior of the cooler. This bag will be your “outer liner.” No water or ice should be placed outside this bag.
Pour in some wet ice in a single layer to cover the bottom of the outer liner.
Add a second large garbage bag to the cooler so that it fits inside the outer liner. The second bag will be your “inner liner.”
Place bagged samples inside the inner liner.
Tie a knot at the top of the inner bag around the sample containers.
Pour ice onto and around the inner liner to fill up any empty spaces on the outside of the inner liner until the cooler is full. The ice should fill up about 30-50% of the content of cooler. Make sure that there are enough loose ends to tie the outer liner in a knot.
Tie a knot at the top of the outer liner in a manner that ensures there will be no leakage.
Place completed COC in a Ziploc bag and place in the cooler on top of the outer liner.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.8 Produce
3.8.1 Field Equipment
KimWipes
Stainless-steel knife
250 mL HDPE wide-mouth bottles, supplied by the lab
Stable, PFAS-free surface for chopping produce
Paper towels
Deionized water
Field forms
COC forms, supplied by the lab
Powderless nitrile gloves
Coolers, supplied by the lab
Ice
3.8.2 Sample Selection Point
Produce samples will be collected from markets throughout the EA sampling frame. Venues selling locally grown produce will be identified before the sampling campaign begins. Local contacts and various organizations can help to identify local food and farm resources (e.g., https://www.localfoodma.org/). The sampling team will identify “local” produce based on labeling in the markets or knowledge of suppliers to community-based farmers’ markets. To the extent possible, sampling will target produce grown in the community itself. If not available, the sampling team will seek produce grown (per labels) within the state/region. Ideally, samples not pre-packaged should be selected.
3.8.3 Analyte and Method Selection
Produce samples will be tested for targeted PFAS by LC-MS/MS. The laboratory will process (homogenize) the as received sample. See Section 4.0 for more information.
3.8.4 Sample Collection
Available data suggest that PFAS accumulation varies by plant part. The shorter chain length PFAS tend to accumulate in the shoots (leaves and fruits), whereas the longer chain length PFAS tend to accumulate in the roots (Blaine et al. 2014; Felizeter et al. 2012; Ghisi et al. 2019; Navarro et al. 2017). Uptake also appears to vary depending on vegetative structure (e.g. presence/absence of barriers) (Blaine et al. 2014; Liu et al. 2019) and protein content (Wen et al. 2016). In some cases, higher concentrations are found in the leaves/foliage than the edible parts of plants (e.g., potatoes, carrots, and cucumbers) (Lechner and Knapp 2011); higher concentrations have been reported in the lettuce heart versus the leaves (Bizkarguenaga et al. 2016). Acknowledging this expected variance, the EI sampling goal will be to collect a cross-section of edible portions of leafy greens, root/shoot vegetables, and fruit vegetables. The following produce serves as a guide for sampling based on several considerations, including documented PFAS accumulation in the edible portion of the plant and likelihood of being grown locally. The methods and analysis of processed foods from FDA’s total diet study will be considered a reference for the produce collection and analysis (Genualdi et al. 2021).
Leafy Greens |
Root/Shoot Vegetables |
”Fruit” |
1. Lettuce 2. Cabbage 3. Spinach 4. Other |
1. Celery 2. Carrots 3. Radishes 4. Potatoes |
1. Tomatoes 2. Melons 3. Pears or apples 4. Corn |
In each community, the sampling team will collect 21 samples—ideally, three samples of seven different types of produce. The seven types of produce may vary depending on sampling location and season. However, an effort should be made to select a variety of produce types and within each produce type, the specific produce that is expected to have the highest PFAS levels, broadly ranked in the list above.
The following sampling procedure is adapted from the MDH soil and garden study (Scher et al. 2018).
At each market, don a pair of nitrile gloves.
Obtain produce sample and place in paper or thin-film plastic bag provided in produce aisle of market.
