0277ss20
0277ss20.docx
Application for New and Amended Pesticide Registration (Renewal)
OMB: 2070-0060
September 24, 2020
SUPPORTING STATEMENT
FOR AN INFORMATION COLLECTION REQUEST (ICR)
1. IDENTIFICATION OF THE INFORMATION COLLECTION
1(a) Title of the Information Collection
Application for New and Amended Pesticide Registration
OMB No.: 2070-0060 EPA No.: 0277.20
Docket ID No.: EPA-HQ-OPP-2019-0644
1(b) Short Characterization/Abstract
This is a renewal of an existing information collection request (ICR) designed to provide the Environmental Protection Agency (EPA) with the necessary information to evaluate an application for the registration of a pesticide product, as required under section 3 of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and section 408 of the Federal Food, Drug, and Cosmetic Act (FFDCA) (see Attachment A). FIFRA provides EPA with the authority to regulate the distribution, sale and use of pesticides in the United States and ensure that pesticides will not pose unreasonable adverse effects to human health and the environment. Pesticides that meet this test receive a license or "registration."
An individual or entity wanting to obtain a registration for a pesticide product must submit an application package consisting of information relating to the identity and composition of the product, proposed labeling, and supporting data (or compensation for others’ data) for the product, as outlined in 40 CFR part 158. The EPA bases registration decisions for pesticides on its evaluation of a battery of test data provided primarily by applicants for registration. Required studies include testing to show whether a pesticide has the potential to cause unreasonable adverse human health or environmental effects. The Agency currently collects data on physical chemistry, toxicology, environmental fate, ecological effects, worker exposure, residue chemistry, environmental chemistry, and product performance. The information required is dependent on the use and type of product. If EPA’s evaluation of the data shows that the statutory requirements of FIFRA are met, a registration is approved.
Registrants may seek, at their discretion, to amend a registration by submitting data and revised, proposed labeling to EPA. Also, registrants of EPA-registered pesticide products can become subject to regulations or guidance which necessitate labeling revisions. The revised labeling is submitted to the Agency as an amendment along with the completed application (EPA Form 8570-1 and other forms as needed; see Attachment C).
Under FFDCA, EPA sets tolerances, or maximum residue limits, for pesticide residues on foods. EPA may grant exemptions in cases where the pesticide residues do not pose a dietary risk under reasonably foreseeable circumstances. Information collection activities associated with the tolerance petition process, including generation of residue chemistry data required in 40 CFR part 158 with registration applications for a new food use of a registered pesticide active ingredient, are covered separately under OMB Control No. 2070-0024.
In the context of its conventional pesticide registration activities, EPA operates a reduced risk program. This program offers an incentive through a shortened regulatory review schedule for proposed uses that might be beneficial to the public and the environment owing to their risk profile, compared to alternatives for pest control. The reduced risk program is described in a policy notice known as the Reduced-Risk Initiative (PR Notice 97-3, “Guidelines for Expedited Review of Conventional Pesticides under Reduced-Risk Initiative and for Biological Pesticides;” (see Attachment D).
In addition, several new proposed actions or programs are included in this ICR. These actions are anticipated to come online in the next three years. The respondent burden associated with the following activities will be estimated in this ICR and will be further updated in future iterations as the details of these programs are finalized and data is collected about the programs. While agency burden for the development of these programs is captured in section 6 of this ICR below, this burden will not be broken out separately.
Pesticide Registration Improvement Act
Several new fee-for-service categories came online after the Pesticide Registration Improvement Act (PRIA) was reauthorized in March 2019. Due to the novelty of these categories, EPA has not yet defined their scope, but anticipates, among other actions, that they will allow applicants to seek an EPA determination of whether an active ingredient or product would require regulation under FIFRA. Examples of these determinations may include, but are not limited to, the following: pesticide device determinations; pesticidal active ingredient determinations; treated article exemption evaluations; minimum risk pesticide determinations; barrier evaluations, and plant growth regulators/bio-stimulants. EPA will reassess the implications of these new PRIA categories and their associated burden with data generated as the program matures. The paperwork burden associated with PRIA fees is covered under a separate ICR, OMB Control No. 2070-0179.
Antimicrobial Performance Evaluation Program Strategy
Based on recommendations from the 2016 Office of Inspector General’s report, “EPA Needs a Risk-Based Strategy to Assure Continued Effectiveness of Hospital-Level Disinfectants” (No. 16-P-0316), EPA developed the risk-based strategy titled, the “Antimicrobial Performance Evaluation Program” (APEP). This program seeks to ensure the continued effectiveness of currently registered hospital disinfectants with hospital-level disinfectant public health label claims. The draft strategy limits the number of products tested per year to less than 100.
The Agency is currently evaluating comments submitted on the draft strategy during a 60-day public comment period. These comments could result in changes to how the program is implemented and the burden imposed on the regulated community. Once the program is finalized, the Agency will reevaluate the associated burden under this ICR. The draft strategy can be found under docket # EPA-HQ-OPP-2018-0265.
Interim Process for Evaluation of Potential Synergistic Effects of Pesticides During the Registration Process
EPA developed a draft guidance for an interim process to obtain, analyze, and document patent claims of greater-than-additive (GTA) effects in mixtures of pesticide active ingredients as part of the pesticide registration process. EPA will request registrants to provide GTA-effect information on approved patents and conduct appropriate statistical analysis for new, conventional pesticide active ingredients and other new products or active ingredients for which EPA has specific concerns about the potential for GTA effects. The purpose of the process is to evaluate the utility of collecting and reviewing GTA patent information for use in conducting risk assessments, and to determine if such data, where applicable, affect risk assessments. EPA will reassess the burden associated with this guidance once it is finalized. This process is described in a guidance document titled “Interim Process for Evaluating Potential Synergistic Effects of Pesticides During the Registration Process” and can be found under docket # EPA-HQ-OPP-2017-0433.
2. NEED FOR AND USE OF THE COLLECTION
2(a) Need/Authority for the Collection
Authorizing legislation is contained in section 3 of FIFRA (7 USC § 136a) and section 408 of FFDCA (21 USC 346a). Governing regulations and guidelines are contained in 40 CFR parts 152, 156, 158 (Attachments E, F and G, respectively), and in PR Notice (PRN) 97-3. Label amendments under 40 CFR part 156, may be required to maintain continued registration following a regulatory review (e.g., registration review). Labeling amendments pertaining to groups of products may be implemented through a PRN or notice in the Federal Register.
2(b) Practical Utility/Users of the Data
The information collected under this ICR is used to support registration regulatory decisions for new or amended pesticides and currently registered hospital-level pesticides available in the marketplace. It is also used to support preapplication determinations of whether products need regulation under FIFRA. Once the product is determined to need regulation, the data reviews are completed satisfactorily, the labeling is determined to be adequate, and the product is determined to meet the statutory standards of FIFRA, a registration is issued to the applicant.
3. NON-DUPLICATION, CONSULTATIONS, AND OTHER COLLECTION CRITERIA
3(a) Non-duplication
Duplication will not occur in this program, as each applicant must submit information unique to the particular product being offered for registration or that is selected for evaluation under APEP. If the product is not unique, existing data may be referenced by the applicant as described in unit 5(c) of this ICR, entitled “Small Entity Flexibility.” On amended applications, the applicant may refer to any previously submitted information that they own, thereby satisfying data requirements without the burden of providing duplicate information or additional data development. EPA’s registration program allows for collaboration through data citation and ensuring that original data generators/submitters are compensated when their data are cited in another application.
To avoid potential overlap between the requirement of study data in support of an application for the registration for a new food use pesticide active ingredient under FIFRA, and developing data to support a tolerance petition, EPA allows the use of data required to support a tolerance petition that are already archived in EPA records for use as part of a FIFRA registration of a pesticide to be used in a like manner and in the same use pattern. Information collection activities to establish tolerance limits are covered under the Tolerance ICR (OMB Control No. 2070-0024), and therefore not included in this ICR.
3(b) Public Notice Required Prior to ICR Submission to OMB
Pursuant to 5 CFR § 1320.8(d), in proposing to renew this ICR, the EPA published a Federal Register Notice (85 FR 6944; February 6, 2020) providing a 60-day public comment period. The Agency received one comment from a consortium of industry stakeholders, Bayer CropScience LP, Corteva Agriscience, and Valent U.S.A. LLC. (Attachment M)
The commenter recommended several minor changes to EPA form 8570-27,
Formulator’s Exemption Statement, including changing a
reference to the former 40 CFR § 158.50(e) to the current
location of the relevant language at 40 CFR § 152.85(c). The
Agency appreciates the commenter’s input and will be revising
paragraph (2) of the Formulator’s Exemption Statement to
read as follows (Attachment H):
“(2) Of
these, each active ingredient listed in paragraph (4) is present
solely as the result of the use of that active ingredient in the
manufacturing, formulation or repackaging of another product which
contains that active ingredient which is registered under FIFRA
Section 3, is purchased by us from another person, and meets the
requirements of 40 CFR § 152.85(c)(2) or (3).”
