Supporting Statement Part B jb
Supporting Statement Part B jb.docx
Online Submission Form for Supplemental Evidence and Data for Systematic Reviews for the Evidence-based Practice Center Program
OMB: 0935-0231
SUPPORTING STATEMENT
Part B
Online Submission Form for Supplemental Evidence and Data for Systematic Reviews for the Evidence-based Practice Center Program
Version: December 21, 2018
Agency of Healthcare Research and Quality (AHRQ)
Table of Contents
B. Collections of Information Employing Statistical Methods 3
B1. The Potential Respondent Universe and any Sampling or other Respondent Selection Methods to be Used……………………………………………………………………….. 3
B2. The Procedures for the Collection of Information 3
B3. Methods to Maximize Response Rates and to Deal with Issues of Non-response…… 3
B4. Tests of Procedures or Methods to be Undertaken…………………………………… 3
Appendixes
Appendix A. Website Portal for Submission of Supplemental Evidence and Data for Systematic
Reviews
Appendix B. Opportunity to Submit Scientific Information E-mail
Appendix C. Federal Register Notice
B. Collections of Information Employing Statistical Methods
B1. The Potential Respondent Universe and any Sampling or other Respondent Selection Methods to be Used
AHRQ contacts industry stakeholders such as investigators, pharmaceutical and device manufacturers, app developers, and other non-governmental institutions and professional associations through the Effective Health Care Program (EHC) listserv for the purposes of supplementing evidence and data (SEADS) collected from published and grey literature searches. 95,834 people are included on this listserv. In some cases, we also post a note about the opportunity to submit SEADS in the Federal Register notice.
B2. The Procedures for the Collection of Information
The online submission form (OSF; see Attachment A) is set up to retrieve this information without creating a heavy burden on the responder. However, other than the OSF, stakeholders are given other ways to respond to the request: email, postal or package services.
The OSF was developed to provide stakeholders with flexibility in how they respond to the request. At a minimum, respondents are requested to input their name along with the information packet.
Each request is a single unique event reflecting the topic chosen by AHRQ and its partners that is not repeated unless it is deemed ready for an update at an undetermined future date.
B3. Methods to Maximize Response Rates and to Deal with Issues of Non-response