Take notes on any relevant details (e.g., where grown, organic/not organic)
Change out gloves between each produce sample collected.
After all produce samples have been collected, purchase produce.
At vehicle, set up a surface that can be used for cutting / preparing samples.
For sample preparation, follow these procedures:
At vehicle or other designated team meeting location, set up a surface that can be used for cutting / preparing samples.
Use a KimWipe to clean the stainless-steel knife and the surface used for cutting / preparing samples.
Remove first produce sample from paper or thin-film plastic bag.
Remove any non-edible portions of the produce (e.g., stems, leaves). Take notes on what parts of the produce are removed.
If the produce has any visible soil on it, wipe away the soil with a paper towel and with deionized water if necessary.
Chop the produce sample into 1 in3 or smaller pieces.
Place each chopped produce sample into pre-labeled jar.
Change out gloves between each sample preparation.
Use a KimWipe to clean the knife and surface in between each sample preparation.
Place all chopped produce samples on ice.
Any deviations to the sampling plan will be documented on the environmental sample collection form and communicated to CDC/ATSDR. Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will also be recorded on the environmental sample collection form.
3.8.5 Sample Labeling and Shipping
A coded label provided by the laboratory will be affixed to each sample container that provides the sample ID and project ID.
All samples will be packed according to the following guidelines and then shipped priority overnight via FedEx. The sampling team anticipates shipping samples once per week, the specific days on which samples are shipped will be determined by the sampling schedule. Any samples that are not shipped the same day they are collected will be held by the sampling team in a secure location (e.g., locked hotel room, office, or vehicle) and stored at <4°C under low light conditions (i.e., in coolers on wet ice) until they are shipped. Samples will be shipped using the instructions below.
After sampling, insert sample containers into pre-cleaned HDPE bottles.
Ensure sample labels are firmly affixed to the HDPE bottles, then tape over the bottles with clear packing tape.
Place the bottles inside a one-gallon PFAS-free resealable bag.
Place a large garbage bag in the interior of the cooler. This bag will be your “outer liner.” No water or ice should be placed outside this bag.
Pour in some wet ice in a single layer to cover the bottom of the outer liner.
Add a second large garbage bag to the cooler so that it fits inside the outer liner. The second bag will be your “inner liner.”
Place bagged samples inside the inner liner.
Tie a knot at the top of the inner bag around the sample containers.
Pour ice onto and around the inner liner to fill up any empty spaces on the outside of the inner liner until the cooler is full. The ice should fill up about 30-50% of the content of cooler. Make sure that there are enough loose ends to tie the outer liner in a knot.
Tie a knot at the top of the outer liner in a manner that ensures there will be no leakage.
Place completed COC in a Ziploc bag and place in the cooler on top of the outer liner.
Ensure contents will not move too much when cooler is closed.
Secure shut the cooler with packing tape before you ship it out.
3.9 PFAS Cross Contamination Prevention Best Practices
Sampling teams will conduct all sampling activities in a manner to minimize potential contamination and cross-contamination of samples. Sampling staff will wear new nitrile gloves at each sampling point to avoid exposure to pollutants and other chemical, physical, and biological hazards, and to prevent cross-contamination of samples. Sampling staff will take care not to touch sampler “cartridges” or the insides of storage bags or sample bottles/jars.
Due to the potential presence of PFAS in common consumer products and in equipment typically used to collect water and dust samples, special handling and care must be taken when collecting samples for PFAS analysis to avoid sample contamination. Below is a summary of items that are likely to contain PFAS and therefore will not be used by the personnel conducting sampling. Where appropriate, acceptable substitutions are provided in italics.