The commenter also recommended the following significant policy changes to EPA’s formulator’s exemption program (40 CFR § 152.85) and the associated Formulator’s Exemption Statement:
Require that applicant submit a signed letter with their registration package from the registrant of each product referenced stating that the registrant does not object to the applicant identifying the registrant’s product(s) as the source of the active ingredient(s) in the product for which the formulator’s exemption is claimed.
EPA updates their 21 Day Content Screen Review Worksheet internal checklist to include an additional item under Item 4 “Formulator’s Exemption” as follows: Letters of non-objection from registrant of each identified registered source product are attached to Formulator’s Exemption Statement.
EPA updates and supersedes the guidance provided in the 1997 Memorandum issued by the Office of Pesticide Program’s Registration Division titled: Formulator’s Exemption and Letters or Petitions to Deny Registrations.
The commenter asserts that implementing the recommendations “will improve the efficiency and effectiveness of EPA’s review and approval of formulator’s exemption applications while minimizing burdens on the Agency, applicants, and the registrants whose products they cite.” Adopting these recommendations would require additional analysis and stakeholder engagement that cannot be accomplished in the ICR consultation process because they are outside the scope of this ICR renewal. However, the Agency intends to pursue a future rulemaking to consider amendments to the formulator’s exemption and will take these recommendations under consideration at that time.
3(c) Consultations
Under 5 CFR § 1320.8(d)(1), agencies are required to consult with respondents about specific aspects of information collection before submitting approval requests to OMB. In accordance with this regulation, EPA staff contacted representatives from a cross section of stakeholders to seek feedback on the burden estimates in this ICR, and on the clarity of the information collection process (Attachment L). The following three companies agreed to receive consultation questions for their responses:
Corteva Agriscience
The Clorox Company
BPIA
A consortium of industry stakeholders, which included Corteva Agriscience, submitted a letter with several recommendations to the formulator’s exemption statement process which the Agency addressed in section 3(b) of this ICR.
3(d) Effects of Less Frequent Collection
Not applicable. The section 3 information collection activities are initiated by applicants for registration. Information is submitted in conjunction with the application. There is no set means by which the EPA can reduce the frequency. If the information were not submitted, EPA would be unable to fulfill its statutory responsibilities relative to the review and registration of pesticides and protection of human health and the environment.
3(e) General Guidelines
Third-party disclosure of label information: Labeling regulations under 40 CFR part 156 require registrants to display product-specific information to potential users and the general public through the pesticide label that is approved by EPA. The required information for the label is gathered and prepared by the registrant and submitted as part of the application for registration. The burden and cost of preparing and submitting the label information to EPA are included in this ICR as part of the burden associated with preparing the application for registration.
This ICR does not, however, include any third-party burden or cost estimates for providing the pesticide label on each product to disclose safety information to potential users and the general public. The use of the pesticide label to accomplish this disclosure is not a collection of information as defined by the Paperwork Reduction Act (PRA) and OMB implementing regulations (5 CFR § 1320.3(c)(2)). This determination was made by OMB in the context of implementing the 1995 PRA amendments and related OMB final regulations of 1995. In general, OMB explained that the new third-party disclosure provision added to the PRA in 1995 was not intended to require agencies to estimate burden for the required disclosure of important health and safety information on product labels, in particular if the label or language for the label is specifically provided by law (e.g., the Surgeon General warnings required to be on the labels of alcohol or tobacco products), or required to be approved by an agency (e.g., EPA labels on pesticide products or PCB containing transformers).
Long-term recordkeeping: The recordkeeping activities briefly described in this ICR exceed OMB’s guideline that agencies not require that records be retained for more than three years (5 CFR § 1320.5(d)(2)(iv)). As authorized under FIFRA section 8, EPA regulations under 40 CFR § 169.2(k) require that registrants retain records containing research data relating to a registered pesticide, including all data submitted to EPA in support of a registration, for as long as the registration is valid, and the producer is in business. However, the burden related to the recordkeeping requirements is covered under another ICR (see OMB Control No. 2070-0028, Recordkeeping Requirements for Producers of Pesticides under Section 8 of FIFRA).
Electronic submissions: While mechanisms and guidance are under development, EPA encourages electronic submissions for the following types of regulatory actions:
New pesticide active ingredients.
New pesticide products containing already-registered pesticide active ingredients.
Amendments to registered pesticide products.
Experimental use permits.
Inert ingredient requests.
Pre-application.
6(a)(2) data.
Petitions for food tolerance.
Distributor products.
Data Call-Ins (DCIs).
Endocrine Disruptor Screening Program (EDSP) Orders.
Resubmit previously submitted 90-day responses.
Voluntary submissions.
The Government Paperwork Elimination Act required agencies to make available to the public electronic reporting alternatives to paper-based submissions by 2003, unless there is a strong reason for not doing so. In the past, the Agency was unable to ensure the security of CBI material that might be transmitted over the Internet. In September 2015, the EPA started offering the Pesticide Submission Portal (PSP), a fully electronic alternative as an option for submitting registration forms electronically, as well as the ability to sign and submit confidential information using Central Data Exchange (CDX) technology. The development of a web-based submission portal is a critical step in the realization of EPA’s long-term vision for secure data exchange between registrants and the Agency. (See Attachment N for screen shots of PSP, along with available online instructions and guidance).
The new electronic submission process allows document uploads and information from paper forms to be submitted through an online form. The PSP leverages the Agency’s existing Central Data Exchange (CDX) to provide a secure method of submitting these documents and information within a secure online environment. CDX does require initial user registration for which the paperwork burden estimate is covered under “Cross-Media Electronic Reporting Rule” ICR, OMB No. 2025-0003.
Application packages and files for pesticide registration can be submitted via PSP. This includes forms, studies, and draft product labeling. Applicants need not submit multiple electronic copies of any pieces of their applications. In PR Notice 2011-3, EPA made clear that the requirement to submit multiple copies of data is applicable only to paper submissions. Similarly, EPA interprets the requirement to submit five copies of draft labeling in 40 CFR § 152.50(e) to apply only to applications made on paper. As electronic submissions are easily reproducible, EPA will accept electronic applications containing one electronic copy of all the required elements.
EPA will continue to accept paper applications but encourages applicants to take advantage of this new, more efficient option and forego the courier costs to send to EPA. For electronic submissions, applicants do not need to submit multiple copies of any pieces of their application, the requirement for multiple copies of data and five copies draft labeling only applies to paper submissions.
Whereas existing registration application forms require an ink signature, the web-based portal uses EPA’s Central Data Exchange (CDX) to allow applicants to electronically sign and securely submit the required information. The use of CDX is intended to save the registrants’ and Agency’s time and resources by simplifying data submissions, receipt confirmation, information access and reporting. The electronic reporting option for CBI-related data through EPA’s CDX reporting site has been in place for three years. Therefore, the Agency has not yet evaluated whether respondents are more inclined to use the new submission portal, PSP, for that purpose.
While it is too early to be able to quantify any change in burden, the Agency's pesticide program, along with the pesticide industry, recognizes the advantages in terms of accuracy, speed and costs from electronic data transfer technologies. The Agency believes that the ability to electronically submit information required for registration reduces the burden of sending, receiving, and archiving paper submissions, minimizes errors, and eliminates the need for multiple data entries across forms.
Additional benefits of using the Portal include a status indicator that allows registrants to track the movement of their submissions and automatically generated MRID numbers. Extensive guidance regarding available electronic submission options is available to registrants via the Office of Pesticide Program’s (OPP) website at http://www2.epa.gov/pesticide-registration/electronic-submissions-pesticide-applications.
Overall, the EPA is continuing to investigate opportunities for technological improvements which focus on using fully electronic tools for information collections. OPP continues to consult with industry associations and other federal agencies regarding this issue and is participating in an Agency-wide workgroup to develop electronic reporting standards. One of OPP’s priority areas in developing such electronic tools is related to electronic submission of data and labels. Some of the electronic tools being developed under this initiative include: software that supports a fully-online electronic Confidential Statement of Formula (eCSF), formerly the Confidential Statement of Product Specifications (CSPS), and the Office of Pesticide Programs Electronic Label (OPPEL), formerly SmartLabel. The former CSPS was originally a collaboration between Canada and the U.S. EPA with an effort toward a harmonized single portal usable for electronic submissions to either Agency. The joint U.S. and Canadian efforts have since ended; however, the U.S. still intends to share the data schema with Canada once the software development is complete.
The Agency anticipates using these electronic tools as they become fully operational. EPA anticipates that submissions via these electronic tools will take less time to prepare than paper or current web-based submissions. It is anticipated that the burden associated with these collections will decrease as these electronic tools become a part of standard business practice. Any burden modifications associated with the anticipated OPPEL and eCSF tools will be evaluated in future iterations of this ICR when the tools are fully implemented and once EPA has data on the frequency of use of these tools for electronic submissions and the time required to complete this type of submission. It is important to note that the proposed or draft changes to the PSP are not covered by the ICR until they are submitted to and approved by OMB.
Other information submission methods include:
Paper submissions. Registrants are required to submit three paper copies of study data to EPA, or two paper copies if they submit the required study data in Adobe Acrobat Portable Document Format (PDF).