The information collection process is initiated with an email notification of the opportunity to submit SEADS via the EHC listserv (see Attachment B). In cases where AHRQ determines that a more general notice to the public is necessary, such as when there are not specific manufacturers of products under review, AHRQ may issue a Federal Register notice (see Attachment C) of the opportunity to submit SEADS.
B4. Tests of Procedures or Methods to be Undertaken
This approach has been used by AHRQ for several years. Tests have been done to ensure that the OSF or user interface does not cause any confusion.
Attachment A -- Website Portal for Submission of Supplemental Evidence and Data for Systematic Reviews
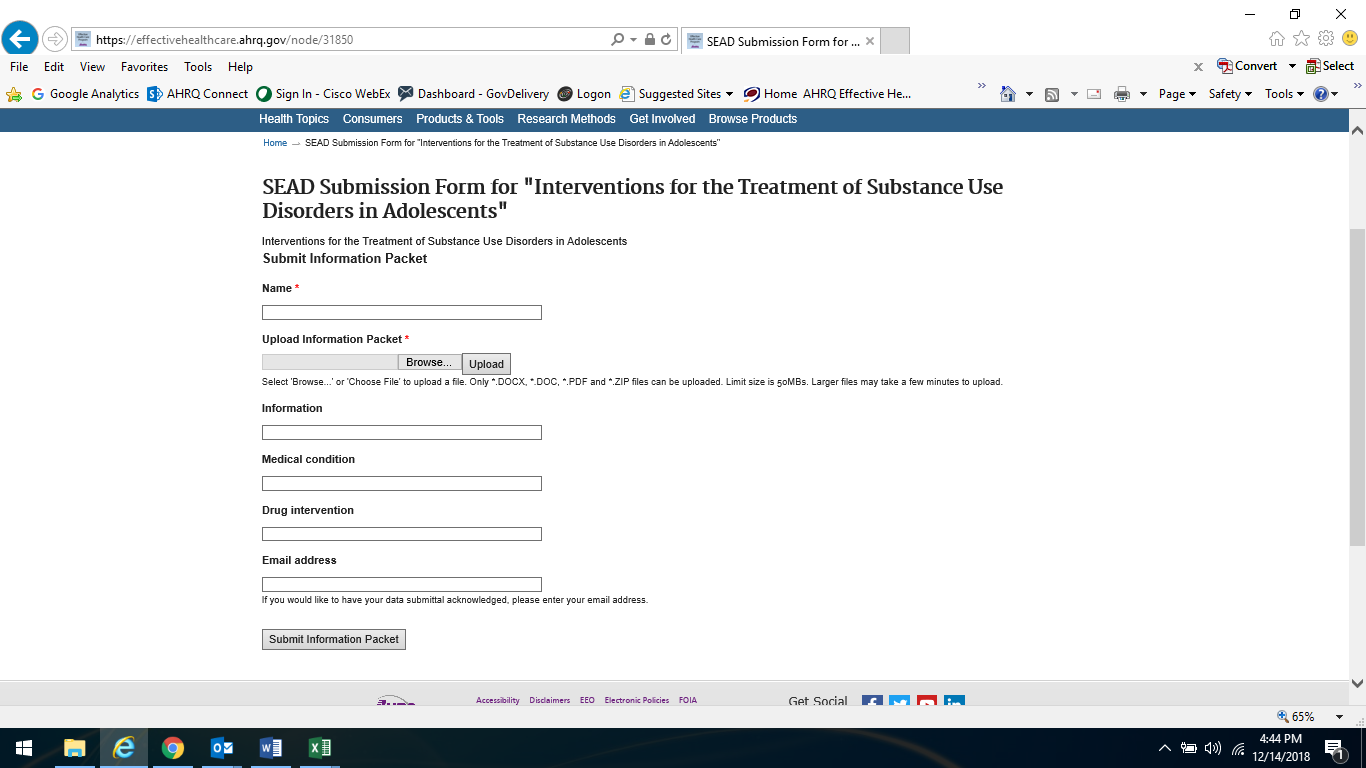
Attachment B -- Opportunity to Submit Scientific Information E-mail
From:
Agency for Healthcare Research and Quality (AHRQ)
<[email protected]>
Sent:
Thursday, December 13, 2018 5:23 PM
Subject:
EHC Program Update: SEADs Request for Treatment of Substance Use in
Adolescents
|
Opportunity to Submit Scientific InformationThe Effective Health Care (EHC) Program is interested in receiving supplemental evidence and data (SEADs) for systematic reviews that are relevant to the questions in our evidence reports. To ensure that it has full access to relevant research, whether or not it is published, the EHC Program is interested in receiving SEADs containing detailed study-specific information. Opportunities to submit scientific information are available for: Interventions
for the Treatment of Substance Use Disorders in Adolescents
About us: AHRQ’s Effective Health Care Program is committed to providing the best available evidence on the outcomes, benefits and harms, and appropriateness of drugs, devices, and health care services and by helping health care professionals, patients, policymakers, and health care systems make informed health care decisions. The program partners with research centers, academic institutions, health professional societies, consumer organizations, and other stakeholders to conduct research, evidence synthesis, evidence translation, dissemination, and implementation of research findings. To learn more: https://www.effectivehealthcare.ahrq.gov Contact us at: [email protected] |
Attachment C -- Federal Register Notice
Billing Code: 4160-90-M
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Agency for Healthcare Research and Quality
Supplemental Evidence and Data Request on Interventions for Substance Use Disorders in Adolescents: A Systematic Review
AGENCY: Agency for Healthcare Research and Quality (AHRQ), HHS.