Decontamination: Decon 90 (instead use Alconox® or Liquinox®, potable water followed by deionized PFAS-free water rinse)
Sample storage and preservation: LDPE or glass bottles, Teflon™-lined caps, chemical ice packs (i.e., Blue ice®) (instead use HDPE or polypropylene containers with HDPE or polypropylene caps, regular ice in Ziploc bags)
Field documentation: waterproof/treated paper or field books, plastic clipboards, Sharpie® markers, “sticky” notes and other adhesive paper products (instead use loose plain paper, metal clipboard, ballpoint pens)
Clothing: clothing or boots with Gore-Tex® or other synthetic water-resistant and/or stain-resistant materials, Tyvek material, fabric softener (old, well laundered (at least 6 washings since purchase) clothing made of cotton preferred)
Personal care products on day of sample collection: cosmetics, moisturizers, hand cream, sunscreen, and other related products
Aluminum foil
Anything with fluoro in the name
Fluorinated ethylene propylene (FEP)
Ethylene tetrafluoroethylene (ETFE)
Low density polyethylene (LDPE), polyvinylidene fluoride (PVDF)
Food and beverage: pre-packaged food, fast food wrappers or containers
To further prevent contamination, hands must be thoroughly washed after handling fast food, carryout food, or snacks, as many food and snack products are packaged in wrappers treated with PFAS. Pre-wrapped food or snacks must not be in the possession of the sampling personnel during sampling. When field personnel require a break to eat or drink, they should remove their gloves and move to an appropriate location. When finished, field personnel should clean up and don a new pair of gloves. No food or drink shall be brought into sample collection or work areas.
3.10 Field Documentation
Field documentation will include CDC/ATSDR’s Environmental Sample Collection Form (Appendix D.1) and COC records. Field notes will include:
Sample locations and description;
General conditions of the sampling point;
Date and time of sample collection;
Field observations and details related to analysis or integrity of samples (e.g., condition in homes, noticeable odors or coloring of samples, etc.); and
Notes regarding other relevant information collected on-site pertaining to the sample location.
3.11 Sample Handling and Custody
To maintain scientifically defensible sampling data, sample integrity must be maintained at all times from sample collection through analysis. Correct and detailed sample labeling will be used and appropriate COC forms will be maintained. In order to stay within appropriate holding times and ensure safe delivery, the samples that are collected will be shipped using FedEx Priority Overnight to ensure they arrive at the analytical laboratories the morning after being shipped. Coolers and ice will be used to transport the samples and maintain holding temperatures. The COC will be correctly signed during the transfer of these samples and at the time of delivery to the laboratory.
To maintain a record of sample collection, transfer between personnel, shipment, and receipt by the laboratory, a COC is completed for each sample set at each sampling location. These forms are used to document sample custody transfer from the field to the laboratory. The COC forms are completed for all samples sent to the laboratory.
In addition to the transfer of custody, the COC provides information to the laboratory on the sample number, type of sample, sample description, the sample collection date and time, and the analyses requested. The comment section is used to provide special notes or instructions to the laboratory. Additionally, deviations from standard sampling protocols are to be noted in the comments section.
3.12 Inspection of Supplies and Consumables
Sampling supplies and consumables for this project include those that contact samples, such as laboratory-provided sample containers and sampling equipment. The sampling staff will be responsible for acquiring required materials from the contract laboratory and specified vendors and inspecting those materials upon receipt to ensure they are usable for this project (e.g., inspecting for breakage, evidence of tampering, or contamination). The sampling team will use new sampling equipment materials, such as nitrile gloves, which will be dedicated to a single sampling point and then disposed.
4.0 Analytes and Method Selection
Eurofins (Test America) is the ATSDR-contracted laboratory that will complete all the PFAS analysis in the environmental samples. Eurofins uses state-of-the-art LC/MS/MS instrumentation in support of trace-level reporting of PFAS contaminants as well as GC/MS/MS for the analysis of the more volatile PFAS.
Samples of bulk dust, soil and silicone wristbands remaining after laboratory analysis will be send to EPA at Research Triangle Park for storage and potential future non-targeted analysis of PFAS.
All the methods to be used are based on Eurofins in-house SOPs. The primary method used for the analysis of PFAS is referred to as 537 Modified. The SOPs for the analysis of TOF and FTOHs are considered proprietary but have been reviewed by the EI team.