XML files. Registrants can construct an XML file for inclusion in an electronic submission package that can be submitted to the EPA via magnetic media-based submissions such as a diskette, CD-ROM, etc.
e-Dossier Builder system. This system allows registrants to create packages for electronic
submission via a “builder” application and presents packages in a specified format. Additional information about e-submission methods can be found at: http://www2.epa.gov/pesticide-registration/assembly-electronic-packages-and-discs.
3(f) Confidentiality
Although the EPA urges the submitter to minimize the amount of claimed Confidential Business Information (CBI), in accordance with FIFRA Section 10 and 40 CFR Part 2, Subpart B, the EPA will protect from disclosure all data and/or information brought to the Agency in conjunction with this information collection that may be claimed as trade secret, commercial, or financial information.
3(g) Sensitive Questions
Not applicable. No information of a sensitive or private nature is requested in conjunction with this collection activity. In addition, this information collection activity complies with the provisions of the Privacy Act of 1974 and OMB circular A-108.
3(h) OMB Terms of Clearance
In September 2019, OMB concluded on EPA’s “Non-Substantive Change Request Justification” approving information collection activities and their associated burden stemming from new statutory requirements imposed on the Agency by amendments to the recently reauthorized Pesticide Registration Improvement Act ICR.
4. THE RESPONDENTS AND THE INFORMATION REQUESTED
4(a) Respondents - NAICS Codes
The information collection under this ICR affects individuals or entities engaged in activities related to the registration of pesticide products. There are approximately 1,751 pesticide respondents holding at least one pesticide registration. The North American Industrial Classification System (NAICS) assigned to the parties responding to this information are as follows:
Category |
NAICS codes |
Examples of potentially affected Entities |
Pesticide and other agricultural chemical manufacturing |
32532 |
Individuals or entities engaged in activities related to the registration of a pesticide product. |
4(b) Information Requested
(i) Data items, including recordkeeping requirements
Application Materials
Forms for pesticide registration applications.
Supporting data may be required as part of the submission.
Draft labeling that meets the regulatory requirements set out in 40 CFR § 152.50.
There are two main categories of applicants for registration: those requiring submission of a full complement of supporting data (e.g., new active ingredients); and those requiring submission of less data (e.g., amendments, for currently registered chemicals). These have been described as Types A and B, respectively. In “Type A” activities, applicants for new active ingredients will be required to submit administrative forms, product labeling, a CSF, as well as a full complement of physical chemistry, toxicology, environmental fate, ecological effects, worker exposure, residue chemistry, environmental chemistry, and product performance data, as identified in 40 CFR part 158. (Note: Residue chemistry study development is accounted for in a separate ICR for tolerance petitions, as explained in Sections 1(b), 3(a) and 6 of this ICR.)
"Type B" activities involve a registrant or applicant assembling and submitting an application for registration of a new or amended product that contains a currently registered active ingredient. Generally, "Type B" activities involve less data and complexity than "Type A" activities. “Type B” activities include a range of actions from Fee-for-Service to the less involved label amendments and notifications. The items that must be submitted or cited in this application include product specific data, administrative forms, product labeling, and a CSF. The product specific data specified in 40 CFR part 158 must be generated by the registrant/applicant or cited from an identical or substantially similar product. There are several types of amendments, or “Type B” activities, including “me-too” products that require little or no data. Applicants for “me-too” products (i.e., pesticide products claimed to be identical or substantially similar in composition and use to a product currently registered by the EPA) may be required only to use the forms listed below to certify that the applicant intends to rely on data previously submitted to the EPA by another producer, has contacted the appropriate company (owning the data that the applicant is referencing), and offered to pay reasonable compensation for the use of the data.
In addition to the two main categories, as described in Section 1(b) of this ICR, the EPA operates a reduced risk program that offers an incentive through an expedited review timeframe for new ingredients or proposed new uses of conventional pesticides that might be beneficial to the public and the environment owing to their risk profile, compared to alternatives for pest control. These activities are described as “Type C” in this ICR.
"Type C" activities involve a registrant or applicant assembling and submitting an reduced risk application for registration of a new active ingredient or a new use for a currently registered active ingredient. In addition to the registration application itself, “Type C” activities require that the registrant provide a “reduced risk” rationale document addressing risk reduction parameters described in PR Notice 97-3. The items required to be submitted in applications for “reduced risk” chemicals include generic data, product specific data, administrative forms, product labeling, and a CSF. Administrative forms usually include the application for registration, data compensation form, a data matrix, the CSF, and copies of the complete labeling. Applicants for “Type C” registrations provide an explanation accompanied by relevant supporting information, including associated tolerance petitions for special consideration based on these factors. (The paperwork burden for tolerance petitions is covered in a separate ICR under OMB No. 2070-0024.) Products that are successfully classified as presenting potentially reduced risk will likely receive earlier registration and consequent earlier marketability.
Response Type
|
Description/Example |
Type A
New AIs & New Uses |
Description: “Type A” activities support the registration of new active ingredients and new uses. They involve a registrant or applicant assembling and submitting an application for registration of a new active ingredient or a new use for a currently registered active ingredient. The items required to be submitted in this application include generic data, product specific data, as specified in 40 CFR part 158. Administrative forms usually include the application for registration and the data compensation forms, a data matrix, the CSF, and copies of the complete labeling. EPA encourages electronic submission of these application types. |
Example: An example of a "Type A" activity would be an application for registration of a new active ingredient (AI). Typically, for new AIs, applications must be submitted for at least two new products -- the manufacturing use product (either imported or made in the U.S. that may be formulated into end-use products) and at least one end-use product (that bears directions for the intended end uses). An applicant would need to determine generic and product specific data required by 40 CFR part 158 for the new AI (taking into account the use patterns sought), generate those data, and submit them with the application. For a new AI the generic data consists of certain acute, sub-chronic, and chronic toxicology; environmental fate; ecological effects (birds, fish, invertebrates); and product chemistry. The applicant would format the complete data and submit along with the other items required for an application, as described above. |
|
Type B
New or Amended Products Using Currently Registered AIs |
Description: "Type B" activities involve a registrant or applicant assembling and submitting an application for a pre-application determination or a registration of a new or amended product that contains a currently registered active ingredient. Generally, "Type B" activities involve less data and complexity than "Type A" activities. “Type B” activities include both Fee-for-Service actions, as well as less involved label amendments and notifications. Individually, “Type B” activities often present low burden, but the number of such submissions is high, translating to a large work load for the Agency. The items that must be submitted or cited in this application include product specific data, administrative forms, product labeling, and a CSF. The product specific data specified in 40 CFR part 158 must be generated by the registrant/applicant or cited from an identical or substantially similar product. If submitted, the data must be formatted properly and with the correct number of copies. Administrative forms usually include the application for registration, data compensation form, a data matrix, the CSF, and copies of the complete labeling. EPA encourages electronic submission of these application types. |
Example: An applicant might seek registration of a new product containing an active ingredient that is already registered. Often, the formulation of this product is identical or substantially similar to that of a currently registered end-use product. This is called a "me-too" registration. In this case, the applicant only needs to cite data from another product (selective method) or from all products containing that AI (cite-all method) to support the new product. The applicant also submits the labeling and other administrative forms without submitting any data. If a product is not substantially similar to another product, the applicant must submit product specific data (acute toxicity and product chemistry) for that product. Nevertheless, this kind of application is far less complicated than a "Type A" application. |
|
Type C
Reduced Risk AIs & Uses |
Description: “Type C” activities support the registration of new active ingredients and new uses that may qualify as "reduced risk" and be given expedited processing. "Type C" activities involve a registrant or applicant assembling and submitting an application for registration of a new active ingredient or a new use for a currently registered active ingredient. The items required to be submitted in this application include generic data, product specific data, administrative forms, product labeling, and a CSF. The generic and product specific data specified in 40 CFR part 158 must be generated by the registrants, formatted properly, and submitted with the correct number of copies. Administrative forms usually include the application for registration, data compensation form, a data matrix, the CSF, and copies of the complete labeling. In addition to the registration application itself, “Type C” activities require that the registrant provide a “reduced risk” rationale document addressing risk reduction parameters described in PR Notice 97-3. EPA encourages electronic submission of these application types. |
Example: An example of a "Type C" activity would be an application for registration of a new active ingredient (AI) where a “reduced risk” rationale per PR Notice 97-3 is also submitted with the registration application. All of the data requirements and administrative forms described under “Type A” applications must be addressed for “Type C” applications, and in addition the “reduced risk” rationale document must be provided. A new AI or new use associated with “Type C” activity is less likely to have risk concerns that would require refined risk assessment on the Agency’s part or require additional information on the registrant’s part to address risk concerns. An application determined by the Agency to be "reduced risk” is provided an expedited decision time frame under PRIA. This kind of application is of equal complexity to the "Type A" activity, but more complex than "Type B." |
The completion and submission of the following forms is necessary to register a pesticide product (see Attachment C):
EPA Form 8570-1, Application for Pesticide Registration, Amendment, Other;
EPA Form 8570-4, Confidential Statement of Formula (CSF)
EPA Form 8570-27, Formulator’s Exemption Statement
EPA Form 8570-34, Certification with Respect to Citation of Data
EPA Form 8570-35, Data Matrix
EPA Form 8570-36, Summary of the Physical/chemical Properties
EPA Form 8570-37, Self-certification Statement for the Physical/Chemical Properties1
(ii) Respondent Activities
Respondent Paperwork Activity |
Description |
1. Read instructions |
Read germane FIFRA legislation, 40 CFR regulations, application form instructions, the Reduced-Risk policy, applicable guidance and correspondence, and germane labeling PR and FR notices;
|
2. Plan activities |
Decide whether the product is a pesticide seeking registration, a “me-too” pesticide, or a product seeking a determination if FIFRA registration is required; this will determine succeeding activities; |
3. Gather information |
Canvass/contact other companies holding EPA registrations or marketing similar products, if any, to determine whether it would be appropriate to share or rely on testing data already submitted by another company; |
4. Create information |
If submitting study data, arrange for testing of any physical chemistry, toxicological, environmental fate, ecological effects, worker exposure, residue chemistry, environmental chemistry, product performance, and efficacy data required by germane regulations to support registration;
|
5. Compile and review |
Assemble data, evaluate for accuracy, appropriateness, and completeness;
|
6. Complete paperwork |
Complete all appropriate application documents;
|
7. Submit registration information (application-related material) |
Using preferred option for registration applications, submit required information to the EPA. |