ACTION: Request for Supplemental Evidence and Data Submissions
SUMMARY: The Agency for Healthcare Research and Quality (AHRQ) is seeking scientific information submissions from the public. Scientific information is being solicited to inform our review of Interventions for Substance Use Disorders in Adolescents: A Systematic Review, which is currently being conducted by the AHRQ’s Evidence-based Practice Centers (EPC) Program. Access to published and unpublished pertinent scientific information will improve the quality of this review.
DATES: Submission Deadline on or before [INSERT DATE 30 DAYS AFTER DATE OF PUBLICATION IN THE FEDERAL REGISTER].
ADDRESSES:
E-mail submissions: [email protected].
Print submissions:
Mailing Address:
Center for Evidence and Practice Improvement
Agency for Healthcare Research and Quality
ATTN: EPC SEADs Coordinator
5600 Fishers Lane
Mail Stop 06E53A
Rockville, MD 20857
Shipping Address (FedEx, UPS, etc.):
Center for Evidence and Practice Improvement
Agency for Healthcare Research and Quality
ATTN: EPC SEADs Coordinator
5600 Fishers Lane
Mail Stop 06E77D
Rockville, MD 20857
FOR FURTHER INFORMATION CONTACT:
Jenae Benns, Telephone: 301-427-1496 or Email: [email protected].
SUPPLEMENTARY INFORMATION:
The Agency for Healthcare Research and Quality has commissioned the Evidence-based Practice Centers (EPC) Program to complete a review of the evidence for Interventions for Substance Use Disorders in Adolescents: A Systematic Review. AHRQ is conducting this systematic review pursuant to Section 902(a) of the Public Health Service Act, 42 U.S.C. 299a(a).
The EPC Program is dedicated to identifying as many studies as possible that are relevant to the questions for each of its reviews. In order to do so, we are supplementing the usual manual and electronic database searches of the literature by requesting information from the public (e.g., details of studies conducted). We are looking for studies that report on Interventions for Substance Use Disorders in Adolescents: A Systematic Review, including those that describe adverse events. The entire research protocol, including the key questions, is also available online at: https://effectivehealthcare.ahrq.gov/topics/substance-use-disorders-adolescents/protocol.
This is to notify the public that the EPC Program would find the following information on Interventions for Substance Use Disorders in Adolescents: A Systematic Review helpful:
A list of completed studies that your organization has sponsored for this indication. In the list, please indicate whether results are available on ClinicalTrials.gov along with the ClinicalTrials.gov trial number.
For completed studies that do not have results on ClinicalTrials.gov, a summary, including the following elements: study number, study period, design, methodology, indication and diagnosis, proper use instructions, inclusion and exclusion criteria, primary and secondary outcomes, baseline characteristics, number of patients screened /eligible /enrolled /lost to follow-up /withdrawn /analyzed, effectiveness/efficacy, and safety results.
A list of ongoing studies that your organization has sponsored for this indication. In the list, please provide the ClinicalTrials.gov trial number or, if the trial is not registered, the protocol for the study including a study number, the study period, design, methodology, indication and diagnosis, proper use instructions, inclusion and exclusion criteria, and primary and secondary outcomes.
Description of whether the above studies constitute ALL Phase II and above clinical trials sponsored by your organization for this indication and an index outlining the relevant information in each submitted file.
Your contribution is very beneficial to the Program. Materials submitted must be publicly available or able to be made public. Materials that are considered confidential; marketing materials; study types not included in the review; or information on indications not included in the review cannot be used by the EPC Program. This is a voluntary request for information, and all costs for complying with this request must be borne by the submitter.
The draft of this review will be posted on AHRQ’s EPC Program website and available for public comment for a period of 4 weeks. If you would like to be notified when the draft is posted, please sign up for the e-mail list at: https://www.effectivehealthcare.ahrq.gov/email-updates.
The systematic review will answer the following questions. This information is provided as background. AHRQ is not requesting that the public provide answers to these questions.