Below is a summary of the methods that Eurofins has developed and validated to capture the variety of matrices and target analytes to be used in the EI.
D.1
Matrix |
Target Analytes/Method |
SOP Name |
Soil, Bulk Dust, Filtered Dust, Surface Wipes and Wristbands |
Extraction and Analytical Method for Targeted list of 40 semivolatile PFAS |
WS-LC-0025 r4.0 |
Soil, Bulk Dust, Filtered Dust, Surface Wipes and Wristbands |
Analytical Method for FTOHs |
TSSGWI7750 r5 |
Soil, Bulk Dust, Filtered Dust, Surface Wipes and Wristbands |
Extraction Method for FTOHs |
TSSGWI41726 r1 |
Soil, Bulk Dust, Filtered Dust, Surface Wipes and Wristbands |
Extraction and Analytical Method for Extractable Organic Fluorine (EOF) |
T-PFAS-WI43681 rA |
Bulk Dust |
Sample Prep and Analytical Method for TOP Assay |
WS-LC-0025 r4.0 |
Soil |
Incremental Sample Management (ISM) Sample Prep Method |
WS-QA-0028 r4.8 |
Produce |
Analytical Method for Targeted list of 40 semivolatile PFAS |
TPFASWI12031 r10 |
Produce |
Extraction Method via FDA C-010.01 prescribed QuEChERS method |
T-PFAS-WI36393 |
Indoor/Outdoor Air |
Analytical Method for Targeted list of 30 semivolatile PFAS |
WS-LC-0025 r4.0 |
Indoor/Outdoor Air |
Extraction Method for Targeted list of 30 semivolatile PFAS from PUF/XAD cartridges |
WS-WI-0065 r1.1 |
Indoor/Outdoor Air |
Extraction and Analytical Method for FTOHs |
Modified EPA TO-17 |
Soil, Produce, Bulk Dust, Surface Wipes and Wristbands
Targeted PFAS will be extracted and analyzed according to the in-house SOP method referred to as 537 Modified with isotope dilution.
For produce, PFAS will be extracted using methods consistent with FDA methodology, with isotope dilution.
Incremental Sample Management (ISM) preparation will be applied to soil samples only and will be completed using Eurofins standard procedures.
Approximately 1 Kg of sample is received
Disaggregate, spread on sheet to air dry
Sieve through 2 mm sieve
Spread on sheet into 2-D slab cake
Grid into approximately 30 units
Using flat ended spoon, take approximately equal subsamples from each
grid division
Subsamples combined into one aliquot for each analysis quoted.
FTOHs will be extracted and analyzed according to the in-house SOP method referred to as FTOHs by GCMSMS.
The TOP Assay is a pre-treatment technique which will be applied to the Bulk Indoor Dust only. The pre-treatment is an oxidation step conducted prior to analysis of a split sample. The analysis is conducted using 537 Modified.
Organic Fluorine will be reported as Extractable Organic Fluorine or EOF for all solid materials. Samples will be extracted using the same solvent system as 537 Modified and analyzed by the in-house method referred to as TOF-CIC. for non-potable water and other environmental media, which will eventually be published as standard methods.2
Indoor Dust (filter)
Targeted PFAS will be extracted and analyzed from a filter cassette according to the in-house SOP method referred to as 537 Modified.
FTOHs will be extracted and analyzed from a filter cassette according to the inhouse SOP method referred to as FTOHs by GCMSMS.
Organic Fluorine will be reported as Extractable Organic Fluorine or EOF. Samples will be extracted from a filter cassette using the same solvent system as 537 Modified and analyzed by the in-house method referred to as TOF-CIC.
Indoor and Outdoor Air
Targeted PFAS will be extracted from a PUF/XAD cartridge by an in-house SOP method using KOH/MeOH extraction and sonication. The analysis is completed by 537 Modified which is identical to the water/solids method.