|
File and maintain copies of all registration data submitted to the Agency. |
5. THE INFORMATION COLLECTED – AGENCY ACTIVITIES, COLLECTION METHODOLOGY, AND INFORMATION MANAGEMENT.
5(a) Agency Activities
In general, the degree of Agency activities in the review of data submissions will depend on the complexity of the product being registered, the pre-application determinations being made, and/or whether it is identical or substantially similar to other products already registered. Products containing active ingredients present in currently registered products and proposed for uses currently registered (“me-too” registrations) may require only a minimal review for completeness of the application, the adequacy of the labeling, and the satisfaction of data compensation requirements.
New Registrations
A product containing a new active ingredient will require multiple data reviews related to physical chemistry, toxicology, environmental fate, ecological effects, worker exposure, residue chemistry, environmental chemistry, and product performance prior to approval. Therefore, many divisions may be actively involved in the data analysis and Agency determination of OPP registration actions. For conventional pesticides, the application is reviewed by ITRMD, the Registration Division (RD), the Health Effects Division (HED), and the Environmental Fate and Effects Division (EFED), and Biological and Economic Analysis Division (BEAD). For biological/biopesticide pesticides, the application is reviewed by ITRMD, and the Biopesticides and Pollution Prevention Division (BPPD). Applications for antimicrobial pesticide products are reviewed by ITRMD and the Antimicrobials Division (AD). The Agency notifies an applicant when an application is incomplete or is found to be deficient. The applicant is permitted to correct the deficiencies and submit the corrections.
Reduced Risk Registrations
Registrants submitting registration applications for new pesticide products that may fall within the scope of the Reduced-Risk Initiative may provide a written rationale with any supporting information on why their pesticide may qualify for special consideration. This rationale with supporting information will be reviewed and evaluated and, if the pesticide demonstrates the opportunity for risk reduction, the EPA uses this finding as a factor in determining a shorter review time. This policy specifies the standard format for registrants to use when providing justification for a reduced-risk pesticide to facilitate efficient processing within OPP.
Amendments
Once issued, a registration also may be amended in various ways, such as adding or deleting uses, modifying the labeling, or altering the product composition in minor ways. To request these changes, the registrant is required to submit an application for amended registration on EPA Form 8570-1, along with all appropriate additional forms, labeling and supporting data.
Notifications
Notifications are registration modifications that require the shortest review and approval or denial time. Unlike a new active ingredient or a new use, Notifications are reviewed only by the division responsible for registering the product.
Exemptions
EPA reviews applications for exemptions and, if it is determined that the application qualifies, the product will be exempted from the requirements of registration under FIFRA and/or receive a tolerance or tolerance exemption under FFDCA.
Label Approvals
Label reviews are most often accomplished by a Product Manager, or Team Leader, in one of the three regulatory divisions within EPA’s Office of Pesticide Programs responsible for pesticide registration: RD, AD, and BPPD. These divisions ensure that revisions comply with the applicable labeling requirement or guidance.
A general category of OPP’s activities related to the information collection described in this ICR is summarized below:
Receive Application
The pesticide registration application package, complete with the required forms, necessary data, and proposed labels, is received by the Front-End Processing Unit in the Information Technology and Resources Management Division (ITRMD). After screening the application for administrative completeness, ITRMD refers the complete application and any accompanying data to the appropriate regulatory division. ITRMD is responsible for entering the registration action into OPP’s central tracking database system, called OPPIN. If the application form is accompanied by data to support the registration application (e.g., new active ingredients and new uses), ITRMD will forward the registration data package to a contractor for inputting into the tracking database. After this is completed, the data package is routed to the appropriate regulatory division for processing.
Review Application
The regulatory divisions, based on the registration action, assign the packages for appropriate evaluation. Each scientific discipline reviews the data and may develop a Data Evaluation Report (DER) and appropriate risk assessments that summarize the data review.
If the registration application is clearly for a “me-too” pesticide product or use, then the product may be registered on an expedited basis by the reviewer. If its similarity to a pesticide currently registered by the EPA is questionable, it may be sent for a short interdisciplinary review. The Program Manager or Team Leader ensures that the database is updated by identifying where it is sent for review. If the registration action is clearly not for a “me-too” pesticide product or use, then action is taken to correct the assignment of the registration action and to route the data to the appropriate scientific evaluation group for full data reviews.
Make Registration Decision
The Program Manager or Team Leader examines all the scientific reviews and proposed labeling and determines whether the product may be registered. If the product contains an active ingredient not currently registered by EPA, the review summary is included as part of a decision package and referred to the Director of OPP for a final decision on whether to register a pesticide. When a new food use is sought, a tolerance or exemption is established for an already registered active ingredient (e.g., new use), the final decision is made by the Division Director of the registering division.
If the registration action is for revised labeling in response to a Pesticide Registration Notice, the revised labeling submitted along with appropriate EPA forms will be reviewed by a Program Manager or Team Leader for compliance with the applicable Pesticide Registration Notice and, following the registration decision, entered into the tracking database.
Notify Applicant/Registrant
OPP sends a Notice of Registration or Determination to the applicant informing the applicant that the product has been registered and specifying any conditions of registration or has received a determination on whether it requires regulation under FIFRA. For labeling amendments, a letter is sent to the applicant stating approval/disapproval. If the label amendment is approved, a stamped master label is sent to the registrant. For exemptions, a letter will be sent to the applicant that the product is exempt from the requirements of registration under FIFRA and/or a tolerance or tolerance exemption under FFDCA.
Under APEP, OPP will notify and work with the registrant to address and resolve failures. As proposed, a “failure” is defined as a product not meeting newly set efficacy standards for hospital disinfectants with hospital-level disinfectant public health label claims. To resolve failures, the Agency may utilize processes such as: reformulations, label amendments, removal of use sites, modifications of use directions, retesting, product cancellations, and/or removal of product from marketplace, etc. For any outstanding unaddressed failures, OPP will coordinate and consult with OECA and the Office of General Counsel (OGC) as needed.
Store and Maintain Data
OPP stores, files, and maintains copies of any registration notices and labeling information.
5(b) Collection Methodology and Management
All registration actions are entered into OPP’s central database system, called OPPIN, to track progress toward registration. Registration actions accompanied by data (e.g., products containing new active ingredients or new uses) are also entered into the database to track progress toward registration. Once a product has been registered, pertinent status information regarding the product is revised in the tracking database.
The system contains the following types of information: new or amended product registrations, suspensions, cancellations, product active ingredients, product uses, use deletions and preapplication determinations. ITRMD maintains official registration file jackets, in which copies of the application, EPA’s reviews, registration approvals, correspondence, label, the CSF and other related information are all retained.
5(c) Small Entity Flexibility
Per 40 CFR § 152.85(c), the formulator’s exemption provision reduces the data submission burden on an applicant for registration of a product that uses an EPA-registered pesticide product as the source of its active ingredient. Submission of EPA Form 8570-27 (“Formulator’s Exemption Statement”) exempts the applicant from furnishing the generic data that already were submitted by the company registering the source product.
The Agency also cataloged and computerized its pesticide database so that one can easily determine whether a particular study has been submitted, by whom it was submitted, and what product or active ingredient it supports. This identifies, by chemical each item of data in the EPA files. As a result, applicants encounter little difficulty in identifying available data needed to support an application for registration.
5(d) Collection Schedule
Not applicable. The activity is conducted only as a registration application is received for consideration. There is no set schedule for the submission of this information to EPA.