The
Key Questions
KQ 1: What are the effects of behavioral, pharmacologic, and combined interventions compared with placebo or no active treatment for substance use disorders and problematic substance use1 in adolescents to achieve abstinence, reduce quantity and frequency of use, improve functional outcomes, and reduce substance-related harms?
How do benefits and adverse outcomes of interventions vary by subpopulations?2
How do benefits and adverse outcomes of interventions vary by intervention characteristics?3
KQ 2 What are the comparative effects of active interventions for substance use disorders and problematic substance use1 in adolescents to achieve abstinence, reduce quantity and frequency of use, improve functional outcomes, and reduce harms?
How do comparative benefits and adverse outcomes of interventions vary by subpopulations? 2
How do comparative benefits and adverse outcomes of interventions vary by intervention characteristics? 3
PICOTS (Populations, Interventions, Comparators, Outcomes, Timing, Settings)
Population (all KQs)
Age: Adolescents (12 – 20 years inclusive)
Exclude if > 20 percent of study sample (or identifiable subgroup) is <12 or >20 years, combined
SUD or problematic use of:
Alcohol
Exclude primary studies of treatment of alcohol use disorder/problematic alcohol use in the college setting (we will include existing systematic reviews)
Cannabis
Opioids
Nonmedical prescription drug use (codeine, hydrocodone, oxycodone)
Illicit (e.g., heroin, illicit synthetics)
Sedatives, hypnotics, or anxiolytics (e.g., benzodiazepines, carbamates, barbiturates, methaqualone)
Stimulants
Nonmedical prescription drug use (e.g., methylphenidate)
Illicit (e.g., cocaine, methamphetamine)
Inhalants
Hallucinogens (e.g., phencyclidine, ketamine, MDMA, LSD)
Unspecified or polysubstance use
Exclude if predominately tobacco/nicotine use
Exclude tobacco/nicotine use disorder or problematic tobacco/nicotine use
Exclude limited (or experimental) substance use that has not been deemed to be at least “problematic”
Subpopulations of interest (not necessary for eligibility)
Psychiatric comorbidities
Attention deficit hyperactivity disorder (ADHD), depression, other internalizing and externalizing disorders.
Age
Early adolescence (12 – 14 years)
Middle adolescence (15 – 17 years)
Late adolescence (18 – 20 years)
Sex and gender
Male vs. female
Gender identity (cis vs. transgender)
Sexual orientation
Racial/ethnic minority
Socioeconomic status and related characteristics (e.g., homelessness, poverty)
Pregnant, postpartum, and parenting adolescents
Demographic/family characteristics
Demographics
Family and community dynamics (i.e. substance using family member)
Involvement with child protection services.
Interventions
Behavioral health treatments (major intervention models are indicated by arrowhead bullets, in bold)
Family Therapies
Family behavioral therapy (FBT)
Family systems therapy (FST)
Brief strategic family therapy (BSFT)
Functional family therapy (FFT)
Ecological family therapy
Multidimensional family therapy (MDFT)
Ecologically based family therapy (EBFT)
Family systems network (FSN)
Educational family therapy
Multi-systemic therapy (MST)
Cognitive behavioral therapy (CBT)
Adolescent community reinforcement approach (ACRA)
Dialectical behavior therapy
Cognitive therapy
Contingency management
Motivational interviewing/ Motivation enhancement therapy
Multi-component interventions consisting of two or more models (e.g., MST + CBT; FFT + CBT)
Psychoeducation
Treatment as usual (does not meet criteria for any of the above categories)
Integrated interventions for substance use and a co-occurring disorder
Other
Culturally sensitive interventions
Recovery support
12-step programs
Peer-based and/or peer supports
Assertive continuing care (ACC)
Exclude primary (universal) and secondary preventive interventions.
Exclude interventions used in population that do not aim to reduce substance use (e.g., needle exchange).