FTOHs will be extracted from a thermal desorption sorbent tube and analyzed according to the in-house SOP method referred to as FTOHs by TD-GCMSMS.
Validation and method testing will be performed prior to deploying to the field to determine the most appropriate length of time for data collection to achieve appropriate MDLs.
Appendix D.2 provides a list of analyses to be performed in each medium along with appropriate method detection limits (MDLs).
4.1 Quality Control Samples
The primary field quality control method for sampling will be the use of field equipment blanks, duplicate samples, and field controls. Table D.1 presents the proposed number of samples, duplicates, field blanks, and field control samples to be collected as part of this EI (MA and DE locations combined).
Table D.1. Proposed sample and quality control sample counts
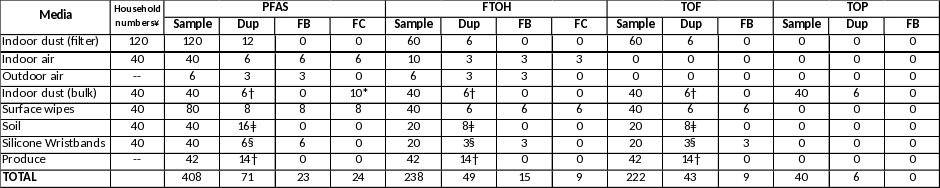
¥ A total of 80 homes will be sampled in MA and 40 in DE. 20 homes at each location will be sampled for filtered dust and all other media
Dup = Duplicate; FB = Field Blank; FC = Field Control
*Lab will purchase 10g of NIST SRM 2585 to prepare 10 field controls for PFAS in the bulk dust sample
** two wipe samples will be taken at each of the 20 homes at each location
† split sample and not a duplicate for bulk dust and produce samples
ǂ triplicate analysis for incremental sampling protocol
§ if only a portion of the wristband is used for the analyses, the laboratory will extract a second portion of the wristband as a duplicate sample
4.2 Field and Equipment Blanks
Field equipment blanks will be used to evaluate possible contamination arising from equipment or containers used during air, filtered dust, surface wipes, and wristband sampling. One unused filter cassette will be connected to the tubing, disconnected, and sent to the contracted lab at each of the EI locations (MA and DE). This will be to ensure the filter cassettes used to collect the samples are PFAS-free. Similarly, an unused cartridge/sorbent tube use for air sampling will be connected to tubing, disconnected, and sent to the contracted lab for analysis at each of the EI locations (MA and DE). For surface wipes, a wipe will be removed from its jar, moistened with methanol, place back in its jar, and sent to the contracted lab for analysis. For wristbands, a wristband will be removed from its jar briefly, placed back in its jar, and sent to the contracted lab for analysis.
4.3 Duplicate and Split Samples
For filtered dust and surface wipes, duplicate samples will be collected in an immediately adjacent area under identical conditions into separate sample containers. It is uncertain whether truly homogenous samples can be collected. For bulk dust, 15% of samples will be split at the lab after the homogenization process. Each split sample will be evaluated independently. For indoor air, at 15% of households, a co-located cartridge and pump will be set up next to the original sample. For outdoor at half of all sampling locations, a co-located cartridge and pump will be set up next to the original sample. For soil, triplicate samples will be collected at 40% of homes. As each original ISM sample is collected within the same location of each increment grid, so will the replicate samples. For wristbands, 15% of samples will be selected for split sampling at the lab. Each split sample will be evaluated independently. Finally, for produce, one third of samples will be split at the lab after the homogenization process. Each split sample will be evaluated independently.
4.4 Field Controls
The laboratory will prepare matrix spikes for the wipe and air media as an additional measure of data quality assurance and control.
5.0 Sampling Logistics
This section of the SAP details specifics of the sampling team, site visit preparation, and contact information
5.1 Field Sampling Schedule
The general anticipated workflow for the 2-week data collection period will be some combination of the following and will be dictated in part by participant availabilities. The goal will be to conduct the more extensive sampling and required set ups in the subset of 20 households per location in the early parts of Week 1 with pickup of samples early in Week 2. The numbers in Table D.2 below reflect the total homes tested per day (2 teams unless noted).