6. ESTIMATING BURDEN AND COST OF THE COLLECTION
The paperwork burden from pesticide registration comes from two sources: the burden that results from preparing and filing the registration application and the PRA burden associated with scientific study data generation. Most of the previous versions of this ICR did not contain estimates of burden associated with data generation; the previous ICR renewal was the first one to include this burden component. Estimates of the paperwork burden hours and costs from both sources are provided in this section. Tables 1-A and 1-B show the total annual paperwork burden estimates (hours) for this information collection. It should be noted that the number of responses for the application process paperwork burden do not directly correlate with the number of responses for the data generation burden.
The paperwork burden from the application process and from data generation varies by the type of application. For the burden from the application process, applications are grouped into three types—Type A, Type B, and Type C—as previously described. Estimates of the burden and cost from the application process is described for each of these three categories in Section 6(a) of this ICR.
The paperwork burden from study data generation does not occur with all types of registrations, only those that require submission of data. Only new active ingredients (AI), new uses, new products, and some label amendments require such data. The majority of data required for registration of new uses are limited to residue chemistry studies necessary to establish a new tolerance. As indicated in Sections 1(b) and 3(a) of this ICR, the Agency has chosen to account for the burden for generation of residue chemistry data used for tolerances in the tolerance petition ICR, whether the data is submitted with an application for a new food use of a pesticide active ingredient or as part of a petition seeking a tolerance. The EPA believes that is the best place for it – as most of that data is generated with petitions and not for the initial application.
Therefore, the paperwork burden from generation study data for new uses would be covered by the Tolerance ICR (OMB Control No. 2070-0024) and is not discussed in this ICR. Estimates of the paperwork burden and the costs of data generation associated with new AIs and new products is discussed in Section 6(b) of this ICR.
Annual aggregate paperwork burden for all respondent Section 3 activities is estimated to be 1.6 million hours: 145,646 hours for application activities and 1.42 million hours from data generation. There are currently an estimated 1,808 pesticide registrants holding at least one pesticide registration. The number of pesticide registrants has increased since the last ICR renewal from 1,751 to 1,808, an increase of 57 registrants.
The total annual costs associated with paperwork burden from the application process are estimated to be approximately $14.2 million per year.
“Type A” activities are estimated to cost approximately $3.24 million per year.
“Type B” activities are estimated to cost approximately $10.74 million per year.
“Type C” activities are estimated to cost approximately $264 thousand per year.
Table 1-A: Annual Information Collection Paperwork Burden Estimates for Registration Application Process
Application Category |
Number of registrants (respondents) |
Average annual responses |
Average annual responses per respondent |
Burden per response (hours) |
Average annual burden per respondent |
Average annual burden (hours) |
Type A |
1,808 |
213 |
0.118 |
194 |
23 |
41,322 |
Type B |
1,808 |
7,221 |
3.994 |
14 |
56 |
101,094 |
Type C |
1,808 |
5 |
0.003 |
646 |
2 |
3,230 |
All Types |
1,808 |
7,439 |
4.114 |
|
81 |
145,646 |
*Numbers may not sum due to rounding.
The total annual costs associated with the paperwork burden from data generation are estimated to be approximately $106.32 million per year.
The paperwork burden from data generation for new active ingredients (AI) is estimated to cost approximately $62.64 million per year.
The paperwork burden from data generation for new products is estimated to cost approximately $43.68 million per year.
Table 1-B: Annual Information Collection Paperwork Burden Estimates for Data Generation
Registration Type |
Number of registrants (respondents) |
Average annual responses |
Average annual responses per respondent |
Average Burden per response (hours) 1 |
Average annual burden per respondent (hours) |
Average annual burden (hours) 2 |
New AI |
1,808 |
27 |
0.015 |
38,057 |
568 |
1,027,536 |
New Product |
1,808 |
762 |
0.421 |
695 |
293 |
529,714 |
All Types |
1,808 |
789 |
0.436 |
|
861 |
1,557,250 |
*Numbers may not sum due to rounding. 1 Average burden hours per response across the three registering divisions. See Tables 6-A and 6-B for details. 2 Average annual burden hours are calculated as the sum of average annual burden hours for each registration type and cannot be computed from the values in this table. See Tables 6-B and 7-B for details. |
To calculate the costs of the paperwork burden from the application process and from data generation, the burden hours were multiplied by current wages. Agency economists revised the estimated wages, benefits and overhead for all labor categories for the affected industry and EPA employees based on publicly available data from the US Bureau of Labor Statistics. The formulas used to estimate the labor rates and formulas used to derive the fully loaded wage rates and overhead costs for this ICR renewal are presented in Attachment J. Cost estimates are provided in the following sections.
6(a) Respondent Paperwork Burden Hours and Cost from Application Process
This section describes the methodologies and provides estimates of respondent paperwork burden hours and costs from the application process. The reporting and recordkeeping burden associated with the Section 3 application process for registering of pesticides may be thought of in terms of three general categories of burden (including most registration actions except those pertaining to setting tolerances and inert ingredients).
To determine the appropriate number of applications (responses) for each category, the EPA averaged data on pesticide registration activities from 2015-2017. When the Agency receives applications for registrations and amendments to registrations, these actions are tracked in OPP’s central database system, called OPPIN. From this system, the Agency can provide reporting on actual numbers of applications, broken down by several major types. Each registering division further tracks registration-related applications in greater detail. Information from the central database and supplemental divisional tracking is used as the basis of the burden estimates.
The average number of responses annually has changed since the last ICR renewal from 7,478 to 7,439, a decrease of less than one percent. Table 2 shows the average number of applications per year by type and division. Across all registering divisions, there were 7,292 Section 3 registration actions annually, on average, during the years 2015-2017. These included an average of 205 “Type A” activities, 7,082 “Type B” activities, and 5 “Type C” activities.
Additionally, in this iteration of the ICR, the Agency calculates the expected annual application burden of three proposed programs that are anticipated to come online in the next three years. As many of these programs are not finalized, the impact of these programs is estimated. Further, many of the proposed programs do not fit neatly into Type A, Type B, or Type C actions. When the description of a proposed program does not directly match any specific Type of action, the Agency calculates burden as equal to whichever Type of action most closely resembles the requirements of the proposed program.
EPA estimates that the addition of several new fee-for-service PRIA categories will increase the annual number of Type B actions by 40 – the Agency expects BPPD to receive 20 actions, AD to receive 10 actions, and RD to receive 10 actions. The Antimicrobial Performance Evaluation Program may impact up to 99 products annually, with an application burden for the registrant of each product. The Agency expects this burden to be similar to a Type B action. The Agency anticipates that the “Interim Process for Evaluating Potential Synergistic Effects of Pesticides During the Registration Process” will not cause a change in the number of product registrations, but will impose an additional Type A burden on registrants equivalent to about 8 active ingredients annually. In total, the Agency anticipates an additional 8 “Type A” activities and 139 “Type B” activities resulting from the three new programs.
The total number of annual responses from the established programs 7,292 (205+7,082+5) and from the three new programs 147 (139 +8) is 7,439.
Table 2: Average Annual Number of Actions by Type and Division, 2015-2017 Average, Plus Anticipated Actions Resulting from Proposed Activities
|
AD |
BPPD |
RD |
All Divisions |
Type A |
11 |
23 |
179 |
213 |
Type B |
2,670 |
663 |
3,888 |
7,221 |
Type C |
0 |
0 |
5 |
5 |
All Action Types |
2,681 |
686 |
4,072 |
7,439 |
*Numbers may not sum due to rounding.
Table 3-A presents estimates for burden hours and costs per “Type A” registration application. Each “Type A” application is estimated to require 26 management hours, 128 technical hours, and 40 clerical hours for a total of 194 hours per application at a cost of $15,230.
Table 3-A: Estimated Burden/Cost per “Type A” Registration Application
Collection
Activities, |
Burden (Hours) |
Total |
|||
Mgmt. |
Technical |
Clerical |
Hours |
Costs |
|
$143.29/hr |
$74.95/hr |
$47.78/hr |
|||
Read Instructions |
18 |
0 |
0 |
18 |
$2,579 |
Plan activities |
4 |
0 |
0 |
4 |
$573 |
Gather/create information |
0 |
120 |
0 |
120 |
$8,994 |
Compile and review |
4 |
8 |
0 |
12 |
$1,173 |
Complete paperwork |
0 |
0 |
30 |
30 |
$1,434 |
Submit information |
|
|
|
|
|
Store/maintain data |
0 |
0 |
10 |
10 |
$478 |
Third party disclosure |
|
|
|
|
|
TOTAL |
26 |
128 |
40 |
194 |
$15,230 |
*Numbers may not sum due to rounding.
Table 3-B presents the total annual burden hours and costs by division for “Type A” registration applications. Registrants spend a total of 2,134 burden hours at a cost of $168 thousand to prepare and submit “Type A” applications to the Antimicrobial Division, 4,462 burden hours at a cost of $350 thousand to prepare and submit “Type A” applications to the Biopesticides and Pollution Prevention Division, and 34,726 hours at a cost of $2.726 million to prepare and submit “Type A” applications to the Registration Division. The total paperwork burden and cost associated with preparing and submitting “Type A” registration applications to EPA is estimated at $3.244 million per year.