Pharmacologic interventions
Exclude medications being used to treat overdose (e.g., naloxone)
Exclude pharmacologic management of acute withdrawal symptoms
Medications to reduce and/or eliminate substance use and to prevent relapse
(See Appendix B for details of FDA approvals)
Alcohol
Gabapentin
Naltrexone
Acamprosate
Disulfiram
Topiramate
Ondansetron
Cannabis
N-acetylcysteine (NAC)
Opioids
Methadone
Buprenorphine
Buprenorphine/Naloxone
Naltrexone
Medications to treat co-occurring psychiatric disorders in patients in patients with concurrent problematic substance use or SUD.
Comparators
KQ 1
No active treatment
Wait list
Placebo (for medications)
Usual care (if not a clearly defined behavioral intervention)
KQ 2
Active interventions (we will evaluate other comparisons if the evidence allows)
Pharmacologic plus behavioral vs. behavioral or pharmacologic alone
Between major behavioral intervention models (e.g. family therapy, cognitive behavioral therapy)
Multicomponent interventions vs. single behavioral intervention model
Outcomes
Abstinence
Urine drug test results (from substance identified on admission to treatment, abstinence from all substances, duration of abstinence)
Quantity, frequency, or severity of use (of primary substance identified on entry to treatment and other substances)
Days of use/abstinence over specified time period
Quantity of use over specified time period
Substance-related problems/symptom count scales
Functional outcomes
School performance and educational attainment
Attendance
Grades / academic performance
Graduation rates
Entering higher education (including trade schools)
Social relationships
Family functioning
Peer relationships
Harmful consequences associated with SUD
Mental health outcomes
Suicidal ideation and behavior
Physical health outcomes
Mortality
All-cause
Drug-related, including fatal overdose
Morbidity
Injuries (non-fatal)
Infections
HIV
Hepatitis C
Other sexually transmitted infections
Legal outcomes
Arrests
Drunk or impaired driving
Contact with juvenile justice system
Adverse effects of intervention(s)
Side effects of pharmacologic interventions
Loss of privacy/confidentiality
Stigmatization/discrimination
Iatrogenic effects of group therapy due to peer deviance
Other reported adverse effects ascribed to interventions
Study Designs and Information Sources
Published, peer reviewed articles and data from clinicaltrials.gov
Randomized controlled trials (including cross-over trials)
N ≥10 participants per study group
Large nonrandomized comparative studies with longitudinal follow-up
N ≥ 100 participants per study group
Must report multiple regression, other adjustment, matching, propensity scoring, or other method to account for confounding.
Single arm pharmacologic studies with at least 200 participants and longitudinal follow-up (to identify side-effects of medications)
We will summarize information from existing systematic reviews specific to treatment of alcohol SUD on college campuses
SR eligible if inclusion criteria for individual studies consistent with our PICOTS criteria for individual studies.
Exclusions
Case-control studies
Cross-sectional studies
Single-arm studies of behavioral interventions
Conference abstracts letters, and other non-peer reviewed reports
Timing
Any duration of treatment
Duration of follow-up of at least a month (but must be longitudinal with separation in time between intervention and outcomes)
Setting
Any setting, including (but not limited to) primary care, school, outpatient, emergency department, in-patient, intensive outpatient, partial hospitalization, intensive inpatient/residential, juvenile justice
Exclude: laboratory-based assessments.
Dated:
Gopal Khanna
Director
1 Substances considered: alcohol, cannabis, opioids, sedatives/hypnotics/anxiolytics, stimulants, inhalants and hallucinogens. Tobacco is excluded.
2 Subpopulations considered: psychiatric co-morbidities, age (early, middle and late adolescence), sex and gender, race/ethnicity, socioeconomic status and related characteristics (e.g., homelessness, poverty), pregnant, postpartum, and parenting adolescents, demographic/family characteristics. Factors in bold will be prioritized if necessary.
3 Intervention characteristics: target (e.g. teen, family or group of teens), duration and setting.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | McKenna, Ryan T (Portland) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-16 |
© 2026 OMB.report | Privacy Policy