Table D.2 Anticipated Data Collection Schedule
Week 1 * |
||||||
Sunday |
Monday |
Tuesday |
Wednesday |
Thursday |
Friday |
Saturday |
6 (robust 1†) |
6 (robust 1†) |
6 (robust 1†) |
2 (robust 1†) |
10 (dust only §– DE and MA) |
10 (dust only §– DE and MA) |
Produce and take down outdoor air |
WEEK 2 |
||||||
6 (robust 2ǂ) |
6 (robust 2ǂ) |
6 (robust 2ǂ) |
6 (robust 2ǂ) |
15 (dust only §– MA only) |
15 (dust only §– MA only) |
10 (dust only – MA only) |
|
|
|
|
|
|
|
* Set up outdoor air sampling the Saturday before sampling begins
† Robust 1 = questionnaire, set indoor air, wipes, soil and wristbands
ǂ Robust 2 – take down indoor air, collect wristbands, indoor dust (filter), bulk dust
§ Dust only = questionnaire, indoor dust (filter) only – 20 for DE (2 teams) and 60 for MA (3 teams)
5.2 Project Contacts
This section summarizes contacts and addresses for personnel involved in the assessment.
Karen Scruton
770-488-1325
Peter Kowalski
770-488-0776
James Durant
770-488-0668
Elaine Cohen Hubal
Kent Thomas
Laboratory Contacts
Taryn McBride, Eurofins (TestAmerica)
Air Sampling Contractor Contacts
Naida Gavrelis, Eastern Research Group (ERG)
6.0 References
Bizkarguenaga E. Zabeleta I, Mijangos L, Iparraguirre A, Ferdandez LA, Prieto A, Zuloaga O. 2016. Uptake of perfluorooctanoic acid, perfluorooctane sulfonate and perfluorooctane sulfonamide by carrot and lettuce from compost amended soil. Science of the Total Environment. 571: 444-451.
Blaine AC, Rich CD, Sedlacko EM, Hundal LS, Kumar K, Lau C, Mills MA, Harris KM, Higgins CP. 2014. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environmental Science and Technology. 48(14): 7858-7865.
EPA [U.S. Environmental Protection Agency]. 2017. Field Collection Standard Operating Procedures (SOPs) for an EPA Pilot Study Evaluating Personal, Housing, and Community Factors Influencing Children’s Potential Exposures to Indoor Contaminants at Various Lifestages (EPA Pilot Study Add-Oon to the Green Housing Study). EPA/600/R-17/064. March 2017. Available at: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=335764&Lab=NERL
EPA. 1999. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Air Using Gas Chromatography/Mass Spectrometry (GC/MS); EPA Compendium Method TO-13A. In Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air (Second Edition). EPA/625/R-96/010b. . Office of Research and Development. Cincinnati, OH. Available at: https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-13arr.pdf
Felizeter S, McLachlan MS, deVoogt P. 2012. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa). Environmental Science and Technology. 46(21): 11735-11743
Genualdi, Susan & Beekman, Jessica & Carlos, Katherine & Fisher, Christine & Young, Wendy & Dejager, Lowri & Begley, Timothy. (2021). Analysis of per- and poly-fluoroalkyl substances (PFAS) in processed foods from FDA’s Total Diet Study. Analytical and Bioanalytical Chemistry. 414. 10.1007/s00216-021-03610-2.
Ghisi R, Vamerali T, Manzetti S. 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ Res. 169: 326-341
Hawaii Department of Health. 2016. Use of Multi Incremental Samples to Characterize DU’s. http://www.hawaiidoh.org/tgm.aspx?p=0402a.aspx
HUD. n.d. Wipe Sample of Settled Dust for Lead Determination. https://www.hud.gov/sites/documents/LBPH-40.PDF
Interstate Technology Regulatory Council [ITRC]. 2018. Site Characterization Considerations, Sampling Precautions, and Laboratory Analytical Methods for Per- and Polyfluoroalkyl Substances (PFAS).