Table 3-B: Total Annual Burden and Cost by Division for "Type A" Registration Applications 1
Labor Category 2 |
AD |
BPPD |
RD |
Total |
||||
Hrs. |
Cost |
Hrs. |
Cost |
Hours |
Cost |
Hours |
Cost |
|
Management |
286 |
$40,981 |
598 |
$85,687 |
4,654 |
$666,868 |
5,538 |
$793,536 |
Technical |
1,408 |
$105,525 |
2,944 |
$220,644 |
22,912 |
$1,717,184 |
27,264 |
$2,043,353 |
Clerical |
440 |
$21,025 |
920 |
$43,962 |
7,160 |
$342,140 |
8,520 |
$407,128 |
TOTAL |
2,134 |
$167,531 |
4,462 |
$350,293 |
34,726 |
$2,726,193 |
41,322 |
$3,244,017 |
*Numbers may not sum due to rounding. 1 Hours are calculated by multiplying hours per labor category from Table 3-A by the average number of applications for that division from Table 2. For example, 286 management hours for AD is calculated as 26 management hours per “Type A” application multiplied by 11 “Type A” applications in AD per year. 2 Hours and wages used to calculate the totals for each labor category are from Table 3-A. |
Table 4-A presents estimates for burden hours and costs per “Type B” applications/notifications. Each “Type B” application is estimated to require 8 management hours, 2 technical hours, and 4 clerical hours for a total of 14 hours per application at a cost of $1,487.
Table 4-A: Estimated Burden/Cost per “Type B” Registration Application/Notification
Collection
Activities, |
Burden (Hours) |
Total |
|||
Mgmt. |
Technical |
Clerical |
Hours |
Costs |
|
$143.29/hr |
$74.95/hr |
$47.78/hr |
|||
Read Instructions |
7 |
0 |
0 |
7 |
$1,003 |
Plan activities |
0.5 |
0 |
0 |
0.5 |
$72 |
Gather/create information |
0 |
1.5 |
0 |
1.5 |
$112 |
Compile and review |
0.5 |
0.5 |
0 |
1 |
$109 |
Complete paperwork |
0 |
0 |
3 |
3 |
$143 |
Submit information |
|
|
|
|
|
Store/maintain data |
0 |
0 |
1 |
1 |
$48 |
Third party disclosure |
|
|
|
|
|
TOTAL |
8 |
2 |
4 |
14 |
$1,487 |
*Numbers may not sum due to rounding.
Table 4-B presents the total annual burden hours and costs by division for “Type B” applications/notifications. Registrants spend a total of 37,380 burden hours at a cost of $3.971 million to prepare and submit “Type B” applications to the Antimicrobial Division, 9,282 burden hours at a cost of $986 thousand to prepare and submit “Type B” applications to the Biopesticides and Pollution Prevention Division, and 54,432 hours at a cost of $5.783 million to prepare and submit “Type B” applications to the Registration Division. The total paperwork burden and cost associated with preparing and submitting “Type B” applications to EPA is estimated at $10.74 million per year.
Table 4-B: Total Annual Burden and Cost by Division for "Type B" Registration Applications 1
Labor Category 2 |
AD |
BPPD |
RD |
Total |
||||
Hrs. |
Cost |
Hrs. |
Cost |
Hrs. |
Cost |
Hours |
Cost |
|
Management |
21,360 |
$3,060,659 |
5,304 |
$760,006 |
31,104 |
$4,456,870 |
57,768 |
$8,277,535 |
Technical |
5,340 |
$400,217 |
1,326 |
$99,380 |
7,776 |
$582,787 |
14,442 |
$1,082,384 |
Clerical |
10,680 |
$510,343 |
2,652 |
$126,726 |
15,552 |
$743,152 |
28,884 |
$1,380,221 |
Total |
37,380 |
$3,971,219 |
9,282 |
$986,112 |
54,432 |
$5,782,809 |
101,094 |
$10,740,140 |
*Numbers may not sum due to rounding. 1 Hours are calculated by multiplying hours per labor category from Table 4-A by the average number of applications for that division from Table 2. For example, 20,488 management hours for AD is calculated as 8 management hours per “Type B” application multiplied by 2,561 “Type B” applications in AD per year. 2 Hours and wages used to calculate the totals for each labor category are from Table 4-A. |
Table 5-A presents estimates for burden hours and costs per “Type C” reduced risk application. Each “Type C” application is estimated to require 102 management hours, 448 technical hours, and 96 clerical hours for a total of 646 hours per application at a cost of $53 thousand.
Table 5-A: Estimated Burden/Cost per “Type C” Reduced Risk Registration Application
Collection
Activities, |
Burden (Hours) |
Total |
|||
Mgmt. |
Technical |
Clerical |
Hours |
Costs |
|
$143.29/hr |
$74.95/hr |
$47.78/hr |
|||
Read Instructions |
22 |
0 |
0 |
22 |
$3,152 |
Gather Information |
0 |
368 |
0 |
368 |
$27,580 |
Process, Compile, and Review Info |
80 |
80 |
0 |
160 |
$17,459 |
Record and Report Info |
0 |
0 |
72 |
72 |
$3,441 |
Store, File, and Maintain Info |
0 |
0 |
24 |
24 |
$1,147 |
TOTAL |
102 |
448 |
96 |
646 |
$52,779 |
*Numbers may not sum due to rounding.
Table 5-B presents the total annual burden hours and costs for “Type C” reduced risk applications. Registrants spend a total of 3,230 burden hours at a cost of $264 thousand to prepare and submit “Type C” applications to the Registration Division. The Antimicrobial Division and the Biopesticides and Pollution Prevention Division do not receive “Type C” applications.
|
|||||
Table 5-B: Total Annual Burden and Cost for "Type C" Reduced Risk Registration Applications 1
Labor Category 2 |
RD |
|
Hours |
Cost |
|
Management |
510 |
$73,078 |
Technical |
2,240 |
$167,881 |
Clerical |
480 |
$22,937 |
Total |
3,230 |
$263,895 |
*Numbers may not sum due to rounding. 1 Hours and costs are calculated using the same method as Tables 3-B and 4-B. 2 Hours and wages used to calculate the totals for each labor category are from Table 5-A. |
6(b) Respondent Paperwork Burden Hours and Cost from Data Generation
To calculate the burden and costs associated with the paperwork activities involved in data generation, the Agency starts with the cost of the test, typically the market price for the test as identified by laboratories that offer testing services. The Agency maintains an archive of the basic FIFRA study cost estimates that were developed through surveys of independent testing laboratories, Agency economic analyses, and registrant comments during ICR renewal periods. To the greatest extent possible, EPA uses multiple sources to provide test cost estimates, which are updated as needed.
EPA uses 35% of the estimated total test cost to calculate the total potential cost for the paperwork activities related to data generation. The 35% of test cost is disaggregated by labor category, and then burden hours are extrapolated by using the loaded labor rates. To disaggregate by labor category, the Agency considered the estimated distribution of paperwork activity across the labor categories represented and the existing methodology assumption that paperwork activities for data generation mostly involve the technical staff to perform the tests, with fewer activities related to management and clerical staff.
Figure 1 illustrates the method for calculating the paperwork burden of data generation.
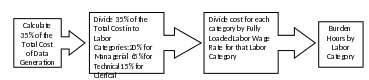
Figure 1: Method for Calculating Paperwork Burden from Test Costs
Similar to the data call-in or DCI ICR (OMB Control No. 2070-0174), this approach assumes and incorporates the following core considerations:
Registrants generate all the data as specified in the CFR without any changes, and none of the data is waived.
All data generation is performed by an independent laboratory.
(3) Paperwork burden is disaggregated by labor category as follows:
a. Managerial (20%)
b. Technical (65%)
c. Clerical (15%)
(4) Labor rates are fully loaded, meaning that they include the estimated costs of wages, overhead, and benefits paid to an employee. See Attachment J.
Using this methodology, Tables 6-A and 6-B below estimate the paperwork burden associated with registering a new AI and a new product for each of the three registering divisions: the Antimicrobial Division (AD), the Biopesticides and Pollution Prevention Division (BPPD), and the Registration Division (RD). The costs of registering a new AI vary depending on use parameters, e.g., food or non-food use, indoor or outdoor use pattern. The data costs used to calculate paperwork burden are an average of total costs across different types of uses that might be registered in that division.
Table 6-A shows that the average cost of data for registering a new AI ranges from an average of $5.1 million for a new biopesticide to an average of $9.85 million for a new conventional chemical. The paperwork burden, 35% of the cost of data generation, ranges from $1.78 million to $3.45 million or between 24 thousand and 46 thousand hours of burden.