Interstate Technology Regulatory Council [ITRC]. 2020. Incremental Sampling Methodology Update. https://ism-2.itrcweb.org/
Lechner M, Knapp H. 2011. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. Sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis Sativus). Journal of Agricultural and Food Chemistry. 59(20): 11011-11018.
Levasseur JL, Hammel SC, Hoffman K, Phillips AL, Zhang S, Ye X, Calafat AC, Webster TF, Stapleton HM. 2021. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environment International (147). https://doi.org/10.1016/j.envint.2020.106317
Liu Z, Lu Y, Song X, Jones K, Sweetman AJ. Johnson AC, Zhang M, Lu X, Su C. 2019. Multiple crop bioaccumulation and human exposure of perfluoroalkyl substances around a mega fluorochemical industrial park, China: Implication for planting optimization and food safety. Environ Int. 127: 671-684
Minnesota Department of Health [MDH]. 2010. House Dust Field Sampling Protocol for Perfluorochemical (PFC) Analysis.
National Children’s Study. n.d. Environmental Vacuum Bag Dust Technician Collect SOP V1.0.
National Institute of Environmental Health Sciences. 2016. Perfluorinated Chemicals (PFCs). National Institutes of Health, Research Triangle Park, NC.
Navarro I, de la Torre A, Sanz P, Porcel MÁ, Pro J, Carbonell G, de los Angeles Martínez M. 2017. Uptake of perfluoroalkyl substances and halogenated flame retardants by crop plants grown in biosolids-amended soils. Environmental Research. 152: 199-206.
Roth J, Abusallout I, Hill T, Holton C, Thapa U, Hanigan D. 2020. Release of volatile per- and polyfluoroalkyl substances from aqueous film-forming foam. Environ. Sci. Technol. Lett. 7:164−170. https://pubs.acs.org/doi/suppl/10.1021/acs.estlett.0c00052/suppl_file/ez0c00052_si_001.pdf