Table 6-A: Estimates of Paperwork Burden Hours from Data Generation for New AIs
Division |
Data Cost ($000s) |
Paperwork Cost ($000s) |
Managerial |
Technical |
Clerical |
Total |
||||
Hours |
FTEs |
Hours |
FTEs |
Hours |
FTEs |
Hours |
FTEs |
|||
AD |
$9,382,000 |
$3,284,000 |
4,654 |
2.2 |
28,916 |
13.9 |
10,467 |
5.0 |
44,038 |
21.2 |
BPPD |
$5,097,000 |
$1,784,000 |
2,528 |
1.2 |
15,708 |
7.6 |
5,686 |
2.7 |
23,923 |
11.5 |
RD |
$9,847,000 |
$3,446,000 |
4,883 |
2.3 |
30,343 |
14.6 |
10,984 |
5.3 |
46,210 |
22.2 |
*Numbers may not sum due to rounding.
Table 6-B shows the calculation of average annual burden hours and costs from paperwork from data generation for new AIs. The burden per registration is taken from Table 6-A. Average annual burden hours is calculated from the average annual responses and burden per registration. Average annual costs are calculated from the average annual responses and average paperwork cost from Table 6-A.
Table 6-B: Estimates of Annual Paperwork Burden Hours and Cost from Data Generation for New AIs |
||||
|
Burden per Registration 1 |
Average Annual Responses |
Average Annual Burden Hours |
Average Annual Cost |
Antimicrobial |
44,038 |
3 |
132,113 |
$9,852,000 |
Biochemical |
23,923 |
18 |
430,614 |
$32,112,000 |
Conventional |
46,210 |
6 |
277,260 |
$20,676,000 |
TOTAL |
38,057 |
27 |
839,987 |
$62,640,000 |
*Numbers may not sum due to rounding. 1 Burden per registration for New AIs from Table 1-B is the average of these values.
|
While we expect that the paperwork burden for application under the draft GTA guidance will look like Type A actions, the Agency does not anticipate a paperwork burden from data generation for new active ingredients for the draft GTA guidance. It is expected that the registrants would have this data on hand already due to their synergy-related applications to other U.S. Agencies. Additionally, the Agency also does not anticipate any paperwork burden from data generation for new active ingredients resulting from the other two proposed programs that will be included in future iterations of this ICR.
Table 7-A shows that the cost of data for registering a new product ranges from $113 thousand to $210 thousand. The paperwork burden, 35% of the cost of data generation, ranges from $40 thousand to $74 thousand or between 527 and 973 hours of burden.
Table 7-A: Estimates of Paperwork Burden Hours from Data Generation for New Products
Division |
Data Cost1 |
Paperwork Cost1 |
Managerial |
Technical |
Clerical |
Total |
||||
Hours |
FTEs |
Hours |
FTEs |
Hours |
FTEs |
Hours |
FTEs |
|||
AD |
$113,900 |
$39,900 |
56 |
0.03 |
346 |
0.17 |
125 |
0.06 |
527 |
0.25 |
BPPD |
$126,700 |
$44,300 |
62 |
0.03 |
384 |
0.18 |
139 |
0.07 |
585 |
0.28 |
RD |
$210,700 |
$73,700 |
103 |
0.05 |
639 |
0.31 |
231 |
0.11 |
973 |
0.47 |
*Numbers may not sum due to rounding.
1 Rounded to nearest hundred.
Table 7-B shows the calculation of average annual burden hours and costs from paperwork from data generation for new AIs. The burden per registration is taken from Table 7-A. Average annual burden hours is calculated from the average annual responses and burden per registration. Average annual costs is calculated from the average annual responses and average paperwork cost from Table 7-A.
Table 7-B: Estimates of Annual Paperwork Burden Hours and Cost from Data Generation for New Products |
||||
|
Burden per Registration 1 |
Average Annual Responses |
Average Annual Burden Hours |
Average Annual Cost |
Antimicrobial |
527 |
325 |
171,270 |
$12,967,500 |
Biochemical |
585 |
51 |
29,840 |
$2,259,300 |
Conventional |
973 |
386 |
375,734 |
$28,448,200 |
TOTAL |
695 |
762 |
576,844 |
$43,675,000 |
*Numbers may not sum due to rounding. 1 Burden per registration for New Products from Table 1-B is the average of these values.
|
EPA expects there may be a data generation burden for up to 99 products annually under the “Antimicrobial Performance Evaluation Program” (APEP). APEP has not been finalized, but EPA conservatively estimates the paperwork burden for data generation for APEP may look similar to the burden for an Antimicrobial new product. These 99 future response burdens are included in Table 7-B in the Average Annual Responses column for Antimicrobial products. While the new PRIA fee-for-service categories are expected to have application paperwork burden equal to 40 Type B actions, the Agency does not anticipate any paperwork burden from data generation for new products resulting from these new PRIA categories. The Agency also does not expect any paperwork burden from data generation for new products for the draft GTA guidance.
The costs used to estimate paperwork burden of data generation in this analysis are conservative because they assume that all new active ingredients generate and submit all data required in 40 CFR Part 158 when, in practice, some of the data requirements may be waived based on specific use patterns or label restrictions. Data waivers would make the average cost of data generation (and the resulting paperwork burden) lower for both new active ingredients and new products. Also, all new products in this analysis are assumed to require toxicology data and product chemistry data to register. In cases where products are substantially similar to other products, they can cite other data rather than generating data. Again, this approach returns conservative estimates of paperwork burden for data generation.
6(c) Estimating Agency Burden and Cost
The Agency is using FIFRA Section 3 registration activity data from the Time and Attendance Information System (TAIS) and from PeoplePlus, which archives the Agency’s Full Time Equivalents (FTEs) for most OPP program activities. The projected burden figures use 2015-2017 data, which include burden hours from internal OPP Divisions that provide significant support and analysis for the FIFRA Section 3 registrations including the Registration Division (RD), the Biopesticides and Pollution Prevention Division (BPPD), the Antimicrobial Division (AD), the Health and Effects Division (HED), the Biological and Economic Analysis Division (BEAD), and the Environmental Fate and Effects Division (EFED). Thus, six OPP Divisions work together to complete the activities related to OPP registration actions. The Agency changed the software it uses for recording FTEs starting in 2017. The categories for tracking and recording time for OPP program activities (e.g., FIFRA Section 3 registrations and other OPP activities) do not map exactly between the two software systems (i.e., TAIS and PeoplePlus); however the general operating procedures (see section 5 of this ICR above for more details) for the Section 3 registrations in the divisions have not changed significantly from 2015-2016 and 2017, therefore it is expected that the Agency burden hours will not change significantly due to this change.
The estimated number of Agency FTEs dedicated to Section 3 registration and registration support activities is approximately 24 managerial FTEs, 146 technical FTEs, and 7 clerical FTEs as shown in Table 8 below. The aggregated Agency estimated FTE dedicated to Section 3 activities is 177 and the burden hours are 369,127 (2,080 hours/FTE X 177 FTEs). These numbers are inclusive of Agency burden required for initiating the three proposed programs which will be included in future iterations of this ICR.
To determine Agency costs, the Agency used the Bureau of Labor Statistics estimates of 2018 labor rates for the North American Industry Classification System (NAICS) code for the Federal Executive Branch (NAICS 999100). The managerial labor rate is based on the Standard Occupational Code (SOC) for management occupations; the technical labor rate is based on the SOC for life, physical and social science occupations; and the clerical labor rate is based on the SOC for office and administrative support occupations. The fully loaded hourly mean wage rate estimate is $132.14 for managerial occupations, $87.24 for technical occupations, and $48.83 for clerical occupations. (See Attachment J for wage calculations.)
To calculate the Agency’s estimated annual cost of Section 3 activities, the number of FTE’s allocated to registration activities (Table 8) is multiplied by these fully loaded labor rates and by 2,080 hours per FTE, which is estimated to be about $6.68 million for management; $26.56 million for technical; and $689 thousand for clerical. The total estimated Agency cost is $33.93 million.
Table 8: Distribution of Agency FTEs Supporting FIFRA Section 3 Registration Activities
|
BEAD |
RD |
EFED |
HED |
AD |
BPPD |
Total FTEs |
Hourly wage |
Total Annual Cost1 |
Managerial |
1.8 |
8.7 |
3.7 |
5.3 |
3.2 |
1.6 |
24 |
$132.1358 |
$6,680,257 |
Technical |
8.9 |
49.3 |
14.7 |
28.5 |
20.9 |
24.1 |
146 |
$87.24 |
$26,560,884 |
Clerical |
0.5 |
2.4 |
1.1 |
1.1 |
1.1 |
0.7 |
7 |
$48.83 |
$688,839 |
Total |
11.2 |
60.4 |
19.4 |
34.9 |
25.2 |
26.4 |
177 |
|
$33,929,979 |
*Numbers may not sum due to rounding. 1 Total annual cost is calculated using the number of hours per FTE multiplied by the number of FTEs and the hourly wage. For example, total annual cost of managerial FTES is calculated as 2,080 hours/FTE x 24 FTEs x $132.14. |
Future Agency burden may increase as a result of implementing the three new proposed programs which will be covered by future ICRs.
6(e) Bottom Line Burden Hours and Cost
Table 9 presents the estimates for total annual hours and cost, for both respondents and the Agency, associated with Section 3 activities. The total respondent burden is 1.56 million hours annually at a cost of $120.56 million. The majority of this paperwork burden, 1.42 million hours, is associated with data generation.
Table 9: Estimated Annual Total Hours and Cost
|
ANNUAL TOTAL |
||
Responses |
Hours |
Costs |
|
TOTAL RESPONDENT BURDEN |
8,228 |
1,562,477 |
$120,563,052 |
Application Process |
7,439 |
145,646 |
$14,248,052 |
Annual “Type A” Responses |
213 |
41,322 |
$3,244,017 |
Annual “Type B” Responses |
7,221 |
101,094 |
$10,740,140 |
Annual “Type C” Responses |
5 |
3,230 |
$263,895 |
Data Generation |
789 |
1,416,831 |
$106,315,000 |
New AIs |
27 |
839,987 |
$62,640,000 |
New Products |
762 |
576,844 |
$43,675,000 |
TOTAL AGENCY BURDEN |
|
369,127 |
$33,929,979 |
*Numbers may not sum due to rounding.
6(f) Reasons for Change in Burden
The activities in this ICR increase net respondent burden by 37,624 hours annually over the levels approved in the currently approved collection. While burden per response levels remain unchanged, the number of responses expected in certain categories has shifted as a result of using an updated data set (Section 3 registration actions annually, on average, during the years 2015-2017) to predict future registration application levels. Additionally, in this iteration of the ICR, the Agency calculates the expected annual application burden of three proposed programs that are anticipated to come online in the next three years.
The average annual number of Section 3 registration actions dropped during the years 2015-2017 from 7,478 to 7,292. As a result, baseline registration levels, exclusive of the new programs, were reset at an annual averages of 205 “Type A” activities, 7,082 “Type B” activities, and 5 “Type C” activities.
EPA estimates that the addition of several new fee-for-service PRIA categories will increase the annual number of Type B actions by 40. The Agency does not anticipate any paperwork burden from data generation for new products or for new active ingredients resulting from these new PRIA categories.
The Antimicrobial Performance Evaluation Program (APEP) may impact up to 99 products annually, with an application burden for the registrant of each product. The Agency expects this burden to be similar to a Type B action. Furthermore, there may be a data generation burden for these products annually under this program. EPA conservatively estimates the paperwork burden for data generation under APEP may look similar to the burden for an Antimicrobial new product and has included this burden in the calculations for the Data Generation IC in this ICR.
The Agency anticipates that the “Interim Process for Evaluating Potential Synergistic Effects of Pesticides During the Registration Process” will not cause a change in the number of product registrations, but will impose an additional Type A burden on registrants equivalent to about 8 active ingredients annually. The Agency does not anticipate a paperwork burden from data generation for new active ingredients or new products for the draft GTA guidance.
6(g) Burden Statement
The annual average reporting and recordkeeping burdens for a registration application are estimated to range from 14 hours to 646 hours, depending upon the type of activity. Estimates for the respondent’s application burden for this collection of information average 194 hours per application for “Type A” activities (which include new active ingredients and new uses) and 14 hours per application for “Type B” activities (which include amendments and notifications). The burden estimate for “Type C” reduced risk applications, which are handled only by RD, is an average of 646 hours per response.
The annual average reporting and recordkeeping burdens for data generation associated with registration of new active ingredients and new products are estimated to range from 527 hours to 46,000 hours, depending on the type of activity and division. For new active ingredients, estimates for the respondent’s paperwork burden for data generation average 46,210 hours for a new conventional, 44,038 for a new antimicrobial, and 23,923 for a new biopesticide. For new products, estimates for the respondent’s paperwork burden for data generation average 973 hours for a new conventional product, 527 hours for a new antimicrobial, and 585 hours for a new biopesticide.
Burden is defined in 5 CFR § 1320.3(b). An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a current and valid OMB control number. The OMB control numbers for EPA’s regulations in title 40 of the CFR, after appearing in the Federal Register, are listed in 40 CFR part 9 and included on the related collection instrument or form, if applicable.
6(h) Docket Information
The Agency has established a docket for this ICR under Docket ID No. EPA-HQ-OPP-2019-0644, which is available for online viewing at http://www.regulations.gov or in person viewing at the EPA Docket Center Public Reading Room, EPA West Building, Room 3334, 1301 Constitution Avenue, NW, Washington, DC 20004. The EPA/DC is open from 8:30 a.m. to 4 p.m., Monday through Friday, excluding federal holidays. The docket telephone number is (202) 566-1744.
You may submit comments regarding the Agency's need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden, including the use of automated collection techniques. Submit your comments, referencing Docket ID No. EPA-HQ-OPP-2019-0644 and OMB Control No. 2070-0060, to both EPA and OMB as follows:
To EPA online using http://www.regulations.gov (our preferred method) or by mail to: EPA Docket Center, Environmental Protection Agency, Mail Code 28221T, 1200 Pennsylvania Ave., NW, Washington, DC 20460, and
To OMB via email to [email protected]. Address comments to OMB Desk Officer for EPA.
ATTACHMENTS TO THE SUPPORTING STATEMENT
Attachments to the supporting statement are available in the public docket established for this Information Collection Request (ICR) under the docket identification number EPA-HQ-OPP-2019-0644. These attachments are available for online viewing at www.regulations.gov or otherwise accessed as described in the sections below.
Attachment A: |
7 U.S.C. 136a – Section 3 of FIFRA. Available at US Code website, https://uscode.house.gov/view.xhtml?path=/prelim@title7/chapter6&edition=prelim |
Attachment B: |
7 U.S.C. 136w-8 – Section 33 of FIFRA. Pesticide registration service fees. Available online at US Code website, https://uscode.house.gov/view.xhtml?hl=false&edition=prelim&path=%2Fprelim%40title7%2Fchapter6%2Fsubchapter2&req=granuleid%3AUSC-prelim-title7-section136w-8&num=0&saved=L3ByZWxpbUB0aXRsZTcvY2hhcHRlcjYvc3ViY2hhcHRlcjI%3D%7CZ3JhbnVsZWlkOlVTQy1wcmVsaW0tdGl0bGU3LWNoYXB0ZXI2LXN1YmNoYXB0ZXIy%7C%7C%7C0%7Cfalse%7Cprelim |
Attachment C: |
Forms for Pesticide Registration. Available online at https://www.epa.gov/pesticide-registration/pesticide-registration-manual-blank-forms |
C – 1. EPA Form 8570-1 - Application for Pesticide, Registration/Amendment C – 2. EPA Form No. 8570-4 - Confidential Statement of Formula C – 3. EPA Form No. 8570-27 - Formulator's Exemption Statement C – 4. EPA Form No. 8570-34 - Certification with Respect to Citation of Data C – 5. EPA Form No. 8570-35 - Data Matrix C – 6. EPA Form No. 8570-36 - Summary of the Physical/Chemical Properties C – 7. EPA Form No. 8570-37 - Self-Certification Statement for the Physical/Chemical Properties |
|
Attachment D: |
Pesticide Registration (PR) Notice 97-3 – Guidelines for Expedited Review of Conventional Pesticides under the Reduced-Risk Initiative and for Biological Pesticides. Available at online at http://www.epa.gov/PR_Notices/pr97-3.html |
Attachment E: |
40 CFR part 152 – Pesticide Registration and Classification Procedures. Available online at https://www.ecfr.gov/cgi-bin/text-idx?SID=acde416ef7c416ec92c63b02c82911b8&mc=true&node=pt40.24.152&rgn=div5 |
Attachment F: |
40 CFR part 156 – Labeling Requirements for Pesticides and Devices. Available at https://www.ecfr.gov/cgi-bin/text-idx?SID=acde416ef7c416ec92c63b02c82911b8&mc=true&node=pt40.24.156&rgn=div5 |
Attachment G: |
40 CFR part 158 – Data Requirements For Registration. Available online at thttps://www.ecfr.gov/cgi-bin/text-idx?SID=acde416ef7c416ec92c63b02c82911b8&mc=true&node=pt40.24.158&rgn=div5 |
Attachment H: |
Revised Formulator’s Exemption Statement Form 8570-27 |
Attachment I: |
Proposed New e-CSF |
Attachment J: |
EPA ICR Number 0277.17 Labor Wage Formulas. Work Sheets used to Calculate Pesticide Registrant Industry Labor Costs; Work Sheets used to Calculate EPA and Federal Government Labor Costs |
Attachment K: |
Display Related to OMB Control #2070-0060 – Listings of Related Regulations in 40 CFR § 9.1. Available at https://www.ecfr.gov/cgi-bin/text-idx?SID=acde416ef7c416ec92c63b02c82911b8&mc=true&node=pt40.1.9&rgn=div5 |
Attachment L: |
Consultation Questions |
Attachment M |
Public Comment Received for this ICR |
Attachment N |
Pesticide Submission Portal (PSP): Screen Shots, Instructions and Related Guidance |
1 The Agency anticipates revising existing language in EPA Form 8570-37 within the next three years to better clarify processes for registrants.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | newuser |
| File Modified | 0000-00-00 |
| File Created | 2021-01-13 |
© 2026 OMB.report | Privacy Policy