Scher, D.P., Kelly, J.E., Huset, C.A., Barry, K.M., Hoffbeck, R.W., Yingling, V.L., and Messing, R.B. (2018) Occurrence of perfluoroalkyl substances (PFAS) in garden produce at homes with a history of PFAS-contaminated drinking water. Chemosphere. 196: 548-555
U.S. Environmental Protection Agency [EPA]. 2017. Field Collection Standard Operating Procedures (SOPs) for an EPA Pilot Study Evaluating Personal, Housing, and Community Factors Influencing Children’s Potential Exposures to Indoor Contaminants at Various Lifestages (EPA Pilot Study Add-On to the Green Housing Study). [SOP for Collection of Indoor Air Samples using Active Samplers]. Office of Research and Development. https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=335764&Lab=NERL
Wen, B., Wu, Y., Zhang, H., Liu, Y., Hu, X., Huang, H., and Zhang, S. (2016) The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environmental Pollution. 216: 682-688
Appendix D.1
PFAS Data Collection Forms
Forms part of the OMB package (Attachment 18 and 19)
Appendix D.2 Analyte List and Method Detection Limits (MDLs)
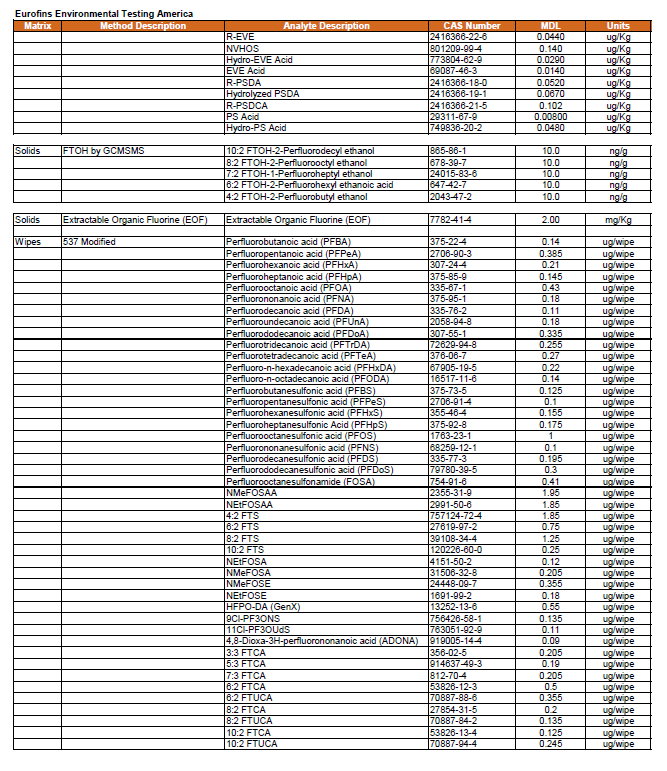
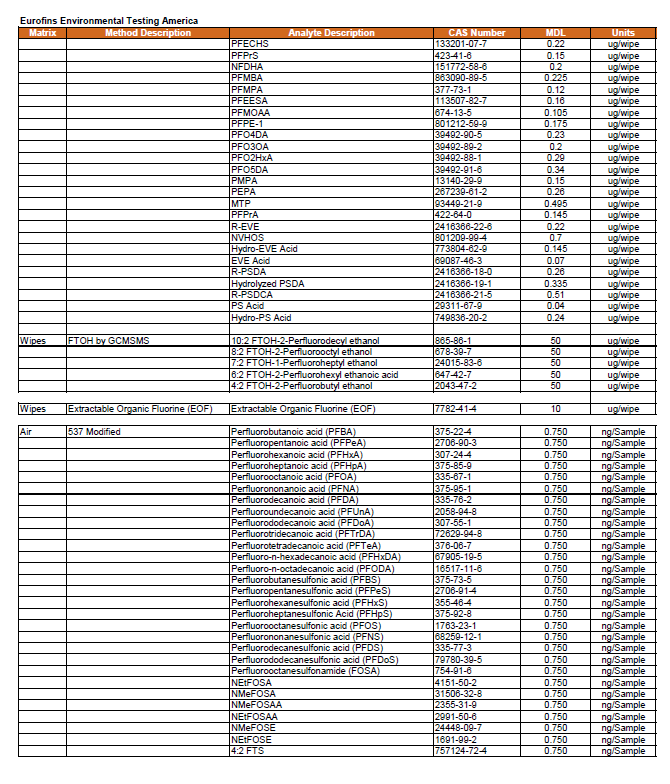
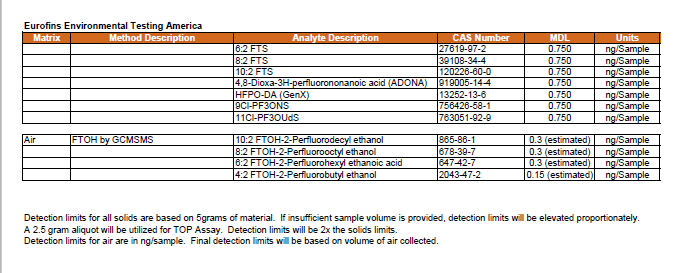
1 ISM samples are designed to provide representative contaminant concentrations over a specific volume of soil. Several agencies use the term “decision unit” to refer to the smallest volume of soil for which a decision will be made with ISM data. The boundaries of decision units can be based on human health exposure areas, ecological exposure areas, source areas, and more. An ISM decision unit may not always align with the exposure unit. For this program, ISM samples will be collected within identified exposure units (i.e., geographically defined areas where a person contacts an environmental medium at random throughout the area over time).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2022-03-18 |
© 2026 OMB.report | Privacy Policy